Abstract
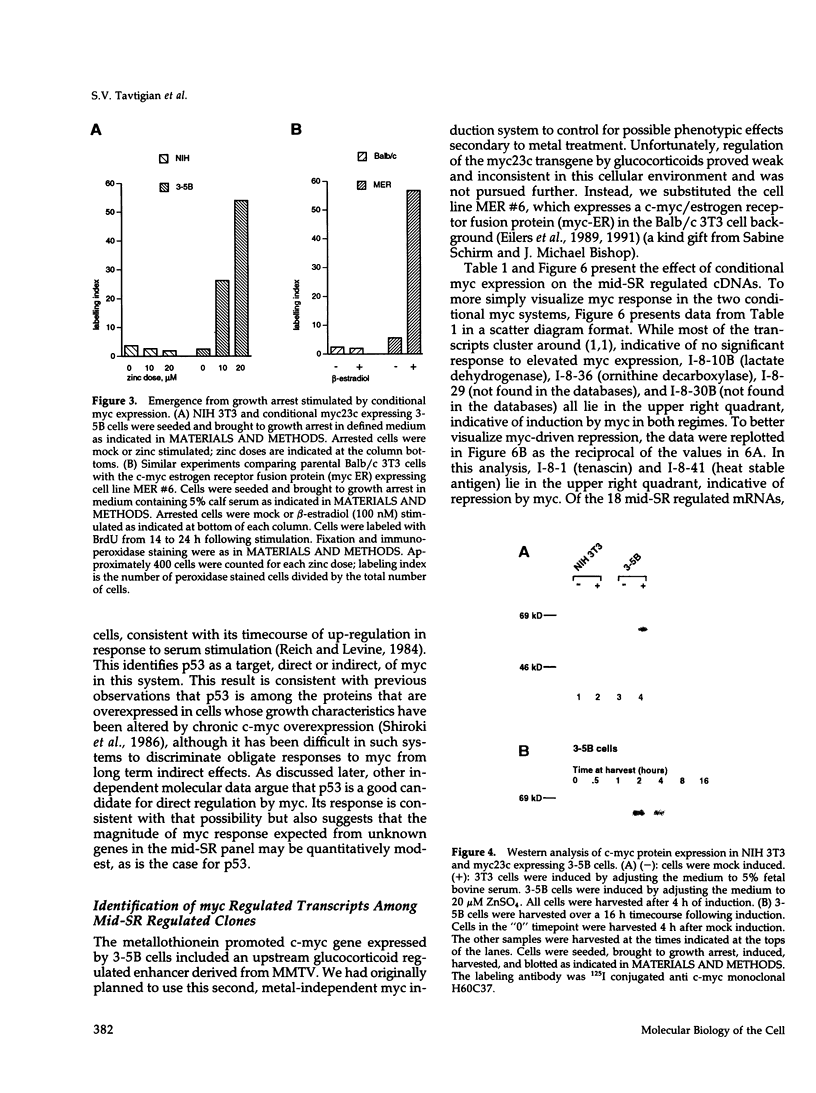
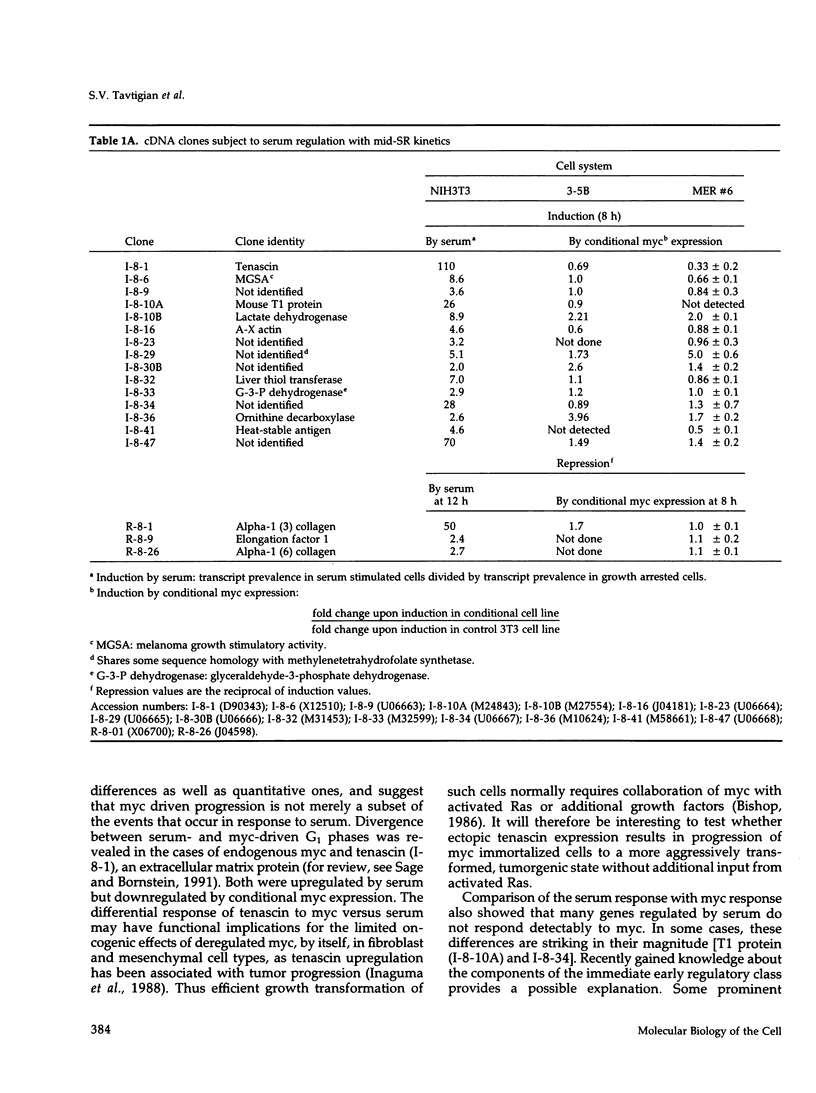
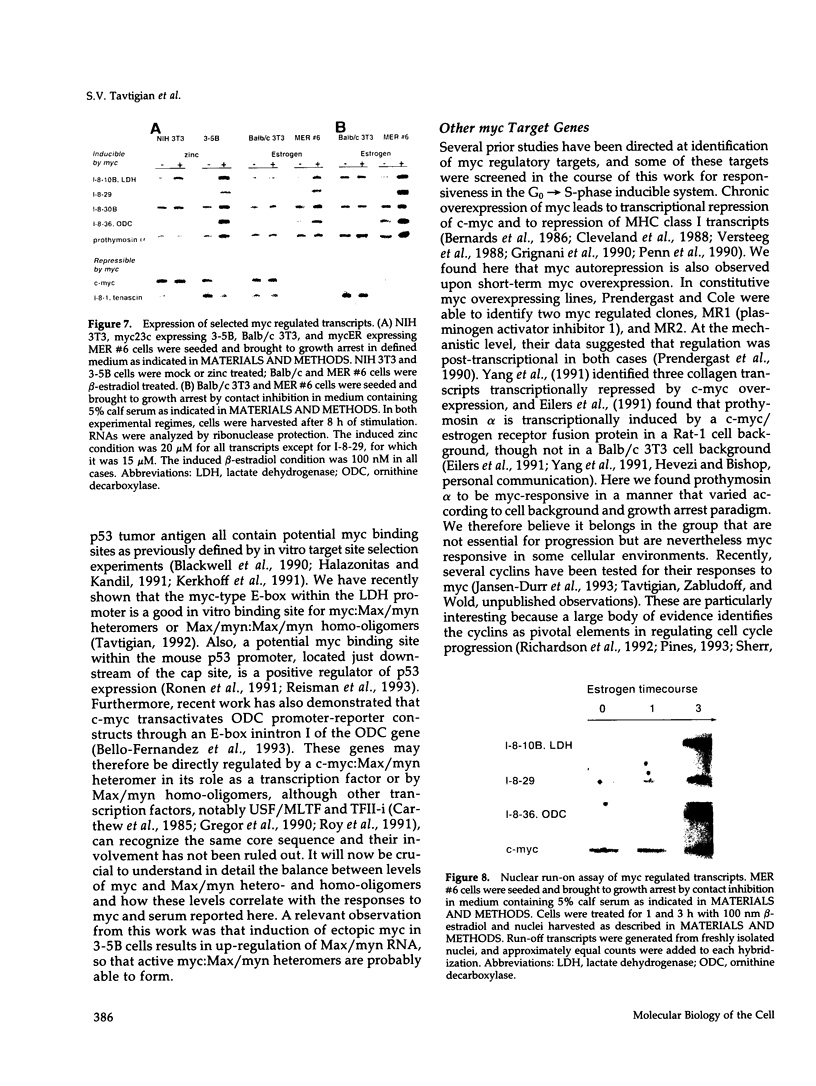
The emergence of cells from a quiescent G0 arrested state into the cell cycle is a multistep process that begins with the immediate early response to mitogens and extends into a specialized G1 phase. Many immediate early serum response genes including c-fos, c-myc, and c-jun are transcriptional regulators. To understand their roles in regulating cell cycle entry and progression, the identities of their regulatory targets must be determined. In this work we have cloned cDNA copies of messenger RNAs that are either up- or down-regulated at a mid-G1 point in the serum response (midserum-response [mid-SR]). The mid-SR panel is expected to include both direct and indirect targets of immediate early regulators. This expectation was confirmed by the identification of several transcriptional targets of conditional c-myc activity. In terms of cellular function, the mid-SR class is also expected to include execution genes needed for progression through G1 and into S-phase. DNA sequence data showed that the mid-SR panel included several genes already known to be involved in cell cycle progression or growth transformation, suggesting that previously unknown cDNAs in the same group are good candidates for other G1 execution functions. In functional assays of G0-->S-phase progression, c-myc expression can bypass the requirement for serum mitogens and drive a large fraction of G0 arrested cells through G1 into S-phase. However, beyond this general similarity, little is known about the relation of a serum-driven progression to a myc-driven progression. Using the mid-SR collection as molecular reporters, we found that the myc driven G1 differs qualitatively from the serum driven case. Instead of simply activating a subset of serum response genes, as might be expected, myc regulated some genes inversely relative to serum stimulation. This suggests that a myc driven progression from G0 may have novel properties with implications for its action in oncogenesis.
Full text
PDF










Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Allen-Hoffmann B. L., Crankshaw C. L., Mosher D. F. Transforming growth factor beta increases cell surface binding and assembly of exogenous (plasma) fibronectin by normal human fibroblasts. Mol Cell Biol. 1988 Oct;8(10):4234–4242. doi: 10.1128/mcb.8.10.4234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Almendral J. M., Sommer D., Macdonald-Bravo H., Burckhardt J., Perera J., Bravo R. Complexity of the early genetic response to growth factors in mouse fibroblasts. Mol Cell Biol. 1988 May;8(5):2140–2148. doi: 10.1128/mcb.8.5.