Abstract
The role of NMDA receptors (NMDARs) expressed by primary afferent neurons in nociception remains controversial. The aim of this study was to develop mice with a tissue selective knockdown of NMDARs in these neurons and to evaluate their behavioral responses to different types of painful stimuli. Mice with floxed NMDAR NR1 subunit gene (fNR1) were crossed with mice expressing Cre recombinase under the control of the peripherin promotor (Prph-Cre). Male Prph-Cre+ floxed NR1 mice were compared to Cre− littermates. Both quantitative RT/PCR and Western blotting indicated a ~75 % reduction in NR1 expression in DRG extracts with no effect on NR1 expression in spinal cord, brain or the enteric nervous system. Immunocytochemistry with antibodies to NR1 revealed decreased staining in all size classes of DRG neurons. NMDA produced a detectable increase in [Ca2+]i in 60 % of DRG neurons cultured from Cre− mice, but only 15% of those from Cre+ mice. Furthermore, the peak [Ca2+]i responses were 64% lower in neurons from Cre+ mice. There was no significant difference between Cre+ and Cre− mice in response latencies to the hotplate or tail withdrawal tests of thermal nociception, nor was there a difference in withdrawal thresholds to mechanical stimuli of the tail or paw. However, compared to Cre− littermates, Cre+ knockdown mice had a 50% decrease in the phase 2 response to formalin injection (p<0.001). There was no effect on phase 1 responses. These results suggest that NMDA receptors expressed by primary afferent nerves play an important role in the development of sensitized pain states.
Keywords: NMDA receptor, dorsal root ganglia, hyperalgesia, nociception, presynaptic
Introduction
N-methyl-D-aspartate receptors (NMDARs) are ionotropic glutamate receptors that have long been known to play an important role in nociceptive transmission. NMDARs residing on dorsal horn neurons are critical in mediating spinal cord wind-up leading to central sensitization to noxious stimuli, particularly under conditions of injury (Davies and Lodge, 1987, Dickenson and Sullivan, 1987a, South et al., 2003). However, NMDARs are also expressed on sensory afferent neurons that innervate peripheral tissues and make synapses with dorsal horn neurons (Lovinger and Weight, 1988, Sato et al., 1993, Liu et al., 1994, Carlton et al., 1995, Ma and Hargreaves, 2000, McRoberts et al., 2001, Marvizon et al., 2002). Stimulation of these peripheral receptors with selective agonists evokes pain behaviors that are blocked by NMDAR antagonists (Zhou et al., 1996, Cairns et al., 2003, Du et al., 2003, Cairns et al., 2006). In addition, peripheral injection of antagonists of NMDARs inhibit behavioral responses to subcutaneous injection of formalin (Davidson et al., 1997, Davidson and Carlton, 1998) , although the specificity of this effect has been questioned (Sawynok and Reid, 2002). In the spinal cord, NMDARs on presynaptic afferent terminals have been shown to mediate neuropeptide release, particularly that of substance P (Liu et al., 1997, Marvizon et al., 1997, Malcangio et al., 1998), although this too has been recently disputed (Nazarian et al., 2008). Since release of neuropeptides such as calcitonin gene-related peptide (CGRP) and substance P are involved in enhanced pain states, facilitation of neuropeptide release would be pro-nociceptive. On the other hand, there is evidence that presynaptic NMDARs decrease glutamate release (Bardoni et al., 2004), which would act as a brake on nociceptive transmission. However in opioid tolerant rats, presynaptic NMDARs increased glutamate release, indicating a pro-nociceptive role under these conditions (Zeng et al., 2006). Thus, the role of NMDARs on primary afferent nerves and their terminals remains controversial.
Part of the problem in dissecting the role of NMDARs on primary afferents is that most of the studies have relied on a pharmacological approach where it is difficult to discriminate between NMDARs on primary afferent terminals and those on dorsal horn neurons. An alternate approach is tissue-targeted deletion of the NMDAR using the Cre-Lox system. Since NMDARs are heteromeric assemblies of an obligatory NR1 subunit together with various combinations of NR2 or NR3 subunits, Cre recombinase-mediated deletion of a floxed NR1 gene will knockout NMDARs. This approach has been used to demonstrate that NMDARs expressed by dorsal horn neurons in the spinal cord are required for central sensitization (South et al., 2003). Using previously developed mice engineered to express Cre recombinase under the control of the peripherin promoter which drives expression of Cre in nearly all sensory neurons (Zhou et al., 2002), we used this approach to ascertain the function of NMDARs on primary afferent nerves in nociception.
Materials and Methods
Animals
Mice engineered with loxP sites flanking exons 11–22 of the NR1 subunit gene (floxed-NR1; backcrossed 8 times into the C57BL/6Tac background) were crossed with mice engineered to express Cre recombinase under the control of the peripherin promotor (Prph-Cre; obtained from MMRRC, Santa Cruz, CA, strain #0120; backcrossed >10 times in the C57BL/6Tac background). The resulting Prph1-Cre x floxed-NR1 mice (denoted Prph-fNR1 in this paper) were viable and fertile. Pairings to produce the mice tested were between male Cre−/Cre− fNR1/fNR1 and Cre+/− fNR1/fNR1 female mice. Genotyping was carried out using standard PCR conditions and primers for floxed and wild type NR1 and Cre recombinase. The primer sequences were NR1mut1: CTTGGGTGGAGAGGCTATTC and NR1mut2: AGGTGAGATGACAGGAGATC for floxed NR1; NR1Ctrl1: GTGAGCTGCACTTCCAGAAG and NR1Ctrl2: GACTTTCGGCATGTGAAATG for wild type NR1; and CreDN2 GATCTCCGGTATTGAAACTCCAGC and CreUP2 GCTAAACATGCTTCATCGTCGG for Cre recombinase. All mice were housed 4 or less per cage, provided unlimited access to food and water, maintained on a 12 h light / dark cycle, and tested during the light phase. All experiments were conducted in accordance with the National Institutes of Health Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of California, Los Angeles.
