Abstract
One prominent component of aging is a defect in memory stabilization. To understand how the formation of enduring memories is altered in the aged brain, long-term markers of the biological events that may mediate memory consolidation were used to examine the activity dynamics of hippocampal circuits over extended intervals. The immediate-early gene Arc, which is implicated in both durable memory and synaptic plasticity, is expressed in the fascia dentata (FD) for long periods following behavioral experience. To test the hypothesis that aging alters long-term Arc transcription in the FD, a region critical for spatial memory and impaired with progressive age, young and aged rats explored a novel environment twice, separated by an 8 hour interval, and FD Arc transcription was assessed. Relative to young rats, (a) fewer granule cells in the aged FD transcribe Arc 8 hours after spatial exploration, and (b) this decrease is correlated with impaired spatial memory. These findings are consistent with behavioral evidence of age-related decline in hippocampal-dependent memory processing long after an event is to be remembered, and re-affirm the integral role of the FD in the neural circuits supporting durable memory.
Keywords: Arg3.1, dentate gyrus, IEG, granule cell, consolidation, long-term memory
1. Introduction
Age-related deficits in hippocampal-dependent memory occur in a wide variety of species, including humans (Erickson and Barnes, 2003; Gallagher and Rapp, 1997; Dickerson and Eichenbaum, 2010). Thus, understanding long-term memory formation in the aged brain may provide insights into the mechanisms by which memories become resilient over time and provide markers for the impairment of this process with progressive age.
Durable memory is thought to require gradual refinement of fragile representations into more permanent states (e.g., Squire, 1992; Nadel and Moscovitch, 1997). The notion that memory traces are replayed to effect consolidation has existed for several decades (e.g., Hebb, 1949; Marr, 1971). More recently, experiments provide compelling evidence for these hypotheses by showing that behavioral patterns of hippocampal activity persist during subsequent quiet waking states and slow wave sleep in several brain regions and species (e.g., Pavlides and Winson 1989; Wilson and McNaughton, 1994; Skaggs and McNaughton, 1996; Qin et al. 1997; Nadasdy et al., 1999; Hoffman and McNaughton 2002; Peigneux et al. 2003; Tatsuno et al., 2006; Lansink et al., 2009). Moreover, the temporally-coherent replay of recent experiences is abnormal in aged rats, and the extent of the deficit in this process correlates with memory decline (Gerrard et al., 2008). However, the majority of data (reviewed in Sutherland and McNaughton, 2000; Rasch and Born, 2007), with a notable exception (Ribeiro et al., 2004), indicate that this recapitulation of experience decays quickly. Given these discrepant results, there is arguably no definitive evidence for hippocampal reactivation at intervals greater than 1 hr. Because the established time course falls short of explaining observations that memory can be disrupted several hours after acquisition (e.g., Ribeiro and Nicolelis, 2004), it remains important to identify other long-term markers of the biological events that mediate lasting memory.
Because electrical activity initiates signaling cascades that may serve to stabilize memory, transcription of immediate-early genes (IEGs) may reflect an important trigger in the process that forms lasting memory. Several lines of evidence implicate the IEG Arc (Link et al., 1995; Lyford et al., 1995) in the sustained modification of neuronal networks (Guzowski et al., 2000; Plath et al., 2006). The fascia dentata (FD), a region critical to learning and memory, is unique within the hippocampal formation in that granule cells show a prolonged expression of Arc transcription that occurs for at least 8 hours following a spatial behavioral experience (Ramirez-Amaya et al., 2005). Moreover, this long-term Arc expression has been functionally linked to enduring changes in synaptic efficacy following the induction of long-term potentiation (LTP). Inhibiting the translation of Arc significantly enhances the decay of LTP (Guzowski et al., 2000), even if this knockdown occurs hours after stimulation of the perforant path (Messaoudi et al., 2007). Thus, analysis of long-term Arc expression in the FD may provide a signaling cascade that aligns with the time course of memory disruption.
In this regard, it is critical to examine animals with memory impairments that may be functionally linked to the FD. It is known that the FD is particularly vulnerable to the effects of aging in humans (Small et al., 2002; Stark et al., 2010; Yassa et al., 2010), monkeys and rats (Small et al., 2004). Moreover, fewer granule cells in the aged FD express Arc immediately after behavioral experience (Small et al., 2004; Penner et al., 2010). Given the importance of continuing Arc expression for LTP maintenance (Messaoudi et al., 2007), which is deficient in aged memory-impaired rats (for review, Burke and Barnes, 2010), we hypothesize that long-term Arc transcription may also be altered in the aged FD, providing an additional mechanism for age-related memory decline.
2. Materials and methods
2.1 Subjects
Seventeen Adult (10-12 months) and 17 aged (24-27 months) male Fischer-344 rats (Harlan-Sprague Dawley, Indianapolis, IN) were used in these experiments. The rats were individually housed on a 12:12 hour light cycle with ad lib access to food and water, and all procedures were carried out at least 2 hours after the commencement of the dark cycle.
2.2 Assessment of Spatial Learning Impairment
Following 7 days of handling, the rats' spatial learning abilities were assessed using the Morris swim task (Shen and Barnes, 1996). Rats were given six spatial trials per day with a hidden platform for 4 consecutive days, and their location was tracked (sampled at 10 Hz) using an overhead video camera connected to a VP114 tracking unit (HVS image, England). This system was used to calculate the rat's corrected integrated path length (CIPL), which is the sum of the rat's distances from the platform, corrected for the rat's initial distance from the platform and speed (Gallagher et al., 1993). Following the four days of spatial training, retention of the platform location was assessed using a probe trial in which the platform is removed. The time spent in the target quadrant (previously containing the platform) vs. the opposite quadrant of the maze was quantified. The probe trial was followed by 12 visual discrimination trials in which the platform was marked and clearly visible above the waterline. These control trials ensure that any age-related differences observed in spatial trials are due to deficits in spatial learning, rather than to deficits in visual acuity. Any aged animals failing the visible platform portion of the Morris swim task (i.e., performing outside the range of performance in young animals) was excluded from behavioral analysis, and became part of the positive technical control group.
2.3 Spatial Exploration
Seven days after completing behavioral testing in the swim task, animals were divided into 3 groups: (1) caged controls (CC; n = 4 young and 4 aged) were sacrificed directly from their home cages as negative controls; (2) behavioral animals (n = 9 young and 9 aged) that explored the same environment twice, with an intervening rest period of 8 hrs in their home cages; and (3) animals that received maximal electroconvulsive shock (MECS; n = 4 young and 4 aged) as positive controls (Cole et al., 1990). The procedure for the spatial exploration trials is similar to that previously described (Guzowski et al., 1999). Briefly, rats were shuttled to a dimly lit room containing several distinct local and distal cues, and placed within a 61 × 61 cm square box divided into 9 equal grids. During exploration, rats were randomly placed within a different grid every 15 seconds until every grid was visited. While this “assisted exploration” procedure produces identical IEG expression profiles relative to free exploration (Marrone et al., 2010; Vazdarjanova and Guzowski, 2004), it ensures that both young and aged animals sampled all aspects of the environment uniformly. Rats were then shuttled back to their colony room cages, where they remained undisturbed for 8 hrs. Following this delay, the exploration treatment was repeated, and rats were then immediately sacrificed.