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Altschul S. F., Gish W., Miller W., Myers E. W., Lipman D. J. Basic local alignment search tool. J Mol Biol. 1990 Oct 5;215(3):403–410. doi: 10.1016/S0022-2836(05)80360-2. [DOI] [PubMed] [Google Scholar]
- Auvinen M., Paasinen A., Andersson L. C., Hölttä E. Ornithine decarboxylase activity is critical for cell transformation. Nature. 1992 Nov 26;360(6402):355–358. doi: 10.1038/360355a0. [DOI] [PubMed] [Google Scholar]
- Bello-Fernandez C., Packham G., Cleveland J. L. The ornithine decarboxylase gene is a transcriptional target of c-Myc. Proc Natl Acad Sci U S A. 1993 Aug 15;90(16):7804–7808. doi: 10.1073/pnas.90.16.7804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benezra R., Davis R. L., Lockshon D., Turner D. L., Weintraub H. The protein Id: a negative regulator of helix-loop-helix DNA binding proteins. Cell. 1990 Apr 6;61(1):49–59. doi: 10.1016/0092-8674(90)90214-y. [DOI] [PubMed] [Google Scholar]
- Bernard O., Cory S., Gerondakis S., Webb E., Adams J. M. Sequence of the murine and human cellular myc oncogenes and two modes of myc transcription resulting from chromosome translocation in B lymphoid tumours. EMBO J. 1983;2(12):2375–2383. doi: 10.1002/j.1460-2075.1983.tb01749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernards R., Dessain S. K., Weinberg R. A. N-myc amplification causes down-modulation of MHC class I antigen expression in neuroblastoma. Cell. 1986 Dec 5;47(5):667–674. doi: 10.1016/0092-8674(86)90509-x. [DOI] [PubMed] [Google Scholar]
- Bishop J. M. Viral oncogenes. Cell. 1985 Aug;42(1):23–38. doi: 10.1016/s0092-8674(85)80098-2. [DOI] [PubMed] [Google Scholar]
- Blackwell T. K., Kretzner L., Blackwood E. M., Eisenman R. N., Weintraub H. Sequence-specific DNA binding by the c-Myc protein. Science. 1990 Nov 23;250(4984):1149–1151. doi: 10.1126/science.2251503. [DOI] [PubMed] [Google Scholar]
- Blackwood E. M., Eisenman R. N. Max: a helix-loop-helix zipper protein that forms a sequence-specific DNA-binding complex with Myc. Science. 1991 Mar 8;251(4998):1211–1217. doi: 10.1126/science.2006410. [DOI] [PubMed] [Google Scholar]
- Blackwood E. M., Lüscher B., Eisenman R. N. Myc and Max associate in vivo. Genes Dev. 1992 Jan;6(1):71–80. doi: 10.1101/gad.6.1.71. [DOI] [PubMed] [Google Scholar]
