Abstract
There is widespread evidence that memory deteriorates with aging, however the exact mechanisms that underlie these changes are not well understood. Given the growing size of the aging population, there is an imperative to study age-related neurocognitive changes in order to better parse healthy from pathological aging. Using a behavioral paradigm that taxes pattern separation (the ability to differentiate novel yet similar information from previously learned information and thus avoid interference), we investigated age-related neural changes in the human hippocampus using high-resolution (1.5 mm isotropic) BOLD fMRI. Recent evidence from animal studies suggests that hyperactivity in the CA3 region of the hippocampus may underlie behavioral deficits in pattern separation in aged rats. Here, we report evidence that is consistent with findings from the animal studies. We found a behavioral impairment in pattern separation in a sample of healthy older adults compared to young controls. We also found a related increase in CA3/dentate gyrus activity levels during an fMRI contrast that stresses pattern separation abilities. In a detailed analysis of behavior, we also found that the pattern of impairment was consistent with the predictions of the animal model, where larger changes in the input (greater dissimilarity) were required in order for elderly adults to successfully encode new information as distinct from previously learned information. These findings are also consistent with recent fMRI and behavioral reports in healthy aging, and further suggest that a specific functional deficit in the CA3/dentate network contributes to memory difficulties with aging.
Keywords: fMRI, recognition memory, aging, medial temporal lobe, computational model
Introduction
It is commonly known that memory function declines with aging. Older adults report memory complaints more frequently than problems with any other cognitive skill (Newson and Kemps, 2006). Aging is associated with a decline in the ability to form new episodic memories (Craik and Simon, 1980; Small et al., 1999; Hedden and Gabrieli, 2004; Hedden and Gabrieli, 2005), spatial memory and navigation (Newman and Kaszniak, 2000), and contextual source memory (Henkel et al., 1998) all functions thought to be subserved by the medial temporal lobes (MTL). This region, which includes the hippocampus and surrounding cortices, plays an essential role in declarative (fact and event) memory (Milner et al., 1998; Squire et al., 2004). Alterations in this system with healthy aging have been documented in animal studies (Burke and Barnes, 2006; Wilson et al., 2006) and human neuroimaging studies (Raz et al., 2005; Chee et al., 2006; Sperling, 2007; Murty et al., 2008). Identifying the neural mechanisms in the MTL that underlie these deficits is crucial to understanding the nature of the changes associated with neurocognitive aging.
Previous neuroimaging studies of the MTL in older adults have reported disparate findings; some have observed decreased hippocampal activity concomitant with increased prefrontal activity (Cabeza et al., 2004; Daselaar et al., 2006; Dennis et al., 2008; Murty et al., 2008), while others have reported no differences in hippocampal activity between young and old (Rand-Giovannetti et al., 2006; Sperling, 2007; Duverne et al., 2008). A recent study by Miller et al. (2008a) reported hippocampal hyperactivity in low-performing older adults during a face-name association task, a result that is often reported in patients with mild cognitive impairment (Dickerson et al., 2004; Dickerson et al., 2005; Celone et al., 2006; Miller et al., 2008b), and has also been observed in cognitively intact ApoE4 carriers (Bookheimer et al., 2000) as well as asymptomatic offspring of autopsy-confirmed Alzheimer's disease patients (Bassett et al., 2006). The lack of consensus across fMRI studies is likely due to the wide variation across tasks and contrasts used to tap into hippocampal function. Functional MRI based on the blood-oxygenation level-dependent (BOLD) effect is a contrastive measure and can only index differences between conditions. This limitation may be a source of the variability in the results. If aging is associated with increased levels of activity in conditions A and B compared to younger adults but not in condition C, variable results could be obtained. The A minus B contrast could show no hyperactivity (despite both being elevated), while the A minus C and B minus C contrasts could show hyperactivity.
