Summary
Notable new applications of antibodies for imaging involve genetically extracting the essential molecular recognition properties of an antibody, and in some cases enhancing them by mutation, before protein expression. The classic paradigm of intravenous administration of a labeled antibody to image not only its target but also its metabolism can be improved on. Protocols in which molecular targeting with an engineered unlabeled protein derived from an antibody, followed by capture of a small probe molecule that provides a signal, are being developed to a high level of utility. This is accompanied by new strategies for probe capture such as irreversible binding, incorporation of engineered enzyme active sites, and antibody-ligand systems that generate a signal only upon binding or uptake.
Introduction
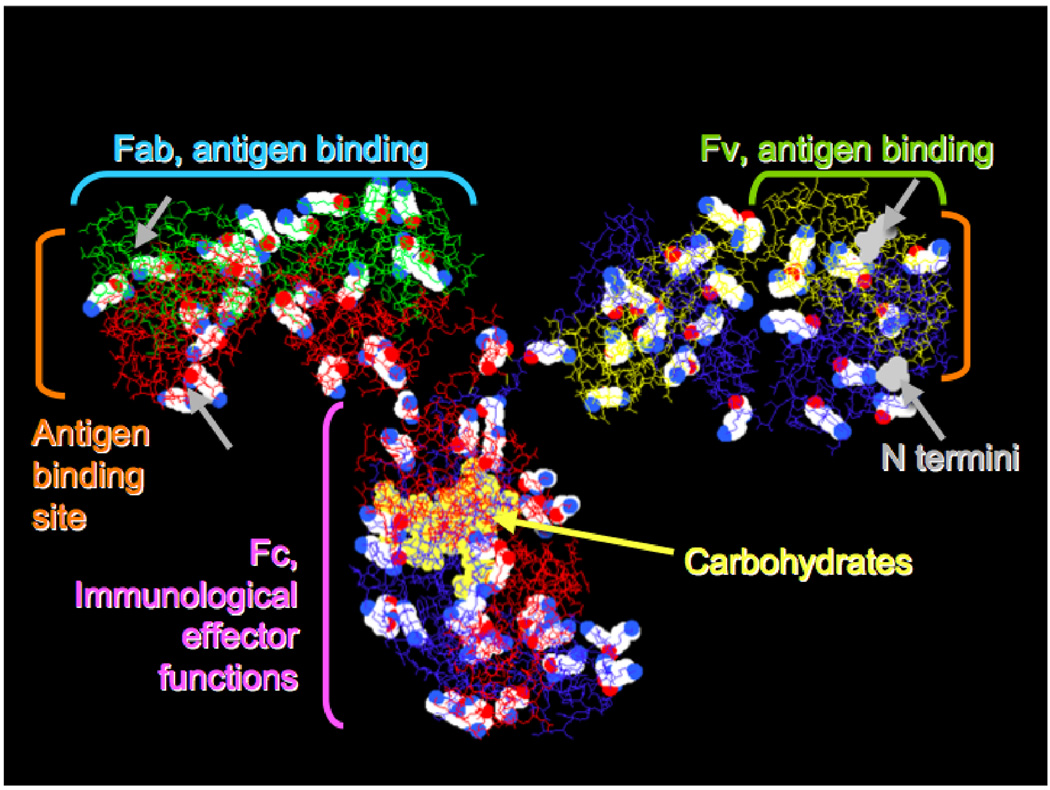
Most of us think of an antibody molecule as an immunoglobulin G (IgG) protein, a Y-shaped macromolecule composed of two identical polypeptide heavy chains (each ≈440 residues) paired with two identical light chains (each ≈214 residues), with an overall molecular weight of ≈150KD (Figure 1). Well-established methods are available to prepare new antibodies that specifically bind to a chosen group of atoms as small as a dinitrophenyl group or as large as a 1,000 Å2 region on another macromolecule.
Figure 1.
An example monoclonal antibody structure (pdb 1IGT, mouse IgG2a), showing 82 lysine residues in cpk spacefill, carbohydrates in yellow spacefill, and N-terminal residues in gray spacefill (visible on the right side only). Heavy chains are red and blue; light chains green and yellow. Important functional regions and fragments are also noted with single brackets.
The organic chemistry of natural antibodies begins with nucleophilic primary amines on lysine side chains, of which there may be 80–90 on the IgG surface. Because most lysines are available for reaction, it is a common strategy to statistically label a small average number of lysines per antibody with the reagent of interest and use the resulting mixture in biological experiments. This practical but untidy procedure can be replaced by site-specific chemistry as discussed below.
Even more nucleophilic than lysine are the N-terminal amines of the four polypeptide chains, but these may be blocked; for example, N-terminal glutamine can eliminate ammonia and form a cyclic amide. IgG molecules contain glycosylation sites at heavy-chain position 297, located well away from the antigen-binding sites; their distinctive chemistry makes these carbohydrates useful attachment sites for enzymes or other macromolecules. IgG molecules also have 16 or more pairs of cysteine residues, practically always occurring in disulfide bonds. Special techniques to selectively reduce some of these disulfides to yield reactive thiols are useful in preparing antibody-drug conjugates [1]. The C-terminal half of each antibody heavy chain (the Fc region), including the carbohydrate, is involved in a variety of interactions important to the behavior of the antibody in vivo [2].
