Abstract
Activation of the dopaminergic (DA) neurons of the ventral tegmental area (VTA) by ethanol has been implicated in its rewarding and reinforcing effects. We previously demonstrated that ethanol enhances GABA release onto VTA-DA neurons via activation of 5-HT2C receptors and subsequent release of calcium from intracellular stores. Here we demonstrate that excitation of VTA-DA neurons by ethanol is limited by an ethanol-enhancement in GABA release.
In this study, we performed whole-cell voltage clamp recordings of miniature inhibitory postsynaptic currents (mIPSCs) and cell-attached recordings of action potential firing from VTA-DA neurons in midbrain slices from young Long Evans rats. Acute exposure to ethanol (75 mM) transiently enhanced the firing rate of VTA-DA neurons as well as the frequency of mIPSCs. Simultaneous blockade of both GABAA and GABAB receptors (Picrotoxin (75 μM) and SCH50911 (20 μM)) disinhibited VTA-DA firing rate whereas a GABAA agonist (muscimol, 1 μM) strongly inhibited firing rate. In the presence of picrotoxin, ethanol enhanced VTA-DA firing rate more than in the absence of picrotoxin. Additionally, a sub-maximal concentration of muscimol together with ethanol inhibited VTA-DA firing rate more than muscimol alone. DAMGO (3 μM) inhibited mIPSC frequency but did not block the ethanol-enhancement in mIPSC frequency. DAMGO (1 and 3 μM) had no effect on VTA-DA firing rate. Naltrexone (60 μM) had no effect on basal or ethanol-enhancement of mIPSC frequency. Additionally, naltrexone (20 and 60 μM) did not block the ethanol-enhancement in VTA-DA firing rate. Overall, the present results indicate that the ethanol enhancement in GABA release onto VTA-DA neurons limits the stimulatory effect of ethanol on VTA-DA neuron activity and may have implications for the rewarding properties of ethanol.
Keywords: reward, mesolimbic, alcohol dependence, electrophysiology, inhibitory synaptic transmission
Activation of dopaminergic (DA) neurons of the ventral tegmental area (VTA) by ethanol and other drugs of abuse is believed to be a critical component in the development and expression of drug dependence and addiction (Gatto et al., 1994; Robbins and Everitt, 1996; Wise, 1996; Koob et al., 1998; Schultz, 2002; Appel, 2004). The VTA is situated in the midbrain and sends projections to the nucleus accumbens (NAc), prefrontal cortex, basolateral amygdala and a variety of other corticolimbic structures (Albanese and Minciacchi, 1983; Oades and Halliday, 1987). The VTA also receives reciprocal excitatory and inhibitory innervations from multiple brain regions. Local VTA GABAergic neurons (interneurons) and GABAergic projections arising from the NAc and ventral pallidum synapse onto VTA-DA neurons and represent a potential target for ethanol modulation of DA activity.
While relatively little controversy surrounds the direct actions of ethanol to enhance DA signaling in the midbrain (Gessa et al., 1985; Brodie et al., 1990; Weiss et al., 1993; Brodie and Appel, 2000), quite disparate findings concerning ethanol modulation of VTA GABAergic transmission have been documented. We and others have reported that acute ethanol exposure enhances GABA release onto midbrain DA neurons (Melis et al., 2002; Criswell et al., 2008; Theile et al., 2008; Theile et al., 2009). Conversely, other groups have presented opposite results. Important observations by Ye and colleagues demonstrated that ethanol (10–40 mM) may have differential effects on VTA action potential-dependent GABA release. Although under control conditions ethanol decreased GABA release, an increase in spontaneous inhibitory postsynaptic current (sIPSC) frequency was observed in the presence of saturating concentrations of the mu-opioid receptor (MOR) agonist DAMGO (Xiao and Ye, 2008). Ye and colleagues also show that ethanol decreased the firing rate of GABA interneurons (Xiao et al., 2007). Steffensen and colleagues demonstrated an ethanol inhibition of GABAergic interneuron excitability believed to be mediated through inhibition of NMDA receptors (Stobbs et al., 2004). However, they also demonstrated that low dose intravenous ethanol (0.01–0.03 g/kg) enhanced VTA-GABA neuron firing rate in rats (Steffensen et al., 2009). GABAA receptors are present on VTA-DA neurons and tonic GABA release inhibits these neurons (Yim and Mogenson, 1980; Johnson and North, 1992a; Johnson and North, 1992b; Westerink et al., 1996). Therefore, we hypothesized that if ethanol enhances GABAergic transmission onto VTA DA neurons, then stimulation or blockade of GABA receptors may modulate the overall stimulatory effect of ethanol on VTA-DA neuron activity.
Furthermore, it has been demonstrated that activation of MORs on GABAergic interneurons in the VTA disinhibits VTA-DA neuron activity (Johnson and North, 1992a; Margolis et al., 2003; Xiao et al., 2007; Xiao and Ye, 2008). The MOR agonist, DAMGO, reduced the frequency of miniature and spontaneous IPSCs through inhibition of the secretory process at the nerve terminal of GABAergic cells (Bergevin et al., 2002). In the central amygdala (CeA), ethanol increased GABA release and this effect was enhanced in delta-opioid receptor (DOR) knock-out mice and in the presence of DOR antagonists (Kang-Park et al., 2007). That study suggests that endogenous opioid release negatively modulates spontaneous and ethanol-induced GABA release in the CeA. It is possible that blocking opioid activation in the VTA may also enhance spontaneous and ethanol-mediated GABA release. As a result, we hypothesize that the ethanol-induced increase in GABA release we observe is limited by basal opioid- activation of presynaptic MORs, thus inhibiting GABA release. Therefore, inhibition of presynaptic MORs with the non-selective opioid antagonist, naltrexone, may remove tonic inhibition of GABA release, thus amplifying the ethanol-induced increase in GABA release. The resultant increase in GABA transmission may then overcome the direct effect of ethanol on DA neuron excitability, thereby providing a possible mechanism to explain the ability of naltrexone to block ethanol-induced increases in dialysate dopamine levels in the NAc of male Wistar rats (Gonzales and Weiss, 1998), and possibly may contribute to the clinical efficacy of naltrexone on preventing relapse in recovering alcoholics.
EXPERIMENTAL PROCEDURES
Slice preparation
All experiments were carried out in accordance with NIH animal use guidelines and were approved by the University of Texas Institutional Animal Care and Use Committee. Slices used in this study were prepared from male Long Evans rats (postnatal day 21 to 28). Rats were anesthetized with halothane, decapitated, and the brain was rapidly removed and placed in an ice-cold choline-based, oxygenated artificial cerebrospinal fluid (aCSF) containing (in mM): 110 choline Cl, 25 NaHCO3, 1.25 NaH2PO4, 2.5 KCl, 25 dextrose, 7 MgSO4, 0.5 CaCl2, 11.6 Na-ascorbate and 3.1 Na-pyruvate, bubbled with 95% O2/5% CO2 (all chemicals obtained from Sigma-Aldrich, St Louis, MO). Horizontal midbrain slices (210 μm) were prepared using a vibrating slicer (VT1000S; Leica, Nussloch, Germany). The slices were then maintained at 32°C before electrophysiological recordings for a minimum of 60 minutes in aCSF containing (in mM): 120 NaCl, 25 NaHCO3, 3.3 KCl, 1.23 NaH2PO4, 10 dextrose, 2.4 MgSO4, and 1.8 CaCl2, bubbled with 95%O2/5%CO2.
Electrophysiological recordings of VTA-DA neurons
Individual slices were transferred to a recording chamber and perfused with oxygenated aCSF (30–32°C) at a flow rate of ~2 ml/min. Recording aCSF was as described above except it contained 0.9 mM MgSO4 and 2 mM CaCl2. Cells were visualized using IR-DIC optics on an Olympus BX-50WI microscope (Leeds Instruments, Irving, TX). The VTA was identified as being medial to the medial terminal nucleus of the accessory optic tract (MT) and rostral to the oculomotor nerve and the medial lemniscus. The majority of recordings were conducted in the lateral VTA, just medial to the MT. For action potential recordings, putative DA neurons were identified by their characteristic slow (1–5 Hz) pacemaking activity. Additionally, whole-cell access was obtained and putative DA neurons were further identified by the presence of a large hyperpolarization-induced Ih current (>200 pA) that was measured immediately following break-in by application of a 1.5-s hyperpolarizing step from −60 to −110 mV (Johnson and North, 1992b). For mIPSC recordings, DA neurons were identified via the presence of an Ih current before the experiment was carried out. Recording electrodes were made from thin-walled borosilicate glass (TW 150F-4, WPI, Sarasota, Florida, 1.5–2.5 MΩ) and contained (in mM): 135 KCl, 12 NaCl, 0.5 EGTA, 10 HEPES, 2 Mg-ATP, and 0.3 Tris-GTP, pH 7.3 with KOH (all chemicals obtained from Sigma, St Louis, MO). Data were collected by an Axon Instruments Model 200B amplifier filtered at 1 kHz and digitized at 20 kHz (for mIPSCs) and 50 kHz (for action potentials) with a Digidata interface using pClamp v10.2 (Molecular Devices, Sunnyvale, California).
