Abstract
In the bluebanded goby, Lythrypnus dalli, removal of the male from a social group results in a rapid behavioral response where one female becomes dominant and changes sex to male. In a previous study, within hours of male removal, aromatase activity in the brain (bAA) of dominant females was almost 50% lower than that of control females from a group in which the male had not been removed. For those females that displayed increased aggressive behavior after the male was removed, the larger the increase in aggressive behavior, the greater the reduction in bAA. To investigate whether decreased bAA leads to increased aggression, the present study used a more rapid time course of behavioral profiling and bAA assay, looking within minutes of male removal from the group. There were no significant differences in bAA between control females (large females from groups with the male still present), females that doubled their aggressive behavior by 10 or 20 minutes after male removal, or females that did not double their aggressive behavior within 30 minutes after male removal. Further, individual variation in bAA and aggressive behavior were not correlated in these fish. Whole brain decreases in aromatase activity thus appear to follow, rather than precede, rapid increases in aggressive behavior, which provides one potential mechanism underlying the rapid increase in androgens that follows aggressive interactions in many vertebrate species. For fish species that change sex from female to male, this increase in androgens could subsequently facilitate sex change.
Keywords: vertebrate, plasticity, estrogen, teleost, 11-ketotestosterone, testosterone, challenge hypothesis, brain aromatase
Introduction
During social interactions, an advantage can often be gained by generating rapid and appropriate responses. However, the mechanisms by which animals regulate their responses and responsiveness to important social cues are not fully understood. One well-established mechanism is through the modulation of steroid levels (Wingfield et al., 1990), since steroid hormones can rapidly alter brain function and social behavior (reviewed in Remage-Healey and Bass, 2006). Many vertebrates show a rapid increase in circulating androgens following aggressive interactions (reviewed in Remage-Healey and Bass, 2005), including rodents (Batty, 1978; Macrides et al., 1975), humans (Bernhardt et al., 1998; Roney et al., 2003), amphibians (Burmeister and Wilczynski, 2000), lizards (Greenberg and Crews, 1990; Yang and Wilczynski, 2002), birds (Wingfield, 1985; Wingfield and Wada, 1989), and teleost fishes (Cardwell and Liley, 1991; Oliveira et al., 1996). However, the definitive source of the rapidly elevated steroids has not been identified in these studies. In some vertebrates, the brain can be a significant source of steroids (Schlinger and Arnold, 1991; Pradhan et al., 2010). Wingfield and Wada (1989) demonstrated in male song sparrows, Zonotrichia melodia, that the rise in testosterone following a social challenge precedes an increase in luteinizing hormone release from the pituitary, supporting the idea that the increased peripheral testosterone might come from an extragonadal source (e.g., the brain). One way to modulate steroid levels is by regulating the activity of enzymes involved in steroid biosynthesis. An enzyme that converts testosterone into estradiol, aromatase, can decrease or increase activity in minutes in the Japanese quail brain following exposure to social stimuli (Balthazart et al., 2001a,b, 2003; Cornil et al, 2005). These rapid changes in brain aromatase activity regulate the conversion of testosterone to estrogen and further correlate with changes in quail behavior (Balthazart et al. 2004; 2006, Balthazart and Ball, 2006). Thus, it is possible that social challenges result in changes in brain steroid biosynthesis, which then provides a mechanism for the increase in androgen levels associated with aggressive social interactions (the challenge hypothesis). Similar rapid effects of steroids have been obtained for the Gulf toadfish, which can rapidly (within 5–20 minutes) change 11-ketotestosterone (11-KT) levels and vocalization behavior in response to simulated territorial intrusion, and in midshipman fish, where exogenous application of 11-KT or 17β-estradiol results in rapid changes in calling behavior (Remage-Healy and Bass, 2004,Remage-Healy and Bass, 2005). These studies demonstrate that steroids can change very rapidly, that these changes in steroid levels are associated with fast changes in behavior, and that social interactions can influence both steroid levels and subsequent behavior.
Steroid levels and the activity of steroidogenic enzymes, like aromatase, can be investigated at many levels, from the whole organ to organelles (Sharma et al., 2004; Peterson et al., 2005; Gonçalves et al., 2008). In this study, we were interested in the whole brain as a site of aromatase activity (AA) and the possible role of modulation of AA in the generation of rapid changes in behavior. This was based on preliminary studies showing little differences between macro dissected brain regions (unpublished data) and a previous study that found profound differences in whole brain aromatase activity (bAA) of fish that were in the process of changing sex from female to male, fish that had recently changed sex to male, and established males of bluebanded gobies, Lythrypnus dalli.
