Abstract
OBJECTIVES
Previous investigators have demonstrated the ability to use serum prostate-specific antigen (PSA) levels to estimate prostate volume (PV) in men without prostate cancer; however, the ability of additional clinical variables to further enhance PV estimation in these men remains unclear. Motivated by this, we utilized two population-based samples of prostate cancer-free men to develop and validate a novel, multivariable equation for estimating PV.
METHODS
We applied linear regression modeling to data from an 80% random sample (n=366) of the baseline cohort from the Olmsted County Study of Urinary Symptoms and Health Status among Men (OCS) to develop an equation for estimating PV in men without prostate cancer. We then evaluated the predictive ability of this equation by comparing estimated and measured PV values in three additional validation sets of men.
RESULTS
The final linear regression model included PSA, age, and weight as independent predictors of PV. For prediction in baseline OCS men, the multiple correlation coefficients increased from 0.62 PSA alone to 0.71 full model. Additionally, the area under the curve estimates from the receiver operating characteristic curves increased from 0.79 PSA alone to 0.85 full model for predicting PV >30 mL.
CONCLUSIONS
Our data suggest that PV can be estimated with easily obtained clinical variables. Moreover, we demonstrate that age and weight can be added to PSA level to achieve greater accuracy in predicting PV. This methodology may prove useful for estimating PV in men in settings where costs and practicality preclude the use of imaging techniques.
Keywords: Prostate volume, PSA, prediction
INTRODUCTION
Obtaining accurate measures of prostate volume (PV) is often important in clinical and epidemiologic studies of benign prostatic enlargement and lower urinary tract symptoms as PV has been associated with increased risk of acute urinary retention and need for treatment.1,2 That being said, currently available techniques for assessing PV tend to be inappropriate for large studies of healthy men. While digital rectal exams (DREs) are simple to perform and are useful for estimating PV, authors of previous studies have indicated that this method underestimates actual PV.3 Transrectal ultrasound (TRUS) or imaging techniques are considered the gold standard for accurately assessing PV.4–6 Unfortunately, these procedures are expensive, time consuming and uncomfortable, limiting their use for large-scale epidemiologic studies. An alternative, less-invasive method of estimating PV would be useful for research studies, particularly among men who do not have significant clinical urologic symptoms.
Elevated serum prostate-specific antigen (PSA) levels are currently used clinically as a screening tool for prostate cancer. In addition, investigators have also reported that serum PSA levels show a positive correlation with PV,7–9 and therefore provide a means of estimating PV among men without prostate cancer.10–15 Citing the reported correlation between PSA and PV, previous authors have created simple equations for predicting PV based on serum PSA level and age. To expand on these findings, we used data from a cohort of community-based white men with no history of prostate cancer to develop and validate a multivariable prediction model to estimate PV and further ascertained the utility of that model in a community-based sample of cancer-free black men.
METHODS
Data from men participating in the Olmsted County Study of Urinary Symptoms and Health Status among Men (OCS) and the Flint Men’s Health Study (FMHS) were used in the analyses described for this report. These studies received approval from the Mayo Clinic, Olmsted Medical Center and University of Michigan Institutional Review Boards.
The OCS is an ongoing cohort study of urologic conditions in a community population of white men. Details of the study have been previously published.16,17 Briefly, the community medical records of a random sample of potential participants were screened for indications of prostate surgery, denervated or surgically treated bladder, urethral stricture, or debilitating central nervous system disease. Following these exclusions, 3,874 men were invited to participate, and 2,115 (55%) were enrolled in the cohort and completed surveys that included questions similar to the American Urological Association Symptom Index (AUASI). A 25% random sub-sample 476/537 (88%) participated in a full urologic exam which included measurement of weight and height, a transrectal ultrasound (TRUS) to measure PV, and a blood draw to measure serum PSA level.
The cohort has been followed biennially since 1990. Men who died or were lost to follow-up were replaced during rounds 2 and 3. These replacement men (n=159) comprised the second validation set of the predictive models (described below).
The FMHS cohort consists of a probability sample of black men from households located in Genesee County, Michigan in 1996.18 Eligible men were stratified into ten-year age groups: 40–49, 50–59, 60–69, and 70–79. Subjects were ineligible if they reported a history of prostate cancer or a prior operation on the prostate gland. A trained interviewer contacted each sample household, identified 730 eligible subjects, and performed a detailed in-home interview which included completion of the AUASI as well as demographic and other lifestyle information. All participants were invited to participate in a comprehensive urologic examination, similar to that received by men participating in the OCS study. Of the 730 men, 379 (52%) completed the exam phase of the study. Men participating in this study comprised the third validation set (described below).
