Abstract
This study examines the role of APE1/Ref-1 in the retina and its potential as a therapeutic target for inhibiting retinal angiogenesis. APE1/Ref-1 expression was quantified by Western blot. The role of APE1/Ref-1 redox function in endothelial cell in vitro angiogenesis was examined by treating retinal vascular endothelial cells (RVECs) with APX3330, a small molecule inhibitor of APE1/Ref-1 redox activity. In vitro methods included a proliferation assay, a transwell migration assay, a Matrigel tube formation assay, and a Real Time Cell Analysis (RTCA) using the xCELLigence System. In vivo functional studies of APE1/Ref-1 were carried out by treating very low density lipoprotein (VLDL) receptor knockout mice (Vldlr−/−) with intravitreal injection of APX3330, and subsequent measurement of retinal angiomatous proliferation (RAP)-like neovascularization for one week. APE1/Ref-1 was highly expressed in the retina and in RVECs and pericytes in mice. APX3330 (1 to 10 µM) inhibited proliferation, migration and tube formation of RVECs in vitro in a dose-dependent manner. Vldlr−/− RVECs were more sensitive to APX3330 than wild-type RVECs. In Vldlr−/− mice, a single intravitreal injection of APX3330 at the onset of RAP-like neovascularization significantly reduced RAP-like neovascularization development. APE1/Ref-1 is expressed in retinal vascular cells. APX3330 inhibits RVEC angiogenesis in vitro and significantly reduces RAP-like neovascularization in Vldlr−/− mice. These data support the conclusion that APE1/Ref-1 redox function is required for retinal angiogenesis. Thus, APE1/Ref-1 may have potential as a therapeutic target for treating neovascular age-related macular degeneration and other neovascular diseases.
Keywords: age-related macular degeneration, RAP-like neovascularization, angiogenesis, APE1/Ref-1, reduction-oxidation regulation, redox inhibitor, APX3330
INTRODUCTION
Neovascularization in the retina is a key pathology in several ocular diseases such as age-related macular degeneration (AMD), diabetic retinopathy (DR), and retinopathy of prematurity (ROP). It is a primary cause of severe vision loss. The most advanced anti-angiogenic treatments, including anti-VEGF therapy, are effective, but only improve vision significantly in 25–40% patients (Hernandez-Pastor et al., 2008, Rosenfeld et al., 2006). While VEGF is a dominant factor in pathological neovacularization, retinal neovascularization has a complex etiology, possibly including multiple genetic and non-genetic factors. These factors include expression and/or activities of complement, integrin, platelet-derived growth factor, inflammatory cytokines, oxidants, and macrophages, which play important roles in various stages of retinal neovascularization (Klein, 2007). Clinical management of retinal neovascularization remains a great challenge, and novel treatment strategies that target multiple components of the angiogenesis cascade hold great potential to improve clinical outcomes.
APE1/Ref-1 (also known as Ref-1, HAP1, hAPE, APE or APEX) is a multi-functional protein with apurinic/apyrimidinic endonuclease activity, encoded by an evolutionarily conserved C-terminal domain, and reduction-oxidation (redox) activity, encoded by a functionally-independent N-terminal domain unique to mammalian members of this protein family (Tell et al., 2005, Xanthoudakis et al., 1994). APE1/Ref-1 redox activity stimulates numerous transcription factors that are involved in cancer promotion and progression, such as PAX, HIF-1α and NFκB, AP-1 (Fos/Jun), HLF, p53, and others (Evans et al., 2000, Fishel and Kelley, 2007, Tell et al., 2005). Several of these transcription factors also play roles in angiogenesis (i.e., HIF-1α (Carmeliet et al., 1998, Gariano and Gardner, 2005), NFκB (Huang et al., 2001, Huang et al., 2000, Yoshida et al., 1999), and AP-1 (Tischer et al., 1991); for review see (Maulik, 2002). In addition, a high level of APE1/Ref-1 expression is associated with more active angiogenesis and chemotherapy resistance in tumors (Bicknell and Harris, 2004, Bilton and Booker, 2003). Selective inhibition of the APE1/Ref-1 redox function by APX3330 (E3330/BQP) inhibits the growth of tumor endothelium and endothelial progenitor cells (Zou et al., 2008). Our recent report that APX3330 significantly attenuates proliferation and tube formation of retinal vascular endothelial cells (RVECs) suggests that APE1/Ref-1 could play a role in retinal angiogenesis (Luo et al., 2008).
This study explores the role of the APE1/Ref-1 redox function during retinal angiogenesis in vitro in RVEC cells and in vivo in Vldlr−/− mice. The Vldlr−/− mouse develops spontaneous age-related RAP-like neovascularization evolving from the retina which mimics the essential histological and angiographic characteristics of retinal angiomatous proliferation (RAP), a subtype of neovascular AMD (Heckenlively et al., 2003, Hu et al., 2008, Li et al., 2007). The results show that APX3330 potently inhibits the growth of RVEC in vitro and significantly reduces RAP-like neovascularization in Vldlr−/− mice, suggesting that APE/Ref-1 is required for retinal angiogenesis in the mouse. Therefore, inhibiting the redox function of APE1/Ref-1 may offer a novel approach to treat retinal angiogenesis.
MATERIALS AND METHODS
Experimental animals
All animals used in the study were maintained and treated with strict adherence to the guidelines for animal care and experimentation prepared by the Association for Research in Vision and Ophthalmology and approved by the Indiana University Animal Care and Use Committee. Breeding pairs of mutant mice with targeted deletion of the Vldlr gene (B6; 129S7-Vldlrtm1Her/J; Vldlr−/−) (Frykman et al., 1995) were obtained from the Jackson Laboratory (Bar Harbor, Maine, USA). Breeding pairs of an ImmortoMouse® transgenic line expressing a temperature-sensitive SV40 large T antigen were obtained from the Charles River Laboratories (Wilmington, MA). Both wild-type (C57BL/6J; +/+) and Vldlr−/− mice were crossbred with the transgenic ImmortoMouse, respectively. Cells isolated from the littermates produced from these crosses can be immortalized by turning on the SV40 gene in vitro. All offspring were subjects for PCR genotype confirmation of SV40, wild-type Vldlr, and Vldlr knockout genes.