In haremic groups of bluebanded gobies, a critical social challenge confronts the highest-ranking (alpha) female when the dominant male is removed from a group. In response to the absence of the male, the alpha female in the group will rapidly and dramatically increase her rates of aggression towards other group members to assert her dominance and thereby ensure her opportunity to change sex (Black et al., 2005a, b; Reavis and Grober, 1999; Rodgers et al., 2007). The doubling of baseline levels of aggression in the alpha female is usually the first observable change that follows male removal, and is an unambiguous predictor that the fish will change sex (Black et al., 2005a, b; Reavis and Grober, 1999; Rodgers et al., 2007). This increased aggressive behavior is followed by dramatic shifts in the physiological, anatomical and behavioral components of reproductive function over the next 1–2 weeks. This predictable cascade of events allows us to investigate the mechanisms that drive socially regulated adult sexual plasticity (Black et al, 2004, 2005a, b; Carlisle, 2001; Reavis and Grober, 1999). The earliest physiologic change detected thus far in L. dalli is a decrease in brain aromatase activity, which happens at a much earlier time point than any detectable change in waterborne steroids, a proxy for plasma steroids (Black et al., 2005a; Earley et al., unpublished data). In waterborne steroid assays, L.dalli display no significant sex differences in testosterone or 11-ketotestosterone concentrations, but males produce significantly lower estradiol levels than females, suggesting a possible role for steroids in the sex change process (Lorenzi et al., 2008). Since behavior obviously changes before gonadal morphology and presumably physiology, it can be speculated that the increased aggression in the dominant female after male removal results from an early change in the brain. A potential rapid-response candidate, serotonin, does not appear to be involved in the onset of the sex change process (Lorenzi et al., 2009), leaving bAA as a potential candidate. In a previous experiment (Black et al., 2005a), within three hours following male removal, large dominant females had lower levels of bAA than large females from groups with the male still present. Further, the changes in aggressive behavior of dominant females were negatively correlated with their bAA levels. As bAA decreases, the pool of available androgens should increase as their conversion to estrogen decreases. Before beginning studies to determine causality, we wanted to test whether there is a close temporal association between changes in whole brain aromatase and increases in aggressive behavior that indicate the onset of sex change. By investigating changes in both bAA and aggressive behavior in the dominant female minutes after male removal from the group, this study tests the hypothesis that there is a close correlation between lower whole brain aromatase and increased aggressive behavior at the onset of the sex change process.
Materials and methods
Fishes were collected off the coast of Catalina Island, California (permit #SC- 801200-01) using hand nets and a 1:10 solution of quinaldine in ethanol, and then shipped to and maintained in a fish facility in Atlanta, Georgia. All protocols were in accordance with the Institutional Animal Care and Use Committee regulations at Georgia State University. Fish groups were tested from July 2003 to January 2004. Each group had one large male (SL=35.46 ± 0.33 mm, mean± SEM), one large female at least 3 mm smaller than the male (SL=29.76 ± 0.31 mm), and two females at least 3 mm smaller than the large female (SL=24.15 ± 0.22 mm). This group structure assures male dominance over all fish and the largest female’s dominance over all other females. Each group was placed in a 40-liter aquarium with a PVC nesting tube and given five days to acclimate to their social conditions. On the fourth day, the largest female’s behavior was observed for 10 min in both the morning and afternoon, a recording frequency that was previously shown to be adequate to characterize the behavior of these fishes and their variation in time (Reavis and Grober, 1999). Recorded behavior included approaches, displacements, and jerk swims (Reavis and Grober, 1999). Approaches consist of one fish moving within five centimeters of another fish and displacements are approaches where the other fish moves away within a second after the approach. Jerk swims are a male-typical courtship behavior that involves swimming with abrupt starts and stops with fins erect, alternating between moving slightly to the left and slightly to the right of the general direction of movement. Each behavior from morning and afternoon observations was averaged for the day as baseline frequency. Subjects were haphazardly assigned to experimental or control groups prior to behavioral observation to avoid selection bias after observation. In experimental groups, the male was removed at an average time of 9:49 AM on the fifth day (range 8:07–11:31am). The males are almost always associated with the nest tube and can therefore easily be removed from the tank, minimizing stress to the other fishes. The dominant female was then observed in consecutive 10-min time periods until it showed a doubling of displacement behavior relative to pre-removal baseline frequency or until 30 min had passed without the doubling in behavior. The dominant female was sacrificed at a point when one of those criteria was met. Three fish did not appear to display dominance. Given that we wanted to study fish initiating the sex change process, their behavioral profiles were not predictive of sex change, and they were excluded from all analyses. Two fish that were designated for experimental groups showed no displacements prior to male removal and were removed from all analyses because they could not pass the criteria of doubling behavior, regardless of the increases in displacements.
After sacrifice, brains were quickly removed (within 10 minutes), weighed, and frozen on dry ice. The genital papilla (external genitalia) of each fish was photographed before and after the experiment and length:width ratios were calculated (Carlisle et al., 2000). The ratios were used to assess the subjects’ sex at the experiment’s start. As the genital papilla of L. dalli is not a perfect predictor of functional sex (St. Mary, 1993) and gonads in this fish do not change as quickly as the time course of this experiment (Black et al, 2005b), gonad inspection was used to verify initial genital-based sex assignment. Two fish coming from two different groups were found to have gonads that were not consistent with their initial sex assignment and were removed from the experiment before the analysis of bAA. After gross anatomical inspection, gonads were weighed and then frozen on dry ice.
All frozen brain and gonad samples were homogenized and assayed for AA by measuring tritiated water production from [1β-3H]-androstenedione (NEN Life Science Products, Boston, MA), as described by Roselli and Resko (1991), with minor modifications (Baillien and Balthazart, 1997). Homogenates containing about 1 mg of fresh weight tissue per assay were incubated with 25 nM androstenedione at 37°C for 1 hour for brain and 15 minutes for gonadal tissue. The incubation durations were selected based on preliminary experiments to limit the amount of substrate metabolized so that the enzymatic reactions could proceed linearly during the entire incubation period (data not shown). Preliminary assays had confirmed that the substrate concentration used here is saturating (at least 5 times Km) in L. dalli as it is in goldfish (Zhao et al., 2001).
