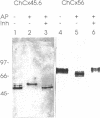
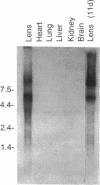
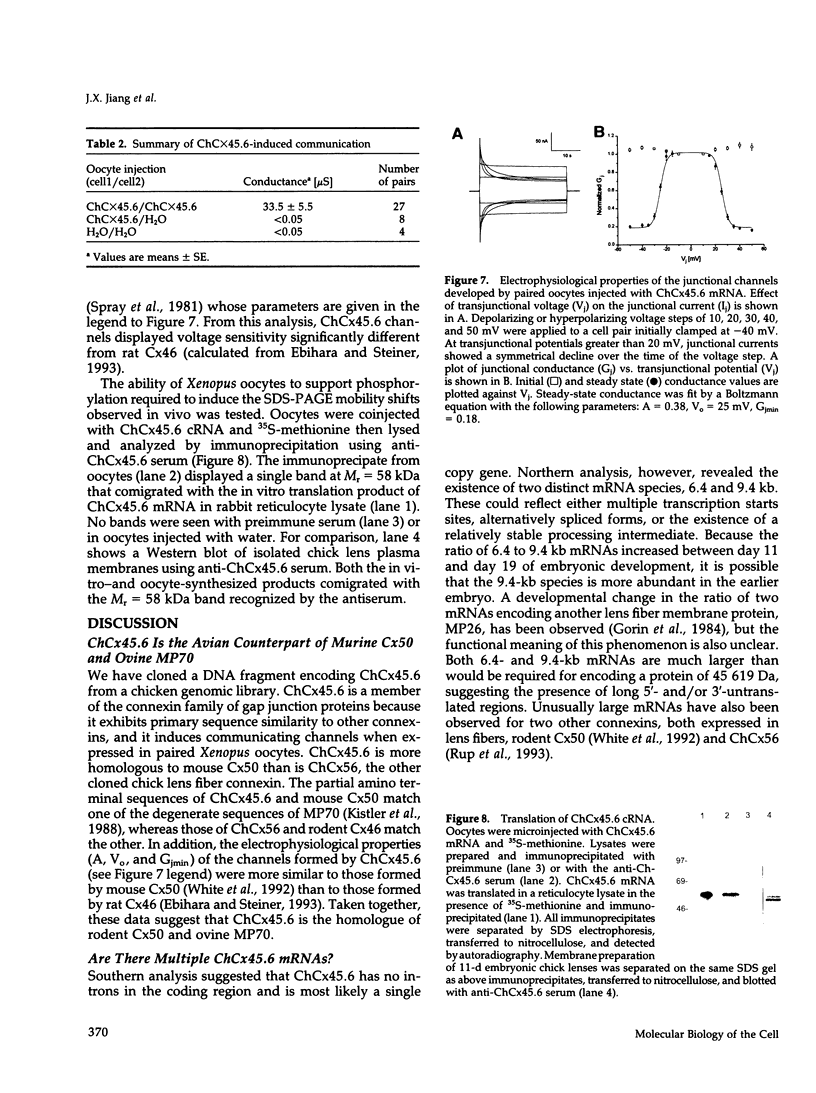
Abstract
The avian lens is an ideal system to study gap junctional intercellular communication in development and homeostasis. The lens is experimentally more accessible in the developing chick embryo than in other organisms, and chick lens cells differentiate well in primary cultures. However, only two members of the connexin gene family have been identified in the avian lens, whereas three are known in the mammalian system. We report here the molecular cloning and characterization of the third lens connexin, chick connexin45.6 (ChCx45.6), a protein with a predicted molecular mass of 45.6 kDa. ChCx45.6 was encoded by a single copy gene and was expressed specifically in the lens. There were two mRNA species of 6.4 kilobase (kb) and 9.4 kb in length. ChCx45.6 was a functional connexin protein, because expression in Xenopus oocyte pairs resulted in the development of high levels of conductance with a characteristic voltage sensitivity. Antisera were raised against ChCx45.6 and chick connexin56 (ChCx56), another avian lens-specific connexin, permitting the examination of the distribution of both proteins. Immunofluorescence localization showed that both ChCx45.6 and ChCx56 were abundant in lens fibers. Treatment of lens membranes with alkaline phosphatase resulted in electrophoretic mobility shifts, demonstrating that both ChCx45.6 and ChCx56 were phosphoproteins in vivo.
