Abstract
The prefrontal cortex is highly vulnerable to traumatic brain injury (TBI) and its structural and/or functional alterations as a result of TBI can give rise to persistent working memory (WM) dysfunction. Using a rodent model of TBI, we have described profound WM deficits following TBI that are associated with increases in prefrontal catecholamine (both dopamine and norepinephrine) content. In this study, we examined if enhanced norepinephrine signaling contributes to TBI-associated WM dysfunction. We demonstrate that administration of α1 adrenoceptor antagonists, but not α2A agonist, at 14 days post-injury significantly improved WM performance. mRNA analysis revealed increased levels of α1A, but not α1B or α1D, adrenoceptor in the medial prefrontal cortex (mPFC) of brain-injured rats. As α1A and 1B adrenoceptor promoters contain putative cAMP response element (CRE) sequences, we therefore examined if CRE-binding protein (CREB) actively engages these sequences in order to increase receptor gene transcription following TBI. Our results show that the phosphorylation of CREB is enhanced in the mPFC at time points during which increased α1A mRNA expression was observed. Chromatin immunoprecipitation (ChIP) assays using mPFC tissue from injured animals indicated increased phospho-CREB binding to the CRE sites of α1A ,but not α1B, promoter compared to that observed in uninjured controls. To address the translatability of our findings, we tested the efficacy of the FDA-approved α1 antagonist Prazosin and observed that this drug improves WM in injured animals. Taken together, these studies suggest that enhanced CREB-mediated expression of α1 adrenoceptor contributes to TBI-associated WM dysfunction, and therapies aimed at reducing α1 signaling may be useful in the treatment of TBI-associated WM deficits in humans.
Keywords: catecholamines, neurotransmitter signaling, protein kinase A, CREB-mediated gene expression, chromatin immunoprecipitation
INTRODUCTION
The PFC is highly vulnerable to traumatic brain injury (TBI), and damage to this structure can cause cognitive deficits including problems with working memory (WM). WM, the ability to transiently hold information in mind in order to guide goal-directed behavior, is critical for many higher cognitive functions. While overt damage (as detected using brain imaging) results in WM deficits, many people with TBI show WM dysfunction in the absence of detectable PFC damage. Identification of the mechanisms underlying these deficits is an intense area of investigation. Using a rodent model, we have shown that TBI can cause persistent WM problems in the absence of detectable neuronal loss in the medial prefrontal cortex (mPFC), a structure anatomically and functionally equivalent to the dorsolateral prefrontal cortex (dlPFC) in humans and non-human primates (Moghaddam and Homayoun, 2008). Interestingly, these deficits were found to be associated with increased PFC catecholamine levels, suggesting that exaggerated dopamine and/or norepinephrine signaling may contribute to the WM deficits seen after TBI. We have previously shown that both systemic and intra-mPFC administration of D1 receptor antagonists to brain-injured animals reduce WM dysfunction (Kobori et al., 2006, Kobori and Dash, 2006). The consequence of elevated norepinephrine on WM dysfunction following TBI has not been examined.
It has been shown that both excessive and insufficient norepinephrine levels impair WM, indicating that an optimal amount of norepinephrine is required for normal WM function (Arnsten and Li, 2005). For example, the WM dysfunction caused by chronic stress is associated with increased norepinephrine levels, and this deficit can be reversed by administration of the α1 antagonist urapidil (Birnbaum et al., 1999, Southwick et al., 1999). In contrast, the WM deficits observed as a result of normal aging are associated with decreased norepinephrine levels. These deficits can be relieved by administration of the α2A agonist guanfacine, an effect thought to be due to stimulation of post-synaptic α2A receptors (Arnsten and Goldman-Rakic, 1985). Since α2A receptors are Gi coupled, this suggests that stimulation of these receptors would result in a decrease in cAMP signaling.
In this study, we investigated if excessive norepinephrine in the mPFC following TBI contributes to WM dysfunction. Our data shows that administration of α1 antagonists improves WM function in brain-injured animals. We further demonstrate that TBI increases the levels of α1 adrenoceptor mRNA within the mPFC, that was associated with enhanced binding of phosphorylated CREB to the α1A adrenoceptor promoter regions. Our findings suggest that therapies aimed at modulating norepinephrine signaling may have benefit in the treatment of TBI-induced WM dysfunction.
EXPERIMENTAL PROCEDURES
Materials
Phospho-CREB (Ser133) and pan-specific CREB antibodies were obtained from Cell Signaling Technology (Danvers, MA). Monoclonal anti-glutamic acid decarboxylase 67 (GAD67) and monoclonal anti-NeuN antibodies were obtained from Millipore (Billerica, MA). The α1 adrenoceptor antagonist prazosin (1-(4-Amino-6,7-dimethoxy-2-quinazolinyl)-4-(2-furanylca rbonyl)piperazine hydrochloride), α1 adrenoceptor antagonist HEAT (2-{[b-(4-Hydroxyphenyl)ethyl]aminomethyl}-1-tetralone hydrochloride), and the α2A adrenoceptor agonist guanfacine ([(2,6-Dichlorophenyl)acetyl]guanidine hydrochloride) were purchased from Tocris Bioscience (Ellisville, MO).
Brain Injury
All protocols involving the use of animals were in compliance with NIH's Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee. Male Sprague Dawley rats (260-300g) were purchased from Harlan (Indianapolis, IN). An electromagnetic controlled cortical impact (CCI) device (Virginia Commonwealth University Custom Design & Fabrication) was used to administer unilateral brain injury as previously described (Lyeth et al., 1990, Dixon et al., 1991, Smith et al., 1995). Rats were anesthetized with 4% isoflurane and a 2:1 mixture of N2O:O2, then mounted in a stereotaxic frame. The head was held in a horizontal plane, and a 7mm craniectomy was performed on the right cranial vault. The center of the craniectomy was placed at 3.0mm posterior of the bregma and 3.5mm lateral to the midline. Animals received a single impact of 3.3mm deformation with an impact velocity of 4.0m/sec at an angle of 10° from the vertical plane using a 6mm diameter impactor tip. The impact was delivered onto the parietal association cortex. The body temperature was maintained at 37°C by the use of a heating pad.