Firing rate recordings were conducted in the absence of any channel/receptor drugs except where mentioned. Action potential firing rate was measured using tight-seal current clamp recordings because rupture of the cell membrane under traditional whole-cell recordings significantly disrupts the pacemaking activity of DA neurons (Morikawa et al., 2003). Following formation of a tight seal and a stable 10-minute baseline (control), drugs were bath applied through the aCSF perfusion line and a continuous 15–17 minute recording epoch was used to detect changes in firing rate. A 10-minute washout followed drug application.
GABAergic mIPSCs were pharmacologically isolated with kynurenic acid (1 mM) to inhibit AMPA- and NMDA receptor-mediated currents. Tetrodotoxin (TTX; 0.5 μM) and eticlopride (250 nM) were included to block Na+ currents and D2 receptor-mediated currents, respectively. Under these conditions, mIPSCs were inward at a holding potential of −60 mV and in an initial set of experiments their identity as GABAergic events was verified by testing for block with picrotoxin or bicuculline (data not shown). Following break-in and a stable 10-minute baseline (control) recording, drugs were bath-applied through the aCSF perfusion line and a continuous 10–15 minute recording epoch was used to detect changes in mIPSC frequency and amplitude. A four-minute drug wash-on preceded the start of data collection in each treatment condition, and a 12-min washout period followed drug application. The number of neurons used per each treatment condition is represented as n with only one neuron used per slice. SCH50911, muscimol and DAMGO were obtained from Tocris Bioscience (Ellisville, MO). Kynurenic acid, naltrexone hydrochloride and picrotoxin were obtained from Sigma-Aldrich (St. Louis, MO). TTX was obtained from Alomone Labs (Jerusalem, Israel).
Data analysis
For firing rate recordings, action potentials (30–50 sweeps each condition, 20 sec/sweep) were detected using a threshold detection protocol contained with pClampfit (pClamp v10.2, Axon Inst., Molecular Devices). The peak 5 minutes of the drug effect and the last 5 minutes of washout were normalized to the last 5 minutes of the baseline (control) and represented as a percentage of the control. Averaged values for all data sets are expressed as mean ± SEM and were compared statistically using paired student’s t test, unpaired student’s t test and one-way analysis of variance (ANOVA) test where mentioned.
For mIPSC recordings, quantal events (90–120 sweeps each condition, 5 sec/sweep) were detected using the template mIPSC detection protocol contained within pClampfit. Access resistance ranged from 8 to 25 MΩ and was monitored throughout the experiment. Experiments where access resistance changed (>20% at anytime during the experiment) were excluded from this study. To minimize detecting small noise deflections as mIPSCs (false positives), events <10 pA were discarded. Treatment and washout groups were normalized to the baseline (control) frequency or amplitude and represented as a percentage of the control. Averaged values for all data sets are expressed as mean ± SEM and were compared statistically using paired student’s t test and unpaired student’s t test where mentioned.
RESULTS
Ethanol transiently enhances firing rate of VTA-DA neurons in vitro
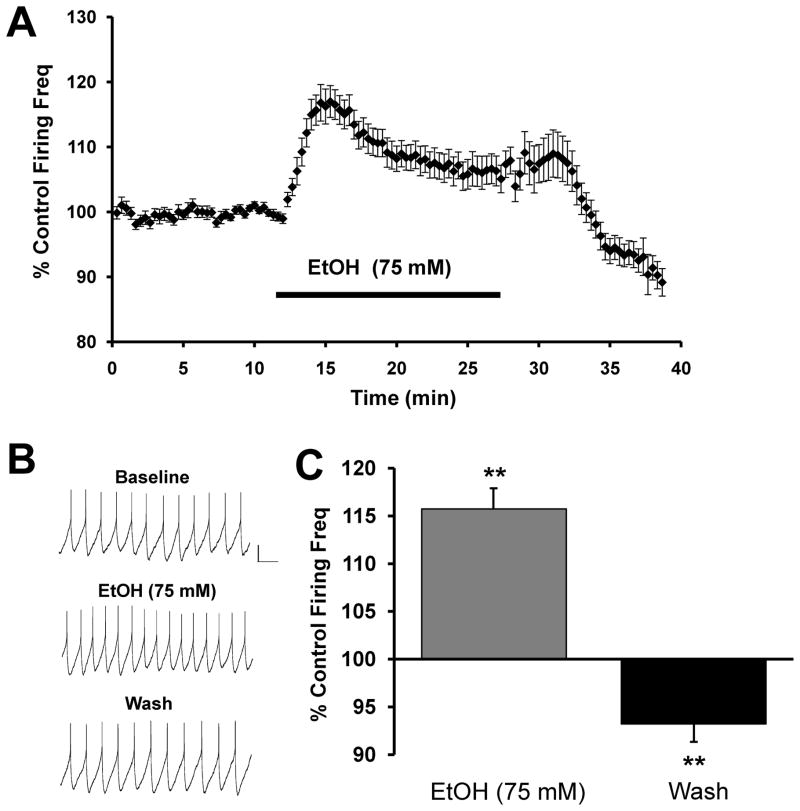
Acute ethanol has been demonstrated to increase the firing rate of DA neurons in the VTA of Sprague-Dawley rats both in vivo (Gessa et al., 1985) and in vitro (Brodie et al., 1990), as well as in mice (Brodie and Appel, 2000). In Long Evans rats, ethanol administration produced a robust and transient increase in dopamine release in the NAc as measured via microdialysis (Howard et al., 2008). Here, we demonstrate electrophysiologically a similar response in acute brain slices from Long Evans rats. Acute application of ethanol (75 mM) produced a transient increase in the firing rate of VTA-DA neurons that reversed upon washout (Fig. 1). This transient increase in firing rate reached a peak of 15.7 ± 2.1% above baseline within the first five minutes of ethanol application, and this was followed by stabilization at an intermediate level between baseline and the initial peak response.
FIGURE 1. Ethanol produces transient enhancement of VTA-DA neuron firing rate.
A. Cumulative graph displaying average firing rate of VTA-DA neurons treated with 75 mM ethanol (n = 30). Data points for individual cells represent 20-sec sweeps in which the average firing rate (Hz) was calculated. Each data point is normalized to the last 5 mins of baseline. The normalized data points were combined to obtain the graph above. Error bars represent the SEM of averaged time points. B. Sample traces from a representative neuron. C. A bar graph representing the % change ± SEM above control firing rate. The bar graph represents the peak 5-min ethanol response and the last 5 mins of washout (n = 30, ** indicates p < 0.01 by a student’s t test different from control). The average baseline firing rate was 2.3 ± 0.1 Hz.
GABA modulates VTA-DA neuron firing rate
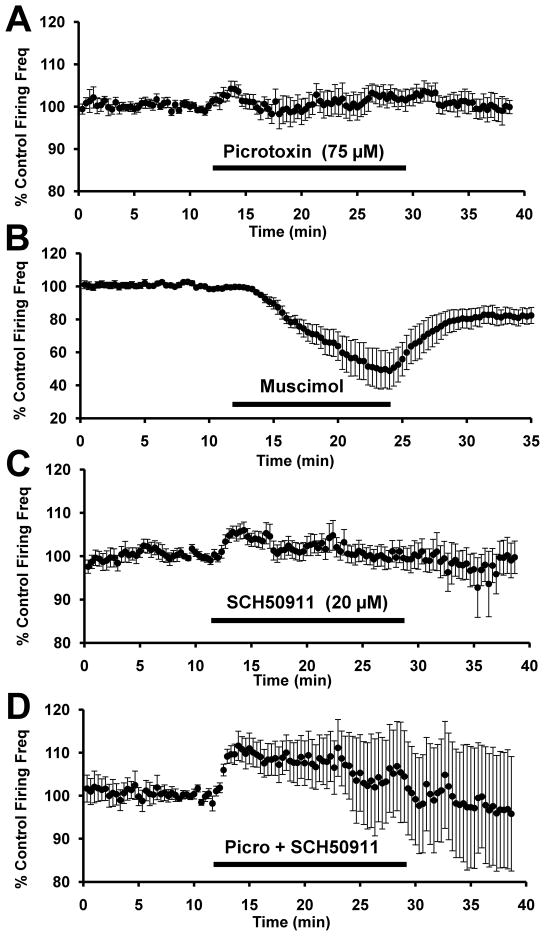
Tonic activation of GABAA receptors present on VTA-DA neurons has been shown to inhibit these neurons (Yim and Mogenson, 1980; Johnson and North, 1992a; Johnson and North, 1992b; Westerink et al., 1996). Conversely, some reports suggest that GABAA inhibition on GABAergic cells themselves is relatively more powerful and overall activation GABAA receptor tone in the VTA results in disinhibition, rather than inhibition, of VTA-DA neurons (Kalivas et al., 1990; Churchill et al., 1992; Xi and Stein, 1998; Laviolette and van der Kooy, 2001; Doherty and Gratton, 2007). Therefore, we sought to determine the actions of GABA receptor/channel antagonists and agonists in our midbrain slice preparation. Picrotoxin (75 μM) alone, the GABAA channel antagonist, had a slight but significant disinhibitory effect to increase VTA-DA firing rate (Fig. 2A). Picrotoxin increased the firing rate to a peak of about 4.1 ± 1.9% above baseline (n = 14, p<0.05 by student’s t-test). Conversely, application of the potent GABAA receptor agonist muscimol (1 μM) substantially inhibited the firing of VTA-DA neurons (Fig. 2B). Muscimol decreased firing rate to about 56.4 ± 10.4% of baseline (n = 8, p<0.05 by student’s t-test).