Matrigel Tube Formation Assay
Early passage (2–4 days) of human umbilical cord blood-derived endothelial colony forming cells (ECFCs) were starved in EBM2 (Lonza, Walkersville, MD) + 0.5% FBS (Hyclone, Logan, UT) for 16 hours. ECFCs were detached with trypsin EDTA (Invitrogen, Carlsbad, CA) and a viable cell count obtained via trypan blue exclusion. Cells were suspended in EBM2 + 0.5% FBS with vehicle only or varying concentrations of APX3330 dissolved in DMSO or Avastin® (Genentech, San Francisco, CA). DMSO/vehicle controls were routinely included in the assay. 7500 cells per well were plated into 96 well tissue culture plates precoated with matrigel. Each condition was plated in triplicate. Plates were incubated at 37°C in a 5% CO2, humidified incubator and examined after 6–8 hours for tube formation. Low magnification images were captured to quantify the total number of closed network units formed per well. The same assay was also performed in RVECs isolated from wild-type and Vldlr−/− mice (Nagata et al., 2003).
TUNEL Assay
TdT mediated dUTP-fluorescein nick end-labeling (TUNEL) assay which allows us to determine the percentage of cells undergoing apoptosis. The reactions were performed following the manufacture’s protocol (Roche, Indianapolis, IN). Cells were treated with various concentrations of APX3330 for 24 hours. A 100 µM H2O2 group was included to serve as a positive control. Random fields of cells are photographed under phase microscopy and scored as percent positive cells for the terminal transferase labeling reaction.
RVEC primary culture
Mouse RVECs and retinal pericytes (RPCs) were isolated as described before with modifications (Su et al., 2003). Briefly, retinal tissues from young adult wild-type and Vldlr−/− mice carrying a SV40 transgene were digested with collagenase type I (Worthington, Lakewood, NJ) in DMEM. The dissociated cells were incubated with sheep anti-rat magnetic beads (Dynal Biotech, Lake Success, NY) pre-coated with a rat anti-mouse PECAM-1 monoclonal antibody (BD Biosceinces, San Jose, CA) for affinity binding of RVECs. Bead-bound cells were seeded in a 24-well plate pre-coated with fibronectin (BD Biosciences, San Jose, CA) in an endothelial cell growth medium (EBM/EGM-2MV, Cambrex). Detailed characterization of these cells (>95% endothelial cells) are described in our recent publication (Jiang et al., 2009). A similar protocol was used to isolate retinal pericyte but using anti-mouse α-SMA antibody to label the magnetic beads, Cells were maintained in culture at 33°C with interferon to turn on the SV40 gene, but were assayed under ordinary conditions at 37°C in the absence of interferon.
Western blot
Whole cell/tissue extract were prepared using RIPA buffer supplemented with protease inhibitor cocktail. The protein extract were separated by 10% SDS-PAGE electrophoresis, and transferred to a nitrocellulose membrane. After blocking in 5% non-fat milk in TBS/T buffer, blots were probed with an anti-APE1/Ref-1 monoclonal antibody (Novus Biologicals) and an anti-tubuline monoclonal antibody (1:400 for both) at 4°C overnight. Blots were then incubated with HRP-conjugated secondary antibodies (Amersham Pharmacia, Arlington Heights, IL) at room temperature for 1 hour, and were visualized by enhanced chemiluminescence (ECL; PerkinElmer).
Cell proliferation assay
RVECs were starved in basal medium supplemented with 0.2% FBS (EBM) at 37°C overnight. After trypsinization, cells were resuspended in basal medium, and seeded at 2,000 cells/well in 96-well plates. APX3330 at various concentrations was added three hours later. After 48 hours incubation at 37°C, the total number of cells was assayed by using the CellTiter 96® AQueous One Solution (Promega Corporation, Madison, WI). The absorbance value at 490 nm was read by a microplate reader (GENios Pro, Tecan Trading AG, Switzerland) after 1 hour incubation. Experiments were performed in 5 to 6 wells per group each time and repeated three times.
Migration assay
Migration was performed using modified Boyden chambers containing polycarbonate membrane (Transwell, 5.0 µm pore size; Costar, Cambridge, MA) with slight modification (Cai et al., 2000). Wild-type and Vldlr−/− RVECs at the same confluence were starved in basal medium overnight and then seeded at 5 × 103 cells/well into Transwell plates coated with fibronectin (10 µg/ml). Basal medium supplemented with 10 ng/ml bFGF, with or without APX3330 at different concentrations were added to the lower chamber and incubated at 37°C for 16 hours. Viable cells were stained with the CellTracker™ Green BODIPY®, fixed in 1% PFA, and counterstained with DAPI. The upper surface of the Transwell member was scraped with a cotton swab to remove un-migrating cells. Seven images of migrating cells were taken randomly from each membrane with a 10× objective. The average number of nuclei in each image was determined. The experiment was performed in triplicate.
