Abstract
Integrins on both tumor cells and the supporting host stromal cells in bone (osteoclasts, new blood vessels, inflammatory cells, platelets and bone marrow stromal cells) play key roles in enhancing bone metastasis. Tumor cells localize to specific tissues through integrin-mediated contacts with extracellular matrix and stromal cells. Integrin expression and signaling are perturbed in cancer cells, allowing them to “escape” from cell-cell and cell matrix tethers, invade, migrate and colonize within new tissues and matrices. Integrin signaling through αvβ3 and VLA-4 on tumor cells can promote tumor metastasis to and proliferation in the bone microenvironment. Osteoclast (OC) mediated bone resorption is a critical component of bone metastasis and can promote tumor growth in bone and αvβ3 integrins are critical to osteoclast function and development. Tumors in the bone microenvironment can recruit new blood vessel formation, platelets, pro-tumor immune cells and bone marrow stromal cells that promote tumor growth and invasion in bone. Integrins play critical roles in platelet aggregation (αvβ3 and αIIbβ3), hematopoietic cell mobilization (VLA-4, osteopontin), neoangiogenesis (αvβ3,αvβ5, α6β4, β1 integrin) and stromal function (osteopontin, VLA-4). Integrins are involved in the pathogenesis of bone metastasis at many levels and further study to define integrin dysregulation by cancer will yield new therapeutic targets for the prevention and treatment of bone metastasis.
Introduction
The development of bone metastasis is common in many cancers, occurring in virtually all patients with multiple myeloma, in 65%–75% of patients with advanced breast and prostate cancers, and in 30%–40% of patients with lung cancer[1–3]. The consequences of bone metastases are often devastating and can cause pain, pathologic fractures, spinal cord and other nerve-compression syndromes and life-threatening hypercalcemia[4]. Both osteolytic lesions and osteoblastic bone metastases are associated with increased osteoclast (OC) activity and disrupted bone micro-architecture[5, 6]. In the bone microenvironment, tumor cells secrete soluble factors that promote bone remodeling resulting in the release of additional bone matrix-bound growth factors which further activates OCs and osteoblasts (OB) and tumor growth[3, 4, 7–16]. Anti-resorptive therapy, e.g. with bisphosphonates or denosumab, significantly decreases skeletal complications of cancer and is a standard of care for patients with bone metastases[4, 8, 17–19]. Beyond their effects on bone, tumors in the bone microenvironment recruit new blood vessel formation, platelets, immune cells and stromal cells that promote tumor growth and invasion in bone. Integrin-mediated cell signaling plays a critical role in many of these processes during bone metastasis, including platelet aggregation (αIIbβ3), hematopoietic/immune cell mobilization (VLA-4, osteopontin), neoangiogenesis (αvβ3,αvβ5, α6β4, β1 integrin) and stromal function (osteopontin, VLA-4) (see Figure 1). For these reasons, the mechanisms by which integrin signaling mediate the pathogenesis of bone metastasis has been an area of active research.
Fig. 1.
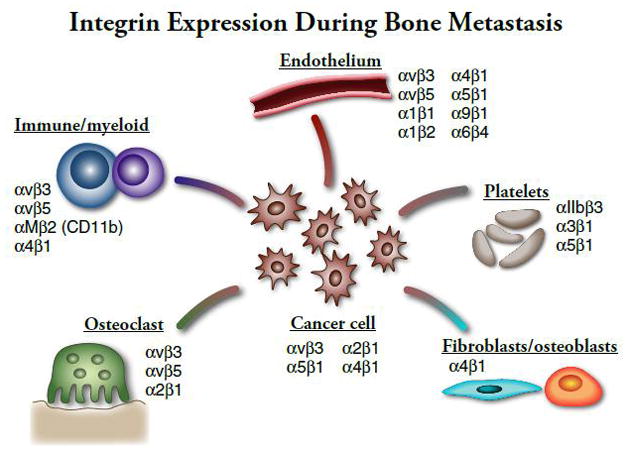
Integrin Expression During Bone Metastatis
Integrin structure, activation and signaling
Integrins are heterodimeric transmembrane glycoproteins that facilitate cell-cell and cell-extracellular matrix (ECM) adhesion and cell migration[20]. Integrins recruit many intracellular signaling molecules and can activate survival, proliferation, and motility signaling pathways[21]. There are 8 beta and 18 alpha integrin subunits that assemble into 24 different known combinations in different cell types, each characterized by distinct ligand binding specificities (including collagen, osteopontin, fibronectin, laminin, and others, depending on the integrin family), signaling abilities, and regulatory mechanisms[22]. Integrins are activated by conformational changes in the integrin extracellular domains (“inside-out” signaling). When the integrin α and β subunit cytoplasmic and transmembrane domains remain closely juxtaposed, the extracellular domains are held in a closed conformation. Activation by intracellular signals to the cytoplasmic tails results in separation of the α and β cytoplasmic and transmembrane domains and exposure of the extracellular ligand binding domain[23] (“inside-out” signaling). The open conformation, facilitates high affinity ligand binding and triggers integrin-mediated cell signaling cascades (outside-in signaling)[24, 25].
Many proteins play critical roles in the activation of specific integrins, but two cytoplasmic proteins, talin and kindlin, are necessary for inside-out signaling required for the activation of all integrin subtypes[23, 26–29]. Talin binds to the proximal end of the beta cytoplasmic tail via a phosphotyrosine-binding (PTB) domain within its FERM domain[27] and links the integrin to the actin cytokeleton[23]. Kindlin 1, 2, or 3, are necessary for talin-induced integrin activation[26, 30, 31]. Kindlin, like talin, also interacts with intracellular proteins including focal adhesion kinase resulting in cytoskeleton reorganization and adhesion[32]. G-protein coupled receptors such as the ADP receptor P2Y12, also play critical roles in the inside-out signaling required for integrin activation[25, 33, 34]. Structure-function analyses on β3 integrins have shown that a “membrane-proximal” region is important for “inside-out” signaling required [28, 35–40].
In addition to activation by inside-out signaling, ligand binding and integrin clustering can be significantly modulated by growth factor receptor interactions and other integrin interacting proteins, as reviewed in[22, 23, 41]. For example, integrin associated protein, CD47, augments integrin activation and affects the ability of αvβ3 integrin to cluster upon ligand binding[42]. Ligation of the integrin then stimulates outside-in signaling that leads to the activation of numerous signals critical for growth, migration, survival, and other functions, including FAK phosphorylation, ERK signaling, and NF-κB activation. Thus, integrin signaling in cancer cells and in associated stromal, endothelial and hematopoietic cells can be influenced by intracellular signaling proteins, growth factors, chemokines and other receptors that participate in regulating integrin function through effects on integrin activation, ligand binding, ligand affinity and integrin clustering.
Maintaining adhesion to the ECM, in part through integrin signaling, is critical to cell survival[43]. Altered cell-cell or cell-matrix interactions can result in disruption of downstream survival signaling and anchorage-dependent non-transformed cells undergo anoikis[43]. Under normal conditions, because each cell type expresses a unique set of integrins that recognize underlying ECM ligands, this form of apoptosis ensures that detached cells do not colonize inappropriate locations[43]. Cells that resist anoikis, such as metastatic cells, take advantage of several different mechanisms, including aberrant integrin expression so that the cell can adhere to a novel ECM[44], constitutive activation of molecules usually activated via integrin signaling including FAK[45], EGFR[46], and SRC[47], and lack of activation of pro-apoptotic pathways[48], among others. The integrin family of adhesion receptors link extracellular matrix to the cytoskeleton through a complex and regulated network of activation, interaction with numerous growth factor, GPCR, chemokine and cytokine receptors and induction of complex signaling cascades.
Integrin expression and signaling on tumor cells that metastasize to bone
Tumor progression, invasion, and eventual metastasis require the activity of many adhesion proteins, including the integrin superfamily. At each stage of cancer progression, subsets of integrin heterodimers are activated, providing the necessary signaling pathways for adhesion, migration, and cell survival. Metastatic tumor cells show differential integrin heterodimerization and activation compared to non-metastatic tumor cells that enable the cell to home to and colonize in a metastatic site, such as the bone marrow cavity [49, 50]. In order for primary epithelial cancers to metastasize, the tumor cells must become resistant to anoikis and detach from the primary tumor site ECM, enter the vasculature, and eventually colonize a distant site. Upon reaching a successful metastatic site, however, tumor cells use both anoikis and anoikis-resistance to their advantage, in some cases forming micro-metastases that are resistant to cancer treatment via integrin binding to the underlying bone ECM as reviewed in[51]. In addition to evading apoptosis, tumor cells must also form interactions between the tumor cell and bone stroma to establish and maintain skeletal metastasis. Many integrins have been implicated in tumor cell-host bone stroma interactions during bone metastasis and tumor growth in bone (Figure 1, Table 1), including the β1 and β3 integrin family members.
Table 1.
Extracellular matrix proteins and the main integrins that participate in bone metastasis and tumor growth in bone
| Integrin | ECM Ligands |
|---|---|
| αvβ3 | vitronectin, osteopontin, bone sialoprotein, fibronectin, TSP-1 |
| α2β1 | collagen I, laminin |
| α4β1 (VLA-4) | VCAM-1 Fibronectin, osteopontin |
| αvβ1 | fibronectin, vitronectin, |
| αvβ5 | vitronectin, osteonectin, bone sialoprotein, fibronectin |
| αIIbβ3 | fibrinogen, |
| β2 | VCAM-1, ICAM-1, fibrinogen |
| α1β1 | collagen |
| α5β1 | fibronectin |
| α9β1 | TSP1 |
| α6β4 | laminin, TSP-1 |
αvβ3 is receptor for osteopontin, fibronectin, and vitronectin, ECM proteins that are important bone matrix proteins, and αvβ3 has been identified as a critical integrin in breast cancer and prostate cancer skeletal metastasis[50, 52–56]. Interestingly, although αvβ3 has been shown to bind to fibronectin in other locations with high affinity, tumor αvβ3 integrins do not bind fibronectin in bone marrow stroma, indicating that αvβ3-expressing tumor cells bind to the bone stromal ligands vitronectin and osteopontin[57]. In breast cancer, αvβ3 binding of host osteoponin is necessary for tumor cell colonization to bone[58]. Bone metastatic cells have a higher expression of αvβ3 than the primary tumor[53], promoting adherence to the bone matrix by binding osteopontin expressed by bone stromal cells[58]. Breast cancer cells that overexpress αvβ3 have increased levels of bone metastasis and associated tumor burden and osteolysis[52, 59–62]. This overexpression of αvβ3 in the tumor cells leads to increased tumor cell adhesion, migration, and invasion to bone as well as enhanced OC recruitment within the bone microenvironment[60, 61], implicating a role of tumor-specific αvβ3 expression in breast cancer metastasis to bone as well as tumor-associated osteolysis. Likewise, in prostate cancer cells, active αvβ3 is necessary for the adherence and migration to bone matrix proteins at early stages of skeletal metastasis. This tumor cell αvβ3 integrin expression allows cancer cells to adhere to the bone matrix and interact directly with the native bone cells, osteoblasts and osteoclasts, as well as with the bone matrix itself [59].
The β1 family member, α5β1, has been identified as the primary integrin receptor for fibronectin on human bone marrow stroma[57]. α5β1 expression on leukemia, prostate and breast cancer cells facilitates interaction with bone stroma[57, 63–65]. Antibody inhibition of α5, β1, or fibronectin block prostate cancer tumor cell binding to bone stroma, indicating necessary roles for both integrin α5α1 on tumor cells and fibronectin on bone marrow stromal cells[57]. In breast cancer skeletal metastasis, the interaction between malignant cell α5β1 and host stromal cell fibronectin contributes to the survival of growth-arrested tumor cells, a potential mechanism through which tumor cells can become sequestered and “dormant” within the bone marrow cavity and may later begin to proliferate to establish a skeletal metastasis[64]. Upon FGF-2 growth factor stimulation, breast cancer cells undergo growth arrest and up regulate α5β1 expression. In most cases, these cells die, but cells that bind fibronectin via α5β1 and initiate cell survival signaling cascades survive[64].
Another β1 family member, α2β1, a collagen type I receptor, is expressed by prostate tumor cells, and its activity promotes invasion and adherence to the bone stroma. The presence of collagen I, the most abundant protein in bone, significantly increases prostate epithelial cell adhesion in culture, and antibody inhibition of integrin subunits α2 and β1 significantly inhibits tumor cell binding to stroma[66]. Hall et al showed that a skeletal metastatic prostate cancer cell line, but not cell lines that are metastatic to other organs, binds to collagen I and that this collagen I binding is α2β1 dependent in vivo [67]. Interestingly, stromal expression of collagen I does not increase tumor growth, but instead promotes tumor cell migration[67]. Tumor cell α2β1 binding of host bone marrow stromal collagen I activates RhoC GTPase which instigates a signaling cascade responsible for cytoskeleton reorganization, migration, and, eventually, collagen-stimulated invasion and preferential skeletal metastasis[68].
α4β1/vascular cell adhesion molecule-1 (VCAM-1) binding has been identified as important for cell-cell contact between α4β1 expressing myeloma cells and VCAM-1 expressing bone marrow stroma[69]. This interaction contributes to bone tumor growth, OC stimulation and resultant osteolysis[69, 70]. Likewise, epithelial tumor cells (CHO) that overexpress α4β1 developed significantly more bone metastases than mice inoculated with CHO cells alone[71]. Bone metastases, but not other metastases, were inhibited by antibodies against α4 and/or VCAM-1, suggesting a role for α4β1/VCAM-1 binding in the skeletal metastases of solid tumors[71]. The role of integrins and chemokine cross talk in tumor cell homing to bone will be discussed below. While many aspects of tumor–bone stromal interactions remain unknown, it is clear that specific interactions between tumor cell integrins and bone stromal cell ligands are essential for successful homing and metastasis to bone.
Integrin expression and signaling and osteoclast function and bone metastasis
Bone invading metastatic tumor cells co-opt integrin signaling pathways that enhance osteoclast (OC) function and recruitment. As part of bone remodeling, OC bind to the bone matrix, form an actin ring mediated sealing zone, secrete enzymes and acid to degrade bone, and then migrate to a new site. Each of these functions is regulated in part by integrins located on the membrane surface of the OC, interacting with neighboring cells and with the extracellular matrix[72].
Several integrins are involved in OC binding to bone, including αvβ3 (osteopontin, vitronectin, bone sialoprotein), avβ5 (fibronectin), and α2β1 (collagen) [73, 74]. Of these, αvβ3 is the predominant integrin found on OCs, and antibody inhibition of αvβ3 inhibits OC attachment to the bone matrix as well as OC mediated bone resorption[75]. In addition, mice with targeted disruption of β3 integrin (β3−/−) have defective osteoclast function [76] and are protected from tumor associated osteolysis[77]. vβ3 is responsible for mediating OC-bone recognition [53, 75, 78, 79] and subsequent attachment to the bone matrix[75, 80], signaling to create the characteristic resorptive ruffled membrane, regulation of osteoclast spreading, and overall organization of the cytoskeleton[76, 81]. Activation of αvβ3 regulates OC adhesion and migration on osteoponin, important for OC polarization and bone resorption[82]. Osteopontin ligand binding of αvβ3 causes a reduction of OC cytosolic calcium, inducing podosome formation and subsequent resorption[83]. In addition, αvβ3 is critical for the activation of c-Src, c-Cbl, and GTPases Rho and Rac, signaling that is necessary for the cytoskeletal reorganization important in OC function [81, 84, 85].
OC targeted therapy is a standard of care for the treatment of bone metastasis and myeloma bone disease. Tumor cells recruit OCs resulting in bone destruction and pain[3, 86, 87]. Because of its known role in OC function and its high expression in skeletal metastatic tumors as discussed above, much research has focused on αvβ3 integrin and its ligands. An important characteristic of αvβ3 cell adhesion, both in OCs and tumor cells, is the requirement of osteopontin, an αvβ3 ligand[58]. Osteopontin is a non-collagenous bone matrix protein that is produced by osteoblasts, OCs, and macrophages and is found in the extracellular matrix adjacent to calcified bone [88–90]. Expression of osteopontin in both the tumor cell and in the bone microenvironment can promote skeletal metastasis[91, 92]. Osteopontin-deficient mice have reduced bone metastasis and tumor induced osteolysis than wild type controls in a mouse model of tumor metastasis using syngeneic B16 melanoma cells[93, 94], confirming a role for host cell osteopontin expression during bone metastasis. Recombinant osteopontin induces cell migration of B16 cells that is inhibited by repressing the ERK/MAPK pathway, suggesting that the ERK/MAPK pathway regulates bone microenvironment osteopontin levels[91]. Overexpression of osteopontin in B16 melanoma cells increases cell proliferation and migration, indicating that the ligand also plays an important role in the tumor cell itself [91]. It has been demonstrated using a prostate cancer cell line over-expressing osteopontin that tumor-cell osteopontin regulates MMP-9 secretion and subsequent CD44/MMP-9 interaction, important for the migration of prostate cancer cells, contributing to metastasic potential[95]. Osteopontin-producing tumor cells enhance osteopontin production by osteoblasts[96] and OCs[97], stimulating osteoclastogenesis, OC adherence, migration, and bone resorption via host αvβ3 binding[88, 98]. Osteopontin activation of αvβ3 integrin leads to downstream activation of FAK, c-Src kinase, and Ras-ERK, among other signaling molecules, resulting in cytoskeletal reorganization, focal adhesion formation, basolateral membrane differentiation, and osteoclastic resorption[59, 99].