Within each experiment, controls using boiled brain or gonad samples with an excess (final concentration 40 μM) of the potent and specific aromatase inhibitor, R76713 (racemic Vorozole™, Janssen Pharmaceutica, Beerse, Belgium) never exceeded 300–600 dpm while active control samples had radioactivities ranging between 2,000 to 150,000 dpm. Assays were performed so that each run had controls and samples from each of the experimental groups. A recovery of 93 ± 2 % was usually obtained from samples of 10,000 dpm tritiated water conducted throughout the entire purification procedure (incubation, centrifugation, and Dowex column). Protein content of all homogenates was determined in triplicate by a micromodification of the Bradford method (Bradford, 1976). Enzyme activity was expressed in pmol/hr/mg protein after correction of the counts for quenching, recovery, blank values, and percentage of tritium in β-position in the substrate (see Baillien and Balthazart, 1997 for detail).
We could not control how many fish doubled their rates of aggression at any of the three sampling times or if the fish doubled within the time frame of the experiment. As most of the fish that doubled their rates of aggression did so after the first 10 minutes, our numbers for the 20-minute doublers and 30-minute doublers were low. There were only two thirty minute doublers, so their data are included in the figures for informational value, but were only used for pooled statistical analyses. Also because of the small sample size of the 20-minute doublers, the distribution for this group is not normal so the four 20-minute doublers are included in one set of analyses and removed from a second set, both reported. Statistical analyses were performed using JMP 5.0.1 (SAS Institute, Cary, NC). One-way ANOVA was used to compare differences between groups in bAA and behavior and was followed when appropriate by Tukey-Kramer HSD tests for comparisons among treatment groups. T-tests were used to compare pooled samples (see above) to controls or those that showed no change in behavior after 30 minutes. Simple linear regression was used to analyze relationships between bAA, rates of behavior, and latency between male removal and sacrifice. All data in the text are presented as means ± SEM.
Results
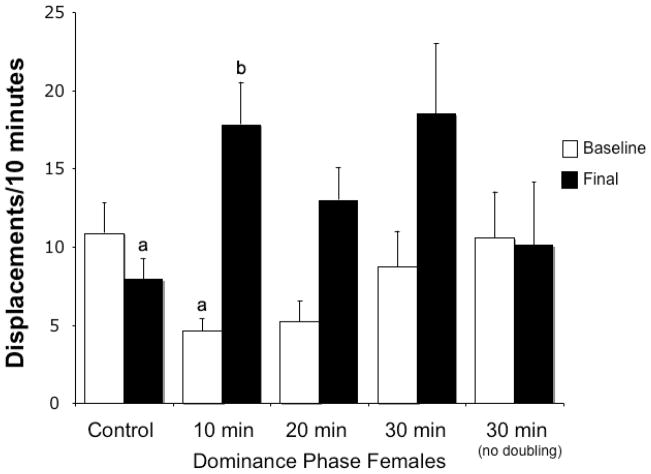
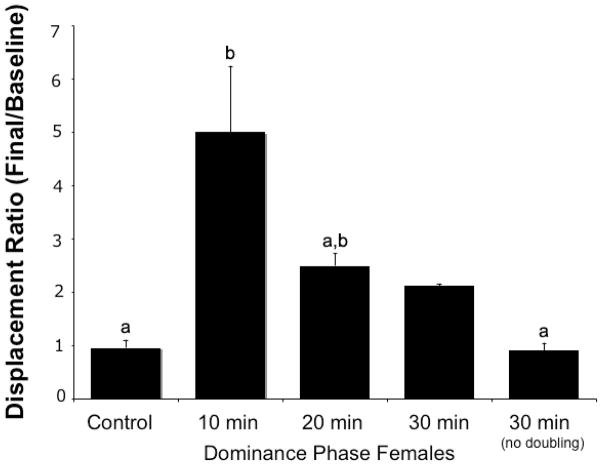
Of the groups that experienced male removal, 46.2% (n=12) doubled their baseline displacement behavior at 10 min, 15.4% (n=4) doubled at 20 min, 7.7% (n=2) doubled at 30 min, and 30.7% (n=8) did not double by the end of 30 min. The baseline rates of displacement for these groups and control females (n=19) were not significantly different (F3,39=2.49, p>0.07 with the 20-minute doublers; F2,36=3.00, p>0.06 without; Fig. 1). Although the baseline displacement rates among all groups were not significantly different, those fish that did not double their displacement rates after male removal and controls that did not experience male removal had higher average baseline displacements than those that doubled displacement rates later. Because groups were haphazardly assigned, these non-significant higher average baseline values were by chance. The higher baseline may have increased the challenge to double behavior and resulted in some individuals taking longer to double displacements or not doubling by 30 minutes. The baseline displacements were not directly related to brain aromatase levels (see below). When comparing all baseline and final displacements across all groups, there were significant differences (F7,78=2.49, p<0.004; Fig. 1). Posthoc comparisons indicated the 10 min baseline and 10 min final groups were significantly different from each other (p<0.002) as well as the 10 min final and control final displacements (p<0.02), but all others were not significantly different from one another (p>0.05; Fig. 1). The ratio of final displacements divided by the baseline displacements showed a statistically significant overall effect with 10-minute doublers being different from controls and 30-minute nondoublers, but 20-minute doublers were not different from any of the groups (ANOVA, F3,38=8.10, p<0.001 with the 20-minute doublers; F2,35=3.00, p<0.001 without; Fig. 2). Females that did not double their displacements by 30 minutes had final rates of displacements that were nearly identical to baseline rates.
