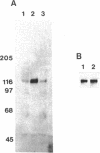
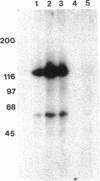
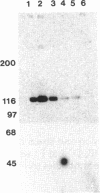
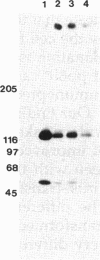
Abstract
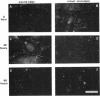
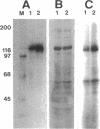
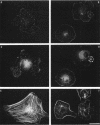
Tyrosine phosphorylation of cytoskeletal proteins occurs during integrin-mediated cell adhesion to extracellular matrix proteins. We have investigated the role of tyrosine phosphorylation in the migration and initial spreading of human umbilical vein endothelial cells (HUVEC). Elevated phosphotyrosine concentrations were noted in the focal adhesions of HUVEC migrating into wounds. Anti-phosphotyrosine Western blots of extracts of wounded HUVEC monolayers demonstrated increased phosphorylation at 120-130 kDa when compared with extracts of intact monolayers. The pp125FAK immunoprecipitated from wounded monolayers exhibited increased kinase activity as compared to pp125FAK from intact monolayers. The time to wound closure in HUVEC monolayers was doubled by tyrphostin AG 213 treatment. The same concentration of AG 213 interfered with HUVEC focal adhesion and stress fiber formation. AG 213 inhibited adhesion-associated tyrosine phosphorylation of pp125FAK in HUVEC. Tyrphostins AG 213 and AG 808 inhibited pp125FAK activity in in vitro kinase assays. pp125FAK immunoprecipitates from HUVEC treated with both of these inhibitors also had kinase activity in vitro that was below levels seen in untreated HUVEC. These findings suggest that tyrosine phosphorylation of cytoskeletal proteins may be important in HUVEC spreading and migration and that pp125FAK may mediate phosphotyrosine formation during these processes.
Full text
PDF





Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Albelda S. M., Buck C. A. Integrins and other cell adhesion molecules. FASEB J. 1990 Aug;4(11):2868–2880. [PubMed] [Google Scholar]
- Albelda S. M., Daise M., Levine E. M., Buck C. A. Identification and characterization of cell-substratum adhesion receptors on cultured human endothelial cells. J Clin Invest. 1989 Jun;83(6):1992–2002. doi: 10.1172/JCI114109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basson C. T., Kocher O., Basson M. D., Asis A., Madri J. A. Differential modulation of vascular cell integrin and extracellular matrix expression in vitro by TGF-beta 1 correlates with reciprocal effects on cell migration. J Cell Physiol. 1992 Oct;153(1):118–128. doi: 10.1002/jcp.1041530116. [DOI] [PubMed] [Google Scholar]
- Bockholt S. M., Otey C. A., Glenney J. R., Jr, Burridge K. Localization of a 215-kDa tyrosine-phosphorylated protein that cross-reacts with tensin antibodies. Exp Cell Res. 1992 Nov;203(1):39–46. doi: 10.1016/0014-4827(92)90037-9. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Burridge K., Petch L. A., Romer L. H. Signals from focal adhesions. Curr Biol. 1992 Oct;2(10):537–539. doi: 10.1016/0960-9822(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Burridge K., Turner C. E., Romer L. H. Tyrosine phosphorylation of paxillin and pp125FAK accompanies cell adhesion to extracellular matrix: a role in cytoskeletal assembly. J Cell Biol. 1992 Nov;119(4):893–903. doi: 10.1083/jcb.119.4.893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bussolino F., Di Renzo M. F., Ziche M., Bocchietto E., Olivero M., Naldini L., Gaudino G., Tamagnone L., Coffer A., Comoglio P. M. Hepatocyte growth factor is a potent angiogenic factor which stimulates endothelial cell motility and growth. J Cell Biol. 1992 Nov;119(3):629–641. doi: 10.1083/jcb.119.3.629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb B. S., Schaller M. D., Leu T. H., Parsons J. T. Stable association of pp60src and pp59fyn with the focal adhesion-associated protein tyrosine kinase, pp125FAK. Mol Cell Biol. 1994 Jan;14(1):147–155. doi: 10.1128/mcb.14.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coomber B. L., Gotlieb A. I. In vitro endothelial wound repair. Interaction of cell migration and proliferation. Arteriosclerosis. 1990 Mar-Apr;10(2):215–222. doi: 10.1161/01.atv.10.2.215. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A. The behaviour of fibroblasts migrating from chick heart explants: changes in adhesion, locomotion and growth, and in the distribution of actomyosin and fibronectin. J Cell Sci. 1979 Oct;39:149–165. doi: 10.1242/jcs.39.1.149. [DOI] [PubMed] [Google Scholar]