RT/PCR
Tissue samples dissected from the frontal cortex, spinal cord, T12 through S2 DRG, 2–3 cm of distal ileum and proximal colon were preserved in RNA-later (Ambion) at −20°C until use. Total RNA was isolated from ~100 mg samples of tissue using the TRIZOL reagent kit (Gibco-ultra pure) and reverse-transcribed with Superscript II RT (Gibco-BRL) using random hexamers and following the manufacturer’s instructions. Quantitative real time PCR was performed using an Applied Biosystems Geneamp 5700 sequence detection system and SYBR® Green reagents (Applied Biosystems, Foster City, CA). The PCR program was: stage 1, 50°C, 2 min; stage 2, 95°C, 10 min; stage 3, 40 cycles, each consisting of 15 s at 95°C and 60 s at 60°C; ending at 25°C. Primer pairs for NR1 and a set of housekeeping genes were selected that crossed intron-exon boundaries as predicted from the mouse Genome Browser at UCSC (Feb 2006 assembly; http://genome.ucsc.edu/). The sequences were (forward/reverse): ACTCCCAACGACCACTTCAC / GTAGACGCGCATCATCTCAA for NR1 (186 bp product), TAAGGCCAACCGTGAAAAGA / ACCAGAGGCATACAGGGACA for beta-actin (103 bp product), ACCACACTCTGGGGAACATC/ CTCGCTGATGAGGTCTGTGA for RNA polymerase II, polypeptide J (Polr2j, 176 bp product), and GGAGTTAGCGGTGCAGATTC / AGCATCTTGATAGCCCGTGT for DEAD box polypeptide 48 (Ddx48, 204 bp product). Primer pairs were optimized using stock RNA prepared from mouse brain. Detection of PCR products was monitored by the increase in fluorescence caused by the binding of SYBR® Green to double stranded DNA. To verify the homogenous nature of the PCR product, melting point determinations were evaluated at the end of each reaction. The ΔCT method was used to compare mRNA expression of each target gene relative to that of several “housekeeping” genes. We used the geometric mean (Vandesompele et al., 2002) of three housekeeping genes (Actb, β-actin. Polr2j, RNA polymerase II polypeptide J, and Ddx48, DEAD box polypeptide 48) reported to be stably expressed in neuronal tissue under a variety of conditions (Méchaly et al., 2006). ΔCT indicates the difference between the number of cycles necessary to just detect linear amplification of the NR1 PCR product relative to that of the housekeeping genes. Data were expressed as 2−ΔCT in order to give a linear estimate of the amount of NR1 mRNA. For graphical presentation, data were expressed as 2−ΔCT, where the expression levels of NR1 in Cre+ mice were normalized to the average expression level in the same tissue from control, Cre− mice.
Tissue extraction and Western blotting
Integral membrane proteins from T12 through S2 DRG were prepared by addition of a 3-fold volume of ice-cold solubilization buffer (150 mM NaCl, 50 mM Tris-HCl, pH 7.5, 1 mM EDTA, 1% Triton X-100, 0.5% sodium deoxycholate and 0.1% SDS) containing protease inhibitors (1 mM phenyl-methyl-sulfonyl fluoride, and 5 μg/ml each pepstatin, leupeptin, chymostatin, antipain, aprotinin). The tissue was finely minced and then homogenized with a Dounce homogenizer using 10 strokes each with a loose-fitting and then a tight-fitting pestle. After setting on ice for 30 minutes, the homogenate was centrifuged at 10,000xg for 5 minutes at 4°C. The supernatant was collected and assayed for protein content using the BCA assay method (Thermo Fisher Scientific, Rockford, IL), and stored at −20°C until used. Twenty μg of protein was electrophoresed on 3–8% NuPAGE Tris-Acetate SDS gels (InVitrogen, Carlsbad, CA) using sample buffer, running buffer and molecular weight standards suggested by the manufacturer. After electrophoresis, proteins were transferred to nitrocellulose membranes, blocked with 3% non-fat dry milk (BioRad Corp., Hercules, CA) and the membranes probed with a goat antibody to NR1 (1:200, Santa Cruz Biotechnology, Santa Cruz, CA). Protein bands were visualized using a horseradish peroxidase-conjugated secondary antibody (Chemicon brand, Millipore, Billerica, MA) and an enhanced chemoluminescent detection system (Thermo Fisher Scientific, Rockford, IL). To control for protein loading, the membranes are stripped (Restore Western Blotting Stripping Buffer; Thermo Fisher Scientific) and then re-probed with a monoclonal antibody to β-actin (1:4000; Cell Signaling Technology, Danvers, MA). The relative expression of NR1 was calculated from the ratio of NR1 band intensity to that of β-actin.
Immunocytochemistry
DRG (L5, L6) were isolated from adult male mice, fixed overnight in 4% paraformaldehyde, 0.18% picric acid in phosphate buffer (PB, 0.1 M sodium phosphate, pH 7.4) then cryoprotected in 20% sucrose, PB. Free-floating sections (25 μm thick) were prepared with a cryostat after embedding the DRG in a drop of Tissue-Tek (Sakura Finetek USA, Inc., Torrance, CA) and freezing it on dry ice. Sections were washed twice with PBS and twice with PBS containing 0.5% Triton X-100, 0.01% thimerosal (PBS/Triton) and 10% normal donkey serum (NS, Jackson ImmunoResearch, West Grove, PA). Sections were incubated with a goat antibody to NR1 (1:100, Santa Cruz Biotech) in 10% NS, PBS/Triton at room temperature for 1 hr and overnight at 4°C. We have used this antibody previously to demonstrate expression and localization of NR1 in the cell body and peripheral terminals of primary afferents (McRoberts et al., 2001, Marvizon et al., 2002). After three washes with 1% NS, PBS/Triton, sections were incubated at room temperature for 2 hr with Alexa Fluor 488 -labeled secondary antibody (1:100, Molecular Probes, Eugene, OR) diluted in 10% NS, PBS/Triton. Sections were washed four more times with PBS and mounted in Prolong (Molecular Probes, Eugene, OR) to reduce photobleaching.
Microscopy and Analysis
Sections were examined with a Zeiss Axioskop epifluorescent microscope using a Zeiss 40x Plan-apo objective (1.0 NA, Carl Zeiss Inc., Jena, Germany). Images were acquired in a blinded fashion using a SPOT CCD digital camera (Diagnostic Instruments, Inc., Sterling Heights, MI) with identical exposure settings (300 msec, gain =1). Images were analyzed using ImageJ (NIH) following the method of Hoffman et al. (Hoffman et al., 2009). Each neuron with a visible nucleus was outlined on a Cintiq 21UX interactive pen display (Wacom; Kita Saitama-Gun, Saitama, Japan). Nuclei were excluded, making each region of interest appear as a donut covering cytoplasm of a single DRG neuron. The mean gray value, perimeter and Ferret diameter in μm2 for each cytoplasmic profile was measured and copied to a spreadsheet. A threshold value of 50% above background as measured over an unstained area of the slide was subtracted from all values. Neurons were classified according to size using and average of the Ferret diameter and the calculated diameter from the perimeter.
Primary culture of DRG neurons and Ca2+ imaging
Isolation and culture of adult mouse DRG neurons followed the method previously described for mouse DRG neurons (Chaban et al., 2001) except that 0.2 mM ketamine (Sigma Chemicals) was added to the final isolation buffer and culture media in order to preserve NMDAR function. Neurons were cultured on Matrigel-coated 15 mm glass coverslips (Collaborative Research Co., Bedford, PA) and studied after 48–72 hours in culture. Changes in [Ca2+]i in response to 250 μM NMDA and 10 μM glycine were determined by ratiometric imaging of the Ca2+-sensitive fluorescent dye, Fura-2 as described previously (Li et al., 2004).