Measures of PV
Total PV for the OCS participants was measured via transrectal ultrasound (TRUS) using a 7.5 MHz biplanar endorectal transducer. In addition to assessing the echogenic pattern of the prostate gland, three measurements were made to calculate the total volume of the prostate assuming an ellipsoid shape.5
Similarly, total PV for the FMHS participants was also measured via TRUS (B&K Medical, Denmark; Hitachi Medical Systems, Tarrytown, NY), and two urologists independently measured prostate length, width, and height. If a measurement differed by more than 50% between the two readers, a third independent expert measured the prostate again. The final PV represents the mean volume calculated by the two closest volume estimates, assuming an ellipsoid shape.18,19
Measures of PSA
Serum PSA levels for the OCS cohort were determined in one laboratory. Baseline samples were tested with the tandem-R monoclonal chemi-radiometric PSA assay (Hybritech Incorporated San Diego, California) according to manufacturer specifications. For the second and third biennial rounds, samples were tested with the Abbott IMx assay (Abbott Diagnostics, Abbott Park, Illinois). During this time period, the coefficient of variation averaged 3–4% (GG Klee, personal communication). Serum samples were obtained prior to any prostatic manipulations, including digital rectal examination and transrectal ultrasound. We have previously reported that PSA determinations were consistent across different assays and laboratories.20
Serum PSA levels for men in the FMHS were assayed using the Abbott AxSYM polyclonal-monoclonal immunoassay (Abbott Diagnostics, Abbott Park, IL). The lower limit of detection for this assay is reported to be 0.1 ng/mL.21
Anthropometric Measures
Participant weights were measured using a beam balance scale, while heights were measured using a stadiometer without shoes. Measurements were obtained by trained study coordinators using a standardized protocol.
Analyses
A split-sample design, with one estimation set and three validation sets was used. The estimation set consisted of an 80% random sample of cancer-free men participating in the baseline OCS (n=376). Using data from this estimation set, we performed least-squares regression to estimate PV based on serum PSA level alone. Subsequently, we developed a forward selection multivariable prediction model to select independent predictors of PV using the following covariates: serum PSA level, age, weight, height, AUA symptom score and all two-way interactions. Due to skewed distributions, PV and serum PSA measures were natural log-transformed in all analyses.
Our first two validation sets consisted of the remaining 20% random sample of OCS baseline men (n=100) and the OCS replacement men (n=159). The third validation set was obtained from the FMHS (n=379). The equation from the estimation set was used to estimate PV in each of the three validation sets and the estimated results were compared with the measured values. An approximate R2 for prediction was obtained from [1 – (sum of squares for the prediction (observed – model predicted) error divided by the corrected total sum of squares in the prediction data set)].22 Linear regression R2 values from the estimation data set were compared to R2 values for prediction in the validation data sets. Multivariable linear logistic regression models were used to determine area under the curve estimates from the receiver operating characteristic (AUC ROC) curves predicting volume >30 mL and volume >40 mL for each data set. Men with known prostate cancer were excluded from all analyses.