Figure 1.
Rates of displacement behavior shown by the largest female in a social group of L. dalli before (baseline) and after (final) removal of the dominant male in the four experimental groups and the control group. Displacement behavior on Day 4 (white bars; prior to male removal) was not statistically different in the five groups of largest females in the different treatment groups. On Day 5 (black bars), the male was removed from all but the control group. Immediately thereafter, control females (n=19) showed the lowest levels of displacements. Experimental females were then assigned to 4 groups based on whether they doubled their displacement frequency within 10 min (n=12), 20 min (n=4), 30 min (n=3) or did not show such a doubling after 30 min (n=8). In this latter group no increase at all in behavior frequency was observed. Displacements were significantly different between the 10 min baseline and 10 min and between the 10 min final and control final displacements. Different letters indicate significant differences.
Figure 2.
Ratio of behavior changes (final frequency divided by baseline) after removal of the dominant male in the experimental groups compared to control groups. Females that doubled their displacement behaviors in the first ten minutes after male removal (10-minute doublers) were statistically different from controls and 30-minute nondoublers, but 20-minute doublers were not different from any of the groups. Different letters indicate significant differences.
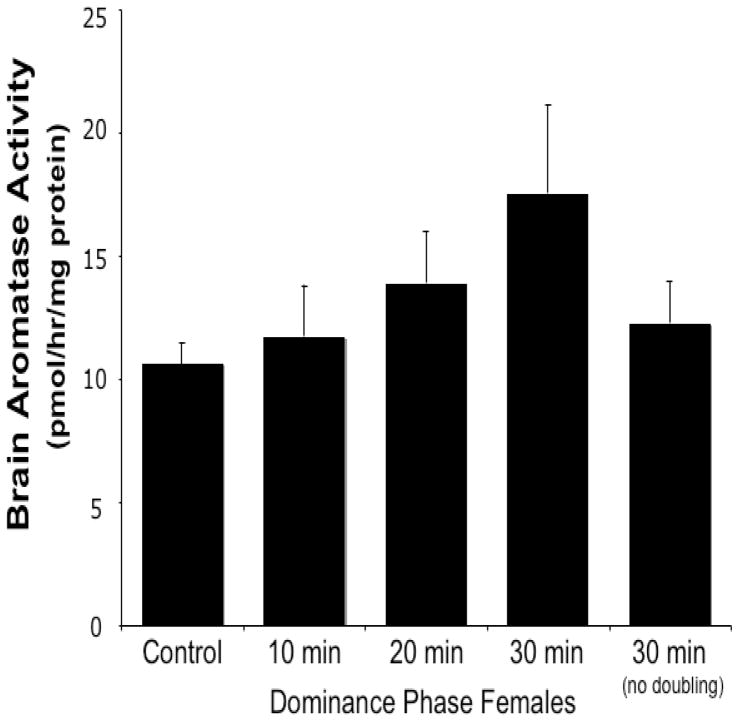
Fish that doubled their displacement behavior within 30 minutes after male removal showed a slight increase in bAA relative to control fish, but this was not statistically significant (Fig. 3). There was no significant difference in bAA between the female groups within thirty minutes of male removal, independent of their behavior (i.e. whether they doubled their baseline behavior or not, or if they were controls or exposed to male removal) (F3,39=0.54, p>0.65 with the 20-minute doublers; F2,36=0.34, p>0.71 without; Fig. 3). If pooled together, all fish that had shown an increase in aggressive behavior (doubler groups at 10, 20 and 30 min) were not significantly different in bAA when compared with control fish (t:−1.284, df=35, p>0.21) or fish exposed to the same social conditions that had not doubled their displacement behavior (group 30 min no double; t: −0.221, df=24, p>0.82).
Figure 3.
Brain aromatase activity for control females and females euthanized at 10, 20 and 30 min after male removal. These females doubled their baseline displacement behavior in the 10-min period just prior to sacrifice, except for the last black bar that represents the females that were sacrificed at 30 minutes but did not double their displacement behavior in any of the three 10-min periods. There are no significant differences between groups.
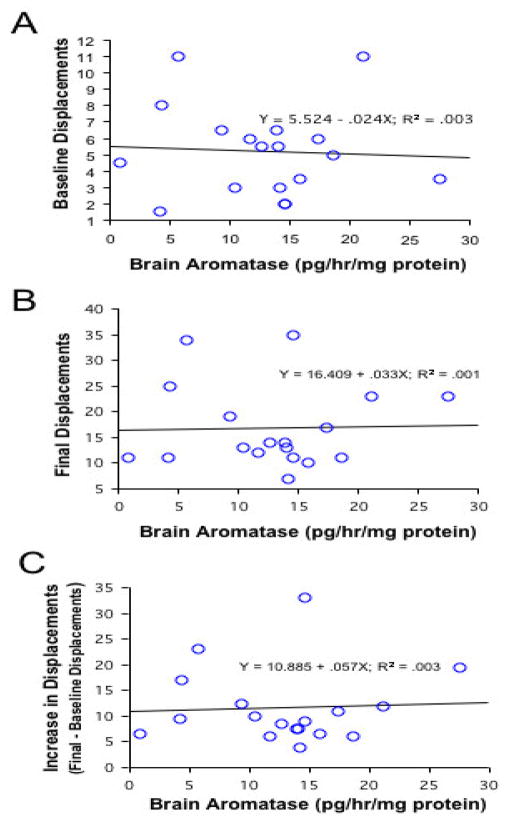
Further, there was no direct relationship between bAA and baseline or final displacement behavior, final/baseline displacement behavior, or increases in displacement behavior among groups that doubled at 10, 20 and 30 min (all R2<0.01, p>0.76; Fig. 4) or among all females (controls and experimentals; all R2<0.04, p>0.20). There was also no difference between any of the female groups in gonadal aromatase activity (gAA; F4,35=1.37, p>0.27; data not shown). None of the experimental or control females displayed jerk swim behavior during observations.