- Davies P. F., Robotewskyj A., Griem M. L. Endothelial cell adhesion in real time. Measurements in vitro by tandem scanning confocal image analysis. J Clin Invest. 1993 Jun;91(6):2640–2652. doi: 10.1172/JCI116503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Defilippi P., Silengo L., Tarone G. Alpha 6.beta 1 integrin (laminin receptor) is down-regulated by tumor necrosis factor alpha and interleukin-1 beta in human endothelial cells. J Biol Chem. 1992 Sep 15;267(26):18303–18307. [PubMed] [Google Scholar]
- Dvir A., Milner Y., Chomsky O., Gilon C., Gazit A., Levitzki A. The inhibition of EGF-dependent proliferation of keratinocytes by tyrphostin tyrosine kinase blockers. J Cell Biol. 1991 May;113(4):857–865. doi: 10.1083/jcb.113.4.857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eriksson A., Siegbahn A., Westermark B., Heldin C. H., Claesson-Welsh L. PDGF alpha- and beta-receptors activate unique and common signal transduction pathways. EMBO J. 1992 Feb;11(2):543–550. doi: 10.1002/j.1460-2075.1992.tb05085.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraker P. J., Speck J. C., Jr Protein and cell membrane iodinations with a sparingly soluble chloroamide, 1,3,4,6-tetrachloro-3a,6a-diphrenylglycoluril. Biochem Biophys Res Commun. 1978 Feb 28;80(4):849–857. doi: 10.1016/0006-291x(78)91322-0. [DOI] [PubMed] [Google Scholar]
- Fuortes M., Jin W. W., Nathan C. Adhesion-dependent protein tyrosine phosphorylation in neutrophils treated with tumor necrosis factor. J Cell Biol. 1993 Feb;120(3):777–784. doi: 10.1083/jcb.120.3.777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gimbrone M. A., Jr, Cotran R. S., Folkman J. Human vascular endothelial cells in culture. Growth and DNA synthesis. J Cell Biol. 1974 Mar;60(3):673–684. doi: 10.1083/jcb.60.3.673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gotlieb A. I., May L. M., Subrahmanyan L., Kalnins V. I. Distribution of microtubule organizing centers in migrating sheets of endothelial cells. J Cell Biol. 1981 Nov;91(2 Pt 1):589–594. doi: 10.1083/jcb.91.2.589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guan J. L., Shalloway D. Regulation of focal adhesion-associated protein tyrosine kinase by both cellular adhesion and oncogenic transformation. Nature. 1992 Aug 20;358(6388):690–692. doi: 10.1038/358690a0. [DOI] [PubMed] [Google Scholar]
- Guan J. L., Trevithick J. E., Hynes R. O. Fibronectin/integrin interaction induces tyrosine phosphorylation of a 120-kDa protein. Cell Regul. 1991 Nov;2(11):951–964. doi: 10.1091/mbc.2.11.951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanks S. K., Calalb M. B., Harper M. C., Patel S. K. Focal adhesion protein-tyrosine kinase phosphorylated in response to cell attachment to fibronectin. Proc Natl Acad Sci U S A. 1992 Sep 15;89(18):8487–8491. doi: 10.1073/pnas.89.18.8487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992 Apr 3;69(1):11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- Ingber D. E., Folkman J. How does extracellular matrix control capillary morphogenesis? Cell. 1989 Sep 8;58(5):803–805. doi: 10.1016/0092-8674(89)90928-8. [DOI] [PubMed] [Google Scholar]
- Juliano R. L., Haskill S. Signal transduction from the extracellular matrix. J Cell Biol. 1993 Feb;120(3):577–585. doi: 10.1083/jcb.120.3.577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch A. E., Polverini P. J., Kunkel S. L., Harlow L. A., DiPietro L. A., Elner V. M., Elner S. G., Strieter R. M. Interleukin-8 as a macrophage-derived mediator of angiogenesis. Science. 1992 Dec 11;258(5089):1798–1801. doi: 10.1126/science.1281554. [DOI] [PubMed] [Google Scholar]