Real-Time Cell Impedance Analysis
Dynamic cellular biology was monitored using Real-Time Cell Analyzer single-plate (RTCA SP) Instrument, xCELLigence System (Roche Applied Science, Mannheim, Germany). The system monitors cellular events in real time measuring electrical impedance across interdigitated micro-electrode integrated on the bottom of tissue culture E-Plates. The impedance measurement provides quantitative information about the biological status of the cells, including cell number, viability, and morphology. A dimensionless parameter called Cell Index (CI) is derived as a relative change in measured electrical impedance to reflect the integrated cellular status in the culture. For experiments, both wild-type and Vldlr−/− RVEC were starved in EBM/0.2% FBS overnight before being seeded on an E-Plate 96. Two hours after seeding, different concentrations of various compounds (APX3330, Avastin®, or Lucentis®) were added in tetraplicate. Dynamic CI values were monitored in 30-minute intervals from the time of plating until the end of the experiment (77 hours). CI values were calculated and plotted on the graph. Standard deviation of tetraplicates of wells for the two types cells with different treatments were analyzed with the RTCA Software.
Intravitreal treatment
Intravitreal treatment was performed Vldlr−/− mice at P14 after anaesthetization. Each animal received a single intravitreal injection of 1 µl volume of BSS® (balanced saline solution, Alcon Laboratories, Fort Worth, Texas) as a vehicle control and the fellow eye received 1 µl of 200 µM APX3330. The target concentration of APX3330 in the retina is approximately 20 µM. An Avastin® (Genentech, South San Francisco, California) treatment group was included for comparison.
Subretinal neovascluarization quantification
A conjugate of isolectin IB4 from Griffonia simplicifolia (isolectin IB4 Alexa Fluor 488, Invitrogen) was used for retinal blood vessel labeling in whole flat mounts. One week after intravitreal treatment, the eyes were harvested and fixed in 1% PFA at 4°C overnight. Retinas were separated, with 4–6 radial cuts to flatten the retina. After blocking, the entire neural retina was incubated in a 1:200 diluted isolectin solution containing 3% donkey serum for 2 days at 4°C, washed with PBS, and mounted with a ProLong Gold antifade reagent (Invitrogen). Retinal images focusing on the subretinal space were photographed under an inverted fluorescence microscope at 10× magnification (Leica DM IRB, Leica Microsystems Inc., Bannockburn, IL). A whole retinal montage was used to quantify the total number of RAP-like neovascularization with masked label.
Statistical analysis
All the statistical analyses were performed using SPSS11.0 (SPSS, Chicago, IL). Data are presented as mean ± SD. Differences were assessed using one-way or two way analysis of variance (ANOVA) or the student t test. A value of p < 0.05 is considered statistically significant.
RESULTS
APX3330 inhibits endothelial cell tube formation
Previous studies suggest that APX3330 inhibits downstream functions of HIF-1α in angiogenesis (Luo et al., 2008). Therefore we examined the effect of APX3330 and combined effect of APX3330 and Avastin®, a known anti-angiogenic compound on Matrigel tube formation assay using human ECFCs. As shown in Figure 1, APX3330 impaired the ability of these cells to form tubules. At 10 µM, APX3330 reduced tube formation by 61%, while Avastin® (500 µg/ml) had little effect on tube formation. However, the combination of these two agents at these doses completely inhibited tube formation. A similar result was observed with Avastin® (500 µg/ml) and a lower dose of 5 µM APX3330. These data suggest that the effects of APX3330 and Avastin® on endothelial cell tube formation are at least additive or maybe even synergistic.
Figure 1.
In vitro Matrigel Tube Formation. APX3330 inhibits formation of blood-vessel like tubules in human umbilical cord blood-derived ECFCs. The addition of APX3330 to Avastin® results in a dramatic decrease in the tube formation ability of these cells at levels much greater than either agent alone. Avastin® (500 µg/ml) had little effect on tube formation, 10 µM APX3330 reduced tube formation by 61%, and the combination of these two agents at these doses completely inhibited tube formation. A similar result was observed with Avastin® (500 µg/ml) and 5 µM APX3330.
APX3330 does not induce apoptosis
APX3330 could reduce the amount of endothelial cell tube formation by inducing cell death. Therefore, TdT mediated dUTP-fluorescein nick end-labeling (TUNEL) assay was performed to quantify cell death of ECFCs in the presence and absence of APX3330. Figure 2 shows that 24 hour exposure to APX3330 does not induce apoptosis, but exposure to H2O2, a positive control, does induce apoptosis under the same condition tested. These data are consistent with the possibility that the effect of APX3330 on endothelial cell tube formation is mediated by inhibition of APE1/Ref-1 redox activity. A similar result was obtained previously (Tell et al., 2005).
Figure 2.
TUNEL (apoptosis) assay performed with various doses of APX3330 on human umbilical cord ECFCs. Exposure to APX3330 at the dose range between 2.5 to 10 µM for 24 hours does not induce apoptosis. H2O2 as a positive control agent does induce significant apoptosis under the condition tested.
Expression of APE1/Ref-1 in the retina and retinal vascular cells
Previous studies reported that APE1/Ref-1 is expressed in the developing retina (Chiarini et al., 2000, Chiarini and Linden, 2000). Here, the expression of APE1/Ref-1 in retinal vascular cells was examined for the first time. Western blot analysis indicated APE1/Ref-1 protein was abundantly expressed in the adult neural retina, as well as in purified RVECs and retinal pericytes (RPCs) (Figure 3). It also revealed that levels of APE1/Ref-1 protein were comparable in retinal tissues and vascular cells from wild-type and Vldlr−/− mice (Figure 3). No genotypic difference was noticed.
Figure 3.
Western analysis of APE1/Ref-1 expression in the retina, RVEC and RPC. High levels of APE1/Ref-1 protein were detected by a monoclonal anti-APE1/Ref-1 antibody in the retinal tissue and in purified retinal vascular cells, RVEC and RPC from wild-type (+/+) and Vldlr−/− (−/−) mice. APE1/Ref-1 expression level was similar in the two mice strains. Anti-tubulin antibody was used as an internal control. The result is representative of 3 replicates.