Full text
PDF









Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Beebe D. C., Piatigorsky J. Translational regulation of delta-crystallin synthesis during lens development in the chicken embryo. Dev Biol. 1981 May;84(1):96–101. doi: 10.1016/0012-1606(81)90374-2. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Barrio L. C., Bargiello T. A., Spray D. C., Hertzberg E., Sáez J. C. Gap junctions: new tools, new answers, new questions. Neuron. 1991 Mar;6(3):305–320. doi: 10.1016/0896-6273(91)90241-q. [DOI] [PubMed] [Google Scholar]
- Bennett M. V., Goodenough D. A. Gap junctions, electrotonic coupling, and intercellular communication. Neurosci Res Program Bull. 1978 Sep;16(3):1–486. [PubMed] [Google Scholar]
- Beyer E. C., Kistler J., Paul D. L., Goodenough D. A. Antisera directed against connexin43 peptides react with a 43-kD protein localized to gap junctions in myocardium and other tissues. J Cell Biol. 1989 Feb;108(2):595–605. doi: 10.1083/jcb.108.2.595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beyer E. C. Molecular cloning and developmental expression of two chick embryo gap junction proteins. J Biol Chem. 1990 Aug 25;265(24):14439–14443. [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin family of gap junction proteins. J Membr Biol. 1990 Jul;116(3):187–194. doi: 10.1007/BF01868459. [DOI] [PubMed] [Google Scholar]
- Beyer E. C., Paul D. L., Goodenough D. A. Connexin43: a protein from rat heart homologous to a gap junction protein from liver. J Cell Biol. 1987 Dec;105(6 Pt 1):2621–2629. doi: 10.1083/jcb.105.6.2621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bruzzone R., Haefliger J. A., Gimlich R. L., Paul D. L. Connexin40, a component of gap junctions in vascular endothelium, is restricted in its ability to interact with other connexins. Mol Biol Cell. 1993 Jan;4(1):7–20. doi: 10.1091/mbc.4.1.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chirgwin J. M., Przybyla A. E., MacDonald R. J., Rutter W. J. Isolation of biologically active ribonucleic acid from sources enriched in ribonuclease. Biochemistry. 1979 Nov 27;18(24):5294–5299. doi: 10.1021/bi00591a005. [DOI] [PubMed] [Google Scholar]
- Dahl G., Miller T., Paul D., Voellmy R., Werner R. Expression of functional cell-cell channels from cloned rat liver gap junction complementary DNA. Science. 1987 Jun 5;236(4806):1290–1293. doi: 10.1126/science.3035715. [DOI] [PubMed] [Google Scholar]
- Duncan G. The site of the ion restricting membranes in the toad lens. Exp Eye Res. 1969 Oct;8(4):406–412. doi: 10.1016/s0014-4835(69)80006-0. [DOI] [PubMed] [Google Scholar]
- Ebihara L., Steiner E. Properties of a nonjunctional current expressed from a rat connexin46 cDNA in Xenopus oocytes. J Gen Physiol. 1993 Jul;102(1):59–74. doi: 10.1085/jgp.102.1.59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisenberg R. S., Rae J. L. Current-voltage relationships in the crystalline lens. J Physiol. 1976 Nov;262(2):285–300. doi: 10.1113/jphysiol.1976.sp011596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans C. W., Eastwood S., Rains J., Gruijters W. T., Bullivant S., Kistler J. Gap junction formation during development of the mouse lens. Eur J Cell Biol. 1993 Apr;60(2):243–249. [PubMed] [Google Scholar]
- FitzGerald P. G., Goodenough D. A. Rat lens cultures: MIP expression and domains of intercellular coupling. Invest Ophthalmol Vis Sci. 1986 May;27(5):755–771. [PubMed] [Google Scholar]