2.4 Maximal Electroconvulsive Shock
Animals that received maximal electroconvulsive shock as positive controls were treated as previously published (Cole et al., 1990). Breifly, animals were shuttled from the colony room to a separate procedure room containing An ECT electrical stimulator (Model 57800, Ugo Basile, Comerio, Italy) which was connected to the animal via auricular electrodes. The animal then received 1 sec of stimulation (100 Hz positive square-wave pulses, 0.5 ms pulse width, 85mA). This resulted in a tonic-clonic seizure lasting ~60 sec. Following this, the animal was returned to his home cage for 8 hours, before repeating the procedure. Animals were sacrificed immediately following seizure activity from the second stimulation.
2.5 Histology
Rats were decapitated under isofluorane anesthesia. Brains were rapidly removed and quick-frozen in a beaker of isopentane bathed in dry ice/ethanol within 180 seconds to maintain mRNA integrity. Before sectioning, hemisections containing the right dorsal hippocampus from 8-10 rats were mounted together with Tissue-Tek OCT compound (Miles, Elkhart, IN) such that all experimental conditions were present on each slide. Coronal sections (20 μm) were cut through the dorsal hippocampus (−3.2 to −3.8 mm from bregma: Paxinos and Watson, 1986), thaw-mounted on Superfrost Plus slides (VWR), dried, and stored at −70°C.
2.6 Fluorescence in situ Hybridization
Fluorescence in situ hybridization was performed as described elsewhere (Guzowski et al., 1999). Briefly, full-length riboprobes of Arc were synthesized using a commercially available transcription kit (Maxiscript; Ambion, Auston TX) and a nucleotide mix containing digoxigenin-labeled UTP (Roche Molecular Biochemicals, Nutley, NJ), purified in mini Quick spine RNA columns (Roche Molecular Biochemicals) and verified by gel electrophoresis. Slides were thawed, fixed in buffered 4% paraformaldehyde, treated with 0.5% acetic anhydride/1.5% triethanolamine, incubated in methanol and acetone (1:1), equilibrated in saline-sodium citrate (SSC), and incubated with 100μl of prehybridization buffer (Sigma, St. Louis, MO) for 30 min at room temperature. This was followed by application ~150 ng of riboprobe, diluted in hybridization buffer (Sigma), denatured at 90°C, and chilled on ice. Each slide was then incubated in a humid chamber overnight at 56°C. Following a graduated series of post-hybridization washes in SSC, slides were bathed in RNase A (10 mg/ml) at 37°C to degrade any single-stranded RNA. Endogenous peroxidases were quenched in 2% H2O2, and slides were blocked with TSA blocking buffer (Perkin Elmer, Boston, MA) 5% normal sheep serum, and then incubated with anti-digoxigenin-HRP antibody (Roche Molecular Biochemicals) at room temperature for 2 hours. Slides were then washed with Tris-buffered saline containing 0.05% Tween-20, and the HRP-antibody conjugate was detected using a Cyanine-3 (CY3) signal amplification kit (Perkin Elmer, Boston, MA). After nuclear counterstaining with Sytox green (Molecular Probes, Eugene, OR), coverslips were applied to the slides with Vectashield anti-fade media (Vector Labs, Burlingame, CA) and sealed.
2.7 Image Acquisition and Analysis
As described previously (Chawla et al., 2005; Ramirez-Amaya et al., 2005; Vazdarjanova et al., 2006), images were collected using an Olympus FV1000 laser scanning confocal microscope at 40x. For consistency, photomultiplier tube assignments, pinhole size, and contrast values remained constant for each slide. Images of CA1 and CA3 were acquired by taking z-stacks (20 μm thickness, 1.1 μm optical planes, 0.7 μm interval) in 3-5 random locations in each of 4-6 slides per rat. In each region, cells identified as principle cells based on size and intensity throughout the median 20% of each optical stack were classified as (a) Arc-negative, (b) Arc-positive in the nucleus, (c) Arc-positive in the surrounding cytoplasm, or (d) expressing Arc in both the nucleus and cytoplasm. These analyses were conducted independently for the suprapyramidal blade [also called the dorsal or inner/enclosed blade (Amaral et al., 2007; Witter, 2007)] and the infrapyramidal blade [also called the ventral or outer/exposed blade (Amaral et al., 2007; Witter, 2007)] of the FD. This analysis was conducted because previous data (Ramirez-Amaya et al., 2005; Vazdarjanova et al., 2006) indicate that each blade exhibits unique long-term transcriptional responses to spatial exploration. Following exploration, the suprapyramidal blade exhibits sustained Arc expression for ~8 hrs (Ramirez-Amaya et al., 2005). The infrapyramidal blade, however, exhibits a late-emerging Arc transcriptional response exclusively 6-8 hrs following behavior (Ramirez-Amaya et al., 2005).
2.8 Statistical Analysis
Spatial learning trials and visual trials in the Morris swim task was analyzed with a repeated measures ANOVA, using training day as the repeated factor. Probe trial performance was assessed using a one-way ANOVA. Analysis of Arc expression was conducted using a general factorial ANOVA for CA regions and the FD with age, behavioral group (i.e., exploration vs. CCs), and region (i.e., suprapyramidal vs. infrapyramidal blade, or CA1 vs. CA3) as factors. Correlation analyses were conducted using data that were normalized by computing the z-score across all animals for both Arc expression and the mean CIPL in the spatial trials of the Morris swim task.
3. Results
3.1 Behavioral training
Consistent with previous data (e.g., Gage et al., 1984; Gallagher et al., 1993; Rosenzweig et al., 1997; Shen et al., 1997), aged animals were significantly impaired in the spatial version of the Morris swim task (Fig. 1), but did not differ on the cued version of the task.
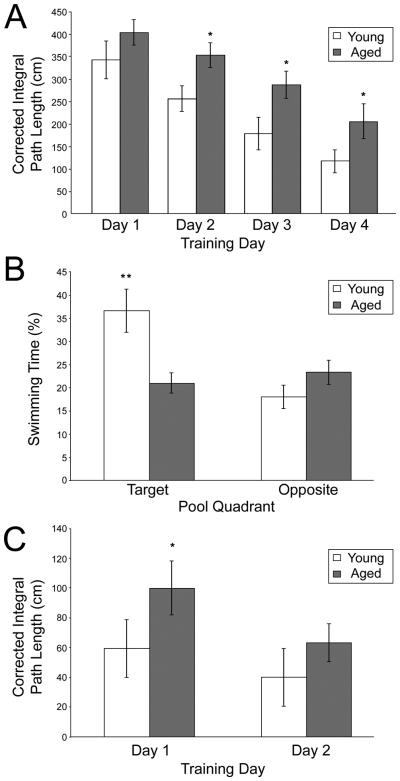
Figure 1.
Aged rats are impaired in the Morris swim task. Young adult rats swam a significantly shorter paths to reach the hidden platform than did the aged rats (A), and this difference was significant on days 2, 3 and 4 of training. During the probe trial (B), young adult rats spent significantly more time in the target quadrant, but not the opposite quadrant, compared to aged rats. During visible platform trials (C), young adult rats took significantly shorter paths to the visible platform than did aged rats on day 1, but this difference was no longer apparent by day 2 of training (all data are mean ± SEM; *p < 0.05; **p < 0.01, young vs. aged animals).