Western blotting
Fourteen days post-injury, animals were sacrificed and brains were dissected while submerged under ice-cold artificial CSF (10mM HEPES pH 7.2, 1.3mM NaH2PO4, 3mM KCl, 124mM NaCl, 10mM dextrose, 26mM NaHCO3 and 2mM MgCl2). The mPFC tissues ipsilateral to the side of impact were quickly removed and snap-frozen on dry ice. Sham-operated animals were sacrificed at 14 days following surgery and used as controls. The mPFC brain tissue was homogenized using a motorized tissue grinder in a lysis-buffer containing 10mM Tris pH 7.4, 1mM EGTA, 1mM EDTA, 0.5mM DTT, 10μg/mL leupeptin, 10μg/mL aprotinin, 1mM PMSF, and 0.1μM okadaic acid, followed by centrifugation at 10,000 × g for 10min. The pelletized material was rinsed twice with the lysis-buffer, then re-suspended in lysis-buffer containing 0.5% Triton X-100. Following sonication (Sonic Dismembrator 100; Fisher Scientific, Pittsburgh, PA), the Triton X-100 soluble fraction was collected and used as a membrane and nuclear fraction extract. The protein concentration was measured using a NanoOrange protein quantification kit (Invitrogen, Carlsbad, CA). Extracts were denatured in 1X Laemmli buffer, resolved in a SDS-PAGE and transferred to an Immobilon-P membrane (Millipore, Bedford, MA) followed by blocking overnight in TBST (10mM Tris pH 7.5, 150mM NaCl, 0.05% Tween-20) plus 5% BSA. Membranes were then incubated with primary antibody (0.2μg/mL) for 3hr at room temperature. Following incubation with the primary antibody, membranes were washed three times and immunoreactivity was assessed by an alkaline phosphatase-conjugated secondary antibody and a CDP-Star chemiluminescent substrate (Cell Signaling Technology). The optical density of the immunoreactive bands was measured utilizing ImageJ software (http://rsb.info.nih.gov/ij/index.html). Prior to reprobing, blots were stripped by two 10min washes in 50mM NaOH at room temperature. The membranes were then washed extensively with TBST and reblocked for an hour in 2% BSA prior to immunodetection.
Chromatin Immunoprecipitation (ChIP)
The mPFC tissue of injured (ipsilateral to the injury) and sham animals were dissected at 14 days after the surgery as described in western blots section. The tissue was minced in small pieces and incubated in 1% paraformaldehyde in PBS at room temperature for 15min to crosslink DNA binding proteins to the DNA. The tissue was then sonicated on ice for 5sec × 5 times with 70% power using Sonic Dismembrator 100 (Fisher Scientific, Pittsburgh, PA). This sonication condition was pre-determined to fragment the majority of genomic DNA to 200 to 600 base pair lengths. DNA concentration in samples was measured using a PicoGreen assay kit (Invitrogen) and a CytoFluor Multi Well Plate Reader (Applied Biosystems) and was normalized prior to carrying out the ChIP assay. ChIP was carried out using EZ-ChIP assay kit with procedures recommended by the manufacturer (Upstate, VA). The sonicated lysates was incubated with either pan-specific anti-CREB or anti-phospho CREB antibodies in ChIP dilution buffer (0.01% SDS, 1.1% Triton X-100, 1.2mM EDTA, 16.7mM Tris-HCl pH 8.1, 167mM NaCl) at 4°C overnight. Precipitation was performed by incubating the samples with Protein G agarose for an hour at 4°C. After brief centrifugation, the precipitated materials were washed sequentially with Low Salt Immune Complex Washing Buffer (0.1% SDS, 1% Triton X-100, 2mM EDTA, 20mM Tris-HCl pH 8.1, 150mM NaCl), High Salt Immune Complex Washing Buffer (0.1% SDS, 1% Triton X-100, 2mM EDTA, 20mM Tris-HCl pH 8.1, 500mM NaCl), LiCl Immune Complex Washing Buffer (0.25M LiCl, 1% IGEPAL-CA630, 1% deoxycholic acid, 1mM EDTA, 20mM Tris-HCl pH 8.1) for one time each, and TE Buffer two times. The immunoprecipitated material was eluted from Protein G in elution buffer (1% SDS, 100mM NaHCO3) for 15min at room temperature two times. For negative control, IP was performed in an absence of antibody or using IgG non-immune antibody (Santa Cruz Biotechnology, CA). After the completion of IP, the crosslink between DNA and protein was reversed by incubating the DNA-protein complex in 200 mM NaCl at 65°C for 6hr, followed by degradation of RNA and proteins with RNase A and proteinase K, respectively. The DNA was purified using a spin column system provided as part of the EZ-ChIP kit. IP was performed in triplicate and final output DNA (purified DNA) was pooled for each sample.
RNA extraction and quantification
The mPFC tissue ipsilateral to the brain injury was removed as described above. The tissue was sonicated in Trizol (Invitrogen) and total RNA was extracted according to the manufacturer's protocol. The concentration of the total RNA was determined using RiboGreen RNA quantification kit (Invitrogen) and a CytoFluor Multi Well Plate Reader (Applied Biosystems). A 1μg sample of total RNA was reverse transcribed for 2h at 36°C in a 20μL reaction containing 50mM Tris pH 8.3, 75mM KCl, 3mM MgCl2, 10mM DTT, 2.5μM random hexamer, 1mM each dNTP, 40U RNase inhibitor, and 400U Superscript II reverse transcriptase (Invitrogen). The level of expression of each target mRNA was quantified by amplification of the cDNA in triplicate using a StepOne real-time PCR system (Applied Biosystems) as described below. Sequences of primers for α1A, α1B, and α1D adrenoceptors were reported previously by others (Marti et al., 2005). Primer sequences for neuron-specific tubulin, which were used as an internal control, were: 5’-ctcaaccaccttgtgtct-3’ (forward), 5’-catgaagaaatgcaagcg-3’ (reverse). A cDNA produced from normal rat cerebral cortex was used to generate a standard curve in real-time PCR.
Quantitative-PCR (qPCR)
DNA fragments of the adrenergic α1A, α1B, and α1D receptors promoter regions that interact with CREB or phospho-CREB were quantified using a StepOne real-time PCR system (Applied Biosystems, Foster City, CA). Sequences of the primers that were used in this study were: α1A CRE1: forward: 5’-ttgtcccgttttgccta-3’, reverse: 5’-gatccattctgtgcatcc-3’; α1A CRE2: forward: 5’-gaaagagaaggacaataaccc-3’, reverse: 5’-caaatacagcgagccaata-3’; α1B CRE1: forward: 5’-tggcttctttcttttgc-3’, reverse: 5’-tcatctttcaactctccc-3’; α1D control: forward: 5’-gaattcagacttcagag-3’, reverse: 5’-cacgaggttatttatgg-3’. PCR reaction was performed in triplicate in a 15μL reaction volume containing 7.5μL Power SYBR Green PCR Master Mix (Applied Biosystems), 0.5μM of each forward and reverse primer, and sample or control DNA. The PCR master mix contains SYBR green 1 and 6-carboxy-X-rhodamine (ROX) for a detection of the double strand DNA, and as a reference dye, respectively. The amplification protocol consisted of one cycle at 95°C for 10min followed by 40 cycles at 95°C for 15 sec, at annealing temperature ranging from 48 to 60°C for 30sec, and 72°C for 30sec. The annealing temperature was pre-determined for each primer set. A single amplified product was confirmed by melt curve analysis. For quantification, a standard curve was generated for each amplification using increasing concentration of sonicated DNA derived from the cerebral cortex of sham animals to determine the linear range and amplification efficiency. The threshold cycle of each sample was fitted to the standard curve to calculate the relative DNA abundance in the sample. The reaction conditions were optimized for each primer set, such that the amplification efficiency was within 80- to-100% and the regression coefficient R2 was > 0.90.