Figure 4.
Regressions of brain aromatase activity and baseline displacements (A), final displacements (B), and the increase in displacements (final-baseline displacements, C). Graphs A–C include all females that showed increases in behavior (n=18). There were no significant correlations between brain aromatase activity and any of these behavioral parameters.
Discussion
The results of this study demonstrate that the rapid increase in aggressive (displacement) behavior observed in the largest female in a group of bluebanded gobies following removal of the dominant male is not associated with a rapid decrease in bAA. If the increased aggression was caused by a decrease in bAA, then a decrease in bAA should have been observed along with or preceding the behavioral change. This would, in turn, leave more testosterone available to activate aggression either directly or through transformation into 11-ketotestosterone. These data thus do not support the idea that a rapid down-regulation of bAA represents a key causal factor leading to rapid increases in aggressive behavior in the largest female of a group after removal of the male, as females that doubled their aggressive behavior within 10 to 30 min did not show lower bAA levels. It is possible that localized changes in bAA within 30 minutes still precede increased levels of aggression, but that these changes were diluted by larger amounts of unresponsive brain tissue so that the whole brain assay was not sensitive enough to detect these local differences. However, this seems unlikely because the total bAA tended to be higher in later sampling time points rather than lower.
Considering the correlation between bAA and aggressive behavior observed in our previous study in L. dalli (Black et al., 2005a), we had suggested that social signals rapidly block bAA (presumably by mechanisms similar to those described in quail, decreasing enzyme activity by phosphorylating aromatase; see Balthazart et al. 2006; Balthazart and Ball, 2006) and that this enzymatic change in turn causes an increase in available androgens and a subsequent marked increase in aggressive behavior. Based on the current study that used a finer temporal scale, the changes that take place in the focal female after removal of the male are not consistent with this interpretation.
One alternative hypothesis is that the increase in aggressive behavior of the dominant female as it begins to change sex is driven by mechanisms that are independent of changes in brain estrogen concentrations and that the change in behavior is the cause of the decrease in bAA. This endocrine change triggered by the performance of a specific behavior would be very reminiscent of the stimulation of ovarian growth observed in female ring doves as a result of their own cooing activity (Cheng, 2003). Such “self-stimulation” occurs when an individual’s behavioral response to an external cue causes a change in the hormone levels rather than the external cue itself, a process demonstrated in both lizards and birds (Cheng, 2003; Cheng et al., 1998; Yang and Wilczynski, 2002). The correlation between increased aggression and bAA in our previous study of L. dalli may be another example of “self-stimulation,” but instead of detecting a change in the plasma hormones concentrations like in these other studies, we detected changes in their rates of synthesis, i.e. in brain steroid metabolism. This change in steroid metabolism may secondarily affect downstream processes either through changes in whole body steroid levels, as would be the case for the change in gonadal sex, or possibly independently of such global changes by more local brain responses leading, for example, to increases in male courtship behavior. Teleost fishes have many examples of changes in sex-typical characteristics not being tightly correlated with plasma steroids or each other. For example, in L.dalli, exogenous steroids have dramatic effects on tissues (e.g. gonads) but not on behavior (Denman, 2005), and non-parenting males and females do not have significant differences in androgen (11-ketotestosterone) levels, but do have sexually dimorphic genitalia (Rodgers et al., 2006). With aggressive behavior changing first, changes in steroid levels that result from aggressive interactions could then begin the process of gonadal rearrangement during sex change (Carlisle et al., 2000).
The above idea is consistent with the fact that many vertebrates show a rapid increase in androgens following aggressive interactions (the Challenge Hypothesis; Cardwell and Liley, 1991; Hirschenhauser and Oliveira, 2006; Oliveira et al., 1996; Wingfield, 1985; Wingfield and Wada, 1989), but the definitive source of the rapidly elevated steroids has not been identified. It may be that increases in aggression result in subsequent changes in bAA as a means of altering steroids levels, consistent with the increase in steroids that follows aggressive interactions. Because of their naturally high levels of aromatase compared to other vertebrates (Callard et al., 1990; Gelinas et al., 1998), fish are an excellent model system in which to study bAA as a mechanism associated with rapid changes in aggressive behavior as well as bAA effects on circulating levels of steroids.
A final alternative to our original hypothesis is that decreases in bAA and increases in aggressive behavior in alpha females are happening in parallel and do not have any direct causal connection. The independent control of these two responses could involve identical signals that turn on both pathways (decreasing bAA and increasing aggressive behavior) or different signals that each triggers a different pathway.