Behavioral testing
Rotorod test of motor coordination
Mice were placed on a computer controlled rotating rod (TSE Systems, Midland, MI) initially rotating at 5 rpm. The speed of rotation was programmed to increase to 60 rpm over a 3 min period and the time until the mice fell off the rod recorded. The test was repeated twice on the same day with a >30 min interval between tests, and again 5–7 days later. The best times for each day were averaged and recorded as the latency to fall off the rod
Hot plate test of thermal nociception
The hot plate assay involved placing the mice on all four feet onto a hot aluminum surface (52.5°C) and recording the latency to lick or lift one of the hindpaws or jump with all 4 feet leaving the hotplate to the nearest 0.1 sec. A cutoff time of 60 sec was used prevent burn injury. (Bryant et al., 2006)
Tail withdrawal test of thermal nociception
The tail withdrawal test was done by placing mice in a soft cloth restraint with a plastic base and dipping the distal third of their tails into a circulating water bath (49°C). The latency to flick the tail out of the water was recorded with a stopwatch to the nearest 0.1 sec. A cut-off of 15 sec was employed to prevent injury.
Tail pressure test of mechanical nociception
The tail pressure test was employed to measure mechanical nociception (Martin et al., 2003). Mice were gently held in a cloth restrainer and increasing pressure was applied locally to the tail by using an analgesia meter that increased pressure at a constant rate (model 7200; Ugo Basile, Italy). The pressure was applied to a surface of 1 mm square of the tail skin by placing the tail of the animal between the metal base and the Teflon plunger. Pressure was increased by depressing a foot pedal until a tail withdrawal response was elicited. The cut-off was established at 400 g. Three consecutive determinations were made in the distal, medial and proximal part of the tail, separated by 15 s. The test was repeated after a 30 min interval, and again 1 week later. The average of the three responses was determined for each animal.
Paw withdrawal threshold to mechanical probing
The mice were placed in a clear plastic, wire mesh-bottomed cage, divided into individual compartments and allowed to acclimate 30 min prior to testing. Baseline mechanical sensitivity was determined by applying a series of logarithmically spaced calibrated von Frey filaments (0.02–2 g, Stoelting, Wood Dale, IL) to the plantar surface of the left hind paw. A positive response was indicated by a sharp withdrawal of the hind paw. Testing began with the 0.4 g filament and proceeded according to the up-down method described by Chaplan et al.(Chaplan et al., 1994). The 50% probability paw withdrawal threshold was calculated using the formula given by Dixon (Dixon, 1980).
Formalin test
Mice were acclimated to mirror backed cages in a darkened room. A dilute solution of formalin (5%) was then administered in a volume of 20 μl into the footpad of the left hindpaw of a lightly restrained mouse. Immediately after the formalin injection, the mouse was returned to the test chamber and videotaped continuously for the next 60 min. An individual, unaware of the genotype, scored the videotape. The time spent licking or lifting the left hindpaw was recorded during each of the 5 min intervals using the periodic sampling method (visual scoring every 5 sec) (Helmstetter and Fanselow, 1987).
Statistical Analysis
Except where noted, data were analyzed using one or two-way ANOVA with Tukey or Bonferroni post-test comparisons or as unpaired Student’s t-tests as appropriate. A P value of ≤ 0.05 was taken as statistically significant. Prism software was used (GraphPad, San Diego, CA).
Results
Decreased expression of NR1 and functional NMDARs in Prph-NR1 mice
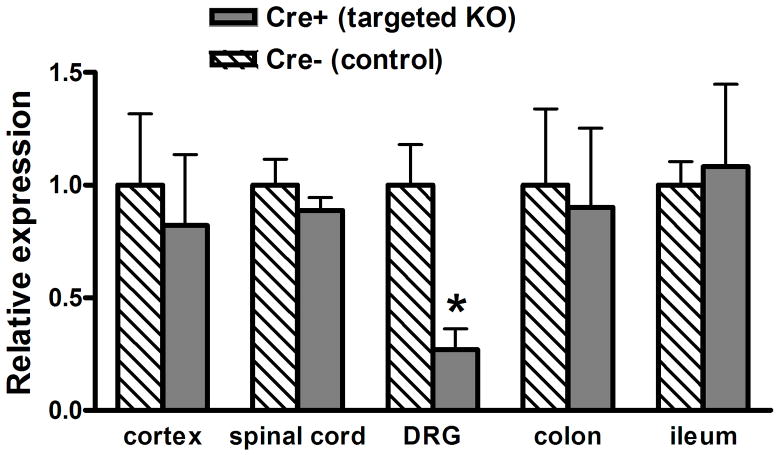
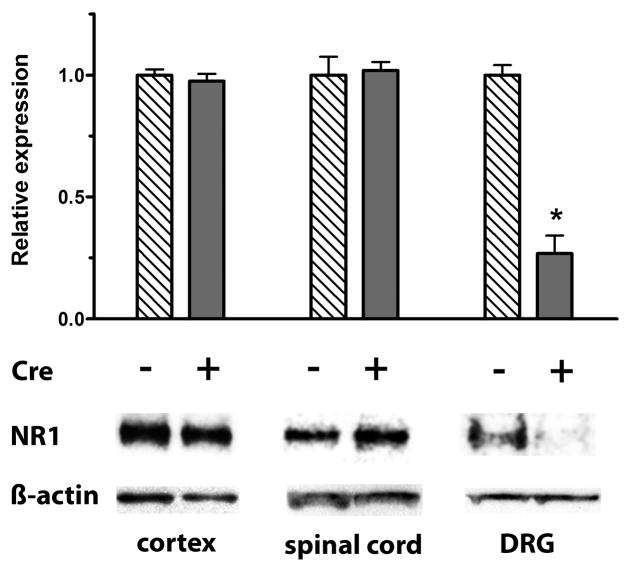
Quantitative RT/PCR of mRNA was used to estimate the relative expression levels of NR1 in different tissues from Cre+ Prph-fNR1 (Prph-Cre+ x floxed-NR1) mice compared to wild type (Cre−) littermates. RNA was extracted from DRG, spinal cord, cortex, ileum and colon and relative expression levels were calculated as using the ΔΔCT method. As shown in Figure 1, there was a 75 ± 20% (n=4 of each genotype) reduction of NR1 mRNA extracted from DRG, with no effect on NR1 mRNA expression in the spinal cord, brain or enteric nervous system. Western blots of protein extracted from all levels of DRG confirmed these results. Normalizing the expression of NR1 to β-actin indicated a 73 ± 8% (n=4 each) decrease in NR1 expression in DRG with no effect on expression in spinal cord or cortex (Figure 2).
Figure 1. Selective decrease in NR1 mRNA in DRG from Cre+ mice.
Quantitative RT/PCR of mRNA was used to estimate the relative expression levels of NR1 in different tissues from 4 Cre+ Prph-fNR1 mice compared to 4 WT (Cre−) littermates. RNA was extracted from DRG (T12-S2), spinal cord, cortex, ileum and colon and amplified with primers specific for NR1 and three housekeeping genes. The 2−ΔCt values in Cre− mice, which reflect NR1 expression levels relative to the housekeeping genes, were 1.15, 0.208, 0.127, 0.00150, 0.000785 for cortex, spinal cord, DRG, colon and ileum, respectively. Expression levels are graphed as the 2−ΔΔCT values which normalizes expression in each tissue from Cre+ mice to that of the same tissue from Cre− mice. * t6 = 2.925, P = 0.0265 Cre+ vs. Cre− mice, unpaired t-test.
Figure 2. Decreased NR1 protein in DRG from Cre+ mice.