3.1.1 Spatial Learning Trials
Although all rats showed behavioral improvement over trials (main effect of training day: F3, 72 = 30.88; P < 0.001), aged rats took longer paths to the platform than younger rats (main effect of age: F1, 24 = 15.32; p = 0.001). No significant interaction was observed between age and training day (F3, 72 = 1.33; p = 0.27), suggesting that age-related differences in spatial learning were consistent across training. Post-hoc analyses confirmed that although age-related differences in CIPL grew larger as training time progressed, aged animals took significantly longer paths on all training days other than day 1 (day 1: p = 0.072; day 2: p = 0.029; day 3: p = 0.041; day 4: p = 0.021). These data demonstrate that the aged rats in the present study had impaired spatial memory that is not accounted for by changes in visual acuity or motor abilities (Fig. 1A).
3.1.2 Probe Trial
The age-related deficit in spatial memory was further confirmed on the probe trial (Fig. 1B). Young rats spent significantly more time in the target quadrant of the pool that previously held the escape platform than did the old rats (F1, 24 = 7.64; p = 0.011). Moreover, this effect was restricted to the quadrant with the platform, since these two groups of rats showed no difference in the time spent in the maze quadrant opposite to the platform location (F1, 24 = 2.01; p = 0.170). Similarly, young adult rats crossed the location of the target platform significantly more than did aged rats (F1, 24 = 10.48; p = 0.004) and swam a greater distance in the immediate vicinity of the target platform location (F1, 24 = 5.80; p = 0.024).
3.1.3 Visual Discrimination Trials
Consistent with performance during spatial trials, both young and aged rats showed significant learning in the visible platform version of the task, assessed by a significant effect of training day (F1, 24 = 14.12; p = 0.001). No main effect of age on CIPL over the training period was observed (F1, 24 = 2.30; p = 0.14), and no significant interaction was observed between age and training day (F1, 24 = 1.33; p = 0.26). Although post-hoc analyses indicate that aged animals took significantly longer paths to reach the visible platform on day 1 (p = 0.047), these differences were no longer apparent on day 2 (p = 0.24). These data confirm that the age-related differences in spatial trials are largely due to deficits in spatial learning, rather than to deficits in visual acuity or motor ability (Fig. 1C).
3.2 Behaviorally-induced Arc in pyramidal cells of CA1 and CA3
Aged animals do not show changes in the proportions of pyramidal cells that express Arc at short intervals (Small et al., 2004; Penner et al., 2010). The current analysis seeks to verify that these same effects occur in animals engaging in spatial processing over longer time intervals. Because previous studies have demonstrated that Arc clears the cytoplasmic compartment surrounding the nucleus within 30 minutes of behavioral activation in pyramidal cells (e.g., Guzowski et al., 1999), Arc expression in response to an exploration occurring 8 hours prior to sacrifice will no longer be present in CA regions. However, since virtually all of the same cells will be activated again if the animal returns to the same environment (e.g., Guzowski et al., 1999; Vazdarjanova et al., 2002), age-related alterations in the transcriptional response of pyramidal cells can be tested by having the animals return to the same environment immediately prior to sacrifice. Based on these data, only intranuclear Arc, which reflects activity from the second exploration, should be observed. Consistent with these previous data, relatively few cells express Arc in the cytoplasm, or in both the cytoplasm and nucleus of cells of the CA fields of young and aged rats (Fig. 2).
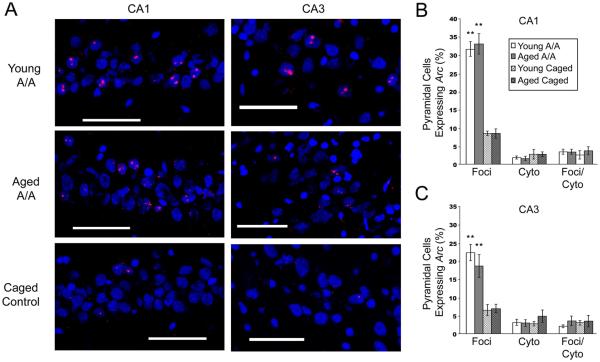
Figure 2.
Aging does not alter the pattern of Arc expression in pyramidal cells of CA1 or CA3. (A) Representative confocal images (scale bar = 60 μm) demonstrate that two exploration sessions separated by 480 minutes induce a robust increase in the number of pyramidal cells that express Arc in CA1 (left) and CA3 (right) in both young (top) and aged (middle) animals relative to caged controls (bottom). No significant differences in Arc expression were observed between young and aged, memory-impaired animals in either CA1 (B) and CA3 (C). Exploration induced a significant increase in the number of cells expressing intra-nuclear Arc (reflecting the second exploration occurring immediately prior to sacrifice) relative to caged controls in both age groups (all data are mean ± SEM; * p < 0.05, ** p < 0.01 vs. caged controls).
A significant main effect of exploration was observed across both CA1 and CA3 on intra-nuclear Arc expression, reflecting the transcriptional response to the second exploration immediately prior to sacrifice (F1, 42 = 82.252; p < 0.001). Post-hoc analyses revealed that both young (p = 0.011) and aged (p = 0.013) animals that engaged in exploration showed a significantly greater proportion of cells expressing Arc relative to CCs.
A main effect of region was also observed on the expression of intra-nuclear Arc expression (F2, 42 = 6.449; p = 0.004), as well as a significant interaction between region and exploration (F1, 42 = 4.324; p = 0.044). Consistent with previous reports (Vazdarjanova et al., 2002; 2005), post-hoc analyses showed that in both young and aged animals showed a greater proportion of cells expressing intra-nuclear Arc in CA1 than CA3 following spatial exploration (p = 0.033). In CCs, comparable numbers of cells in CA1 and CA3 expressed intra-nuclear Arc (p = 0.884; Fig. 2C).
No significant main effect of age on hippocampal pyramidal cell Arc expression was observed (F1, 42 = 0.042; p = 0.838). No significant interactions occurred between age and CA region or between age and condition, consistent with previous data (Small et al., 2004; Penner et al., 2010).
Collectively, these data replicate the result that the number of CA1 pyramidal cells transcribing Arc does not change with age (Penner et al., 2010). In addition, the current study extends this result by showing that the number of cells transcribing Arc in area CA3 also does not change with age. This conclusion must be made with caution, however, since Penner et al (2010) showed that although the number of CA1 pyramidal neurons transcribing Arc does not change across the lifespan, the relative abundance of Arc transcripts decreases with progressive age. It remains to be seen if this decrease in abundance also occurs in CA3.