- Goodenough D. A., Dick J. S., 2nd, Lyons J. E. Lens metabolic cooperation: a study of mouse lens transport and permeability visualized with freeze-substitution autoradiography and electron microscopy. J Cell Biol. 1980 Aug;86(2):576–589. doi: 10.1083/jcb.86.2.576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodenough D. A., Paul D. L., Jesaitis L. Topological distribution of two connexin32 antigenic sites in intact and split rodent hepatocyte gap junctions. J Cell Biol. 1988 Nov;107(5):1817–1824. doi: 10.1083/jcb.107.5.1817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gorin M. B., Yancey S. B., Cline J., Revel J. P., Horwitz J. The major intrinsic protein (MIP) of the bovine lens fiber membrane: characterization and structure based on cDNA cloning. Cell. 1984 Nov;39(1):49–59. doi: 10.1016/0092-8674(84)90190-9. [DOI] [PubMed] [Google Scholar]
- Green C. R., Harfst E., Gourdie R. G., Severs N. J. Analysis of the rat liver gap junction protein: clarification of anomalies in its molecular size. Proc R Soc Lond B Biol Sci. 1988 Mar 22;233(1271):165–174. doi: 10.1098/rspb.1988.0016. [DOI] [PubMed] [Google Scholar]
- Gruijters W. T., Kistler J., Bullivant S., Goodenough D. A. Immunolocalization of MP70 in lens fiber 16-17-nm intercellular junctions. J Cell Biol. 1987 Mar;104(3):565–572. doi: 10.1083/jcb.104.3.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu Y., Rosenblatt J., Morgan D. O. Cell cycle regulation of CDK2 activity by phosphorylation of Thr160 and Tyr15. EMBO J. 1992 Nov;11(11):3995–4005. doi: 10.1002/j.1460-2075.1992.tb05493.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haefliger J. A., Bruzzone R., Jenkins N. A., Gilbert D. J., Copeland N. G., Paul D. L. Four novel members of the connexin family of gap junction proteins. Molecular cloning, expression, and chromosome mapping. J Biol Chem. 1992 Jan 25;267(3):2057–2064. [PubMed] [Google Scholar]
- Helms C., Graham M. Y., Dutchik J. E., Olson M. V. A new method for purifying lambda DNA from phage lysates. DNA. 1985 Feb;4(1):39–49. doi: 10.1089/dna.1985.4.39. [DOI] [PubMed] [Google Scholar]
- Hertzberg E. L., Disher R. M., Tiller A. A., Zhou Y., Cook R. G. Topology of the Mr 27,000 liver gap junction protein. Cytoplasmic localization of amino- and carboxyl termini and a hydrophilic domain which is protease-hypersensitive. J Biol Chem. 1988 Dec 15;263(35):19105–19111. [PubMed] [Google Scholar]
- Jiang J. X., Paul D. L., Goodenough D. A. Posttranslational phosphorylation of lens fiber connexin46: a slow occurrence. Invest Ophthalmol Vis Sci. 1993 Dec;34(13):3558–3565. [PubMed] [Google Scholar]
- Kistler J., Christie D., Bullivant S. Homologies between gap junction proteins in lens, heart and liver. Nature. 1988 Feb 25;331(6158):721–723. doi: 10.1038/331721a0. [DOI] [PubMed] [Google Scholar]
- Kistler J., Kirkland B., Bullivant S. Identification of a 70,000-D protein in lens membrane junctional domains. J Cell Biol. 1985 Jul;101(1):28–35. doi: 10.1083/jcb.101.1.28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krieg P. A., Melton D. A. Functional messenger RNAs are produced by SP6 in vitro transcription of cloned cDNAs. Nucleic Acids Res. 1984 Sep 25;12(18):7057–7070. doi: 10.1093/nar/12.18.7057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mathias R. T., Rae J. L., Eisenberg R. S. The lens as a nonuniform spherical syncytium. Biophys J. 1981 Apr;34(1):61–83. doi: 10.1016/S0006-3495(81)84837-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Menko A. S., Klukas K. A., Johnson R. G. Chicken embryo lens cultures mimic differentiation in the lens. Dev Biol. 1984 May;103(1):129–141. doi: 10.1016/0012-1606(84)90014-9. [DOI] [PubMed] [Google Scholar]
