Abstract
Decreased cognitive performance reduces independence and quality of life for aging individuals. Healthy brain aging does not involve significant neuronal loss, but little is known about the effects of aging at synaptic terminals. Age-related cognitive decline likely reflects the manifestation of dysregulated synaptic function and ineffective neurotransmission. In this study, hippocampal synaptosomes were enriched from Young-adult (3 months), Adult (12 months), and Aged (26 months) Fischer 344 × Brown Norway rats, and quantitative alterations in the synaptoproteome were examined by 2-DIGE and MS/MS. Bioinformatic analysis of differentially expressed proteins identified a significant effect of aging on a network of neurotransmission-regulating proteins. Specifically, altered expression of DNM1, HPCA, PSD95, SNAP25, STX1, SYN1, SYN2, SYP, and VAMP2 was confirmed by immunoblotting. 14-3-3 isoforms identified in the proteomic analysis were also confirmed due to their implication in the regulation of the synaptic vesicle cycle and neurotransmission modulation. The findings of this study demonstrate a coordinated downregulation of neurotransmission-regulating proteins that suggests an age-based deterioration of hippocampal neurotransmission occurring between adulthood and advanced age. Altered synaptic protein expression may decrease stimulus-induced neurotransmission and vesicle replenishment during prolonged or intense stimulation, which are necessary for learning and the formation and perseverance of memory.
Keywords: aging, proteomic, hippocampus, synapse, SNARE, neurotransmission
Introduction
Even in the absence of overt disease, increasing age is associated with decreasing cognitive function of varying severity in humans, significantly affecting as much as 60% of the aged population. In otherwise healthy humans, age-related declines in cognitive capabilities represent a detriment to the health-span, diminishing independence and quality of life and imparting a costly burden to society and to the health care system. Despite advances in understanding both age-related neurodegenerative diseases and nonpathological age-related changes in the brain, little is known about the etiology of age-related cognitive decline. As a result, development of targeted therapeutics designed to prevent or reverse loss of cognitive function in aging individuals has proven difficult, and currently, few effective treatments for age-related cognitive impairment exist.
In light of the fact that healthy brain aging does not include significant neurodegeneration (Rapp and Gallagher 1996;Rasmussen et al. 1996), diminished cognitive function with increased age is likely a manifestation of dysregulated synaptic function and ineffective neurotransmission. A growing literature details age-related perturbations in hippocampal activity coincident with deficits of learning and memory in healthy humans (Beeri et al. 2009;Daselaar et al. 2006;Dennis et al. 2008) that likely reflect dysregulated neurotransmission and neuromodulation. Similarly, rodent models of healthy aging demonstrate strong correlations between impaired performance of aged rats on behavioral tests of hippocampus-dependent learning and memory and aberrant hippocampal neuron ensemble activity. These age-related deficits include delayed formation and decreased stability of cognitive spatial field maps (Barnes et al. 1997;Wilson et al. 2004), rigidity of existing ensemble activity patterns that fail to encode novel information (Wilson et al. 2003), and altered ensemble reactivation of temporal sequence patterns following learning trials (Gerrard et al. 2008). Ex vivo electrophysiological studies provide additional evidence of age-related disturbances in hippocampal plasticity involving multiple neuron types and pathways [e.g., long-term potentiation (LTP), long-term depression (LTD), paired-pulse facilitation (PPF)]. For example, aged mice exhibit decreased basal synaptic transmission and PPF in perforant pathway-granule cell synapses (Gureviciene et al. 2009). Further, LTP is more difficult to establish in aged rats than in young or adult rats (Norris et al. 1996), and occurs via alternate mechanisms (Boric et al. 2008), persists for shorter durations (Sierra-Mercado et al. 2008), and is prone to reversal (Norris et al. 1998). Hippocampal LTD and depotentiation are more easily facilitated in aged animals than in their younger counterparts (Foster 2007;Norris et al. 1996;Rosenzweig and Barnes 2003). These changes suggest an age-related decline in neuronal function and synaptic efficacy that likely plays a critical role in cognitive impairment.
The mechanisms underlying decreases in neural processing as the brain ages remain to be fully determined, but likely include changes at the molecular, cellular, and/or structural levels. In animal models of aging, various aspects of dendritic and synaptic morphology, including perforated postsynaptic densities and multiple spine bouton complexes, undergo atrophy (Adams et al. 2008;Brunso-Bechtold et al. 2000;Shi et al. 2005;Sonntag et al. 1997), and synapse-to-neuron ratios decrease in distinct hippocampal regions (Shi et al. 2007), suggesting a decline in synaptic integrity. Previous proteomic reports reveal regional dysregulation of multiple regulatory processes including metabolism, glutamate processing and protein synthesis (Poon et al. 2006b), protein folding and accumulation (Paz Gavilan et al. 2006), and cytoprotection (Calabrese et al. 2004). Additional reports have examined mitochondrial function, oxidative stress, and proteolysis (Poon et al. 2006b) with aging, while proteomic studies have addressed hippocampal protein expression and posttranslational modification (Butterfield et al. 2006;Poon et al. 2006a;Weinreb et al. 2007). Similarly, there are numerous changes in hippocampal expression of specific mRNAs with increasing age (Blalock et al. 2003;Kumar et al. 2007;Rowe et al. 2007). We have previously reported alterations in the unfractionated hippocampal proteome both related to general aging and specific to age-related cognitive decline, suggesting abnormalities in hippocampal glycolysis/gluconeogenesis and protein processing (Freeman et al. 2009). Together, these reports demonstrate a number of important hippocampal gene and protein expression changes with increasing age, but are only partially informative of potential age-related changes in the subcellular environment of synaptic terminals, which likely contribute to synaptic dysfunction and impaired cognitive capabilities.
The aim of this work was to specifically target the hippocampal synaptic proteome to profile changes in hippocampal synaptic protein composition with increasing age across three timepoints spanning young-adulthood to old age. This work demonstrates a coordinated age-related downregulation of neurotransmission-regulating proteins that could impair synaptic exocytosis/endocytosis machinery, and also demonstrates the importance of subcellular fractionation in proteomic investigations of low-abundance or compartment-specific proteins.
Materials and Methods
Animals
Thirty, pathogen-free Fischer 344 × Brown Norway (F1) hybrid male rats (Young-adult: 3 months; Adult: 12 months; Aged: 26 months; n=10 per group) were obtained from the National Institute on Aging colony at Harlan Industries (Indianapolis, IN). Animals were singly housed, to eliminate potential variability in social/environmental interactions, in laminar flow cages (Polysulfone) in the OUHSC Barrier Facility with free access to food (Purina Mills, Richmond, IN) and water. The animal rooms were kept at a constant temperature and humidity, and maintained on a 12 hour light/dark cycle. The animal facilities at OUHSC are fully accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, and all animal procedures were approved by the Institutional Animal Care and Use Committee in accordance with the Public Health Service Policy on Humane Care and Use of Laboratory Animals and the national Research Council’s Guide for the Care and Use of Laboratory Animals. Animals were sacrificed by decapitation without anesthesia, and the hippocampi rapidly dissected for synaptosome enrichment.
Hippocampal synaptosome enrichment
Synaptosomes were enriched from rat hippocampi by a procedure adapted from previously published methods (VanGuilder et al. 2008). Immediately following sacrifice, individual hippocampi from each animal were rapidly dissected into ice-cold sucrose buffer (320mM sucrose, 4mM HEPES, 1mM Na3VO4, pH 7.4). Samples were incubated on ice for 30 min, with buffer replaced three times at 10 minute intervals, prior to homogenization using a mechanically-driven dounce. First, whole homogenates were centrifuged to pellet the nuclear/cytoskeletal fraction (4°C, 1000 × g, 12 min). The resulting supernatants were then centrifuged to pellet the synaptosomal fraction (4°C, 25,000 × g, 16 min). Synaptosome samples were resuspended in a detergent-based protein lysis buffer (100mM NaCl, 20mM HEPES, 1mM EDTA, 1mM dithiothreitol, 1.0% Tween20, 1mM Na3VO4, 1 Complete Mini EDTA-free Protease Inhibitor Cocktail Tablet for every 10mL lysis buffer) and stored at −80°C for subsequent experimentation. To examine the quality of hippocampal synaptosomes, two synaptosome samples each from Young-adult, Adult, and Aged rats were examined by electron microscopy as described in the Supporting Information available online.