Recent electrophysiological studies in aged rodents have offered new insight into the potential neural mechanisms in the MTL that underlie age-related memory impairment, and a potential source of at least some this hyperactivity. These studies observed “rigid” place cell firing patterns in the aged rats' CA3 region that did not distinguish between two similar but not identical environments (Wilson et al., 2005a; Wilson et al., 2005b). That age-related alteration, thought to be due to a failure to encode new information while navigating similar environments, appeared to result from a reliance on pre-existing familiar representations (Wilson et al., 2006). This can be thought of as a deficit in pattern separation, or the ability to differentiate similar representations into distinct, non-overlapping representations (McClelland et al., 1995; O'Reilly and Norman, 2002; Norman and O'Reilly, 2003) and therefore discriminate between the current environment and a previously encountered similar environment. Aged rats exhibit a shift in the computational bias from pattern separation to pattern completion, or the process of reactivating pre-existing representations in response to partial or degraded cues. The degree of completion (measured as similarity in place cell firing patterns in old and new environments) was also observed to strongly correlate with spatial learning deficits (Wilson et al., 2003). Furthermore, place cell firing was abnormally elevated in the aged rats' CA3, a likely result of reduced inhibitory input into the CA3's autoassociative recurrent collateral network (Wilson et al., 2006). This hyperactivity, coupled with synaptic loss in the perforant path input, could be the underlying mechanism for a shift from separation to completion and the learning deficit observed. The model by Wilson and colleagues, incorporating the findings from a well characterized model of neurocognitive impairment, postulates that aging is associated with a shift in the pattern separation / pattern completion tuning curve in the CA3, such that larger changes in input (i.e., greater dissimilarity) are necessary for separation to occur.
To address this question in humans, we have utilized a method that improves the spatial resolution of fMRI scans. Previous neuroimaging studies of aging have used traditional functional MRI methods, which operate at resolutions of 3-6 mm, precluding examination at the level of hippocampal subfields. Here, we used high-resolution (1.5 mm isotropic) BOLD fMRI to investigate changes in activity within hippocampal subfields. Recently, our laboratory has used these methods to demonstrate the first evidence for pattern separation signals in the human CA3 and/or dentate gyrus (DG) (Bakker et al., 2008). The purpose of the current study is to test the following three hypotheses: (A) older adults suffer from a behavioral impairment in pattern separation with a shift instead to pattern completion, (B) consistent with the rodent work, there will be elevated activity levels in the CA3 and/or dentate regions of the hippocampus, and (C) the behavioral deficit will be consistent with the pattern hypothesized by Wilson and colleagues in which older adults need larger changes in input (i.e., greater dissimilarity) in order to remember that a prior experience is different from the current experience.
To address these hypotheses, we used a continuous recognition task (Kirwan and Stark, 2007) to behaviorally investigate pattern separation as well as related BOLD activity in the hippocampus. Participants were shown novel items, repeated items, and similar items (i.e., “lures”) (Fig. 1) and were asked to indicate whether each either “new”, “old” or “similar”. The contrast of lure trials that were correctly endorsed as “similar” (lure correct rejections – LCR) vs. lure trials incorrectly endorsed as “old” (lure false alarms – LFA) was used as our critical contrast for testing young vs. old MTL activity, since it is heavily biased towards separation-related activity. We used this task to test our first two hypotheses. To test the third hypothesis, we used recognition performance from a large number of young participants to derive an empirical measure of the mnemonic similarity of each pair of items. This provided us with a continuous metric by which older participants' performance could be assessed.
Figure 1.
Sample behavioral task stimuli ranked in order of mnemonic similarity with highly dissimilar object pairs at the far left and highly similar object pairs at the far right.