- Kolega J., Shure M. S., Chen W. T., Young N. D. Rapid cellular translocation is related to close contacts formed between various cultured cells and their substrata. J Cell Sci. 1982 Apr;54:23–34. doi: 10.1242/jcs.54.1.23. [DOI] [PubMed] [Google Scholar]
- Kornberg L. J., Earp H. S., Turner C. E., Prockop C., Juliano R. L. Signal transduction by integrins: increased protein tyrosine phosphorylation caused by clustering of beta 1 integrins. Proc Natl Acad Sci U S A. 1991 Oct 1;88(19):8392–8396. doi: 10.1073/pnas.88.19.8392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kornberg L., Earp H. S., Parsons J. T., Schaller M., Juliano R. L. Cell adhesion or integrin clustering increases phosphorylation of a focal adhesion-associated tyrosine kinase. J Biol Chem. 1992 Nov 25;267(33):23439–23442. [PubMed] [Google Scholar]
- Kubota Y., Kleinman H. K., Martin G. R., Lawley T. J. Role of laminin and basement membrane in the morphological differentiation of human endothelial cells into capillary-like structures. J Cell Biol. 1988 Oct;107(4):1589–1598. doi: 10.1083/jcb.107.4.1589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Levitzki A. Tyrphostins--potential antiproliferative agents and novel molecular tools. Biochem Pharmacol. 1990 Sep 1;40(5):913–918. doi: 10.1016/0006-2952(90)90474-y. [DOI] [PubMed] [Google Scholar]
- Lipfert L., Haimovich B., Schaller M. D., Cobb B. S., Parsons J. T., Brugge J. S. Integrin-dependent phosphorylation and activation of the protein tyrosine kinase pp125FAK in platelets. J Cell Biol. 1992 Nov;119(4):905–912. doi: 10.1083/jcb.119.4.905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madri J. A., Pratt B. M., Yannariello-Brown J. Matrix-driven cell size change modulates aortic endothelial cell proliferation and sheet migration. Am J Pathol. 1988 Jul;132(1):18–27. [PMC free article] [PubMed] [Google Scholar]
- Molony L., Armstrong L. Cytoskeletal reorganizations in human umbilical vein endothelial cells as a result of cytokine exposure. Exp Cell Res. 1991 Sep;196(1):40–48. doi: 10.1016/0014-4827(91)90454-3. [DOI] [PubMed] [Google Scholar]
- Nakao-Hayashi J., Ito H., Kanayasu T., Morita I., Murota S. Stimulatory effects of insulin and insulin-like growth factor I on migration and tube formation by vascular endothelial cells. Atherosclerosis. 1992 Feb;92(2-3):141–149. doi: 10.1016/0021-9150(92)90273-j. [DOI] [PubMed] [Google Scholar]
- Nuckolls G. H., Romer L. H., Burridge K. Microinjection of antibodies against talin inhibits the spreading and migration of fibroblasts. J Cell Sci. 1992 Aug;102(Pt 4):753–762. doi: 10.1242/jcs.102.4.753. [DOI] [PubMed] [Google Scholar]
- Pelletier A. J., Bodary S. C., Levinson A. D. Signal transduction by the platelet integrin alpha IIb beta 3: induction of calcium oscillations required for protein-tyrosine phosphorylation and ligand-induced spreading of stably transfected cells. Mol Biol Cell. 1992 Sep;3(9):989–998. doi: 10.1091/mbc.3.9.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M. S., Belin D., Montesano R., Orci L., Vassalli J. D. Transforming growth factor-beta 1 modulates basic fibroblast growth factor-induced proteolytic and angiogenic properties of endothelial cells in vitro. J Cell Biol. 1990 Aug;111(2):743–755. doi: 10.1083/jcb.111.2.743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pepper M. S., Sappino A. P., Montesano R., Orci L., Vassalli J. D. Plasminogen activator inhibitor-1 is induced in migrating endothelial cells. J Cell Physiol. 1992 Oct;153(1):129–139. doi: 10.1002/jcp.1041530117. [DOI] [PubMed] [Google Scholar]
- Regen C. M., Horwitz A. F. Dynamics of beta 1 integrin-mediated adhesive contacts in motile fibroblasts. J Cell Biol. 1992 Dec;119(5):1347–1359. doi: 10.1083/jcb.119.5.1347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romer L. H., Burridge K., Turner C. E. Signaling between the extracellular matrix and the cytoskeleton: tyrosine phosphorylation and focal adhesion assembly. Cold Spring Harb Symp Quant Biol. 1992;57:193–202. doi: 10.1101/sqb.1992.057.01.024. [DOI] [PubMed] [Google Scholar]
