Clinically many orofacial pain complaints including trigeminal neuralgia, tooth extractions, and pain associated with orthodontic tooth movement involve a mechanical component (i.e., touch sensitivity or pain with injury). Although the characteristics of clinical orofacial pain are well described, evaluation of orofacial pain in animals is challenging. Previously, assessments of trigeminal nerve-mediated nociceptive responses have been limited to methods that assess processing within the brain stem [5, 10, 17, 23], utilizing unlearned behaviors that were elicited by mechanical sensitivity using von Frey filaments [23] or thermal stimulation [9]. Our lab has previously reported the development of a novel thermal operant facial testing system that provides an investigator-independent behavioral assessment system (Fig 1A). We use operant conflict paradigms to establish a behavioral outcome in which an animal can decide between receiving a reward and escaping an aversive stimulus [13,18]. The current report utilizes a modification of our original operant test system by adding a simple mechanical component. This new modification provides investigators with an opportunity to assess both mechanical and thermal assessments of facial allodynia, hyperalgesia, and pain using the same operant system that we developed previously [15]. This novel mechanical behavioral assessment strategy of orofacial pain could further provide a key step for the advancement of translational pain research, as we now demonstrate the ability to directly assess and compare mechanical versus thermal pain using the same outcomes.
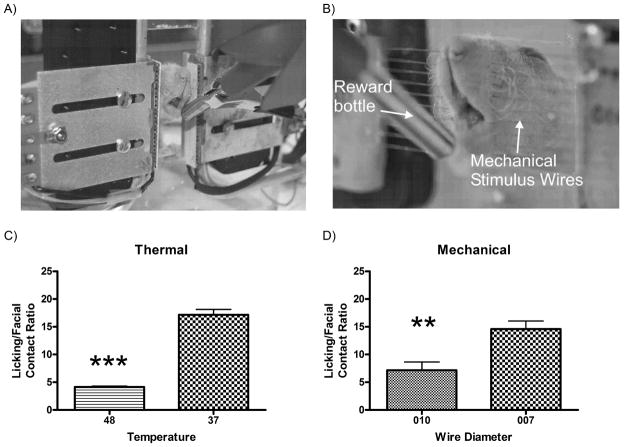
Figure 1. Comparison of a thermal and a mechanical operant testing system.
Photograph of a rat performing a thermal (A) and mechanical (B) facial operant test. In each test, the rats’ cheeks must contact the thermode or stimulus wires in order to reach the reward bottle containing sweetened condensed milk. Comparison of the Lick/Face contact pain ratio for thermal (C) and mechanical (D) stimuli demonstrated that there was a significant decrease in the Lick/Face ratio when the stimulus thermode was set at 48°C (N=134) as compared to 37°C (N=82; ***p<0.0001). There was a similar decrease in this ratio when comparing NiTi wires with a diameter of 0.010” (N=10) and 0.007” (N=10; ***p<0.005).
The objective of this study was to characterize behavioral responses to facial mechanical stimulation and to see how these responses change under conditions of inflammation and analgesia. Since we are using an adaptation of our established thermal facial operant testing paradigm, we also compared baseline thermal stimuli data (unpublished) collected over the past 6 months using our standard thermal operant system, as described in detail previously [15]. In this current study, we modified standard nickel titanium (NiTi) wires similar to those used in orthodontics for providing tooth movement. The advantage of nickel titanium wires is they have tremendous memory and strength, thus allowing for standardized repeated use of the same wires. Additionally, these wires can conduct electricity, so we were able to modify our existing cage to detect and record actual facial contacts, which is one of the keys to the operant testing. We examined the influence of two diameters of nickel titanium wires (0.010” and 0.007”) on the performance of a facial reward/conflict paradigm testing system [15, 16, 19]. We have previously published and presented some of this data in abstract form [8] and this study builds on this prior work by directly comparing the effects of thermal versus mechanical stimuli on the same operant outcome measure.