It has become common practice to use molecular biology to improve properties by engineering fragments or analogs of antibodies. This generally preserves the antigen-binding site while decreasing the protein size and deleting other immunologically active sites such as the Fc region. Therefore the papers discussed below only occasionally involve intact IgG molecules. Often the antigen-binding function is expressed from genes coding for the Fv fragment (Figure 1), comprising the N-terminal regions of the heavy and light chains, with additional DNA codons for a peptide linker inserted to form a single gene coding for a single-chain Fv (scFv) protein [3]. A further refinement is pretargeting an engineered protein to a desired site on a cell or tissue, and then using it to capture a small probe molecule [4]. References [5, 6••] describe an important recent example.
Pretargeting for In Vivo Imaging
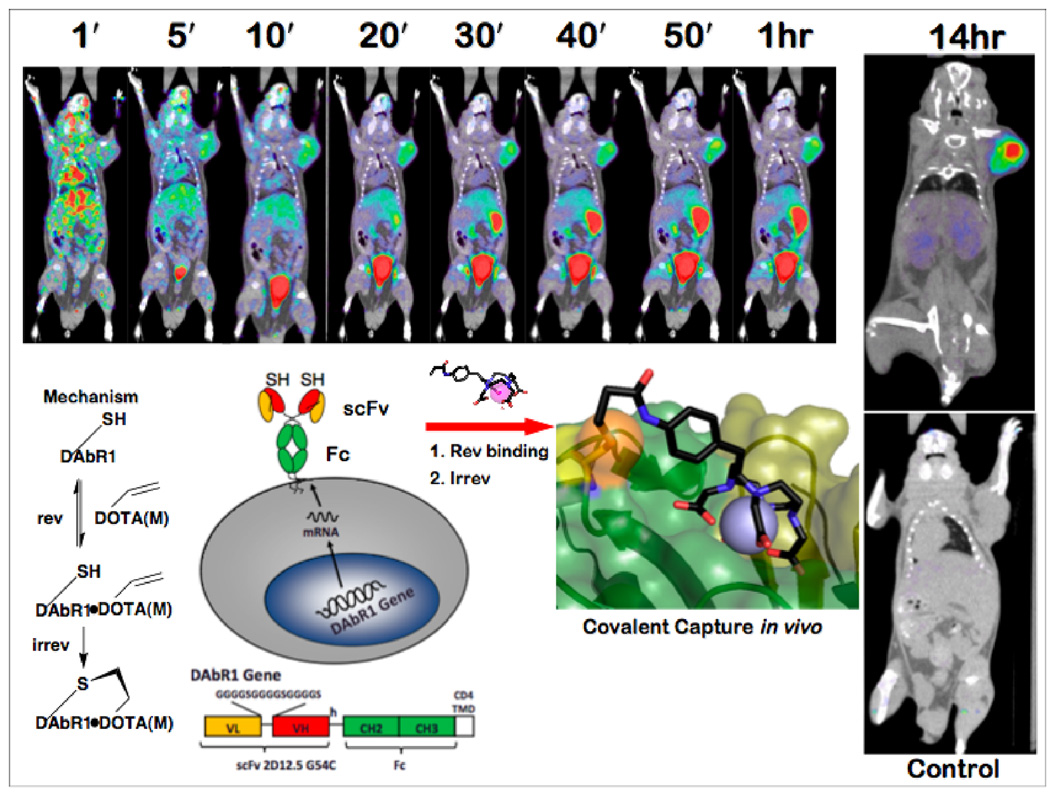
An approach for imaging has been evaluated in animal models, using an antibody-based reporter gene whose receptor product is capable of binding irreversibly to metal chelate reporter probes by Michael addition [7••, 8•]. The reporter gene, named DOTA Antibody Reporter 1 (DAbR1), consists of the scFv fragment of the mutant anti-DOTA(Y) antibody 2D12.5 G54C [9], genetically fused to the hinge region of a human IgG4 Fc fragment and the T-cell CD4 transmembrane domain (Figure 2). Transfected human glioma U-87 tumors, expressing ≈106 DAbR1 sites per cell on their surface, were xenografted into scid mice [7••]. The ability of DAbR1 to capture and bind to the reporter probe ligand acrylamidobenzyl-DOTA(86Y) (AABD(86Y)) was studied using positron emission tomography (PET). The images revealed substantial uptake of AABD(86Y) in DAbR1-expressing tumors versus tumors lacking the DabR1 gene, and low background in non-target tissues.
Figure 2.