Immunohistochemical staining
Injured and sham animals at 14 days post-surgery were anesthetized by an intra-peritoneal (i.p.) injection of 0.7% chloral hydrate followed by perfusion-fixation using trans-cardiac infusion of 0.1M phosphate-buffered saline (PBS) plus 1,000units/mL heparin sulfate followed by 0.1M PBS containing 4% paraformaldehyde and 0.2% glutaraldehyde. Brain tissue was removed and post-fixed with 4% paraformaldehyde in 0.1M PBS at 4°C overnight. Following cryo-protection in sucrose, 30μm-thick frozen sections of the mPFC region were prepared. Every 150μm, sections were chosen for immunostaining. Sections were incubated in 2% normal goat serum, 0.2% Triton X-100 in 0.1M PBS at room temperature for 2hr, followed by incubation with the primary antibody (0.5μg/mL for anti-phospho CREB (Ser133), 0.25μg/mL for anti-pan CREB and anti-GAD67, and 1:2000 dilution for anti-NeuN). Sections were washed three times in PBS and incubated with biotin or alkaline phosphatase-conjugated secondary antibody as suggested by the vendor. For double immuno-staining, biotin labeling was detected by incubation with avidin-biotin-peroxidase complex using an ABC kit (Vector Laboratories, CA). The complex was reacted with 3,3’-diaminebenzidine (DAB, Vector Laboratories). Alkaline phosphatase (AP) activity was detected using an AP-substrate kit (Vector Laboratories).
Cell Counts
Cell counts were performed using the optical dissector technique (Coggeshall and Lekan, 1996, Dash et al., 2001, Kobori and Dash, 2006). Sections of every 150μm interval were immunostained as described in the Immunohistochemistry section. Cells were quantified using Stereo Investigator, Optical Dissector Probe (MicroBrightField, Colchester, VT) within a defined boundary of 2.15×10-2 mm3 encompassing the mPFC at 40x magnification. The corpus callosum was used as a marker in order to locate mPFC. Cell counts were performed by two independent researchers who were blind to the treatment groups. The results obtained from five sections per animal were averaged.
Working Memory (WM) Testing
All behavioral tests were performed by an experimenter blind to the treatment groups. Spatial WM was assessed using a WM version of the Morris water maze task (delayed match-to-place task) as previously described (Hamm et al., 1996, Steele and Morris, 1999, Kline et al., 2002). Briefly, 2 days before testing (day 12 post-injury), animals were given seven training trials in order to familiarize them with the task (Kobori and Dash, 2006). Each testing session consisted of a location trial, a 5sec delay, and a match trial. Each location trial was initiated by placing the rat in a random start location. Once the animal found the platform, it was allowed to remain there for 10sec. The animal was then removed from the water tank for a 5sec delay period. The rat was then placed back into the maze at the same start position as used in the location trial and allowed to search for the hidden escape platform (the match trial). If the animal failed to locate the platform within 60 sec on any given trial, it was led there by the experimenter. After each location-match pair, animals were given a 4min intertrial interval (iti), during which time the platform was moved to a new position. For testing the influence of drugs on WM performance (day 14 post-injury), animals were injected then after a 30min recovery period, tested in four location-match trials with each pair having a unique start position and platform location. The WM testing was repeated 24 hr and 3 days after the drug treatment.
Statistical analysis
Student's t-test for unpaired variables or a one-way analysis of variance (ANOVA) was used for the analysis of western blot, PCR and stereological cell count data, when appropriate. A repeated measures two-way ANOVA was used for evaluating WM performance and treatment effects. Results were considered significant at p<0.05. Data are presented as the mean ± standard error of the mean (S.E.M.).
RESULTS
α1 antagonist improves spatial working memory (WM) in injured rats
To test if norepinephrine signaling contributes to TBI-associated WM deficits, the influences of the α1 selective antagonist HEAT (0.1 mg/kg) and the α2A selective agonist guanfacine (0.1 mg/kg) were examined on day 14 post-injury, a time point when WM deficits have been observed following cortical impact injury.
Prior to testing the influence of adrenergic receptor agonist/antagonist, injured animals were given a series of training trials on day 12 post-injury (Figure 1a). Figure 1b shows summary data of WM performance of typical sham-operated control animals on day 14 after the surgery (n=8, location trial, 47.7±2.53 sec, match trial, 19.5±3.41 sec). On day 14 post-TBI, drugs were systemically administered and WM tested 30 min post-injection (n=8/condition). Figure 1c shows that prior to drug administration, there was no difference in performance between the animals to be treated with either HEAT or vehicle. Following drug administration, however, the HEAT-treated injured animals performed significantly better in the spatial WM task than did vehicle-treated injured animals as indicated by a decrease in latency during the match trial compared with the location trial (location trial, 50.4±2.69 sec, match trial, 33.7±2.78 sec, P<0.001). Vehicle-treated, injured animals, by comparison, remained dysfunctional and had similar latencies in both the location and the match trials (location trial 51.4±1.41 sec, match trial 45.9±3.19 sec, P=0.125). Comparison of the performance curves between vehicle- and HEAT-treated injured animals revealed a significant interaction between trial number and treatment (two-way repeated measures ANOVA: F(1,14)=5.727, P=0.032). In contrast to the effect of HEAT, the performance curves of animals treated with guanfacine was not significantly different from that seen in vehicle-treated, injured controls (two-way repeated measures ANOVA: F(1, 14)=0.825, P=0.382) (Figure 1d).
Figure 1. Systemic administration of α1 antagonists, but not α2A agonists, improve WM performance in TBI animals.
a) Rats were injured, then trained (on day 12 post-injury) in the WM version of the Morris water maze task. On day 14 post-injury, rats were injected with either the α1 antagonist HEAT (0.1 mg/kg i.p.), the α2A agonist guanficine (0.1 mg/kg i.p.) or vehicle, then tested for WM 30min later. b) Summary data showing the latency to locate the hidden platform in the location (Loc) and match trials (Match) in sham-operated control animals. c) Summary data showing the latency to locate the hidden platform in the location (Loc) and match trials (Match) during the last 3 trials of training for rats to be treated with HEAT (or vehicle). Rats treated with HEAT had improved WM performance when tested 30min post-injection. d) Summary data showing the latency to locate the hidden platform in the location (Loc) and match trials (Match) during the last 3 trials of training for animals to be treated with guanfacine (or vehicle). Rats treated with guanfacine remained impaired in the WM task when tested 30min post-injection.  Significant interaction between trial number and treatment. *Significant difference in latency between location and match trial.