- Bond V. C., Wold B. Poly-L-ornithine-mediated transformation of mammalian cells. Mol Cell Biol. 1987 Jun;7(6):2286–2293. doi: 10.1128/mcb.7.6.2286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bravo R. Growth factor-responsive genes in fibroblasts. Cell Growth Differ. 1990 Jun;1(6):305–309. [PubMed] [Google Scholar]
- Carthew R. W., Chodosh L. A., Sharp P. A. An RNA polymerase II transcription factor binds to an upstream element in the adenovirus major late promoter. Cell. 1985 Dec;43(2 Pt 1):439–448. doi: 10.1016/0092-8674(85)90174-6. [DOI] [PubMed] [Google Scholar]
- Cavalieri F., Goldfarb M. Growth factor-deprived BALB/c 3T3 murine fibroblasts can enter the S phase after induction of c-myc gene expression. Mol Cell Biol. 1987 Oct;7(10):3554–3560. doi: 10.1128/mcb.7.10.3554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chomczynski P., Sacchi N. Single-step method of RNA isolation by acid guanidinium thiocyanate-phenol-chloroform extraction. Anal Biochem. 1987 Apr;162(1):156–159. doi: 10.1006/abio.1987.9999. [DOI] [PubMed] [Google Scholar]
- Cleveland J. L., Huleihel M., Bressler P., Siebenlist U., Akiyama L., Eisenman R. N., Rapp U. R. Negative regulation of c-myc transcription involves myc family proteins. Oncogene Res. 1988;3(4):357–375. [PubMed] [Google Scholar]
- Eilers M., Picard D., Yamamoto K. R., Bishop J. M. Chimaeras of myc oncoprotein and steroid receptors cause hormone-dependent transformation of cells. Nature. 1989 Jul 6;340(6228):66–68. doi: 10.1038/340066a0. [DOI] [PubMed] [Google Scholar]
- Eilers M., Schirm S., Bishop J. M. The MYC protein activates transcription of the alpha-prothymosin gene. EMBO J. 1991 Jan;10(1):133–141. doi: 10.1002/j.1460-2075.1991.tb07929.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregor P. D., Sawadogo M., Roeder R. G. The adenovirus major late transcription factor USF is a member of the helix-loop-helix group of regulatory proteins and binds to DNA as a dimer. Genes Dev. 1990 Oct;4(10):1730–1740. doi: 10.1101/gad.4.10.1730. [DOI] [PubMed] [Google Scholar]
- Grignani F., Lombardi L., Inghirami G., Sternas L., Cechova K., Dalla-Favera R. Negative autoregulation of c-myc gene expression is inactivated in transformed cells. EMBO J. 1990 Dec;9(12):3913–3922. doi: 10.1002/j.1460-2075.1990.tb07612.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halazonetis T. D., Kandil A. N. Determination of the c-MYC DNA-binding site. Proc Natl Acad Sci U S A. 1991 Jul 15;88(14):6162–6166. doi: 10.1073/pnas.88.14.6162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inaguma Y., Kusakabe M., Mackie E. J., Pearson C. A., Chiquet-Ehrismann R., Sakakura T. Epithelial induction of stromal tenascin in the mouse mammary gland: from embryogenesis to carcinogenesis. Dev Biol. 1988 Aug;128(2):245–255. doi: 10.1016/0012-1606(88)90288-6. [DOI] [PubMed] [Google Scholar]