2-DIGE
Eight animals each from the Young-adult, Adult, and Aged groups were analyzed using a quantitative 2-DIGE protocol similar to that previously reported (Freeman et al. 2009). For additional details on the 2-DIGE labeling, electophoresis, and image analysis methods please see the Supporting Information available online.
Quantitation of each protein spot represents the ratio of background-subtracted sample-specific signal (Cy3 or Cy5) to the normalization pool signal (Cy2), allowing between-gel comparison. Only protein spots matched across >67% of spot maps were included in data analysis. One-way ANOVA (p <0.05) was used to determine protein spots significantly regulated between the three age groups. To minimize inclusion of false-positive protein spot changes, protein expression data were filtered by the following criteria (Allison et al. 2006): one-way ANOVA (p <0.05; significant within entire experiment), Student’s unpaired t-test post-hoc (p <0.05; pair-wise comparison between groups), and 1.1-fold change. We used a 1.1-fold change filter as described previously (Freeman et al. 2009). This magnitude was chosen based on 1) previous technical reports demonstrating the ability of 2DIGE to routinely detect small magnitude changes in protein expression (Marouga et al. 2005;Viswanathan et al. 2006), and 2) previous behavioral neuroscience studies detailing small-magnitude (10%–20%) but biologically relevant changes in neurotransmission-related proteins associated with functional alterations (English et al. 2009;Morton et al. 2001;Rubino et al. 2009). Additionally we have found that the implementation of this fold change filter in DIGE studies that include confirmation by orthogonal techniques provides the best balance of minimizing false negatives while avoiding false positives.
Mass spectrometry
692 protein spots, including all spots significantly altered in expression with age and matched to a preparative gel, as well as a sample of unchanged proteins included for completeness, were robotically excised from the preparative gels using an ETTAN Spot Picker (GE Healthcare). Of these protein spots, 155 spots were excised from both preparative gels (for a total of 847 gel plugs) to maximize the opportunity for protein identification and to enable assessment of the reproducibility of protein identifications. Sample preparation for mass spectrometry and MALDI-TOF/TOF methods were performed similarly to that previously described (Freeman et al. 2009). For additional details of the mass spectrometry methods please see the Supporting Information available online.
Bioinformatics
Proteomic profiles of age groups were analyzed with GeneSpring GX 10 software (Agilent Technologies, Palo Alto, CA) to generate heatmap, Venn and Principal component analysis (PCA) plots. The Ingenuity Pathway Analysis system (Ingenuity Systems, Redwood City, CA) was used to identify protein networks/pathways regulated with age as described previously (Freeman et al. 2009). For additional details on bioinformatic methods please see the Supporting Information available online.
Immunoblotting
Soluble protein was extracted from synaptosome samples by sonicating synaptosomes in protein lysis buffer on ice, incubating suspensions with rocking for 15 minutes at 4°C, and centrifuging at 10,000 × g for 12 minutes at 4°C. Supernatant protein concentrations were determined using the BCA protein assay (Pierce). After BCA quantitation, all protein samples were brought to a concentration of 2µg/µL and diluted in LDS sample buffer (Invitrogen, Carlsbad, CA) in volumes sufficient to load all gels from one preparation per sample to avoid aliquot-to-aliquot variation. 10µg of each sample (n=10/ group) was separated by SDS-PAGE using Criterion Tris-HCl precast gels (4%–20% gradient, 1mm, 26 wells; BioRad, Hercules, CA), and quantitated by Deep Purple total protein staining performed as described above. Likewise, 10µg of each sample (n=10/group) was loaded onto gels for immunoblot experiments. For immunoblotting, proteins were transferred to PVDF membranes (HyBond PVDF-LFP; GE Healthcare), blocked with 3.0% BSA in phosphate buffered saline with 1.0% Tween-20, probed with primary antibodies (Supplemental Table 2) overnight at 4°C, and visualized using species-appropriate fluorescent Cy- or HRP-conjugated secondary antibodies. Blots were imaged using a multimode Typhoon 9410 or developed with ECL substrate (GE Healthcare) and imaged on film, and quantitated using automated digital densitometry (ImageQuant TL software; Molecular Dynamics, Inc., Sunnyvale, CA). Resultant immunoblot data for each sample/target protein were standardized to the corresponding whole-lane densitometric volume of the total protein stained gel. For data comparison between multiple membranes, individual bands were standardized to the mean “Adult” value on each membrane for a given antibody. All immunoblot experiments were analyzed by one-way ANOVA with Student-Newman-Keuls post hoc tests.
Results
Increasing age does not effect synaptosome enrichment or characteristics
Hippocampal synaptosome samples enriched from Young-adult, Adult, and Aged rats (n=10 per group) yielded an average of 1667 ± 53µg of detergent-soluble protein, with no difference in protein yield between age groups. Quality control assessment of synaptosome preparations was conducted in two ways. To evaluate synaptosomal enrichment, representative pools containing equal amounts of protein from each sample (n=10) for each age group were immunoblotted for the SNARE protein SNAP25. Compared to the nuclear/cytosolic fractions (P1), SNAP25 content in the synaptosomal fractions (P2) was enriched by an average of 84%, with no difference in enrichment between Young-adult, Adult, and Aged synaptosome samples. Because SNAP25 is also highly expressed in extrasynaptic and axonal membranes (Hagiwara et al. 2005;Tao-Cheng et al. 2000), more specifically localized proteins (PSD95 and synapsin 1) were also assessed for synaptosomal enrichment. These proteins demonstrated significant enrichment in the synaptosomal fractions (P2 PSD95: 298 ± 12% of P1; P2 synapsin 1: 225 ± 29% of P1). Additionally, synaptosomal profiles from Young-adult, Adult, and Aged groups were assessed by electron microscopy with sample identities masked. The general abundance and quality of intact synaptosomes, vesicle containing presynaptic terminals, and postsynaptic densities were examined, with no notable qualitative differences observed between synaptosome samples (Figure 1). These preparations also contained membrane fragments and notable mitochondria, with both synaptosomal and free mitochondria visible in electron micrographs of all age groups.
Figure 1. Synaptosome quality assessment.
Synaptosomes from Young-adult, Adult, and Aged rats were evaluated by electron microscopy. Synaptosomes from each age group were intact and qualitatively similar, with presynaptic terminals containing numerous neurotransmitter vesicles (open arrows) and connected postsynaptic terminals (black arrows).
Quantitative hippocampal synaptoproteomic analysis reveals age-related alterations in protein expression patterns
Eleven of the 12 analytical gels and both preparative gels produced consistent, high-resolution protein spot patterns (Supplemental Figure 2). One analytical gel (Gel #1, Supplemental Figure 1) failed to focus properly in the first dimension, and was therefore excluded from further analysis. Of the protein spots detected using the DeCyder 6.5 DIA module, 1209 spots were matched across at least eight out of the eleven analytical gels (i.e., ≥5 animals/group) in the BVA module. Spots which matched across fewer gels (<8) were excluded as they did not provide sufficient sample sizes for rigorous statistical analysis. Cy3- and Cy5-labeled protein spots were normalized to signal intensities of analogous spots in the Cy2-labeled normalization pool for inter-sample and inter-gel data comparison. Statistical analysis of 2-DIGE results revealed a total of 273 age-related protein changes (Supplemental Table 3).