Expression of the reporter gene for engineered probe-capture antibody with infinite affinity DAbR1 on the surface of glioma cells leads to excellent images of tumors implanted in scid mice [7••]. Probe binding to DAbR1 followed by attachment of cysteine thiol to the acryloyl group of the probe leads to durable labeling. Serial small-animal PET/CT images from a dynamic scan of mice bearing DAbR1-expressing tumor on the right shoulder show uptake in target tumor, bladder, and small bowel up to 1 hr after injection of 3.7 MBq AABD(86Y) probe in the tail vein. Image after 14 hr shows the tumor is labeled while other organs are clear. Control animal at lower right was treated identically, including 86Y probe injection, except its tumor did not receive the reporter gene. Credit: Wei LH, Olafsen T, Radu C, Hildebrandt IJ, McCoy MR, Phelps ME, Meares CF, Wu AM, Czernin J, Weber WA. Engineered Antibody Fragments with Infinite Affinity as Reporter Genes for PET Imaging. J. Nucl. Med. 2008;49:1828–1835. Copyright Society of Nuclear Medicine. Adapted with permission. PET is positron emission tomography, showing probe distribution in color; CT is computed x-ray tomography, showing anatomy in grayscale.
A recent extension of the Michael addition strategy has led to preparation of an acryloyl-bearing affibody protein that forms a covalent bond with an appropriately positioned cysteine, lysine, or histidine on its specific protein target, even in complex biological mixtures [10]. An affibody is a 58-residue peptide based on Staphylococcus aureus Protein A [11].
A novel bio-orthogonal reaction that employs an inverse-electron-demand Diels-Alder reaction involving cycloaddition of an s-tetrazine probe to a trans-cyclooctene tagged antibody, presents an alternative to the strategy above [12,13]. Antibody CC49 conjugated with trans-cyclooctene ex vivo was administered to nude mice bearing LS174T human colon cancer xenografts, followed 27 hr later by injection with a tetrazine-DOTA(111In) probe. Single-photon tomography images indicate that the covalent adduct was formed at the surface of the tumor, while very high levels of probe are found in the bladder [14]. In contrast to the approach of [7••], the probe was attached by direct reaction with the antibody-bound cyclooctene without affinity capture at a probe-binding site.
18F is commonly used for PET imaging in the form of fluorodeoxyglucose (FDG). Due to the 110 min radioactive half-life of 18F, its applications to imaging are constrained by the time required for the synthesis and purification of 18F-labeled probes. A new method of using 18F has been demonstrated by the chelation of the aluminum fluoride ([Al18F]2+) complex by the macrocyclic chelator 1,4,7-triazacyclononane-1,4,7-triacetic acid (NOTA) [5, 15••]. A NOTA-labeled peptide is able to bind the [Al18F]2+ stably in the presence of human serum. Starting from 18F−, the preparation and purification of the radiolabeled peptide can be accomplished within 1 hr, as opposed to 18FDG protocols which may take several hours [15••]. This NOTA-peptide can also be labeled with 68Ga(III), another radionuclide used for PET imaging [6••].
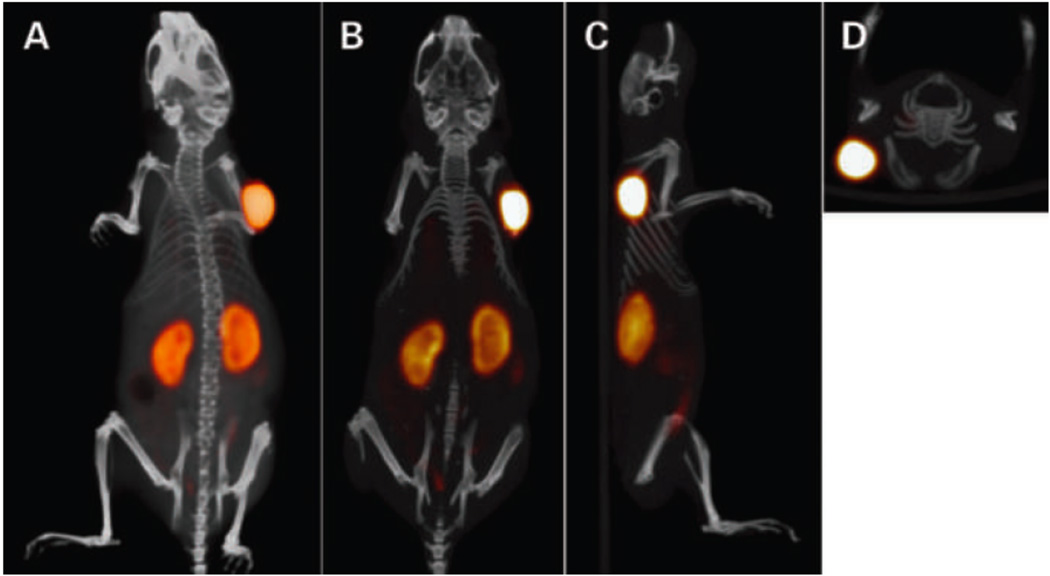
Using the “dock and lock” (DNL) approach, a trivalent bispecific antibody has been constructed to bind to an antigen-bearing tumor and to an [Al18F]2+ labeled peptide-chelate (Figure 3). Previous work describes the dock and lock approach in detail [16]. Pretargeting involves the intravenous administration of the modified antibody, and a later injection of the Al18F-NOTA-peptide. This takes advantage of quick clearance of the excess radiolabeled peptide compared to an antibody [17].
