Abstract
Commensal bacteria are crucial for maturation and function of the mucosal immune system. However, the mechanisms of these interactions are poorly understood. In addition, the role of the composition of the microbiota and the importance of individual species in this community in stimulating different types of immunity are major unanswered questions. We recently demonstrated that the balance between two major effector T cell populations in the intestine, IL-17+ Th17 cells and Foxp3+ Tregs, requires signals from commensal bacteria and is dependent on the composition of the intestinal microbiota. Comparison of microbiota from Th17 cell-deficient and Th17 cell-sufficient mice identified segmented filamentous bacteria (SFB) as capable of specifically inducing Th17 cells in the gut. SFB represent the first example of a commensal species that can skew the mucosal effector T cell balance and thus affect the immune fitness of the individual.
Keywords: Commensal bacteria, intestinal microbiota, SFB, Th17, Taconic B6, intestinal epithelium
The main function of the immune system is to recognize foreign antigens and to initiate responses appropriate for the offending microorganisms. The mucosal immune system, however, faces unique challenges due to the nature of the foreign antigens that it encounters. While pathogenic microorganisms need to be neutralized, they represent only a small fraction of the intestinal antigenic load. Most intestinal antigens consist of food substances and large numbers of resident bacteria, known as commensal microbiota, which must be tolerated because their presence is necessary for the normal function of the organism. Food antigens are crucial for survival and induce oral tolerance. Commensal bacteria, similarly, play an integral part in host metabolism, physiology, and immunity and thus need to be preserved.
The knowledge that microorganisms are present in our bodies is as old as the study of microbiology itself. Approximately 350 years ago Leeuwenhoek visualized the first bacteria by examining samples from his oral cavity and feces noting in his letters to the Royal Society of London that in the scurf of a man’s teeth “there might have been 1000 in a quantity of matter no bigger than 1/100 part of sand”. We now know that resident bacteria in the gut outnumber the cells of the host by two orders of magnitude. Although labeled “commensal”, these bacteria (and other microorganisms) do more than to simply “share our table”.
The gut microbiota are absolutely required for normal host function. Intestinal bacteria provide necessary enzymes for the digestion and fermentation of certain nutrients and non-digestible dietary residues; synthesize important vitamins; metabolize dietary carcinogens; participate in the processing of endogenously derived mucus; help with ion absorption and the salvage of energy; and control epithelial cell proliferation. In addition, commensal bacteria participate in protection against intestinal pathogens. They occupy important ecological niches and compete with ingested pathogens for nutrients; they strengthen the epithelial barrier and occupy epithelial cell adhesion receptors, decreasing the invasive capacity of pathogens; they produce ligands that activate pattern recognition receptors (PRRs), e.g. TLRs, resulting in secretion of host bactericidal substances, such as antimicrobial peptides, and in immuno-stimulatory signals that help recruit B and T cells in the lamina propria, organize secondary lymphoid structures and induce IgA secretion into the lumen; and they secrete molecules, such as lactic acid, that inhibit the growth of competing microorganisms. Thus, the intestinal microbiota represent an integral and vital part of the host organism and are therefore referred to as the “microbial organ”.
The aforementioned functions of the microbial organ could be considered general properties of established microbial communities, which can be performed by a large number of bacterial taxons. However, how the composition of the microbiota affects host metabolic and immune functions has remained relatively unexplored. Pioneering studies from Jeff Gordon’s group demonstrated the importance of the repertoire of commensal taxons in modulating host metabolism and energy uptake by identifying an obesity-associated microbiome1. Changes in the representation of bacterial groups have also been described in inflammatory bowel disease (IBD) patients and in mouse colitis models and there have been efforts to identify an IBD-associated microbiome. These and other studies have led to the hypothesis that shifts in the composition of intestinal communities may contribute to disease and that preventing such changes or antagonizing the relevant mechanisms may lead to new therapies.