Volcano plots were generated from DeCyder Cy3/Cy2 and Cy5/Cy2 protein spot ratios to provide visualization of the equivalent distribution of both unchanged and up- and down-regulated proteins in each age-based group comparison (Supplemental Figure 3). A heatmap was also generated from these data to depict the relative expression of these proteins between animal groups (Figure 2A). Distinct patterns of protein expression were apparent, including both increases and decreases with age. A protein expression-based group condition tree demonstrated a similarity between protein expression patterns of Young-Adult and Adult groups, and a more distant relationship of these groups with the Aged group. A number of proteins were significantly altered in abundance in each pairwise age group comparison: 79 in the Young-adult versus Adult comparison, 160 proteins in the Adult versus Aged comparison, and 248 proteins in the Young-adult versus Aged comparison. A set analysis was performed to assess the intersection of these protein sets and to visualize the overlap of proteins differentially expressed in two or more group comparisons (Figure 2B). Of these protein changes, 4 were unique to the Young-adult/Adult comparison, 14 were specific to the Adult/Aged comparison, 79 were specific to the Young-adult/Aged comparison, and 34 were common to all three age-group comparisons (see Supplemental Table 3 for full data set). To depict the relationship of age group-based proteomic profiles to each other, a Principal Component Analysis (PCA) was performed, using Adult mean-standardized expression values for the 273 proteins significantly regulated in this study (Figure 2C). These expression data were combined for all animals within an age group to yield one data point per group. The first component (x-axis; Age) accounted for 88% of study variance, and segregated Young-adult and Adult rats from Aged rats. The second component accounted for only 12% of variance with the Young-Adult group separating from Adult and Aged groups. This visualization further demonstrates that the Young-adult and Adult synaptoproteomes are more similar to each other than to the Aged synaptoproteome.
Figure 2. Quantitative analyses of synaptoproteomic profiles across age groups.
(A) A heatmap was generated from relative expression values of the 273 proteins differentially expressed with age between animal groups [Young-adult (3 months), Adult (12 months), Aged (26 months)]. Protein expression patterns indicate both upregulation and downregulation with age, as well as a number of proteins with biphasic expression patterns. An expression-based condition tree demonstrates a primary relationship between Young-adult (3 months) and Adult (12 months) groups, indicating that synaptoproteomic profiles of animals from these groups are more similar to each other than to those from Aged rats. (B) A Venn diagram was generated to visualize commonalities and differences in protein expression changes between age groups using the 273 proteins significantly regulated with aging in this study. Considerable overlap was observed between Aged versus Adult and Aged versus Young-adult comparisons, with limited commonality between other age group comparisons. (C) Principal component analysis was performed using Adult mean-standardized expression values of the 273 proteins regulated with aging. The first component (x-axis) accounted for 88% of study variance and segregated Young-adult and Adult animal groups from the Aged group, demonstrating a primary effect of aging on hippocampal synaptoproteomic profiles. The second component (y-axis) accounted for 12% of study variance.
Identification of proteins through tandem mass spectrometry
Four hundred and four protein spots were identified with a minimum MASCOT confidence interval >95% by either MALDI- or LTQ-mass spectrometry. The reproducibility of the protein identification was also tested. We observed a 98% agreement (84/86 spots) in protein identification between matched protein spots excised and identified from both preparative gels. For the two spots with differing protein identifications, the disagreement was between two isoforms of the same protein, and the isoform with the largest number of highly confident MS/MS spectra was accepted as the protein identity (spot 482: enolase; spot 1155: v-ATPase; Supplemental Table 4). Identified proteins had an average molecular weight of 52kDa and an average GRAVY (hydropathicity) score of −0.3 (Supplemental Figure 4). A full list of identified proteins and relevant peptide information is provided in Supplemental Table 4.
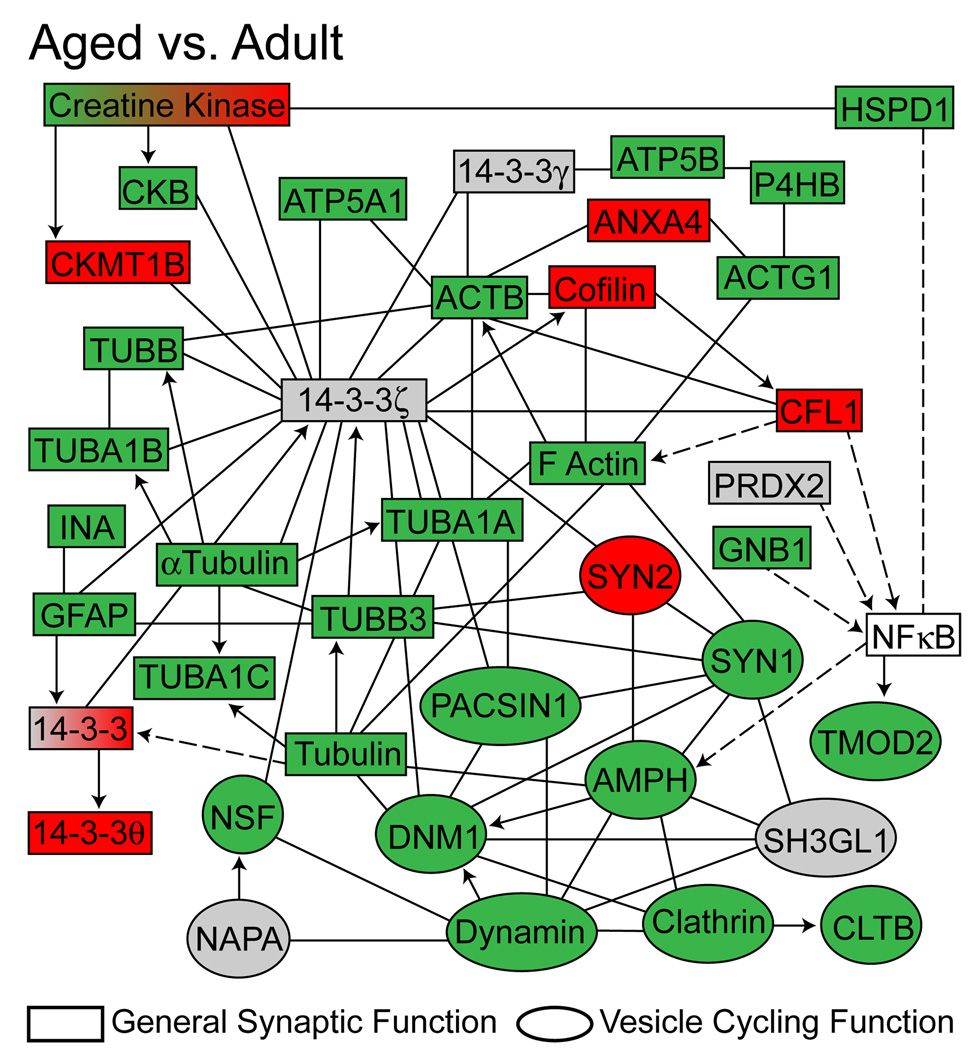
Bioinformatic analysis of proteins regulated with age identifies a network of neurotransmission-regulating proteins
Ingenuity Pathway Analysis was also conducted on proteins with significantly different expression levels within the experiment. The most highly regulated network identified (p <0.0001, Fisher’s Exact Test) was a nervous system function network of proteins involved in regulation of synaptic transmission and synaptic vesicle quantity, association, and recycling. To visualize the effects of aging on this neurotransmission-regulating protein network, relative expression values for each age group-based comparison were overlayed onto the network (Aged versus Adult, Figure 3; Adult versus Young-adult and Aged versus Young-adult, Supplemental Figure 5A and 5B).
Figure 3. Age-related dysregulation of a neuronal function protein network.