APX3330 inhibits retinal endothelial cell proliferation
To test the specific effect of APX3330 on retinal vascular cells, a serious of in vitro assays was carried out using magnetic beads-purified RVECs. We have previously showed that APX3330 inhibits proliferation of wild-type RVECs in vitro (Luo et al., 2008), which suggests that it is likely to inhibit angiogenesis in vivo. In the present study, we have found that APX3330 inhibited the proliferation of not only wild-type but also Vldlr−/− RVECs (Figure 4A). This was associated with a slightly higher efficacy in Vldlr−/− RVECs (61.58±7.66%) than that in wild-type RVECS (84.15±9.23%). Because Vldlr−/− RVECs grow at a much faster rate (Jiang et al., 2009), Vldlr−/− RVECs are more sensitive to APX3330 than wild-type RVECs.
Figure 4.
Effects of APX3330 on RVEC proliferation (A) and migration (B). APX3330 inhibited proliferation of wild-type and Vldlr−/− RVECs in a dose-dependent manner (A). The inhibition was more potent in Vldlr−/− RVEC than that in wild-type RVEC. APX3330 inhibited the migration of RVECs in a similar fashion. Again, while Vldlr−/− RVEC was more pro for migration in the control condition, Vldlr−/− RVEC was more sensitive to APX3330 than wild-type RVEC at the same dosage (B). The effective dosage of APX3330 inhibiting migration (5 µM reduced by ~80–90%) was lower than that for proliferation (10 µM reduced by ~20–40%). *, p < 0.05 when compared with wild-type RVEC normal control (NC); **, p < 0.05 when compared with Vldlr−/− RVEC NC group; #, p < 0.01 between the two NC groups.
APX3330 inhibits retinal endothelial cell migration
Endothelial cell migration is essential for angiogenesis. Therefore, the effect of APX3330 on migration of wild-type and Vldlr−/− RVECs was examined using a dual chamber transwell assay. Cells were seeded in the upper chamber and basic fibroblast growth factor (bFGF) (10 ng/ml) was added to the lower chamber as a chemoattractant. In the absence of APX3330, migrating Vldlr−/− RVECs (311.93±4.75) were more abundant than migrating wild-type RVECs (228.28±7.07) (Figure 4B; p < 0.01), as reported previously (Jiang et al., 2009). Furthermore, APX3330 (5 µM) significantly inhibited migration of RVECs, reducing the number of migrating wild-type RVECs by 69% to 70.21±17.68 per field (p < 0.01) and reducing the number of migrating Vldlr−/− RVECs by 90% to 34.71±3.23. Similar results were obtained when cells were treated with 10 and 20 µM APX3330.
APX3330 inhibits retinal endothelial cell angiogenesis in vitro
Results presented in Figures 1 show that APX3330 inhibits human ECFC tube formation in a dose-dependent manner. Here, we show that despite a much higher basal level of tube formation in Vldlr−/− RVECs than wild-type RVECs, addition of APX3330 was still able to inhibit such profound ability to form capillary-like structures dose-dependently (Figure 5). The inhibitory effects of APX3330 on Vldlr−/− RVEC tube formation was already significant (p < 0.05) at 1 µM concentration (Figure 5C) and reached 78% reduction at 20 µM APX3330 (p < 0.01; Figure 5B, C). The much enhanced tube formation in Vldlr−/− RVECs is consistent with our previous report (Jiang et al., 2009).
Figure 5.
Matrigel tube formation assay of wild-type (+/+) and Vldlr−/− (−/−) RVECs with or without various doses of APX3330. A, B, Representative images showing that APX3330 at 20 µM abolished most of capillary-like structures in Vldlr−/− RVECs (B) comparing to the control condition without APX3330 treatment (A). C, Quantitative measurement of the number of tubes showed a striking genotypic difference in the basal level of tube formation with much more tubules in Vldlr−/− RVECs than those in wild-type RVECs. Nevertheless, addition of APX3330 was still able to inhibit such profound tube formation dose-dependently. The inhibition effect on Vldlr−/− RVECs was significant at all doses from 1 to 20 µM of APX3330. The effects of APX3330 on wild-type RVECs were significant at 10 and 20 µM of APX3330. *, p < 0.05 when compared with wild-type RVEC NC; **, p < 0.05 when compared with Vldlr−/− RVEC NC group; #, p < 0.01 between the two NC groups.
APX3330 reduces CI of retinal endothelial cells
A Real-Time Cell Analyzer (RTCA) was also used to monitor dynamic changes in the properties of retinal endothelial cells during prolonged exposure to APX3330. RTCA evaluates the ionic environment at an electrode/solution interface in a cell culture. RTCA output is a dimensionless parameter called Cell Index (CI), which integrates information on cell viability, number, morphology, and adhesion. RTCA assays were initiated by seeding wild-type and Vldlr−/− RVECs on an E-Plate 96 at the same density. APX3330 was added at 5, 10 and 20 µM 2 h after seeding and CI values were monitored at 30-minute intervals for 72 h. As shown in Figures 7A and 7B, APX3330 reduced CI values in wild-type and Vldlr−/− RVECs in a dose- and time-dependent manner. The effect was statistically significant in all three doses in both genotype RVECs (p < 0.05). Despite a significant upsurge of the growth curve, the response of Vldlr−/− RVECs to APX3330 was more dramatic than that of corresponding wild-type RVECs. This is consistent with our above findings of the more potent effects of APX3330 on Vldlr−/− RVEC proliferation (Figure 6A) and migration than the wild-type (Figure 6B). These in vitro results indicate that activated endothelial cells are more sensitive to APX3330 treatment. Such selective effect would be favorable for future therapeutic usage if proven.
Figure 7.