3.3 Age-related changes in behaviorally-induced Arc expression in the fascia dentata
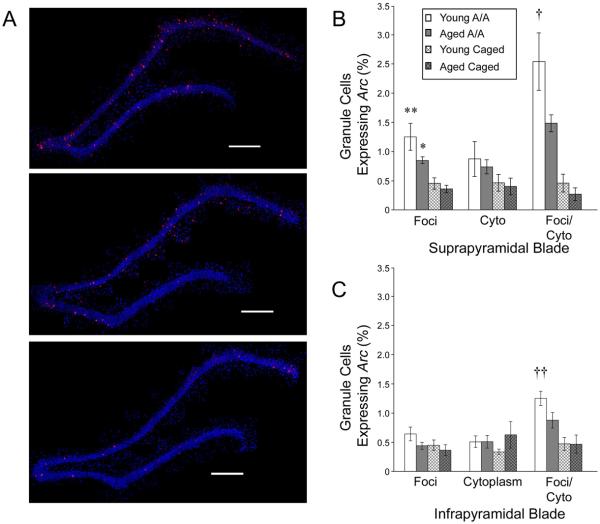
Unlike pyramidal cells, granule cells of the FD show persistent Arc expression for 8 hours following spatial exploration (Ramirez-Amaya et al., 2005), thus the majority of granule cells express Arc within both the nucleus and surrounding cytoplasm. Animals that explored a novel environment showed significantly more granule cells that expressed Arc solely within the nucleus (main effect of condition: F1, 44 = 17.726; p < 0.001) as well as both the nucleus and cytoplasm (main effect of condition: F1, 44 = 60.498; p < 0.001) relative to CCs (Fig. 3). Behavioral exploration had no effect on the number of cells expressing Arc in the cytoplasm only (F1, 44 = 1. 521; p < 0.224), consistent with previous reports of long-term Arc expression in the FD. Similarly, a significant main effect of region (i.e., suprapyramidal versus infrapyramidal blade of the FD) was also apparent in the number of cells expressing Arc in the nucleus only (F1, 44 = 7.780; p = 0.008), as well as both the nucleus and surrounding cytoplasm (F1, 44 = 14.238; p < 0.001), but not in the number of cells expressing Arc in the cytoplasm only (F1, 44 = 1.512; p = 0.225). These differences between the suprapyramidal and infrapyramidal blades also showed a significant interaction with condition. Relative to the infrapyramidal blade, significantly more granule cells in the suprapyramidal blade expressed Arc solely in the nucleus (F1, 44 = 7.908; p = 0.007), or within the nucleus and surrounding cytoplasm (F1, 44 = 22.352; p < 0.001), and the increase in Arc expression induced by exploration was greater in the suprapyramidal blade, consistent with previous reports (Temple et al., 2003; Chawla et al., 2005; Ramirez-Amaya et al., 2005; Vazdarjanova et al., 2006).
Figure 3.
Fewer granule cells express Arc in the fascia dentata (FD) of aged animals 480 minutes following spatial exploration. (A) Representative reconstructed overlapping confocal images (scale bar = 500 μm) showing that, although exploration induces a robust increase in the proportion of granule cells expressing Arc in the FD in both young (top) and aged (middle) exploring animals relative to caged controls (bottom), the increase in the proportion of granule cells expressing Arc is significantly less in aged, memory-impaired animals relative to young ones. Quantitative data for the suprapyramidal (B) and infrapyramidal (C) blades of the FD show this attenuation of gene expression (all data are mean ± SEM; * p < 0.05, ** p < 0.01 vs. age-matched caged controls; † p < 0.05, †† p < 0.01 young vs. aged exploring animals).
A main effect of age was observed in double-labeled cells expressing Arc in the nucleus and surrounding cytoplasm (F1, 44 = 4.702; p = 0.10). In addition, a significant interaction between condition and age was observed for the number of double-labeled granule cells (F1, 44 = 5.643; p = 0.022). Post-hoc tests reveal that, young and aged CC groups showed no significant differences (both blades: p > 0.9). However, following an exploration episode, young animals show a significantly greater proportion of double-labeled granule cells than CCs in both the suprapyramidal (p = 0.023) and infrapyramidal (p = 0.007) blades. Although aged animals that explored a novel environment also consistently showed an increase in the number of double-labeled granule cells, this increase was attenuated such that in post-hoc comparisons they were not significantly different relative to CCs (suprapyramidal blade: p = 0.284; infrapyramidal blade: p = 0.255).
3.4 Spatial memory performance is correlated with Arc expression
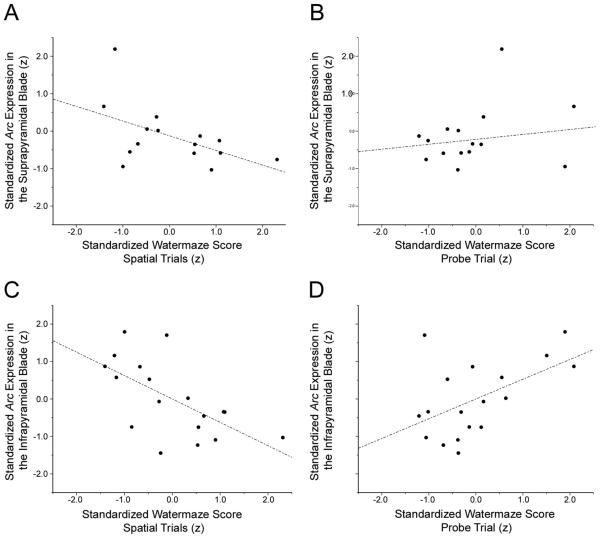
After normalizing both Morris swim task performance and Arc expression by z-score transformation, the total number of granule cells expressing Arc in the suprapyramidal blade of the FD negatively correlated with CIPL during spatial learning trials (r = -0.448; p = 0.047). These data indicate that animals with poorer spatial memory performance (and thus both longer path lengths) express Arc in fewer granule cells during exploration. Moreover, this significant correlation is accounted for largely by the number of cells that are double-labeled for Arc both in the nucleus and surrounding cytoplasm. These double-labeled cells show a similar significant negative correlation with CIPL scores (r = -0.515; p = 0.025) in the spatial trials of the Morris swim task (Fig. 4A). No significant correlations were found between performance in the Morris swim task and the number of granule cells expressing Arc solely in the cytoplasm (p = 0.326) or nucleus (p = 0.471). In addition, no significant correlation was found between animals' performance during the probe trial and Arc expression in the suprapyramidal blade (p = 0.165; Fig. 4B). Similar analyses of the infrapyramidal FD indicate that the number of granule cells expressing Arc in both the nucleus and cytoplasm (r = -0.623; p = 0.003) significantly predicts spatial performance in the Morris swim task (Fig. 4C). This correlation was not seen between CIPL scores in the Morris swim task and the number of cells expressing Arc exclusively in the nucleus (p = 0.273) or the cytoplasm (p = 0.349). Moreover, Arc expression in the infrapyramidal blade also significantly predicted performance in the probe trial of the Morris swim task (r = 0.527; p = 0.012, Fig. 4D).
Figure 4.
The expression of Arc in the fascia dentata (FD) is correlated with spatial memory performance in the Morris swim task. Within individual animals, the proportion of granule cells in the suprapyramidal blade of the FD expressing Arc 480 minutes following spatial processing is significantly correlated with performance during spatial trials (A), but not probe trials (B) in the Morris swim task. The number of granule cells expressing Arc in the infrapyramidal blade significantly correlated with both spatial trial (C) and probe trial (D) performance.
3.5 Arc expression induced by electrical stimulation
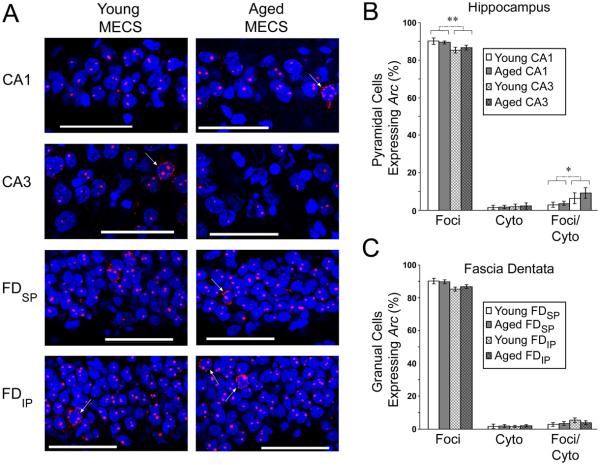
Under maximally activating conditions, such as MECS, Arc transcription occurred within the nucleus of nearly all cells in the hippocampal formation in young and aged animals, with cytoplasmic signal only occasionally present (Fig. 5A).