CD47, integrin associated protein, is expressed constitutively and interacts with integrins, including αvβ3, as part of inside-out signaling cascades and also operates in an integrin-independent manner. CD47 plays a role in osteoclast and macrophage biology and CD47−/− mice have decreased OC number and function[100, 101] which can be rescued in vitro by inhibiting nitric oxide synthase[101]. CD47−/− mice have decreased bone metastases and tumor-associated osteolysis compared to wild type[101]. During the early stages of osteoclastogenesis, namely, macrophage fusion, CD47 binds with SIRP1α, a molecule that is transiently induced in myeloid cells and that likely participates in early fusion events[102]. In the event of tumor cell metastasis to bone, however, it has been reported that cancer cells may utilize this macrophage self-recognition signaling to fuse with macrophages[103], leading to mature OCs with tumor cell nuclei and subsequent overexpression of OC stimulation factors, thus leading to increased OC function[104].
These data underscore the importance of integrins, especially αvβ3, and its adaptor proteins in OC biology and bone metabolism and point to the role of osteoclast integrins in regulating growth of cancer cells in the bone.
Integrins and tumor neovasculature and bone metastasis
Tumor neovascularization is essential for tumor cell invasion and metastasis. Access to the host blood supply provides the tumor cells with nutrients and connects the tumor to the circulation, facilitating the dissemination of metastatic cells. The angiogenic process begins with the de-stabilization and de-differentiation of local vessels, followed by activation of endothelial cells (EC), EC migration and proliferation into the tumor extracellular matrix (ECM), and finally organization of ECs into functional vessels. The ability of tumor cells to activate the normally quiescent vasculature is proposed to be controlled by an “angiogenic switch” mechanism, whereby tumor or stromal cells induce changes in the relative balance of inducers (e.g. vascular endothelial growth factor (VEGF) or TGFβ, PDGF, TNFα, bFGF) and inhibitors (e.g. thrombospondin-1 [TSP-1]) of angiogenesis reviewed in[41, 105–109]. Activated platelets, tumor cells, and fibroblasts secrete many of these pro-angiogenic factors. It has recently been appreciated that macrophage lineage cells play important roles in promoting tumor-associated angiogenesis[110–113]. Bone metastasis and bone residing tumors like myeloma also modify and recruit endothelial cells to enhance neoangiogenesis[114, 115].
Many integrin heterodimers have been implicated in tumor-associated angiogenesis [41, 105–109, 116]. The first integrin found to regulate angiogenesis, αvβ3, is expressed at high levels on tumor-associated vasculature[117, 118] and tumor-associated angiogenesis can be inhibited by β3 integrin neutralizing antibodies [119–122]. αvβ3 has been specifically implicated in the angiogenesis associated with prostate cancer bone metastases; antibody inhibition of αvβ3 decreases tumor-associated blood vessels in mice[123]. Interestingly, Reynolds et al. demonstrated enhanced (not reduced) tumor-associated angiogenesis in subcutaneous tumors in β3−/− mice[124]. Elevated levels of VEGFR2 were found on tumor-associated blood vessels in β3−/− mice, and a VEGFR2 inhibitor could block the enhanced blood vessel formation[125]. It should be noted that an inhibitor of integrin binding and signaling might have different consequences than loss of integrin expression. For example, apoptotic machinery is activated in certain cells expressing integrins that are not ligand-bound[126–129]. Recent reports that low dose integrin antagonists can increase tumor growth and angiogenesis while higher doses suppress tumor growth and angiogenesis[130] underscore the complexity of targeting β3 integrins for angiogenesis and cancer therapy.
Another αv integrin, αvβ5, also shows increased expression on tumor-associated vasculature, and αvβ5 antibodies inhibit VEGF-induced tumor-associated angiogenesis[131]. In contrast, the β3/β5 −/− double knock out mice show enhanced tumor-associated angiogenesis, as was seen in β3−/− mice[125]. Several hypotheses have been proposed that reconcile the contradictory results involving the αv integrin family that outline the roles of the integrins as pro-angiogenic, anti-angiogenic, and/or working through different pathways as reviewed in[41, 108]. It is clear, however, that αvβ3 and αvβ5 have distinct roles in regulation of tumor-associated angiogenesis and associated metastasis. The bone targeted bisphosphonate, zoledronic acid, alters endothelial cell integrin-mediated adhesion by reduced expression of αvβ3 and αvβ5 integrin on endothelial cells in vitro in one observation[132]. This observation provides a possible mechanism for osteoclast-independent anti-tumor actions for bisphosphonates that have been observed in some animal models[133–135] and clinically[136–139]. Evaluation of the effects of bisphosphonates on integrin signaling in the tumor bone microenviroment are underway.
While much of the research in integrin-mediated angiogenesis has been focused on the αv integrins, there is evidence that other heterodimers play a role in angiogenic regulation, particularly the β1 and β4 families. The β1 integrin family (α1β1, α2β1, α5β1, α4β1) has a critical role in angiogenesis with β1 −/− mice having severe vascular defects. α1β1 (a collagen receptor) and α2β1 (a laminin receptor) have been shown to be important for mediating cell adhesion in VEGF-stimulated endothelial cells[140]. In vivo, function-blocking antibodies to α1 and α2 significantly inhibited VEGF-induced angiogenesis, indicating a positive regulatory role for α1β1 and α2β1 expression in tumor-associated angiogenesis [141]. Genetic data further support a role for the integrin α1β1 as a positive regulator of angiogenesis as α1-deficent mice show reduced angiogenesis[142].
Fibronectin receptor α5β1 has also been implicated as a positive regulator of angiogenesis: α5β1 antagonists inhibit tumor-associated angiogenesis in mice by promoting endothelial cell migration and regulating proliferation and apoptosis[143, 144]. Importantly, the α5β1 antagonists did not inhibit angiogenesis induced by VEGF, indicating that the integrin α5β1 (together with αvβ3) may act in a VEGF-independent pathway[144]. α4β1, together with its ligand, VCAM-1, expressed in vessel mural cells, plays an important role in adhesion of endothelial cells and vascular smooth muscle cells during blood vessel formation[145]. Both anti-α4β1 antibodies and anti-VCAM-1 antibodies inhibit angiogenesis in vivo. Another integrin, laminin receptor α6β4 is reported to regulate several aspects of tumor angiogenesis. Genetic studies reveal that α6β4 promotes endothelial cell migration in culture; in addition, the integrin is involved in the translational regulation of VEGF, having a pro-angiogenic effect[146, 147].
In many cases, integrins influence angiogenesis through their interaction with the integrin ligand, thrombospondin 1 (TSP-1). Mice with a TSP-1 deficiency have increased tumor burden and tumor-associated vasculature, both in capillary size and number, while mice that over-express TSP-1 have delayed or absent tumor growth and reduced tumor-associated vasculature[148]. These data indicate that TSP-1 can contribute to tumor burden via negative regulation of angiogenesis. In contrast, in a human breast cancer cell line, TSP-1 stimulation up-regulates both integrin subunit α6 mRNA levels and protein levels which leads to elevated adhesion to ECM protein laminin in vitro, suggesting that TSP-1 facilitates pathogenic angiogenesis[149]. TSP-1 also interacts with α9β1 via its N-terminal domain and has a positive effect on proliferation and motility in culture and on angiogenesis in vivo that can be reduced by α9β1 inhibitors. This binding of the microvasculature-associated integrin in endothelial cells with TSP-1 activates signaling cascades including ERK and paxillin. Thus, TSP-1 can play both pro- and anti-angiogenic roles, depending on its specific integrin interaction.
The roles of integrins in tumor-associated angiogenesis are complex, not only involving integrin-ligand interactions and associated signaling pathways, but also specific temporal regulation and indirect effects through proteins such as TSP-1, and are important for the progression of angiogenesis and eventual metastasis.
Integrins and hematopoietic and tumor-induced mobilization and modulation of bone marrow cells
The bone marrow is the primary site of hematopoeisis in the adult. Osteoblasts and bone marrow stromal cells regulate hematopoeitic stem cell (HSC) growth, differentiation and bone marrow retention through numerous signaling pathways including integrin VLA-4/VCAM[150], chemokine SDF-1/CXCR4, BMPs and Notch[151–156]. Hematopoietic progenitors and stem cells express the integrin, VLA-4 and the chemokine receptor, CXCR4. Osteoblast and bone marrow stromal cells produce VCAM-1, SDF-1 and osteopontin, all important compontents of the “hematopoeitic stem cell niche”[157–159]. Integrin and chemokine signaling work in concert to promote HSC and progenitor cell homing and mobilization in the bone marrow [160]. Disruption of VLA-4/VCAM-1 and SDF-1/CXCR4 result in mobilization of HSC into the circulation[159]. G-CSF mobilization of HSC acts in part through disruption of VLA-4/VCAM-1 and CXCR4/SDF1[158, 161]. Osteoclast resorption can also regulate HSC mobilization and the stem cell niche [162].
Diverse integrins are expressed on hematopoietic progenitor cells in specific patterns and at distinct time points[163]. Integrins not only mediate the binding of normal progenitor cells to stroma and matrix molecules, but may also regulate expansion, maturation and differentiation of those cells[164], [165]. For example, α4β1 integrin regulates hematopoietic progenitor cell fate through changes in integrin expression and activity levels during cell maturation and differentiation into erythrocytes and neutrophils[165–167]. α4 containing integrins mediate adhesion of hematopoietic progenitors to stromal cells likely through binding to matrix components such as fibronectin[168] or cellular receptors such as VCAM-1[169]. The integrin subunits α5, α6 and α9 have also been shown to be expressed by progenitor cells[170–172]. Studies using blocking antibodies demonstrated that α6 subunit cooperates in collaboration with the α4 subunit is regulating homing of progenitor cells[171]. α9β1 integrin is also important for adhesion of progenitor cells to osteoblasts in the bone marrow[172], illustrating the fact that hematopoiesis takes place in three dimensional matrices, the so-called bone marrow niches. These niches are located either at the endosteum near osteoblasts and also in the vascular niche close to marrow blood vessels [173].
Tumor cells both in the bone microenvironment and at distant sites can modulate and mobilize hematopoietic progenitor and immune cells to promote bone and visceral metastasis and local tumor growth. Tumor-induced mobilization of VEGFR+ and Sca+kit-bone marrow derived cells have been implicated in enhancing distant tumor and metastatic growth[174]. These mobilized VEGFR+ cells also express α4β1and can migrate to sites of increased synthesis of matrix components such as fibronectin and establish a “pre-metastatic niche” that can favor tumor metastasis and growth[174]. β2 integrins on bone marrow derived endothelial progenitors can also mediate the adhesion and VEGF-induced migration of the progenitors to the mature endothelium of actively remodeling vasculature[175].
Tumor cells from a primary lesion can act at a distance to influence bone marrow hematopoeisis through secreted factors such as the integrin ligand, osteopontin[176]. Primary epithelial tumors can “instigate” growth of indolent tumors through modulation of the bone marrow microenvironment and mobilization and recruitment of bone marrow cells to distant tumor sites[176, 177]. McAllister et al. found that tumor secretion of osteopontin is necessary but not sufficient in xenograft models to modulate the bone microenvironment and promote bone marrow cell recruitment to tumor metastasis[176]. Pazolli et al. found that osteopontin secreted by senescent fibroblasts promoted tumorigenesis in animal models of skin cancer[178].
Thus tumors cells both in bone and at distant sites can modulate hematopoeisis in part through osteopontin and bone marrow cell integrins resulting in the mobilization and recruitment of bone marrow derived cells that will enhance local and metastatic tumor growth.
Integrins and tumor cell homing/colonization of bone
The site of metastasis is tumor cell specific depending on their integrin, chemokine receptor and cytokine/receptor expression profiles[50, 179–181]. At the metastatic site, normal physiology is changed towards increased secretion of cytokines and activation of integrins to support recruitment, survival and growth of tumor cells. Metastasizing cancer cells can co-opt the same mechanisms used in physiological hematopoietic progenitor cell homing to bone through expression of integrins and chemokines [150, 152, 153]. CXCR4 expressed on cancer cells can direct those cells to bone[181–186]. The migration of myeloma cells to and across bone marrow stromal cells is in part regulated by SDF-1α/CXCR4 ligation and up-regulation of α4β1 (VLA-4) resulting in adhesion of myeloma cells to the underlying bone marrow stroma[187]. Likewise, CXCR4 ligation can increase αvβ3 expression and aggressiveness of metastatic prostate cancer cells and disruption of CXCR4 can inhibit prostate cancer bone metastases[183–185].
It has been recently shown that β3 integrin activity on circulating CXCR4 positive bone marrow derived cells is important for their migration and recruitment to sites of angiogenesis. In mice with mutated tyrosine residues “knocked in” to the β3 integrin locus to inhibit proper phosphorylation (DiYF mice)[188], CXCR4 positive bone marrow derived cells were higher in number and defective in recruitment to subcutaneously implanted tumors or wounds, where SDF-1 levels were also lower[189]. These data demonstrate that β3 integrin on bone marrow derived cells may be critical for the CXCR4/SDF-1 gradient, and thus maybe important for localization of tumor cells to the bone microenvironment and also localization of myeloid/endothelial cells to tumors. Interestingly, CXCR4 deletion on bone marrow cells can enhance osteoclast activity which could counteract some of the beneficial effects of CXCR4 inhibition on bone metastases[9]. Integrins expressed by tumor cells, in concert with bone microenvironment chemokine secretion and further integrin activation, determines the osteotropic characteristics of metastasizing cancer cells and represent an ideal target for skeletal metastatic cancer therapy.
Integrins and myeloid/immune cell function during tumor growth in bone
Myeloid cell integrins are involved in tumor evasion from immune responses and tumor induced angiogenesis. Bone marrow derived myeloid cells (macrophages, monocytes, myeloid derived suppressor cells, myeloid dendritic cells) migrate to tumors and contribute to in tumor growth, invasion, and angiogenesis[190–194]. Macrophages within tumors are called tumor-associated-macrophages (TAM) [127], and are recruited by chemoattractants secreted by the tumor such as MCP-1[195] and then differentiate into tissue macrophages[196]. The anti-tumor M1 phenotype, represents a classical activation that is induced by pathogens, lipopolysaccharides (LPS) or interferon gamma resulting in secretion of proinflammatory cytokines such as tumor necrosis factor α (TNFα), interleukin 1β (IL-1 β) and others. M1 macrophages can act in an anti-tumor fashion by secretion of cytotoxic cytokines and antigen presentation to lymphocytes[197]. The pro-tumor M2 phenotype, represents alternative activation induced by IL-4 or IL-10[198]. M2 polarized macrophages, promote tumor cell proliferation and survival, suppress immune responses, and drive tumor neoangiogenesis[197, 199–201]. Studies have shown that the TAM content of tumors and prognosis of patients are inversely correlated[192, 202, 203].
β2 integrins are involved in monocyte/myeloid cell migration through endothelium and in phagocytosis, while β1 integrins mediate adhesion to matrix proteins and the induction of inflammatory genes[204]. α4β1 and αvβ3 integrins have been implicated in myeloid cell homing, adhesion, and migration to tumors. α4β1 promotes endothelial progenitor cells and monocyte homing and adhesion to sites of active pathological angiogenesis[205]. Inhibition of α4β1 leads to suppressed monocyte and macrophage colonization of tumors and associated vasculature and decreased angiogenesis[194].
The αvβ3 integrin is downregulated during differentiation of bone marrow myeloid progenitor cells to monocytes, but induced in macrophages during inflammation[206, 207]. αvβ3 promotes myeloid homing and adhesion and migration of bone marrow derived cells through the endothelium to sites of tumor angiogenesis[189]. β3 integrins are involved in phagocytosis of apoptotic cells[208, 209] and limit the secretion of inflammatory mediators[207]. Defective macrophage tumor infiltration is observed in TAM from β3 integrin−/− knockout bone marrow, myeloid specific β3KOM−/− mice and in the signaling defective DiYF β3 knock-in mice (knock-in mice with two mutated tyrosine residues) [111, 189, 210–213], suggesting that defective cytoskeletal (re)organization or lack of appropriately polarized macrophages[212] within tumors may be due to β3 integrin deficiency.
Myeloid derived suppressor cell (MDSC)[214] are a subpopulation of immature myeloid cells that are roughly characterized by GR1+ and by the αMβ2(CD11b) integrin adhesion marker[214]. The MDSC suppress T-cell antigen receptor mediated immune responses[190]. MDSC can promote TAM M2 polarizaiton[215] MDSC from myeloma bearing mice had a greater capacity to become bone resorbing cells compared to MDSC from control mice[191]. The role of integrins in MDSC differentiation, recruitment and function is underway. Thus, integrins are involved in monocyte/macrophage differentiation and recruitment to tumors and can influence local and metastatic tumor growth.