Network analysis (Ingenuity Pathway Analysis v8.5) was conducted using the full set of proteins differentially expressed with age. A nervous system function network, including proteins involved in regulation of synaptic transmission and synaptic vesicle quantity, association and recycling, was determined to contain an overrepresentation of age-related changes in the hippocampal synaptic proteome (p <0.0001, Fisher’s Exact Test). Specific proteins are colored according to their statistically significant changes in expression between the Aged (26 months) versus Adult (12 months) groups. Expression key: green=decreased, red=increased, grey=detected but unchanged, white=not detected. Relationship key: solid arrow= acts directly on target, dashed arrow= acts indirectly on target, solid line=direct protein-protein interaction, dashed line=indirect protein-protein interaction. Expression data for Aged versus Young-adult and Adult versus Young-adult group comparisons are presented in Supplemental Figure 5.
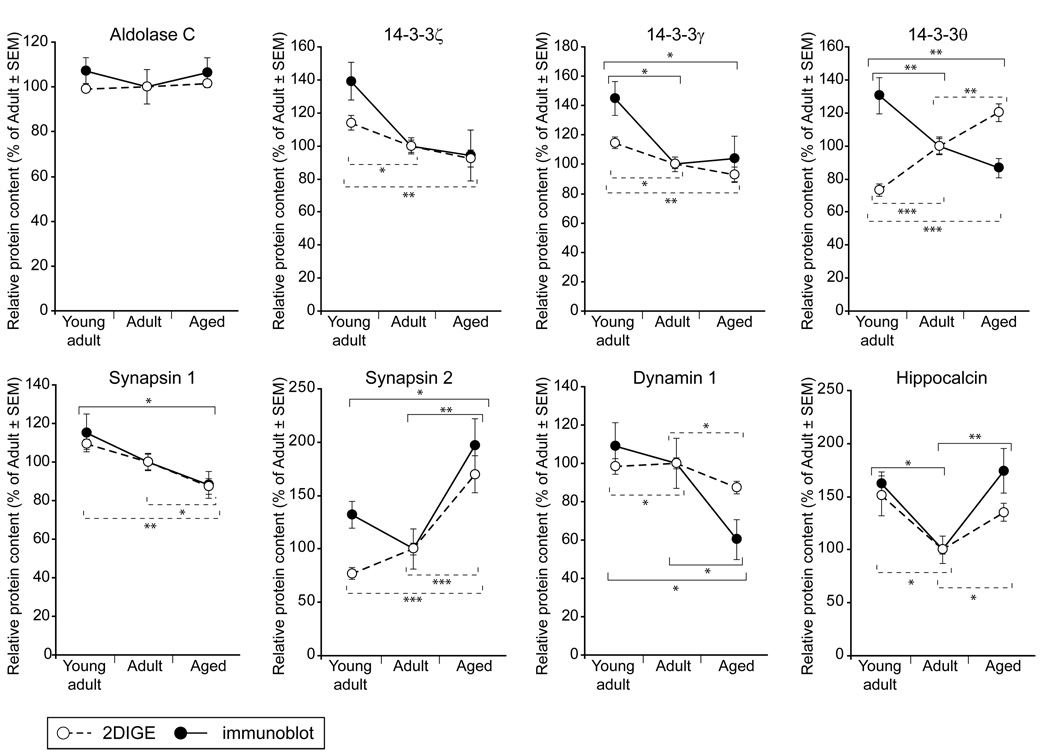
Neurotransmission-regulating protein expression changes identified by proteomic analysis are confirmed by an orthogonal method
To confirm the 2-DIGE quantitation and examine the neurotransmission-regulating network identified in the bioinformatic analysis, one unchanged protein and seven differentially expressed proteins were quantified by immunoblotting and standardized to a corresponding Deep-Purple total protein-stained gel to normalize any protein loading variation (Supplemental Figure 6). To validate the quantitative accuracy of 2-DIGE measurements, we first probed synaptosomal lysates (n=10 per group), including the samples included in 2-DIGE analysis, for aldolase C, which was included as an unchanged negative control. In agreement with findings from the 2-DIGE analysis, there was no difference in aldolase C content between the three age groups (Figure 4).
Figure 4. Immunoblot confirmation of 2-DIGE quantitation.
Seven differentially expressed proteins, and one unchanged negative control protein (Aldolase C), were confirmed by immunoblotting. Equal amounts of hippocampal synaptosome protein (10µg) were loaded in equal volumes from Young-adult (3 months), Adult (12 months) and Aged (26 months) rats (n=10 for all groups). Immunoblot (dashed lines) and 2-DIGE (solid lines) quantitation demonstrated highly similar values, with the exception of 14-3-3θ. Immunoblot data were normalized to total protein staining, 2-DIGE data were normalized to the Cy2 channel. Data are presented as mean±SEM, percent of Adult. Statistical analysis was performed by one-way ANOVA with pair-wise Student-Newman-Keuls post hoc testing; * p <0.05, ** p <0.01, *** p<0.001.
Multiple 14-3-3 (tyrosine 3-monooxygenase/tryptophan 5-monooxygenase activation protein) isoforms (γ,θ,ζ) were significantly regulated with age in the 2-DIGE study, and were selected as targets for immunoblot confirmation due to their regulatory functions in neurotransmission and central placement in the nervous system function network identified determined to change significantly with aging (IPA analysis, Fisher’s Exact test, p<0.001). Compared to Young-adults, there was significantly less 14-3-3θ and 14-3-3γ content in Adult and Aged rats (~30% less), with no differences between Adult and Aged rats themselves (Figure 4). 14-3-3ζ demonstrated a highly similar pattern which did not reach statistical significance (ANOVA p=0.08). Interestingly, 14-3-3θ was significantly changed in expression in a direction opposite to that observed by 2-DIGE.
Selected members of the network identified by 2-DIGE/bioinformatic analysis with more direct roles in neurotransmission regulation (synapsin 1, synapsin 2, dynamin 1, and hippocalcin) were also confirmed by immunoblotting and standardized to total protein (Figure 4). Consistent with 2-DIGE findings, synapsin 1 was significantly decreased in Aged rats compared to Young-adults, and synapsin 2 was significantly increased in Aged rats compared to both Adults and Young-adults. Also in agreement with expression changes determined by 2-DIGE, dynamin 1 was significantly decreased (by ~40%) in Aged rats compared to both Adults and Young-adults, while hippocalcin exhibited a biphasic expression pattern, decreasing significantly in Adult rats compared to Young-adults, and returning to Young-adult levels in Aged rats. Fold-change magnitudes quantified by immunoblotting were highly similar to those identified in the 2-DIGE screen. The differences observed are likely due to what the two technical approaches measure [i.e., total protein quantitation (immunoblotting) vs. quantitation of individual protein post-translationally modified species (2-DIGE)] and the inclusion of additional samples in the immunoblotting experiments that were not present in the 2-DIGE analysis. Overall, we observed a high degree of reproducibility between the 2-DIGE and immunoblot quantitation approaches.
Additional neurotransmission-regulating proteins decline with age
To expand these findings to include additional regulators of neurotransmission, protein targets for additional immunoblot evaluation were selected based on their known roles in neuronal function. Synaptosomal lysates were immunoblotted for the anti-apoptosis, pro-longevity protein 14-3-3 epsilon; the vesicular protein synaptophysin; the postsynaptic scaffolding protein PSD95; and the SNARE proteins VAMP2, syntaxin 1, and SNAP25. These proteins were significantly decreased between animal groups of increasing age (Figure 5), with SNARE proteins decreasing by as much as 30% between Adult and Aged rats.
Figure 5. Age-related alterations in expression of neurotransmission-regulating proteins.
To expand analysis of synaptosomal protein expression to include additional neurotransmission-related proteins not detected by 2-DIGE, 14-3-3ε, the vesicle-associated protein synaptophysin (SYP), the postsynaptic density protein PSD95, and the SNARE proteins VAMP2, syntaxin 1 (Stx1), and SNAP25 were quantified in synaptosomal lysates by immunoblotting as described above. These targets were consistently downregulated with increasing age, with significant expression decreases ranging from ~15% to ~35%. Immunoblot data were normalized to total protein staining. Data are presented as mean±SEM, percent of Adult. Statistical analysis of all immunoblot data was performed by one-way ANOVA with pair-wise Student-Newman-Keuls posthoc testing; * p <0.05, ** p <0.01, *** p <0.001.