Figure 3.
Static PET/CT imaging study of a BALB/c nude mouse with LS174T tumor (0.1 g) on the right side, which received 6.0 nmol TF2 and 0.25 nmol 18F-IMP-449 (5 MBq) intravenously with a 16-hr interval [6••]. The animal was imaged 1 hr after injection of 18F-IMP-449. The panel shows the three-dimensional volume rendering (posterior view; A) and cross-sections at the tumor region: coronal (B), sagittal (C), and transverse (D). Credit: Schoffelen R, Sharkey RM, Goldenberg DM, Franssen G, McBride WJ, Rossi EA, Chang C-H, Laverman P, Disselhorst JA, Eek A, et al.: Pretargeted Immuno-Positron Emission Tomography Imaging of Carcinoembryonic Antigen-Expressing Tumors with a Bispecific Antibody and a 68Ga- and 18F-Labeled Hapten Peptide in Mice with Human Tumor Xenografts. Molecular Cancer Therapeutics 9:1019–1027. ©2010 American Association for Cancer Research. Adapted with permission.
Optical imaging
A method for imaging cells has recently been developed using antibody-based Fluorogen-Activating Proteins (FAPs) that bind to fluorogenic dye molecules, causing them to fluoresce. Familiar examples of fluorescence activation of dye molecules include DNA binding with the intercalating dyes ethidium bromide or thiazole orange (TO), or RNA binding with malachite green (MG). The mechanism responsible for the activation of the dye is thought to involve structural constraints on the bound fluorophore [18••].
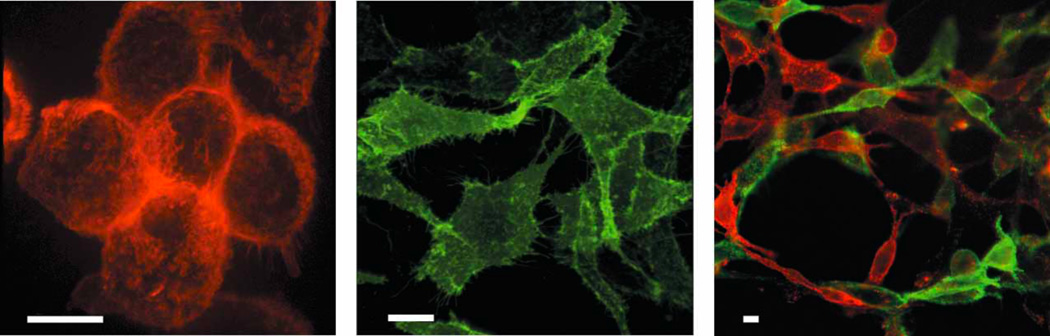
FAPs have been isolated that bind to and activate fluorogens such as MG, TO and dimethyl indole red (DIR). FAPs displayed on yeast or mammalian cell surfaces have been found to bind their respective fluorogens with nanomolar affinity, and increased fluorescence by as much as a thousand-fold (Figure 4). A “promiscuous” FAP that binds with high affinity to several fluorogenic cyanine dyes has also been reported [19]. In some cases, an entire scFv is unnecessary for fluorogen activation, and a heavy or light chain fragment may activate a fluorogen [20].
Figure 4.
(left) Surface labeling of human tumor cells with a malachite green (MG) fluorogen-activating protein (FAP). Stably transformed M21 melanoma cells expressing fluorogen-activating protein HL4-MG fused to platelet-derived growth factor receptor (PDGFR) were imaged as a confocal stack at 488-nm excitation using 10 nM impermeant malachite green derivative MG-11p [18••]. Photomicrograph is a three-dimensional reconstruction of the stack. (center) Surface labeling of fibroblasts with a thiazole orange (TO1) FAP. Stably transformed NIH3T3 cells expressing fluorogen-activating protein HL1.1-TO1 fused to PDGFR and imaged using 40 nM impermeant thiazole orange derivative TO1-2p. (right) Simultaneous surface labeling of fibroblasts with MG and TO1 FAPs. NIH3T3 cells respectively expressing the FAPs of left and center panels were mixed 1:1 and imaged using 10 nM MG-2p and 40 nM TO1-2p. The transparency of surface-labeled cells allows fine discrimination of contact surfaces between cells of different colors. Scale bars, 10 µm. Adapted by permission from Macmillan Publishers Ltd: Nature Biotechnology Vol 26 Issue 2, pp 235–240 (2008), copyright 2007.