RESULTS
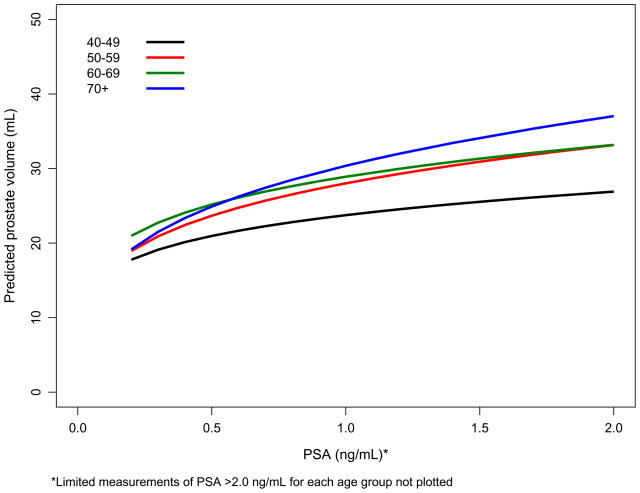
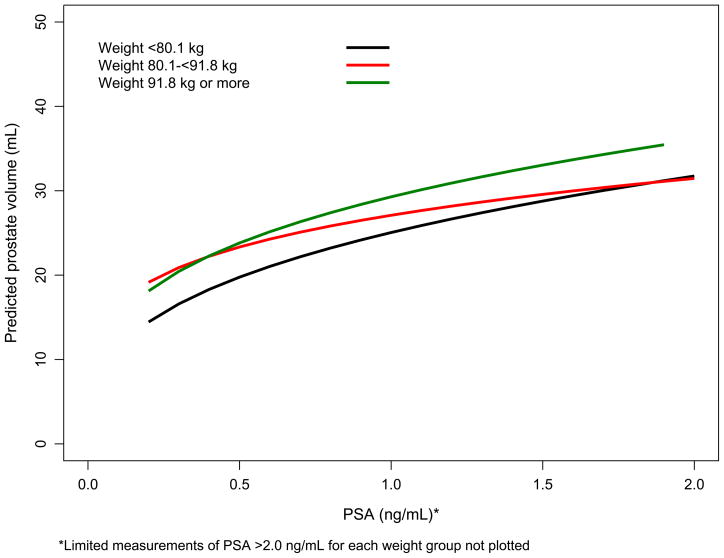
Complete data were available for 466 (98%) men in the OCS baseline clinic subset, 149 (94%) of OCS replacement men, and 344 (91%) men from the FMHS. Baseline characteristics of the participants in each of the data sets are shown in Table 1. OCS replacement men were slightly younger, had smaller prostates, and lower serum PSA levels than the other three cohorts. PV increases with PSA were greater for men who were older and with higher baseline weight (Figure 1a and 1b).
Table 1.
Baseline characteristics of the estimation and validation data sets: Olmsted County and Flint Men’s Health studies
| Estimation Data Set | Remaining OCS Baseline Men (20%) | Validation Data Sets | FMHS Men | |
|---|---|---|---|---|
| OCS Baseline Men (80%) | OCS Replacement Men | |||
| n = 366 | n = 100 | n = 149 | n = 344 | |
| Characteristic | Median (Q1, Q3) | Median (Q1, Q3) | Median (Q1, Q3) | Median (Q1, Q3) |
| Race | White | White | White | Black |
| Age (years) | 54.25 (47.12, 64.45) | 55.06 (45.43, 62.75) | 46.20 (43.99, 54.41) | 55.00 (48.00, 65.00) |
| AUA Symptom Score | 5.70 (3.00, 9.00) | 6.00 (3.00, 9.00) | 5.00 (2.00, 8.00) | 6.00 (2.00, 11.00) |
| Prostate Volume (mL) | 26.36 (20.92, 33.80) | 27.46 (20.94, 37.04) | 22.76 (19.30, 27.82) | 25.96 (20.10, 33.18) |
| Serum PSA (ng/mL) | 1.00 (0.60, 1.70) | 1.00 (0.60, 1.80) | 0.70 (0.50, 1.20) | 0.95 (0.56, 1.71) |
| Height (cm) | 178.00 (173.00, 182.00) | 177.00 (173.00, 181.00) | 177.00 (172.00, 182.00) | 180.34 (172.72, 182.88) |
| Weight (kg) | 85.70 (76.80, 96.80) | 86.75 (78.30, 96.25) | 86.00 (78.80, 98.20) | 85.50 (76.28, 96.98) |
Figure 1.
Figure 1a. Predicted PV versus serum PSA level by age decade based on OCS baseline men (80%; Figure 1b. Predicted PV versus serum PSA level by weight tertiles based on OCS baseline men (80%).
The final linear regression model, based on data from 366 men in the baseline OCS cohort, included log serum PSA (p<0.001), age (p<0.001), and weight (p<0.001) as independent predictors of PV. Based on this model, estimated natural log (PV (mL)) = 2.25881 (standard error (se)=0.13810) + 0.2385 (se=0.02079) natural log (serum PSA level (ng/mL)) + 0.0096 (se=0.00152) age (in years) + 0.0058 (se=0.00107) weight (kg). Hence, a man aged 62 years, weighing 74 kg with a serum PSA level of 2.1 ng/mL, had an estimated PV of 31.8 mL. The regression of log PV on log serum PSA level had a multiple correlation coefficient (R) of 0.57 in the estimation data set, which increased to 0.65 when age and weight were added to the prediction model.