Behavioral observations of fish implanted with an aromatase inhibitor such as fadrozole could be used to further test for causal links between changes in bAA and behavior. Kroon et al. (2005) demonstrated that whole body fadrozole treatment, which should decrease whole body estradiol levels, resulted in female to male sex change in another goby that can change sex in both directions, the coral goby, Gobiodon erythrospilus. Moreover, increasing estradiol resulted in male to female sex change in this species. These data clearly imply a role for estrogen in the sex change process. However, these fish were exposed to altered steroid levels for a long period (six weeks) and there was no information on the actual estradiol concentrations in these fish and on the direct effects of these treatments on their social behavior. Since it is also well known that steroids directly affect gonadal function in a wide range of fishes (for review see Devlin and Nagahama, 2002), these results do not provide any indication concerning the potential role of acute changes in brain aromatase activity or estradiol levels in initiating the process of socially controlled sex change.
Two recent studies investigated the effect of fadrozole on teleost social behavior. Hallgren et al. (2006) demonstrated that blocking aromatase reduced two of three male sexual displays in guppies, Poecilia reticulata. An experiment with sneaker male peacock blennies, Salaria pavo, showed that estradiol decreased the frequency of aggressive displays towards females, but fadrozole treatment, which decreased estradiol levels, had no effect on female-like courtship displays, time displaying female-like nuptial coloration or aggressive displays towards females (Gonçalves et al., 2007). The direct behavioral effects of experimental manipulations of aromatase activity and brain estrogen concentrations remain uncertain. These studies also examined the effects of decreases in whole body aromatase levels, rather than changes in aromatase within the brain.
In conclusion, the evidence from this study does not support the hypothesis that a decrease in whole brain aromatase activity leads to increases in aggressive behavior in dominant females during the initiation of sex change. The rapid change observed in aggressive behavior is likely caused by a neural mechanism independent or upstream of the changes in brain aromatase. As such, changes in bAA are more likely a result of aggressive interactions or dominant status, and may then drive downstream processes that are critical for accomplishing adult sex change.
Acknowledgments
We thank E. Ball, E. Broadwater, J. Edwards, J. Hicks, T. Holloman, V. Lorenzi, T. Mills, and C. Mizell for help with behavioral observations, J. Pylkkanen for help catching fish, and E. Broadwater for papilla measurements. This paper is contribution no. 246 from the Wrigley Marine Science Center, Catalina Island. This material is based upon work supported in part by the STC Program of the National Science Foundation under Agreement No. IBN-9876754, the Georgia Research Alliance and GSU-RPE program, NIH NRSA F32MH079529 to MPB, NSF-IBN 9723817 and IOB 0548567 to MSG, and NIMH (MH50388) and the Belgian FRFC (2.4537.9) to JB.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baillien M, Balthazart J. A direct dopaminergic control of aromatase activity in the quail preoptic area. J Steroid Biochem Mol Biol. 1997;63:99–113. doi: 10.1016/s0960-0760(97)00080-0. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Phosphorylation processes mediate rapid changes of brain aromatase activity. J Steroid Biochem Mol Biol. 2001a;79:261–277. doi: 10.1016/s0960-0760(01)00143-1. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Ball GF. Rapid and reversible inhibition of brain aromatase activity. J Neuroendocrinol. 2001b;13:61–71. doi: 10.1046/j.1365-2826.2001.00598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Charlier TD, Ball GF. Calcium-dependent phosphorylation processes control brain aromatase in quail. Eur J Neurosci. 2003;17:1591–1606. doi: 10.1046/j.1460-9568.2003.02598.x. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Baillien M, Cornil CA, Ball GF. Preoptic aromatase modulates male sexual behavior: slow fast mechanisms of action. Physiol Behav. 2004;83:247–70. doi: 10.1016/j.physbeh.2004.08.025. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Ball GF. Is brain estradiol a hormone or a neurotransmitter? Trends Neurosci. 2006;29:241–9. doi: 10.1016/j.tins.2006.03.004. [DOI] [PubMed] [Google Scholar]
- Balthazart J, Cornil CA, Taziaux M, Charlier TD, Baillien M, Ball GF. Rapid changes in production and behavioral action of estrogens. Neuroscience. 2006;138:783–91. doi: 10.1016/j.neuroscience.2005.06.016. [DOI] [PubMed] [Google Scholar]
- Batty J. Acute changes in plasma testosterone levels and their relation to measures of sexual-behavior in male house mouse (Mus musculus) Anim Behav. 1978;26:349–357. doi: 10.1016/0003-3472(78)90053-2. [DOI] [PubMed] [Google Scholar]