Intravitreal treatment effects of APX3330 or Avastin® on RAP-like neovascularization in Vldlr−/− mice. At the onset of RAP-like neovascularization (P14), each mouse received a single intravitreal injection of 1 µl volume of BSS as a vehicle control and the fellow eye received 1 µl of 200 µm APX3330, or 25 µg Avastin®. Total number of RAP-like neovascularization was quantified one week after the treatment in the whole mount retina after isolectin-FITC staining. Representative images of isolectin stained retinal whole mount showing RAP-like neovascularization spots after BSS control (A) or APX3330 (B) injection. Quantitative measurement revealed that 17/20 individuals had reduced number of RAP-like neovascularization in the eyes treated with APX3330 (C). The effective rate is 85%. The significant improved rate (> 20% reduction) is 75%. Paired t-test analysis showed a very significant (p = 0.000772) inhibition effect of APX3330 on the total number of RAP-like neovascularization in Vldlr−/− mice. In contrast, the effect of 25 µg Avastin® treatment on total number of RAP-like neovascularization was inconsistent (D, p > 0.05).
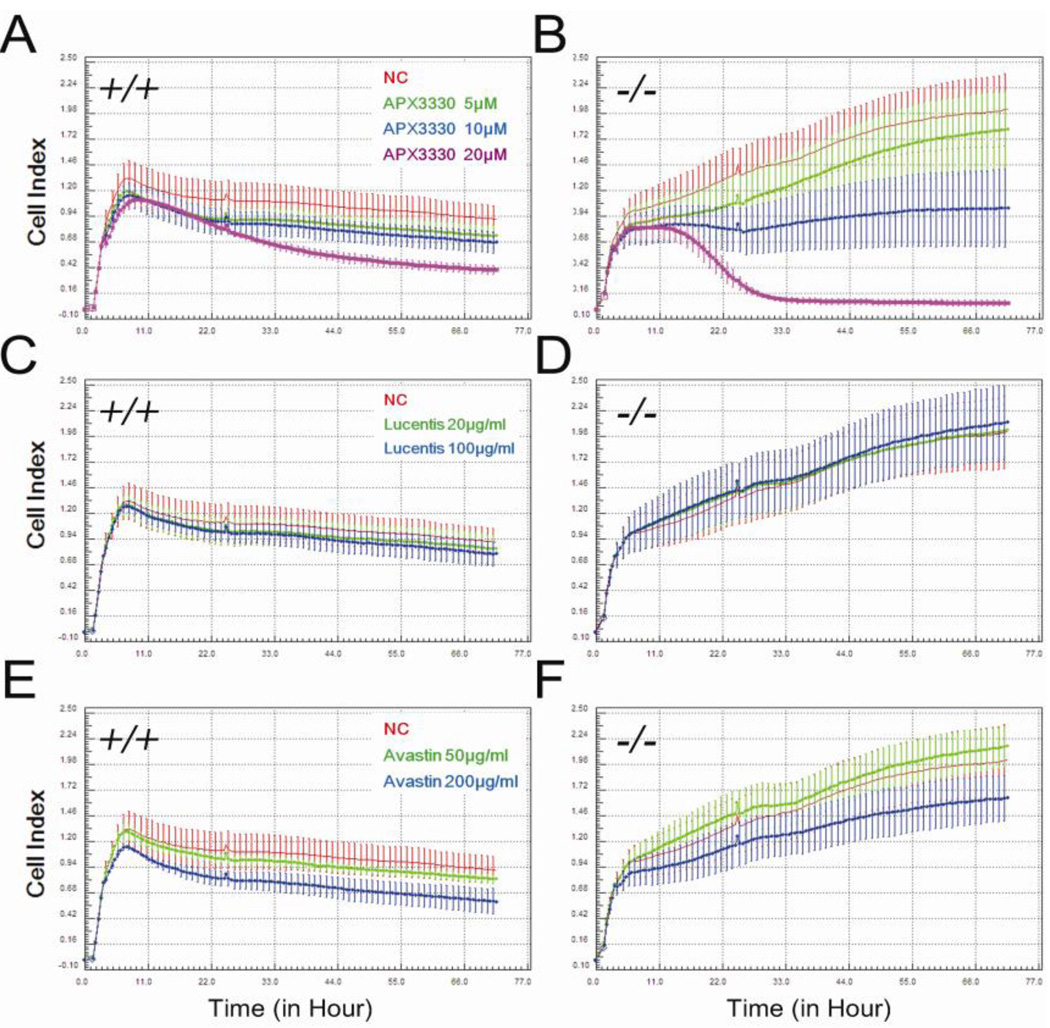
Figure 6.
Real time analysis of the effect of APX3330 (A, B), Lucentis® (C, D) or Avastin® (E, F) on wild-type (A, C, E) and Vldlr−/− (B, D, F) RVECs using the xCELLigence system. All treatment or vehicle control agents were added 2 h after seeding. Cell Index (CI), which integrally reflects an overall property of endothelial cells including proliferation, morphology, adhesion, and etc, was monitored and recorded at 30-minute intervals for 72 h. The administration of APX3330 (5, 10 or 20 µM) significantly reduced CI values in both wild-type and Vldlr−/− RVECs in a dose- and time-dependent manner (p < 0.05). Despite a significant upsurge of the growth curve, Vldlr−/− RVECs were quit sensitive to APX3330 (B). The CI value after 20 µM APX3330 treatment in Vldlr−/− RVECs reached a much lower level than that of the corresponding dosage in wild-type RVECs. In contrast, Lucentis® (10 or 100 µg/ml) or Avastin® (50 µg/ml) had little or no effect to RVECs of both genotypes (p > 0.05). A significant inhibitory effect was only seen at a high dose of 200 µg/ml Avastin® in wild-type and Vldlr−/− RVECs (p < 0.05).