Integrins and tumor recruited platelets and bone metastasis
Cancer cells co-exist with platelets and mononuclear hematopoietic cells in thrombi located throughout the organs of patients with metastatic cancer[216], [217, 218]. Platelet aggregation and activation enhances tumor growth and metastasis to bone[77, 219]. Platelets are anuclear, metabolically active cells that are formed from bone marrow megakaryocytes. Platelet aggregation is stimulated by soluble factors such as ADP and thromboxane (TXA2), or membrane proteins collagen or von Willebrand factor that are produced by injured endothelial cells, inflammatory cells and tumor cells. αIIbβ3 plays a central role in the initiation of arterial thrombosis and platelet aggregation[220, 221]. αIIbβ3 integrins are expressed on the surface of megakaryocytes and platelets and are undetectable on any other non-cancerous cell type. Mice globally deficient for the β3 integrin have prolonged bleeding times, defects in platelet aggregation and clot retraction, cutaneous and gastrointestinal bleeding, all characteristics of Glanzmann’s thrombasthenia, [222] a disease characterize by functional reduction or absence of αIIb β3 in humans. Targeting β3 integrins by monoclonal antibodies to the receptor (abciximab/Reopro) or by inhibiting the binding of the ligand fibrinogen to the receptor (tirofiban/Integrilin) are used in patients with acute coronary and cerebral vascular syndromes but have significant bleeding risks that prevent their usefulness for chronic uses such as cancer.
Tumor cell lines have been shown to induce platelet aggregation and adhesion in vitro through mechanisms involving aIIbb3integrin, ADP, thrombin, von Willebrand factor, and selectins[77, 223–229]. The metastatic potential of tumor cell lines is markedly diminished in mice with defective platelet aggregation (β3 integrin−/−, Gaq−/−, Par4−/−, NFE2−/−, and fibrinogen−/−) [77, 219, 223, 226, 228–244]. 3−/− mice are protected from bone metastasis in part through a mechanism involving defective platelet aggregation[77]. Additionally, tumor cells engineered to respond to platelet-derived lysophosphatidic acid (LPA) have enhanced bone metastatic potential in mice[219]. Platelets also represent a significant source of proangiogenic (VEGF) and antiangiogenic factors (TSP-1) and are recruited to sites of tumor where their aggregation could affect local tumor growth[245]. Platelet specific integrin targeting is a promising therapeutic approach for inhibiting bone metastasis, especially to prevent or slow metastasis.
On the other hand, bone marrow megakaryocytes can inhibit prostate cancer tumor growth in bone[246]. Megakaryocytes can indirectly inhibit bone resorption by inhibiting osteoclast formation[247]. The negative effect of megakaryocytes on bone resorption is likely mediated in part through the osteoclast inhibitory factor, osteoprotegrin, that is contained in secretory granules of platelets and megakaryocytes[248, 249]. Adhesion of mature polyploid megakaryocytes to fibronectin is also mediated by β1 subunit containing integrins [250, 251]. Megakaryocytes may also influence bone remodeling and resorption through effects on osteoblast proliferation that are mediated by the α3β1, α5β1 and glycoprotein IIb integrins[252]. Given the location of mature megakaryocytes at vascular sinusoids, they are also among the first cells to physically encounter cancer cells as they enter the bone marrow, so that direct mechanism of action involving integrin mediated signal transduction could be involved. Interestingly, bisphosphonates (BP) increase megakaryocyte proliferation and increase the platelet concentration of the anti-angiogeneic integrin ligand TSP1[253–255] which suggest non-osteoclast mechanisms of bisphosphonates’ impact in decreasing tumor growth in bone. Thus, platelets and their megakaryocytic precursors interact with cancer cells before, during and after metastasis to bone through interactions mainly determined by integrins and their ligands.
Integrins and bone metastasis: Therapeutic aspects
Because of the wide range of functions in physiological and pathological processes, the integrin family of adhesion receptors has been adopted as a promising target for metastatic bone diseases. Several tumor cell types express an abnormal integrin profile compared to non-tumor cells [41, 51, 256], providing an opportunity for specific targeting. Targeting integrins on both tumor and/or host cells has proven to be effective not only in blocking local cancer progression, but also in reducing tumor cell detachment from their primary site in preclinical models[257–259].
In recent years, integrins on the tumor cells and/or on the endothelium have been targeted by monoclonal antibodies and RGD peptides in order to reduce tumor angiogenesis[109, 260]. Integrin antagonists, including humanized monoclonal antibodies, small molecule antagonists, and cyclic peptides, have been developed based on the recognition sequences of integrin physiological ligands[261]. Several compounds are already in clinical use or undergoing their clinical evaluation for various diseases.
For the future treatment of skeletal metastasis, the αvβ3 integrin has become an attractive target because of its expression in tumor and angiogenic cells, its role in OC differentiation and function, and its role in tumor cell homing to bone[53, 60, 61, 183, 262–267]. The multiple expected beneficial effects on endothelial, cancer, and osteoclastic cells instigated a significant effort to develop drug candidates that target the αvβ3 integrin for therapy of skeletal complications of cancer. These strategies resulted predominantly in antagonists of αvβ3, αvβ5, and αIIβ3 integrins that showed efficacy in animal models. Peptidomimentic antagonists of the αvβ3 and αvβ5 integrin were successfully used to inhibit OC in vitro and to reduce bone loss in a rat osteoporosis model [268]. An active nonpeptide αvβ3 integrin antagonist and anti-αvβ3 antibodies were shown to hinder cancer induced bone loss [79, 268–270]. It is possible that the current treatment for bone metastasis, bisphosphonates, may also exert an effect on αvβ3 on both endothelial cells[132] as well as OCs in a similar way.
Many drugs candidates targeting integrin αvβ3 have advanced to the clinics for the treatment of osteoporosis and cancer, though none have specifically targeted patients with bone metastases. A lipophilic isoester of RGD (L000845704), developed by Merck, is effective in increasing bone mineral density (BMD) in postmenopausal women[271]. Another inhibitor, RGD-mimetic cyclic peptide Cilengitide (EMD-1219974) directed at both αvβ3 and αvβ5[272] and currently produced by MerckSerono, is in advanced stages of clinical testing for the treatment of glioblastoma multiforme and is under investigation for the treatment of squamous cell carcinoma, prostate cancer, and lung cancer (Phase II).
Clinical trials of function-blocking antibodies are also ongoing, including Vitaxin (LM609), a humanized monoclonal IgG1 antibody against the extracellular domain of the αvβ3 integrin heterodimer. Vitaxin had substantial anti-angiogenic effects in preclinical models[119, 262] and has shown direct anti-tumor effects as well as impaired bone resorption by inhibiting OC attachment to the bone surface [273]. Another monoclonal antibody (CNTO95), directed against the αv subunit, is under development by Centocor and is in phase I–II testing for solid tumors. Two other additions to this therapeutic family are planned to be more specifically evaluated for their effects on bone metastasis [62], organic small molecule GLPG0187 [62] and peptide antagonist S247[257].
Given the participation of the osteoclasts, blood vessels and platelets in bone metastases, it may be beneficial to block both αvβ3 and αIIβ3 integrins on host cells. This concept of combination inhibition relies on the common RGD ligand binding domains of αvβ3, αvβ5, and αIIβ3. In fact, many of the synthetically designed αvβ3 integrin inhibitors display some selectivity towards αvβ5 integrin, and, in the case of Cilengitide, this dual antagonism is part of the mechanism to treat cancer by inhibiting neoangiogenesis as well as invasion[274, 275]. The strategy to combine multiple targets also bears some risks with regards to the desired high therapeutic specificity and low off-target toxicity. This issue is further complicated by the differential function of the integrins as determined by their location, expression level, activation status, and ligand binding. Studies in animal models and xenograft tumor models have demonstrated that low concentrations of αvβ3 integrin antagonists can act as integrin agonists[130, 276, 277]. Further research is necessary to identify optimal drug dosing and targeting that overcome the problem of generalized integrin inhibition to reduce or prevent skeletal metastasis.
Another area of active research in bone metastasis therapeutics is the specific targeting of integrins on hematopoietic stem cells or progenitors that prepare the metastatic niche and enhance bone marrow colonization by cancer cells which then instigate the vicious cycle of bone metastasis[278, 279]. Interfering with integrin mediated homing of cancer cells to the bone or to hijack the bone cells represents an early option for intervention. siRNA against the αv integrin subunit was used to prevent the progression of prostate cancer to bone by interfering with the ECM-integrin interaction[280]. In another approach, a disintegrin and a neutralizing antibody to VCAM-1 or its receptor α4β1 integrin reduced metastasis of melanoma cells and diminished osteolysis by decreasing osteoclast activity in a myeloma in vitro model[69, 281]. These strategies, however, are not yet in clinical trials. An exciting new approach to cancer therapy takes advantage of the fact that cancer cells use CXCR4 and VLA-4 to home to and “engraft” in the marrow. HSC mobilizing agents such as AMD3100 and anti-VLA-4 targeted agents can be used to mobilize leukemia and myeloma cells into the blood from the bone marrow which leads to increased sensitivity to chemotherapy [282–284] in mice. This approach is now being tried in clinical trials.
Future perspectives
Despite the high level of complexity of the integrin family, the β3 integrin remains a major target in the search for effective therapies for skeletal metastasis. In recent years, a steady increase in knowledge has led several interesting compounds into clinical testing. There remains, however, a lack of clarity concerning the exact roles of the integrins in different cell types. In the initiated clinical studies using αvβ3 integrin antagonists, the overall effect in reducing tumor growth and pathological angiogenesis in fast progressing deadly tumors may outweigh potential undesired effects in tissues or cells other than tumor or endothelial origin. Drugs designed to tackle skeletal complications of cancer must be “targeted” to the bone microenvironment as underscored by the clinical successes of the bone matrix targeted bisphosphonates and the osteoclast targeted denosumab in treating and preventing skeletal complications of bone metastases and myeloma. Because of the complexity of cells recruited in the tumor microenvironment and the pro-and anti-tumor effects of integrins depending on the cellular context, a detailed understanding of the role of integrin regulation in both the metastatic tumor cells and the tumor-associated stroma will allow for a more targeted and focused approach to treat bone metastases.
Acknowledgments
The authors sincerely thank Dr. Michael Tomasson for his help, guidance and critical reading of this manuscript. This work was supported by the NIH-NIHR0152152 to KNW and by the St. Louis Men’s group against cancer (SAH). JGS was supported by an IZKF start up grant from the University of Wuerzburg, Germany.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Lipton A. Pathophysiology of bone metastases: how this knowledge may lead to therapeutic intervention. J Support Oncol. 2004;2:205–13. discussion 213–4, 216–7, 219–20. [PubMed] [Google Scholar]
- 2.Coleman RE. Metastatic bone disease: clinical features, pathophysiology and treatment strategies. Cancer Treat Rev. 2001;27:165–76. doi: 10.1053/ctrv.2000.0210. [DOI] [PubMed] [Google Scholar]
- 3.Mundy GR. Metastasis to bone: causes, consequences and therapeutic opportunities. Nat Rev Cancer. 2002;2:584–93. doi: 10.1038/nrc867. [DOI] [PubMed] [Google Scholar]
- 4.Roodman GD. Mechanisms of bone metastasis. N Engl J Med. 2004;350:1655–64. doi: 10.1056/NEJMra030831. [DOI] [PubMed] [Google Scholar]
- 5.Lipton A, Costa L, Ali S, Demers L. Use of markers of bone turnover for monitoring bone metastases and the response to therapy. Semin Oncol. 2001;28:54–9. doi: 10.1016/s0093-7754(01)90233-7. [DOI] [PubMed] [Google Scholar]
- 6.Coleman RE, Major P, Lipton A, Brown JE, Lee KA, Smith M, Saad F, Zheng M, Hei YJ, Seaman J, Cook R. Predictive value of bone resorption and formation markers in cancer patients with bone metastases receiving the bisphosphonate zoledronic acid. J Clin Oncol. 2005;23:4925–35. doi: 10.1200/JCO.2005.06.091. [DOI] [PubMed] [Google Scholar]
- 7.Guise TA, Mohammad KS, Clines G, Stebbins EG, Wong DH, Higgins LS, Vessella R, Corey E, Padalecki S, Suva L, Chirgwin JM. Basic mechanisms responsible for osteolytic and osteoblastic bone metastases. Clin Cancer Res. 2006;12:6213s–6216s. doi: 10.1158/1078-0432.CCR-06-1007. [DOI] [PubMed] [Google Scholar]
- 8.Hirbe A, Morgan EA, Uluckan O, Weilbaecher K. Skeletal complications of breast cancer therapies. Clin Cancer Res. 2006;12:6309s–6314s. doi: 10.1158/1078-0432.CCR-06-0652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hirbe AC, Rubin J, Uluckan O, Morgan EA, Eagleton MC, Prior JL, Piwnica-Worms D, Weilbaecher KN. Disruption of CXCR4 enhances osteoclastogenesis and tumor growth in bone. Proc Natl Acad Sci U S A. 2007;104:14062–7. doi: 10.1073/pnas.0705203104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bendre MS, Montague DC, Peery T, Akel NS, Gaddy D, Suva LJ. Interleukin-8 stimulation of osteoclastogenesis and bone resorption is a mechanism for the increased osteolysis of metastatic bone disease. Bone. 2003;33:28–37. doi: 10.1016/s8756-3282(03)00086-3. [DOI] [PubMed] [Google Scholar]
- 11.Pfeilschifter J, D’Souza SM, Mundy GR. Effects of transforming growth factor-beta on osteoblastic osteosarcoma cells. Endocrinology. 1987;121:212–8. doi: 10.1210/endo-121-1-212. [DOI] [PubMed] [Google Scholar]