Figure 5.
Aging does not alter Arc expression induced by maximal electroconvulsive shock (MECS). (A) Representative confocal images (scale bar = 60 μm) show that, following MECS, the vast majority of cells express Arc within punctuate intranuclear foci, although a small number of cells can be seen expressing Arc within the cytoplasm (arrows). This general trend is true for CA1 and CA3 of the hippocampus, as well as the suprapyramidal (FDSP) and infrapyramidal (FDIP) blades of the fascia dentata of both young and aged animals, and can be clearly seen in quantitative data, showing that approximately 90% of principal cells in the hippocampus proper (B) and the fascia dentata (C) express Arc within the nucleus (all data are mean ± SEM; * p < 0.05, ** p < 0.01 CA1 vs. CA3).
Following MECS, approximately 90% of pyramidal cells expressed Arc within the nucleus, reflecting the transcription induced by the second stimulation given immediately prior to sacrifice of the animal (Fig. 5B). Relative to young animals, comparable of pyramidal cells expressed Arc solely within the nucleus (F1,12 = 0.230; p = 0.641), solely within the cytoplasm (F1,12 = 0.207; p = 0.657), or within both cellular compartments (F1,12 = 0.509; p = 0.489). This pattern of Arc transcription, however, was slightly different in CA3 relative to CA1. In CA3, fewer cells expressed Arc solely in the nucleus (F1, 12 = 11.180; p = 0.006) and more cells expressed Arc within both compartments (F1, 12 = 5.265; p = 0.041). It should be noted, however, that this difference is quite small, and if all of the cells that transcribed Arc in response to the second MECS stimulation are quantified (i.e., the sum of all cells expressing Arc within the nucleus, regardless of whether they also express Arc in the surrounding cytoplasm), this sum is comparable between regions. The number of cells expressing Arc solely within the cytoplasm was not different between regions (F1,12 = 0.477; p = 0.503). No significant interactions between age and region were present.
Similar data were obtained from the FD (Fig. 5C). No significant age difference was present in the number of granule cells expressing Arc solely within the nucleus (F1,12 = 0.550; p = 0.472), solely within the cytoplasm (F1,12 = 0.299; p = 0.595), or within both cellular compartments (F1,12 = 0.507; p = 0.490). When comparing the infrapyramidal and suprapyramidal blades of the FD, the infrapyramidal blade appeared to contain slightly fewer cells expressing Arc solely within the nucleus, along with slightly more cells expressing Arc in both the nucleus and cytoplasm. None of these trends, however, were significantly different (Nucleus only: F1,12 = 3.236; p = 0.098; Cytoplasm only: F1,12 = 0.072; p = 0.784; Double-labeled: F1,12 = 2.562.; p = 0.135). No significant interactions between age and region were present.
It should be noted that, unlike Arc induced by behavior, MECS did not induce long-lasting accumulation of cytoplasmic Arc within the FD. The majority of granule cells expressed Arc solely within the nucleus, reflecting the transcription driven by the second stimulation, while Arc induced by the first stimulation, which would have been present within the surrounding cytoplasm, is largely absent. This result is consistent with several previous studies showing that Arc is exquisitely sensitive to the precise level and pattern of stimulation that a cell receives. When comparing different preparations (e.g., French et al., 2001), stimulation patterns (e.g., Steward et al., 2007), or electrical stimulation to behavioral induction (e.g., Chotiner et al., 2010), different experimental protocols have repeatedly been shown to induce different signaling cascades, often with fundamentally different timecourses. Despite these differences between the patterns of Arc induced by MECS and behavior, these data demonstrate that young and aged animals have similar maximum capacities for transcription. Comparable Arc transcription in the young and aged hippocampal formation after MECS indicates that alterations in behaviorally-induced Arc transcription that occur with progressive age likely do not represent a transcriptional deficit per se, rather they likely represent a dysfunction further upstream. This conclusion must be made with caution, however, since the abundance of transcripts produced with comparable stimulation can change with progressive age, even in the absence of changes in the number of cells transcribing Arc (Penner et al., 2010).
4. Discussion
The current findings further the observations that the functional integrity of the FD declines with progressive age. In fact, a decline in Arc expression in this region can persist for at least 8 hours after spatial behavioral experience.
These data also demonstrate that a commensurate decline of Arc expression can be found in both the suprapyramidal and infrapyramidal blade of the FD of aged rats long after behavioral induction, suggesting a persistent deficit across this region. Moreover, those animals with superior spatial memory showed a greater level of long-term Arc expression across the FD 8 hours following spatial processing. This decline in the number of cells expressing Arc following behavior was apparent despite the fact that young and aged animals showed comparable numbers of granule cells transcribing Arc following MECS. This result indicates that granule cells maintain their capacity to express Arc across the lifespan, given sufficient stimulation. This finding is consistent reports that age-related deficits in the induction of perforant path LTP only consistently emerge following stimulus parameters close to LTP induction threshold, while high-intensity stimulation well above the threshold for LTP produces no induction deficits (see Burke and Barnes, 2010 for review). These findings also complement recent data combining in situ hybridization and PCR methods to measure both the recruitment of cells to a state of active transcription, as well as the relative abundance of the transcripts produced (Penner et al., 2010). This study showed that (unlike pyramidal cells) the number of granule cells transcribing Arc following behavior is reduced, but the relative abundance of Arc transcripts per activated cell remained comparable in young and aged animals. These data collectively suggest that the transcriptional machinery within granule cells remains intact, but fewer synapses are being activated, functionally reducing the probability that a granule cell will receive sufficient stimulation to induce Arc transcription. This is likely caused by the reduction in synaptic innervation of the FD from the entorhinal cortex that is known to occur with progressive aging (e.g., Geinisman et al., 1977; 1986; 1992).
In addition, the present data show that poor performance in the Morris Swim task was predicted not only by the number of cells expressing Arc, but also by the consistency with which the same cells expressed Arc again upon return to the same environment. Similarly, Penner et al (2010) showed that, following two explorations in a single environment separated by 20 minutes, significantly fewer granule cells transcribed Arc on the second exposure. Moreover, this decline is largely accounted for by a dramatic reduction in the number of cells expressing Arc simultaneously within both the nucleus and surrounding cytoplasm. A similar pattern of results in the aged FD occurs in the expression of another IEG, zif268 (Marrone et al., 2010). The current data extend these results by demonstrating that, in young animals, granule cells are able to reliably transcribe Arc in the same cells following multiple visits to the same environment even when these visits are separated by several hours. With progressive aging, however, a deficit in the ability of granule cells to consistently express Arc upon multiple visits to the same environment emerges that is predictive of spatial memory decline.