When applied to the validation samples, the prediction R values were 0.71, 0.69, and 0.63 for the remaining baseline sample, OCS replacement men, and men from the FMHS, respectively (all p<0.001) using the model based on log PSA, age and weight. The pooled average standard deviation about the predicted line (root mean square error) was 0.28. The percent of the variability (R2) using the validations data sets, as compared to the variability explained by using the full prediction equation from the estimation data set was 54% vs. 51%, 54% vs. 48%, and 42% vs. 40%, for the remaining OCS baseline, OCS replacement and FMHS validation data sets, respectively.
The full model performed better than PSA alone at predicting which men had a PV >30 mL and >40 mL (Table 2). Overall, the AUC ROC ranged from 0.76 to 0.85 for the model predicting PV >30 mL with PSA alone and consistently increased to over 0.80 when age and weight were added to the prediction equation. The AUC ROC for the models predicting PVs >40 mL were ≥0.79 for both models. To minimize |sensitivity – specificity| in prediction of PV >40 mL, age-specific cut-points were serum PSA >1.3 ng/mL, >2.0 ng/mL and >2.2 ng/mL for men in their 50’s, 60’s and 70’s, respectively. At these cut-points, sensitivity and specificity were 70% for men in their 50’s and increased to 80% for men in their 60’s and 70’s.
Table 2.
Area under the curve (AUC) estimates from the receiver operating characteristic curves predicting prostate volume >30 or >40 mL
| Estimation Data Set | Remaining OCS Baseline Men (20%) | Validation Data Sets | FMHS Men | |
|---|---|---|---|---|
| OCS Baseline Men (80%) | OCS Replacement Men | |||
| n = 366 | n = 100 | n = 149 | n = 344 | |
| AUC (std err) | AUC (std err) | AUC (std err) | AUC (std err) | |
| Volume >30 mL | ||||
| PSA alone | 0.76 (0.03) | 0.79 (0.04) | 0.85 (0.05) | 0.79 (0.02) |
| PSA, weight, age | 0.81 (0.02) | 0.85 (0.04) | 0.91 (0.03) | 0.83 (0.02) |
| Volume >40 mL | ||||
| PSA alone | 0.88 (0.02) | 0.86 (0.04) | 0.97 (0.01) | 0.79 (0.04) |
| PSA, weight, age | 0.89 (0.02) | 0.89 (0.04) | 0.98 (0.01) | 0.85 (0.03) |
COMMENT
The ability to obtain accurate estimates of PV without the need for invasive, uncomfortable TRUS procedures has the potential to increase participation rates in research studies and thereby reduce concerns regarding potential participation bias.
Related to this, previous investigators have shown that PSA can be used to estimate PV. In this report, we confirm that PSA level is the single strongest predictor of PV; however, age and weight can be added to the prediction model to further enhance the accuracy of PV prediction. More importantly, we show that our multivariate prediction model maintains a higher level of accuracy compared to PSA alone when applied to three separate validation data sets, including a population of community-based black men.
Our study results are in agreement with previous studies that have estimated PVs from PSA levels. Roehrborn and colleagues have previously found, in a sample of 4,448 men with benign prostatic hyperplasia, that serum PSA level predicted PV with an AUC ROC of 0.76 for PV greater than 40 mL with age-specific cut-points of PSA >1.6 ng/mL, >2.0 ng/mL and >2.3 ng/mL for men in their 50’s, 60’s and 70’s, respectively.10 In a community-based sample of Dutch men, serum PSA level was also found to be the strongest predictor of PV and a more accurate predictor than DRE,11 with an AUC ROC of 0.86 for PV greater than 40 mL. In studies of Korean men, serum PSA level was predictive of PV >40 mL with an AUC ROC of >0.80; however, the serum PSA thresholds for detecting a PV >40 mL were lower than in white men.12,15
In this study, we also saw a consistent 10% improvement in the percent of variability explained when using the full predictive model compared to serum PSA level alone. While the level of discrimination using serum PSA level alone was in the range considered to be reasonable or acceptable in detecting men with a prostate volume >30 mL (AUC ROC range from 0.76 to 0.85),23 the level of discrimination increased with the inclusion of age and weight in the model to a good to excellent area under the curve level (0.81 to 0.91).23
Multiple techniques were used to assess the validity of the prediction model. The sign and magnitude of the empirically-derived model coefficients were reasonable and there was little difference in the model coefficients in the validation models as compared the coefficients in the estimation model (data not shown). Additionally, there was only a small decrease in the percent of explained variability in the actual data, as compared to that from the prediction equation, which provides evidence that the least squares model can be used in prediction. However, we also note that although approximately half of the variability in PV (as measured by TRUS) may be explained by these three variables, half the variability remains unexplained.