- Menko A. S., Klukas K. A., Liu T. F., Quade B., Sas D. F., Preus D. M., Johnson R. G. Junctions between lens cells in differentiating cultures: structure, formation, intercellular permeability, and junctional protein expression. Dev Biol. 1987 Oct;123(2):307–320. doi: 10.1016/0012-1606(87)90389-7. [DOI] [PubMed] [Google Scholar]
- Milks L. C., Kumar N. M., Houghten R., Unwin N., Gilula N. B. Topology of the 32-kd liver gap junction protein determined by site-directed antibody localizations. EMBO J. 1988 Oct;7(10):2967–2975. doi: 10.1002/j.1460-2075.1988.tb03159.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T. M., Goodenough D. A. Evidence for two physiologically distinct gap junctions expressed by the chick lens epithelial cell. J Cell Biol. 1986 Jan;102(1):194–199. doi: 10.1083/jcb.102.1.194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller T., Dahl G., Werner R. Structure of a gap junction gene: rat connexin-32. Biosci Rep. 1988 Oct;8(5):455–464. doi: 10.1007/BF01121644. [DOI] [PubMed] [Google Scholar]
- Moreno A. P., Fishman G. I., Spray D. C. Phosphorylation shifts unitary conductance and modifies voltage dependent kinetics of human connexin43 gap junction channels. Biophys J. 1992 Apr;62(1):51–53. doi: 10.1016/S0006-3495(92)81775-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Beyer E. C., Goodenough D. A. Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol. 1990 Jun;116(2):163–175. doi: 10.1007/BF01868674. [DOI] [PubMed] [Google Scholar]
- Musil L. S., Cunningham B. A., Edelman G. M., Goodenough D. A. Differential phosphorylation of the gap junction protein connexin43 in junctional communication-competent and -deficient cell lines. J Cell Biol. 1990 Nov;111(5 Pt 1):2077–2088. doi: 10.1083/jcb.111.5.2077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musil L. S., Goodenough D. A. Biochemical analysis of connexin43 intracellular transport, phosphorylation, and assembly into gap junctional plaques. J Cell Biol. 1991 Dec;115(5):1357–1374. doi: 10.1083/jcb.115.5.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okada T. S., Eguchi G., Takeichi M. The expression of differentiation by chicken lens epithelium in in vitro cell culture. Dev Growth Differ. 1971 Dec;13(4):323–336. doi: 10.1111/j.1440-169x.1971.00323.x. [DOI] [PubMed] [Google Scholar]
- Paul D. L., Ebihara L., Takemoto L. J., Swenson K. I., Goodenough D. A. Connexin46, a novel lens gap junction protein, induces voltage-gated currents in nonjunctional plasma membrane of Xenopus oocytes. J Cell Biol. 1991 Nov;115(4):1077–1089. doi: 10.1083/jcb.115.4.1077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paul D. L. Molecular cloning of cDNA for rat liver gap junction protein. J Cell Biol. 1986 Jul;103(1):123–134. doi: 10.1083/jcb.103.1.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Philipson B. T., Hanninen L., Balazs E. A. Cell contacts in human and bovine lenses. Exp Eye Res. 1975 Sep;21(3):205–219. doi: 10.1016/0014-4835(75)90091-3. [DOI] [PubMed] [Google Scholar]