In particular, Arc expression in the infrapyramidal blade of the FD of aged rats show a more striking attenuation at 8 hours following spatial processing than that observed in the suprapyramidal blade. In fact, aged animals show no significant increase in Arc expression relative to CCs, despite a significant increase in young animals. The observations of dramatically reduced Arc transcription in the infrapyramidal blade of the FD with progressive age is remarkable, given that only a single study has shown a modest response in the infrapyramidal blade several hours following behavior (Ramirez-Amaya et al., 2005), while several previous studies have reported almost no short-term transcriptional response in this region following spatial behavior (Chawla et al., 2005; Ramirez-Amaya et al., 2006; Vazdarjanova et al., 2006). Given this novel long-term response to spatial processing, several important questions remain concerning the composition and function of this activity. The fact that the infra- and suprapyramidal blade each show a unique time course of Arc expression following behavior supports the idea that the functional roles of the blades of the FD may be distinct (Chawla et al., 2005; Ramirez-Amaya et al., 2005, 2006; Vazdarjanova et al., 2006). Even long after behavioral induction, Arc expression remains modest in the infrapyramidal blade relative the neighboring suprapyramidal blade, despite the fact that the two blades exhibit comparable Arc expression following MECS. These findings suggest that the infrapyramidal blade remains capable to Arc expression, but it may not be adequately engaged by a spatial memory task. This notion, however, remains speculative at this point, since no suitable behavioral paradigm yet exists to induce a robust activity-dependent signal in this region. The finding that spatial exploration can induce a modest response in this region in young rats given sufficient survival time, however, has made this problem tractable.
Given the correlation between Arc expression and age-related memory impairment, it is tempting to relate the lasting accumulation of Arc in the FD to memory consolidation. Consistent with this notion, the induction of Arc in the infrapyramidal FD at 8 hours following exploration coincides with a robust increase in Arc protein across both the hippocampus and cortex (Ramirez-Amaya, 2005), suggesting a coordinated bout of memory processing. Because a second exploration was required to capture Arc expression in pyramidal cells from the same animals, however, it is not possible to dissociate long-term stability of Arc transcripts generated within the previous 8 hours from de novo delayed transcription based on the current experiment. Regardless of which of these two possibilities accounts for the expression of Arc within granule cells long after spatial processing, the demonstration that Arc expression is attenuated long after behavioral induction provides an important insight into the dysfunction of this component of the hippocampal circuit. This is particularly interesting in light of the accumulating evidence that short- and long-term expression of Arc may have dissociable functional roles in regulating synaptic plasticity (Chowdhury et al., 2006; Rial Verde et al., 2006; Shepherd et al., 2006; Messaoudi et al., 2007; Park et al., 2008; Waung et al., 2008). For instance, LTP consolidation in the FD requires sustained Arc synthesis lasting for hours (Messaoudi et al. 2007). Knockdown of Arc 2 hours post-stimulation permanently reverses LTP as well as Arc mRNA and protein expression. Brief knockdown of Arc immediately after stimulation, however, reverses both LTP and Arc expression only transiently. Similarly, late phase LTP is selectively occluded in Arc knockout mice (Plath et al. 2006). These data suggest that the relative lack of long-term Arc expression in the aged FD may play a critical role in the enhanced decay rate of plasticity that occurs in this region with age. Several studies have shown that age-related deficits in LTP are largely time-dependent. The rate of LTP decay in the short-term (i.e., 1 h) is not different between aged and young rats (e.g., Landfield and Lynch, 1977; Landfield et al., 1978; Dupree et al., 1991), but over longer periods, LTP decays much faster in aged animals (e.g. Barnes, 1979; Barnes and McNaughton, 1980; for review see Barnes, 2003). These data suggest that the deficit in late-phase Arc expression may selectively mediate the age-related deficit in late-phase plasticity within the FD.
This, however, may only be one of many functional roles for late-phase Arc expression. In addition to the induction and maintenance of LTP, Arc protein is critical to long-term depression, neurogenesis, and synaptic scaling (reviewed in Bramham et al., 2008; 2010). Functionally, Arc has been implicated in the trafficking of AMPA receptors, the promotion of actin polymerization, and the regulation of translational machinery, and unique binding partners are implicated in each of these processes (reviewed in Bramham et al., 2008). Thus, the multiple potential mechanisms by which Arc can regulate neuronal function are thought to be mediated by the “molecular context” created by the presence of other plasticity-related molecules (reviewed in Miyashita et al., 2008). Although the molecular interactions involved in these processes have not yet been fully described, these data suggest that the immediate and long-term down-regulation of Arc may give rise to different changes in neuronal responses, depending on which other molecules are present for Arc to interact with. It will require electrophysiological recordings over long intervals, which are notoriously difficult, to verify this hypothesis directly. In this regard, IEG-based techniques may be particularly valuable for detecting activation of neurons over long intervals, particularly in structures such as the FD where the number of cells active at any given time is low.
Behavioral studies do suggest a decline in hippocampal-dependent memory processing in aged rats long after an initial exposure to a stimulus. When exposed to contextual fear conditioning, the freezing behavior exhibited by rats becomes progressively stronger with time in the absence of further training, a phenomenon referred to as memory incubation (Eysenck, 1968). Following a single day of contextual fear conditioning, the freezing response exhibited by young rats upon returning to the training environment continues to increase for at least 80 days. This incubation effect, however, is largely absent in aged animals, despite comparable levels of freezing behavior during training or when exposed to the conditioning tone (Houston et al., 1999). While the time course examined is qualitatively different, it confirms that the transformation of memories over time that normally depend on the hippocampus for their initial acquisition declines with age. Although the nature of memory transformation over time remains an area of debate, the current data points to the FD as a prime candidate to participate in the neural circuits that underlie such transformations.
Acknowledgments
This work was supported by the McKnight Brain Research Foundation and NIA grant AG009219, (C.A.B, P.F.W.) the state of Arizona and ADHS (C.A.B), NSERC (D.F.M.), and OMHF (D.F.M., E.S.).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Disclosure statement
The authors declare no actual or potential conflicts of interest.