Discussion
Age-related cognitive decline affects a significant portion of the healthy aging population but its causes remain to be determined. Since neuronal communication underlies cognitive function, faulty synaptic communication leading to impaired neurotransmission is likely a key factor in age-related cognitive deficits. In the aged hippocampus, neuronal activation during behavioral tests of spatial learning and memory capacities is significantly depressed, and electrophysiological correlates of learning and memory are dysregulated (Norris et al. 1996;Norris et al. 1998;Rosenzweig and Barnes 2003). Age-related alterations in hippocampal transcript and protein content that may contribute to impaired synaptic function have been identified for synaptic signaling proteins (Baxter et al. 1999;Jiang et al. 2008;Liu et al. 2008;Majdi et al. 2009;Sato et al. 2005;Shi et al. 2007).
Hippocampal synaptosomes isolated from Wistar rats demonstrated significant decreases in SNAP25 and synaptophysin expression between adult (12 months) and aged (18–24 months) animals, with magnitudes of change similar to those observed here (Canas et al. 2009). Likewise, synaptic vesicle glycoprotein 2B, SV2-related protein, Homer 1, and synaptoporin were recently demonstrated to decrease in expression throughout aging in Fischer 344 rats of ages similar to those in this study (i.e., 3 months, 12 months, 23 months) (Kadish et al. 2009). These findings are in agreement with reports of dysregulated synaptic connectivity and functionality with aging (Kumar et al. 2007;Sametsky et al. 2008;Thibault et al. 2001). Many of these studies, however, have focused on specific transcripts and protein species rather than on the entire proteomic profile of hippocampal synapses. The work presented here adds to the understanding of age-related alterations in the unfractionated hippocampus by focusing on the proteomic composition of the hippocampal synaptoproteome.
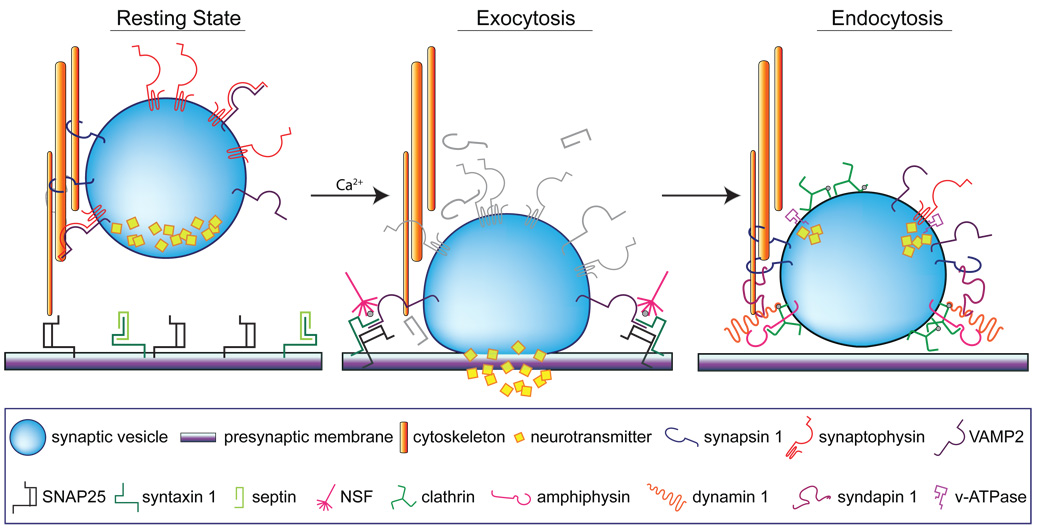
In this study, age-related downregulation of 14-3-3 signaling protein isoforms and a number of proteins with neurotransmission-regulating functions were identified by bioinformatic analysis to be members of a protein network implicated in nervous system function. Alterations in synaptic protein expression have the potential to decrease stimulus-induced neurotransmission and replenishment of synaptic vesicle pools during instances of prolonged or intense stimulation, such as those necessary for learning and the formation and maintenance of memory (Figure 6).
Figure 6. Functional roles of neurotransmission-regulating proteins altered with increasing age.
Under normal, healthy conditions, synergistic protein-protein interactions maintain synaptic function at rest and facilitate stimulus-evoked vesicle exocytosis and endocytosis. At rest, vesicles are tethered to the cytoskeleton by synapsin 1, and synaptophysin interacts with the vesicular SNARE protein VAMP2 to prevent spontaneous vesicle mobilization. The target SNARE proteins syntaxin and SNAP25 remain unassociated at the presynaptic plasma membrane, in part due to the interaction of septins with syntaxins, which precludes the formation of the SNARE docking complex. Neuronal depolarization and calcium influx trigger vesicle exocytosis and neurotransmitter release by altering protein interactions in the synaptic terminal. Calcium-dependent phosphorylation of synapsin 1 releases vesicles from the cytoskeleton, which dissociation of synaptophysin and VAMP2 enables vesicle mobilization to the plasma membrane. Syntaxin and SNAP25 interact to form the SNARE docking complex, and binding of VAMP2 creates the intact SNARE fusion complex. This creates a pore in the plasma membrane to allow neurotransmitter release into the synaptic cleft, following which, NSF rapidly disassembles the SNARE complex. Vesicles are then recycled through endocytosis, mediated by formation of a clathrin coat along components of the vesicular membrane. The vesicle-associated protein amphiphysin interacts with clathrin and recruits the cytosolic protein dynamin, which simultaneously associates with cytoskeleton-associated syndapin and triggers vesicle fission. Targeting signals from VAMP2 and interaction of dephosphorylated synapsin 1 with syndapin and the cytoskeleton mobilize recycled vesicles away from the presynaptic plasma membrane, after which they are re-tethered to the cytoskeleton. Vacuolar ATPases and synaptophysin modulate vesicle reloading with neurotransmitter, priming vesicles for subsequent rounds of exocytosis.
A salient finding of this work was the biphasic expression pattern of hippocalcin, a calcium-binding protein required for spatial learning and memory (Kobayashi et al. 2005). Hippocalcin facilitates calcium-mediated LTD in ex vivo hippocampal slices, in which calcium signaling and calcium channel expression are also dysregulated (Brewer et al. 2007;Landfield and Pitler 1984). Additionally, hippocalcin functions as a diffusible calcium sensor critical in calcium gating of slow afterhyperpolarization in hippocampal neurons (Tzingounis et al. 2007). Although specific mechanisms underlying the involvement of hippocalcin in synaptic plasticity are not fully understood, depolarization-sensitive calcium-induced translocation along hippocampal dendrites and axons and interactions with clathrin-mediated endocytic machinery and glutamate receptors are likely contributing factors (Markova et al. 2008;Palmer et al. 2005). Interestingly, we observed a significant decrease in synaptosomal hippocalcin expression in Adult rats compared to Young-adult rats, at ages prior to the development of hippocampal cognitive deficits. In contrast, in Aged rats, hippocalcin returned to levels observed in Young-adults. It is possible that, in conjunction with abnormal calcium dynamics and decreased neurotransmission-regulating proteins, increased synaptic expression of hippocalcin facilitates the susceptibility of aged rodents to LTD and impaired spatial learning and memory.