Significant interaction between trial number and treatment. *Significant difference in latency between location and match trial.
TBI increases the mRNA levels of α1A adrenoceptor in the mPFC
Previous in vitro studies have shown that chronic exposure of cells to norepinephrine can increase the expression of α1 adrenoceptors. We therefore examined if the levels of these adrenoceptors in the mPFC are increased after injury. Because none of the commercially available antibodies have been demonstrated to be specific for α1 receptors (Jensen et al., 2009), we examined the mRNA levels of these receptors. Total RNA was extracted from mPFC tissues on day 14 post-injury. Quantification using real-time PCR revealed that α1A (Figure 2a; Student's t-test: P=0.002), but not α1B (Figure 2b; Mann-Whitney Rank Sum Test: P=0.597) or α1D (Figure 2c; Student's t-test: P=0.754), adrenoceptor mRNA levels were significantly increased in the mPFC at this time point. It has been reported that the mRNA expression of α1A adrenoceptor can be enhanced by cAMP elevating agents (Gao et al., 1997, Razik et al., 1997). In order to examine if the cAMP cascade contributes to the increased α1A mRNA levels we observe, Rp-cAMP (10, 20 and 40 nmol) was infused into the PFC on day 14 post-injury and tissue harvested 6 hr later (n=4/dose). Figure 2d shows that Rp-cAMP significantly reduced α1A mRNA levels in a dose-dependent manner compared to vehicle-treated, injured controls (One Way ANOVA F(3,18)=10.68, P<0.001). No effect on α1D mRNA levels was observed as a result of Rp-cAMP treatment (One Way ANOVA F(3,18)=0.231, P=0.873).
Figure 2. Brain injury increases the mRNA levels of α1A adrenoceptor in the mPFC.
Summary results of quantitative real-time PCR showing the fold-changes of mRNA levels of a) α1A, b) α1B and c) α1D adrenoceptors in sham and brain-injured animals on day 14 after the surgery. *Significant difference between sham and brain injury. d) The levels of α1A, but not α1D, could be reduced by intra-mPFC infusions of Rp-cAMP. * Significant difference between vehicle and Rp-cAMP treatments.
CREB phosphorylation is enhanced in the mPFC following TBI
Since α1A mRNA appeared to be responsive to PKA, and the transcription factor CREB is a known target of PKA, western blots were performed using an antibody which specifically detects CREB when phosphorylated at Ser133. Figure 3a shows pictures of representative western blots of phospho- and total CREB immunoreactivities from mPFC tissue samples prepared from sham and 14 day injured animals. Quantification of the immunoreactive bands indicate that TBI (n=6) significantly increased phospho-CREB immunoreactivity compared to that observed in sham-operated controls (n=5) (Figure 3b; Student's t-test: P=0.014). When the blots were re-probed using the pan-specific CREB antibody that recognizes CREB regardless of its phosphorylation status, there was no difference between the injury and sham samples (Figure 3c; Student's t-test: P=0.280).
Figure 3. CREB phosphorylation is increased in the mPFC on day 14 post- injury.

a) Representative western blots for phospho-CREB and pan-specific CREB immunoreactivities in mPFC in sham and brain-injured animals on day 14 after injury. Summary results showing that b) the phosphorylation, but not c) the total levels, of CREB are increased in the mPFC following injury. *Significant difference between sham and brain injury.
TBI increases CREB phosphorylation in mPFC GABAergic neurons
To identify the neuronal cell types with enhanced CREB phosphorylation, double-label immunohistochemical staining followed by stereological cell counting was performed. Figure 4a and 4b show representative photomicrographs indicating phospho-CREB staining co-labeled with the neuronal marker NeuN and the marker of inhibitory neurons GAD67, respectively. The number of NeuN+, phospho-CREB+ neurons was slightly increased, although this increase did not reach statistical significance (Figure 4c; Student's t-test: P=0.190). Phospho-CREB+, non-neuronal cells (NeuN-) were not significantly different between the two groups (Figure 4d, Student's t-test: P=0.733). The number of phospho-CREB and GAD67 double immunopositive cells, however, was found to be significantly increased as a result of brain injury (Figure 4e, Student's t-test: P=0.029). No significant differences were found in the total number of CREB (phosphorylated plus non-phosphorylated) positive cells (data not shown).
Figure 4. Brain injury increases CREB phosphorylation in mPFC GABAergic neurons.
Representative photomicrographs of double-immunostaings for a) phospho-CREB (brown) and NeuN (blue) and b) phospho-CREB (brown) and GAD67 (blue). Arrow, examples of double positive cells. Arrowheads, examples of mono-positive cells. Bar, 20μm. Summary results of the unbiased stereological cell counting for c) total phospho-CREB positive cells, d) phospho-CREB and NeuN double-positive cells, and e) phospho-CREB and GAD67 double positive cells. *Significant difference between sham and brain injury.
TBI increases the binding of phospho-CREB to the α1A promoter
The results presented above suggest that CREB may be responsible, at least in part, for the observed increase in α1A mRNA. In order to directly examine if the increased α1A adrenoceptor mRNA levels result from enhanced CREB-driven transcription, chromatin immunoprecipitation (ChIP) assays were performed using mPFC tissue samples on day 14 post-injury. It has been previously shown that the promoter regions of the α1A and α1B adrenoceptor genes contain putative CRE sequences (Gao and Kunos, 1993, Michelotti et al., 2003) (Figure 5a). Although the α1D adrenoceptor promoter does not contain the typical consensus CRE sequence, a putative sequence with two base-pair mismatch was identified between -1591 and-1584. Binding to this region was used as a negative control for the ChIP assay (Figure 5a). Immunoprecipitation (IP) was performed using an anti-phospho-CREB (Ser133) antibody and a pan-specific total anti-CREB antibody. The summary results of real-time qPCR shown in Figure 5b indicate the fold-changes in amplified target from each of the promoter regions relative to the amount detected in sham controls. Binding of phosphorylated CREB to the α1A promoter regions, but not to the α1B promoter region, as a result of injury (n=7 for both injury and sham samples) was found to be significantly enhanced (Figure 5b, α1A CRE1 and 2, p < 0.001 for CRE1, p = 0.005 for CRE2). No amplified products were detected when the α1D primer pairs were used for qPCR analysis. When pre-IP samples were used for qPCR analysis, the α1D promoter region was amplified comparable to that detected for α1A and α1B promoters. When the pan-specific total CREB antibody was used for IP, neither α1A nor α1B promoter region in brain injury samples showed significant changes compared with the samples obtained from sham animals (Figure 5b). Taken together, these results indicate that CREB-driven gene expression contributes to the observed increase in α1A mRNA levels in the mPFC following brain injury.