The activatable fluorogen, indocyanine green (ICG), has been used in combination with radiolabeling to test a multimodal approach for imaging human epidermal receptors HER1 and HER2 on xenograft tumors in mice. ICG is used for in vivo imaging, with emission in the range of 700–850 nm [21••]. Antibodies have been labeled with 111In and ICG and injected into tumor-bearing mice; comparing the optical and nuclear images may have promise for use with whole-body nuclear imaging followed by optically guided surgery.
Site-Specific Conjugation
The simplest labeling methods for antibodies can yield complex mixtures due to random reactions with subsets of cysteine or lysine residues, which can affect antibody function adversely. The following examples demonstrate recently reported ways of attaching probes while retaining antigen-binding activity. These selective conjugations are flexible, and may be extended to yield antibody molecules with imaging capabilities for in vitro assays and future in vivo studies. The copper-catalyzed azide-alkyne "click" reaction is discussed elsewhere (e.g., [22]).
SNAP-tag [23] and Covalin [24•] are small proteins that react specifically and stoichiometrically with synthetic linkers. A human DNA-repair enzyme O(6)-alkylguanine DNA alkyltransferase (SNAP-Tag) containing a reactive thiol in its binding pocket reacts with its substrate, para-substituted O(6)-benzylguanine (BG). BG can be derivatized with probes such as biotin, fluorophores, reporter proteins, or nanoparticles. Various SNAP-Tag-scFvs have been cloned to image cell surfaces using BG-derivatized probes. Covalin is a SNAP-Tag/Halo-Tag fusion protein for cross-linking orthogonally tagged molecules, small or large. The Halo-tag protein contains a reactive carboxylate in the binding pocket that forms a stable ester linkage with primary chloroalkane groups conjugated to a chosen molecule, orthogonal to the SNAP-tag reaction.
An aldehyde tag peptide sequence, genetically engineered into the Fc region of an IgG [25], facilitates the conversion of a cysteine residue to formylglycine by a formylglycine generating enzyme. The product can react covalently with aminooxy or hydrazide probes. After enzymatic removal of terminal galactose residues, a sugar residue with a functional group C2-keto-Gal (modified ketone on Galactose) can be placed on the N-glycan moiety of the IgG by a galactosyltransferase mutant [26]. An alternative strategy to attach multiple copies of C2-keto-Gal to an engineered antibody is to genetically add several threonine residues to the C terminus [27] and use human polypeptide-alpha-N-acetylgalactosaminyltransferase II to transfer C2-keto-Gal to the side-chain hydroxyl group of threonine.
Genetic insertion of selenocysteine into the C-termini of whole antibodies, Fab fragments [28] or Fc fragments [29] facilitates stoichiometric conjugation to electrophilic moieties. At mildly acidic pH the selenocysteine can be labeled selectively in the presence of free cysteine residues. In another unusual reaction, herceptin has been conjugated via the phenol side chain of tyrosine to an RGD conjugate of a cyclic diazodicarboxamide, 4-phenyl-3H-1,2,4-triazole-3,5(4H)-dione [30•]. This provides an alternative to lysine tagging, and may be useful where essential lysines must be preserved without modification.
A variation on chemically modified antibodies is to covalently attach the Fc fragment to non-antibody receptor ligands. These ligands bind to highly expressed receptors on cancer cells and are designed to direct the Fc to the receptors [31]. Antibody 38C2 produced by reactive immunization of mice with a 1,3-diketone [32] has been chemically programmed by reacting the beta-lactam conjugates of biotin or cyclic RGD peptide [25]. Similarly, a glycosylated IgG1 Fc fragment expressed with N-terminal cysteine residues has been selectively modified with a thioester-containing cyclic RGD peptide through native chemical ligation [33].
Improving Biodistribution of Engineered Antibody Fragments
Among the other functional sites in the Fc region of an IgG molecule is one that binds the FcRn receptor, which is involved in extending the half-life of the antibody in circulation [34]. Proteins that lack binding sites for the FcRn receptor often clear too quickly, before adequate target uptake has been achieved. Improvement of the pharmaceutical properties of recombinant scFv-F8 antibody fragments by extension of the serum half-life has recently been accomplished using site-specific conjugation of "Albu tag", a small organic molecule — 2-(3-maleimidopropanamido)-6-(4-(4-iodophenyl)butanamido)hexanoate — designed to bind reversibly to the abundant, long-lived protein serum albumin [35••, 36]. Comparison of Albu-tagged and unmodified proteins in animal models shows that antibody fragments conjugated with Albu tag exhibit an ≈10-fold increase in tumor uptake and an ≈35-fold reduction in blood clearance rate. Cloning an albumin-binding peptide to an antibody fragment can also dramatically improve biodistribution properties, as shown earlier by Dennis et al. [37,38]. Aspects of this strategy have been compared to conjugation with polyethylene glycol (PEG) [39–41]. A PEGylated scFv occupies a large volume and may exhibit decreased binding affinity, while a tagged scFv complexed with albumin forms a reservoir with which free scFv is in equilibrium.