Western blot analysis of NR1 protein in DRG, spinal cord and cortex from 4 Cre+ Prph-fNR1 male mice compared to 4 WT (Cre−) littermates. Results were normalized to β-actin and indicate a 73 ± 8% (n=4) knockdown in NR1 expression in DRG with no effect on expression in spinal cord or cortex. * t6 = 8.665, P = 0.001 Cre+ vs. Cre− mice, unpaired t-test.
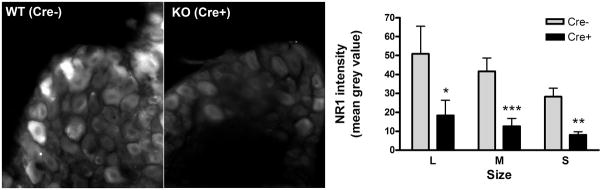
In sections of DRG from targeted knock out (Cre+) mice and phenotypically wild type (Cre−) littermates, immunocytochemistry revealed much less NR1 immunoreactivity in most DRG neurons from the mutant mice (Figure 3). NIH ImageJ was used to quantitatively measure the intensity of the NR1-ir in images taken from 2 non-adjacent sections per mouse from 3 mice of each genotype. Overall the mean grey density was decreased 72 ± 7% in sections from the Cre+ mutant mice compared to the wildtype Cre− mice (t64 = 5.875, P<0.0001, t-test). As shown in the bar graph in Figure 3, there was a significant decrease in staining across all size classes of neurons. Two way analysis of variance of the staining intensity indicated a significant effect of size (P<0.03), but a much stronger effect of genotype (P<0.0001). There was a slightly higher degree of knockdown in small (S, <30μm diameter; 74% decease) compared to medium size (M, 30–40μm; 70%) and to large size neurons (L, >40μm diameter; 64%), however these differences were not significant. These results indicate that NR1 was knocked down to a similar degree in the three afferent fiber types, C, Aδ, and Aβ.
Figure 3. Decreased NR1 immunoreactivity in DRG from Cre+ mice.
Representative fluorescent micrographs of sections of L5 and L6 DRG from Cre− wild type mice (WT, left) and Cre+ Prph-fNR1 knockdown mice (KO, right) are shown together with a bar graph depicting the relative degree of staining in different size classes of neurons. The intensity of the NR1-immunoreactivity over the cytoplasm of neurons with visible nuclei was quantified using NIH ImageJ as described in the methods section. Two sections each from 3 mice of each genotype were quantified. Neurons were binned into three classes according to size: small (S; <30μm diameter) and medium (M, 30–40μm) and large neurons (L, >40μm diameter). A total of 117 neuron profiles were quantified (72 from Cre+ and 45 from Cre− mice) of which 63 were classified as small, 44 as medium and 14 as large. Two-way ANOVA indicated that both neuron size (F2, 115 = 3.92, P = 0.023) and mouse genotype (F1, 115 =30.38, P < 0.0001) had a significant effect on NR1 immunoreactivity, however interaction between the two variables did not reach significance (P = 0.497) meaning that there was no significant effect of size on the degree of knockdown in the Cre+ mice. Bonferroni post-test comparisons within each size class indicated that NR1 immunoreactivity was significantly decreased in all size classes of neurons from Cre+ mice compared to Cre− littermates: * P<0.05; ** P<0.01; *** P<0.001.
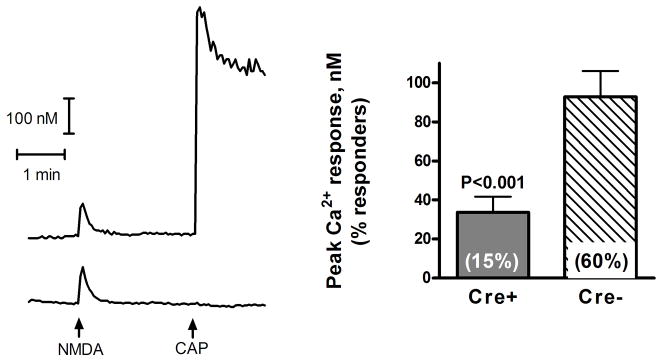
To determine if the decrease in mRNA and protein expression resulted in a decrease in the functional expression of NMDARs on the cell surface, DRG neurons were isolated, placed in primary culture and NMDAR-mediated [Ca2+]i transients quantified. Short (4 sec) application of saturating concentrations of the two co-agonists, NMDA (250 μM) and glycine (10 μM), in Mg2+-deficient Hank’s buffer led to detectable (≥10 nM) increases in intracellular [Ca2+]i in 38 out of 63 (60%) small to medium size (< 40μm diameter) DRG neurons from Cre− (WT) mice, but only 12 of 81 (15%) neurons from Cre+ (KO) mice (difference between proportions = 45%, P<0.001, two-sided Fisher's exact test). Furthermore, as shown in Figure 4, the mean change in peak [Ca2+]i response was significantly smaller in neurons from Cre+ mice compared to Cre− mice (t47 =3.828, P=0.0004, Welch-corrected t-test). There was no significant difference between the neurons from Cre+ and Cre− mice in the frequency (~50% of the neurons) or magnitude (~200 nM) of the response to subsequent application of 10 μM capsaicin indicating that the knockdown of [Ca2+]i responses was specific for NMDARs.
Figure 4. Decreased NMDAR-mediated [Ca2+]i transients in DRG neurons from Cre+ mice.
DRG neurons from Cre+ and Cre− mice were placed in primary culture and loaded with Fura-2. [Ca2+]i increases in small to medium size (<40 μm diameter) neurons were measured in response to short (4 sec) application of 250 μM NMDA and 10 μM glycine in Mg2+-deficient Hank’s buffer. As an internal control we also quantified [Ca2+]i responses to subsequent addition of 10 μM capsaicin (CAP). Traces on the left are representative of the responses of two neurons from Cre− mice, one of which responded to capsaicin (top trace), while the other did not (lower trace). The response of neurons from Cre+ mice to NMDA and glycine was similar to that of Cre− mice, except there were significantly fewer neurons that responded to NMDA and the peak [Ca2+]i response of those that did respond was smaller. The bar graph on the right shows the peak [Ca2+]i response of 38 neurons from Cre− and 12 neurons from Cre+ mice (t47 = 3.828, P=0.0004, Welch-corrected t-test). Values in parenthesis inside the bars indicate the portion of neurons that responded with a detectable increase in [Ca2+]i (≥10 nM).There was no significant difference between the 2 groups in the frequency (~50%; 46 of 81 neurons from Cre+ mice, 29 of 63 neurons from Cre− mice) or magnitude (~200 nM) of the response to subsequent application of 10 μM capsaicin.