Strengths of this study include the ability to measure serum PSA level and PV in a community-based sample of white men and a split-sample design which allowed for two validation data sets from the same population of men. Additionally, a third data set allowed for validation in a community-based sample of black men. Further generalizability may be limited as additional racial groups were not assessed and men with known prostate cancer were excluded. Finally, our model may not be applicable to specially selected clinical-based samples with different baseline characteristics.
CONCLUSION
These data suggest that prostate volume can be estimated with easily obtained clinical variables. While our data confirm that serum PSA level is the single strongest predictor of prostate volume, we demonstrate that age and weight can be added to serum PSA level to achieve greater accuracy when estimating prostate volume. In cases where the goal is to determine whether the prostate is enlarged, serum PSA tests, along with routine clinical exam measures (age and weight), and provided formulas offer an easy, cost-effective, and less-invasive mechanism to estimate prostate volume.
Acknowledgments
This project was supported by research grants from the Public Health Service, National Institutes of Health (DK58859, AR30582 and 1UL1 RR024150-01), and Merck Research Laboratories.
The authors thank the members of the Mayo Clinic research team for their excellent work and Ms. Sondra Buehler for her assistance with manuscript preparation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Natural history of prostatism: risk factors for acute urinary retention. J Urol. 1997;158:481–487. doi: 10.1016/s0022-5347(01)64508-7. [DOI] [PubMed] [Google Scholar]
- 2.Jacobsen SJ, Jacobson DJ, Girman CJ, et al. Treatment for benign prostatic hyperplasia among community dwelling men: the Olmsted County study of urinary symptoms and health status. J Urol. 1999;162:1301–1306. [PubMed] [Google Scholar]
- 3.Roehrborn CG, Girman CJ, Rhodes T, et al. Correlation between prostate size estimated by digital rectal examination and measured by transrectal ultrasound. Urology. 1997;49:548–557. doi: 10.1016/s0090-4295(97)00031-9. [DOI] [PubMed] [Google Scholar]
- 4.Tong S, Cardinal HN, McLoughlin RF, et al. Intra- and inter-observer variability and reliability of prostate volume measurement via two-dimensional and three-dimensional ultrasound imaging. Ultrasound Med Biol. 1998;24:673–681. doi: 10.1016/s0301-5629(98)00039-8. [DOI] [PubMed] [Google Scholar]
- 5.Terris MK, Stamey TA. Determination of prostate volume by transrectal ultrasound. J Urol. 1991;145:984–987. doi: 10.1016/s0022-5347(17)38508-7. [DOI] [PubMed] [Google Scholar]
- 6.Torp-Pedersen S, Juul N, Jakobsen H. Transrectal prostatic ultrasonography. Equipment, normal findings, benign hyperplasia and cancer. Scand J Urol Nephrol Suppl. 1988;107:19–25. [PubMed] [Google Scholar]
- 7.Oesterling JE, Jacobsen SJ, Chute CG, et al. Serum prostate-specific antigen in a community-based population of healthy men. Establishment of age-specific reference ranges. JAMA. 1993;270:860–864. [PubMed] [Google Scholar]
- 8.Collins GN, Lee RJ, McKelvie GB, et al. Relationship between prostate specific antigen, prostate volume and age in the benign prostate. Br J Urol. 1993;71:445–450. doi: 10.1111/j.1464-410x.1993.tb15990.x. [DOI] [PubMed] [Google Scholar]
- 9.Lim KB, Ho H, Foo KT, et al. Comparison of intravesical prostatic protrusion, prostate volume and serum prostatic-specific antigen in the evaluation of bladder outlet obstruction. Int J Urol. 2006;13:1509–1513. doi: 10.1111/j.1442-2042.2006.01611.x. [DOI] [PubMed] [Google Scholar]