Acknowledgements
Work in the Meares lab described here has been supported by research grants CA016861 and CA136639 from the National Cancer Institute, National Institutes of Health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Jeffrey J. Day, Email: jefday@ucdavis.edu.
Bernadette V. Marquez, Email: bmarquez@ucdavis.edu.
Heather E. Beck, Email: hbeck@ucdavis.edu.
Tolulope A. Aweda, Email: taaweda@ucdavis.edu.
Prasad D. Gawande, Email: pdgwande@ucdavis.edu.
References and recommended reading
Papers of particular interest, published within the annual period of review, have been highlighted as:
• of special interest
•• of outstanding interest
- 1.Senter PD. Potent antibody drug conjugates for cancer therapy. Curr Op Chem Biol. 2009;13:235–244. doi: 10.1016/j.cbpa.2009.03.023. [DOI] [PubMed] [Google Scholar]
- 2.Jefferis R, Lund J, Pound J. IgG-Fc-mediated effector functions: molecular definition of interaction sites for effector ligands and the role of glycosylation. Immunological Rev. 1998;163:59–76. doi: 10.1111/j.1600-065x.1998.tb01188.x. [DOI] [PubMed] [Google Scholar]
- 3.Olafsen T, Sirk SJ, Betting DJ, Kenanova V, Bauer KB, Ladno W, Raubitschek A, Timmerman JM, Wu AM. ImmunoPET imaging of B-cell lymphoma using 124I-anti-CD20 scFv dimers (diabodies) Protein Eng Des Selec. 2010;23:243–249. doi: 10.1093/protein/gzp081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Walter R, Press OW, Pagel JM. Pretargeted Radioimmunotherapy for Hematologic and Other Malignancies. Cancer Biotherapy Radiopharm. 2010;25:125–142. doi: 10.1089/cbr.2010.0759. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.McBride WJ, D'Souza CA, Sharkey RM, Karacay H, Rossi EA, Chang C-H, Goldenberg DM. Improved 18F Labeling of Peptides with a Fluoride-Aluminum-Chelate Complex. Bioconjugate Chem. 2010;21 doi: 10.1021/bc100137x. web June 14, 2010 DOI 2010.1021/bc100137x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Schoffelen R, Sharkey RM, Goldenberg DM, Franssen G, McBride WJ, Rossi EA, Chang C-H, Laverman P, Disselhorst JA, Eek A, et al. Pretargeted Immuno-Positron Emission Tomography Imaging of Carcinoembryonic Antigen-Expressing Tumors with a Bispecific Antibody and a 68Ga- and 18F-Labeled Hapten Peptide in Mice with Human Tumor Xenografts. Molec Cancer Therap. 2010;9:1019–1027. doi: 10.1158/1535-7163.MCT-09-0862. •• Compared chelated Al-18F to 18FDG for PET imaging, demonstrating higher specificity to tumors.
- 7. Wei L, Olafsen T, Radu C, Hildebrandt IJ, McCoy MR, Phelps ME, Meares CF, Wu AM, Czernin J, Weber WA. Engineered Antibody Fragments with Infinite Affinity as Reporter Genes for PET Imaging. J Nucl Med. 2008;49:1828–1835. doi: 10.2967/jnumed.108.054452. •• Demonstration of the importance of irreversible probe capture for targeted imaging.
- 8. Aweda T, Beck HE, Wu AM, Wei LH, Weber WA, Meares CF. Rates and Equilibria for Probe Capture by an Antibody with Infinite Affinity. Bioconjugate Chem. 2010;21:784–791. doi: 10.1021/bc100046p. • Quantitative model for the system in [7], based on in vitro calorimetry, luminescence, and in vivo biodistribution experiments.
- 9.Corneillie T, Lee KC, Whetstone PA, Wong JP, Meares CF. Irreversible engineering of the multielement-binding antibody 2D12.5 and its complementary ligands. Bioconjugate Chem. 2004;15:1392–1402. doi: 10.1021/bc049824m. [DOI] [PubMed] [Google Scholar]
- 10.Holm L, Moody P, Howarth M. Electrophilic Affibodies Forming Covalent Bonds to Protein Targets. J Biol Chem. 2009;284:32906–32913. doi: 10.1074/jbc.M109.034322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nord K, Nilsson J, Nilsson B, Uhlen M, Nygren PA. A combinatorial library of an alpha-helical bacterial receptor domain. Protein Eng. 1995;8:601–608. doi: 10.1093/protein/8.6.601. [DOI] [PubMed] [Google Scholar]
- 12.Blackman M, Royzen M, Fox JM. Tetrazine Ligation: Fast Bioconjugation Based on Inverse-Electron-Demand Diels-Alder Reactivity. J Amer Chem Soc. 2008;130:13518–13519. doi: 10.1021/ja8053805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Devaraj N, Upadhyay R, Haun JB, Hilderbrand SA, Weissleder R. Fast and Sensitive Pretargeted Labeling of Cancer Cells through a Tetrazine/trans-Cyclooctene Cycloaddition. Angew Chem Intl Ed. 2009;28:7013–7016. doi: 10.1002/anie.200903233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Rossin R, Verkerk PR, van den Bosch SM, Vulders RCM, Verel I, Lub J, Robillard MS. In Vivo Chemistry for Pretargeted Tumor Imaging in Live Mice. Angew Chem Intl Ed. 2010;49:3375–3378. doi: 10.1002/anie.200906294. [DOI] [PubMed] [Google Scholar]
- 15. McBride WJ, Sharkey RM, Karacay H, D'Souza CA, Rossi EA, Laverman P, Chang C-H, Boerman OC, Goldenberg DM. A Novel Method of 18F Radiolabeling for PET. J Nucl Med. 2009;50:991–998. doi: 10.2967/jnumed.108.060418. •• New approach to radiolabeling: an Al-18F complex.