Adaptation of the operant orofacial system was accomplished by adding two horizontal rows of NiTi wires embedded in an acrylic module that could be easily adjusted, attached and removed from our existing operant testing boxes. Unrestrained hairless male Sprague-Dawley rats (250–300g, Charles River, Raleigh, NC) were housed in a standard environment. During testing, animals were placed separately in each operant cage and the reward bottle containing diluted (1:2 with water) sweetened condensed milk solution (Nestle, room temperature) was positioned in proximity to the cage such that the animal will be allowed access to the reward bottle when simultaneously contacting the NiTi wires (either 0.010” or 0.007” in diameter, (Small Parts, Lexington, KY)) with its face (Fig 1B). Both the metal spout on the watering bottle and the NiTi wires of the faceplate was connected to a DC power supply and, in series, to a multi-channel data acquisition module (WinDaq Data Acq DI-710-UH, DATAQ Instruments, Inc). As with our prior thermal test, when the animal completed the task and drank from the bottle, the animal's tongue contacted the metal spout on the water bottle, completing an electrical circuit. The closed circuit was registered in the computer and each spout contact was recorded as a licking event. A separate circuit was established from the NiTi mechanical wires to the animal by grounding the floor with an aluminum sheet for recording of facial contact events. We previously tried unsuccessfully to use proximity sensors to detect actual facial contacts, as animals are able to modify their strategy to accessing the reward and thus leading to potential false positive responses. Therefore this latter facial contact circuit is critical and necessary to determine if the animal is discouraged by the stimulus. The duration of each facial contact and the total number of events (licking, facial contact) were recorded. During offline data analysis the cumulative duration and frequency of events were determined for both the licking (reward) contact data and the facial stimulus data. A licking/facial contact ratio was calculated for each animal and presented as mean ± s.e.m., as described previously [15]. When considering this ratio in the context of behavioral pain testing, a non-nociceptive stimulus (e.g., 37°C) typically results in a high number of licking events per single facial contact for the animal. Under nociceptive conditions (e.g., 48°C), this ratio significantly decreases, as the animal is only able to achieve a low number of licking events per single facial contact. Alternatively, the animal must significantly increase the number of facial stimulus contacts to achieve the same number of reward licking events. We compared the effects of each wire (0.007” vs. 0.010”) and neutral and hot thermal stimuli on these outcome measures.
We also examined the effect of neurogenic inflammation and opioid administration on the performance of the operant test for each wire diameter. A capsaicin cream (0.075%, Thomson Micromedix, CO) was liberally applied to the cheek region of lightly anesthetized (inhaled isoflurane, 1–2%) rats and left on for 5 min to produce nociceptive sensitization [14]. The capsaicin was then removed and the face was wiped clean with a moist paper towel and returned to their home cage for 30 min prior to behavioral testing. The final experiment studied the effect of morphine analgesia on performance in the operant test. Morphine (Baxter Healthcare Corporation, Deerfield, IL) was diluted in phosphate buffered saline pH 7.4 (PBS) and injected subcutaneously (1 mg/kg, volume of 200–250 μl) 30 min prior to testing. All rats had ad libitum access to food and water between testing sessions and their weights were monitored weekly but were food fasted for 16 h prior to each testing session and trained (n=9 sessions) to drink sweetened condensed milk. This lead-in training period was necessary to acquaint the animals with the task of locating the reward bottle.
Statistical analysis included an unpaired t-test to compare thermal (37°C vs. 48°C) and a paired t-test to compare mechanical (0.007” vs. 0.010”) baseline data. Additionally, comparison of the 0.007” and the 0.010” data was performed using a one-way ANOVA with a Dunnett’s post-test with the baseline data of each group used as the control for comparison. All statistical evaluations were made using GraphPad Prism (v. 4.02, GraphPad Software, San Diego, CA). Significance was set at p < 0.05.
The first outcome evaluated was to demonstrate how the thermal success ratios at 48° C and 37° C (Fig. 1C) compare with the mechanical baseline data for the 0.007” and 0.010” wires (Fig. 1D). Note that we utilized our database of animal experiments to yield the thermal data (unpublished) by selecting all animals tested by our group over the past 6 months. Our thermal operant testing system shows a significant decrease in the licking/facial contact ratio at 48° C compared to 37° C, demonstrating a thermal pain response. Similarly, a wire diameter of 0.010” produced a significantly decreased licking/facial contact ratio when compared to a wire diameter of 0.007”, demonstrating a mechanical pain response of the thicker wire relative to the thinner one. These results suggest that adaptation of our thermal operant testing system for mechanical operant testing produces comparable results under these stimulus conditions, thus providing a system that easily produces data for both mechanical and thermal operant testing and makes them comparable using the licking/facial contact ratio.