The 14-3-3 isoform family of scaffolding adaptor proteins is highly enriched in brain and is implicated in modulation of neurotransmission, with potential roles in learning and memory (Broadie et al. 1997;Li et al. 2006;Philip et al. 2001). Through association with hundreds of binding partners, 14-3-3 isoforms mediate numerous processes including phosphatase signaling, protein trafficking and conformational changes, and facilitation of signal transduction of exocytic pathways (Pozuelo et al. 2004). In hippocampal neurons, 14-3-3 activity reduces short term synaptic depression by modulating calcium channel inactivation dynamics (Li et al. 2006). Recent proteomic analyses of 14-3-3 binding proteins suggest a postsynaptic component of 14-3-3 function in neurotransmission. PSD95, a postsynaptic scaffolding protein highly expressed in hippocampal glutamatergic synapses, binds 14-3-3 and enables indirect interaction of 14-3-3 with numerous glutamate receptors and synaptic signaling proteins (Fernandez et al. 2009). Similarly, 14-3-3 modifies postsynaptic glutamate receptor signaling through interaction with Homer 3, a postsynaptic scaffolding protein that links receptor signaling targets and receptors (Angrand et al. 2006). Decreased synaptosomal expression of multiple 14-3-3 isoforms was identified by proteomic and immunoblotting techniques in this study, indicating a potential mechanism for disrupted protein-protein interactions required for maintenance of healthy neurotransmission.
A large subset of protein expression changes in this study, such as NSF, SNAP25, syntaxin 1, VAMP2, synaptophysin, synapsin 1, dynamin 1, amphiphysin, clathrin, syndapin 1, and syntaxin-binding protein 1, represent both effectors and regulators of neurotransmission. For example, SNAP25, syntaxin 1, and VAMP2 interact to form the SNARE complex critical for vesicle docking and fusion. Heterozygous loss of SNAP25 decreases stimulus-evoked membrane fusion and impairs short term plasticity and spatial learning at both excitatory and inhibitory neurons (Tafoya et al. 2006;Washbourne et al. 2002). Similarly, disrupted expression of VAMP2, the vesicular SNARE component, nearly abolishes calcium-induced exocytosis and endocytic replenishment of the synaptic vesicle pool (Deak et al. 2004;Schoch et al. 2001). Synapsin 1, also decreased with age, crosslinks neurotransmitter-primed synaptic vesicles to the cytoskeleton in the resting state to effectively restrain the reserve pool and minimize spontaneous vesicle mobilization in a phospho-dependent manner. Synapsin 1-knockout mice exhibit demonstrate impaired cognitive function and spatial memory with age (Corradi et al. 2008). Synaptophysin and PSD95 which undergo age-related changes in expression in both humans and animal models (Adams et al. 2008;Head et al. 2009;Majdi et al. 2009), were also reduced in synaptosomal expression with age in this work. Together, the synaptoproteomic changes observed in this study suggest an age-related impairment of exocytosis (SNARE proteins, NSF, synapsin 1, tropomodulin 2), endocytosis (clathrin, dynamin, amphiphysin, syndapin, etc.), and receptor aggregation (PSD95). Additionally, several of these proteins are implicated in activity-dependent synaptic maintenance.
This study describes a coordinated downregulation of a network of synaptosomal proteins with functions in neurotransmitter vesicle exocytosis and recycling dynamics. These proteins are often absent from proteomic reports of aging, perhaps due to their relatively low whole-tissue expression levels. The combination of synaptosome enrichment and 2-DIGE proteomic methods represents a technical advance that enabled the assessment of the protein composition of an isolated subcellular region, the synaptic terminal, allowing quantitation of neurotransmission-related proteins in their functional niche. Synaptosomal enrichment also increased sensitivity for less-abundant protein species that often fall below the limit of detection in proteomic studies of unfractionated tissue. Additional novel pathways and networks revealed by this synaptoproteomic analysis remain to be pursued in future studies. Also, in agreement with previous reports, we identified a number of metabolic and mitochondrial proteins differentially regulated in comparisons of Young-adult, Adult, and Aged rats (Freeman et al. 2009;Poon et al. 2006b). The synaptosomal preparation used in this study, although highly enriched for synaptic terminals, also contains both synaptic and non-synaptic membrane fragments and mitochondria. Definitive localization studies are required to determine whether the changes in expression of the specific mitochondrial proteins occur throughout the cell or are restricted to specific subcellular compartment.
Together, observed decreases in effectors and regulators of neurotransmission, including SNARE and SNARE-associated proteins and 14-3-3 isoforms, suggest an age-based deterioration of hippocampal neurotransmission that occurs between adulthood and advanced age. These alterations in synaptic protein expression have the potential to decrease stimulus-induced neurotransmission and replenishment of synaptic vesicle pools during instances of prolonged or intense stimulation, such as those necessary for learning and the formation and perseverance of memory.
Supplementary Material
Acknowledgments
The authors thank the Penn State Mass Spectrometry Facility, Roland Myers of the Penn State Microscopy and Histology Core Facility, Matthew Mitschelen and the Oklahoma Medical Research Foundation staff, and NextGen Sciences for their technical assistance and Ms. Colleen Van Kirk and Mr. Dante Smith for valuable editorial guidance. This work was supported by R01AG026607 and P01AG11370 (W.E.S).
Footnotes
The authors have no competing interests to declare.
References
- Adams MM, Shi L, Linville MC, et al. Caloric restriction and age affect synaptic proteins in hippocampal CA3 and spatial learning ability. Exp. Neurol. 2008;211:141–149. doi: 10.1016/j.expneurol.2008.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allison DB, Cui X, Page GP, Sabripour M. Microarray data analysis: from disarray to consolidation and consensus. Nat. Rev. Genet. 2006;7:55–65. doi: 10.1038/nrg1749. [DOI] [PubMed] [Google Scholar]
- Angrand PO, Segura I, Volkel P, et al. Transgenic mouse proteomics identifies new 14-3-3-associated proteins involved in cytoskeletal rearrangements and cell signaling. Mol. Cell Proteomics. 2006;5:2211–2227. doi: 10.1074/mcp.M600147-MCP200. [DOI] [PubMed] [Google Scholar]
- Barnes CA, Suster MS, Shen J, McNaughton BL. Multistability of cognitive maps in the hippocampus of old rats. Nature. 1997;388:272–275. doi: 10.1038/40859. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Frick KM, Price DL, Breckler SJ, Markowska AL, Gorman LK. Presynaptic markers of cholinergic function in the rat brain: relationship with age and cognitive status. Neuroscience. 1999;89:771–779. doi: 10.1016/s0306-4522(98)00374-1. [DOI] [PubMed] [Google Scholar]