Behavioral effects of NMDAR knockdown
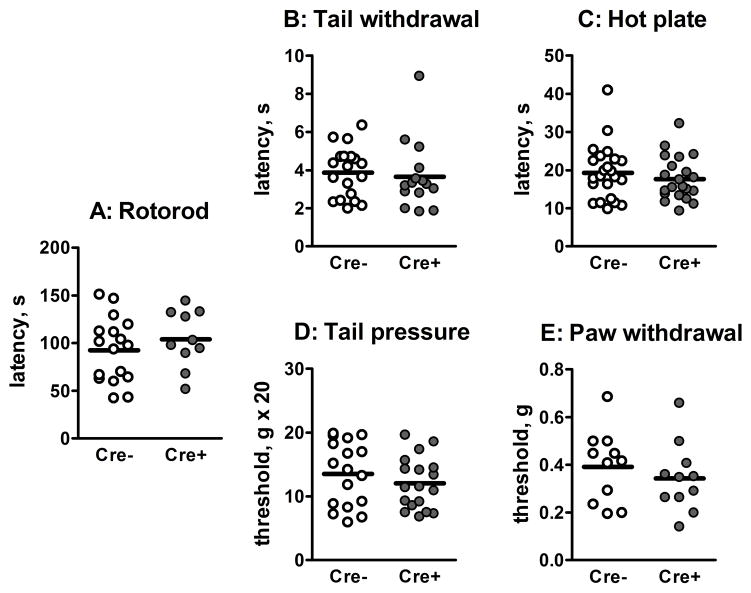
Mice were tested for differences in coordination using the rotorod assay which involves placing the mouse on a slowly turning rod that was programmed to increase in speed. The latency to fall off is an indication of gross motor coordination. We found no differences between the Cre+ mice and their wild type siblings (Figure 5A). To determine whether knockdown of NMDARs on sensory neurons had an effect on behavioral nociceptive responses, we examined responses to both acute thermal and mechanical stimuli. Thermal nociceptive responses were measured using the tail flick assay and the hot plate assay. The former response involves a spinal reflex while the latter involves a supraspinal response. Response latencies in both these tests were not different between the two groups of mice (Figure 5B, C). Mechanical nociceptive responses were made using the tail pinch and paw withdrawal assays. Again there was no significant difference between Cre+ and Cre− mice in the threshold values to either stimuli (Figure 5D, E).
Figure 5. Comparison of motor coordination and basal nociceptive responses between Cre− and Cre+ Prph-fNR1 mice.
A: Rotorod test of gross motor coordination. Values are the latencies for the mice to fall off a slowly accelerating rod. B: Thermal tail withdrawal test. Times are the latency to withdrawal the tail from a 48.5 ºC hot water bath. C: Hot plate test. Values are the latency to lick the hind paw or jump after the mice were placed on a 52.5°C hot plate. D: Tail pressure test. Measurements were made with a Ugo Basile Analgesia-Meter at 3 points along the tail and averaged. Values are the pressure, in grams, at which the mouse twitched or withdrew its tail. E: Mechanical paw withdrawal test. Paw withdrawal thresholds to application of a graded series of von Frey fibers were estimated using the up-down method. Values are in grams of applied force. For A–E, n=10–17 mice of each genotype.
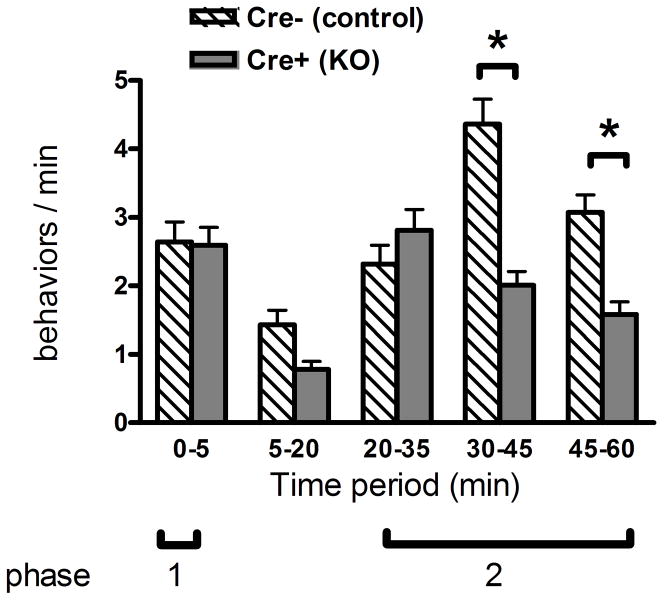
The formalin test involves injecting one hind paw of the mouse with a dilute solution of formalin (20 μl 5% in PBS) and counting the nociceptive behaviors (licking, lifting or shaking of the injected hind paw) over the next hour. Behavioral responses occur in two phases. There is an early phase (phase 1) occurring during the first 5 min after injection, followed by a short quiescent period and the development of a prolonged phase 2 response. The first phase is thought to be due to direct effects of formalin on the peripheral nerve terminals, probably mediated by activation of the TrpA1 channel (McNamara et al., 2007). The second phase involves central sensitization of dorsal horn neurons or “spinal cord wind-up” (Herrero et al., 2000). In addition, at the concentration used in this study, formalin causes a localized inflammatory response which is thought to contribute to peripheral sensitization of the nociceptive terminals (Yashpal and Coderre, 1998). As shown in Figure 6, we found no difference in the phase 1 response to formalin between Cre+ Prph-fNR1 mice compared to their Cre− littermates, however, there was a significant ~50% decrease in later 2 periods (30–45 and 45–60 min) of the phase 2 response of the Cre+ mice (P<0.001).
Figure 6. Cre+ Prph-fNR1 have decreased response in phase 2 of the formalin test.
Male mice (3–9 months old; 9 Cre+, 9 Cre− littermates) were injected with a dilute solution of formalin in the left hind paw and spontaneous pain behaviors were recorded over the next hour. Cre+ Prph-fNR1 mice had a ~50% decrease in the later part of the phase 2 (inflammatory hyperalgesia) response compared with Cre− mice. A two-way ANOVA indicated an extremely significant effect of genotype (F1,80 = 25.05, P<0.0001) and time (F4,80 = 18.05, P<0.0001) with a highly significant degree of interaction between these two variables (F4,80 = 9.79, P<0.0001). Bonferroni post-test comparisons indicated a significant difference between Cre+ mice and their Cre− littermates in the number of responses during the last two time periods (30–45, 45–60 min; *P<0.001).
Discussion
Targeted knock down of NMDARs using Cre expression driven by the peripherin promoter produced a ~75% decrease in NR1 expression in DRG neurons with no change in NR1 expression in the CNS, spinal cord, or enteric nervous system. The decrease in NR1 expression occurred across all size classes of DRG neurons indicating a similar degree of knockdown in C, Aδ and Aβ fibers. We also demonstrated a decrease in NMDA-mediated Ca2+ influx, indicating that reduced expression of the NR1 subunit caused a decrease in functional cell surface NMDARs. There was no difference between Cre+ Prph-fNR1 knockdown mice and their Cre− wild type littermates in motor coordination or acute nociceptive responses to thermal or mechanical stimuli, indicating that these receptors do not participate in primary nociception. However the knockdown mice had a 50% decrease in the phase 2 of the formalin test. This result suggests that NMDARs on primary afferent neurons have an important role in the development of hyperalgesia associated with peripheral inflammation and central sensitization. Our results are similar to those of South et al. (South et al., 2003) who used the same floxed NR1 mice and viral vector-mediated Cre recombinase expression to selectively knockdown NMDARs in dorsal horn neurons, but not DRG neurons. These mice had normal acute pain responses to mechanical or thermal nociceptive stimuli, and showed a similar, but more robust (75%) decrease in formalin-induced phase 2 pain behaviors. Together, these studies demonstrate that NMDAR expression in both dorsal horn neurons and sensory nerves are important for the development of hyperalgesia following formalin injection.