Figure 5. TBI increases the binding of phospho-CREB to the α1A adrenoceptor promoter.
a) Illustration of the promoter regions of α1 adrenoceptors and the locations of CRE consensus sequences. b) Summary results of ChIP assay. Amplicons (CRE1, 2 or control) correspond to that shown in a). Results are shown as fold-changes in amplified target from each of the promoter regions relative to the amount detected in sham controls. *Significant difference between sham and brain injury.
Prazosin improves WM in injured rats
Our results suggest that α1 antagonist may have clinical utility. Since HEAT is not a FDA approved drug, we tested the efficacy of Prazosin, an FDA-approved α1 selective antagonist. Prazosin (0.5mg/kg i.p.) or vehicle was injected on day 14 post-injury and WM assessed at various time points following injection (Figure 6a, n=9/group). Figure 6b shows that there was a trend towards improved WM performance 30 minutes post-injection of Prazosin, although this did not reach statistical significance (two-way repeated measures ANOVA: F(1,16)=1.13, P=0.304). However, the Prazosin-treated group showed significant improvement in WM compared to vehicle-treated controls at 24hr post-injection (Figure 6c; two-way repeated measures ANOVA: F(1,16)=15.2, P=0.001). This is indicated by a significant decrease in latency during the match trial compared with the location trial (location trial, 48.3±3.30 sec, matching trial, 25.0±3.45 sec, P<0.001). This benefit could be observed for at least 3 days following the injection (Figure 6d; F(1,16)=11.9, P=0.004).
Figure 6. Systemic administration of Prazosin on day 14 improves WM performance.
a) Rats were injured, then trained (on day 12 post-injury) in the WM version of the Morris water maze task. On day 14 post-injury, rats were injected with the α1 antagonist Prazosin or vehicle, then tested for WM at various time points. Summary data showing the latency to locate the hidden platform in the location (Loc) and match trials (Match) assessed b) 30min, c) 24hr, and d) 3 days after injection.  Significant interaction between trial number and treatment. *Significant difference in latency between location and matching trial.
Significant interaction between trial number and treatment. *Significant difference in latency between location and matching trial.
DISCUSSION
This study revealed four key findings: (1) TBI increases α1A, but not α1B or α1D, adrenoceptor mRNA levels in mPFC; (2) TBI increases the phosphorylation of CREB on Ser133 predominantly in GABAergic interneurons; (3) TBI enhances the recruitment of phospho-CREB to the α1A promoter; and (4) systemic administration of α1 antagonists improves WM performance in injured animals. Taken together, these findings support the clinical usefulness of α1 receptors antagonists as a treatment for WM dysfunction following TBI.
In the current study, we observed that TBI increases the mRNA levels of α1A, but not α1B or α1D, adrenoceptors in the mPFC (Figure 2). Although the intracellular signaling that is responsible for these increases in mRNA is not known, α1 expression has been reported to be enhanced upon prolonged stimulation of the receptor by norepinephrine. For example, chronic treatment of cardiac myocytes with either α1 specific agonists or norepinephrine has been shown to be associated with increased α1A, but not α1B or α1D, adrenoceptor mRNA and protein levels (Rokosh et al., 1996, Autelitano and Woodcock, 1998). As we have previously reported that brain injury causes a prolonged increase in prefrontal norepinephrine levels (Kobori et al., 2006), the elevated α1A mRNA levels we observed may be a direct result of norepinephrine-mediated altered gene expression.
Several promoter regulatory elements that can alter α1A receptor expression have been identified. For example, it has been shown that the basal transcription of α1A adrenoceptor is controlled by Sp1 in humans and transcriptional enhancer factor-1 (TEF-1) in rodents (Razik et al., 1997, O'Connell et al., 2001). Potential regulatory elements for activating protein-1 (AP-1), activating protein-2 (AP-2), insulin, glucocorticoids, estrogen and CREB were also identified (Lee et al., 1998). Although treatments that increase the phosphorylation and activation of CREB result in up-regulation of α1 adrenoceptors in various cell lines (Gao et al., 1997, Razik et al., 1997), direct interaction of CREB with the α1A promoter has not been demonstrated. Using ChIP assays, we demonstrated that injury-induced upregulation of α1A adrenoceptors is associated with a significant increase in binding of phosphorylated CREB to the CRE-like sequences within its promoter (Figure 5). These results, coupled with our findings that PKA inhibitor administration reduces α1A mRNA levels, suggest that cAMP-mediated activation of CREB is responsible, at least in part, for the enhanced expression of α1A mRNA we observed. The roles of the other regulatory sequences in inducing α1A mRNA following brain injury remain to be determined.
Our observation that phospho-CREB immunoreactivity was enhanced in GAD67-positive cells, coupled with our findings that there was increased phospho-CREB binding to the α1 promoter, implies that α1 receptor expression is increased in GABAergic interneurons. These findings suggest that enhanced NE signaling as a result of TBI would stimulate the interneurons, thereby reducing the activity of the resident pyramidal neurons. Consistent with this, it has been shown that stimulation of post-synaptic α1 adrenoceptors increases inhibitory postsynaptic currents (IPSC) (Bergles et al., 1996, Kawaguchi and Shindou, 1998, Yuan et al., 2009) and suppresses evoked and miniature excitatory post-synaptic currents (eEPSC and mESPS)(Kobayashi et al., 2009). Specific subtypes of interneurons, distinguishable based on their anatomical location, morphology, physiology and expression of specific calcium-binding proteins, make synaptic connections at distinct locations on pyramidal neurons and can differentially regulate their activity. For example, parvalbumin-positive chandelier cells exhibit a fast-spiking firing pattern and are thought to regulate the synchronized oscillatory activity of cortical pyramidal neurons necessary for WM via widespread inhibition (Whittington and Traub, 2003). In contrast, the calretinin-expressing double bouquet and Cajal-Retzius cells make synapses on the distal dendrites of pyramidal neurons as well as with other interneurons, and are thought to cause localized disinhibition of pyramidal cells through their interaction with other interneurons (Wang et al., 2004). Calbindin-expressing double bouquet, neurogliaform and Martinotti interneurons make synapses at the distal segment of pyramidal dendrites, and have been hypothesized to help filter out distracting stimuli (Wang et al., 2004). Although we have previously shown that TBI increases GAD67 expression in parvalbumin-positive interneurons (Kobori and Dash, 2006), it remains to be shown if the same population of interneurons also have increased levels of α1 adrenoceptors. When α1-specific antibodies become available, this issue can be addressed.