- Jansen-Dürr P., Meichle A., Steiner P., Pagano M., Finke K., Botz J., Wessbecher J., Draetta G., Eilers M. Differential modulation of cyclin gene expression by MYC. Proc Natl Acad Sci U S A. 1993 Apr 15;90(8):3685–3689. doi: 10.1073/pnas.90.8.3685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jendrisak J., Young R. A., Engel J. D. Cloning cDNA into lambda gt10 and lambda gt11. Methods Enzymol. 1987;152:359–371. doi: 10.1016/0076-6879(87)52043-2. [DOI] [PubMed] [Google Scholar]
- Kay R., Takei F., Humphries R. K. Expression cloning of a cDNA encoding M1/69-J11d heat-stable antigens. J Immunol. 1990 Sep 15;145(6):1952–1959. [PubMed] [Google Scholar]
- Kerkhoff E., Bister K., Klempnauer K. H. Sequence-specific DNA binding by Myc proteins. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4323–4327. doi: 10.1073/pnas.88.10.4323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kristensen P., Nielsen L. S., Grøndahl-Hansen J., Andresen P. B., Larsson L. I., Danø K. Immunocytochemical demonstration of tissue-type plasminogen activator in endocrine cells of the rat pituitary gland. J Cell Biol. 1985 Jul;101(1):305–311. doi: 10.1083/jcb.101.1.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lanahan A., Williams J. B., Sanders L. K., Nathans D. Growth factor-induced delayed early response genes. Mol Cell Biol. 1992 Sep;12(9):3919–3929. doi: 10.1128/mcb.12.9.3919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lau L. F., Nathans D. Expression of a set of growth-related immediate early genes in BALB/c 3T3 cells: coordinate regulation with c-fos or c-myc. Proc Natl Acad Sci U S A. 1987 Mar;84(5):1182–1186. doi: 10.1073/pnas.84.5.1182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowe D. G., Moran L. A. Molecular cloning and analysis of DNA complementary to three mouse Mr = 68,000 heat shock protein mRNAs. J Biol Chem. 1986 Feb 15;261(5):2102–2112. [PubMed] [Google Scholar]
- Mirkes P. E. Simultaneous banding of rat embryo DNA, RNA, and protein in cesium trifluoroacetate gradients. Anal Biochem. 1985 Aug 1;148(2):376–383. doi: 10.1016/0003-2697(85)90242-8. [DOI] [PubMed] [Google Scholar]