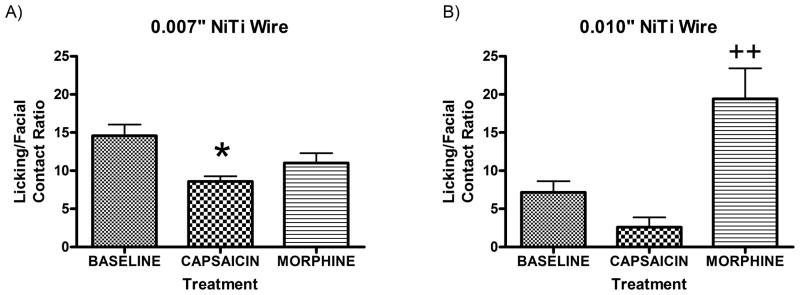
Following baseline testing, we examined the effect of capsaicin and morphine treatments on licking/facial contact ratios using either the 0.007” or the 0.010” NiTi wires. Treatment with capsaicin produced a significant decrease in licking/facial contact ratio compared to baseline when the animals were tested on the 0.007” NiTi wires indicative of a mechanical allodynic response (Fig. 2A). Morphine injection prior to testing on the 0.007” NiTi wires did not increase the licking/facial contact ratio compared to baseline, providing further evidence that this wire diameter is not aversive and is comparable to at the 37° C stimulus. Additionally, we examined the effect of capsaicin treatment and morphine injection on the licking/facial contact ratios using the 0.010” NiTi wire (Fig. 2B). Following capsaicin treatment, there was a clear reduction in the licking/facial contact ratio outcome, but this difference was not significant. The results may be confounded by the floor effect because of the already low ratio observed at baseline. However, morphine treatment did significantly increase the licking/facial contact ratio compared to baseline, suggesting that the 0.010” NiTi wires are aversive and that the morphine provided sufficient analgesia to overcome the mechanical pain produced by these wires.
Figure 2. Effect of capsaicin and morphine on mechanical pain sensitivity.
Mechanical stimulation using the 0.007” NiTi wire showed that the capsaicin treatment (N=4) produced a significantly reduced (*p<0.05) lick/face ratio compared to baseline (N=10)(F2,21=3.831) and no significant change following morphine treatment (N=10) (A). Mechanical stimulation using the 0.010” NiTi wire showed that morphine (N=10) resulted in a significantly increased (++p<0.005) lick/face contact ratio compared to baseline (N=10) (F2,19=6.96) with no significant change following capsaicin (N=3) treatment (B). Note that the already low lick/face contact ratio of baseline may exhibit a floor effect preventing capsaicin treatment to drive performance lower and that 1 animal avoided contacting the wires by pushing them down with its’ paw (data not included).
Previous studies of orofacial pain using mechanical stimulation have relied on reflexive behavioral tests that only address changes in brainstem neurochemistry and physiology. Classical pain evaluation methodologies of animal models often utilize segmental reflexes that involve links between sensory inputs and motor neurons. One advantage of reflexive testing is that they are relatively easy to perform; however, these tests do not necessarily measure pain [3]. Kauppila et al. have shown that tail-reflex and limb withdrawal response can be elicited in spinalized animals [11]. Even complex, unlearned behaviors mediated by brainstem processing such as paw licking, face-rubbing, limb-guarding, vocalization, grooming [1, 2, 6, 7, 12, 20] have been shown to be present in decerebrate animals [24]. Adaptation of these tests for the assessment of orofacial pain has been challenging and do not provide information about higher order cerebral processing.
Operant responses involve complex behaviors and are advantageous over reflexive tests since the animal has control over the amount of nociceptive stimulation and can modify its behavior based on cortical processing [13, 21]. Additionally, the use of a conflict paradigm illustrates the animals’ choices between receiving a positive reward or escaping aversive stimuli [15, 22]. There are several advantages this system provides over the traditional reflexive mechanical tests. The first is that this system is completely investigator independent providing a better assessment of novel therapies. Secondly, it removes perceived investigator bias that may affect the outcome measures from investigator initiated reflexive responses [4], which represents a major advance in preclinical testing. Lastly, this operant system reduces stress and anticipation because the animal is unrestrained during the testing session.
Our current study introduces a novel adaptation of our thermal operant testing system [15] to test mechanical orofacial pain. To our knowledge, this is the first detailed written report characterizing a novel operant system that measures both mechanical and thermal sensitivity and this model is a natural extension of our prior studies [8,14,15,16,19]. With the two different stimuli, one can assess the influence of mechanical versus thermal pain on operant behavior in the context of the same experiment, and in fact, can directly compare the relative pain across these two modalities. As can be seen in Fig. 1, the 37° C and 0.007” NiTi wires produce relatively the same licking/facial contact ratio of ~15, while the noxious stimuli of 48° C and 0.010” produce a significant 2–3 fold decrease in this measure. These values are consistent with our previous studies evaluating thermal sensitivity, with a 37° C stimulus typically providing a ratio between 12–18 and hot stimuli (45–48° C) producing values of less than 4–5 [15, 19]. For these comparisons, we typically will define a known, non-nociceptive stimulus, such as 37° C, as our baseline licking/facial stimulus value and compare this to values obtained under nociceptive or analgesic conditions. This comparison could be accomplished in greater detail using a dual-chambered place preference setup that we describe previously [19]. This would be particularly useful especially in the context of pharmacologic testing to determine if a potential analgesic is more effective for reducing mechanical or thermal pain.