FIGURE 2. GABAA receptor activation and blockade modulate VTA-DA neuron activity.
Cumulative graph displaying average firing rate of VTA-DA neurons treated with (A) 75 μM picrotoxin (n = 14), (B) 1 μM muscimol (n = 8; note different scale), (C) 20 μM SCH50911 (n = 13) and (D) picrotoxin and SCH50911 (n = 12). Data points for individual cells represent 20-sec sweeps in which the average firing rate (Hz) was calculated. Each data point is normalized to the last 5 mins of baseline. The normalized data points were combined to obtain the graph above. Error bars represent the SEM of averaged time points. The average baseline firing rates were for (A) 2.2 ± 0.3 Hz, (B) 2.5 ± 0.3 Hz (C) 2.4 ± 0.3 Hz and (D) 2.1 ± 0.2 Hz.
GABAB receptors are located at pre- and postsynaptic sites in the VTA with activation of presynaptic GABAB receptors resulting in a decrease in GABA release (Melis et al., 2002; Theile et al., 2008). However, some argue that the majority of GABAB receptors are at postsynaptic sites on DA cells (Xi and Stein, 1998) and activation of these receptors with baclofen decreases DA output to the NAc (Westerink et al., 1996; Amantea and Bowery, 2004). Therefore we measured the effect of the GABAB receptor antagonist, SCH50911 (20 μM), on VTA-DA neuron firing rate. In the presence of SCH0911 alone, firing rate was moderately increased (Fig. 2C). SCH50911 increased the firing rate to a peak of about 4.4 ± 1.9% above baseline (n = 13, p<0.05 by student’s t-test). This suggests that there may indeed be some tonic activation of postsynaptic GABAB receptors regulating VTA-DA neuron activity. Lastly, complete blockade of both GABAA and GABAB receptors with dual application of SCH50911 and picrotoxin produced an even greater increase in VTA-DA firing rate than with either alone (Fig. 2D). Co-application of picrotoxin and SCH50911 increased the firing rate to a peak of about 12.1 ± 3.3% above baseline (n = 12, p<0.05 by student’s t-test). Overall, these results further support the notion that endogenous GABAergic tone is sufficient to modulate VTA-DA activity in vitro, and that tonic GABA release inhibits VTA-DA neuron activity via activation of both GABAA and GABAB receptors.
Ethanol-enhancement of VTA-DA neuron firing rate is self-limiting
We have previously demonstrated that acute ethanol application results in a long-lasting (several minutes) increase in GABA release onto VTA-DA neurons in Sprague-Dawley rats (Theile et al., 2008; Theile et al., 2009). Here we observed a transient enhancement in VTA-DA firing rate in acute slices from Long Evans rats in the presence of ethanol. We hypothesized that an ethanol-enhancement in GABA release may play a role in the transient nature of the excitatory effect of ethanol on firing rate. As in Sprague-Dawley rats, acute application of ethanol (75 mM) markedly increased mIPSC frequency for several minutes while having no effect on amplitude in slices from Long Evans rats (Fig. 3). Therefore, ethanol enhances DA neuron activity while concurrently increasing inhibitory drive onto those same neurons.
FIGURE 3. Ethanol enhances mIPSC frequency.
A. mIPSCs recorded from a VTA-DA neuron under control conditions, in the presence of 75 mM ethanol and a washout. B. A cumulative bar graph representing the % change ± SEM above control mIPSC frequency for the conditions shown in A. Event frequency under control conditions was 1.1 ± 0.1 Hz (n = 11, ** indicates p < 0.01 by a student’s t test different from control, * indicates p < 0.05 by a student’s t test different from EtOH).
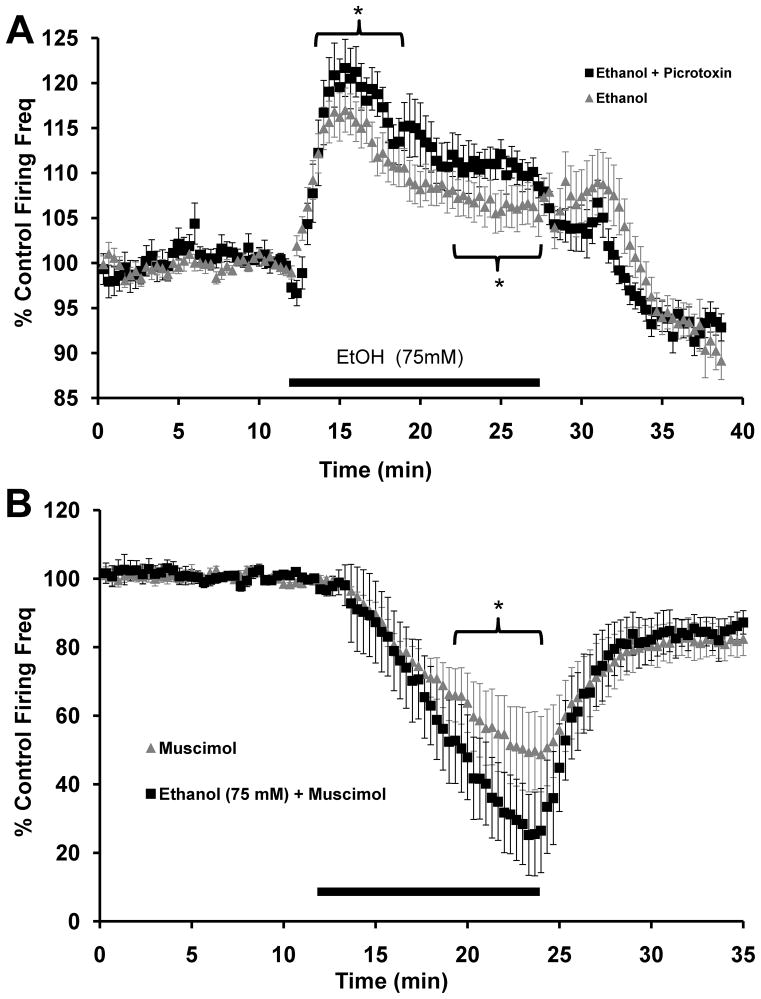
In light of these results, we hypothesized that the simultaneous action of ethanol to enhance GABA release partially offsets the direct effect of ethanol to increase in VTA-DA firing rate. To test this possibility, we measured the effects of ethanol on VTA-DA firing rate in the continued presence of a saturating concentration of picrotoxin (75 μM). In the absence of picrotoxin, the peak ethanol-induced increase in VTA-DA firing rate was approximately 16% above baseline (Fig. 1). In the presence of picrotoxin (Fig. 4A), the peak ethanol-induced increase was 19.3 ± 2.7% above baseline (n=14, p<0.01 by student’s t-test). When comparing the peak 5 minute ethanol effect between ethanol alone and in the presence of picrotoxin, this difference was significant (One-way ANOVA, p<0.01, F = 20.55, df = 1,28). Additionally, in the presence of picrotoxin, the elevated firing rate during the plateau phase was higher than with ethanol alone. When comparing the last 5 minutes of ethanol exposure between ethanol alone and in the presence of picrotoxin, this difference was also significant (One-way ANOVA, p<0.01, F = 195.79, df = 1,28). These results suggest that one of the factors reducing the plateau phase of the ethanol response may be a prolonged ethanol-induced enhancement in GABA release.