- Mohn K. L., Laz T. M., Hsu J. C., Melby A. E., Bravo R., Taub R. The immediate-early growth response in regenerating liver and insulin-stimulated H-35 cells: comparison with serum-stimulated 3T3 cells and identification of 41 novel immediate-early genes. Mol Cell Biol. 1991 Jan;11(1):381–390. doi: 10.1128/mcb.11.1.381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moshier J. A., Dosescu J., Skunca M., Luk G. D. Transformation of NIH/3T3 cells by ornithine decarboxylase overexpression. Cancer Res. 1993 Jun 1;53(11):2618–2622. [PubMed] [Google Scholar]
- Mueller P. R., Salser S. J., Wold B. Constitutive and metal-inducible protein:DNA interactions at the mouse metallothionein I promoter examined by in vivo and in vitro footprinting. Genes Dev. 1988 Apr;2(4):412–427. doi: 10.1101/gad.2.4.412. [DOI] [PubMed] [Google Scholar]
- Mäkelä T. P., Koskinen P. J., Västrik I., Alitalo K. Alternative forms of Max as enhancers or suppressors of Myc-ras cotransformation. Science. 1992 Apr 17;256(5055):373–377. doi: 10.1126/science.256.5055.373. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Ohtsubo M., Roberts J. M. Cyclin-dependent regulation of G1 in mammalian fibroblasts. Science. 1993 Mar 26;259(5103):1908–1912. doi: 10.1126/science.8384376. [DOI] [PubMed] [Google Scholar]
- Palazzolo M. J., Hamilton B. A., Ding D. L., Martin C. H., Mead D. A., Mierendorf R. C., Raghavan K. V., Meyerowitz E. M., Lipshitz H. D. Phage lambda cDNA cloning vectors for subtractive hybridization, fusion-protein synthesis and Cre-loxP automatic plasmid subcloning. Gene. 1990 Mar 30;88(1):25–36. doi: 10.1016/0378-1119(90)90056-w. [DOI] [PubMed] [Google Scholar]
- Palvimo J. J., Eisenberg L. M., Jänne O. A. Protein-DNA interactions in the cAMP responsive promoter region of the murine ornithine decarboxylase gene. Nucleic Acids Res. 1991 Jul 25;19(14):3921–3927. doi: 10.1093/nar/19.14.3921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pardee A. B. G1 events and regulation of cell proliferation. Science. 1989 Nov 3;246(4930):603–608. doi: 10.1126/science.2683075. [DOI] [PubMed] [Google Scholar]
- Payvar F., DeFranco D., Firestone G. L., Edgar B., Wrange O., Okret S., Gustafsson J. A., Yamamoto K. R. Sequence-specific binding of glucocorticoid receptor to MTV DNA at sites within and upstream of the transcribed region. Cell. 1983 Dec;35(2 Pt 1):381–392. doi: 10.1016/0092-8674(83)90171-x. [DOI] [PubMed] [Google Scholar]