- 10.Roehrborn CG, Boyle P, Gould AL, et al. Serum prostate-specific antigen as a predictor of prostate volume in men with benign prostatic hyperplasia. Urology. 1999;53:581–589. doi: 10.1016/s0090-4295(98)00655-4. [DOI] [PubMed] [Google Scholar]
- 11.Bohnen AM, Groeneveld FP, Bosch JL. Serum prostate-specific antigen as a predictor of prostate volume in the community: the Krimpen study. Eur Urol. 2007;51:1645–1652. doi: 10.1016/j.eururo.2007.01.084. discussion 1652-1643. [DOI] [PubMed] [Google Scholar]
- 12.Chung BH, Hong SJ, Cho JS, et al. Relationship between serum prostate-specific antigen and prostate volume in Korean men with benign prostatic hyperplasia: a multicentre study. BJU Int. 2006;97:742–746. doi: 10.1111/j.1464-410X.2006.06016.x. [DOI] [PubMed] [Google Scholar]
- 13.Mochtar CA, Kiemeney LA, van Riemsdijk MM, et al. Prostate-specific antigen as an estimator of prostate volume in the management of patients with symptomatic benign prostatic hyperplasia. Eur Urol. 2003;44:695–700. doi: 10.1016/s0302-2838(03)00384-1. [DOI] [PubMed] [Google Scholar]
- 14.Hochberg DA, Armenakas NA, Fracchia JA. Relationship of prostate-specific antigen and prostate volume in patients with biopsy proven benign prostatic hyperplasia. Prostate. 2000;45:315–319. doi: 10.1002/1097-0045(20001201)45:4<315::aid-pros5>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 15.Shim HB, Lee JK, Jung TY, et al. Serum prostate-specific antigen as a predictor of prostate volume in Korean men with lower urinary tract symptoms. Prostate Cancer Prostatic Dis. 2007;10:143–148. doi: 10.1038/sj.pcan.4500937. [DOI] [PubMed] [Google Scholar]
- 16.Chute CG, Panser LA, Girman CJ, et al. The prevalence of prostatism: a population-based survey of urinary symptoms. J Urol. 1993;150:85–89. doi: 10.1016/s0022-5347(17)35405-8. [DOI] [PubMed] [Google Scholar]
- 17.Jacobsen SJ, Guess HA, Panser L, et al. A population-based study of health care-seeking behavior for treatment of urinary symptoms. The Olmsted County Study of Urinary Symptoms and Health Status among Men. Arch Fam Med. 1993;2:729–735. doi: 10.1001/archfami.2.7.729. [DOI] [PubMed] [Google Scholar]
- 18.Wei JT, Schottenfeld D, Cooper K, et al. The natural history of lower urinary tract symptoms in black American men: relationships with aging, prostate size, flow rate and bothersomeness. J Urol. 2001;165:1521–1525. [PubMed] [Google Scholar]
- 19.Joseph MA, Wei JT, Harlow SD, et al. Relationship of serum sex-steroid hormones and prostate volume in African American men. Prostate. 2002;53:322–329. doi: 10.1002/pros.10154. [DOI] [PubMed] [Google Scholar]
- 20.Jacobsen SJ, Klee GG, Lilja H, et al. Stability of serum prostate-specific antigen determination across laboratory, assay, and storage time. Urology. 1995;45:447–453. doi: 10.1016/S0090-4295(99)80014-4. [DOI] [PubMed] [Google Scholar]
- 21.Cooney KA, Strawderman MS, Wojno KJ, et al. Age-specific distribution of serum prostate-specific antigen in a community-based study of African-American men. Urology. 2001;57:91–96. doi: 10.1016/s0090-4295(00)00873-6. [DOI] [PubMed] [Google Scholar]
- 22.Montgomery D, Peck E. Validation of regression models. In: Bradley RA, Hunter JS, Kendall DG, Watson GS, editors. Introduction to Linear Regression Analysis. New York: John Wiley & Sons; 1982. pp. 424–444. [Google Scholar]
- 23.Hanley JA, McNeil BJ. The meaning and use of the area under a receiver operating characteristic (ROC) curve. Radiology. 1982;143:29–36. doi: 10.1148/radiology.143.1.7063747. [DOI] [PubMed] [Google Scholar]