Similarly, shifts in the composition of the microbiota may modulate the mucosal immune balance at steady state, thereby strengthening immune responses against intestinal pathogens. How specific commensals fulfill this role has received little attention. Recently, however, the human commensal, Bacteroides fragilis, was shown to induce the maturation of systemic Th1 and intestinal regulatory responses following colonization of germ-free (GF) mice, through the production of unique types of polysaccharide A2,3. The presence of B. fragilis induced IL-10-mediated protection from colitis initiated by Helicobacter hepaticus infection3. Further, we recently discovered that the composition of the intestinal microbiota can profoundly affect the balance between IL-17-producing effector T helper (Th17) cells, which sustain mucosal immune responses, and Foxp3+ regulatory T cells, which downregulate excessive inflammation in the lamina propria4. Commensal bacteria were required for induction of Th17 cells, as these cells were absent in GF animals. At the same time GF mice possessed higher proportions (although not total numbers) of Foxp3+ Tregs than conventionally raised animals. Introduction of wildtype microbiota into GF mice reversed the ratio between Th17 and Treg cells. Most importantly, colonization of GF mice with microbiota isolated from different colonies of conventionally raised mice had different effects on the Th17:Treg balance. Colonization with microbiota from Taconic C57BL/6 (B6) mice induced robust Th17 cell differentiation and decreased Treg percentages. In contrast, colonization with Jackson B6 microbiota led to only a modest increase in the proportion of Th17 cells and did not affect Treg percentages. The effects of the microbiota on Th17 cell induction were independent of the presence of any single known microbe-associated molecular pattern (MAMP) pathway4. In addition to suggesting the existence of a Th17 cell-inducing component of the microbiota, these studies for the first time demonstrated that the nature of the pre-existing mucosal T cell response in the same host can be modulated by the composition of intestinal bacteria. Together, all these results suggest that it may be possible to target the mechanisms of specific commensal-host interactions to modulate mucosal immune responses for therapeutic purposes.
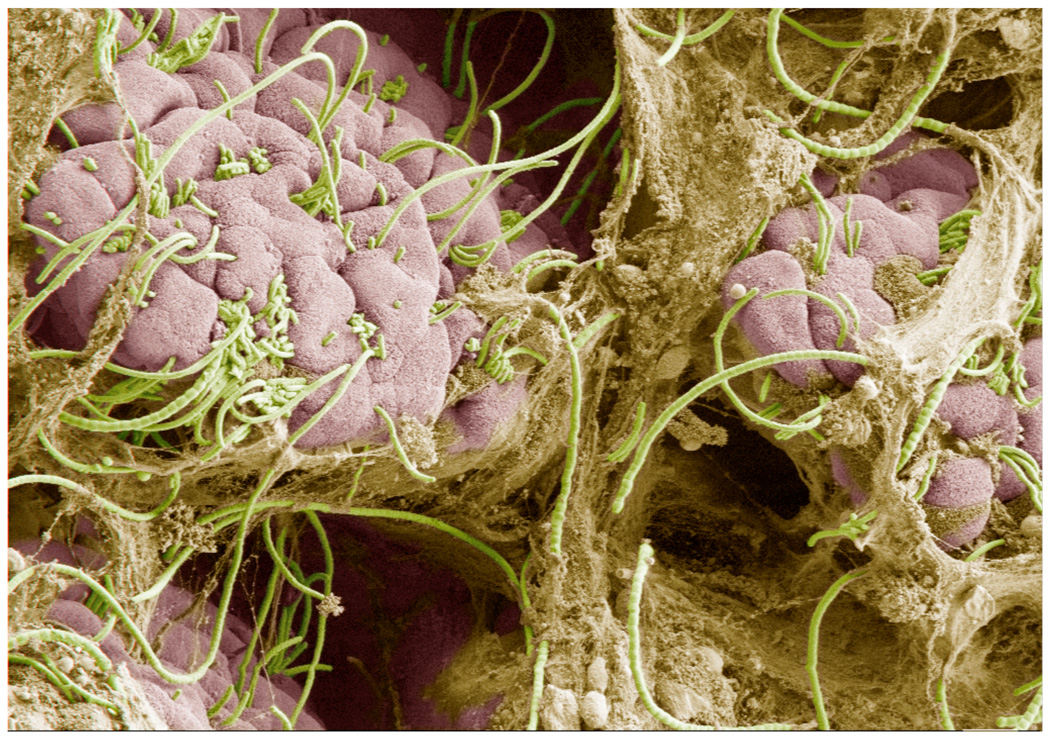
With the goal of identifying such interactions, we compared the composition of microbiota of Taconic and Jackson B6 mice5. We aimed to identify Th17 cell-inducing commensal bacteria and therefore concentrated on bacterial taxons that were overrepresented in the Taconic microbiota. Notably, the differences between the two colonies did not cluster to specific phyla, but were represented as individual taxons across diverse bacterial classes, indicating that in addition to general MAMPs, products of individual bacteria may be important for the function of the microbial organ. One member, segmented filamentous bacteria (SFB), was abundant in Th17 cell-sufficient Taconic B6 mice, but absent in Th17 cell-deficient Jackson B6 mice. SFB were described more than 40 years ago as commensals in a number of mammalian and insect species. They are currently defined by their unique morphology and by recently