Materials and Methods
Subjects
Experiment 1
Seventeen right-handed, healthy young adults (5 males, 12 females; mean age=23, SD=7) and 10 right-handed, healthy older adults (2 males, 8 females; mean age=75, SD=7) were included. Written informed consent was obtained from all participants. Participants were screened for any health conditions that may interact with their neurological status, as well as contraindications for MRI scanning. Young adults were recruited from the Johns Hopkins University graduate and undergraduate student population using IRB-approved flyers. Older subjects were referred to us by the Department of Neurology where they had undergone a series of medical, neurological, psychiatric and cognitive assessments to establish that they were cognitively normal. Exclusion criteria included any major medical conditions, (e.g. diabetes, heart disease) any neurologically active medication use, any history of mental or psychiatric disorder, as well as any MRI contraindications including any metal in the body and claustrophobia. The Telephone Interview for Cognitive Status (Brandt et al., 1988) was also conducted with the older adult sample at or around the time of testing to further ascertain mental status. All participants scored in the normal range (33-41) with a mean score of 36.3 (SD 1.4).
Experiment 2
114 young participants (mean age = 20 SD = 2) and 18 older adults (mean age = 71 SD = 7) were recruited for the behavioral study. For the perceptual similarity / working memory task, we selected a subset of young (n = 16, mean age = 20 SD = 3) and older participants (n = 13, mean age = 70 SD = 8). All participants were recruited by flyer advertising and by direct referral through a large longitudinal study of healthy aging at the University of California, Irvine.
fMRI Behavioral Task
The paradigm was an explicit three-alternative forced choice task, in which participants viewed novel, repeated and similar stimuli (i.e., “lures”). Stimuli were color photographs of common objects (Figure 1). Each participant underwent six functional runs, each with 16 identical pairs (i.e., “repetitions”), 16 similar pairs (i.e., “lures”) and 44 unrelated novel items (i.e., “foils”), fully randomized throughout the run. The number of trials separating similar and identical pairs was randomly varied between 10 and 40 trials. Participants were instructed to make a judgment as to whether the object seen was new (novel items), old (repeated items) or similar but not identical (lure items). Of critical interest were the participants' responses on the lure items. An “old” response given a lure item would suggest that the participant was more biased towards pattern completion, whereas a “similar” response would suggest a bias towards pattern separation instead. Response rates in each of the 9 potential categories (new items called new, new items called old, new items called similar, lures called new, lures called similar, lures called old, repetitions called new, repetitions called similar, repetitions called old) were calculated to determine the overall change in performance associated with age. We used the Cogent 2000 Toolbox (http://www.fil.ion.ucl.ac.uk) in Matlab 7.0 (The MathWorks, Natick, MA) for stimulus presentation and behavioral data collection. A separation bias metric was calculated using [p(“Similar∣Lure) – p(“Similar∣Foil)], which quantifies the likelihood that the item was successfully separated, and should correct for any response bias across groups. Statistical tests were conducted on this metric using two-tailed independent samples t-tests.
Mnemonic Similarity Task
Participants viewed 128 pictures of common objects (2000 ms each, 500 ms inter-stimulus-interval). After a delay of several minutes, participants were shown 64 of the studied objects, 64 new objects, and 64 lure items that were similar to but not identical to studied objects. Like the study phase, each stimulus was presented for 2000 ms (500 ms inter-stimulus-interval). In a three-alternative forced choice recognition task, similar to the one used above for the fMRI task, participants were asked to decide for each item, is it old (repetition), similar (lure), or new (new). In order to norm the stimuli, we tested 114 college-age undergraduates to generate a mnemonic similarity rating for each pair (rather than a simple perceptual similarity rating). Figure 1 shows examples of lower-similarity and higher-similarity pairs as determined by their probability of responding “old” to an item when the lure version was presented. In constructing the stimuli this way, we were able to present stimuli with no delta-input (repetitions), large amounts of delta-input (new items), and known, variable amounts of delta-input (lures). Lure trials were then sorted into five bins based on the degree of mnemonic similarity. The metric used in this analysis was the same as the separation bias metric previously used to analyze behavior [p(“Similar”∣Lure)-p(“Similar”∣Foil)] which corrects for potential response bias. This was conducted as a qualitative analysis to investigate the shape of the functions describing behavior.