- 12.Kozlow W, Guise TA. Breast cancer metastasis to bone: mechanisms of osteolysis and implications for therapy. J Mammary Gland Biol Neoplasia. 2005;10:169–80. doi: 10.1007/s10911-005-5399-8. [DOI] [PubMed] [Google Scholar]
- 13.Kingsley LA, Fournier PG, Chirgwin JM, Guise TA. Molecular biology of bone metastasis. Mol Cancer Ther. 2007;6:2609–17. doi: 10.1158/1535-7163.MCT-07-0234. [DOI] [PubMed] [Google Scholar]
- 14.Clines GA, Guise TA. Molecular mechanisms and treatment of bone metastasis. Expert Rev Mol Med. 2008;10:e7. doi: 10.1017/S1462399408000616. [DOI] [PubMed] [Google Scholar]
- 15.Yin JJ, Selander K, Chirgwin JM, Dallas M, Grubbs BG, Wieser R, Massague J, Mundy GR, Guise TA. TGF-beta signaling blockade inhibits PTHrP secretion by breast cancer cells and bone metastases development. J Clin Invest. 1999;103:197–206. doi: 10.1172/JCI3523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kakonen SM, Selander KS, Chirgwin JM, Yin JJ, Burns S, Rankin WA, Grubbs BG, Dallas M, Cui Y, Guise TA. Transforming growth factor-beta stimulates parathyroid hormone-related protein and osteolytic metastases via Smad and mitogen-activated protein kinase signaling pathways. J Biol Chem. 2002;277:24571–8. doi: 10.1074/jbc.M202561200. [DOI] [PubMed] [Google Scholar]
- 17.Hamdy NA. Denosumab: RANKL inhibition in the management of bone loss. Drugs Today (Barc) 2008;44:7–21. doi: 10.1358/dot.2008.44.1.1178467. [DOI] [PubMed] [Google Scholar]
- 18.Body JJ, Lipton A, Gralow J, Steger GG, Gao G, Yeh H, Fizazi K. Effects of Denosumab in Patients with Bone Metastases, with and without Previous Bisphosphonate Exposure. J Bone Miner Res. 2009 doi: 10.1359/jbmr.090810. [DOI] [PubMed] [Google Scholar]
- 19.Fizazi K, Lipton A, Mariette X, Body JJ, Rahim Y, Gralow JR, Gao G, Wu L, Sohn W, Jun S. Randomized phase II trial of denosumab in patients with bone metastases from prostate cancer, breast cancer, or other neoplasms after intravenous bisphosphonates. J Clin Oncol. 2009;27:1564–71. doi: 10.1200/JCO.2008.19.2146. [DOI] [PubMed] [Google Scholar]
- 20.Schwartz MA, Schaller MD, Ginsberg MH. Integrins: emerging paradigms of signal transduction. Annu Rev Cell Dev Biol. 1995;11:549–99. doi: 10.1146/annurev.cb.11.110195.003001. [DOI] [PubMed] [Google Scholar]
- 21.Hynes RO. Integrins: versatility, modulation, and signaling in cell adhesion. Cell. 1992;69:11–25. doi: 10.1016/0092-8674(92)90115-s. [DOI] [PubMed] [Google Scholar]
- 22.Hynes RO. Integrins: bidirectional, allosteric signaling machines. Cell. 2002;110:673–87. doi: 10.1016/s0092-8674(02)00971-6. [DOI] [PubMed] [Google Scholar]
- 23.Shattil SJ, Kim C, Ginsberg MH. The final steps of integrin activation: the end game. Nat Rev Mol Cell Biol. 2010;11:288–300. doi: 10.1038/nrm2871. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Qin J, Vinogradova O, Plow EF. Integrin bidirectional signaling: a molecular view. PLoS Biol. 2004;2:e169. doi: 10.1371/journal.pbio.0020169. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Offermanns S. Activation of platelet function through G protein-coupled receptors. Circ Res. 2006;99:1293–304. doi: 10.1161/01.RES.0000251742.71301.16. [DOI] [PubMed] [Google Scholar]
- 26.Ma YQ, Qin J, Wu C, Plow EF. Kindlin-2 (Mig-2): a co-activator of beta3 integrins. J Cell Biol. 2008;181:439–46. doi: 10.1083/jcb.200710196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tadokoro S, Shattil SJ, Eto K, Tai V, Liddington RC, de Pereda JM, Ginsberg MH, Calderwood DA. Talin binding to integrin beta tails: a final common step in integrin activation. Science. 2003;302:103–6. doi: 10.1126/science.1086652. [DOI] [PubMed] [Google Scholar]
- 28.Vinogradova O, Velyvis A, Velyviene A, Hu B, Haas T, Plow E, Qin J. A structural mechanism of integrin alpha(IIb)beta(3) “inside-out” activation as regulated by its cytoplasmic face. Cell. 2002;110:587–97. doi: 10.1016/s0092-8674(02)00906-6. [DOI] [PubMed] [Google Scholar]
- 29.Harburger DS, Calderwood DA. Integrin signalling at a glance. J Cell Sci. 2009;122:159–63. doi: 10.1242/jcs.018093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Montanez E, Ussar S, Schifferer M, Bosl M, Zent R, Moser M, Fassler R. Kindlin-2 controls bidirectional signaling of integrins. Genes Dev. 2008;22:1325–30. doi: 10.1101/gad.469408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Moser M, Nieswandt B, Ussar S, Pozgajova M, Fassler R. Kindlin-3 is essential for integrin activation and platelet aggregation. Nat Med. 2008;14:325–30. doi: 10.1038/nm1722. [DOI] [PubMed] [Google Scholar]
- 32.Lai-Cheong JE, Parsons M, McGrath JA. The role of kindlins in cell biology and relevance to human disease. Int J Biochem Cell Biol. 2010;42:595–603. doi: 10.1016/j.biocel.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 33.Kamae T, Shiraga M, Kashiwagi H, Kato H, Tadokoro S, Kurata Y, Tomiyama Y, Kanakura Y. Critical role of ADP interaction with P2Y12 receptor in the maintenance of alpha(IIb)beta3 activation: association with Rap1B activation. J Thromb Haemost. 2006;4:1379–87. doi: 10.1111/j.1538-7836.2006.01941.x. [DOI] [PubMed] [Google Scholar]
- 34.Woulfe D, Jiang H, Mortensen R, Yang J, Brass LF. Activation of Rap1B by G(i) family members in platelets. J Biol Chem. 2002;277:23382–90. doi: 10.1074/jbc.M202212200. [DOI] [PubMed] [Google Scholar]
- 35.Chen YP, O’Toole TE, Ylanne J, Rosa JP, Ginsberg MH. A point mutation in the integrin beta 3 cytoplasmic domain (S752-->P) impairs bidirectional signaling through alpha IIb beta 3 (platelet glycoprotein IIb-IIIa) Blood. 1994;84:1857–65. [PubMed] [Google Scholar]
- 36.Hughes PE, Diaz-Gonzalez F, Leong L, Wu C, McDonald JA, Shattil SJ, Ginsberg MH. Breaking the integrin hinge. A defined structural constraint regulates integrin signaling. J Biol Chem. 1996;271:6571–4. doi: 10.1074/jbc.271.12.6571. [DOI] [PubMed] [Google Scholar]
- 37.O’Toole TE, Katagiri Y, Faull RJ, Peter K, Tamura R, Quaranta V, Loftus JC, Shattil SJ, Ginsberg MH. Integrin cytoplasmic domains mediate inside-out signal transduction. J Cell Biol. 1994;124:1047–59. doi: 10.1083/jcb.124.6.1047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Vinogradova O, Haas T, Plow EF, Qin J. A structural basis for integrin activation by the cytoplasmic tail of the alpha IIb-subunit. Proc Natl Acad Sci U S A. 2000;97:1450–5. doi: 10.1073/pnas.040548197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Vinogradova O, Vaynberg J, Kong X, Haas TA, Plow EF, Qin J. Membrane-mediated structural transitions at the cytoplasmic face during integrin activation. Proc Natl Acad Sci U S A. 2004;101:4094–9. doi: 10.1073/pnas.0400742101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ylanne J, Huuskonen J, O’Toole TE, Ginsberg MH, Virtanen I, Gahmberg CG. Mutation of the cytoplasmic domain of the integrin beta 3 subunit. Differential effects on cell spreading, recruitment to adhesion plaques, endocytosis, and phagocytosis. J Biol Chem. 1995;270:9550–7. doi: 10.1074/jbc.270.16.9550. [DOI] [PubMed] [Google Scholar]
- 41.Desgrosellier JS, Cheresh DA. Integrins in cancer: biological implications and therapeutic opportunities. Nat Rev Cancer. 10:9–22. doi: 10.1038/nrc2748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Brown EJ, Frazier WA. Integrin-associated protein (CD47) and its ligands. Trends Cell Biol. 2001;11:130–5. doi: 10.1016/s0962-8924(00)01906-1. [DOI] [PubMed] [Google Scholar]
- 43.Frisch SM, Screaton RA. Anoikis mechanisms. Curr Opin Cell Biol. 2001;13:555–62. doi: 10.1016/s0955-0674(00)00251-9. [DOI] [PubMed] [Google Scholar]
- 44.Bissell MJ, Radisky D. Putting tumours in context. Nat Rev Cancer. 2001;1:46–54. doi: 10.1038/35094059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Frisch SM, Vuori K, Ruoslahti E, Chan-Hui PY. Control of adhesion-dependent cell survival by focal adhesion kinase. J Cell Biol. 1996;134:793–9. doi: 10.1083/jcb.134.3.793. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Demers MJ, Thibodeau S, Noel D, Fujita N, Tsuruo T, Gauthier R, Arguin M, Vachon PH. Intestinal epithelial cancer cell anoikis resistance: EGFR-mediated sustained activation of Src overrides Fak-dependent signaling to MEK/Erk and/or PI3-K/Akt-1. J Cell Biochem. 2009;107:639–54. doi: 10.1002/jcb.22131. [DOI] [PubMed] [Google Scholar]
- 47.Shain KH, Landowski TH, Dalton WS. Adhesion-mediated intracellular redistribution of c-Fas-associated death domain-like IL-1-converting enzyme-like inhibitory protein-long confers resistance to CD95-induced apoptosis in hematopoietic cancer cell lines. J Immunol. 2002;168:2544–53. doi: 10.4049/jimmunol.168.5.2544. [DOI] [PubMed] [Google Scholar]
- 48.Simpson KJ, Selfors LM, Bui J, Reynolds A, Leake D, Khvorova A, Brugge JS. Identification of genes that regulate epithelial cell migration using an siRNA screening approach. Nat Cell Biol. 2008;10:1027–38. doi: 10.1038/ncb1762. [DOI] [PubMed] [Google Scholar]
- 49.Edlund M, Miyamoto T, Sikes RA, Ogle R, Laurie GW, Farach-Carson MC, Otey CA, Zhau HE, Chung LW. Integrin expression and usage by prostate cancer cell lines on laminin substrata. Cell Growth Differ. 2001;12:99–107. [PubMed] [Google Scholar]
- 50.Yoneda T. Cellular and molecular basis of preferential metastasis of breast cancer to bone. J Orthop Sci. 2000;5:75–81. doi: 10.1007/s007760050012. [DOI] [PubMed] [Google Scholar]
- 51.Clezardin P. Integrins in bone metastasis formation and potential therapeutic implications. Curr Cancer Drug Targets. 2009;9:801–6. doi: 10.2174/156800909789760348. [DOI] [PubMed] [Google Scholar]
- 52.van der P, Vloedgraven H, Papapoulos S, Lowick C, Grzesik W, Kerr J, Robey PG. Attachment characteristics and involvement of integrins in adhesion of breast cancer cell lines to extracellular bone matrix components. Lab Invest. 1997;77:665–75. [PubMed] [Google Scholar]
- 53.Liapis H, Flath A, Kitazawa S. Integrin alpha V beta 3 expression by bone-residing breast cancer metastases. Diagn Mol Pathol. 1996;5:127–35. doi: 10.1097/00019606-199606000-00008. [DOI] [PubMed] [Google Scholar]
- 54.McCabe NP, De S, Vasanji A, Brainard J, Byzova TV. Prostate cancer specific integrin alphavbeta3 modulates bone metastatic growth and tissue remodeling. Oncogene. 2007;26:6238–43. doi: 10.1038/sj.onc.1210429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Townsend PA, Villanova I, Uhlmann E, Peyman A, Knolle J, Baron R, Teti A, Horton MA. An antisense oligonucleotide targeting the alphaV integrin gene inhibits adhesion and induces apoptosis in breast cancer cells. Eur J Cancer. 2000;36:397–409. doi: 10.1016/s0959-8049(99)00275-0. [DOI] [PubMed] [Google Scholar]
- 56.Gillespie MT, Thomas RJ, Pu ZY, Zhou H, Martin TJ, Findlay DM. Calcitonin receptors, bone sialoprotein and osteopontin are expressed in primary breast cancers. Int J Cancer. 1997;73:812–5. doi: 10.1002/(sici)1097-0215(19971210)73:6<812::aid-ijc7>3.0.co;2-5. [DOI] [PubMed] [Google Scholar]
- 57.Van der Velde-Zimmermann D, Verdaasdonk MA, Rademakers LH, De Weger RA, Van den Tweel JG, Joling P. Fibronectin distribution in human bone marrow stroma: matrix assembly and tumor cell adhesion via alpha5 beta1 integrin. Exp Cell Res. 1997;230:111–20. doi: 10.1006/excr.1996.3405. [DOI] [PubMed] [Google Scholar]
- 58.Takayama S, Ishii S, Ikeda T, Masamura S, Doi M, Kitajima M. The relationship between bone metastasis from human breast cancer and integrin alpha(v)beta3 expression. Anticancer Res. 2005;25:79–83. [PubMed] [Google Scholar]
- 59.Nakamura I, Duong le T, Rodan SB, Rodan GA. Involvement of alpha(v)beta3 integrins in osteoclast function. J Bone Miner Metab. 2007;25:337–44. doi: 10.1007/s00774-007-0773-9. [DOI] [PubMed] [Google Scholar]
- 60.Pecheur I, Peyruchaud O, Serre CM, Guglielmi J, Voland C, Bourre F, Margue C, Cohen-Solal M, Buffet A, Kieffer N, Clezardin P. Integrin alpha(v)beta3 expression confers on tumor cells a greater propensity to metastasize to bone. FASEB J. 2002;16:1266–8. doi: 10.1096/fj.01-0911fje. [DOI] [PubMed] [Google Scholar]
- 61.Sloan EK, Pouliot N, Stanley KL, Chia J, Moseley JM, Hards DK, Anderson RL. Tumor-specific expression of alphavbeta3 integrin promotes spontaneous metastasis of breast cancer to bone. Breast Cancer Res. 2006;8:R20. doi: 10.1186/bcr1398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Zhao Y, Bachelier R, Treilleux I, Pujuguet P, Peyruchaud O, Baron R, Clement-Lacroix P, Clezardin P. Tumor alphavbeta3 integrin is a therapeutic target for breast cancer bone metastases. Cancer Res. 2007;67:5821–30. doi: 10.1158/0008-5472.CAN-06-4499. [DOI] [PubMed] [Google Scholar]
- 63.Martin-Thouvenin V, Gendron MC, Hogervorst F, Figdor CG, Lanotte M. Phorbol ester-induced promyelocytic leukemia cell adhesion to marrow stromal cells involves fibronectin specific alpha 5 beta 1 integrin receptors. J Cell Physiol. 1992;153:95–102. doi: 10.1002/jcp.1041530113. [DOI] [PubMed] [Google Scholar]
- 64.Korah R, Boots M, Wieder R. Integrin alpha5beta1 promotes survival of growth-arrested breast cancer cells: an in vitro paradigm for breast cancer dormancy in bone marrow. Cancer Res. 2004;64:4514–22. doi: 10.1158/0008-5472.CAN-03-3853. [DOI] [PubMed] [Google Scholar]
- 65.Liesveld JL, Dipersio JF, Abboud CN. Integrins and adhesive receptors in normal and leukemic CD34+ progenitor cells: potential regulatory checkpoints for cellular traffic. Leuk Lymphoma. 1994;14:19–28. doi: 10.3109/10428199409049647. [DOI] [PubMed] [Google Scholar]
- 66.Lang SH, Clarke NW, George NJ, Testa NG. Primary prostatic epithelial cell binding to human bone marrow stroma and the role of alpha2beta1 integrin. Clin Exp Metastasis. 1997;15:218–27. doi: 10.1023/a:1018465213641. [DOI] [PubMed] [Google Scholar]
- 67.Hall CL, Dai J, van Golen KL, Keller ET, Long MW. Type I collagen receptor (alpha 2 beta 1) signaling promotes the growth of human prostate cancer cells within the bone. Cancer Res. 2006;66:8648–54. doi: 10.1158/0008-5472.CAN-06-1544. [DOI] [PubMed] [Google Scholar]
- 68.Hall CL, Dubyk CW, Riesenberger TA, Shein D, Keller ET, van Golen KL. Type I collagen receptor (alpha2beta1) signaling promotes prostate cancer invasion through RhoC GTPase. Neoplasia. 2008;10:797–803. doi: 10.1593/neo.08380. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Mori Y, Shimizu N, Dallas M, Niewolna M, Story B, Williams PJ, Mundy GR, Yoneda T. Anti-alpha4 integrin antibody suppresses the development of multiple myeloma and associated osteoclastic osteolysis. Blood. 2004;104:2149–54. doi: 10.1182/blood-2004-01-0236. [DOI] [PubMed] [Google Scholar]