FIGURE 4. Ethanol modulation of VTA-DA activity is regulated by GABAA receptors.
A. Cumulative graph displaying average firing rate of VTA-DA neurons treated with ethanol (75 mM; n = 30; reproduced from Fig. 1) and ethanol (75 mM) in the continued presence of picrotoxin (75 μM; n = 14). The average baseline firing rate for ethanol with picrotoxin was 1.9 ± 0.1 Hz (* indicates p<0.01 by one-way ANOVA). B. Cumulative graph displaying average firing rate of VTA-DA neurons treated with muscimol (1 μM; n = 8; reproduced from Fig. 2B) and ethanol (75 mM) with muscimol (n = 9). The average baseline firing rate for ethanol with muscimol was 2.6 ± 0.6 Hz (* indicates p<0.01 by one-way ANOVA). For both A and B, data points for individual cells represent 20-sec sweeps in which the average firing rate (Hz) was calculated. Each data point is normalized to the last 5 mins of baseline. The normalized data points were combined to obtain the graphs above. Error bars represent the SEM of averaged time points. Note differences in scale.
We further studied this interaction using co-application of the GABA agonist, muscimol, and ethanol and observed evidence of an additive inhibitory effect of ethanol on VTA-DA neuron firing rate. In the presence of muscimol (1 μM) alone, VTA-DA neuron firing rate was depressed to about 56% of baseline (Fig. 2B) with only 1 of 8 neurons tested exhibiting complete cessation in firing. However, co-application of muscimol and ethanol (75 mM) depressed firing rate to 36.5 ± 11.5% of baseline with 5 of 9 neurons tested exhibiting complete cessation of firing (Fig. 4B). When comparing the last 5 minutes of exposure between muscimol alone and muscimol with ethanol, this difference was significant (One-way ANOVA, p<0.01, F = 45.26, df = 1, 26). Thus, under conditions of adjunctive GABAA receptor activation (i.e., in the presence of a sub-maximal concentration of muscimol), ethanol inhibited VTA-DA neuron activity, presumably via an enhancement in GABA release.
MOR activation inhibits GABA release onto VTA-DA neurons
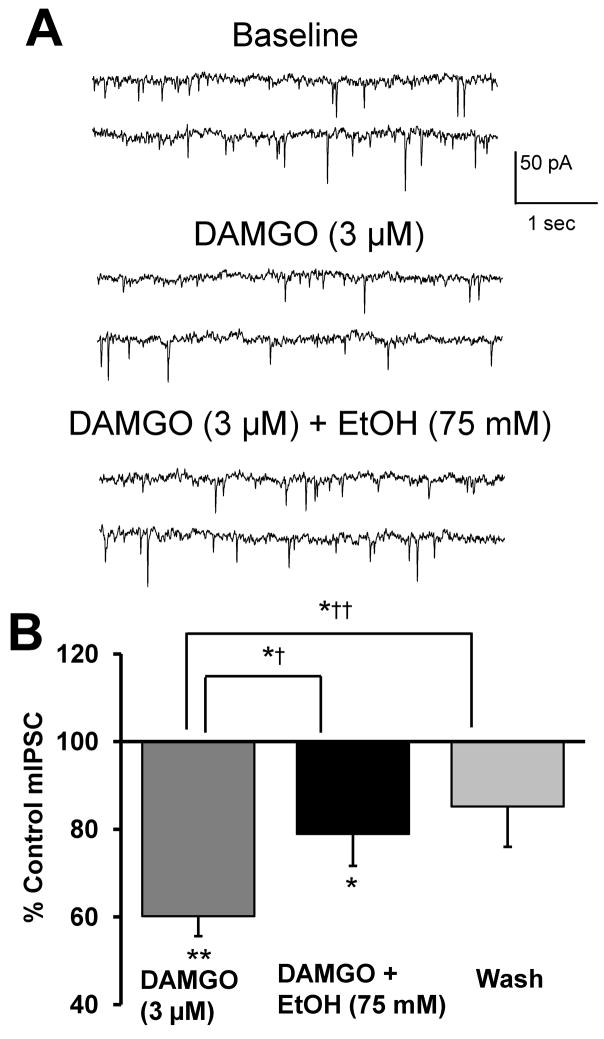
Bergevin et al. (2002) demonstrated a clear effect of DAMGO, a selective MOR agonist, to reduce the frequency, but not amplitude, of both GABAA receptor-mediated sIPSCs and mIPSCs as recorded from VTA-DA neurons of Sprague-Dawley rats. Additionally, acute ethanol has been demonstrated to enhance opioid levels in the VTA in vivo (Jarjour et al., 2009), although it is unknown if ethanol increases opioid levels in an in vitro preparation. To determine whether MOR activation in our preparation could offset the observed ethanol-enhancement in GABA release, we recorded mIPSCs in the presence of ethanol and DAMGO. Application of DAMGO alone (3 μM) significantly decreased mIPSC frequency (Fig. 5) while having no significant effect on mIPSC amplitude (data not shown). These results support the idea proposed by Bergevin et al. (2002) that activation of MORs, likely on GABAergic terminals synapsing onto DA neurons, decreases vesicular release at these terminals via an action-potential independent mechanism. Following the decrease in mIPSC frequency by DAMGO alone, co-application of DAMGO (3 μM) and ethanol (75 mM) potentiated mIPSC frequency (Fig. 5). When normalized to DAMGO alone, ethanol enhanced mIPSC frequency by about 32%. In the absence of DAMGO pretreatment, ethanol enhanced mIPSC frequency by about 55% (Fig. 3B), although not significantly different compared to the 32% increase observed in the presence of DAMGO (p = 0.08 by student’s t-test). Additionally, there was no significant effect on mIPSC amplitude under either condition (data not shown). These results suggest that ethanol acts independently of opioid receptors to enhance GABA release.
FIGURE 5. Ethanol enhances GABA release independently of MOR activation by DAMGO.
A. mIPSCs recorded from a VTA-DA neuron under control conditions, in the presence of 3 μM DAMGO and 75 mM ethanol with 3 μM DAMGO. B. A cumulative bar graph representing the % change ± SEM above control mIPSC frequency for the conditions shown in A and a wash. Event frequency under control conditions was 1.2 ± 0.2 Hz (n = 8, ** indicates p < 0.01 by student’s t test different from control, *† indicates p < 0.05 by a paired student’s t test different from DAMGO, *†† indicates p < 0.05 by a paired student’s t test different from DAMGO, * indicates p < 0.05 by Student’s t test different from control).