developed specific DNA probes. The SFB 16S rRNA gene sequence is most closely related to Clostridia, although proper classification awaits the genomic sequence of this organism. SFB have been associated with a number of immunostimulatory functions and appear in the small intestine after weaning. Studies of SFB biology have been hampered by the current absence of ex-vivo culture methods. Nevertheless, SFB can be propagated as a pure culture by monocolonization of GF mice. The most intriguing feature of SFB is their close interaction with epithelial cells in the terminal ileum and their intimate crosstalk with the host immune system6,7. SFB were present only in mice that had Th17 cell-inducing microbiota, including Taconic B6 mice. In contrast, they were not detected in mice that lacked Th17 cells due to absence of Th17 cell-inducing microbiota, such as Jackson B6 mice or Taconic B6 mice treated with Th17 cell-depleting antibiotics5. In Taconic B6 mice, SFB formed a network of segmented filaments in the terminal ileum and attached to the villous epithelium (Figure 1).
Figure 1. Segmented filamentous bacteria (SFB) in the terminal ileum of an 8-week old Taconic B6 mouse.
SFB (green) are members of the commensal microbiota that colonize wildtype mice at the time of weaning. SFB are endemic for the terminal ileum where they form stable interactions with the villous epithelium (pink). SFB form long filaments that often span the length of several villi. When present, SFB specifically induce the differentiation of effector Th17 cells in the lamina propria. Scanning electron micrograph taken by Alice Liang, NYU and Doug Wei, Carl Zeiss Inc.; artificial coloring by Eric Roth, NYU.
To test if SFB are sufficient to induce Th17 cell differentiation we colonized GF mice as well as SFB-deficient Jackson B6 mice with fecal material from SFB-monocolonized gnotobiotic mice. This treatment, but not colonization with a number of other commensal bacteria, including closely related Clostridia, induced IL-17 and IL-22 production by CD4+ T cells. SFB colonization specifically induced Th17 cells as it did not affect the proportion of IFNγ+ Th1 or Foxp3+ Treg cells5. Therefore SFB represent the first example of a commensal bacterium that specifically induces an effector helper T cell population in the intestinal mucosa. The Th17 cell inductive capacity of SFB was confirmed by two other studies7,8. One study, reported a more general immunostimulatory capacity for SFB, illustrated by relative increases in IFNγ+ and CD25+Foxp3+ cells in the intestine8. These discrepancies may be due to the different source of SFB or may suggest a dependence of the SFB effect on the host, as the studies were performed in different mouse strains. At the same time, they may be explained by the increase in the total number of CD4+ T cells in the gut after SFB colonization4,8. Indeed, more careful examination of the data shows that although SFB colonization recruits T cells in the lamina propria, which leads to a general increase in T cell activation markers, there doesn’t seem to be a proportional increase in IFNγ+ or Foxp3+ cells in the CD4+ T cell subset8. Thus, SFB very specifically affect the proportion of Th17 cells and can potentially skew the balance of mucosal effector T cell responses.