References
- Alvarez P, Zola-Morgan S, Squire LR. Damage limited to the hippocampal region produces long-lasting memory impairment in monkeys. J. Neurosci. 1995;15:3796–3807. doi: 10.1523/JNEUROSCI.15-05-03796.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Amaral DG, Scharfman HE, Lavenex P. The dentate gyrus, fundamental neuroanatomical organization., dentate gyrus for dummies. Prog. Brain Res. 2007;163:3–22. doi: 10.1016/S0079-6123(07)63001-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bach ME, Barad M, Son H, Zhuo M, Lu YF, Shih R, Mansuy I, Hawkins RD, Kandel ER. Age-related defects in spatial memory are correlated with defects in the late phase of hippocampal long-term potentiation in vitro and are attenuated by drugs that enhance the cAMP signaling pathway. Proc. Natl. Acad. Sci. U.S.A. 1999;96:5280–5285. doi: 10.1073/pnas.96.9.5280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA. Memory deficits associated with senescence: a neurophysiological and behavioral study in the rat. J. Comp. Physiol. Psychol. 1979;93:74–104. doi: 10.1037/h0077579. [DOI] [PubMed] [Google Scholar]
- Barnes CA. Long-term potentiation and the ageing brain. Philos Trans R Soc Lond B Biol Sci. 2003;358:765–772. doi: 10.1098/rstb.2002.1244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes CA, McNaughton BL. Physiological compensation for loss of afferent synapses in rat hippocampal granule cells during senescence. J Physiol. 1980;309:473–485. doi: 10.1113/jphysiol.1980.sp013521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Worley PF, Moore MJ, Guzowski JF. The immediate early gene arc/arg3.1, regulation, mechanisms, and function. J. Neurosci. 2008;28:11760–11767. doi: 10.1523/JNEUROSCI.3864-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bramham CR, Alme MN, Bittins M, Kuipers SD, Nair RR, Pai B, Panja D, Schubert M, Soule J, Tiron A, Wibrand K. The Arc of synaptic memory. Exp Brain Res. 2010;200:125–140. doi: 10.1007/s00221-009-1959-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends Neurosci. 2010;33:153–161. doi: 10.1016/j.tins.2009.12.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chawla MK, Guzowski JF, Ramirez-Amaya V, Lipa P, Hoffman KL, Marriott LK, Worley PF, McNaughton BL, Barnes CA. Sparse, environmentally selective expression of Arc RNA in the upper blade of the rodent fascia dentata by brief spatial experience. Hippocampus. 2005;15:579–586. doi: 10.1002/hipo.20091. [DOI] [PubMed] [Google Scholar]
- Cherkin A. Kinetics of memory consolidation, role of amnesic treatment parameters. Proc. Natl. Acad. Sci. U.S.A. 1969;63:1094–1101. doi: 10.1073/pnas.63.4.1094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chotiner JK, Nielson J, Farris S, Lewandowski G, Huang F, Banos K, de Leon R, Steward O. Assessment of the role of MAP kinase in mediating activity-dependent transcriptional activation of the immediate early gene Arc/Arg3.1 in the dentate gyrus in vivo. Learn Mem. 2010;17:117–129. doi: 10.1101/lm.1585910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chowdhury S, Shepherd JD, Okuno H, Lyford G, Petralia RS, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 interacts with the endocytic machinery to regulate AMPA receptor trafficking. Neuron. 2006;52:445–459. doi: 10.1016/j.neuron.2006.08.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacol. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Deupree DL, Turner DA, Watters CL. Spatial performance correlates with in vitro potentiation in young and aged Fischer 344 rats. Brain Res. 1991;554:1–9. doi: 10.1016/0006-8993(91)90164-q. [DOI] [PubMed] [Google Scholar]
- Erickson CA, Barnes CA. The neurobiology of memory changes in normal aging. Exp. Gerontol. 2003;38:61–69. doi: 10.1016/s0531-5565(02)00160-2. [DOI] [PubMed] [Google Scholar]
- Eysenck HJ. A theory of the incubation of anxiety/fear responses. Behav. Res. Ther. 1968;6:309–321. doi: 10.1016/0005-7967(68)90064-8. [DOI] [PubMed] [Google Scholar]
- French PJ, O'Connor V, Jones MW, Davis S, Errington ML, Voss K, Truchet B, Wotjak C, Stean T, Doyère V, Maroun M, Laroche S, Bliss TV. Subfield-specific immediate early gene expression associated with hippocampal long-term potentiation in vivo. Eur J Neurosci. 2001;13:968–976. doi: 10.1046/j.0953-816x.2001.01467.x. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu. Rev. Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Burwell R, Burchinal M. Severity of spatial learning impairment in aging, development of a learning index for performance in the Morris water maze. Behav. Neurosci. 1993;107:618–626. doi: 10.1037//0735-7044.107.4.618. [DOI] [PubMed] [Google Scholar]
- Gage FH, Dunnett SB, Björklund A. Spatial learning and motor deficits in aged rats. Neurobiol. Aging. 1984;5:43–48. doi: 10.1016/0197-4580(84)90084-8. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, Bondareff W, Dodge JT. Partial deafferentation of neurons in the dentate gyrus of the senescent rat. Brain Res. 1977;134:541–545. doi: 10.1016/0006-8993(77)90828-9. [DOI] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F. Loss of perforated synapses in the dentate gyrus: morphological substrate of memory deficit in aged rats. Proc. Natl Acad. Sci. USA. 1986;83:3027–3031. doi: 10.1073/pnas.83.9.3027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, de Toledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Gerrard JL, Burke SN, McNaughton BL, Barnes CA. Sequence reactivation in the hippocampus is impaired in aged rats. J Neurosci. 2008;28:7883–7890. doi: 10.1523/JNEUROSCI.1265-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guzowski JF, McNaughton BL, Barnes CA, Worley PF. Environment-Specific expression of the immediate-early gene Arc in hippocampal neuronal ensembles. Nat. Neurosci. 1999;2:1120–1124. doi: 10.1038/16046. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J. Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Houston FP, Stevenson GD, McNaughton BL, Barnes CA. Effects of age on the generalization and incubation of memory in F344 rat. Learn Mem. 1999;6:111–119. [PMC free article] [PubMed] [Google Scholar]
- Landfield PW, Lynch G. Impaired monosynaptic potentiation in in vitro hippocampal slices from aged, memory-deficient rats. J Gerontol. 1977;32:523–533. doi: 10.1093/geronj/32.5.523. [DOI] [PubMed] [Google Scholar]
- Landfield PW, McGaugh JL, Lynch G. Impaired synaptic potentiation processes in the hippocampus of aged, memory-deficient rats. Brain Res. 1978;150:85–101. doi: 10.1016/0006-8993(78)90655-8. [DOI] [PubMed] [Google Scholar]
- Lansink CS, Goltstein PM, Lankelma JV, McNaughton BL, Pennartz CM. Hippocampus leads ventral striatum in replay of place-reward information. PLoS Biol. 2009;7:e1000173. doi: 10.1371/journal.pbio.1000173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Link W, Konietzko U, Kauselmann G, Krug M, Schwanke B, Frey U, Kuhl D. Somatodendritic expression of an immediate-early gene is regulated by synaptic activity. Proc. Natl. Acad. Sci. U.S.A. 1995;92:5734–5738. doi: 10.1073/pnas.92.12.5734. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyford GL, Yamagata K, Kaufmann WE, Barnes CA, Sanders LK, Copeland NG, Gilbert DJ, Jenkins NA, Lanahan AA, Worley PF. Arc, a growth factor and activity-regulated gene, encodes a novel cytoskeleton-associated protein that is enriched in neuronal dendrites. Neuron. 1995;14:433–445. doi: 10.1016/0896-6273(95)90299-6. [DOI] [PubMed] [Google Scholar]
- Marr D. Simple memory: a theory for archicortex. Philos. Trans. R. Soc. London Ser. B. 1971;262:23–81. doi: 10.1098/rstb.1971.0078. [DOI] [PubMed] [Google Scholar]