MOR activation does not alter VTA-DA neuron firing rate
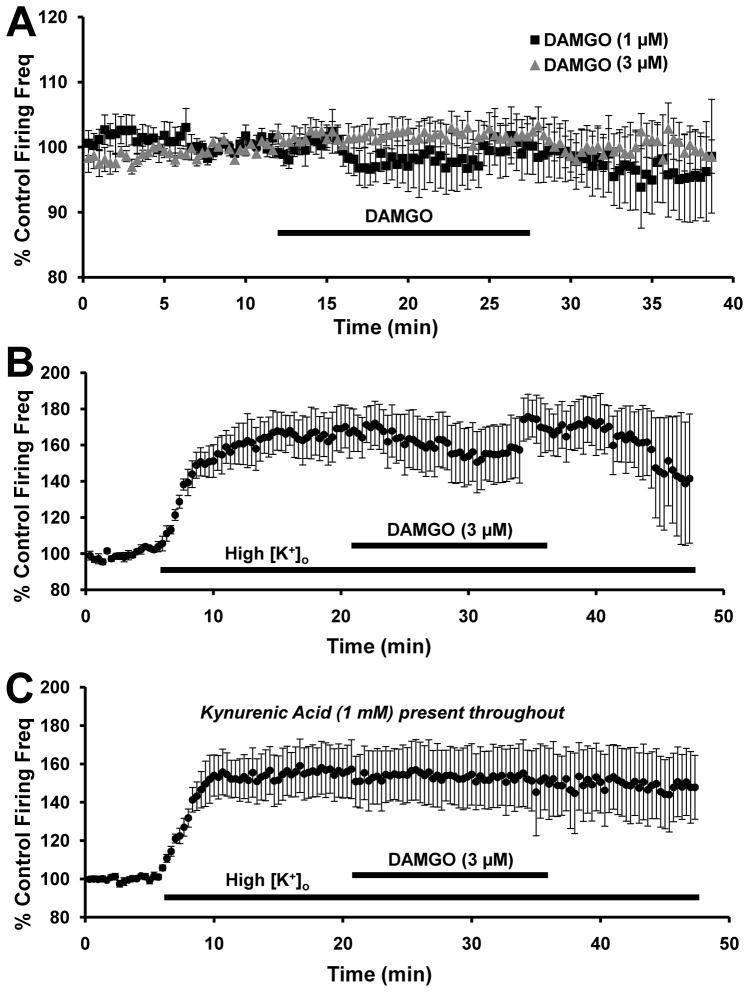
VTA-DA neuron activity is partly regulated via MOR-mediated inhibition of GABA release. Several labs have demonstrated an MOR-mediated disinhibition of VTA-DA neurons in Sprague-Dawley rats (Johnson and North, 1992a; Margolis et al., 2003; Xiao et al., 2007). Since we observed a substantial decrease in GABA release with DAMGO, we would expect that DAMGO would exert a disinhibitory effect on VTA-DA neuron firing rate so therefore we measured the firing rate of VTA-DA neurons in the presence of the MOR-selective agonist, DAMGO. The peak response to DAMGO was not significantly greater than the baseline firing rate at 1 μM (n = 8, p>0.05 by student’s t-test, Fig. 6A) or 3 μM (n = 9, p>0.05 by student’s t-test, Fig. 6A). We speculate that the presence of a strong GABA tone is necessary in order for a disinhibitory effect of DAMGO on VTA-DA neuron firing rate in vitro to be revealed. Since there was no change in firing rate at either 1 or 3 μM DAMGO, we sought to increase GABAergic neuron activity by raising the [K+]o from 3.3 mM to 7.0 mM. After establishing a stable baseline firing frequency, the high [K+]o solution was washed on before application of DAMGO. Although the addition of the higher [K+]o solution dramatically increased the VTA-DA firing rate (73.2 ± 6.8% above baseline, n = 6, p<0.01 by student’s t-test), subsequent addition of DAMGO did not result in any further change in the firing rate (72.9 ± 7.7% above baseline, p>0.05 by paired student’s t-test compared to high [K+]o alone, Fig. 6B). Increasing [K+]o not only increases GABA drive onto VTA-DA neurons, but also increases glutamate release onto VTA DA neurons as well. Therefore, we repeated that experiment with kynurenic acid in the recording solution to block NMDA- and AMPA receptor-mediated currents (Fig. 6C). Under these conditions, addition of the high [K+]o solution increased firing rate to 49.7 ± 11.5% above baseline (n = 7, p<0.01 by student’s t-test). However, application of DAMGO had no further effect on DA neuron firing rate (38.1 ± 14.4% above baseline, p>0.05 by paired student’s t-test compared to high [K+]o alone). The extent of the increase in firing rate with high [K+]o solution in the presence of kynurenic acid did not reach the level as that seen in high [K+]o alone. These observations suggest that the high [K+]o solution increases glutamatergic transmission while also directly depolarizing VTA-DA neurons. Nevertheless, under no conditions did we observe any evidence of MOR regulation of VTA-DA firing rate.
FIGURE 6. DAMGO does not modulate VTA-DA neuron activity.
Cumulative graph displaying average firing rate of VTA-DA neurons treated with (A) 1 μM DAMGO (n = 8) and 3 μM DAMGO (n = 9), (B) 3 μM DAMGO with 7 mM [K+]o (n = 6) and (C) 3 μM DAMGO with 7 mM [K+]o and 1 mM kynurenic acid (n = 7). Data points for individual cells represent 20-sec sweeps in which the average firing rate (Hz) was calculated. Each data point is normalized to the last 5 mins of baseline. The normalized data points were combined to obtain the graph above. Error bars represent the SEM of averaged time points. The average baseline firing rates were for (A) 2.3 ± 0.2 Hz for 1 μM and 2.7 ± 0.3 Hz for 3 μM, (B) 1.7 ± 0.3 Hz and (C) 2.1 ± 0.3 Hz.
MOR blockade has no effect on GABA tone or ethanol-enhancement of VTA-DA neuron activity
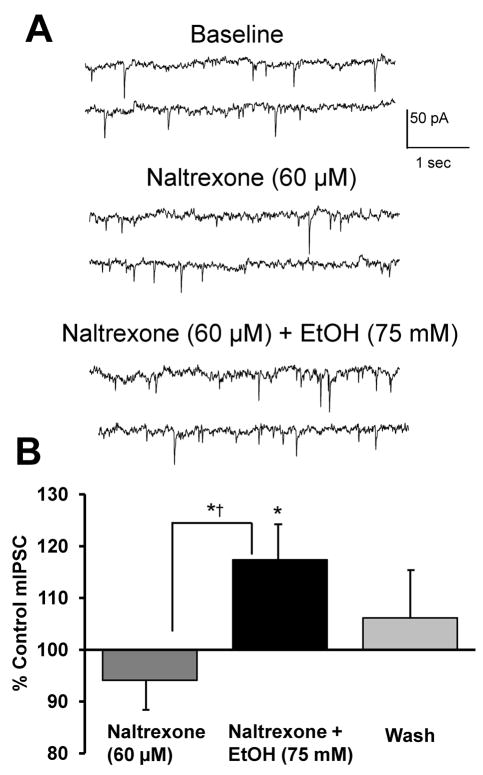
Evidence indicates that the endogenous opioidergic system positively modulates the excitatory effect of ethanol on DA release in vivo (Gonzales and Weiss, 1998; Job et al., 2007), while negatively modulating spontaneous and ethanol-enhanced GABA release in the CeA in vitro (Kang-Park et al., 2007). Therefore, we hypothesized that the ethanol-induced increase in GABA release is limited by endogenous or ethanol-enhanced opioid tone acting on MORs localized to interneurons. Inhibition of MORs with naltrexone may be expected to uncover a tonic inhibition of GABA release. Thus, in the presence of naltrexone, an increase in GABAergic transmission may antagonize the direct excitatory effect of ethanol on DA neuron activity. This may provide a possible mechanism to explain the ability of naltrexone to block ethanol-induced increases in dialysate dopamine levels in the NAc (Gonzales and Weiss, 1998). To test this hypothesis, we first examined the effect of naltrexone (60 μM) alone and with ethanol (75 mM) on mIPSCs (Fig. 7). Naltrexone had no effect on mIPSC frequency. Since we did not observe an enhancement in mIPSC frequency following MOR blockade, this suggests that there is no basal opioid tone in our slice preparation.
FIGURE 7. Naltrexone does not potentiate ethanol-enhancement of mIPSC frequency.
A. mIPSCs recorded from a VTA-DA neuron under control conditions, in the presence of 60 μM naltrexne and 75 mM ethanol with 60 μM naltrexone. B. A bar graph representing the % change ± SEM above control mIPSC frequency for the conditions shown in A and a wash. Event frequency under control conditions was 1.6 ± 0.3 Hz (n = 8, * indicates p < 0.05 by student’s t test different from control, *† indicates p < 0.05 by a paired student’s t test different from naltrexone).
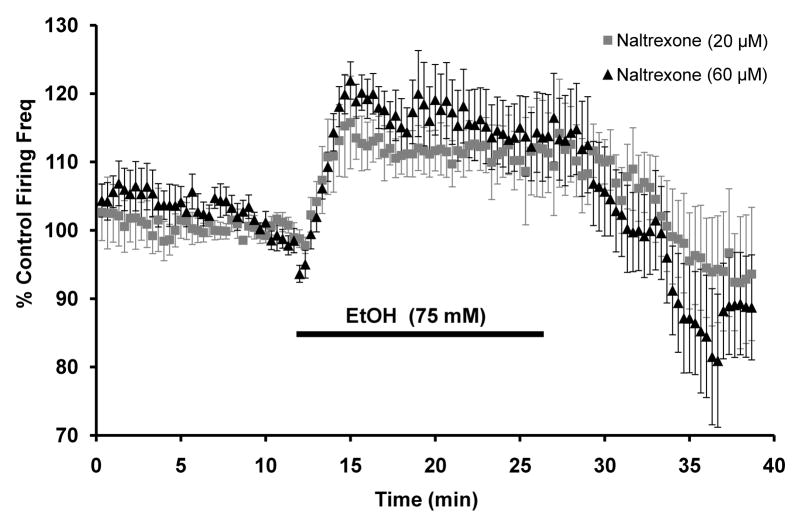
Additionally, we measured VTA-DA firing rate under normal conditions and in the presence of ethanol (75 mM). Application of naltrexone alone at 20 μM (2.1 ± 1.5% above baseline, n = 9, p>0.05 by student’s t-test) and 60 μM (1.6 ± 3.1% of baseline, n = 8, p>0.05 by student’s t-test) had no effect on VTA-DA firing rate. Naltrexone did not block the ethanol-induced enhancement in VTA-DA firing rate (Fig. 8) and in the continued presence of naltrexone (20 μM), ethanol (75 mM) enhanced VTA DA firing rate by 13.7 ± 2.2% above baseline (n = 8, p<0.01 by student’s t-test). In the presence of 60 μM naltrexone, ethanol enhanced firing rate by 21.2 ± 3.2% above baseline (n = 11, p<0.01 by student’s t-test). These results suggest that MOR blockade with naltrexone does not have the same effect on VTA-DA neuron activity in vitro as observed in vivo.