Lucentis® and Avastin® are anti-VEGF antibodies used to treat AMD in the clinic. Therefore, the effect of Lucentis® and Avastin® on CI values of RVECs was also measured using RTCA. In contrast to APX3330, Lucentis® (20 µg/ml and 100 µg/ml) did not reduce CI values of wild-type or Vldlr−/− RVECs (Figure 6C and D). Avastin® significantly reduced CI values of wild-type and Vldlr−/− RVECs at a high dose (200 µg/ml, p < 0.05) but not at a lower dose (50 µg/ml (Figure 6, E and F) and Vldlr−/− RVECs were less sensitive to Avastin® than wild-type RVECs. A much higher dose of Avastin® was required to reduce CI values to the same extent as APX3330 (200 µg/ml Avastin® versus 5–10 µM APX3330).
APX3330 inhibits RAP-like neovascularization in Vldlr−/− mice
As shown in Figures 4–6, APX3330 strongly inhibits proliferation, migration and tube formation of Vldlr−/− RVECs, and the effects of APX3330 are more potent in Vldlr−/− than in wild-type RVECs. To extend these observations to an in vivo model, Vldlr−/− mice at P14 were dosed with 1 µl of 200 µm APX3330 or 25 µg Avastin® (control) by intravitreal injection. The final concentration of APX3330 was equivalent to approximately 20 µM in the retina. To minimize individual variation, one eye was injected with APX3330 or Avastin®, while the fellow eye of the same mouse was injected with vehicle control (BSS). One week after the injection, RAP-like neovascularization were quantified in the whole mount retina stained with isolectin-FITC. In 17/20 mice (85%), the number of RAP-like neovascularization was lower in the APX3330-treated eye than in the untreated eye of the same animal (Figure 7C) with 75% of treated eyes showing > 20% reduction in RAP-like neovascularization (p = 0.000772 in paired t-test). In contrast, Avastin® only reduced RAP-like neovascularization in 1/7 mice (Figure 7D) and the overall results were not significantly different.
DISCUSSION
This study shows that APE1/Ref-1 is highly expressed in the retina, choroid/RPE, and in RVECs and RPCs from the mouse and provides evidence that APE1/Ref-1 redox activity is required for efficient retinal endothelial cell proliferation, migration and tube formation. Furthermore, Vldlr−/− RVECs not only responded in vitro to APX3330, a selective inhibitor of APE1/Ref-1 redox activity, but were more sensitive to APX3330 than wild-type RVECs. The Vldlr−/− mouse model was used to provide complementary in vivo data suggesting that APE1/Ref-1 plays a role in retinal angiogenesis in the mouse. In particular, a single intravitreal injection of APX3330 at the onset of RAP-like neovascularization significantly reduced the total number of RAP-like neovascularization in Vldlr−/− mice after one week.
APE1/Ref-1 in the retina
APE1/Ref-1 expression in the retina has been reported previously (Chiarini et al., 2000, Chiarini and Linden, 2000). For example, Chiarini and colleagues localized the expression of APE1/Ref-1 to ganglion cells and inner nuclear layers of the retina, with relatively less staining in the outer nuclear layer. Staining of the photoreceptors was more intense than in the neuroblastic layer of younger rats. In tissue from both P27 and P45 rats the pattern of immunoreactivity for APE1/Ref-1 was similar to that at P13 (Chiarini et al., 2000). Expression of APE1/Ref-1 is lower in the retina following ischemia/reperfusion injury, and intravitroues bFGF can upregulate the expression of APE1/Ref-1 (Wang et al., 2007). Our findings show strong expression of APE1/Ref-1 in the retina of adult wild-type and Vldlr−/− mice. A high level of APE1/Ref-1 protein was also detected in purified RVECs and pericytes, consistent with an important biological role for APE1/Ref-1 in retinal vascularization, as discussed below.
APE1/Ref-1 as a target for anti-angiogenesis treatment in retinal neovascular disorders
Previous studies using APX3330 suggested that APE1/Ref-1 redox activity is required for in vitro proliferation and tube formation by RVECs (Luo et al., 2008) and for in vitro growth of pancreatic cancer-associated endothelial cells (Zou et al., 2008). This study confirms the earlier finding that APX3330 inhibits proliferation of murine RVECs in vitro. However, an important novel finding of this study is that APX3330 has more potent growth inhibitory effects in vitro on Vldlr−/− RVECs, which are activated with respect to angiogenic function and activity, relative to wild-type RVECs (Jiang et al., 2009). Although the exact mechanism for this effect is not known, it could be that activated endothelial cells are more dependent on the APE1/Ref-1 redox function than not activated endothelial cells. Western blot analysis suggests a similar level of APE1/Ref-1 protein in wild-type and Vldlr−/− RVECs; however, a change in the specific activity of APE1/Ref-1 in Vldlr−/− RVECs could alter sensitivity of these cells to APX3330, and this possibility has not been ruled out at present. Nevertheless, the high sensitivity of Vldlr−/− RVECs to APX3330 in vitro, combined with the demonstration in this study that a single intravitreal injection of APX3330 reduced RAP-like neovascularization in the eyes of Vldlr−/− mice strongly supports a possible role for APE1/Ref-1 in retinal vascularization.