- 16.Goldenberg DM, Rossi EA, Sharkey RM, McBride WJ, Chang C-H. Multifunctional Antibodies by the Dock-and-Lock Method for Improved Cancer Imaging and Therapy by Pretargeting. J Nucl Med. 2008;49:158–163. doi: 10.2967/jnumed.107.046185. [DOI] [PubMed] [Google Scholar]
- 17.Goldenberg DM, Sharkey RM, Paganelli G, Barbet J, Chatal J-F. Antibody Pretargeting Advances Cancer Radioimmunodetection and Radioimmunotherapy. J Clin Oncol. 2006;24:823–834. doi: 10.1200/JCO.2005.03.8471. [DOI] [PubMed] [Google Scholar]
- 18. Szent-Gyorgyi C, Schmidt BF, Creeger Y, Fisher GW, Zakel KL, Adler S, Fitzpatrick JAJ, Woolford CA, Yan Q, Vasilev KV, et al. Fluorogen-activating single-chain antibodies for imaging cell surface proteins. Nat Biotech. 2008;26:235–240. doi: 10.1038/nbt1368. •• Demonstration of cell-surface fusion proteins that cause specific cell-impermeant small molecules to fluoresce.
- 19.Özhalici-Ünal H, Pow CL, Marks SA, Jesper LD, Silva GL, Shank NI, Jones EW, Burnette JM, Berget PB, Armitage BA. A Rainbow of Fluoromodules: A Promiscuous scFv Protein Binds to and Activates a Diverse Set of Fluorogenic Cyanine Dyes. J Amer Chem Soc. 2008;130:12620–12621. doi: 10.1021/ja805042p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Falco CN, Dykstra KM, Yates BP, Berget PB. scFv-based fluorogen activating proteins and variable domain inhibitors as fluorescent biosensor platforms. Biotechnol J. 2009;4:1328–1336. doi: 10.1002/biot.200900075. [DOI] [PubMed] [Google Scholar]
- 21. Ogawa M, Regino CAS, Seidel J, Green MV, Xi W, Williams M, Kosaka N, Choyke PL, Kobayashi H. Dual-Modality Molecular Imaging Using Antibodies Labeled with Activatable Fluorescence and a Radionuclide for Specific and Quantitative Targeted Cancer Detection. Bioconjugate Chem. 2009;20:2177–2184. doi: 10.1021/bc900362k. •• Probes with potential for combined nuclear whole-body imaging and optically guided surgery.
- 22.van Dijk M, Rijkers DTS, Liskamp RMJ, van Nostrum CF, Hennink WE. Synthesis and Applications of Biomedical and Pharmaceutical Polymers via Click Chemistry Methodologies. Bioconjugate Chem. 2009;20:2001–2016. doi: 10.1021/bc900087a. [DOI] [PubMed] [Google Scholar]
- 23.Kampmeier F, Ribbert M, Nachreiner T, Dembski S, Beaufils F, Brecht A, Barth S. Site-Specific, Covalent labeling of Recombinant Antibody Fragments via Fusion to an Engineered Version of 6-O-Alkylguanine DNA Alkyltransferase. Bioconjugate Chem. 2009;20:1010–1015. doi: 10.1021/bc9000257. [DOI] [PubMed] [Google Scholar]
- 24. Chidley C, Mosiewicz K, Johnsson K. A Designed Protein for the Specific and Covalent Heteroconjugation of Biomolecules. Bioconjugate Chem. 2008;19:1753–1756. doi: 10.1021/bc800268j. • Covalin can be used as a highly specific cross-linker to link orthogonally tagged molecules, small or large.