It has been postulated that norepinephrine signaling has an inverted U dose/response, with both excessive and insufficient signaling impairing WM (Arnsten, 2000). For example, WM dysfunction in aged monkeys with naturally occurring catecholamine loss, as well as in catecholamine-depleted young monkeys, is alleviated by systemic treatment with α2 agonists such as clonidine and guanfacine (Arnsten and Goldman-Rakic, 1985, Rama et al., 1996). α2 agonists also improve WM performance in intact, young monkeys, but at higher doses than those needed to improve aged or depleted animals (Franowicz and Arnsten, 1999). Although the density of α1 adrenoceptors in the brain matches that of α2 adrenoceptors, and far exceeds that of the ß adrenoceptors, norepinephrine has a higher affinity for α2A than α1, with the lowest affinity for ß adrenoceptors (α2A receptor, 56 nM; α1 receptor, 330 nM) (Mohell et al., 1983, Arnsten, 2000). All three α1 adrenoceptor subtypes (α1A, α1B, and α1D) are primarily postsynaptic, and are expressed on both excitatory and inhibitory neurons. In normal monkeys, α1 antagonists do not affect spatial WM, demonstrating that these receptors do not play a role in normal WM physiology (Li and Mei, 1994). However, in chronic stress conditions such as post traumatic stress disorder (PTSD), excessive level of norepinephrine release activates these low affinity receptors and impairs PFC function (Southwick et al., 1999, Raskind et al., 2003). Consistent with this, stress-induced prefrontal dysfunction by the anxiogenic drug FG7142 is improved by both α2A agonist and α1 antagonist (Birnbaum et al., 1999, Birnbaum et al., 2000).
We show that administration of the α1 antagonists HEAT and Prazosin significantly improve WM performance following brain injury (Figures 1 and 6). Although we did not see an acute influence of Prazosin, as was seen using HEAT, it was observed that Prazosin-treated animals had reduced swimming speeds. This influence of Prazosin could be related to the transient reinstatement of TBI-associated motor deficits that has been previously observed following prazosin administration (Stibick and Feeney, 2001). In contrast to the influence of α1 antagonists, the α2A agonist guanfacine did not alleviate brain injury-induced WM deficits (Figure 1). While the reason for this lack of effect of guanfacine is not clear, it is possible that receptor saturation or desensitization (Liang et al., 1998) may have contributed. If desensitization of α2A receptor following TBI diminished the effect of guanfacine, higher dose than that was used in the current study may alleviate the WM deficit of injured animals.
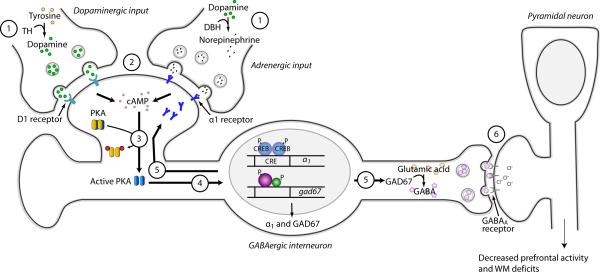
Based on the findings presented in the current report, and on those previously published by our and other laboratories, we propose a working model for TBI-induced WM dysfunction (Figure 7). We propose that (1) TBI increases the expression of tyrosine hydroxylase, the rate-limiting enzyme for catecholamine biosynthesis, leading to (2) increased dopamine and norepinephrine levels within the PFC (Kobori et al., 2004, Kobori et al., 2006). (3) These neurotransmitters stimulate their receptors and either directly or indirectly activate the cAMP-PKA cascade (Missale et al., 1998, Hein, 2006). (4) The catalytic subunit of PKA translocates to the nucleus where it phosphorylates the transcription factor CREB in GABAergic interneurons (Meinkoth et al., 1993). (5) In addition to increasing α1A adrenoceptor expression, TBI also causes a dopamine D1 receptor-dependent elevation of GAD67 levels (Kobori and Dash, 2006). However, the transcription factor mediating this enhancement has not been identified. (6) The combination of elevated α1 levels and enhanced GAD67 activity leads to increased inhibition of pyramidal neurons and WM dysfunction.
Figure 7. Hypothetical model for TBI-induced WM deficits.
(1) TBI increases the activity of tyrosine hydroxylase (TH). This increased activity leads to (2) increased dopamine and norepinephrine content within the mPFC. (3) Gs-protein-coupled receptor stimulation leads to increased cAMP production and the activation of PKA. (4) Activated PKA translocates to the nucleus where it phosphorylates the transcription factor CREB. Increased transcription within GABAergic neurons leads to the (5) enhanced expression of α1 adrenoceptor, as well as GAD67 via a D1-dependent mechanism. (6) Enhanced α1 receptor expression and elevated GAD67 combine to depress mPFC activity and cause WM dysfunction.
CONCLUSIONS
Enhanced CREB-mediated expression of α1 adrenoceptor contributes to TBI associated WM dysfunction, and therapies aimed at reducing α1 signaling may be useful in the treatment of TBI-associated WM deficits.
Acknowledgement
The authors would like to thank Anthony Moore and John Redell for critical review of the manuscript. Then work was supported by grants [NS052313; NS49160; MH072933] from National Institutes of Health.