- Marrone DF, Adams AA, Satvat E. Increased pattern separation in the aged fascia dentata. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.03.021. 10.1016/j.neurobiolaging.2010.03.021. [DOI] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J. Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Kubik S, Lewandowski G, Guzowski JF. Networks of neurons, networks of genes: an integrated view of memory consolidation. Neurobiol Learn Mem. 2008;89:269–84. doi: 10.1016/j.nlm.2007.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadasdy Z, Hirase H, Czurko A, Csicsvari J, Buzsáki G. Replay and time compression of recurring spike sequences in the hippocampus. J Neurosci. 1999;19:9497–9507. doi: 10.1523/JNEUROSCI.19-21-09497.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nadel L, Moscovitch M. Memory consolidation, retrograde amnesia and the hippocampal complex. Curr Opin Neurobiol. 1997;7:217–227. doi: 10.1016/s0959-4388(97)80010-4. [DOI] [PubMed] [Google Scholar]
- Park S, Park JM, Kim S, Kim JA, Shepherd JD, Smith-Hicks CL, Chowdhury S, Kaufmann W, Kuhl D, Ryazanov AG, Huganir RL, Linden DJ, Worley PF. Elongation factor 2 and fragile X mental retardation protein control the dynamic translation of Arc/Arg3.1 essential for mGluR-LTD. Neuron. 2008;59:70–83. doi: 10.1016/j.neuron.2008.05.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pavlides C, Winson J. Influences of hippocampal place cell firing in the awake state on the activity of these cells during subsequent sleep episodes. J Neurosci. 1989;9:2907–2918. doi: 10.1523/JNEUROSCI.09-08-02907.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peigneux P, Laureys S, Fuchs S, Destrebecqz A, Collette F, Delbeuck X, Phillips C, Aerts J, Del Fiore G, Degueldre C, Luxen A, Cleeremans A, Maquet P. Learned material content and acquisition level modulate cerebral reactivation during posttraining rapid-eye-movements sleep. Neuroimage. 2003;20:125–134. doi: 10.1016/s1053-8119(03)00278-7. [DOI] [PubMed] [Google Scholar]
- Penner MR, Roth TL, Chawla MK, Hoang LT, Roth ED, Lubin FD, Sweatt JD, Worley PF, Barnes CA. Age-related changes in Arc transcription and DNA methylation within the hippocampus. Neurobiol Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.009. doi:10.1016/j.neurobiolaging.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plath N, Ohana O, Dammermann B, Errington ML, Schmitz D, Gross C, Mao X, Engelsberg A, Mahlke C, Welzl H, Kobalz U, Stawrakakis A, Fernandez E, Waltereit R, Bick-Sander A, Therstappen E, Cooke SF, Blanquet V, Wurst W, Salmen B, Bösl MR, Lipp HP, Grant SG, Bliss TV, Wolfer DP, Kuhl D. Arc/Arg3.1 is essential for the consolidation of synaptic plasticity and memories. Neuron. 2006;52:437–444. doi: 10.1016/j.neuron.2006.08.024. [DOI] [PubMed] [Google Scholar]
- Qin YL, McNaughton BL, Skaggs WE, Barnes CA. Memory reprocessing in corticocortical and hippocampocortical neuronal ensembles. Philos Trans R Soc Lond B Biol Sci. 1997;352:1525–1533. doi: 10.1098/rstb.1997.0139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression, evidence for selective, network-specific reactivation. J. Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Marrone DF, Gage FH, Worley PF, Barnes CA. Integration of new neurons into functional neural networks. J. Neurosci. 2006;26:12237–12241. doi: 10.1523/JNEUROSCI.2195-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasch B, Born J. Maintaining memories by reactivation. Curr. Opin. Neurobiol. 2007;17:698–703. doi: 10.1016/j.conb.2007.11.007. [DOI] [PubMed] [Google Scholar]
- Rial Verde EM, Lee-Osbourne J, Worley PF, Malinow R, Cline HT. Increased expression of the immediate-early gene arc/arg3.1 reduces AMPA receptor-mediated synaptic transmission. Neuron. 2006;52:461–474. doi: 10.1016/j.neuron.2006.09.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Gervasoni D, Soares ES, Zhou Y, Lin SC, Pantoja J, Lavine M, Nicolelis MA. Long-lasting novelty-induced neuronal reverberation during slow-wave sleep in multiple forebrain areas. P.L.o.S. Biol. 2004;2:E24. doi: 10.1371/journal.pbio.0020024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Nicolelis MA. Reverberation, storage, and postsynaptic propagation of memories during sleep. Learn. Mem. 2004;11:686–696. doi: 10.1101/lm.75604. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenzweig ES, Rao G, McNaughton BL, Barnes CA. Role of temporal summation in age-related long-term potentiation-induction deficits. Hippocampus. 1997;7:549–558. doi: 10.1002/(SICI)1098-1063(1997)7:5<549::AID-HIPO10>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Shen J, Barnes CA. Age-related decrease in cholinergic synaptic transmission in three hippocampal subfields. Neurobiol. Aging. 1996;17:439–451. doi: 10.1016/0197-4580(96)00020-6. [DOI] [PubMed] [Google Scholar]
- Shepherd JD, Rumbaugh G, Wu J, Chowdhury S, Plath N, Kuhl D, Huganir RL, Worley PF. Arc/Arg3.1 mediates homeostatic synaptic scaling of AMPA receptors. Neuron. 2006;52:475–484. doi: 10.1016/j.neuron.2006.08.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skaggs WE, McNaughton BL. Replay of neuronal firing sequences in rat hippocampus during sleep following spatial experience. Science. 1996;271:1870–1873. doi: 10.1126/science.271.5257.1870. [DOI] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span, is memory decline normal or not? Ann. Neurol. 2002;51:290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc. Natl. Acad. Sci. U.S.A. 2004;101:7181–7186. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squire LR. Memory and the hippocampus, a synthesis from findings with rats, monkeys, and humans. Psychol. Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- Stark SM, Yassa MA, Stark CE. Individual differences in spatial pattern separation performance associated with healthy aging in humans. Learn. Mem. 2010;17:284–288. doi: 10.1101/lm.1768110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Huang F, Guzowski JF. A form of perforant path LTP can occur without ERK1/2 phosphorylation or immediate early gene induction. Learn Mem. 2007;14:433–445. doi: 10.1101/lm.554607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sutherland GR, McNaughton BL. Memory trace reactivation in hippocampal and neocortical neuronal ensembles. Curr. Opin. Neurobiol. 2000:180–186. doi: 10.1016/s0959-4388(00)00079-9. [DOI] [PubMed] [Google Scholar]
- Temple M, Worley PF, Steward O. Visualizing changes in circuit activity resulting from denervation and reinnervation using immediate early gene expression. J. Neurosci. 2003;23:2779–2788. doi: 10.1523/JNEUROSCI.23-07-02779.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context, evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24:6489–6496. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, McNaughton BL, Barnes CA, Worley PF, Guzowski JF. Experience-dependent coincident expression of the effector immediate-early genes Arc and Homer 1a in hippocampal and neocortical neuronal networks. J Neurosci. 2002;22:10067–10071. doi: 10.1523/JNEUROSCI.22-23-10067.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazdarjanova A, Ramirez-Amaya V, Insel N, Plummer TK, Rosi S, Chowdhury S, Mikhael D, Worley PF, Guzowski JF, Barnes CA. Spatial exploration induces ARC, a plasticity-related immediate-early gene, only in calcium/calmodulin-dependent protein kinase II-positive principal excitatory and inhibitory neurons of the rat forebrain. J. Comp. Neurol. 2006;498:317–329. doi: 10.1002/cne.21003. [DOI] [PubMed] [Google Scholar]
- Wilson MA, McNaughton BL. Reactivation of hippocampal ensemble memories during sleep. Science. 1994;265:676–679. doi: 10.1126/science.8036517. [DOI] [PubMed] [Google Scholar]
- Winocur G, Moscovitch M, Sekeres M. Memory consolidation or transformation, context manipulation and hippocampal representations of memory. Nature Neurosci. 2007;10:555–557. doi: 10.1038/nn1880. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Muftuler LT, Stark CE. Ultrahigh-resolution microstructural diffusion tensor imaging reveals perforant path degradation in aged humans in vivo. Proc. Natl. Acad. Sci. U.S.A. 2010;107:12687–12691. doi: 10.1073/pnas.1002113107. [DOI] [PMC free article] [PubMed] [Google Scholar]