This study compares and finds significant differences in the effects of APX3330 and Avastin® on the in vitro properties of RVECs and on RAP-like neovascularization in the eyes of Vldlr−/− mice. For example, a much higher concentration of Avastin® than APX3330 was required to reduce CI values of RVECs in vitro, and intravitreal Avastin® did not reduce RAP-like neovascularization in Vldlr−/− mice, while APX3330 strongly reduced RAP-like neovascularization after a single intravitreal injection. The weak response to Avastin® in this model could be due to poor cross-species immunoreactivity with murine VEGF-A (Yu et al., 2008) and it would require higher dosage. Alternatively, APE-1/Ref1 could be a more effective target than VEGF for inhibiting RVEC viability and proliferation in vitro (as measured by CI) or reducing RAP-like neovascularization in Vldlr−/− mice because APE-1/Ref1 is upstream of multiple transcription factors essential for neovascularization. Also, our data suggest that the effects of APX3330 and Avastin® in endothelial cell tube formation are additive or synergistic. This is not surprising as APX3330 blocks APE1/Ref-1 redox function and therefore, would block the ability of HIF-1α to bind to various downstream target promoters such as VEGF gene and other angiogenic molecules (Carmeliet et al., 1998, Gariano and Gardner, 2005). Avastin®, on the other hand, is an antibody that binds to VEGF and prevents it from binding to its target receptor. While APE1/Ref-1 redox inhibition could affect multiple signaling pathways including VEGF, and Avastin® neutralizes VEGF molecule turned on by different mechanisms, these pathways are not necessary overlapping, rather than complementary, to each other.
In summary, this study suggests that agents such as APX3330, which inhibit APE1/Ref-1 redox activity, have potential as therapeutic agents for treating retinal neovascularization. APX3330 may be especially useful for treating neovascularization that is refractory to anti-VEGF agents. The efficacy of APX3330 on choroidal neovascularization is also being explored for treating neovascular AMD.
APE1/Ref-1 is expressed in mouse retina, retinal endothelial cells and pericytes.
APE1/Ref-1 redox inhibition by APX3330 suppresses retinal vascular cell growth.
APE1/Ref-1 redox inhibition by APX3330 suppresses retinal vascular cell growth.
APX3330 significantly reduce retinal neovascularization in Vldlr−/− mice.
ACKNOWLEDGEMENTS
Financial support for this work was provided by a postdoctoral fellowship from the Fight for Sight to A.J., research grants from Reeve’s Foundation, and American Health Assistance Foundation to X.Q. and the NIH, National Cancer Institute CA106298, CA114571 and CA121168 to M.R.K, NIH National Eye Institute R41 EY019784 to both M.R.K. and X.Q and the Riley Children’s Foundation (M.R.K). Additional support was provided by pilot funding through TRAC1 of the CTSA UL1RR025761 to the IU School of Medicine. The corresponding author had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Bicknell R, Harris AL. Novel angiogenic signaling pathways and vascular targets. Annu.Rev Pharmacol.Toxicol. 2004;44:219–238. doi: 10.1146/annurev.pharmtox.44.101802.121650. [DOI] [PubMed] [Google Scholar]
- 2.Bilton RL, Booker GW. The subtle side to hypoxia inducible factor (HIFalpha) regulation. Eur.J Biochem. 2003;270(5):791–798. doi: 10.1046/j.1432-1033.2003.03446.x. [DOI] [PubMed] [Google Scholar]
- 3.Cai W, Rook SL, Jiang ZY, Takahara N, Aiello LP. Mechanisms of hepatocyte growth factor-induced retinal endothelial cell migration and growth. Invest Ophthalmol Vis Sci. 2000;41(7):1885–1893. [PubMed] [Google Scholar]
- 4.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394(6692):485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 5.Chiarini LB, Freitas FG, Petrs-Silva H, Linden R. Evidence that the bifunctional redox factor / AP endonuclease Ref-1 is an anti-apoptotic protein associated with differentiation in the developing retina. Cell Death Differ. 2000;7(3):272–281. doi: 10.1038/sj.cdd.4400639. [DOI] [PubMed] [Google Scholar]
- 6.Chiarini LB, Linden R. Tissue biology of apoptosis. Ref-1 and cell differentiation in the developing retina. Ann N Y Acad Sci. 2000;926:64–78. doi: 10.1111/j.1749-6632.2000.tb05599.x. [DOI] [PubMed] [Google Scholar]
- 7.Evans AR, Limp-Foster M, Kelley MR. Going APE over ref-1. Mutat Res. 2000;461(2):83–108. doi: 10.1016/s0921-8777(00)00046-x. [DOI] [PubMed] [Google Scholar]
- 8.Fishel ML, Kelley MR. The DNA base excision repair protein Ape1/Ref-1 as a therapeutic and chemopreventive target. Mol Aspects Med. 2007;28(3–4):375–395. doi: 10.1016/j.mam.2007.04.005. [DOI] [PubMed] [Google Scholar]
- 9.Frykman PK, Brown MS, Yamamoto T, Goldstein JL, Herz J. Normal Plasma Lipoproteins and Fertility in Gene-Targeted Mice Homozygous for a Disruption in the Gene Encoding Very Low Density Lipoprotein Receptor. Proc Natl Acad Sci U S A. 1995;92(18):8453–8457. doi: 10.1073/pnas.92.18.8453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Gariano RF, Gardner TW. Retinal angiogenesis in development and disease. Nature. 2005;438(7070):960–966. doi: 10.1038/nature04482. [DOI] [PubMed] [Google Scholar]