- Penn L. J., Brooks M. W., Laufer E. M., Land H. Negative autoregulation of c-myc transcription. EMBO J. 1990 Apr;9(4):1113–1121. doi: 10.1002/j.1460-2075.1990.tb08217.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pines J. Cyclins and cyclin-dependent kinases: take your partners. Trends Biochem Sci. 1993 Jun;18(6):195–197. doi: 10.1016/0968-0004(93)90185-p. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Diamond L. E., Dahl D., Cole M. D. The c-myc-regulated gene mrl encodes plasminogen activator inhibitor 1. Mol Cell Biol. 1990 Mar;10(3):1265–1269. doi: 10.1128/mcb.10.3.1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prendergast G. C., Lawe D., Ziff E. B. Association of Myn, the murine homolog of max, with c-Myc stimulates methylation-sensitive DNA binding and ras cotransformation. Cell. 1991 May 3;65(3):395–407. doi: 10.1016/0092-8674(91)90457-a. [DOI] [PubMed] [Google Scholar]
- Prendergast G. C., Ziff E. B. Methylation-sensitive sequence-specific DNA binding by the c-Myc basic region. Science. 1991 Jan 11;251(4990):186–189. doi: 10.1126/science.1987636. [DOI] [PubMed] [Google Scholar]
- Reich N. C., Levine A. J. Growth regulation of a cellular tumour antigen, p53, in nontransformed cells. Nature. 1984 Mar 8;308(5955):199–201. doi: 10.1038/308199a0. [DOI] [PubMed] [Google Scholar]
- Reisman D., Elkind N. B., Roy B., Beamon J., Rotter V. c-Myc trans-activates the p53 promoter through a required downstream CACGTG motif. Cell Growth Differ. 1993 Feb;4(2):57–65. [PubMed] [Google Scholar]
- Richardson H., Lew D. J., Henze M., Sugimoto K., Reed S. I. Cyclin-B homologs in Saccharomyces cerevisiae function in S phase and in G2. Genes Dev. 1992 Nov;6(11):2021–2034. doi: 10.1101/gad.6.11.2021. [DOI] [PubMed] [Google Scholar]
- Ronen D., Rotter V., Reisman D. Expression from the murine p53 promoter is mediated by factor binding to a downstream helix-loop-helix recognition motif. Proc Natl Acad Sci U S A. 1991 May 15;88(10):4128–4132. doi: 10.1073/pnas.88.10.4128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roussel M. F., Cleveland J. L., Shurtleff S. A., Sherr C. J. Myc rescue of a mutant CSF-1 receptor impaired in mitogenic signalling. Nature. 1991 Sep 26;353(6342):361–363. doi: 10.1038/353361a0. [DOI] [PubMed] [Google Scholar]
- Roy A. L., Meisterernst M., Pognonec P., Roeder R. G. Cooperative interaction of an initiator-binding transcription initiation factor and the helix-loop-helix activator USF. Nature. 1991 Nov 21;354(6350):245–248. doi: 10.1038/354245a0. [DOI] [PubMed] [Google Scholar]
- Sage E. H., Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991 Aug 15;266(23):14831–14834. [PubMed] [Google Scholar]
- Sargent T. D. Isolation of differentially expressed genes. Methods Enzymol. 1987;152:423–432. doi: 10.1016/0076-6879(87)52049-3. [DOI] [PubMed] [Google Scholar]