In conclusion, we demonstrate the usefulness of our operant system for assessing both mechanical and thermal facial pain. Mechanical allodynia is a significant concern in both acute and chronic pain conditions, as well in the post-operative surgical setting. Finding a reliable system to evaluate mechanical sensitivity would represent a significant advancement in the development of new analgesics. We have developed such a system that is automated, investigator-independent, and compares with our established thermal operant system. As seen in Fig. 1, we have an equivalent licking/facial contact ratio of ~15 for the 37° C and 0.007” NiTi wire stimuli and ~5 for the 48° C and 0.010” NiTi wire. This uniquely demonstrates similar non-nociceptive and nociceptive outcomes to both thermal and mechanical stimuli using the same assay. While, this may not be the case for all orofacial pain models, in the context of neurogenic inflammatory pain induced by capsaicin, we in fact demonstrate differentiation of mechanical allodynia and hyperalgesia based on these different wire diameters, which is consistent with our previous study using a thermal stimulus [14]. These data help fill a current void in animal operant orofacial pain assessment by providing a behavioral measure that expresses physiological and cortical processing of pain providing an assessment system which allows for the evaluation of physiological and affective components of pain. This system provides a novel assessment of mechanical orofacial pain and in conjunction with our previously reported thermal operant facial testing system provides researchers with a more complete assessment of animal facial pain.
Acknowledgments
Support for this research was provided by grant #2R44DA026220-02A1, National Institute on Drug Abuse, National Institutes of Health, Department of Health and Human Services, Bethesda, MD, USA. Dr. Nolan was supported by National Institute of Neurological Disorders and Stroke training grant T32NS045551, to the University of Florida Comprehensive Center for Pain Research. The technology for this behavioral assay has been licensed from the University of Florida by Stoelting Co. (Wood Dale, IL).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Benoliel R, Wilensky A, Tal M, Eliav E. Application of a pro-inflammatory agent to the orbital portion of the rat infraorbital nerve induces changes indicative of ongoing trigeminal pain. Pain. 2002;99:567–578. doi: 10.1016/S0304-3959(02)00272-5. [DOI] [PubMed] [Google Scholar]
- 2.Berridge KC. Progressive degradation of serial grooming chains by descending decerebration. Behavioural Brain Research. 1989;33:241–253. doi: 10.1016/s0166-4328(89)80119-6. [DOI] [PubMed] [Google Scholar]
- 3.Chapman CR, Casey KL, Dubner R, Foley KM, Gracely RH, Reading AE. Pain measurement: an overview. Pain. 1985;22:1–31. doi: 10.1016/0304-3959(85)90145-9. [DOI] [PubMed] [Google Scholar]
- 4.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neuroscience Biobehavioural Review. 2002;26:907–923. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 5.Clavelou P, Dallel R, Orliaguet T, Woda A, Raboisson P. The orofacial formalin test in rats: effects of different formalin concentrations. Pain. 1995;62:295–301. doi: 10.1016/0304-3959(94)00273-H. [DOI] [PubMed] [Google Scholar]
- 6.Hargreaves K, Dubner R, Brown F, Flores C, Joris J. A new and sensitive method for measuring thermal nociception in cutaneous hyperalgesia. Pain. 1988;32:77–88. doi: 10.1016/0304-3959(88)90026-7. [DOI] [PubMed] [Google Scholar]
- 7.Hartwig AC, Mathias SI, Law AS, Gebhart GF. Characterization and opioid modulation of inflammatory temporomandibular joint pain in the rat. Journal of Oral Maxillofacial Surgery. 2003;61:1302–1309. doi: 10.1016/s0278-2391(03)00732-8. [DOI] [PubMed] [Google Scholar]
- 8.Hester J, Nolan TA, Neubert JK. A novel mechanical operant assay for assessment of orofacial pain. Journal of Dental Research. 2010;89(Spec Iss A) Abstract #1165. [Google Scholar]