- 70.Michigami T, Shimizu N, Williams PJ, Niewolna M, Dallas SL, Mundy GR, Yoneda T. Cell-cell contact between marrow stromal cells and myeloma cells via VCAM-1 and alpha(4)beta(1)-integrin enhances production of osteoclast-stimulating activity. Blood. 2000;96:1953–60. [PubMed] [Google Scholar]
- 71.Matsuura N, Puzon-McLaughlin W, Irie A, Morikawa Y, Kakudo K, Takada Y. Induction of experimental bone metastasis in mice by transfection of integrin alpha 4 beta 1 into tumor cells. Am J Pathol. 1996;148:55–61. [PMC free article] [PubMed] [Google Scholar]
- 72.Teitelbaum SL, Ross FP. Genetic regulation of osteoclast development and function. Nat Rev Genet. 2003;4:638–49. doi: 10.1038/nrg1122. [DOI] [PubMed] [Google Scholar]
- 73.Ross FP, Teitelbaum SL. alphavbeta3 and macrophage colony-stimulating factor: partners in osteoclast biology. Immunol Rev. 2005;208:88–105. doi: 10.1111/j.0105-2896.2005.00331.x. [DOI] [PubMed] [Google Scholar]
- 74.Novack DV, Teitelbaum SL. The osteoclast: friend or foe? Annu Rev Pathol. 2008;3:457–84. doi: 10.1146/annurev.pathmechdis.3.121806.151431. [DOI] [PubMed] [Google Scholar]
- 75.Ross FP, Chappel J, Alvarez JI, Sander D, Butler WT, Farach-Carson MC, Mintz KA, Robey PG, Teitelbaum SL, Cheresh DA. Interactions between the bone matrix proteins osteopontin and bone sialoprotein and the osteoclast integrin alpha v beta 3 potentiate bone resorption. J Biol Chem. 1993;268:9901–7. [PubMed] [Google Scholar]
- 76.McHugh KP. Mice lacking b3 integrins are osteosclerotic because of dysfunctional osteoclasts. Journal of Clinical Investigation. 2000;105:433–440. doi: 10.1172/JCI8905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Bakewell SJ, Nestor P, Prasad S, Tomasson MH, Dowland N, Mehrotra M, Scarborough R, Kanter J, Abe K, Phillips D, Weilbaecher KN. Platelet and osteoclast beta3 integrins are critical for bone metastasis. Proc Natl Acad Sci U S A. 2003;100:14205–10. doi: 10.1073/pnas.2234372100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Zambonin Zallone A, Teti A, Gaboli M, Marchisio PC. Beta 3 subunit of vitronectin receptor is present in osteoclast adhesion structures and not in other monocyte-macrophage derived cells. Connect Tissue Res. 1989;20:143–9. doi: 10.3109/03008208909023882. [DOI] [PubMed] [Google Scholar]
- 79.Crippes BA, Engleman VW, Settle SL, Delarco J, Ornberg RL, Helfrich MH, Horton MA, Nickols GA. Antibody to beta3 integrin inhibits osteoclast-mediated bone resorption in the thyroparathyroidectomized rat. Endocrinology. 1996;137:918–24. doi: 10.1210/endo.137.3.8603604. [DOI] [PubMed] [Google Scholar]
- 80.Chellaiah MA. Regulation of podosomes by integrin alphavbeta3 and Rho GTPase-facilitated phosphoinositide signaling. Eur J Cell Biol. 2006;85:311–7. doi: 10.1016/j.ejcb.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 81.Faccio R, Takeshita S, Zallone A, Ross FP, Teitelbaum SL. c-Fms and the alphavbeta3 integrin collaborate during osteoclast differentiation. J Clin Invest. 2003;111:749–58. doi: 10.1172/JCI16924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Faccio R, Grano M, Colucci S, Zallone AZ, Quaranta V, Pelletier AJ. Activation of alphav beta3 integrin on human osteoclast-like cells stimulates adhesion and migration in response to osteopontin. Biochem Biophys Res Commun. 1998;249:522–5. doi: 10.1006/bbrc.1998.9180. [DOI] [PubMed] [Google Scholar]
- 83.Miyauchi A, Alvarez J, Greenfield EM, Teti A, Grano M, Colucci S, Zambonin-Zallone A, Ross FP, Teitelbaum SL, Cheresh D, et al. Recognition of osteopontin and related peptides by an alpha v beta 3 integrin stimulates immediate cell signals in osteoclasts. J Biol Chem. 1991;266:20369–74. [PubMed] [Google Scholar]
- 84.Rucci N, DiGiacinto C, Orru L, Millimaggi D, Baron R, Teti A. A novel protein kinase C alpha-dependent signal to ERK1/2 activated by alphaVbeta3 integrin in osteoclasts and in Chinese hamster ovary (CHO) cells. J Cell Sci. 2005;118:3263–75. doi: 10.1242/jcs.02436. [DOI] [PubMed] [Google Scholar]
- 85.Zhang Z, Baron R, Horne WC. Integrin engagement, the actin cytoskeleton, and c-Src are required for the calcitonin-induced tyrosine phosphorylation of paxillin and HEF1, but not for calcitonin-induced Erk1/2 phosphorylation. J Biol Chem. 2000;275:37219–23. doi: 10.1074/jbc.M001818200. [DOI] [PubMed] [Google Scholar]
- 86.Clohisy DR, Ramnaraine ML. Osteoclasts are required for bone tumors to grow and destroy bone. J Orthop Res. 1998;16:660–6. doi: 10.1002/jor.1100160606. [DOI] [PubMed] [Google Scholar]
- 87.Honore P, Luger NM, Sabino MA, Schwei MJ, Rogers SD, Mach DB, O’Keefe PF, Ramnaraine ML, Clohisy DR, Mantyh PW. Osteoprotegerin blocks bone cancer-induced skeletal destruction, skeletal pain and pain-related neurochemical reorganization of the spinal cord. Nat Med. 2000;6:521–8. doi: 10.1038/74999. [DOI] [PubMed] [Google Scholar]
- 88.Carlinfante G, Vassiliou D, Svensson O, Wendel M, Heinegard D, Andersson G. Differential expression of osteopontin and bone sialoprotein in bone metastasis of breast and prostate carcinoma. Clin Exp Metastasis. 2003;20:437–44. doi: 10.1023/a:1025419708343. [DOI] [PubMed] [Google Scholar]
- 89.Heinegard D, Andersson G, Reinholt FP. Roles of osteopontin in bone remodeling. Ann N Y Acad Sci. 1995;760:213–22. doi: 10.1111/j.1749-6632.1995.tb44632.x. [DOI] [PubMed] [Google Scholar]
- 90.Katayama Y, House CM, Udagawa N, Kazama JJ, McFarland RJ, Martin TJ, Findlay DM. Casein kinase 2 phosphorylation of recombinant rat osteopontin enhances adhesion of osteoclasts but not osteoblasts. J Cell Physiol. 1998;176:179–87. doi: 10.1002/(SICI)1097-4652(199807)176:1<179::AID-JCP19>3.0.CO;2-2. [DOI] [PubMed] [Google Scholar]
- 91.Hayashi C, Rittling S, Hayata T, Amagasa T, Denhardt D, Ezura Y, Nakashima K, Noda M. Serum osteopontin, an enhancer of tumor metastasis to bone, promotes B16 melanoma cell migration. J Cell Biochem. 2007;101:979–86. doi: 10.1002/jcb.21298. [DOI] [PubMed] [Google Scholar]
- 92.Denhardt DT, Chambers AF. Overcoming obstacles to metastasis --defenses against host defenses: osteopontin (OPN) as a shield against attack by cytotoxic host cells. J Cell Biochem. 1994;56:48–51. doi: 10.1002/jcb.240560109. [DOI] [PubMed] [Google Scholar]
- 93.Nemoto H, Rittling SR, Yoshitake H, Furuya K, Amagasa T, Tsuji K, Nifuji A, Denhardt DT, Noda M. Osteopontin deficiency reduces experimental tumor cell metastasis to bone and soft tissues. J Bone Miner Res. 2001;16:652–9. doi: 10.1359/jbmr.2001.16.4.652. [DOI] [PubMed] [Google Scholar]
- 94.Ohyama Y, Nemoto H, Rittling S, Tsuji K, Amagasa T, Denhardt DT, Nifuji A, Noda M. Osteopontin-deficiency suppresses growth of B16 melanoma cells implanted in bone and osteoclastogenesis in co-cultures. J Bone Miner Res. 2004;19:1706–11. doi: 10.1359/jbmr.2004.19.10.1706. [DOI] [PubMed] [Google Scholar]
- 95.Desai B, Rogers MJ, Chellaiah MA. Mechanisms of osteopontin and CD44 as metastatic principles in prostate cancer cells. Mol Cancer. 2007;6:18. doi: 10.1186/1476-4598-6-18. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Hullinger TG, Taichman RS, Linseman DA, Somerman MJ. Secretory products from PC-3 and MCF-7 tumor cell lines upregulate osteopontin in MC3T3-E1 cells. J Cell Biochem. 2000;78:607–16. doi: 10.1002/1097-4644(20000915)78:4<607::aid-jcb10>3.0.co;2-f. [DOI] [PubMed] [Google Scholar]
- 97.Abe M, Hiura K, Wilde J, Shioyasono A, Moriyama K, Hashimoto T, Kido S, Oshima T, Shibata H, Ozaki S, Inoue D, Matsumoto T. Osteoclasts enhance myeloma cell growth and survival via cell-cell contact: a vicious cycle between bone destruction and myeloma expansion. Blood. 2004;104:2484–91. doi: 10.1182/blood-2003-11-3839. [DOI] [PubMed] [Google Scholar]
- 98.Standal T, Borset M, Sundan A. Role of osteopontin in adhesion, migration, cell survival and bone remodeling. Exp Oncol. 2004;26:179–84. [PubMed] [Google Scholar]
- 99.Chen YJ, Wei YY, Chen HT, Fong YC, Hsu CJ, Tsai CH, Hsu HC, Liu SH, Tang CH. Osteopontin increases migration and MMP-9 up-regulation via alphavbeta3 integrin, FAK, ERK, and NF-kappaB-dependent pathway in human chondrosarcoma cells. J Cell Physiol. 2009;221:98–108. doi: 10.1002/jcp.21835. [DOI] [PubMed] [Google Scholar]
- 100.Lundberg P, Koskinen C, Baldock PA, Lothgren H, Stenberg A, Lerner UH, Oldenborg PA. Osteoclast formation is strongly reduced both in vivo and in vitro in the absence of CD47/SIRPalpha-interaction. Biochem Biophys Res Commun. 2007;352:444–8. doi: 10.1016/j.bbrc.2006.11.057. [DOI] [PubMed] [Google Scholar]
- 101.Uluckan O, Becker SN, Deng H, Zou W, Prior JL, Piwnica-Worms D, Frazier WA, Weilbaecher KN. CD47 regulates bone mass and tumor metastasis to bone. Cancer Res. 2009;69:3196–204. doi: 10.1158/0008-5472.CAN-08-3358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Han X, Sterling H, Chen Y, Saginario C, Brown EJ, Frazier WA, Lindberg FP, Vignery A. CD47, a ligand for the macrophage fusion receptor, participates in macrophage multinucleation. J Biol Chem. 2000;275:37984–92. doi: 10.1074/jbc.M002334200. [DOI] [PubMed] [Google Scholar]
- 103.Rachkovsky M, Sodi S, Chakraborty A, Avissar Y, Bolognia J, McNiff JM, Platt J, Bermudes D, Pawelek J. Melanoma × macrophage hybrids with enhanced metastatic potential. Clin Exp Metastasis. 1998;16:299–312. doi: 10.1023/a:1006557228604. [DOI] [PubMed] [Google Scholar]
- 104.Vignery A. Macrophage fusion: are somatic and cancer cells possible partners? Trends Cell Biol. 2005;15:188–93. doi: 10.1016/j.tcb.2005.02.008. [DOI] [PubMed] [Google Scholar]
- 105.Hanahan D, Weinberg RA. The hallmarks of cancer. Cell. 2000;100:57–70. doi: 10.1016/s0092-8674(00)81683-9. [DOI] [PubMed] [Google Scholar]
- 106.Stupack DG, Cheresh DA. Integrins and angiogenesis. Curr Top Dev Biol. 2004;64:207–38. doi: 10.1016/S0070-2153(04)64009-9. [DOI] [PubMed] [Google Scholar]
- 107.Hood JD, Cheresh DA. Role of integrins in cell invasion and migration. Nat Rev Cancer. 2002;2:91–100. doi: 10.1038/nrc727. [DOI] [PubMed] [Google Scholar]
- 108.Hodivala-Dilke K. alphavbeta3 integrin and angiogenesis: a moody integrin in a changing environment. Curr Opin Cell Biol. 2008;20:514–9. doi: 10.1016/j.ceb.2008.06.007. [DOI] [PubMed] [Google Scholar]
- 109.Silva R, D’Amico G, Hodivala-Dilke KM, Reynolds LE. Integrins: the keys to unlocking angiogenesis. Arterioscler Thromb Vasc Biol. 2008;28:1703–13. doi: 10.1161/ATVBAHA.108.172015. [DOI] [PubMed] [Google Scholar]
- 110.Stockmann C, Doedens A, Weidemann A, Zhang N, Takeda N, Greenberg JI, Cheresh DA, Johnson RS. Deletion of vascular endothelial growth factor in myeloid cells accelerates tumorigenesis. Nature. 2008;456:814–8. doi: 10.1038/nature07445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.Morgan EA, Schneider J, TB, Uluckan O, Heller EA, Hurchla MA, Deng H, Floyd DH, Berdy A, Prior JL, Piwnica-Worms D, Teitelbaum SL, FPR, Weilbaecher K. Dissection of platelet and myeloid cell defects by conditional targeting of the β3 integrin subunit. FASEB. 2009 doi: 10.1096/fj.09-138420. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Cackowski FC, Anderson JL, Patrene KD, Choksi RJ, Shapiro SD, Windle JJ, Blair HC, Roodman GD. Osteoclasts are important for bone angiogenesis. Blood. 115:140–9. doi: 10.1182/blood-2009-08-237628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Capoccia BJ, Shepherd RM, Link DC. G-CSF and AMD3100 mobilize monocytes into the blood that stimulate angiogenesis in vivo through a paracrine mechanism. Blood. 2006;108:2438–45. doi: 10.1182/blood-2006-04-013755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.De Palma M, Naldini L. Role of haematopoietic cells and endothelial progenitors in tumour angiogenesis. Biochim Biophys Acta. 2006;1766:159–66. doi: 10.1016/j.bbcan.2006.06.003. [DOI] [PubMed] [Google Scholar]
- 115.Papaspyridonos M, Lyden D. Chapter 11. The role of bone marrow-derived cells in tumor angiogenesis and metastatic progression. Methods Enzymol. 2008;444:255–69. doi: 10.1016/S0076-6879(08)02811-5. [DOI] [PubMed] [Google Scholar]
- 116.Ramjaun AR, Hodivala-Dilke K. The role of cell adhesion pathways in angiogenesis. Int J Biochem Cell Biol. 2009;41:521–30. doi: 10.1016/j.biocel.2008.05.030. [DOI] [PubMed] [Google Scholar]
- 117.Brooks PC, Clark RA, Cheresh DA. Requirement of vascular integrin alpha v beta 3 for angiogenesis. Science. 1994;264:569–71. doi: 10.1126/science.7512751. [DOI] [PubMed] [Google Scholar]
- 118.Mahabeleshwar GH, Byzova TV. Vascular integrin signaling. Methods Enzymol. 2008;443:199–226. doi: 10.1016/S0076-6879(08)02011-9. [DOI] [PubMed] [Google Scholar]
- 119.Brooks PC, Montgomery AM, Rosenfeld M, Reisfeld RA, Hu T, Klier G, Cheresh DA. Integrin alpha v beta 3 antagonists promote tumor regression by inducing apoptosis of angiogenic blood vessels. Cell. 1994;79:1157–64. doi: 10.1016/0092-8674(94)90007-8. [DOI] [PubMed] [Google Scholar]
- 120.Brooks PC, Silletti S, von Schalscha TL, Friedlander M, Cheresh DA. Disruption of angiogenesis by PEX, a noncatalytic metalloproteinase fragment with integrin binding activity. Cell. 1998;92:391–400. doi: 10.1016/s0092-8674(00)80931-9. [DOI] [PubMed] [Google Scholar]
- 121.Silletti S, Kessler T, Goldberg J, Boger DL, Cheresh DA. Disruption of matrix metalloproteinase 2 binding to integrin alpha vbeta 3 by an organic molecule inhibits angiogenesis and tumor growth in vivo. Proc Natl Acad Sci U S A. 2001;98:119–24. doi: 10.1073/pnas.011343298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Maeshima Y, Yerramalla UL, Dhanabal M, Holthaus KA, Barbashov S, Kharbanda S, Reimer C, Manfredi M, Dickerson WM, Kalluri R. Extracellular matrix-derived peptide binds to alpha(v)beta(3) integrin and inhibits angiogenesis. J Biol Chem. 2001;276:31959–68. doi: 10.1074/jbc.M103024200. [DOI] [PubMed] [Google Scholar]
- 123.Nemeth JA, Cher ML, Zhou Z, Mullins C, Bhagat S, Trikha M. Inhibition of alpha(v)beta3 integrin reduces angiogenesis, bone turnover, and tumor cell proliferation in experimental prostate cancer bone metastases. Clin Exp Metastasis. 2003;20:413–20. doi: 10.1023/a:1025461507027. [DOI] [PubMed] [Google Scholar]
- 124.Reynolds LE, Wyder L, Lively JC, Taverna D, Robinson SD, Huang X, Sheppard D, Hynes RO, Hodivala-Dilke KM. Enhanced pathological angiogenesis in mice lacking beta3 integrin or beta3 and beta5 integrins. Nat Med. 2002;8:27–34. doi: 10.1038/nm0102-27. [DOI] [PubMed] [Google Scholar]
- 125.Reynolds AR, Reynolds LE, Nagel TE, Lively JC, Robinson SD, Hicklin DJ, Bodary SC, Hodivala-Dilke KM. Elevated Flk1 (vascular endothelial growth factor receptor 2) signaling mediates enhanced angiogenesis in beta3-integrin-deficient mice. Cancer Res. 2004;64:8643–50. doi: 10.1158/0008-5472.CAN-04-2760. [DOI] [PubMed] [Google Scholar]