- Beeri MS, Lee H, Cheng H, Wollman D, Silverman JM, Prohovnik I. Memory activation in healthy nonagenarians. Neurobiol. Aging. 2009 doi: 10.1016/j.neurobiolaging.2009.02.022. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blalock EM, Chen KC, Sharrow K, Herman JP, Porter NM, Foster TC, Landfield PW. Gene microarrays in hippocampal aging: statistical profiling identifies novel processes correlated with cognitive impairment. J. Neurosci. 2003;23:3807–3819. doi: 10.1523/JNEUROSCI.23-09-03807.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boric K, Munoz P, Gallagher M, Kirkwood A. Potential adaptive function for altered long-term potentiation mechanisms in aging hippocampus. J. Neurosci. 2008;28:8034–8039. doi: 10.1523/JNEUROSCI.2036-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brewer LD, Thibault O, Staton J, Thibault V, Rogers JT, Garcia-Ramos G, Kraner S, Landfield PW, Porter NM. Increased vulnerability of hippocampal neurons with age in culture: temporal association with increases in NMDA receptor current, NR2A subunit expression and recruitment of L-type calcium channels. Brain Res. 2007;1151:20–31. doi: 10.1016/j.brainres.2007.03.020. [DOI] [PubMed] [Google Scholar]
- Broadie K, Rushton E, Skoulakis EM, Davis RL. Leonardo, a Drosophila 14-3-3 protein involved in learning, regulates presynaptic function. Neuron. 1997;19:391–402. doi: 10.1016/s0896-6273(00)80948-4. [DOI] [PubMed] [Google Scholar]
- Brunso-Bechtold JK, Linville MC, Sonntag WE. Age-related synaptic changes in sensorimotor cortex of the Brown Norway X fischer 344 rat. Brain Res. 2000;872:125–133. doi: 10.1016/s0006-8993(00)02515-4. [DOI] [PubMed] [Google Scholar]
- Butterfield DA, Poon HF, St Clair D, Keller JN, Pierce WM, Klein JB, Markesbery WR. Redox proteomics identification of oxidatively modified hippocampal proteins in mild cognitive impairment: insights into the development of Alzheimer's disease. Neurobiol. Dis. 2006;22:223–232. doi: 10.1016/j.nbd.2005.11.002. [DOI] [PubMed] [Google Scholar]
- Calabrese V, Scapagnini G, Ravagna A, Colombrita C, Spadaro F, Butterfield DA, Giuffrida Stella AM. Increased expression of heat shock proteins in rat brain during aging: relationship with mitochondrial function and glutathione redox state. Mech. Ageing Dev. 2004;125:325–335. doi: 10.1016/j.mad.2004.01.003. [DOI] [PubMed] [Google Scholar]
- Canas PM, Duarte JM, Rodrigues RJ, Kofalvi A, Cunha RA. Modification upon aging of the density of presynaptic modulation systems in the hippocampus. Neurobiol. Aging. 2009;30:1877–1884. doi: 10.1016/j.neurobiolaging.2008.01.003. [DOI] [PubMed] [Google Scholar]
- Corradi A, Zanardi A, Giacomini C, Onofri F, Valtorta F, Zoli M, Benfenati F. Synapsin-I- and synapsin-II-null mice display an increased age-dependent cognitive impairment. J. Cell Sci. 2008;121:3042–3051. doi: 10.1242/jcs.035063. [DOI] [PubMed] [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb. Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deak F, Schoch S, Liu X, Sudhof TC, Kavalali ET. Synaptobrevin is essential for fast synaptic-vesicle endocytosis. Nat. Cell Biol. 2004;6:1102–1108. doi: 10.1038/ncb1185. [DOI] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J. Exp. Psychol. Learn. Mem. Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- English JA, Dicker P, Focking M, Dunn MJ, Cotter DR. 2-D DIGE analysis implicates cytoskeletal abnormalities in psychiatric disease. Proteomics. 2009;9:3368–3382. doi: 10.1002/pmic.200900015. [DOI] [PubMed] [Google Scholar]
- Fernandez E, Collins MO, Uren RT, et al. Targeted tandem affinity purification of PSD-95 recovers core postsynaptic complexes and schizophrenia susceptibility proteins. Mol. Syst. Biol. 2009;5:269. doi: 10.1038/msb.2009.27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foster TC. Calcium homeostasis and modulation of synaptic plasticity in the aged brain. Aging Cell. 2007;6:319–325. doi: 10.1111/j.1474-9726.2007.00283.x. [DOI] [PubMed] [Google Scholar]
- Freeman WM, VanGuilder HD, Bennett C, Sonntag WE. Cognitive performance and age-related changes in the hippocampal proteome. Neuroscience. 2009;159:183–195. doi: 10.1016/j.neuroscience.2008.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes CA. Sequence reactivation in the hippocampus is impaired in aged rats. J. Neurosci. 2008;28:7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gureviciene I, Gurevicius K, Tanila H. Aging and alpha-synuclein affect synaptic plasticity in the dentate gyrus. J. Neural Transm. 2009;116:13–22. doi: 10.1007/s00702-008-0149-x. [DOI] [PubMed] [Google Scholar]
- Hagiwara A, Fukazawa Y, guchi-Tawarada M, Ohtsuka T, Shigemoto R. Differential distribution of release-related proteins in the hippocampal CA3 area as revealed by freeze-fracture replica labeling. J. Comp Neurol. 2005;489:195–216. doi: 10.1002/cne.20633. [DOI] [PubMed] [Google Scholar]
- Head E, Corrada MM, Kahle-Wrobleski K, Kim RC, Sarsoza F, Goodus M, Kawas CH. Synaptic proteins, neuropathology and cognitive status in the oldest-old. Neurobiol. Aging. 2009;30:1125–1134. doi: 10.1016/j.neurobiolaging.2007.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang L, Fang J, Moore DS, Gogichaeva NV, Galeva NA, Michaelis ML, Zaidi A. Age-associated changes in synaptic lipid raft proteins revealed by two-dimensional fluorescence difference gel electrophoresis. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kadish I, Thibault O, Blalock EM, Chen KC, Gant JC, Porter NM, Landfield PW. Hippocampal and cognitive aging across the lifespan: a bioenergetic shift precedes and increased cholesterol trafficking parallels memory impairment. J. Neurosci. 2009;29:1805–1816. doi: 10.1523/JNEUROSCI.4599-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobayashi M, Masaki T, Hori K, Masuo Y, Miyamoto M, Tsubokawa H, Noguchi H, Nomura M, Takamatsu K. Hippocalcin-deficient mice display a defect in cAMP response element-binding protein activation associated with impaired spatial and associative memory. Neuroscience. 2005;133:471–484. doi: 10.1016/j.neuroscience.2005.02.034. [DOI] [PubMed] [Google Scholar]
- Kumar A, Thinschmidt JS, Foster TC, King MA. Aging effects on the limits and stability of long-term synaptic potentiation and depression in rat hippocampal area CA1. J. Neurophysiol. 2007;98:594–601. doi: 10.1152/jn.00249.2007. [DOI] [PubMed] [Google Scholar]
- Landfield PW, Pitler TA. Prolonged Ca2+-dependent afterhyperpolarizations in hippocampal neurons of aged rats. Science. 1984;226:1089–1092. doi: 10.1126/science.6494926. [DOI] [PubMed] [Google Scholar]
- Li Y, Wu Y, Zhou Y. Modulation of inactivation properties of CaV2.2 channels by 14-3-3 proteins. Neuron. 2006;51:755–771. doi: 10.1016/j.neuron.2006.08.014. [DOI] [PubMed] [Google Scholar]
- Liu P, Smith PF, Darlington CL. Glutamate receptor subunits expression in memory-associated brain structures: regional variations and effects of aging. Synapse. 2008;62:834–841. doi: 10.1002/syn.20563. [DOI] [PubMed] [Google Scholar]
- Majdi M, Ribeiro-da-Silva A, Cuello AC. Variations in excitatory and inhibitory postsynaptic protein content in rat cerebral cortex with respect to aging and cognitive status. Neuroscience. 2009;159:896–907. doi: 10.1016/j.neuroscience.2008.11.034. [DOI] [PubMed] [Google Scholar]
- Markova O, Fitzgerald D, Stepanyuk A, Dovgan A, Cherkas V, Tepikin A, Burgoyne RD, Belan P. Hippocalcin signaling via site-specific translocation in hippocampal neurons. Neurosci. Lett. 2008;442:152–157. doi: 10.1016/j.neulet.2008.06.089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marouga R, David S, Hawkins E. The development of the DIGE system: 2D fluorescence difference gel analysis technology. Anal. Bioanal. Chem. 2005;382:669–678. doi: 10.1007/s00216-005-3126-3. [DOI] [PubMed] [Google Scholar]
- Morton AJ, Faull RL, Edwardson JM. Abnormalities in the synaptic vesicle fusion machinery in Huntington's disease. Brain Res. Bull. 2001;56:111–117. doi: 10.1016/s0361-9230(01)00611-6. [DOI] [PubMed] [Google Scholar]