- Piatigorsky J., Rothschild S. S., Milstone L. M. Differentiation of lens fibers in explanted embryonic chick lens epithelia. Dev Biol. 1973 Oct;34(2):334–345. doi: 10.1016/0012-1606(73)90362-x. [DOI] [PubMed] [Google Scholar]
- Rae J. L., Stacey T. Lanthanum and procion yellow as extracellular markers in the crystalline lens of the rat. Exp Eye Res. 1979 Jan;28(1):1–21. doi: 10.1016/0014-4835(79)90101-5. [DOI] [PubMed] [Google Scholar]
- Rae J. L. The electrophysiology of the crystalline lens. Curr Top Eye Res. 1979;1:37–90. [PubMed] [Google Scholar]
- Rup D. M., Veenstra R. D., Wang H. Z., Brink P. R., Beyer E. C. Chick connexin-56, a novel lens gap junction protein. Molecular cloning and functional expression. J Biol Chem. 1993 Jan 5;268(1):706–712. [PubMed] [Google Scholar]
- Saez J. C., Spray D. C., Nairn A. C., Hertzberg E., Greengard P., Bennett M. V. cAMP increases junctional conductance and stimulates phosphorylation of the 27-kDa principal gap junction polypeptide. Proc Natl Acad Sci U S A. 1986 Apr;83(8):2473–2477. doi: 10.1073/pnas.83.8.2473. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuetze S. M., Goodenough D. A. Dye transfer between cells of the embryonic chick lens becomes less sensitive to CO2 treatment with development. J Cell Biol. 1982 Mar;92(3):694–705. doi: 10.1083/jcb.92.3.694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Spray D. C., Harris A. L., Bennett M. V. Equilibrium properties of a voltage-dependent junctional conductance. J Gen Physiol. 1981 Jan;77(1):77–93. doi: 10.1085/jgp.77.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swenson K. I., Jordan J. R., Beyer E. C., Paul D. L. Formation of gap junctions by expression of connexins in Xenopus oocyte pairs. Cell. 1989 Apr 7;57(1):145–155. doi: 10.1016/0092-8674(89)90180-3. [DOI] [PubMed] [Google Scholar]
- Swenson K. I., Piwnica-Worms H., McNamee H., Paul D. L. Tyrosine phosphorylation of the gap junction protein connexin43 is required for the pp60v-src-induced inhibition of communication. Cell Regul. 1990 Dec;1(13):989–1002. doi: 10.1091/mbc.1.13.989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tenbroek E., Arneson M., Jarvis L., Louis C. The distribution of the fiber cell intrinsic membrane proteins MP20 and connexin46 in the bovine lens. J Cell Sci. 1992 Sep;103(Pt 1):245–257. doi: 10.1242/jcs.103.1.245. [DOI] [PubMed] [Google Scholar]
- White T. W., Bruzzone R., Goodenough D. A., Paul D. L. Mouse Cx50, a functional member of the connexin family of gap junction proteins, is the lens fiber protein MP70. Mol Biol Cell. 1992 Jul;3(7):711–720. doi: 10.1091/mbc.3.7.711. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yancey S. B., John S. A., Lal R., Austin B. J., Revel J. P. The 43-kD polypeptide of heart gap junctions: immunolocalization, topology, and functional domains. J Cell Biol. 1989 Jun;108(6):2241–2254. doi: 10.1083/jcb.108.6.2241. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang J. T., Nicholson B. J. Sequence and tissue distribution of a second protein of hepatic gap junctions, Cx26, as deduced from its cDNA. J Cell Biol. 1989 Dec;109(6 Pt 2):3391–3401. doi: 10.1083/jcb.109.6.3391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zimmer D. B., Green C. R., Evans W. H., Gilula N. B. Topological analysis of the major protein in isolated intact rat liver gap junctions and gap junction-derived single membrane structures. J Biol Chem. 1987 Jun 5;262(16):7751–7763. [PubMed] [Google Scholar]