- 126.Carmeliet P. Integrin indecision. Nat Med. 2002;8:14–6. doi: 10.1038/nm0102-14. [DOI] [PubMed] [Google Scholar]
- 127.Martin KH, Slack JK, Boerner SA, Martin CC, Parsons JT. Integrin connections map: to infinity and beyond. Science. 2002;296:1652–3. doi: 10.1126/science.296.5573.1652. [DOI] [PubMed] [Google Scholar]
- 128.Wang XQ, Sun P, Paller AS. Inhibition of integrin-linked kinase/protein kinase B/Akt signaling: mechanism for ganglioside-induced apoptosis. J Biol Chem. 2001;276:44504–11. doi: 10.1074/jbc.M106563200. [DOI] [PubMed] [Google Scholar]
- 129.Zhao H, Ross FP, Teitelbaum SL. Unoccupied alpha(v)beta3 integrin regulates osteoclast apoptosis by transmitting a positive death signal. Mol Endocrinol. 2005;19:771–80. doi: 10.1210/me.2004-0161. [DOI] [PubMed] [Google Scholar]
- 130.Reynolds AR, Hart IR, Watson AR, Welti JC, Silva RG, Robinson SD, Da Violante G, Gourlaouen M, Salih M, Jones MC, Jones DT, Saunders G, Kostourou V, Perron-Sierra F, Norman JC, Tucker GC, Hodivala-Dilke KM. Stimulation of tumor growth and angiogenesis by low concentrations of RGD-mimetic integrin inhibitors. Nat Med. 2009;15:392–400. doi: 10.1038/nm.1941. [DOI] [PubMed] [Google Scholar]
- 131.Friedlander M, Brooks PC, Shaffer RW, Kincaid CM, Varner JA, Cheresh DA. Definition of two angiogenic pathways by distinct alpha v integrins. Science. 1995;270:1500–2. doi: 10.1126/science.270.5241.1500. [DOI] [PubMed] [Google Scholar]
- 132.Bellahcene A, Chaplet M, Bonjean K, Castronovo V. Zoledronate inhibits alphavbeta3 and alphavbeta5 integrin cell surface expression in endothelial cells. Endothelium. 2007;14:123–30. doi: 10.1080/10623320701347187. [DOI] [PubMed] [Google Scholar]
- 133.Hirbe AC, Roelofs AJ, Floyd DH, Deng H, Becker SN, Lanigan LG, Apicelli AJ, Xu Z, Prior JL, Eagleton MC, Piwnica-Worms D, Rogers MJ, Weilbaecher K. The bisphosphonate zoledronic acid decreases tumor growth in bone in mice with defective osteoclasts. Bone. 2009;44:908–16. doi: 10.1016/j.bone.2009.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 134.Ottewell PD, Monkkonen H, Jones M, Lefley DV, Coleman RE, Holen I. Antitumor effects of doxorubicin followed by zoledronic acid in a mouse model of breast cancer. J Natl Cancer Inst. 2008;100:1167–78. doi: 10.1093/jnci/djn240. [DOI] [PubMed] [Google Scholar]
- 135.Gao L, Deng H, Zhao H, Hirbe A, Harding J, Ratner L, Weilbaecher K. HTLV-1 Tax transgenic mice develop spontaneous osteolytic bone metastases prevented by osteoclast inhibition. Blood. 2005;106:4294–302. doi: 10.1182/blood-2005-04-1730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 136.Coleman RE, Guise TA, Lipton A, Roodman GD, Berenson JR, Body JJ, Boyce BF, Calvi LM, Hadji P, McCloskey EV, Saad F, Smith MR, Suva LJ, Taichman RS, Vessella RL, Weilbaecher KN. Advancing treatment for metastatic bone cancer: consensus recommendations from the Second Cambridge Conference. Clin Cancer Res. 2008;14:6387–95. doi: 10.1158/1078-0432.CCR-08-1572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Gnant M, Mlineritsch B, Schippinger W, Luschin-Ebengreuth G, Postlberger S, Menzel C, Jakesz R, Seifert M, Hubalek M, Bjelic-Radisic V, Samonigg H, Tausch C, Eidtmann H, Steger G, Kwasny W, Dubsky P, Fridrik M, Fitzal F, Stierer M, Rucklinger E, Greil R, Marth C. Endocrine therapy plus zoledronic acid in premenopausal breast cancer. N Engl J Med. 2009;360:679–91. doi: 10.1056/NEJMoa0806285. [DOI] [PubMed] [Google Scholar]
- 138.Aft R, Naughton M, Trinkaus K, Watson M, Ylagan L, Chavez-MacGregor M, Zhai J, Kuo S, Shannon W, Diemer K, Herrmann V, Dietz J, Ali A, Ellis M, Weiss P, Eberlein T, Ma C, Fracasso PM, Zoberi I, Taylor M, Gillanders W, Pluard T, Mortimer J, Weilbaecher K. Effect of zoledronic acid on disseminated tumour cells in women with locally advanced breast cancer: an open label, randomised, phase 2 trial. Lancet Oncol. 11:421–8. doi: 10.1016/S1470-2045(10)70054-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 139.Gnant M. The evolving role of zoledronic acid in early breast cancer. Onco Targets Ther. 2009;2:95–104. doi: 10.2147/ott.s4082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 140.Tanjore H, Zeisberg EM, Gerami-Naini B, Kalluri R. Beta1 integrin expression on endothelial cells is required for angiogenesis but not for vasculogenesis. Dev Dyn. 2008;237:75–82. doi: 10.1002/dvdy.21385. [DOI] [PubMed] [Google Scholar]
- 141.Senger DR, Claffey KP, Benes JE, Perruzzi CA, Sergiou AP, Detmar M. Angiogenesis promoted by vascular endothelial growth factor: regulation through alpha1beta1 and alpha2beta1 integrins. Proc Natl Acad Sci U S A. 1997;94:13612–7. doi: 10.1073/pnas.94.25.13612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 142.Pozzi A, Moberg PE, Miles LA, Wagner S, Soloway P, Gardner HA. Elevated matrix metalloprotease and angiostatin levels in integrin alpha 1 knockout mice cause reduced tumor vascularization. Proc Natl Acad Sci U S A. 2000;97:2202–7. doi: 10.1073/pnas.040378497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 143.Kim S, Bell K, Mousa SA, Varner JA. Regulation of angiogenesis in vivo by ligation of integrin alpha5beta1 with the central cell-binding domain of fibronectin. Am J Pathol. 2000;156:1345–62. doi: 10.1016/s0002-9440(10)65005-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 144.Boudreau NJ, Varner JA. The homeobox transcription factor Hox D3 promotes integrin alpha5beta1 expression and function during angiogenesis. J Biol Chem. 2004;279:4862–8. doi: 10.1074/jbc.M305190200. [DOI] [PubMed] [Google Scholar]
- 145.Garmy-Susini B, Jin H, Zhu Y, Sung RJ, Hwang R, Varner J. Integrin alpha4beta1-VCAM-1-mediated adhesion between endothelial and mural cells is required for blood vessel maturation. J Clin Invest. 2005;115:1542–51. doi: 10.1172/JCI23445. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 146.Nikolopoulos SN, Blaikie P, Yoshioka T, Guo W, Giancotti FG. Integrin beta4 signaling promotes tumor angiogenesis. Cancer Cell. 2004;6:471–83. doi: 10.1016/j.ccr.2004.09.029. [DOI] [PubMed] [Google Scholar]
- 147.Chung J, Bachelder RE, Lipscomb EA, Shaw LM, Mercurio AM. Integrin (alpha 6 beta 4) regulation of eIF-4E activity and VEGF translation: a survival mechanism for carcinoma cells. J Cell Biol. 2002;158:165–74. doi: 10.1083/jcb.200112015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 148.Rodriguez-Manzaneque JC, Lane TF, Ortega MA, Hynes RO, Lawler J, Iruela-Arispe ML. Thrombospondin-1 suppresses spontaneous tumor growth and inhibits activation of matrix metalloproteinase-9 and mobilization of vascular endothelial growth factor. Proc Natl Acad Sci U S A. 2001;98:12485–90. doi: 10.1073/pnas.171460498. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 149.John AS, Rothman VL, Tuszynski GP. Thrombospondin-1 (TSP-1) Stimulates Expression of Integrin alpha6 in Human Breast Carcinoma Cells: A Downstream Modulator of TSP-1-Induced Cellular Adhesion. J Oncol. 2010;2010:645376. doi: 10.1155/2010/645376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Eliceiri BP, Cheresh DA. Adhesion events in angiogenesis. Curr Opin Cell Biol. 2001;13:563–8. doi: 10.1016/s0955-0674(00)00252-0. [DOI] [PubMed] [Google Scholar]
- 151.Calvi LM, Adams GB, Weibrecht KW, Weber JM, Olson DP, Knight MC, Martin RP, Schipani E, Divieti P, Bringhurst FR, Milner LA, Kronenberg HM, Scadden DT. Osteoblastic cells regulate the haematopoietic stem cell niche. Nature. 2003;425:841–6. doi: 10.1038/nature02040. [DOI] [PubMed] [Google Scholar]
- 152.Brenner S, Whiting-Theobald N, Kawai T, Linton GF, Rudikoff AG, Choi U, Ryser MF, Murphy PM, Sechler JM, Malech HL. CXCR4-transgene expression significantly improves marrow engraftment of cultured hematopoietic stem cells. Stem Cells. 2004;22:1128–33. doi: 10.1634/stemcells.2003-0196. [DOI] [PubMed] [Google Scholar]
- 153.Kahn J, Byk T, Jansson-Sjostrand L, Petit I, Shivtiel S, Nagler A, Hardan I, Deutsch V, Gazit Z, Gazit D, Karlsson S, Lapidot T. Overexpression of CXCR4 on human CD34+ progenitors increases their proliferation, migration, and NOD/SCID repopulation. Blood. 2004;103:2942–9. doi: 10.1182/blood-2003-07-2607. [DOI] [PubMed] [Google Scholar]
- 154.Zhang J, Niu C, Ye L, Huang H, He X, Tong WG, Ross J, Haug J, Johnson T, Feng JQ, Harris S, Wiedemann LM, Mishina Y, Li L. Identification of the haematopoietic stem cell niche and control of the niche size. Nature. 2003;425:836–41. doi: 10.1038/nature02041. [DOI] [PubMed] [Google Scholar]
- 155.Papayannopoulou T. Mechanisms of stem-/progenitor-cell mobilization: the anti-VLA-4 paradigm. Semin Hematol. 2000;37:11–8. doi: 10.1016/s0037-1963(00)90084-2. [DOI] [PubMed] [Google Scholar]
- 156.Hidalgo A, Peired AJ, Weiss LA, Katayama Y, Frenette PS. The integrin alphaMbeta2 anchors hematopoietic progenitors in the bone marrow during enforced mobilization. Blood. 2004;104:993–1001. doi: 10.1182/blood-2003-10-3702. [DOI] [PubMed] [Google Scholar]
- 157.Stier S, Ko Y, Forkert R, Lutz C, Neuhaus T, Grunewald E, Cheng T, Dombkowski D, Calvi LM, Rittling SR, Scadden DT. Osteopontin is a hematopoietic stem cell niche component that negatively regulates stem cell pool size. J Exp Med. 2005;201:1781–91. doi: 10.1084/jem.20041992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Christopher MJ, Liu F, Hilton MJ, Long F, Link DC. Suppression of CXCL12 production by bone marrow osteoblasts is a common and critical pathway for cytokine-induced mobilization. Blood. 2009;114:1331–9. doi: 10.1182/blood-2008-10-184754. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 159.Adams GB, Martin RP, Alley IR, Chabner KT, Cohen KS, Calvi LM, Kronenberg HM, Scadden DT. Therapeutic targeting of a stem cell niche. Nat Biotechnol. 2007;25:238–43. doi: 10.1038/nbt1281. [DOI] [PubMed] [Google Scholar]
- 160.Mendez-Ferrer S, Frenette PS. Hematopoietic stem cell trafficking: regulated adhesion and attraction to bone marrow microenvironment. Ann N Y Acad Sci. 2007;1116:392–413. doi: 10.1196/annals.1402.086. [DOI] [PubMed] [Google Scholar]
- 161.Eash KJ, Means JM, White DW, Link DC. CXCR4 is a key regulator of neutrophil release from the bone marrow under basal and stress granulopoiesis conditions. Blood. 2009;113:4711–9. doi: 10.1182/blood-2008-09-177287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Kollet O, Dar A, Shivtiel S, Kalinkovich A, Lapid K, Sztainberg Y, Tesio M, Samstein RM, Goichberg P, Spiegel A, Elson A, Lapidot T. Osteoclasts degrade endosteal components and promote mobilization of hematopoietic progenitor cells. Nat Med. 2006;12:657–64. doi: 10.1038/nm1417. [DOI] [PubMed] [Google Scholar]
- 163.Bungartz G, Stiller S, Bauer M, Muller W, Schippers A, Wagner N, Fassler R, Brakebusch C. Adult murine hematopoiesis can proceed without beta1 and beta7 integrins. Blood. 2006;108:1857–64. doi: 10.1182/blood-2005-10-007658. [DOI] [PubMed] [Google Scholar]
- 164.Jiang Y, Prosper F, Verfaillie CM. Opposing effects of engagement of integrins and stimulation of cytokine receptors on cell cycle progression of normal human hematopoietic progenitors. Blood. 2000;95:846–54. [PubMed] [Google Scholar]
- 165.Voura EB, Billia F, Iscove NN, Hawley RG. Expression mapping of adhesion receptor genes during differentiation of individual hematopoietic precursors. Exp Hematol. 1997;25:1172–9. [PubMed] [Google Scholar]
- 166.Hemler ME, Lobb RR. The leukocyte beta 1 integrins. Curr Opin Hematol. 1995;2:61–7. doi: 10.1097/00062752-199502010-00009. [DOI] [PubMed] [Google Scholar]
- 167.Coulombel L, Auffray I, Gaugler MH, Rosemblatt M. Expression and function of integrins on hematopoietic progenitor cells. Acta Haematol. 1997;97:13–21. doi: 10.1159/000203655. [DOI] [PubMed] [Google Scholar]
- 168.Verfaillie CM, McCarthy JB, McGlave PB. Differentiation of primitive human multipotent hematopoietic progenitors into single lineage clonogenic progenitors is accompanied by alterations in their interaction with fibronectin. J Exp Med. 1991;174:693–703. doi: 10.1084/jem.174.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 169.Oostendorp RA, Reisbach G, Spitzer E, Thalmeier K, Dienemann H, Mergenthaler HG, Dormer P. VLA-4 and VCAM-1 are the principal adhesion molecules involved in the interaction between blast colony-forming cells and bone marrow stromal cells. Br J Haematol. 1995;91:275–84. doi: 10.1111/j.1365-2141.1995.tb05290.x. [DOI] [PubMed] [Google Scholar]
- 170.Liesveld JL, Winslow JM, Frediani KE, Ryan DH, Abboud CN. Expression of integrins and examination of their adhesive function in normal and leukemic hematopoietic cells. Blood. 1993;81:112–21. [PubMed] [Google Scholar]
- 171.Qian H, Tryggvason K, Jacobsen SE, Ekblom M. Contribution of alpha6 integrins to hematopoietic stem and progenitor cell homing to bone marrow and collaboration with alpha4 integrins. Blood. 2006;107:3503–10. doi: 10.1182/blood-2005-10-3932. [DOI] [PubMed] [Google Scholar]
- 172.Schreiber TD, Steinl C, Essl M, Abele H, Geiger K, Muller CA, Aicher WK, Klein G. The integrin alpha9beta1 on hematopoietic stem and progenitor cells: involvement in cell adhesion, proliferation and differentiation. Haematologica. 2009;94:1493–501. doi: 10.3324/haematol.2009.006072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 173.Yin T, Li L. The stem cell niches in bone. J Clin Invest. 2006;116:1195–201. doi: 10.1172/JCI28568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Kaplan RN, Riba RD, Zacharoulis S, Bramley AH, Vincent L, Costa C, MacDonald DD, Jin DK, Shido K, Kerns SA, Zhu Z, Hicklin D, Wu Y, Port JL, Altorki N, Port ER, Ruggero D, Shmelkov SV, Jensen KK, Rafii S, Lyden D. VEGFR1-positive haematopoietic bone marrow progenitors initiate the pre-metastatic niche. Nature. 2005;438:820–7. doi: 10.1038/nature04186. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Chavakis E, Aicher A, Heeschen C, Sasaki K, Kaiser R, El Makhfi N, Urbich C, Peters T, Scharffetter-Kochanek K, Zeiher AM, Chavakis T, Dimmeler S. Role of beta2-integrins for homing and neovascularization capacity of endothelial progenitor cells. J Exp Med. 2005;201:63–72. doi: 10.1084/jem.20041402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176.McAllister SS, Gifford AM, Greiner AL, Kelleher SP, Saelzler MP, Ince TA, Reinhardt F, Harris LN, Hylander BL, Repasky EA, Weinberg RA. Systemic endocrine instigation of indolent tumor growth requires osteopontin. Cell. 2008;133:994–1005. doi: 10.1016/j.cell.2008.04.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 177.Lyden D, Hattori K, Dias S, Costa C, Blaikie P, Butros L, Chadburn A, Heissig B, Marks W, Witte L, Wu Y, Hicklin D, Zhu Z, Hackett NR, Crystal RG, Moore MA, Hajjar KA, Manova K, Benezra R, Rafii S. Impaired recruitment of bone-marrow-derived endothelial and hematopoietic precursor cells blocks tumor angiogenesis and growth. Nat Med. 2001;7:1194–201. doi: 10.1038/nm1101-1194. [DOI] [PubMed] [Google Scholar]