- 25.Gavrilyuk JI, Wuellner U, Barbas CF., 3rd Beta-Lactam-based approach for the chemical programming of aldolase antibody 38C2. Bioorg Med Chem Lett. 2009;19:1421–1424. doi: 10.1016/j.bmcl.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Boeggeman E, Ramakrishnan B, Pasek M, Manzoni M, Puri A, Loomis KH, Waybright TJ, Qasba PK. Site specific conjugation of fluoroprobes to the remodeled Fc N-glycans of monoclonal antibodies using mutant glycosyltransferases: application for cell surface antigen detection. Bioconjugate Chem. 2009;20:1228–1236. doi: 10.1021/bc900103p. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Ramakrishnan B, Boeggeman E, Manzoni M, Zhu Z, Loomis K, Puri A, Dimitrov DS, Qasba PK. Multiple Site-Specific in Vitro Labeling of Single-Chain Antibody. Bioconjugate Chem. 2009;20:1383–1389. doi: 10.1021/bc900149r. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hofer T, Skeffington LR, Chapman CM, Rader C. Molecularly defined antibody conjugation through a selenocysteine interface. Biochemistry. 2009;48:12047–12057. doi: 10.1021/bi901744t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hofer T, Thomas JD, Burke TR, Jr, Rader C. An engineered selenocysteine defines a unique class of antibody derivatives. Proc Natl Acad Sci USA. 2008;105:12451–12456. doi: 10.1073/pnas.0800800105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Ban H, Gavrilyuk J, Barbas CF., 3rd Tyrosine bioconjugation through aqueous ene-type reactions: a click-like reaction for tyrosine. J Amer Chem Soc. 2010;132:1523–1525. doi: 10.1021/ja909062q. • An electrophilic reagent that tags tyrosine in proteins, apparently without interference from lysine.
- 31.Meares CF. Targeted instant immunity. Nat Biotech. 2009;27:452–453. doi: 10.1038/nbt0509-452. [DOI] [PubMed] [Google Scholar]
- 32.Popkov M, Gonzalez B, Sinha SC, Barbas CF. Instant immunity through chemically programmable vaccination and covalent self-assembly. Proc Natl Acad Sci USA. 2009;106:4378–4383. doi: 10.1073/pnas.0900147106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Xiao J, Chen R, Pawlicki MA, Tolbert TJ. Targeting a Homogeneously Glycosylated Antibody Fc To Bind Cancer Cells Using a Synthetic Receptor Ligand. J Amer Chem Soc. 2009;131:13616–13618. doi: 10.1021/ja9045179. [DOI] [PubMed] [Google Scholar]
- 34.Ghetie V, Ward ES. Multiple roles for the major histocompatibility complex class I-related receptor FcRn. Annu Rev Immunol. 2000;18:739–766. doi: 10.1146/annurev.immunol.18.1.739. [DOI] [PubMed] [Google Scholar]
- 35. Trüssel S, Dumelin C, Frey K, Villa A, Buller F, Neri D. New Strategy for the Extension of the Serum Half-Life of Antibody Fragments. Bioconjugate Chem. 2009;20:2286–2292. doi: 10.1021/bc9002772. •• "Albu tag" greatly improved the biodistribution of engineered antibody fragments.
- 36.Dumelin CE, Trüssel S, Buller F, Trachsel E, Bootz F, Zhang Y, Mannocci L, Beck SC, Drumea-Mirancea M, Seeliger MW, Baltes C, Muggler T, Kranz F, Rudin M, Melkko S, Scheuermann J, Neri D. A portable albumin binder from a DNA-encoded chemical library. Angew Chem Intl Ed. 2008;47:3196–3201. doi: 10.1002/anie.200704936. [DOI] [PubMed] [Google Scholar]
- 37.Dennis M, Zhang M, Meng YG, Kadkhodayan M, Kirchhofer D, Combs D, Damico LA. Albumin binding as a general strategy for improving the pharmacokinetics of proteins. J Biol Chem. 2002;277:35035–35043. doi: 10.1074/jbc.M205854200. [DOI] [PubMed] [Google Scholar]
- 38.Dennis M, Jin H, Dugger D, Yang R, McFarland L, Ogasawara A, Williams S, Cole MJ, Ross S, Schwall R. Imaging tumors with an albumin-binding Fab, a novel tumor-targeting agent. Cancer Res. 2007;67:254–261. doi: 10.1158/0008-5472.CAN-06-2531. [DOI] [PubMed] [Google Scholar]
- 39.Constantinou A, Chen C, Deonarain MP. Modulating the pharmacokinetics of therapeutic antibodies. Biotechnol Lett. 2010;32:609–622. doi: 10.1007/s10529-010-0214-z. [DOI] [PubMed] [Google Scholar]
- 40.Stork R, Campigna E, Robert B, Müller D, Kontermann RE. Biodistribution of a Bispecific Single-chain Diabody and Its Half-life Extended Derivatives. J Biol Chem. 2009;284:25612–25619. doi: 10.1074/jbc.M109.027078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Thurber GM, Schmidt MM, Wittrup KD. Antibody tumor penetration: Transport opposed by systemic and antigen-mediated clearance. Adv Drug Deliv Rev. 2008;60:1421–1434. doi: 10.1016/j.addr.2008.04.012. [DOI] [PMC free article] [PubMed] [Google Scholar]