What, then, is the functional consequence of the presence of SFB in the commensal microbiota? To address this question we examined the changes in gene expression in the terminal ileum following colonization with SFB. Genes specifically induced by SFB, but not other commensals, were enriched in immune, inflammatory and defense response pathways5. Among the most highly upregulated genes were those encoding a number of epithelial cell-derived anti-microbial peptides (AMPs), such as RegIIIγ. RegIIIγ is a bactericidal C-type lectin that is induced by commensal bacteria and by IL-22 and provides protection in the lumen from intestinal infections9. We therefore hypothesized that colonization with SFB may bolster mucosal immunity through the induction of Th17 cells, IL-22 and AMPs. To directly test this hypothesis, we challenged SFB-colonized and control mice with Citrobacter rodentium, an intestinal pathogen whose clearance requires Th17-related molecules such as IL-23, IL-22 as well as RegIIIγ9. SFB-colonized mice were better protected from infection, as indicated by reduced colon mucosa thickness, as well as a reduced Citrobacter rodentium titer recovery from the colonic wall5. Thus, SFB mediate mucosal protection through the induction of specific host immune mechanisms.
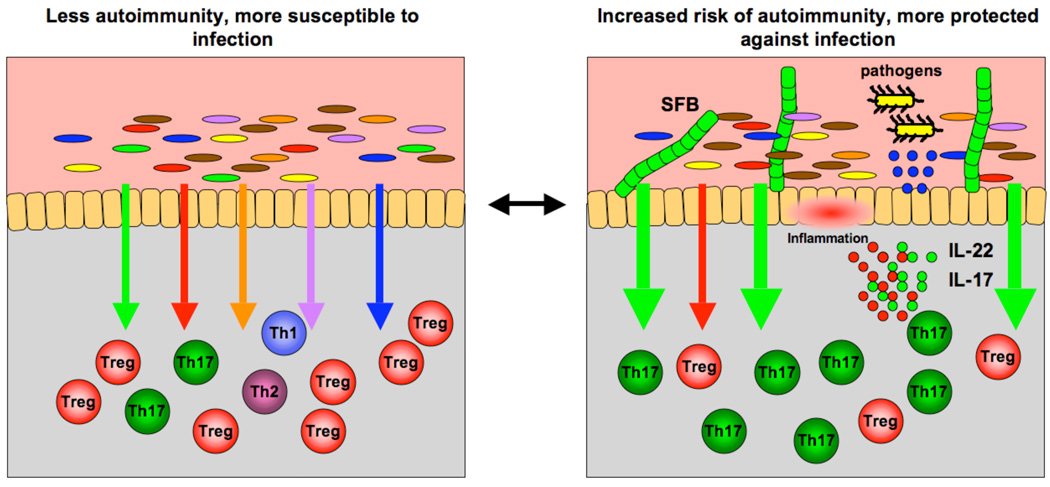
Our results constitute the first evidence that a specific commensal bacterium can induce a defined CD4+ T cell effector population in the gut mucosa. Thus, the composition of the gut microbiota can modulate or predetermine the nature of the immune response of the host and affect protective immunity upon infection. In the case of SFB, this leads to an increase in the relative proportions of Th17 cells and Th17 cell cytokines, which provide protection against mucosal infection. The flipside is the generation of potentially harmful pro-inflammatory cytokines. Therefore SFB-mediated changes may increase the risk of autoimmune inflammation in susceptible hosts (Figure 2).
Figure 2. The composition of intestinal microbiota participates in the regulation of immune homeostasis.
(Left panel) Signals from different components of the microbiota (different color arrows) regulate different branches of the mucosal T cell response in the lamina propria (corresponding color immune cells). (Right panel) Changes in the composition of commensal bacteria, e.g. introduction of segmented filamentous bacteria (SFB), shift the immune homeostasis in a different direction, in this case increase in the signals mediating induction of Th17 cells (green arrows). This changes the immunological fitness of the individual. In the case of SFB, the increased production of Th17 cell effector cytokines, e.g. IL-17 and IL-22, and the consecutive increase in antimicrobial peptide production from epithelial cells (blue circles) augments the ability of the host to fight off intestinal infections. At the same time, this increase in pro-inflammatory cytokines may render the host more susceptible to chronic autoimmune inflammation. In this way, differences in the repertoire of commensal bacteria between individuals of the same species may account for differences in the nature and robustness of their response in the face of similar environmental challenges.