Figure 8. Naltrexone does not block ethanol-enhancement in VTA-DA firing rate.
Cumulative graph displaying average firing rate of VTA-DA neurons treated with 75 mM ethanol with 20 μM naltrexone present throughout (n = 8) and 75 mM ethanol with 60 μM naltrexone present throughout (n = 11). Data points for individual cells represent 20-sec sweeps in which the average firing rate (Hz) was calculated. Each data point is normalized to the last 5 mins of baseline. The normalized data points were combined to obtain the graph above. Error bars represent the SEM of averaged time points. The average baseline firing rates were for (20 μM naltrexone) 2.4 ± 0.3 Hz, (60 μM naltrexone) 1.8 ± 0.1 Hz.
DISCUSSION
In this report we have investigated how our prior observations (Theile et al., 2008; Theile et al., 2009) of ethanol-enhancement of GABA release onto VTA DA neurons may modulate firing of these cells and how our observations relate to those of other investigators (Melis et al., 2002; Stobbs et al., 2004; Xiao et al., 2007; Criswell et al., 2008; Xiao and Ye, 2008; Steffensen et al., 2009). Ethanol (75 mM) application produced a robust and reliable increase in VTA-DA neuron firing rate that consisted of two phases: a rapid peak and a slow plateau. These results suggest that the maximal excitatory effect of ethanol on VTA-DA excitation is not maintained throughout the entire duration of ethanol exposure, but rather reaches an initial peak before some other factor(s) act to diminish the excitation. In this report we demonstrated that GABA modulates VTA-DA firing rate and that ethanol enhances GABA tone. As a result, we hypothesized that an ethanol-induced enhancement in inhibitory drive is one factor that dampens the excitatory effect of ethanol on firing rate. Indeed, in the presence of saturating concentrations of picrotoxin (75 μM), the peak excitatory effect of ethanol was larger, in contrast to observations by Xiao et al. (2007). In that study, they demonstrated that ethanol-enhancement in VTA-DA activity is attenuated by application of the GABAA receptor antagonist, bicuculline. They proposed that ethanol-enhancement in VTA-DA firing is mediated in part via inhibition of local interneurons, and thus by blocking GABA input the disinhibitory effect of ethanol on VTA-DA excitability is reduced. Furthermore, we observed that a sub-saturating concentration of the GABAA receptor agonist muscimol inhibited VTA-DA firing rate and that this inhibition was significantly more pronounced in the presence of ethanol. Thus, our results demonstrate that adjunctive GABAA receptor activation reveals an inhibitory effect of ethanol on VTA-DA neuron activity. Since we have never observed any evidence for ethanol potentiation of post-synaptic GABAA receptor function on VTA DA neurons (Theile et al., 2008; Theile et al., 2009), then the most parsimonious explanation for the reduction in firing rate is that the increase in GABA release produced by ethanol functions in concert with the direct GABAA receptor activation by muscimol to additively increase GABAergic inhibition of VTA DA neurons. Overall, these results support the idea that the excitatory effect of ethanol on VTA-DA neuron activity is self-limiting and that GABAA receptors represent a ‘fine gain switch’ regulating the overall stimulatory/inhibitory effect of ethanol.
Several labs have demonstrated an MOR-mediated disinhibition of VTA-DA neurons in Sprague-Dawley rats (Johnson and North, 1992a; Margolis et al., 2003; Xiao et al., 2007). It should be noted, however, that in the study by Margolis et al. (2003), disinhibition was seen in only 47% of the neurons tested. Additionally, in the study by Johnson and North (1992a), the use of an elevated [K+]o solution was required to observe disinhibition. In line with the results of another study (Bergevin et al., 2002), we demonstrate that DAMGO application decreased mIPSC frequency consistent with an MOR-mediated inhibition of GABA release. Therefore, we investigated whether the DAMGO-mediated decrease in GABA tone would translate to an increase in the excitability of VTA-DA neurons. In the presence of DAMGO (1–3 μM), there was neither a change in DA neuron firing rate under normal recordings conditions nor in the presence of an elevated [K+]o solution. These results are surprising considering that we did observe a considerable reduction in GABA release in the presence of DAMGO. It is possible that the addition of the higher [K+]o solution created a ‘ceiling effect’ on the VTA-DA neuron activity, thus preventing any further excitation upon addition of DAMGO. Interestingly, kynurenic acid appeared to dampen the overall excitability of the neurons in the presence of the higher [K+]o solution. This suggests that glutamatergic terminals are also excited with the higher [K+]o solution. Conversely, Xiao et al. (2007) reported a disinhibitory effect of DAMGO on DA neuron excitability in the VTA of Sprague-Dawley rats under normal recording conditions. We cannot fully explain why we do not see similar results. Although the animals are of similar age, use of coronal slices in that study may partly explain the different observations seen here with horizontal slices. Overall, these results underscore the unique mechanisms by which ethanol and MORs regulate GABA transmission onto VTA-DA neurons.
Naltrexone has been demonstrated to reduce the ethanol-induced enhancement in dopamine release from the NAc (Gonzales and Weiss, 1998). However, the exact mechanism for this is unclear. We demonstrate here that ethanol reliably enhanced GABA release in the VTA and that GABA release is modulated via MOR activation. Therefore, we hypothesized that if there is opioid tone in our slice preparation, then blockade with naltrexone may enhance GABA tone resulting in a greater ethanol effect on GABA release. Indeed, genetic deletion of MORs increased GABA tone in both the CeA and the VTA of mice (Chefer et al., 2009; Kang-Park et al., 2009). Upregulation of basal or ethanol-mediated GABA release could overcome a direct excitatory effect of ethanol on DA neuron activity. However, we do not observe any change in GABA release in the presence of naltrexone (20–60 μM). Moreover, naltrexone did not block the ethanol-induced enhancement in VTA-DA neuron firing rate. Opioid tone is likely substantially reduced or absent in our slice preparation due to deafferentation, thus representing drawbacks to studying opioid modulation of synaptic transmission onto VTA-DA neurons in a slice preparation. Furthermore, in the study by Kang-Park et al. (2009) there is no observable effect of MOR deletion on ethanol-enhancement of GABA release in the CeA, although they previously demonstrated an ethanol interaction with DORs (Kang-Park et al., 2007).
Interestingly, in the presence of naltrexone (60 μM), the ethanol-enhancement in firing rate did not exhibit the pronounced falling phase as seen with ethanol alone. We can only speculate as to the reason for this result. In addition to being localized to cell bodies and axon terminals of GABAergic neurons in the VTA, MORs are also present on a subset of DA cell bodies and dendrites (Garzon and Pickel, 2001). These DA neurons are classified as tertiary neurons and are differentiated from principal neurons in that they are inhibited by MOR agonists (Cameron et al., 1997; Margolis et al., 2003). Therefore, if ethanol application increases opioid release under our conditions, it is possible that MOR blockade would promote a longer lasting ethanol-induced excitation of DA neuron activity. Additionally, KORs are also present on principal DA neurons and activation of these receptors results in inhibition of firing (Margolis et al., 2003). Naltrexone is non-selective, especially at high concentrations, thus KOR blockade by naltrexone may result in a disinhibitory effect on firing rate and could explain our observations. Furthermore, in the VTA, block of Ih results in a biphasic ethanol-mediated enhancement followed by a decrease in DA neuron firing rate that is abolished in the presence of barium, an inhibitor of G protein-linked potassium (GIRK) channels (McDaid et al., 2008). Although we observe a biphasic ethanol-enhancement of firing rate in the absence of Ih inhibition, it remains a possibility that inhibition of an opioid-receptor sensitive GIRK conductance by naltrexone contributes to the reduced ethanol desensitization observed here.
In this study we recorded from slices of young rats (postnatal day 21 to 28). It is well-known that recordings from slices of adult animals are significantly more difficult to obtain than from younger animals. Hence, the overwhelming majority of electrophysiological recordings in all areas of neuroscience using slice patch configuration are from younger animals such as used here. Numerous labs have studied ethanol mediated effects in the midbrain using 2–5 week old animals. Ethanol has been demonstrated to enhance firing rate (Okamoto et al., 2006; McDaid et al., 2008) and increase mIPSCs (Melis et al., 2002) in animals from this age. Other studies have used more mature animals and noted similar ethanol-mediated increases in firing rate (Brodie and Appel, 1998; Appel et al., 2003). Additionally, a recent report has demonstrated that in the hippocampus, ethanol enhances mIPSCs similarly in young and adult animals (Li et al., 2006). Although GABA transmission regulates DA neuron activity in adult animals (Johnson and North, 1992b; Westerink et al., 1996), it is unknown whether ethanol enhances GABA release in the midbrain of adult animals. To put such questions in perspective, it is notable that our basic understanding of the ontogeny of VTA excitatory and inhibitory synaptic transmission itself is largely undefined for that matter.