- Norris CM, Halpain S, Foster TC. Reversal of age-related alterations in synaptic plasticity by blockade of L-type Ca2+ channels. J. Neurosci. 1998;18:3171–3179. doi: 10.1523/JNEUROSCI.18-09-03171.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norris CM, Korol DL, Foster TC. Increased susceptibility to induction of long-term depression and long-term potentiation reversal during aging. J. Neurosci. 1996;16:5382–5392. doi: 10.1523/JNEUROSCI.16-17-05382.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Palmer CL, Lim W, Hastie PG, et al. Hippocalcin functions as a calcium sensor in hippocampal LTD. Neuron. 2005;47:487–494. doi: 10.1016/j.neuron.2005.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paz Gavilan M, Vela J, Castano A, Ramos B, del Rio JC, Vitorica J, Ruano D. Cellular environment facilitates protein accumulation in aged rat hippocampus. Neurobiol. Aging. 2006;27:973–982. doi: 10.1016/j.neurobiolaging.2005.05.010. [DOI] [PubMed] [Google Scholar]
- Philip N, Acevedo SF, Skoulakis EM. Conditional rescue of olfactory learning and memory defects in mutants of the 14-3-3zeta gene leonardo. J. Neurosci. 2001;21:8417–8425. doi: 10.1523/JNEUROSCI.21-21-08417.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poon HF, Calabrese V, Calvani M, Butterfield DA. Proteomics analyses of specific protein oxidation and protein expression in aged rat brain and its modulation by L-acetylcarnitine: insights into the mechanisms of action of this proposed therapeutic agent for CNS disorders associated with oxidative stress. Antioxid. Redox. Signal. 2006a;8:381–394. doi: 10.1089/ars.2006.8.381. [DOI] [PubMed] [Google Scholar]
- Poon HF, Shepherd HM, Reed TT, et al. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: Mitochondrial dysfunction, glutamate dysregulation and impaired protein synthesis. Neurobiol. Aging. 2006b;27:1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- Pozuelo RM, Geraghty KM, Wong BH, Wood NT, Campbell DG, Morrice N, Mackintosh C. 14-3-3-affinity purification of over 200 human phosphoproteins reveals new links to regulation of cellular metabolism, proliferation and trafficking. Biochem. J. 2004;379:395–408. doi: 10.1042/BJ20031797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rapp PR, Gallagher M. Preserved neuron number in the hippocampus of aged rats with spatial learning deficits. Proc. Natl. Acad. Sci. U. S. A. 1996;93:9926–9930. doi: 10.1073/pnas.93.18.9926. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasmussen T, Schliemann T, Sorensen JC, Zimmer J, West MJ. Memory impaired aged rats: no loss of principal hippocampal and subicular neurons. Neurobiol. Aging. 1996;17:143–147. doi: 10.1016/0197-4580(95)02032-2. [DOI] [PubMed] [Google Scholar]
- Rosenzweig ES, Barnes CA. Impact of aging on hippocampal function: plasticity, network dynamics, and cognition. Prog. Neurobiol. 2003;69:143–179. doi: 10.1016/s0301-0082(02)00126-0. [DOI] [PubMed] [Google Scholar]
- Rowe WB, Blalock EM, Chen KC, et al. Hippocampal expression analyses reveal selective association of immediate-early, neuroenergetic, and myelinogenic pathways with cognitive impairment in aged rats. J. Neurosci. 2007;27:3098–3110. doi: 10.1523/JNEUROSCI.4163-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rubino T, Realini N, Braida D, et al. Changes in hippocampal morphology and neuroplasticity induced by adolescent THC treatment are associated with cognitive impairment in adulthood. Hippocampus. 2009;19:763–772. doi: 10.1002/hipo.20554. [DOI] [PubMed] [Google Scholar]
- Sametsky EA, Disterhoft JF, Geinisman Y, Nicholson DA. Synaptic strength and postsynaptically silent synapses through advanced aging in rat hippocampal CA1 pyramidal neurons. Neurobiol. Aging. 2008 doi: 10.1016/j.neurobiolaging.2008.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato Y, Yamanaka H, Toda T, Shinohara Y, Endo T. Comparison of hippocampal synaptosome proteins in young-adult and aged rats. Neurosci. Lett. 2005;382:22–26. doi: 10.1016/j.neulet.2005.02.053. [DOI] [PubMed] [Google Scholar]
- Schoch S, Deak F, Konigstorfer A, Mozhayeva M, Sara Y, Sudhof TC, Kavalali ET. SNARE function analyzed in synaptobrevin/VAMP knockout mice. Science. 2001;294:1117–1122. doi: 10.1126/science.1064335. [DOI] [PubMed] [Google Scholar]
- Shi L, Adams MM, Linville MC, Newton IG, Forbes ME, Long AB, Riddle DR, Brunso-Bechtold JK. Caloric restriction eliminates the aging-related decline in NMDA and AMPA receptor subunits in the rat hippocampus and induces homeostasis. Exp. Neurol. 2007;206:70–79. doi: 10.1016/j.expneurol.2007.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Linville MC, Tucker EW, Sonntag WE, Brunso-Bechtold JK. Differential effects of aging and insulin-like growth factor-1 on synapses in CA1 of rat hippocampus. Cereb. Cortex. 2005;15:571–577. doi: 10.1093/cercor/bhh158. [DOI] [PubMed] [Google Scholar]
- Sierra-Mercado D, Dieguez D, Jr, Barea-Rodriguez EJ. Brief novelty exposure facilitates dentate gyrus LTP in aged rats. Hippocampus. 2008;18:835–843. doi: 10.1002/hipo.20447. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sonntag WE, Lynch CD, Cooney PT, Hutchins PM. Decreases in cerebral microvasculature with age are associated with the decline in growth hormone and insulin-like growth factor 1. Endocrinology. 1997;138:3515–3520. doi: 10.1210/endo.138.8.5330. [DOI] [PubMed] [Google Scholar]
- Tafoya LC, Mameli M, Miyashita T, Guzowski JF, Valenzuela CF, Wilson MC. Expression and function of SNAP-25 as a universal SNARE component in GABAergic neurons. J. Neurosci. 2006;26:7826–7838. doi: 10.1523/JNEUROSCI.1866-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tao-Cheng JH, Du J, McBain CJ. Snap-25 is polarized to axons and abundant along the axolemma: an immunogold study of intact neurons. J. Neurocytol. 2000;29:67–77. doi: 10.1023/a:1007168231323. [DOI] [PubMed] [Google Scholar]
- Thibault O, Hadley R, Landfield PW. Elevated postsynaptic [Ca2+]i and L-type calcium channel activity in aged hippocampal neurons: relationship to impaired synaptic plasticity. J. Neurosci. 2001;21:9744–9756. doi: 10.1523/JNEUROSCI.21-24-09744.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tzingounis AV, Kobayashi M, Takamatsu K, Nicoll RA. Hippocalcin gates the calcium activation of the slow afterhyperpolarization in hippocampal pyramidal cells. Neuron. 2007;53:487–493. doi: 10.1016/j.neuron.2007.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- VanGuilder HD, Brucklacher RM, Patel K, Ellis RW, Freeman WM, Barber AJ. Diabetes downregulates presynaptic proteins and reduces basal synapsin I phosphorylation in rat retina. Eur. J. Neurosci. 2008;28:1–11. doi: 10.1111/j.1460-9568.2008.06322.x. [DOI] [PubMed] [Google Scholar]
- Viswanathan S, Unlu M, Minden JS. Two-dimensional difference gel electrophoresis. Nat. Protoc. 2006;1:1351–1358. doi: 10.1038/nprot.2006.234. [DOI] [PubMed] [Google Scholar]
- Washbourne P, Thompson PM, Carta M, et al. Genetic ablation of the t-SNARE SNAP-25 distinguishes mechanisms of neuroexocytosis. Nat. Neurosci. 2002;5:19–26. doi: 10.1038/nn783. [DOI] [PubMed] [Google Scholar]
- Weinreb O, Drigues N, Sagi Y, Reznick AZ, Amit T, Youdim MB. The application of proteomics and genomics to the study of age-related neurodegeneration and neuroprotection. Antioxid. Redox. Signal. 2007;9:169–179. doi: 10.1089/ars.2007.9.169. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gureviciene I, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Cognitive aging and the hippocampus: how old rats represent new environments. J. Neurosci. 2004;24:3870–3878. doi: 10.1523/JNEUROSCI.5205-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol. Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.