- Bernhardt PC, Dabbs JM, Fielden JA, Lutter CD. Testosterone changes during vicarious experiences of winning and losing among fans at sporting events. Physiol Behav. 1998;65:59–62. doi: 10.1016/s0031-9384(98)00147-4. [DOI] [PubMed] [Google Scholar]
- Black MP, Reavis RH, Grober MS. Socially induced sex change regulates forebrain isotocin in Lythrypnus dalli. Neuroreport. 2004;15:185–189. doi: 10.1097/00001756-200401190-00036. [DOI] [PubMed] [Google Scholar]
- Black MP, Balthazart J, Ballien M, Grober MS. Socially induced and rapid increases in aggression are inversely related to brain aromatase activity in a sex-changing fish, Lythrypnus dalli. Proc R Soc Lond, B, Biol Sci. 2005a;272:2435–2440. doi: 10.1098/rspb.2005.3210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Black MP, Moore B, Canario AVM, Ford D, Reavis RH, Grober MS. Reproduction in context: Field testing a lab model of socially controlled sex change in Lythrypnus dalli (Gilbert) J Exp Mar Biol Ecol. 2005b;318:127–143. [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1006/abio.1976.9999. [DOI] [PubMed] [Google Scholar]
- Burmeister S, Wilczynski W. Social signals influence hormones independently of calling behavior in the treefrog (Hyla cinerea) Horm Behav. 2000;38:201–209. doi: 10.1006/hbeh.2000.1605. [DOI] [PubMed] [Google Scholar]
- Callard GV, Schlinger BA, Pasmanik M. Nonmammalian vertebrate models in studies of brain-steroid interactions. J Exp Zool Suppl. 1990;4:6–16. doi: 10.1002/jez.1402560404. [DOI] [PubMed] [Google Scholar]
- Cardwell JR, Liley NR. Androgen control of social–status in males of a wild population of stoplight parrotfish, Sparisoma-viride (Scaridae) Horm Behav. 1991;25:1–18. doi: 10.1016/0018-506x(91)90035-g. [DOI] [PubMed] [Google Scholar]
- Carlisle SL. MS Thesis. Arizona State University; Tempe: 2001. Androgens mediate changes in sexually dimorphic structures in the bluebanded goby. [Google Scholar]
- Carlisle SL, Marxer-Miller SK, Canario AVM, Oliveira RF, Carneiro L, Grober MS. Effects of 11-ketotestosterone on genital papilla morphology in the sex changing fish Lythrypnus dalli. J Fish Biol. 2000;57:445–456. [Google Scholar]
- Cheng MF. Vocal self-stimulation: From the ring dove story to emotion-based vocal communication. Adv Study Behav. 2003;33:309–353. [Google Scholar]
- Cheng MF, Peng JP, Johnson P. Hypothalamic neurons preferentially respond to female nest coo stimulation: demonstration of direct acoustic stimulation of lutenizing hormone release. J Neurosci. 1998;18:5477–5489. doi: 10.1523/JNEUROSCI.18-14-05477.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Dalla C, Papadopoulu-Daifoti Z, Baillien M, Dejace C, Ball GF, Balthazart J. Rapid decreases in preoptic aromatase activity and brain monoamine concentrations after engaging in male sexual behavior. Endocrinology. 2005;146:3809–3820. doi: 10.1210/en.2005-0441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Denman H. MS Thesis. Georgia State University; Atlanta: 2005. Sex change in the bluebanded goby (Lythrypnus dalli): the interaction of sex steroids and the social environment. [Google Scholar]
- Devlin RH, Nagahama Y. Sex determination and sex differentiation in fish: an overview of genetic, physiological, and environmental influences. Aquaculture. 2002;208:191–364. [Google Scholar]
- Gelinas D, Pitoc GA, Callard GV. Isolation of a goldfish brain cytochrome P450 aromatase cDNA: mRNA expression during the seasonal cycle and after steroid treatment, Mol. Cell Endocrinol. 1998;138:81–93. doi: 10.1016/s0303-7207(98)00015-x. [DOI] [PubMed] [Google Scholar]
- Gonçalves D, Aledrinha J, Teles M, Oliveira RF. Endocrine control of sexual behavior in sneaker males of the peacock blenny Salaria pavo: Effects of castration, aromatase inhibition, testosterone and estradiol. Horm Behav. 2007;51:534–541. doi: 10.1016/j.yhbeh.2007.02.003. [DOI] [PubMed] [Google Scholar]
- Gonçalves D, Teles M, Aledrinha J, Oliveira RF. Brain and gonadal aromatase activity and steroid hormone levels in female and polymorphic males of the peacock blenny Salaria pavo. Horm Behav. 2008;54:717–725. doi: 10.1016/j.yhbeh.2008.07.014. [DOI] [PubMed] [Google Scholar]
- Greenberg N, Crews D. Endocrine and behavioral-responses to aggression and social-dominance in the green anole lizard, Anolis carolinensis. Gen Comp Endocrinol. 1990;77:246–255. doi: 10.1016/0016-6480(90)90309-a. [DOI] [PubMed] [Google Scholar]
- Hallgren SLE, Linderoth M, Olsén KH. Inhibition of cytochrome p450 brain aromatase reduces two male specific sexual behaviours in the male Endler guppy (Poecilia reticulata) Gen Comp Endocrinol. 2006;147:323–328. doi: 10.1016/j.ygcen.2006.02.005. [DOI] [PubMed] [Google Scholar]
- Hirschenhauser K, Oliveira RF. Social modulation of androgens in male vertebrates: meta-analyses of the challenge hypothesis. Anim Behav. 2006;71:265–277. [Google Scholar]