- Sherr C. J. Mammalian G1 cyclins. Cell. 1993 Jun 18;73(6):1059–1065. doi: 10.1016/0092-8674(93)90636-5. [DOI] [PubMed] [Google Scholar]
- Shiroki K., Segawa K., Koita Y., Shibuya M. Neoplastic transformation of rat 3Y1 cells by a transcriptionally activated human c-myc gene and stabilization of p53 cellular tumor antigen in the transformed cells. Mol Cell Biol. 1986 Dec;6(12):4379–4386. doi: 10.1128/mcb.6.12.4379. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tan T. H., Wallis J., Levine A. J. Identification of the p53 protein domain involved in formation of the simian virus 40 large T-antigen-p53 protein complex. J Virol. 1986 Sep;59(3):574–583. doi: 10.1128/jvi.59.3.574-583.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson C. B., Challoner P. B., Neiman P. E., Groudine M. Levels of c-myc oncogene mRNA are invariant throughout the cell cycle. 1985 Mar 28-Apr 3Nature. 314(6009):363–366. doi: 10.1038/314363a0. [DOI] [PubMed] [Google Scholar]
- Van Beveren C., van Straaten F., Curran T., Müller R., Verma I. M. Analysis of FBJ-MuSV provirus and c-fos (mouse) gene reveals that viral and cellular fos gene products have different carboxy termini. Cell. 1983 Apr;32(4):1241–1255. doi: 10.1016/0092-8674(83)90306-9. [DOI] [PubMed] [Google Scholar]
- Versteeg R., Noordermeer I. A., Krüse-Wolters M., Ruiter D. J., Schrier P. I. c-myc down-regulates class I HLA expression in human melanomas. EMBO J. 1988 Apr;7(4):1023–1029. doi: 10.1002/j.1460-2075.1988.tb02909.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wenzel A., Cziepluch C., Hamann U., Schürmann J., Schwab M. The N-Myc oncoprotein is associated in vivo with the phosphoprotein Max(p20/22) in human neuroblastoma cells. EMBO J. 1991 Dec;10(12):3703–3712. doi: 10.1002/j.1460-2075.1991.tb04938.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wigler M., Sweet R., Sim G. K., Wold B., Pellicer A., Lacy E., Maniatis T., Silverstein S., Axel R. Transformation of mammalian cells with genes from procaryotes and eucaryotes. Cell. 1979 Apr;16(4):777–785. doi: 10.1016/0092-8674(79)90093-x. [DOI] [PubMed] [Google Scholar]
- Yang B. S., Geddes T. J., Pogulis R. J., de Crombrugghe B., Freytag S. O. Transcriptional suppression of cellular gene expression by c-Myc. Mol Cell Biol. 1991 Apr;11(4):2291–2295. doi: 10.1128/mcb.11.4.2291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Goldfarb M. Growth factor requirements of oncogene-transformed NIH 3T3 and BALB/c 3T3 cells cultured in defined media. Mol Cell Biol. 1986 Oct;6(10):3541–3544. doi: 10.1128/mcb.6.10.3541. [DOI] [PMC free article] [PubMed] [Google Scholar]