Fine-tuning of intestinal immune responses by the presence or absence of certain members of the microbiota opens the exciting possibility of directionally modulating these responses by either changing the composition of the gut bacteria or modifying relevant signaling pathways to increase resistance to infection or decrease susceptibility to IBD and other inflammatory diseases. Therefore, it will be crucial to elucidate the molecular mechanisms by which commensals, such as SFB, influence mucosal responses.
The specific effect of SFB on the homeostasis of intestinal effector T cell populations is not likely to be an isolated event. On the one hand, other commensals with Th17 cell-inducing capacity may exist. For example, SFB-deficient Jackson microbiota induce low levels of Th17 cells upon transfer into GF animals and the existence of non-SFB ATP-producing microbiota that induce Th17 cells has been reported5,10. On the other hand, other members of the microbiota may skew immune homeostasis in different directions. For example, although colonization of GF mice with total intestinal microbiota both induced Th17 cell differentiation and decreased the proportions of Foxp3+ Tregs, colonization with SFB only affected Th17 cell numbers4,5. Therefore, other microbiota entities may be involved in regulating Treg proportions or function. Identification of more examples of immunomodulatory interactions between individual commensal species and the host, and the underlying mechanisms, promises new and exciting ways to modulate intestinal immune responses for therapeutic purposes.
ACKNOWLEDGMENTS
I.I.I. is supported by Crohn’s and Colitis Foundation of America and D.R.L. by the Howard Hughes Medical Institute and the Helen and Martin Kimmel Center for Biology and Medicine. We also thank MacLean Sellars for critical reading and comments on the manuscript
References
- 1.Turnbaugh PJ, et al. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444(7122):1027–1031. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 2.Mazmanian SK, Liu CH, Tzianabos AO, Kasper DL. An immunomodulatory molecule of symbiotic bacteria directs maturation of the host immune system. Cell. 2005;122(1):107–118. doi: 10.1016/j.cell.2005.05.007. [DOI] [PubMed] [Google Scholar]
- 3.Mazmanian SK, Round JL, Kasper DL. A microbial symbiosis factor prevents intestinal inflammatory disease. Nature. 2008;453(7195):620–625. doi: 10.1038/nature07008. [DOI] [PubMed] [Google Scholar]
- 4.Ivanov II, et al. Specific microbiota direct the differentiation of IL-17-producing T-helper cells in the mucosa of the small intestine. Cell Host Microbe. 2008;4(4):337–349. doi: 10.1016/j.chom.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Ivanov II, et al. Induction of intestinal Th17 cells by segmented filamentous bacteria. Cell. 2009;139(3):485–498. doi: 10.1016/j.cell.2009.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Suzuki K, et al. Aberrant expansion of segmented filamentous bacteria in IgA-deficient gut. Proc Natl Acad Sci U S A. 2004;101(7):1981–1986. doi: 10.1073/pnas.0307317101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Salzman NH, et al. Enteric defensins are essential regulators of intestinal microbial ecology. Nat Immunol. 11(1):76–83. doi: 10.1038/ni.1825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gaboriau-Routhiau V, et al. The key role of segmented filamentous bacteria in the coordinated maturation of gut helper T cell responses. Immunity. 2009;31(4):677–689. doi: 10.1016/j.immuni.2009.08.020. [DOI] [PubMed] [Google Scholar]
- 9.Zheng Y, et al. Interleukin-22 mediates early host defense against attaching and effacing bacterial pathogens. Nat Med. 2008;14(3):282–289. doi: 10.1038/nm1720. [DOI] [PubMed] [Google Scholar]
- 10.Atarashi K, et al. ATP drives lamina propria T(H)17 cell differentiation. Nature. 2008 doi: 10.1038/nature07240. [DOI] [PubMed] [Google Scholar]