- 11.Heckenlively JR, Hawes NL, Friedlander M, Nusinowitz S, Hurd R, Davisson M, Chang B. Mouse model of subretinal neovascularization with choroidal anastomosis. Retina. 2003;23(4):518–522. doi: 10.1097/00006982-200308000-00012. [DOI] [PubMed] [Google Scholar]
- 12.Hernandez-Pastor LJ, Ortega A, Garcia-Layana A, Giraldez J. Ranibizumab for neovascular age-related macular degeneration. Am J Health Syst.Pharm. 2008;65(19):1805–1814. doi: 10.2146/ajhp070342. [DOI] [PubMed] [Google Scholar]
- 13.Hu W, Jiang A, Liang J, Meng H, Chang B, Gao H, Qiao X. Expression of VLDLR in the retina and evolution of subretinal neovascularization in the knockout mouse model's retinal angiomatous proliferation. Invest Ophthalmol Vis Sci. 2008;49(1):407–415. doi: 10.1167/iovs.07-0870. [DOI] [PubMed] [Google Scholar]
- 14.Huang S, Pettaway CA, Uehara H, Bucana CD, Fidler IJ. Blockade of NF-kappaB activity in human prostate cancer cells is associated with suppression of angiogenesis, invasion, and metastasis. Oncogene. 2001;20(31):4188–4197. doi: 10.1038/sj.onc.1204535. [DOI] [PubMed] [Google Scholar]
- 15.Huang S, Robinson JB, Deguzman A, Bucana CD, Fidler IJ. Blockade of nuclear factor-kappaB signaling inhibits angiogenesis and tumorigenicity of human ovarian cancer cells by suppressing expression of vascular endothelial growth factor and interleukin 8. Cancer Res. 2000;60(19):5334–5339. [PubMed] [Google Scholar]
- 16.Jiang A, Hu W, Meng H, Gao H, Qiao X. Loss of VLDL receptor activates retinal vascular endothelial cells and promotes angiogenesis. Invest Ophthalmol Vis Sci. 2009;50(2):844–850. doi: 10.1167/iovs.08-2447. [DOI] [PubMed] [Google Scholar]
- 17.Klein R. Overview of progress in the epidemiology of age-related macular degeneration. Ophthalmic Epidemiol. 2007;14(4):184–187. doi: 10.1080/09286580701344381. [DOI] [PubMed] [Google Scholar]
- 18.Li C, Huang Z, Kingsley R, Zhou X, Li F, Parke DW, 2nd, Cao W. Biochemical alterations in the retinas of very low-density lipoprotein receptor knockout mice: an animal model of retinal angiomatous proliferation. Arch Ophthalmol. 2007;125(6):795–803. doi: 10.1001/archopht.125.6.795. [DOI] [PubMed] [Google Scholar]
- 19.Luo M, Delaplane S, Jiang A, Reed A, He Y, Fishel M, Nyland RL, 2nd, Borch RF, Qiao X, Georgiadis MM, Kelley MR. Role of the multifunctional DNA repair and redox signaling protein Ape1/Ref-1 in cancer and endothelial cells: small-molecule inhibition of the redox function of Ape1. Antioxid Redox Signal. 2008;10(11):1853–1867. doi: 10.1089/ars.2008.2120. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maulik N. Redox signaling of angiogenesis. Antioxid Redox Signal. 2002;4(5):805–815. doi: 10.1089/152308602760598963. [DOI] [PubMed] [Google Scholar]
- 21.Nagata D, Mogi M, Walsh K. AMP-activated protein kinase (AMPK) signaling in endothelial cells is essential for angiogenesis in response to hypoxic stress. J Biol Chem. 2003;278(33):31000–31006. doi: 10.1074/jbc.M300643200. [DOI] [PubMed] [Google Scholar]
- 22.Rosenfeld PJ, Rich RM, Lalwani GA. Ranibizumab: Phase III clinical trial results. Ophthalmol Clin North Am. 2006;19(3):361–372. doi: 10.1016/j.ohc.2006.05.009. [DOI] [PubMed] [Google Scholar]
- 23.Su X, Sorenson CM, Sheibani N. Isolation and characterization of murine retinal endothelial cells. Mol Vis. 2003;9:171–178. [PubMed] [Google Scholar]
- 24.Tell G, Damante G, Caldwell D, Kelley MR. The intracellular localization of APE1/Ref-1: more than a passive phenomenon? Antioxid Redox Signal. 2005;7(3–4):367–384. doi: 10.1089/ars.2005.7.367. [DOI] [PubMed] [Google Scholar]
- 25.Tischer E, Mitchell R, Hartman T, Silva M, Gospodarowicz D, Fiddes JC, Abraham JA. The human gene for vascular endothelial growth factor. Multiple protein forms are encoded through alternative exon splicing. J Biol Chem. 1991;266(18):11947–11954. [PubMed] [Google Scholar]
- 26.Wang Y, Niu Y, Zhao Y, Liu F. Expression of Ref-1 in rat retina with ischemia/reperfusion injury and after bFGF treatment. Chin Ophthal Res. 2007;25(5):4. [Google Scholar]
- 27.Xanthoudakis S, Miao GG, Curran T. The redox and DNA-repair activities of Ref-1 are encoded by nonoverlapping domains. Proc Natl Acad Sci U S A. 1994;91(1):23–27. doi: 10.1073/pnas.91.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Yoshida A, Yoshida S, Ishibashi T, Kuwano M, Inomata H. Suppression of retinal neovascularization by the NF-kappaB inhibitor pyrrolidine dithiocarbamate in mice. Invest Ophthalmol Vis Sci. 1999;40(7):1624–1629. [PubMed] [Google Scholar]
- 29.Yu L, Wu X, Cheng Z, Lee CV, LeCouter J, Campa C, Fuh G, Lowman H, Ferrara N. Interaction between bevacizumab and murine VEGF-A: a reassessment. Invest Ophthalmol Vis Sci. 2008;49(2):522–527. doi: 10.1167/iovs.07-1175. [DOI] [PubMed] [Google Scholar]
- 30.Zou GM, Karikari C, Kabe Y, Handa H, Anders RA, Maitra A. The Ape-1/Ref-1 redox antagonist E3330 inhibits the growth of tumor endothelium and endothelial progenitor cells: Therapeutic implications in tumor angiogenesis. J Cell Physiol. 2008;219(1):209–218. doi: 10.1002/jcp.21666. [DOI] [PubMed] [Google Scholar]