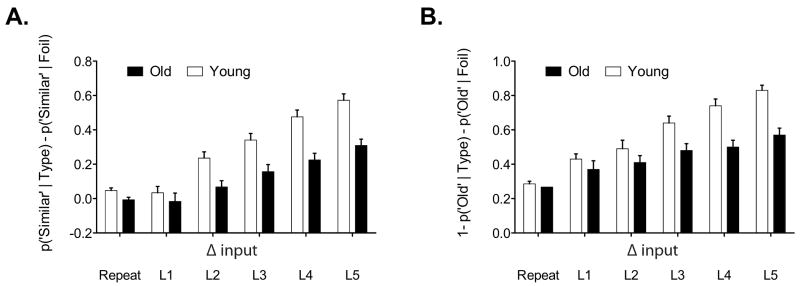
In order to correct for potential response bias, we created two other versions of the behavioral separation bias metric. The first is in the form of [p(“Similar”∣Lure) – p(“Similar”∣Foil)] for Lure bins 1-5 and [p(“Similar”∣Target) – p(“Similar”∣Foil)] for repeated hits. Foils (1st presentations) are not included because they would by definition be zero. This correction should account for the general bias to respond with “similar” regardless of the type of stimulus. Results using this corrected metric are shown in Figure 6A. The second correction is in the form of 1-[p(“Old”∣Lure) – p(“Old”∣ Foil)] for Lure bins 1-5 and 1-[p(“Old”∣Target) – p(“Old”∣Foil)] for repeated hits. Foils are again not included because by definition they would be 1. This correction should account for the general bias to respond with “old” regardless of the type of the stimulus. The metric was inverted to map onto the similarity metrics used elsewhere in the paper. Results using this corrected metric are shown in Figure 6B.
Figure 6.
Response-bias corrected versions of the behavioral performance plot in Figure 4. (A) Bias metric = [p(“Similar”∣Lure) – p(“Similar”∣Foil)] for Lure bins 1-5 and [p(“Similar”∣Target) – p(“Similar”∣ Foil)] for repeated hits. Foils (1st presentations) are not plotted because they would by definition be zero. (B) Bias metric = 1-[p(“Old”∣Lure) – p(“Old”∣Foil)] for Lure bins 1-5 and 1-[p(“Old”∣Target) – p(“Old”∣Foil)] for repeated hits. Foils are again not plotted because by definition they would be 1.
fMRI Scanning Procedures
Imaging data were collected on a Phillips 3 Tesla scanner (Eindhoven, The Netherlands) equipped with an 8-channel SENSE (Sensitivity Encoding) head coil, located at the F.M. Kirby Research Center for Functional Brain Imaging at the Kennedy Krieger Institute (Baltimore, MD). We used a high-resolution scanning protocol developed in our laboratory (Kirwan et al., 2007). Echo-planar images (EPI) were collected using an acquisition matrix of 64 × 64, a repetition time (TR) of 1500 milliseconds, an echo time (TE) of 30 milliseconds, a flip angle of 70 degrees, a SENSE reduction factor of 2, and an isotropic resolution of 1.5 mm × 1.5 mm × 1.5 mm with no gap. Nineteen oblique slices were acquired parallel to the principal longitudinal axis of the hippocampus and covered the entire medial temporal lobe region bilaterally. In addition to the functional runs, a whole-brain MPRAGE structural scan was also acquired (parameters: 150 oblique slices, 1mm isotropic resolution). We used several procedures to correct for fMRI signal distortions. First, we employed higher-order shims, which can directly compensate for local field distortions. Second, we used SENSE parallel imaging which utilizes multiple surface coils to undersample k-space with fewer phase encoding steps. This resulted in significantly reduced acquisition time, which also limited distortion resulting from magnetic susceptibility. Furthermore, the adoption of high-resolution scanning protocols reduced remaining artifacts substantially as these artifacts are a function of number of slices from the inhomogeneity rather than absolute distance (Buxton, 2001).