There are two possible mechanisms by which NMDA receptors on primary afferent neurons can participate in formalin-induced inflammatory pain. First, NMDA receptors are expressed on the peripheral axons and terminals of primary afferents (Coggeshall and Carlton, 1998, McRoberts et al., 2001) and peripheral injection of NMDAR antagonists inhibits phase 2 but not phase 1 responses to subcutaneous formalin (Davidson et al., 1997, Davidson and Carlton, 1998). Thus locally released glutamate, possibly from the afferent terminals themselves, could contribute to continued excitation of afferents, which in turn is necessary for development of for the central sensitization of dorsal horn neurons including the phase 2 response to formalin (Dickenson and Sullivan, 1987b, Puig and Sorkin, 1996). This idea is supported by the observations that formalin injection (Omote et al., 1998) or direct electrical or chemical stimulation of afferents causes release of glutamate into peripheral tissues (deGroot et al., 2000). In fact, formalin injection into the planar surface of the hindpaw caused tissue glutamate to increase rapidly, peaking between 40–45 min, the declining slowly thereafter (Omote et al., 1998). This time period corresponds to the time points at which phase 2 pain behavior was most affected in the knockdown mice. However the direct role of NMDARs on peripheral afferent terminals in mediating pain responses to formalin has been questioned (Sawynok and Reid, 2002). Sawynok & Reid found that peripheral injection of three different NMDAR antagonists (ketamine, dextromethorphan, and memantine) had little or no effect in antagonizing phase 2 responses to formalin, in direct contradiction of the previous studies from Carlton’s group (Davidson et al., 1997, Davidson and Carlton, 1998). These studies differ only in the site of drug and formalin injection, namely the dorsal aspect of the paw in the Sawynok & Reid study versus the plantar surface of the paw in the Davidson & Carlton study. Our studies in mice were done with formalin injection into the plantar surface and thus might be expected to include a glutamate-NMDAR mediated component.
The second possible mechanism whereby loss of NMDARs on peripheral afferents can influence phase 2 responses to formalin is in the spinal cord where primary afferents synapse onto dorsal horn neurons. Several studies have shown that NMDA induces substance P release from the central terminals of primary afferents in the spinal cord of intact rats (Liu et al., 1997) and in rat spinal cord slices (Marvizon et al., 1997, Malcangio et al., 1998). Since substance P and other neuropeptides released by peptidergic afferent terminals such as CGRP and brain derived nerve growth factor are pro-nociceptive, activation of presynaptic NMDARs would be expected to enhance pain responses. However, two other studies were unable to detect NMDA-induced substance P release in vivo (Afrah et al., 2001, Nazarian et al., 2008). In addition, Bardoni et al. (Bardoni et al., 2004) using a neonatal rat spinal cord preparation found that activation of presynaptic NMDARs on primary afferent terminals in the spinal cord inhibited glutamate release as measured by EPSPs in dorsal horn neurons. Inhibition of glutamate release would reduce the transmission of nociceptive signals to dorsal horn neurons and thus be anti-nociceptive. These observations lead to the prediction that our Prph-fNR1 knockout mice should have enhanced pain responses to nociceptive stimuli, which was not found in the present study. One possible explanation for this disparity is that the Bardoni et al. study was done with neonatal rats, while our studies tested adult mice. It is known that the function and subunit composition of NMDARs changes dramatically during development in the CNS (Simeone et al., 2004), although little is known about developmental changes in the peripheral nervous system. Furthermore, Zang et al.(Zeng et al., 2006), using the same neonatal spinal cord preparation as Bardoni et al., found that NMDA increased, rather than decreased, EPSPs recorded in dorsal horn neurons of rats made tolerant to morphine.
There are a number of possibilities that might explain all of these disparate observations from different laboratories including age, species and strain of the animals, or prior exposure to certain drugs, anesthesia and surgical procedures. An explanation being explored in this laboratory could be differences in the activation state of the presynaptic NMDAR which might be influenced by some of the variables suggested above. Working in spinal cord slices, Chen et al. (Chen et al., 2010) found that phosphorylation of presynaptic NMDARs by a Src-family kinase was necessary for NMDA-induced substance P release. These observations are consistent with previous results from our laboratory that found a persistent 3-fold activation of NMDARs in DRG neurons following induction of experimental colitis (Li et al., 2006) which was reversed by addition of a Src family kinase inhibitor. Hence, it is possible that the tyrosine phosphorylation state of these NMDARs changes in different physiological conditions, explaining the ability of NMDAR agonists to induce neurotransmitter release from primary afferents in some studies but not in others. With respect to the present results, presynaptic NMDARs on the central afferent terminals could contribute to nociceptive signaling if they become activated by phosphorylation during phase 2 of the formalin response; whether or not this is the case remains to be determined.
In summary, our observations using targeted knockdown mice support a pro-nociceptive role of NMDARs on primary afferent nerves in the processing of sensitized pain states such as that produced by formalin injection. Whether this effect is mediated at the peripheral or central terminals, or both, remains to be determined. These tissue specific knockdown mice should be useful for further studies elucidating the role of NMDARs on primary afferents in sensitized pain states that follow tissue or nerve injury and inflammation.
Acknowledgments
The authors wish to thank Yash Mittal, Brian Nguyen, Nan Yi Zhang, Murphy Bergstrom, and Samantha Jagannathan for their assistance in the behavioral testing, Mareike Kuypers, T. Cheung, S. Stayte, S. Wu, C. Leong, and Ed Cairns for help with breeding and genotyping, and Dr. E. Matthew Hoffman for help with the image analysis. This work was supported by NIH grants DK 58173 (JM) and 1 P50 DK64539 (EM), VA Rehabilitation Research & Development Service B4766I (JCM), NHMRC Australia Grant 188819 (BV), NSW State Government’s BioFirst Award and a NSW Spinal Cord Injury and Related Neurological Conditions Research Grant (BV), Amadeus Energy Ltd. Western Australia (BV), Bill and Laura Gruy (BV).