Abbreviations
- aCSF
artificial cerebrospinal fluid
- AP-1
activating protein-1
- CRE
cAMP response element
- CREB
cAMP response element binding protein
- ChIP
chromatin immunoprecipitation
- DAB
3,3’-diaminobenzadine
- GAD67
glutamic acid decarboxylase 67
- mPFC
medial prefrontal cortex
- PFC
prefrontal cortex
- PKA
protein kinase A
- qPCR
quantitative polymerase chain reaction
- TBI
traumatic brain injury
- TH
tyrosine hydroxylase
- WM
working memory
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Arnsten AF. (Through the looking glass: differential noradenergic modulation of prefrontal cortical function. Neural Plast. 2000;7:133–146.. doi: 10.1155/NP.2000.133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Goldman-Rakic PS. (Alpha 2-adrenergic mechanisms in prefrontal cortex associated with cognitive decline in aged nonhuman primates. Science. 1985;230:1273–1276. doi: 10.1126/science.2999977. [DOI] [PubMed] [Google Scholar]
- Arnsten AF, Li BM. (Neurobiology of executive functions: catecholamine influences on prefrontal cortical functions. Biol Psychiatry. 2005;57:1377–1384. doi: 10.1016/j.biopsych.2004.08.019. [DOI] [PubMed] [Google Scholar]
- Autelitano DJ, Woodcock EA. (Selective activation of alpha1A-adrenergic receptors in neonatal cardiac myocytes is sufficient to cause hypertrophy and differential regulation of alpha1-adrenergic receptor subtype mRNAs. J Mol Cell Cardiol. 1998;30:1515–1523. doi: 10.1006/jmcc.1998.0717. [DOI] [PubMed] [Google Scholar]
- Bergles DE, Doze VA, Madison DV, Smith SJ. (Excitatory actions of norepinephrine on multiple classes of hippocampal CA1 interneurons. J Neurosci. 1996;16:572–585. doi: 10.1523/JNEUROSCI.16-02-00572.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birnbaum S, Gobeske KT, Auerbach J, Taylor JR, Arnsten AF. (A role for norepinephrine in stress-induced cognitive deficits: alpha-1-adrenoceptor mediation in the prefrontal cortex. Biol Psychiatry. 1999;46:1266–1274. doi: 10.1016/s0006-3223(99)00138-9. [DOI] [PubMed] [Google Scholar]
- Birnbaum SG, Podell DM, Arnsten AF. (Noradrenergic alpha-2 receptor agonists reverse working memory deficits induced by the anxiogenic drug, FG7142, in rats. PharmacolBiochemBehav. 2000;67:397–403. doi: 10.1016/s0091-3057(00)00306-3. [DOI] [PubMed] [Google Scholar]
- Coggeshall RE, Lekan HA. (Methods for determining numbers of cells and synapses: a case for more uniform standards of review. J Comp Neurol. 1996;364:6–15. doi: 10.1002/(SICI)1096-9861(19960101)364:1<6::AID-CNE2>3.0.CO;2-9. [DOI] [PubMed] [Google Scholar]
- Dash PK, Mach SA, Moore AN. (Enhanced neurogenesis in the rodent hippocampus following traumatic brain injury. J Neurosci Res. 2001;63:313–319. doi: 10.1002/1097-4547(20010215)63:4<313::AID-JNR1025>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- Dixon CE, Clifton GL, Lighthall JW, Yaghmai AA, Hayes RL. (A controlled cortical impact model of traumatic brain injury in the rat. J Neurosci Methods. 1991;39:253–262. doi: 10.1016/0165-0270(91)90104-8. [DOI] [PubMed] [Google Scholar]
- Franowicz JS, Arnsten AF. (Treatment with the noradrenergic alpha-2 agonist clonidine, but not diazepam, improves spatial working memory in normal young rhesus monkeys. Neuropsychopharmacology. 1999;21:611–621. doi: 10.1016/S0893-133X(99)00060-3. [DOI] [PubMed] [Google Scholar]
- Gao B, Chen J, Johnson C, Kunos G. (Both the cyclic AMP response element and the activator protein 2 binding site mediate basal and cyclic AMP-induced transcription from the dominant promoter of the rat alpha 1B-adrenergic receptor gene in DDT1MF-2 cells. Mol Pharmacol. 1997;52:1019–1026. doi: 10.1124/mol.52.6.1019. [DOI] [PubMed] [Google Scholar]
- Gao B, Kunos G. (Isolation and characterization of the gene encoding the rat alpha 1B adrenergic receptor. Gene. 1993;131:243–247. doi: 10.1016/0378-1119(93)90300-r. [DOI] [PubMed] [Google Scholar]
- Hamm RJ, Temple MD, Pike BR, O'Dell DM, Buck DL, Lyeth BG. (Working memory deficits following traumatic brain injury in the rat. JNeurotrauma. 1996;13:317–323. doi: 10.1089/neu.1996.13.317. [DOI] [PubMed] [Google Scholar]
- Hein L. (Adrenoceptors and signal transduction in neurons. Cell Tissue Res. 2006;326:541–551. doi: 10.1007/s00441-006-0285-2. [DOI] [PubMed] [Google Scholar]
- Jensen BC, Swigart PM, Simpson PC. (Ten commercial antibodies for alpha-1-adrenergic receptor subtypes are nonspecific. Naunyn Schmiedebergs Arch Pharmacol. 2009;379:409–412. doi: 10.1007/s00210-008-0368-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawaguchi Y, Shindou T. (Noradrenergic excitation and inhibition of GABAergic cell types in rat frontal cortex. J Neurosci. 1998;18:6963–6976. doi: 10.1523/JNEUROSCI.18-17-06963.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kline AE, Massucci JL, Marion DW, Dixon CE. (Attenuation of working memory and spatial acquisition deficits after a delayed and chronic bromocriptine treatment regimen in rats subjected to traumatic brain injury by controlled cortical impact. JNeurotrauma. 2002;19:415–425. doi: 10.1089/08977150252932370. [DOI] [PubMed] [Google Scholar]
- Kobayashi M, Kojima M, Koyanagi Y, Adachi K, Imamura K, Koshikawa N. (Presynaptic and postsynaptic modulation of glutamatergic synaptic transmission by activation of alpha(1)- and beta-adrenoceptors in layer V pyramidal neurons of rat cerebral cortex. Synapse. 2009;63:269–281. doi: 10.1002/syn.20604. [DOI] [PubMed] [Google Scholar]
- Kobori N, Clifton GL, Dash PK. (Enhanced catecholamine synthesis in the prefrontal cortex after traumatic brain injury: implications for prefrontal dysfunction. J Neurotrauma. 2006;23:1094–1102. doi: 10.1089/neu.2006.23.1094. [DOI] [PubMed] [Google Scholar]
- Kobori N, Dash PK. (Reversal of brain injury-induced prefrontal glutamic acid decarboxylase expression and working memory deficits by D1 receptor antagonism. J Neurosci. 2006;26:4236–4246. doi: 10.1523/JNEUROSCI.4687-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kobori N, Waymire JC, Haycock JW, Clifton GL, Dash PK. (Enhancement of tyrosine hydroxylase phosphorylation and activity by glial cell line-derived neurotrophic factor. J Biol Chem. 2004;279:2182–2191. doi: 10.1074/jbc.M310734200. [DOI] [PubMed] [Google Scholar]