CONCLUSIONS
The results presented here do help to reconcile the evidence that ethanol stimulates VTA-DA neuron activity while simultaneously enhancing GABAergic input onto those same neurons. For the first time, we demonstrate an inhibitory effect of ethanol on VTA-DA neuron firing under conditions of additional GABAA receptor activation. This may have implications with regards to the use of GABAA agonists in the treatment of alcohol dependence. GABA, and perhaps opioids, may not be the only factors mediating the plateau phase of the ethanol-enhancement in VTA-DA neuron activity. Ethanol directly excites VTA-DA neurons through a variety of mechanisms including, but not limited to, enhancement of the Ih current (Okamoto et al., 2006) and a reduction in a voltage-dependent K+ current (Koyama et al., 2007).
The switch from ethanol stimulation to inhibition may have implications for the rewarding and reinforcing properties of ethanol. Strong ethanol stimulation of brain regions with GABAergic projections to the VTA in conjunction with local GABAergic stimulation could overcome the direct excitatory effect of ethanol on VTA-DA neuron activity. Additionally, in those that become dependent, basal and/or ethanol-enhanced GABA release may initially be minimal or become weaker after chronic exposure. This would correlate to a more robust excitatory effect of ethanol on VTA-DA neuron activity and thus ethanol consumption could potentially be more reinforcing. Conversely, individuals who are less susceptible to developing dependence might possess more robust basal and/or ethanol-enhanced GABA release, thereby curbing the excitatory effect of ethanol on VTA-DA neuron activity. This in turn could theoretically limit the initial reinforcing effects of ethanol. Animal strains which are genetically prone to drink less or not at all (alcohol non-preferring) may exhibit a strong basal GABA tone and/or a robust ethanol-enhancement in GABA release which would correlate with a weaker reinforcing effect of acute ethanol. Conversely, those strains which are genetically prone to drink more (alcohol preferring) may exhibit a weak GABA tone and/or a weaker ethanol-enhancement in GABA release and thus exhibit a strong reinforcing effect of ethanol. Indeed, recent work using Sardinian alcohol-preferring rats demonstrated that the frequency of mIPSCs recorded from VTA-DA neurons under control conditions was considerably less than that in neurons from non-preferring rats (Melis et al., 2009). Thus, such new insights into the complex effects of ethanol on synaptic and non-synaptic regulation of VTA-DA neurons indicate that our understanding of the mesocorticolmbic neuroadaptive changes critical in the development and expression of alcohol dependence remain largely undefined.
Acknowledgments
Support: This work was supported by the National Institute on Alcohol Abuse and Alcoholism 1F31AA017020 (JWT); and the National Institutes of Health RO1AA14874 (RAG) and RO1AA15167 and U01AA16651 (RAM).
ABBREVIATIONS
- DA
Dopaminergic
- VTA
Ventral tegmental area
- NAc
Nucleus accumbens
- CeA
Central amygdala
- mIPSCs
miniature inhibitory postsynaptic currents
- sIPSCs
spontaneous inhibitory postsynaptic currents
- MOR
mu-opioid receptor
- DOR
delta-opioid receptor
- aCSF
artificial cerebral spinal fluid
- ANOVA
analysis of variance
- TTX
tetrodotoxin
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Albanese A, Minciacchi D. Organization of the ascending projections from the ventral tegmental area: a multiple fluorescent retrograde tracer study in the rat. J Comp Neurol. 1983;216:406–420. doi: 10.1002/cne.902160406. [DOI] [PubMed] [Google Scholar]
- Amantea D, Bowery NG. Reduced inhibitory action of a GABAB receptor agonist on [3H]-dopamine release from rat ventral tegmental area in vitro after chronic nicotine administration. BMC Pharmacol. 2004;4:24. doi: 10.1186/1471-2210-4-24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Appel SB, et al. Ethanol Effects on Dopaminergic “Reward” Neurons in the Ventral Tegmental Area and the Mesolimbic Pathway. Alcoholism: Clinical and Experimental Research. 2004;28:1768–1778. [Google Scholar]
- Appel SB, Liu Z, McElvain MA, Brodie MS. Ethanol excitation of dopaminergic ventral tegmental area neurons is blocked by quinidine. J Pharmacol Exp Ther. 2003;306:437–446. doi: 10.1124/jpet.103.050963. [DOI] [PubMed] [Google Scholar]
- Bergevin A, Girardot D, Bourque MJ, Trudeau LE. Presynaptic mu-opioid receptors regulate a late step of the secretory process in rat ventral tegmental area GABAergic neurons. Neuropharmacology. 2002;42:1065–1078. doi: 10.1016/s0028-3908(02)00061-8. [DOI] [PubMed] [Google Scholar]
- Brodie MS, Appel SB. The effects of ethanol on dopaminergic neurons of the ventral tegmental area studied with intracellular recording in brain slices. Alcohol Clin Exp Res. 1998;22:236–244. [PubMed] [Google Scholar]
- Brodie MS, Appel SB. Dopaminergic neurons in the ventral tegmental area of C57BL/6J and DBA/2J mice differ in sensitivity to ethanol excitation. Alcohol Clin Exp Res. 2000;24:1120–1124. [PubMed] [Google Scholar]
- Brodie MS, Shefner SA, Dunwiddie TV. Ethanol increases the firing rate of dopamine neurons of the rat ventral tegmental area in vitro. Brain Res. 1990;508:65–69. doi: 10.1016/0006-8993(90)91118-z. [DOI] [PubMed] [Google Scholar]
- Cameron DL, Wessendorf MW, Williams JT. A subset of ventral tegmental area neurons is inhibited by dopamine, 5-hydroxytryptamine and opioids. Neuroscience. 1997;77:155–166. doi: 10.1016/s0306-4522(96)00444-7. [DOI] [PubMed] [Google Scholar]
- Chefer VI, Denoroy L, Zapata A, Shippenberg TS. Mu opioid receptor modulation of somatodendritic dopamine overflow: GABAergic and glutamatergic mechanisms. Eur J Neurosci. 2009;30:272–278. doi: 10.1111/j.1460-9568.2009.06827.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Churchill L, Dilts RP, Kalivas PW. Autoradiographic localization of gamma-aminobutyric acidA receptors within the ventral tegmental area. Neurochem Res. 1992;17:101–106. doi: 10.1007/BF00966870. [DOI] [PubMed] [Google Scholar]
- Criswell HE, Ming Z, Kelm MK, Breese GR. Brain regional differences in the effect of ethanol on GABA release from presynaptic terminals. J Pharmacol Exp Ther. 2008;326:596–603. doi: 10.1124/jpet.107.135418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doherty M, Gratton A. Differential involvement of ventral tegmental GABA(A) and GABA(B) receptors in the regulation of the nucleus accumbens dopamine response to stress. Brain Res. 2007;1150:62–68. doi: 10.1016/j.brainres.2007.02.081. [DOI] [PubMed] [Google Scholar]
- Garzon M, Pickel VM. Plasmalemmal mu-opioid receptor distribution mainly in nondopaminergic neurons in the rat ventral tegmental area. Synapse. 2001;41:311–328. doi: 10.1002/syn.1088. [DOI] [PubMed] [Google Scholar]
- Gatto GJ, McBride WJ, Murphy JM, Lumeng L, Li TK. Ethanol self-infusion into the ventral tegmental area by alcohol-preferring rats. Alcohol. 1994;11:557–564. doi: 10.1016/0741-8329(94)90083-3. [DOI] [PubMed] [Google Scholar]
- Gessa GL, Muntoni F, Collu M, Vargiu L, Mereu G. Low doses of ethanol activate dopaminergic neurons in the ventral tegmental area. Brain Res. 1985;348:201–203. doi: 10.1016/0006-8993(85)90381-6. [DOI] [PubMed] [Google Scholar]
- Gonzales RA, Weiss F. Suppression of ethanol-reinforced behavior by naltrexone is associated with attenuation of the ethanol-induced increase in dialysate dopamine levels in the nucleus accumbens. J Neurosci. 1998;18:10663–10671. doi: 10.1523/JNEUROSCI.18-24-10663.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Howard EC, Schier CJ, Wetzel JS, Duvauchelle CL, Gonzales RA. The shell of the nucleus accumbens has a higher dopamine response compared with the core after non-contingent intravenous ethanol administration. Neuroscience. 2008;154:1042–1053. doi: 10.1016/j.neuroscience.2008.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarjour S, Bai L, Gianoulakis C. Effect of Acute Ethanol Administration on the Release of Opioid Peptides From the Midbrain Including the Ventral Tegmental Area. Alcohol Clin Exp Res. 2009 doi: 10.1111/j.1530-0277.2009.00924.x. [DOI] [PubMed] [Google Scholar]