- Kroon FJ, Munday PL, Westcott DA, Hobbs JPA, Liley NR. Aromatase pathway mediates sex change in each direction. Proc R Soc Lond, B, Biol Sci. 2005;272:1399–1405. doi: 10.1098/rspb.2005.3097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lorenzi V, Earley RL, Rodgers EW, Pepper DR, Grober MS. Diurnal patterns and sex differences in cortisol, 11-ketotestosterone, testosterone, and 17b-estradiol in the bluebanded goby (Lythrypnus dalli) Gen Comp Endocrinol. 2008;155:438–446. doi: 10.1016/j.ygcen.2007.07.010. [DOI] [PubMed] [Google Scholar]
- Lorenzi V, Carpenter RE, Summers CH, Earley RL, Grober MS. Serotonin, social status and sex change in the bluebanded goby Lythrypnus dalli. Physiol Behav. 2009;97:476–483. doi: 10.1016/j.physbeh.2009.03.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Macrides F, Bartke A, Dalterio S. Strange females increase plasma testosterone levels in male mice. Science. 1975;189:1104–1106. doi: 10.1126/science.1162363. [DOI] [PubMed] [Google Scholar]
- Oliveira RF, Almada VC, Canario AVM. Social modulation of sex steroid concentrations in the urine of male cichlid fish Oreochromis mossambicus. Horm Behav. 1996;30:2–12. doi: 10.1006/hbeh.1996.0002. [DOI] [PubMed] [Google Scholar]
- Peterson RS, Yarram L, Schlinger BA, Saldanha CJ. Aromatase is pre-synaptic and sexually dimorphic in the adult zebra finch brain. Proc R Soc Lond, B, Biol Sci. 2005;272:2089–2096. doi: 10.1098/rspb.2005.3181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pradhan DS, Newman AE, Wacker DW, Wingfield JC, Schlinger BA, Soma KK. Aggressive interactions rapidly increase androgen synthesis in the brain during the non-breeding season. Horm Behav. 2010;57:381–389. doi: 10.1016/j.yhbeh.2010.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reavis RH, Grober MS. An integrative approach to sex change: social, behavioral and neurological changes in Lythrypnus dalli. Acta Ethologica. 1999;2:51–60. [Google Scholar]
- Remage-Healey L, Bass AH. Rapid hierarchical modulation of vocal patterning by steroid hormones. J Neurosci. 2004;24:5982–5900. doi: 10.1523/JNEUROSCI.1220-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. Rapid elevations in both steroid hormones and vocal signaling during playback challenge: A field experiment in Gulf toadfish. Horm Behav. 2005;47:297–305. doi: 10.1016/j.yhbeh.2004.11.017. [DOI] [PubMed] [Google Scholar]
- Remage-Healey L, Bass AH. A rapid neuromodulatory role for steroid hormones in the control of reproductive behavior. Brain Res. 2006;1126:27–35. doi: 10.1016/j.brainres.2006.06.049. [DOI] [PubMed] [Google Scholar]
- Rodgers EW, Earley RL, Grober MS. Elevated 11-ketotestosterone during paternal behavior in the Bluebanded goby (Lythrypnus dalli) Horm Behav. 2006;49:610–614. doi: 10.1016/j.yhbeh.2006.01.008. [DOI] [PubMed] [Google Scholar]
- Rodgers EW, Earley RL, Grober MS. Social status determines sexual phenotype in the bi-directional sex changing bluebanded goby, Lythrypnus dalli. J Fish Biol. 2007;70:1660–1668. [Google Scholar]
- Roney JR, Mahler SV, Maestripieri D. Behavioral and hormonal responses of men to brief interactions with women. Evol Hum Behav. 2003;24:365–375. [Google Scholar]
- Roselli CE, Resko JA. In vitro assay of aromatase activity in the central nervous system. In: Greenstein B, editor. Neuroendocrine Research Methods. Vol. 2. Harwood Academic Publishers; Chur, Switzerland: 1991. pp. 937–951. [Google Scholar]
- Schlinger BA, Arnold AP. Brain is the major site of estrogen synthesis in a male songbird. Proc Natl Acad Sci USA. 1991;88:4191–4194. doi: 10.1073/pnas.88.10.4191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma TP, Blache D, Roselli CE, Martin GB. Distribution of aromatase activity in brain and peripheral tissues of male sheep: effect of nutrition. Reprod Fertil Dev. 2004;16:709–715. doi: 10.1071/rd04018. [DOI] [PubMed] [Google Scholar]
- St Mary CM. Novel sexual patterns in two simultaneous hermaphroditic gobies, Lythrypnus dalli and Lythrypnus zebra. Copeia. 1993;1993:1062–1072. [Google Scholar]
- Wingfield JC. Short-term changes in plasma-levels of hormones during establishment and defense of a breeding territory in male song sparrows, Melospiza melodia. Horm Behav. 1985;19:174–187. doi: 10.1016/0018-506x(85)90017-0. [DOI] [PubMed] [Google Scholar]
- Wingfield JC, Wada M. Changes in plasma-levels of testosterone during male-male interactions in the song sparrow, Melospiza melodia—Time course and specificity of response. J Comp Physiol A. 1989;166:189–194. [Google Scholar]
- Wingfield JC, Hegner RE, Dufty AM, Ball GF. The “Challenge Hypothesis”: Theorectical implications for patterns of testosterone secretion, mating systems and breeding strategies. Am Nat. 1990;136:829–846. [Google Scholar]
- Yang EJ, Wilczynski W. Relationships between hormones and aggressive behavior in green anole lizards: an analysis using structural equation modeling. Horm Behav. 2002;42:192–205. doi: 10.1006/hbeh.2002.1811. [DOI] [PubMed] [Google Scholar]
- Zhao J, Mak P, Tchoudakova A, Callard G, Chen S. Different catalytic properties and inhibitor responses of the goldfish brain and ovary aromatase isozymes. Gen Comp Endocrinol. 2001;123:180–191. doi: 10.1006/gcen.2001.7661. [DOI] [PubMed] [Google Scholar]