Abbreviations
- NMDA
N-methyl-D-aspartic acid
- NMDAR
NMDA receptor
- DRG
dorsal root ganglia
- CGRP
calcitonin gene-related peptide
- NGF
nerve growth factor
- DMEM
Dulbecco’s modified Eagle’s medium
Footnotes
Conflict of Interest: The authors have no conflicts of interest with statements or conclusions included in this manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Afrah AW, Stiller CO, Olgart L, Brodin E, Gustafsson H. Involvement of spinal N-methyl-D-aspartate receptors in capsaicin-induced in vivo release of substance P in the rat dorsal horn. Neurosci Lett. 2001;316:83–86. doi: 10.1016/s0304-3940(01)02380-1. [DOI] [PubMed] [Google Scholar]
- Bardoni R, Torsney C, Tong CK, Prandini M, MacDermott AB. Presynaptic NMDA receptors modulate glutamate release from primary sensory neurons in rat spinal cord dorsal horn. J Neurosci. 2004;24:2774–2781. doi: 10.1523/JNEUROSCI.4637-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bryant CD, Eitan S, Sinchak K, Fanselow MS, Evans CJ. NMDA receptor antagonism disrupts the development of morphine analgesic tolerance in male, but not female C57BL/6J mice. Am J Physiol Regul Integr Comp Physiol. 2006;291:R315–R326. doi: 10.1152/ajpregu.00831.2005. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Castrillon E, Hupfeld S, Sessle BJ, Arendt-Nielsen L. Ketamine attenuates glutamate-induced mechanical sensitization of the masseter muscle in human males. Exp Brain Res. 2006;169:467–472. doi: 10.1007/s00221-005-0158-z. [DOI] [PubMed] [Google Scholar]
- Cairns BE, Svensson P, Wang K, Hupfeld S, Graven-Nielsen T, Sessle BJ, Berde CB, Arendt-Nielsen L. Activation of peripheral NMDA receptors contributes to human pain and rat afferent discharges evoked by injection of glutamate into the masseter muscle. J Neurophysiol. 2003;90:2098–2105. doi: 10.1152/jn.00353.2003. [DOI] [PubMed] [Google Scholar]
- Carlton SM, Hargett GL, Coggeshall RE. Localization and activation of glutamate receptors in unmyelinated axons of rat glabrous skin. Neurosci Lett. 1995;197:25–28. doi: 10.1016/0304-3940(95)11889-5. [DOI] [PubMed] [Google Scholar]
- Chaban VV, McRoberts JA, Ennes HS, Mayer EA. Nitric oxide synthase inhibitors enhance mechanosensitive Ca(2+) influx in cultured dorsal root ganglion neurons. Brain Res. 2001;903:74–85. doi: 10.1016/s0006-8993(01)02407-6. [DOI] [PubMed] [Google Scholar]
- Chaplan SR, Bach FW, Pogrel JW, Chung JM, Yaksh TL. Quantitative assessment of tactile allodynia in the rat paw. J Neurosci Meth. 1994;53:55–63. doi: 10.1016/0165-0270(94)90144-9. [DOI] [PubMed] [Google Scholar]
- Chen W, Zhang G, Marvizon JC. NMDA receptors in primary afferents require phosphorylation by Src family kinases to induce substance P release in the rat spinal cord. Neuroscience. 2010;166:924–934. doi: 10.1016/j.neuroscience.2010.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coggeshall RE, Carlton SM. Ultrastructural analysis of NMDA, AMPA, and kainate receptors on unmyelinated and myelinated axons in the periphery. J Comp Neurol. 1998;391:78–86. doi: 10.1002/(sici)1096-9861(19980202)391:1<78::aid-cne7>3.3.co;2-8. [DOI] [PubMed] [Google Scholar]
- Davidson EM, Carlton SM. Intraplantar injection of dextrorphan, ketamine or memantine attenuates formalin-induced behaviors. Brain Res. 1998;785:136–142. doi: 10.1016/s0006-8993(97)01396-6. [DOI] [PubMed] [Google Scholar]
- Davidson EM, Coggeshall RE, Carlton SM. Peripheral NMDA and non-NMDA glutamate receptors contribute to nociceptive behaviors in the rat formalin test. Neuroreport. 1997;8:941–946. doi: 10.1097/00001756-199703030-00025. [DOI] [PubMed] [Google Scholar]
- Davies SN, Lodge D. Evidence for involvement of N-methylaspartate receptors in 'wind- up' of class 2 neurones in the dorsal horn of the rat. Brain Res. 1987;424:402–406. doi: 10.1016/0006-8993(87)91487-9. [DOI] [PubMed] [Google Scholar]
- deGroot J, Zhou S, Carlton SM. Peripheral glutamate release in the hindpaw following low and high intensity sciatic stimulation. Neuroreport. 2000;11:497–502. doi: 10.1097/00001756-200002280-00014. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Evidence for a role of the NMDA receptor in the frequency dependent potentiation of deep rat dorsal horn nociceptive neurones following C fibre stimulation. Neuropharmacol. 1987a;26:1235–1238. doi: 10.1016/0028-3908(87)90275-9. [DOI] [PubMed] [Google Scholar]
- Dickenson AH, Sullivan AF. Peripheral origins and central modulation of subcutaneous formalin- induced activity of rat dorsal horn neurones. Neurosci Lett. 1987b;83:207–211. doi: 10.1016/0304-3940(87)90242-4. [DOI] [PubMed] [Google Scholar]
- Dixon WJ. Efficient analysis of experimental observations. Ann Rev Pharmacol Toxicol. 1980;20:441–462. doi: 10.1146/annurev.pa.20.040180.002301. [DOI] [PubMed] [Google Scholar]
- Du J, Zhou S, Coggeshall RE, Carlton SM. N-methyl-D-aspartate-induced excitation and sensitization of normal and inflamed nociceptors. Neuroscience. 2003;118:547–562. doi: 10.1016/s0306-4522(03)00009-5. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Fanselow MS. Effects of naltrexone on learning and performance of conditional fear-induced freezing and opioid analgesia. Physiol Behav. 1987;39:501–505. doi: 10.1016/0031-9384(87)90380-5. [DOI] [PubMed] [Google Scholar]
- Herrero JF, Laird JM, Lopez-Garcia JA. Wind-up of spinal cord neurones and pain sensation: much ado about something? Prog Neurobiol. 2000;61:169–203. doi: 10.1016/s0301-0082(99)00051-9. [DOI] [PubMed] [Google Scholar]
- Hoffman EM, Schechter R, Miller KE. Fixative Composition Alters Distributions of Immunoreactivity for Glutaminase and Two Markers of Nociceptive Neurons, Nav1.8 and TRPV1, in the Rat Dorsal Root Ganglion. J Histochem Cytochem. 2009 doi: 10.1369/jhc.2009.954008. jhc.2009.954008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Ennes HS, Trevisani M, Nicoletti P, Mittal Y, Mayer EA. Experimental colitis modulates the functional properties of NMDA receptors in dorsal root ganglia neurons. Amer J Physiol - Gastrointest Liver Physiol. 2006;291:G219–G228. doi: 10.1152/ajpgi.00097.2006. [DOI] [PubMed] [Google Scholar]
- Li J, McRoberts JA, Nie J, Ennes HS, Mayer EA. Electrophysiological characterization of N- methyl-D-aspartate receptors in rat dorsal root ganglia neurons. Pain. 2004;109:443–452. doi: 10.1016/j.pain.2004.02.021. [DOI] [PubMed] [Google Scholar]