- Job MO, Tang A, Hall FS, Sora I, Uhl GR, Bergeson SE, Gonzales RA. Mu (mu) opioid receptor regulation of ethanol-induced dopamine response in the ventral striatum: evidence of genotype specific sexual dimorphic epistasis. Biol Psychiatry. 2007;62:627–634. doi: 10.1016/j.biopsych.2006.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Opioids excite dopamine neurons by hyperpolarization of local interneurons. J Neurosci. 1992a;12:483–488. doi: 10.1523/JNEUROSCI.12-02-00483.1992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson SW, North RA. Two types of neurone in the rat ventral tegmental area and their synaptic inputs. J Physiol. 1992b;450:455–468. doi: 10.1113/jphysiol.1992.sp019136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalivas PW, Duffy P, Eberhardt H. Modulation of A10 dopamine neurons by gamma-aminobutyric acid agonists. J Pharmacol Exp Ther. 1990;253:858–866. [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Roberto M, Madamba SG, Siggins GR, Moore SD. Mu-opioid receptors selectively regulate basal inhibitory transmission in the central amygdala: lack of ethanol interactions. J Pharmacol Exp Ther. 2009;328:284–293. doi: 10.1124/jpet.108.140749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang-Park MH, Kieffer BL, Roberts AJ, Siggins GR, Moore SD. Presynaptic delta opioid receptors regulate ethanol actions in central amygdala. J Pharmacol Exp Ther. 2007;320:917–925. doi: 10.1124/jpet.106.112722. [DOI] [PubMed] [Google Scholar]
- Koob GF, Roberts AJ, Schulteis G, Parsons LH, Heyser CJ, Hyytia P, Merlo-Pich E, Weiss F. Neurocircuitry targets in ethanol reward and dependence. Alcohol Clin Exp Res. 1998;22:3–9. [PubMed] [Google Scholar]
- Koyama S, Brodie MS, Appel SB. Ethanol inhibition of m-current and ethanol-induced direct excitation of ventral tegmental area dopamine neurons. J Neurophysiol. 2007;97:1977–1985. doi: 10.1152/jn.00270.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laviolette SR, van der Kooy D. GABA(A) receptors in the ventral tegmental area control bidirectional reward signalling between dopaminergic and non-dopaminergic neural motivational systems. Eur J Neurosci. 2001;13:1009–1015. doi: 10.1046/j.1460-9568.2001.01458.x. [DOI] [PubMed] [Google Scholar]
- Li Q, Wilson WA, Swartzwelder HS. Developmental differences in the sensitivity of spontaneous and miniature IPSCs to ethanol. Alcohol Clin Exp Res. 2006;30:119–126. doi: 10.1111/j.1530-0277.2006.00006.x. [DOI] [PubMed] [Google Scholar]
- Margolis EB, Hjelmstad GO, Bonci A, Fields HL. Kappa-opioid agonists directly inhibit midbrain dopaminergic neurons. J Neurosci. 2003;23:9981–9986. doi: 10.1523/JNEUROSCI.23-31-09981.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDaid J, McElvain MA, Brodie MS. Ethanol effects on dopaminergic ventral tegmental area neurons during block of Ih: involvement of barium-sensitive potassium currents. J Neurophysiol. 2008;100:1202–1210. doi: 10.1152/jn.00994.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Camarini R, Ungless MA, Bonci A. Long-lasting potentiation of GABAergic synapses in dopamine neurons after a single in vivo ethanol exposure. J Neurosci. 2002;22:2074–2082. doi: 10.1523/JNEUROSCI.22-06-02074.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Melis M, Pillolla G, Perra S, Colombo G, Muntoni AL, Pistis M. Electrophysiological properties of dopamine neurons in the ventral tegmental area of Sardinian alcohol-preferring rats. Psychopharmacology (Berl) 2009;201:471–481. doi: 10.1007/s00213-008-1309-2. [DOI] [PubMed] [Google Scholar]
- Morikawa H, Khodakhah K, Williams JT. Two intracellular pathways mediate metabotropic glutamate receptor-induced Ca2+ mobilization in dopamine neurons. J Neurosci. 2003;23:149–157. doi: 10.1523/JNEUROSCI.23-01-00149.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oades RD, Halliday GM. Ventral tegmental (A10) system: neurobiology. 1. Anatomy and connectivity. Brain Res. 1987;434:117–165. doi: 10.1016/0165-0173(87)90011-7. [DOI] [PubMed] [Google Scholar]
- Okamoto T, Harnett MT, Morikawa H. Hyperpolarization-activated cation current (Ih) is an ethanol target in midbrain dopamine neurons of mice. J Neurophysiol. 2006;95:619–626. doi: 10.1152/jn.00682.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robbins TW, Everitt BJ. Neurobehavioural mechanisms of reward and motivation. Curr Opin Neurobiol. 1996;6:228–236. doi: 10.1016/s0959-4388(96)80077-8. [DOI] [PubMed] [Google Scholar]
- Schultz W. Getting formal with dopamine and reward. Neuron. 2002;36:241–263. doi: 10.1016/s0896-6273(02)00967-4. [DOI] [PubMed] [Google Scholar]
- Steffensen SC, Walton CH, Hansen DM, Yorgason JT, Gallegos RA, Criado JR. Contingent and non-contingent effects of low-dose ethanol on GABA neuron activity in the ventral tegmental area. Pharmacol Biochem Behav. 2009;92:68–75. doi: 10.1016/j.pbb.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stobbs SH, Ohran AJ, Lassen MB, Allison DW, Brown JE, Steffensen SC. Ethanol suppression of ventral tegmental area GABA neuron electrical transmission involves N-methyl-D-aspartate receptors. J Pharmacol Exp Ther. 2004;311:282–289. doi: 10.1124/jpet.104.071860. [DOI] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Ethanol enhances GABAergic transmission onto dopamine neurons in the ventral tegmental area of the rat. Alcohol Clin Exp Res. 2008;32:1040–1048. doi: 10.1111/j.1530-0277.2008.00665.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Theile JW, Morikawa H, Gonzales RA, Morrisett RA. Role of 5-hydroxytryptamine2C receptors in Ca2+-dependent ethanol potentiation of GABA release onto ventral tegmental area dopamine neurons. J Pharmacol Exp Ther. 2009;329:625–633. doi: 10.1124/jpet.108.147793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss F, Lorang MT, Bloom FE, Koob GF. Oral alcohol self-administration stimulates dopamine release in the rat nucleus accumbens: genetic and motivational determinants. J Pharmacol Exp Ther. 1993;267:250–258. [PubMed] [Google Scholar]
- Westerink BH, Kwint HF, deVries JB. The pharmacology of mesolimbic dopamine neurons: a dual-probe microdialysis study in the ventral tegmental area and nucleus accumbens of the rat brain. J Neurosci. 1996;16:2605–2611. doi: 10.1523/JNEUROSCI.16-08-02605.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wise RA. Neurobiology of addiction. Curr Opin Neurobiol. 1996;6:243–251. doi: 10.1016/s0959-4388(96)80079-1. [DOI] [PubMed] [Google Scholar]
- Xi ZX, Stein EA. Nucleus accumbens dopamine release modulation by mesolimbic GABAA receptors-an in vivo electrochemical study. Brain Res. 1998;798:156–165. doi: 10.1016/s0006-8993(98)00406-5. [DOI] [PubMed] [Google Scholar]
- Xiao C, Ye JH. Ethanol dually modulates GABAergic synaptic transmission onto dopaminergic neurons in ventral tegmental area: role of mu-opioid receptors. Neuroscience. 2008;153:240–248. doi: 10.1016/j.neuroscience.2008.01.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao C, Zhang J, Krnjevic K, Ye JH. Effects of ethanol on midbrain neurons: role of opioid receptors. Alcohol Clin Exp Res. 2007;31:1106–1113. doi: 10.1111/j.1530-0277.2007.00405.x. [DOI] [PubMed] [Google Scholar]
- Yim CY, Mogenson GJ. Electrophysiological studies of neurons in the ventral tegmental area of Tsai. Brain Res. 1980;181:301–313. doi: 10.1016/0006-8993(80)90614-9. [DOI] [PubMed] [Google Scholar]