- 9.Imamura Y, Kawamoto H, Nakanishi O. Characterization of heat-hyperalgesia in an experimental trigeminal neuropathy in rats. Experimental Brain Research. 1997;116:97–103. doi: 10.1007/pl00005748. [DOI] [PubMed] [Google Scholar]
- 10.Imbe H, Iwata K, Zhou QQ, Zou S, Dubner R, Ren K. Orofacial deep and cutaneous tissue inflammation and trigeminal neuronal activation. Implications for persistent temporomandibular pain. Cells Tissues Organs. 2001;169:238–247. doi: 10.1159/000047887. [DOI] [PubMed] [Google Scholar]
- 11.Kauppila T, Kontinen VK, Pertovaara A. Influence of spinalization on spinal withdrawal reflex responses varies depending on the submodality of the test stimulus and the experimental pathophysiological condition in the rat. Brain Research. 1998;797:234–242. doi: 10.1016/s0006-8993(98)00379-5. [DOI] [PubMed] [Google Scholar]
- 12.Kayser V, Guilbaud G. Local and remote modifications of nociceptive sensitivity during carrageenin-induced inflammation in the rat. Pain. 1987;28:99–107. doi: 10.1016/0304-3959(87)91064-5. [DOI] [PubMed] [Google Scholar]
- 13.Mauderli AP, Acosta-Rua A, Vierck CJ. An operant assay of thermal pain in conscious, unrestrained rats. Journal of Neuroscience Methods. 2000;97:19–29. doi: 10.1016/s0165-0270(00)00160-6. [DOI] [PubMed] [Google Scholar]
- 14.Neubert JK, Rossi HL, Malphurs W, Vierck CJ, Jr, Caudle RM. Differentiation between capsaicin-induced allodynia and hyperalgesia using a thermal operant assay. Behavioural Brain Research. 2006;170:308–315. doi: 10.1016/j.bbr.2006.03.008. [DOI] [PubMed] [Google Scholar]
- 15.Neubert JK, Widmer CG, Malphurs W, Rossi HL, Vierck CJ, Jr, Caudle RM. Use of a novel thermal operant behavioral assay for characterization of orofacial pain sensitivity. Pain. 2005;116:386–395. doi: 10.1016/j.pain.2005.05.011. [DOI] [PubMed] [Google Scholar]
- 16.Neubert JK, King C, Malphurs W, Wong F, Weaver JP, Jenkins AC, Rossi HL, Caudle RM. Characterization of mouse orofacial pain and the effects of lesioning TRPV1-expressing neurons on operant behavior. Molecular Pain. 2008;4 doi: 10.1186/1744-8069-4-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pelissier T, Pajot J, Dallel R. The orofacial capsaicin test in rats: effects of different capsaicin concentrations and morphine. Pain. 2002;96:81–87. doi: 10.1016/s0304-3959(01)00432-8. [DOI] [PubMed] [Google Scholar]
- 18.Price DD, Dubner R, Hu JW. Trigeminothalamic neurons in nucleus caudalis responsive to tactile, thermal, and nociceptive stimulation of monkey's face. Journal of Neurophysiology. 1976;39:936–953. doi: 10.1152/jn.1976.39.5.936. [DOI] [PubMed] [Google Scholar]
- 19.Rossi HL, Vierck CJ, Jr, Caudle RM, Neubert JK. Characterization of cold sensitivity and thermal preference using an operant orofacial assay. Molecular Pain. 2006;2 doi: 10.1186/1744-8069-2-37. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van Eick AJ. A change in the response of the mouse in the “hot plate” analgesia-test, owing to a central action of atropine and related compounds. Acta Physiologica et Pharmacologica Neerlandica. 1967;14:499–500. [PubMed] [Google Scholar]
- 21.Vierck CJ, Jr, Kline RH, Wiley RG. Intrathecal substance p-saporin attenuates operant escape from nociceptive thermal stimuli. Neurosci. 2003;119:223–232. doi: 10.1016/s0306-4522(03)00125-8. [DOI] [PubMed] [Google Scholar]
- 22.Vierck CJ, Jr, Hamilton DM, Thornby JI. Pain reactivity of monkeys after lesions to the dorsal and lateral columns of the spinal cord. Experimental Brain Research. 1971;13:140–158. doi: 10.1007/BF00234083. [DOI] [PubMed] [Google Scholar]
- 23.Vos BP, Strassman AM, Maciewicz RJ. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat's infraorbital nerve. Journal of Neuroscience. 1994;14:2708–2723. doi: 10.1523/JNEUROSCI.14-05-02708.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Woolf CJ. Long term alterations in the excitability of the flexion reflex produced by peripheral tissue injury in the chronic decerebrate rat. Pain. 1984;18:325–343. doi: 10.1016/0304-3959(84)90045-9. [DOI] [PubMed] [Google Scholar]