- 178.Pazolli E, Luo X, Brehm S, Carbery K, Chung JJ, Prior JL, Doherty J, Demehri S, Salavaggione L, Piwnica-Worms D, Stewart SA. Senescent stromal-derived osteopontin promotes preneoplastic cell growth. Cancer Res. 2009;69:1230–9. doi: 10.1158/0008-5472.CAN-08-2970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 179.Wei SC, Tsao PN, Yu SC, Shun CT, Tsai-Wu JJ, Wu CH, Su YN, Hsieh FJ, Wong JM. Placenta growth factor expression is correlated with survival of patients with colorectal cancer. Gut. 2005;54:666–72. doi: 10.1136/gut.2004.050831. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Marcellini M, De Luca N, Riccioni T, Ciucci A, Orecchia A, Lacal PM, Ruffini F, Pesce M, Cianfarani F, Zambruno G, Orlandi A, Failla CM. Increased melanoma growth and metastasis spreading in mice overexpressing placenta growth factor. Am J Pathol. 2006;169:643–54. doi: 10.2353/ajpath.2006.051041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 181.Kang Y, Siegel PM, Shu W, Drobnjak M, Kakonen SM, Cordon-Cardo C, Guise TA, Massague J. A multigenic program mediating breast cancer metastasis to bone. Cancer Cell. 2003;3:537–49. doi: 10.1016/s1535-6108(03)00132-6. [DOI] [PubMed] [Google Scholar]
- 182.Muller A, Homey B, Soto H, Ge N, Catron D, Buchanan ME, McClanahan T, Murphy E, Yuan W, Wagner SN, Barrera JL, Mohar A, Verastegui E, Zlotnik A. Involvement of chemokine receptors in breast cancer metastasis. Nature. 2001;410:50–6. doi: 10.1038/35065016. [DOI] [PubMed] [Google Scholar]
- 183.Sun YX, Fang M, Wang J, Cooper CR, Pienta KJ, Taichman RS. Expression and activation of alpha(v)beta(3) integrins by SDF-1/CXC12 increases the aggressiveness of prostate cancer cells. Prostate. 2007;67:61–73. doi: 10.1002/pros.20500. [DOI] [PubMed] [Google Scholar]
- 184.Sun YX, Wang J, Shelburne CE, Lopatin DE, Chinnaiyan AM, Rubin MA, Pienta KJ, Taichman RS. Expression of CXCR4 and CXCL12 (SDF-1) in human prostate cancers (PCa) in vivo. J Cell Biochem. 2003;89:462–73. doi: 10.1002/jcb.10522. [DOI] [PubMed] [Google Scholar]
- 185.Sun YX, Schneider A, Jung Y, Wang J, Dai J, Cook K, Osman NI, Koh-Paige AJ, Shim H, Pienta KJ, Keller ET, McCauley LK, Taichman RS. Skeletal localization and neutralization of the SDF-1(CXCL12)/CXCR4 axis blocks prostate cancer metastasis and growth in osseous sites in vivo. J Bone Miner Res. 2005;20:318–29. doi: 10.1359/JBMR.041109. [DOI] [PubMed] [Google Scholar]
- 186.Smith MC, Luker KE, Garbow JR, Prior JL, Jackson E, Piwnica-Worms D, Luker GD. CXCR4 regulates growth of both primary and metastatic breast cancer. Cancer Res. 2004;64:8604–12. doi: 10.1158/0008-5472.CAN-04-1844. [DOI] [PubMed] [Google Scholar]
- 187.Parmo-Cabanas M, Bartolome RA, Wright N, Hidalgo A, Drager AM, Teixido J. Integrin alpha4beta1 involvement in stromal cell-derived factor-1alpha-promoted myeloma cell transendothelial migration and adhesion: role of cAMP and the actin cytoskeleton in adhesion. Exp Cell Res. 2004;294:571–80. doi: 10.1016/j.yexcr.2003.12.003. [DOI] [PubMed] [Google Scholar]
- 188.Mahabeleshwar GH, Feng W, Phillips DR, Byzova TV. Integrin signaling is critical for pathological angiogenesis. J Exp Med. 2006;203:2495–507. doi: 10.1084/jem.20060807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 189.Feng W, McCabe NP, Mahabeleshwar GH, Somanath PR, Phillips DR, Byzova TV. The angiogenic response is dictated by beta3 integrin on bone marrow-derived cells. J CellBiol. 2008;183:1145–57. doi: 10.1083/jcb.200802179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 190.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–74. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 191.Yang L, Edwards CM, Mundy GR. Gr-1+CD11b+ myeloid-derived suppressor cells: formidable partners in tumor metastasis. J Bone Miner Res. 25:1701–6. doi: 10.1002/jbmr.154. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 192.Bingle L, Brown NJ, Lewis CE. The role of tumour-associated macrophages in tumour progression: implications for new anticancer therapies. J Pathol. 2002;196:254–65. doi: 10.1002/path.1027. [DOI] [PubMed] [Google Scholar]
- 193.Dirkx AE, Oude Egbrink MG, Wagstaff J, Griffioen AW. Monocyte/macrophage infiltration in tumors: modulators of angiogenesis. J Leukoc Biol. 2006;80:1183–96. doi: 10.1189/jlb.0905495. [DOI] [PubMed] [Google Scholar]
- 194.Jin H, Su J, Garmy-Susini B, Kleeman J, Varner J. Integrin alpha4beta1 promotes monocyte trafficking and angiogenesis in tumors. Cancer Res. 2006;66:2146–52. doi: 10.1158/0008-5472.CAN-05-2704. [DOI] [PubMed] [Google Scholar]
- 195.Hume DA. The mononuclear phagocyte system. Curr Opin Immunol. 2006;18:49–53. doi: 10.1016/j.coi.2005.11.008. [DOI] [PubMed] [Google Scholar]
- 196.Sweet MJ, Hume DA. CSF-1 as a regulator of macrophage activation and immune responses. Arch Immunol Ther Exp (Warsz) 2003;51:169–77. [PubMed] [Google Scholar]
- 197.Sica A, Allavena P, Mantovani A. Cancer related inflammation: the macrophage connection. Cancer Lett. 2008;267:204–15. doi: 10.1016/j.canlet.2008.03.028. [DOI] [PubMed] [Google Scholar]
- 198.Gordon S, Taylor PR. Monocyte and macrophage heterogeneity. Nat Rev Immunol. 2005;5:953–64. doi: 10.1038/nri1733. [DOI] [PubMed] [Google Scholar]
- 199.Martinez FO, Sica A, Mantovani A, Locati M. Macrophage activation and polarization. Front Biosci. 2008;13:453–61. doi: 10.2741/2692. [DOI] [PubMed] [Google Scholar]
- 200.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–55. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 201.Chen ZG, Bottazzi B, Wang JM, Mantovani A. Tumor-associated macrophages in metastasizing tumors. Adv Exp Med Biol. 1988;233:61–71. doi: 10.1007/978-1-4899-5037-6_8. [DOI] [PubMed] [Google Scholar]
- 202.Chambers SK, Kacinski BM, Ivins CM, Carcangiu ML. Overexpression of epithelial macrophage colony-stimulating factor (CSF-1) and CSF-1 receptor: a poor prognostic factor in epithelial ovarian cancer, contrasted with a protective effect of stromal CSF-1. Clin Cancer Res. 1997;3:999–1007. [PubMed] [Google Scholar]
- 203.Canioni D, Salles G, Mounier N, Brousse N, Keuppens M, Morchhauser F, Lamy T, Sonet A, Rousselet MC, Foussard C, Xerri L. High numbers of tumor-associated macrophages have an adverse prognostic value that can be circumvented by rituximab in patients with follicular lymphoma enrolled onto the GELA-GOELAMS FL-2000 trial. J Clin Oncol. 2008;26:440–6. doi: 10.1200/JCO.2007.12.8298. [DOI] [PubMed] [Google Scholar]
- 204.Reyes-Reyes M, Mora N, Gonzalez G, Rosales C. beta1 and beta2 integrins activate different signalling pathways in monocytes. Biochem J. 2002;363:273–80. doi: 10.1042/0264-6021:3630273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 205.Jin H, Aiyer A, Su J, Borgstrom P, Stupack D, Friedlander M, Varner J. A homing mechanism for bone marrow-derived progenitor cell recruitment to the neovasculature. J Clin Invest. 2006;116:652–62. doi: 10.1172/JCI24751. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Sato T, Nakai T, Tamura N, Okamoto S, Matsuoka K, Sakuraba A, Fukushima T, Uede T, Hibi T. Osteopontin/Eta-1 upregulated in Crohn’s disease regulates the Th1 immune response. Gut. 2005;54:1254–62. doi: 10.1136/gut.2004.048298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 207.Schneider JG, Zhu Y, Coleman T, Semenkovich CF. Macrophage beta3 integrin suppresses hyperlipidemia-induced inflammation by modulating TNFalpha expression. Arterioscler Thromb Vasc Biol. 2007;27:2699–706. doi: 10.1161/ATVBAHA.107.153650. [DOI] [PubMed] [Google Scholar]
- 208.Savill J, Dransfield I, Hogg N, Haslett C. Vitronectin receptor-mediated phagocytosis of cells undergoing apoptosis. Nature. 1990;343:170–3. doi: 10.1038/343170a0. [DOI] [PubMed] [Google Scholar]
- 209.Savill J, Hogg N, Ren Y, Haslett C. Thrombospondin cooperates with CD36 and the vitronectin receptor in macrophage recognition of neutrophils undergoing apoptosis. J Clin Invest. 1992;90:1513–22. doi: 10.1172/JCI116019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 210.Law DA, DeGuzman FR, Heiser P, Ministri-Madrid K, Killeen N, Phillips DR. Integrin cytoplasmic tyrosine motif is required for outside-in alphaIIbbeta3 signalling and platelet function. Nature. 1999;401:808–11. doi: 10.1038/44599. [DOI] [PubMed] [Google Scholar]
- 211.Taverna D, Moher H, Crowley D, Borsig L, Varki A, Hynes RO. Increased primary tumor growth in mice null for beta3- or beta3/beta5-integrins or selectins. Proc Natl Acad Sci U S A. 2004;101:763–8. doi: 10.1073/pnas.0307289101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 212.Kanamori M, Kawaguchi T, Berger MS, Pieper RO. Intracranial microenvironment reveals independent opposing functions of host alphaVbeta3 expression on glioma growthand angiogenesis. J Biol Chem. 2006;281:37256–64. doi: 10.1074/jbc.M605344200. [DOI] [PubMed] [Google Scholar]
- 213.Galarneau H, Villeneuve J, Gowing G, Julien JP, Vallieres L. Increased glioma growth in mice depleted of macrophages. Cancer Res. 2007;67:8874–81. doi: 10.1158/0008-5472.CAN-07-0177. [DOI] [PubMed] [Google Scholar]
- 214.Gabrilovich DI, Bronte V, Chen SH, Colombo MP, Ochoa A, Ostrand-Rosenberg S, Schreiber H. The terminology issue for myeloid-derived suppressor cells. Cancer Res. 2007;67:425. doi: 10.1158/0008-5472.CAN-06-3037. author reply 426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 215.Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S. Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol. 2007;179:977–83. doi: 10.4049/jimmunol.179.2.977. [DOI] [PubMed] [Google Scholar]
- 216.Honn KV, Tang DG, Crissman JD. Platelets and cancer metastasis: a causal relationship? Cancer Metastasis Rev. 1992;11:325–51. doi: 10.1007/BF01307186. [DOI] [PubMed] [Google Scholar]
- 217.Billroth T. Pathology and therapeutics, in fifty lectures. Clin Orthop. 2003;1871:4–11. doi: 10.1097/00003086-200303000-00002. [DOI] [PubMed] [Google Scholar]
- 218.Gouin-Thibault I, Achkar A, Samama MM. The thrombophilic state in cancer patients. Acta Haematol. 2001;106:33–42. doi: 10.1159/000046587. [DOI] [PubMed] [Google Scholar]
- 219.Boucharaba A, Serre CM, Gres S, Saulnier-Blache JS, Bordet JC, Guglielmi J, Clezardin P, Peyruchaud O. Platelet-derived lysophosphatidic acid supports the progression of osteolytic bone metastases in breast cancer. J Clin Invest. 2004;114:1714–25. doi: 10.1172/JCI22123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 220.Phillips DR, Charo IF, Scarborough RM. GPIIb-IIIa: the responsive integrin. Cell. 1991;65:359–62. doi: 10.1016/0092-8674(91)90451-4. [DOI] [PubMed] [Google Scholar]
- 221.Smyth SS, Reis ED, Vaananen H, Zhang W, Coller BS. Variable protection of beta 3-integrin--deficient mice from thrombosis initiated by different mechanisms. Blood. 2001;98:1055–62. doi: 10.1182/blood.v98.4.1055. [DOI] [PubMed] [Google Scholar]
- 222.Hodivala-Dilke KM, McHugh KP, Tsakiris DA, Rayburn H, Crowley D, Ullman-Cullere M, Ross FP, Coller BS, Teitelbaum S, Hynes RO. Beta3-integrin-deficient mice are a model for Glanzmann thrombasthenia showing placental defects and reduced survival. J Clin Invest. 1999;103:229–38. doi: 10.1172/JCI5487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 223.Jurasz P, Stewart MW, Radomski A, Khadour F, Duszyk M, Radomski MW. Role of von Willebrand factor in tumour cell-induced platelet aggregation: differential regulation by NO and prostacyclin. Br J Pharmacol. 2001;134:1104–12. doi: 10.1038/sj.bjp.0704343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 224.Karpatkin S. Role of thrombin in tumor angiogenesis, implantation, and metastasis. Pathophysiol Haemost Thromb. 2003;33 (Suppl 1):54–5. doi: 10.1159/000073294. [DOI] [PubMed] [Google Scholar]
- 225.Nierodzik ML, Karpatkin S. Thrombin induces tumor growth, metastasis, and angiogenesis: Evidence for a thrombin-regulated dormant tumor phenotype. Cancer Cell. 2006;10:355–62. doi: 10.1016/j.ccr.2006.10.002. [DOI] [PubMed] [Google Scholar]
- 226.Nierodzik ML, Plotkin A, Kajumo F, Karpatkin S. Thrombin stimulates tumor-platelet adhesion in vitro and metastasis in vivo. J Clin Invest. 1991;87:229–36. doi: 10.1172/JCI114976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 227.Gasic GJ, Gasic TB, Stewart CC. Antimetastatic effects associated with platelet reduction. Proceedings of National Academy of Sciences, USA. 1968;61:46–52. doi: 10.1073/pnas.61.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.Izumi Y, Taniuchi Y, Tsuji T, Smith CW, Nakamori S, Fidler IJ, Irimura T. Characterization of human colon carcinoma variant cells selected for sialyl Lex carbohydrate antigen: liver colonization and adhesion to vascular endothelial cells. Exp Cell Res. 1995;216:215–21. doi: 10.1006/excr.1995.1027. [DOI] [PubMed] [Google Scholar]
- 229.Kim YJ, Borsig L, Varki NM, Varki A. P-selectin deficiency attenuates tumor growth and metastasis. Proc Natl Acad Sci U S A. 1998;95:9325–30. doi: 10.1073/pnas.95.16.9325. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 230.Karpatkin S, Pearlstein E, Ambrogio C, Coller BS. Role of adhesive proteins in platelet tumor interaction in vitro and metastasis formation in vivo. J Clin Invest. 1988;81:1012–9. doi: 10.1172/JCI113411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 231.Lerner WA, Pearlstein E, Ambrogio C, Karpatkin S. A new mechanism for tumor induced platelet aggregation. Comparison with mechanisms shared by other tumor with possible pharmacologic strategy toward prevention of metastases. Int J Cancer. 1983;31:463–9. doi: 10.1002/ijc.2910310411. [DOI] [PubMed] [Google Scholar]
- 232.Nierodzik ML, Bain RM, Liu LX, Shivji M, Takeshita K, Karpatkin S. Presence of the seven transmembrane thrombin receptor on human tumour cells: effect of activation on tumour adhesion to platelets and tumor tyrosine phosphorylation. Br J Haematol. 1996;92:452–7. doi: 10.1046/j.1365-2141.1996.d01-1494.x. [DOI] [PubMed] [Google Scholar]
- 233.Mannori G, Crottet P, Cecconi O, Hanasaki K, Aruffo A, Nelson RM, Varki A, Bevilacqua MP. Differential colon cancer cell adhesion to E-, P-, and L-selectin: role of mucin-type glycoproteins. Cancer Res. 1995;55:4425–31. [PubMed] [Google Scholar]