- Rosen E. M., Liu D., Setter E., Bhargava M., Goldberg I. D. Interleukin-6 stimulates motility of vascular endothelium. EXS. 1991;59:194–205. doi: 10.1007/978-3-0348-7494-6_13. [DOI] [PubMed] [Google Scholar]
- Rozengurt E., Sinnett-Smith J. Bombesin stimulation of DNA synthesis and cell division in cultures of Swiss 3T3 cells. Proc Natl Acad Sci U S A. 1983 May;80(10):2936–2940. doi: 10.1073/pnas.80.10.2936. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schaller M. D., Borgman C. A., Cobb B. S., Vines R. R., Reynolds A. B., Parsons J. T. pp125FAK a structurally distinctive protein-tyrosine kinase associated with focal adhesions. Proc Natl Acad Sci U S A. 1992 Jun 1;89(11):5192–5196. doi: 10.1073/pnas.89.11.5192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwartz M. A. Spreading of human endothelial cells on fibronectin or vitronectin triggers elevation of intracellular free calcium. J Cell Biol. 1993 Feb;120(4):1003–1010. doi: 10.1083/jcb.120.4.1003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seckl M., Rozengurt E. Tyrphostin inhibits bombesin stimulation of tyrosine phosphorylation, c-fos expression, and DNA synthesis in Swiss 3T3 cells. J Biol Chem. 1993 May 5;268(13):9548–9554. [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Fukazawa H., Murakami Y., Mizuno S. Irreversible inhibition of v-src tyrosine kinase activity by herbimycin A and its abrogation by sulfhydryl compounds. Biochem Biophys Res Commun. 1989 Sep 15;163(2):803–809. doi: 10.1016/0006-291x(89)92293-6. [DOI] [PubMed] [Google Scholar]
- Uehara Y., Hori M., Takeuchi T., Umezawa H. Phenotypic change from transformed to normal induced by benzoquinonoid ansamycins accompanies inactivation of p60src in rat kidney cells infected with Rous sarcoma virus. Mol Cell Biol. 1986 Jun;6(6):2198–2206. doi: 10.1128/mcb.6.6.2198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uehara Y., Hori M., Takeuchi T., Umezawa H. Screening of agents which convert 'transformed morphology' of Rous sarcoma virus-infected rat kidney cells to 'normal morphology': identification of an active agent as herbimycin and its inhibition of intracellular src kinase. Jpn J Cancer Res. 1985 Aug;76(8):672–675. [PubMed] [Google Scholar]
- Weiner T. M., Liu E. T., Craven R. J., Cance W. G. Expression of focal adhesion kinase gene and invasive cancer. Lancet. 1993 Oct 23;342(8878):1024–1025. doi: 10.1016/0140-6736(93)92881-s. [DOI] [PubMed] [Google Scholar]
- Wong M. K., Gotlieb A. I. In vitro reendothelialization of a single-cell wound. Role of microfilament bundles in rapid lamellipodia-mediated wound closure. Lab Invest. 1984 Jul;51(1):75–81. [PubMed] [Google Scholar]
- Woodford-Thomas T. A., Rhodes J. D., Dixon J. E. Expression of a protein tyrosine phosphatase in normal and v-src-transformed mouse 3T3 fibroblasts. J Cell Biol. 1992 Apr;117(2):401–414. doi: 10.1083/jcb.117.2.401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang E. Y., Moses H. L. Transforming growth factor beta 1-induced changes in cell migration, proliferation, and angiogenesis in the chicken chorioallantoic membrane. J Cell Biol. 1990 Aug;111(2):731–741. doi: 10.1083/jcb.111.2.731. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yost J. C., Herman I. M. Substratum-induced stress fiber assembly in vascular endothelial cells during spreading in vitro. J Cell Sci. 1990 Mar;95(Pt 3):507–520. doi: 10.1242/jcs.95.3.507. [DOI] [PubMed] [Google Scholar]
- Young W. C., Herman I. M. Extracellular matrix modulation of endothelial cell shape and motility following injury in vitro. J Cell Sci. 1985 Feb;73:19–32. doi: 10.1242/jcs.73.1.19. [DOI] [PubMed] [Google Scholar]
- Zachary I., Rozengurt E. Focal adhesion kinase (p125FAK): a point of convergence in the action of neuropeptides, integrins, and oncogenes. Cell. 1992 Dec 11;71(6):891–894. doi: 10.1016/0092-8674(92)90385-p. [DOI] [PubMed] [Google Scholar]
- Zachary I., Sinnett-Smith J., Rozengurt E. Bombesin, vasopressin, and endothelin stimulation of tyrosine phosphorylation in Swiss 3T3 cells. Identification of a novel tyrosine kinase as a major substrate. J Biol Chem. 1992 Sep 25;267(27):19031–19034. [PubMed] [Google Scholar]