- Lee K, Richardson CD, Razik MA, Kwatra MM, Schwinn DA. (Multiple potential regulatory elements in the 5' flanking region of the human alpha 1a-adrenergic receptor. DNA Seq. 1998;8:271–276. doi: 10.3109/10425179809008464. [DOI] [PubMed] [Google Scholar]
- Li BM, Mei ZT. (Delayed-response deficit induced by local injection of the alpha 2-adrenergic antagonist yohimbine into the dorsolateral prefrontal cortex in young adult monkeys. Behav Neural Biol. 1994;62:134–139. doi: 10.1016/s0163-1047(05)80034-2. [DOI] [PubMed] [Google Scholar]
- Liang M, Eason MG, Jewell-Motz EA, Williams MA, Theiss CT, Dorn GW, 2nd, Liggett SB. (Phosphorylation and functional desensitization of the alpha2A-adrenergic receptor by protein kinase C. Mol Pharmacol. 1998;54:44–49. doi: 10.1124/mol.54.1.44. [DOI] [PubMed] [Google Scholar]
- Lyeth BG, Jenkins LW, Hamm RJ, Dixon CE, Phillips LL, Clifton GL, Young HF, Hayes RL. (Prolonged memory impairment in the absence of hippocampal cell death following traumatic brain injury in the rat. Brain Res. 1990;526:249–258. doi: 10.1016/0006-8993(90)91229-a. [DOI] [PubMed] [Google Scholar]
- Marti D, Miquel R, Ziani K, Gisbert R, Ivorra MD, Anselmi E, Moreno L, Villagrasa V, Barettino D, D'Ocon P. (Correlation between mRNA levels and functional role of alpha1-adrenoceptor subtypes in arteries: evidence of alpha1L as a functional isoform of the alpha1A-adrenoceptor. Am J Physiol Heart Circ Physiol. 2005;289:H1923–1932. doi: 10.1152/ajpheart.00288.2005. [DOI] [PubMed] [Google Scholar]
- Meinkoth JL, Alberts AS, Went W, Fantozzi D, Taylor SS, Hagiwara M, Montminy M, Feramisco JR. (Signal transduction through the cAMP-dependent protein kinase. Mol Cell Biochem. 1993;127-128:179–186. doi: 10.1007/BF01076769. [DOI] [PubMed] [Google Scholar]
- Michelotti GA, Bauman MJ, Smith MP, Schwinn DA. (Cloning and characterization of the rat alpha 1a-adrenergic receptor gene promoter. Demonstration of cell specificity and regulation by hypoxia. J Biol Chem. 2003;278:8693–8705. doi: 10.1074/jbc.M211986200. [DOI] [PubMed] [Google Scholar]
- Missale C, Nash SR, Robinson SW, Jaber M, Caron MG. (Dopamine receptors: from structure to function. Physiol Rev. 1998;78:189–225. doi: 10.1152/physrev.1998.78.1.189. [DOI] [PubMed] [Google Scholar]
- Moghaddam B, Homayoun H. (Divergent plasticity of prefrontal cortex networks. Neuropsychopharmacology. 2008;33:42–55. doi: 10.1038/sj.npp.1301554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mohell N, Svartengren J, Cannon B. (Identification of [3H]prazosin binding sites in crude membranes and isolated cells of brown adipose tissue as alpha 1-adrenergic receptors. Eur J Pharmacol. 1983;92:15–25. doi: 10.1016/0014-2999(83)90103-6. [DOI] [PubMed] [Google Scholar]
- O'Connell TD, Rokosh DG, Simpson PC. (Cloning and characterization of the mouse alpha1C/A-adrenergic receptor gene and analysis of an alpha1C promoter in cardiac myocytes: role of an MCAT element that binds transcriptional enhancer factor-1 (TEF-1). Mol Pharmacol. 2001;59:1225–1234. doi: 10.1124/mol.59.5.1225. [DOI] [PubMed] [Google Scholar]
- Rama P, Linnankoski I, Tanila H, Pertovaara A, Carlson S. (Medetomidine, atipamezole, and guanfacine in delayed response performance of aged monkeys. Pharmacol Biochem Behav. 1996;55:415–422. doi: 10.1016/s0091-3057(96)00111-6. [DOI] [PubMed] [Google Scholar]
- Raskind MA, Peskind ER, Kanter ED, Petrie EC, Radant A, Thompson CE, Dobie DJ, Hoff D, Rein RJ, Straits-Troster K, Thomas RG, McFall MM. (Reduction of nightmares and other PTSD symptoms in combat veterans by prazosin: a placebo-controlled study. Am J Psychiatry. 2003;160:371–373. doi: 10.1176/appi.ajp.160.2.371. [DOI] [PubMed] [Google Scholar]
- Razik MA, Lee K, Price RR, Williams MR, Ongjoco RR, Dole MK, Rudner XL, Kwatra MM, Schwinn DA. (Transcriptional regulation of the human alpha1a-adrenergic receptor gene. Characterization Of the 5'-regulatory and promoter region. J Biol Chem. 1997;272:28237–28246. doi: 10.1074/jbc.272.45.28237. [DOI] [PubMed] [Google Scholar]
- Rokosh DG, Stewart AF, Chang KC, Bailey BA, Karliner JS, Camacho SA, Long CS, Simpson PC. (Alpha1-adrenergic receptor subtype mRNAs are differentially regulated by alpha1-adrenergic and other hypertrophic stimuli in cardiac myocytes in culture and in vivo. Repression of alpha1B and alpha1D but induction of alpha1C. J Biol Chem. 1996;271:5839–5843. doi: 10.1074/jbc.271.10.5839. [DOI] [PubMed] [Google Scholar]
- Smith DH, Soares HD, Pierce JS, Perlman KG, Saatman KE, Meaney DF, Dixon CE, McIntosh TK. (A model of parasagittal controlled cortical impact in the mouse: cognitive and histopathologic effects. J Neurotrauma. 1995;12:169–178. doi: 10.1089/neu.1995.12.169. [DOI] [PubMed] [Google Scholar]
- Southwick SM, Bremner JD, Rasmusson A, Morgan CA, 3rd, Arnsten A, Charney DS. (Role of norepinephrine in the pathophysiology and treatment of posttraumatic stress disorder. Biol Psychiatry. 1999;46:1192–1204. doi: 10.1016/s0006-3223(99)00219-x. [DOI] [PubMed] [Google Scholar]
- Steele RJ, Morris RG. (Delay-dependent impairment of a matching-to-place task with chronic and intrahippocampal infusion of the NMDA-antagonist D-AP5. Hippocampus. 1999;9:118–136. doi: 10.1002/(SICI)1098-1063(1999)9:2<118::AID-HIPO4>3.0.CO;2-8. [DOI] [PubMed] [Google Scholar]
- Stibick DL, Feeney DM. (Enduring vulnerability to transient reinstatement of hemiplegia by prazosin after traumatic brain injury. J Neurotrauma. 2001;18:303–312. doi: 10.1089/08977150151070955. [DOI] [PubMed] [Google Scholar]
- Wang XJ, Tegner J, Constantinidis C, Goldman-Rakic PS. (Division of labor among distinct subtypes of inhibitory neurons in a cortical microcircuit of working memory. Proc Natl Acad Sci U S A. 2004;101:1368–1373. doi: 10.1073/pnas.0305337101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittington MA, Traub RD. (Interneuron diversity series: inhibitory interneurons and network oscillations in vitro. Trends Neurosci. 2003;26:676–682. doi: 10.1016/j.tins.2003.09.016. [DOI] [PubMed] [Google Scholar]
- Yuan WX, Chen SR, Chen H, Pan HL. (Stimulation of alpha(1)-adrenoceptors reduces glutamatergic synaptic input from primary afferents through GABA(A) receptors and T-type Ca(2+) channels. Neuroscience. 2009;158:1616–1624. doi: 10.1016/j.neuroscience.2008.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]