- Liu H, Mantyh PW, Basbaum AI. NMDA-receptor regulation of substance P release from primary afferent nociceptors. Nature. 1997;386:721–724. doi: 10.1038/386721a0. [DOI] [PubMed] [Google Scholar]
- Liu H, Wang H, Sheng M, Jan LY, Jan YN, Basbaum AI. Evidence for presynaptic N-methyl-D-aspartate autoreceptors in the spinal cord dorsal horn. Proc Natl Acad Sci USA. 1994;91:8383–8387. doi: 10.1073/pnas.91.18.8383. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lovinger DM, Weight FF. Glutamate induces a depolarization of adult rat dorsal root ganglion neurons that is mediated predominantly by NMDA receptors. Neurosci Lett. 1988;94:314–320. doi: 10.1016/0304-3940(88)90037-7. [DOI] [PubMed] [Google Scholar]
- Ma QP, Hargreaves RJ. Localization of N-methyl-D-aspartate NR2B subunits on primary sensory neurons that give rise to small-caliber sciatic nerve fibers in rats. Neuroscience. 2000;101:699–707. doi: 10.1016/s0306-4522(00)00419-x. [DOI] [PubMed] [Google Scholar]
- Malcangio M, Fernandes K, Tomlinson DR. NMDA receptor activation modulates evoked release of substance P from rat spinal cord. Br J Pharmacol. 1998;125:1625–1626. doi: 10.1038/sj.bjp.0702260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin M, Matifas A, Maldonado R, Kieffer BL. Acute antinociceptive responses in single and combinatorial opioid receptor knockout mice: distinct mu, delta and kappa tones. Eur J Neurosci. 2003;17:701–708. doi: 10.1046/j.1460-9568.2003.02482.x. [DOI] [PubMed] [Google Scholar]
- Marvizon JC, Martinez V, Grady EF, Bunnett NW, Mayer EA. Neurokinin 1 receptor internalization in spinal cord slices induced by dorsal root stimulation is mediated by NMDA receptors. J Neurosci. 1997;17:8129–8136. doi: 10.1523/JNEUROSCI.17-21-08129.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marvizon JC, McRoberts JA, Ennes HS, Song B, Wang X, Jinton L, Corneliussen B, Mayer EA. Two N-methyl-D-aspartate receptors in rat dorsal root ganglia with different subunit composition and localization. J Comp Neurol. 2002;446:325–341. doi: 10.1002/cne.10202. [DOI] [PubMed] [Google Scholar]
- McNamara CR, Mandel-Brehm J, Bautista DM, Siemens J, Deranian KL, Zhao M, Hayward NJ, Chong JA, Julius D, Moran MM, Fanger CM. TRPA1 mediates formalin-induced pain. Proc Natl Acad Sci U S A. 2007;104:13525–13530. doi: 10.1073/pnas.0705924104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McRoberts JA, Coutinho SV, Marvizon JC, Grady EF, Tognetto M, Sengupta JN, Ennes HS, Chaban VV, Amadesi S, Creminon C, Lanthorn T, Geppetti P, Bunnett NW, Mayer EA. Role of peripheral N-methyl-D-aspartate (NMDA) receptors in visceral nociception in rats. Gastroenterology. 2001;120:1737–1748. doi: 10.1053/gast.2001.24848. [DOI] [PubMed] [Google Scholar]
- Méchaly I, Bourane S, Piquemal D, Al-Jumaily M, Ventéo S, Puech S, Scamps F, Valmier J, Carroll P. Gene profiling during development and after a peripheral nerve traumatism reveals genes specifically induced by injury in dorsal root ganglia. Mol Cell Neurosci. 2006;32:217–229. doi: 10.1016/j.mcn.2006.04.004. [DOI] [PubMed] [Google Scholar]
- Nazarian A, Gu G, Gracias NG, Wilkinson K, Hua XY, Vasko MR, Yaksh TL. Spinal N-methyl-d-aspartate receptors and nociception-evoked release of primary afferent substance P. Neuroscience. 2008;152:119–127. doi: 10.1016/j.neuroscience.2007.11.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Omote K, Kawamata T, Kawamata M, Namiki A. Formalin-induced release of excitatory amino acids in the skin of the rat hindpaw. Brain Res. 1998;787:161–164. doi: 10.1016/s0006-8993(97)01568-0. [DOI] [PubMed] [Google Scholar]
- Puig S, Sorkin LS. Formalin-evoked activity in identified primary afferent fibers: systemic lidocaine suppresses phase-2 activity. Pain. 1996;64:345–355. doi: 10.1016/0304-3959(95)00121-2. [DOI] [PubMed] [Google Scholar]
- Sato K, Kiyama H, Park HT, Tohyama M. AMPA, KA and NMDA receptors are expressed in the rat DRG neurones. Neuroreport. 1993;4:1263–1265. doi: 10.1097/00001756-199309000-00013. [DOI] [PubMed] [Google Scholar]
- Sawynok J, Reid A. Modulation of formalin-induced behaviors and edema by local and systemic administration of dextromethorphan, memantine and ketamine. Eur J Pharmacol. 2002;450:153–162. doi: 10.1016/s0014-2999(02)02119-2. [DOI] [PubMed] [Google Scholar]
- Simeone TA, Sanchez RM, Rho JM. Molecular biology and ontogeny of glutamate receptors in the mammalian central nervous system. J Child Neurol. 2004;19:343–360. doi: 10.1177/088307380401900507. [DOI] [PubMed] [Google Scholar]
- South SM, Kohno T, Kaspar BK, Hegarty D, Vissel B, Drake CT, Ohata M, Jenab S, Sailer AW, Malkmus S, Masuyama T, Horner P, Bogulavsky J, Gage FH, Yaksh TL, Woolf CJ, Heinemann SF, Inturrisi CE. A conditional deletion of the NR1 subunit of the NMDA receptor in adult spinal cord dorsal horn reduces NMDA currents and injury-induced pain. J Neurosci. 2003;23:5031–5040. doi: 10.1523/JNEUROSCI.23-12-05031.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vandesompele J, De Preter K, Pattyn F, Poppe B, Van Roy N, De Paepe A, Speleman F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002;3:research0034.0031–research0034.0011. doi: 10.1186/gb-2002-3-7-research0034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yashpal K, Coderre TJ. Influence of formalin concentration on the antinociceptive effects of anti-inflammatory drugs in the formalin test in rats: separate mechanisms underlying the nociceptive effects of low- and high-concentration formalin. Euro J Pain. 1998;2:63–68. doi: 10.1016/s1090-3801(98)90047-7. [DOI] [PubMed] [Google Scholar]
- Zeng J, Thomson LM, Aicher SA, Terman GW. Primary afferent NMDA receptors increase dorsal horn excitation and mediate opiate tolerance in neonatal rats. J Neurosci. 2006;26:12033–12042. doi: 10.1523/JNEUROSCI.2530-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhou L, Nepote V, Rowley DL, Levacher B, Zvara A, Santha M, Mi QS, Simonneau M, Donovan DM. Murine peripherin gene sequences direct Cre recombinase expression to peripheral neurons in transgenic mice. FEBS Lett. 2002;523:68–72. doi: 10.1016/s0014-5793(02)02936-8. [DOI] [PubMed] [Google Scholar]
- Zhou S, Bonasera L, Carlton SM. Peripheral administration of NMDA, AMPA or KA results in pain behaviors in rats. Neuroreport. 1996;7:895–900. doi: 10.1097/00001756-199603220-00012. [DOI] [PubMed] [Google Scholar]