- 234.Bromberg ME, Bailly MA, Konigsberg WH. Role of protease-activated receptor 1 in tumor metastasis promoted by tissue factor. Thromb Haemost. 2001;86:1210–4. [PubMed] [Google Scholar]
- 235.Camerer E, Qazi AA, Duong DN, Cornelissen I, Advincula R, Coughlin SR. Platelets, protease-activated receptors, and fibrinogen in hematogenous metastasis. Blood. 2004;104:397–401. doi: 10.1182/blood-2004-02-0434. [DOI] [PubMed] [Google Scholar]
- 236.Francis JL, Amirkhosravi A. Effect of antihemostatic agents on experimental tumor dissemination. Semin Thromb Hemost. 2002;28:29–38. doi: 10.1055/s-2002-20562. [DOI] [PubMed] [Google Scholar]
- 237.Hu L, Lee M, Campbell W, Perez-Solar R, Karpatkin S. Role of Endogenous Thrombin in Tumor Implantation, Seeding and Spontaneous Metastasis. Blood. 2004 doi: 10.1182/blood-2004-03-1047. [DOI] [PubMed] [Google Scholar]
- 238.Palumbo JS, Talmage KE, Massari JV, La Jeunesse CM, Flick MJ, Kombrinck KW, Jirouskova M, Degen JL. Platelets and fibrin(ogen) increase metastatic potential by impeding natural killer cell-mediated elimination of tumor cells. Blood. 2005;105:178–85. doi: 10.1182/blood-2004-06-2272. [DOI] [PubMed] [Google Scholar]
- 239.Pearlstein E, Ambrogio C, Karpatkin S. Effect of antiplatelet antibody on the development of pulmonary metastases following injection of CT26 colon adenocarcinoma, Lewis lung carcinoma, and B16 amelanotic melanoma tumor cells into mice. Cancer Res. 1984;44:3884–7. [PubMed] [Google Scholar]
- 240.Rickles FR, Patierno S, Fernandez PM. Tissue factor, thrombin, and cancer. Chest. 2003;124:58S–68S. doi: 10.1378/chest.124.3_suppl.58s. [DOI] [PubMed] [Google Scholar]
- 241.Savage B, Almus-Jacobs F, Ruggeri ZM. Specific synergy of multiple substrate-receptor interactions in platelet thrombus formation under flow. Cell. 1998;94:657–66. doi: 10.1016/s0092-8674(00)81607-4. [DOI] [PubMed] [Google Scholar]
- 242.Shi X, Gangadharan B, Brass LF, Ruf W, Mueller BM. Protease-activated receptors (PAR1 and PAR2) contribute to tumor cell motility and metastasis. Mol Cancer Res. 2004;2:395–402. [PubMed] [Google Scholar]
- 243.Amirkhosravi A, Amaya M, Siddiqui FA. Blockade of GpIIb/IIIa inhibits the release of vascular endothelial growth factor (VEGF) from tumor cell-activated platelets and experimental metastasis. Platelets. 1999;10:285–292. doi: 10.1080/09537109975915. [DOI] [PubMed] [Google Scholar]
- 244.Amirkhosravi A, Meyer T, Chang JY, Amaya M, Siddiqui F, Desai H, Francis JL. Tissue factor pathway inhibitor reduces experimental lung metastasis of B16 melanoma. Thromb Haemost. 2002;87:930–6. [PubMed] [Google Scholar]
- 245.Rafii DC, Psaila B, Butler J, Jin DK, Lyden D. Regulation of vasculogenesis by platelet-mediated recruitment of bone marrow-derived cells. Arterioscler Thromb Vasc Biol. 2008;28:217–22. doi: 10.1161/ATVBAHA.107.151159. [DOI] [PubMed] [Google Scholar]
- 246.Li X, Koh AJ, Wang Z, Soki FN, Park SI, Pienta KJ, McCauley LK. Inhibitory effects of megakaryocytic cells in prostate cancer skeletal metastasis. J Bone Miner Res. doi: 10.1002/jbmr.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Beeton CA, Bord S, Ireland D, Compston JE. Osteoclast formation and bone resorption are inhibited by megakaryocytes. Bone. 2006;39:985–90. doi: 10.1016/j.bone.2006.06.004. [DOI] [PubMed] [Google Scholar]
- 248.Grundt A, Grafe IA, Liegibel U, Sommer U, Nawroth P, Kasperk C. Direct effects of osteoprotegerin on human bone cell metabolism. Biochem Biophys Res Commun. 2009;389:550–5. doi: 10.1016/j.bbrc.2009.09.026. [DOI] [PubMed] [Google Scholar]
- 249.Chollet ME, Brouland JP, Bal dit Sollier C, Bauduer F, Drouet L, Bellucci S. Evidence of a colocalisation of osteoprotegerin (OPG) with von Willebrand factor (VWF) in platelets and megakaryocytes alpha granules. Studies from normal and grey platelets. Br J Haematol. 148:805–7. doi: 10.1111/j.1365-2141.2009.07989.x. [DOI] [PubMed] [Google Scholar]
- 250.Leven RM, Tablin F. Extracellular matrix stimulation of guinea pig megakaryocyte proplatelet formation in vitro is mediated through the vitronectin receptor. Exp Hematol. 1992;20:1316–22. [PubMed] [Google Scholar]
- 251.Schmitz B, Thiele J, Otto F, Farahmand P, Henze F, Frimpong S, Wickenhauser C, Fischer R. Evidence for integrin receptor involvement in megakaryocyte-fibroblast interaction: a possible pathomechanism for the evolution of myelofibrosis. J Cell Physiol. 1998;176:445–55. doi: 10.1002/(SICI)1097-4652(199809)176:3<445::AID-JCP1>3.0.CO;2-O. [DOI] [PubMed] [Google Scholar]
- 252.Lemieux JM, Horowitz MC, Kacena MA. Involvement of integrins alpha(3)beta(1) and alpha(5)beta(1) and glycoprotein IIb in megakaryocyte-induced osteoblast proliferation. J Cell Biochem. 109:927–32. doi: 10.1002/jcb.22468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 253.Escudero ND, Lacave M, Ubios AM, Mandalunis PM. Effect of monosodium olpadronate on osteoclasts and megakaryocytes: an in vivo study. J Musculoskelet Neuronal Interact. 2009;9:109–20. [PubMed] [Google Scholar]
- 254.Bernasconi S, Matteucci C, Sironi M, Conni M, Colotta F, Mosca M, Colombo N, Bonazzi C, Landoni F, Corbetta G, et al. Effects of granulocyte-monocyte colony-stimulating factor (GM-CSF) on expression of adhesion molecules and production of cytokines in blood monocytes and ovarian cancer-associated macrophages. Int J Cancer. 1995;60:300–7. doi: 10.1002/ijc.2910600304. [DOI] [PubMed] [Google Scholar]
- 255.Zaslavsky A, Baek KH, Lynch RC, Short S, Grillo J, Folkman J, Italiano JE, Jr, Ryeom S. Platelet-derived thrombospondin-1 is a critical negative regulator and potential biomarker of angiogenesis. Blood. 115:4605–13. doi: 10.1182/blood-2009-09-242065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 256.Mizejewski GJ. Role of integrins in cancer: survey of expression patterns. Proc Soc Exp Biol Med. 1999;222:124–38. doi: 10.1177/153537029922200203. [DOI] [PubMed] [Google Scholar]
- 257.Khalili P, Arakelian A, Chen G, Plunkett ML, Beck I, Parry GC, Donate F, Shaw DE, Mazar AP, Rabbani SA. A non-RGD-based integrin binding peptide (ATN-161) blocks breast cancer growth and metastasis in vivo. Mol Cancer Ther. 2006;5:2271–80. doi: 10.1158/1535-7163.MCT-06-0100. [DOI] [PubMed] [Google Scholar]
- 258.Trikha M, De Clerck YA, Markland FS. Contortrostatin, a snake venom disintegrin, inhibits beta 1 integrin-mediated human metastatic melanoma cell adhesion and blocks experimental metastasis. Cancer Res. 1994;54:4993–8. [PubMed] [Google Scholar]
- 259.Ramos OH, Kauskot A, Cominetti MR, Bechyne I, Salla Pontes CL, Chareyre F, Manent J, Vassy R, Giovannini M, Legrand C, Selistre-de-Araujo HS, Crepin M, Bonnefoy A. A novel alpha(v)beta (3)-blocking disintegrin containing the RGD motive, DisBa-01, inhibits bFGF-induced angiogenesis and melanoma metastasis. Clin Exp Metastasis. 2008;25:53–64. doi: 10.1007/s10585-007-9101-y. [DOI] [PubMed] [Google Scholar]
- 260.Avraamides CJ, Garmy-Susini B, Varner JA. Integrins in angiogenesis and lymphangiogenesis. Nat Rev Cancer. 2008;8:604–17. doi: 10.1038/nrc2353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 261.Fujii H, Komazawa H, Mori H, Kojima M, Itoh I, Murata J, Azuma I, Saiki I. Antimetastatic activities of synthetic Arg-Gly-Asp-Ser (RGDS) and Arg-Leu-Asp-Ser (RLDS) peptide analogues and their inhibitory mechanisms. Biol Pharm Bull. 1995;18:1681–8. doi: 10.1248/bpb.18.1681. [DOI] [PubMed] [Google Scholar]
- 262.Brooks PC, Stromblad S, Klemke R, Visscher D, Sarkar FH, Cheresh DA. Antiintegrin alpha v beta 3 blocks human breast cancer growth and angiogenesis in human skin. J Clin Invest. 1995;96:1815–22. doi: 10.1172/JCI118227. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 263.Vaillant F, Asselin-Labat ML, Shackleton M, Forrest NC, Lindeman GJ, Visvader JE. The mammary progenitor marker CD61/beta3 integrin identifies cancer stem cells in mouse models of mammary tumorigenesis. Cancer Res. 2008;68:7711–7. doi: 10.1158/0008-5472.CAN-08-1949. [DOI] [PubMed] [Google Scholar]
- 264.Feng X, Novack DV, Faccio R, Ory DS, Aya K, Boyer MI, McHugh KP, Ross FP, Teitelbaum SL. A Glanzmann’s mutation in beta 3 integrin specifically impairs osteoclast function. J Clin Invest. 2001;107:1137–44. doi: 10.1172/JCI12040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 265.Putz E, Witter K, Offner S, Stosiek P, Zippelius A, Johnson J, Zahn R, Riethmuller G, Pantel K. Phenotypic characteristics of cell lines derived from disseminated cancer cells in bone marrow of patients with solid epithelial tumors: establishment of working models for human micrometastases. Cancer Res. 1999;59:241–8. [PubMed] [Google Scholar]
- 266.Felding-Habermann B, O’Toole TE, Smith JW, Fransvea E, Ruggeri ZM, Ginsberg MH, Hughes PE, Pampori N, Shattil SJ, Saven A, Mueller BM. Integrin activation controls metastasis in human breast cancer. Proc Natl Acad Sci U S A. 2001;98:1853–8. doi: 10.1073/pnas.98.4.1853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 267.Teti A, Migliaccio S, Baron R. The role of the alphaVbeta3 integrin in the development of osteolytic bone metastases: a pharmacological target for alternative therapy? Calcif Tissue Int. 2002;71:293–9. doi: 10.1007/s00223-001-2071-1. [DOI] [PubMed] [Google Scholar]
- 268.Engelman VW. A peptidomimetic antagonists of the avb3 integrin inhibits bone resorption in vitro and prevents osteoporosis in vivo. Journal of Clinical Investigation. 1997;99:2284–2292. doi: 10.1172/JCI119404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 269.Harms JF, Welch DR, Samant RS, Shevde LA, Miele ME, Babu GR, Goldberg SF, Gilman VR, Sosnowski DM, Campo DA, Gay CV, Budgeon LR, Mercer R, Jewell J, Mastro AM, Donahue HJ, Erin N, Debies MT, Meehan WJ, Jones AL, Mbalaviele G, Nickols A, Christensen ND, Melly R, Beck LN, Kent J, Rader RK, Kotyk JJ, Pagel MD, Westlin WF, Griggs DW. A small molecule antagonist of the alpha(v)beta3 integrin suppresses MDA-MB-435 skeletal metastasis. Clin Exp Metastasis. 2004;21:119–28. doi: 10.1023/b:clin.0000024763.69809.64. [DOI] [PubMed] [Google Scholar]
- 270.Nemeth JA, Nakada MT, Trikha M, Lang Z, Gordon MS, Jayson GC, Corringham R, Prabhakar U, Davis HM, Beckman RA. Alpha-v integrins as therapeutic targets in oncology. Cancer Invest. 2007;25:632–46. doi: 10.1080/07357900701522638. [DOI] [PubMed] [Google Scholar]
- 271.Murphy MG, Cerchio K, Stoch SA, Gottesdiener K, Wu M, Recker R. Effect of L-000845704, an alphaVbeta3 integrin antagonist, on markers of bone turnover and bone mineral density in postmenopausal osteoporotic women. J Clin Endocrinol Metab. 2005;90:2022–8. doi: 10.1210/jc.2004-2126. [DOI] [PubMed] [Google Scholar]
- 272.Smith JW, Ruggeri ZM, Kunicki TJ, Cheresh DA. Interaction of integrins alpha v beta 3 and glycoprotein IIb-IIIa with fibrinogen. Differential peptide recognition accounts for distinct binding sites. J Biol Chem. 1990;265:12267–71. [PubMed] [Google Scholar]
- 273.Gramoun A, Shorey S, Bashutski JD, Dixon SJ, Sims SM, Heersche JN, Manolson MF. Effects of Vitaxin, a novel therapeutic in trial for metastatic bone tumors, on osteoclast functions in vitro. J Cell Biochem. 2007;102:341–52. doi: 10.1002/jcb.21296. [DOI] [PubMed] [Google Scholar]
- 274.Hariharan S, Gustafson D, Holden S, McConkey D, Davis D, Morrow M, Basche M, Gore L, Zang C, O’Bryant CL, Baron A, Gallemann D, Colevas D, Eckhardt SG. Assessment of the biological and pharmacological effects of the alpha nu beta3 and alpha nu beta5 integrin receptor antagonist, cilengitide (EMD 121974), in patients with advanced solid tumors. Ann Oncol. 2007;18:1400–7. doi: 10.1093/annonc/mdm140. [DOI] [PubMed] [Google Scholar]
- 275.Oliveira-Ferrer L, Hauschild J, Fiedler W, Bokemeyer C, Nippgen J, Celik I, Schuch G. Cilengitide induces cellular detachment and apoptosis in endothelial and glioma cells mediated by inhibition of FAK/src/AKT pathway. J Exp Clin Cancer Res. 2008;27:86. doi: 10.1186/1756-9966-27-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 276.Legler DF, Wiedle G, Ross FP, Imhof BA. Superactivation of integrin alphavbeta3 by low antagonist concentrations. J Cell Sci. 2001;114:1545–53. doi: 10.1242/jcs.114.8.1545. [DOI] [PubMed] [Google Scholar]
- 277.Alghisi GC, Ponsonnet L, Ruegg C. The integrin antagonist cilengitide activates alphaVbeta3, disrupts VE-cadherin localization at cell junctions and enhances permeability in endothelial cells. PLoS One. 2009;4:e4449. doi: 10.1371/journal.pone.0004449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 278.Guo W, Giancotti FG. Integrin signalling during tumour progression. Nat Rev Mol Cell Biol. 2004;5:816–26. doi: 10.1038/nrm1490. [DOI] [PubMed] [Google Scholar]
- 279.Sterling JA, Edwards JR, Martin TJ, Mundy GR. Advances in the biology of bone metastasis: How the skeleton affects tumor behavior. Bone. doi: 10.1016/j.bone.2010.07.015. [DOI] [PubMed] [Google Scholar]
- 280.Bisanz K, Yu J, Edlund M, Spohn B, Hung MC, Chung LW, Hsieh CL. Targeting ECM-integrin interaction with liposome-encapsulated small interfering RNAs inhibits the growth of human prostate cancer in a bone xenograft imaging model. Mol Ther. 2005;12:634–43. doi: 10.1016/j.ymthe.2005.05.012. [DOI] [PubMed] [Google Scholar]
- 281.Danen EH, Marcinkiewicz C, Cornelissen IM, van Kraats AA, Pachter JA, Ruiter DJ, Niewiarowski S, van Muijen GN. The disintegrin eristostatin interferes with integrin alpha 4 beta 1 function and with experimental metastasis of human melanoma cells. Exp Cell Res. 1998;238:188–96. doi: 10.1006/excr.1997.3821. [DOI] [PubMed] [Google Scholar]
- 282.Nervi B, Ramirez P, Rettig MP, Uy GL, Holt MS, Ritchey JK, Prior JL, Piwnica-Worms D, Bridger G, Ley TJ, DiPersio JF. Chemosensitization of acute myeloid leukemia (AML) following mobilization by the CXCR4 antagonist AMD3100. Blood. 2009;113:6206–14. doi: 10.1182/blood-2008-06-162123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 283.Lane SW, Scadden DT, Gilliland DG. The leukemic stem cell niche: current concepts and therapeutic opportunities. Blood. 2009;114:1150–7. doi: 10.1182/blood-2009-01-202606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 284.Azab AK, Runnels JM, Pitsillides C, Moreau AS, Azab F, Leleu X, Jia X, Wright R, Ospina B, Carlson AL, Alt C, Burwick N, Roccaro AM, Ngo HT, Farag M, Melhem MR, Sacco A, Munshi NC, Hideshima T, Rollins BJ, Anderson KC, Kung AL, Lin CP, Ghobrial IM. CXCR4 inhibitor AMD3100 disrupts the interaction of multiple myeloma cells with the bone marrow microenvironment and enhances their sensitivity to therapy. Blood. 2009;113:4341–51. doi: 10.1182/blood-2008-10-186668. [DOI] [PMC free article] [PubMed] [Google Scholar]