fMRI Data Preprocessing and Alignment
Data analysis was performed using Analysis of Functional NeuroImages (AFNI: Cox, 1996). Images were first co-registered through time using a three-dimensional registration algorithm, creating motion vectors used to censor time points from further analysis. Acquisitions in which a significant motion event occurred (more than 3 degrees of rotation or 2 mm of translation in any direction relative to prior acquisition), plus and minus one TR were excluded from the analyses. Initial whole-brain spatial normalization was accomplished using each participant's structural MRI scan to transform the data to the atlas of Talairach and Tournoux (1988) to provide a rough alignment. ROI-Demons (Yassa and Stark, 2009) was applied to align subregions of the medial temporal lobe and the hippocampus (CA1, CA3/DG, subiculum, entorhinal cortex, perirhinal cortex, and parahippocampal cortex) across participants. This method achieves optimal overlap in the hippocampus by focusing alignment power where it is needed the most and not wasting any degrees of freedom outside of those regions. Anatomical segmentations of the above regions were conducted on every individual scan as detailed in our previous work (Yassa and Stark, 2009). A model was constructed to represent a central tendency across groups. No spatial smoothing of the statistical maps was used given the degree of alignment accuracy and the need to examine activity differences among the small, closely spaced structures in the MTL. The transformation parameters were then applied to the functional data.
fMRI Data Analysis
Behavioral vectors based on trial type and behavioral responses were used to model the data using a deconvolution approach based on multiple linear regression (3dDeconvolve). The resultant fit coefficients (betas) estimated activity versus baseline (novel foils) for a given time point and trial type in a voxel. The sum of the fit coefficients over the expected hemodynamic response (3-12 s after trial onset) was taken as the model's estimate of the response to each trial type (relative to baseline). Group analyses were performed using a two-way Analysis of Variance (ANOVA) with trial type and group as fixed factors, and participant as a random factor, nested within condition. Each participant's overall F-statistic (i.e. anything that was modulated by any aspect of the task) was thresholded liberally (p<0.05 uncorrected, extent ≥ 10 voxels) and used to remove voxels that are unrelated to the task. Next, the remaining voxels were combined with anatomical ROIs to create new hybrid functional/structural ROIs. Voxels in these ROIs were collapsed and the average activity in each ROI was extracted to conduct more refined statistics. This approach reduces voxel-selection biases and enhances our signal-to-noise ratio. This yielded 6 ROIs in the hippocampus (bilateral CA3/DG, bilateral CA1, bilateral subiculum). A contrast of activity during lures called “similar” versus lures called “old” was calculated. Subsequent testing was conducted using two-tailed independent samples t-tests.
Results
Experiment 1
Behavioral results
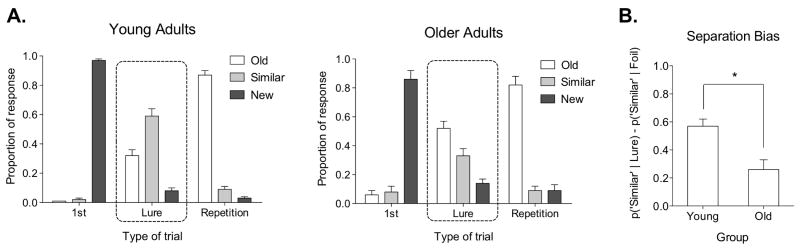
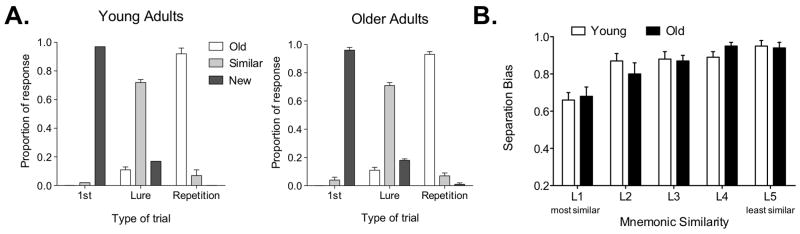
Older adults were much more likely to generate “false alarms” to items that were similar (i.e., “lures”) than young participants (Fig. 2). This shift towards completion (generalization) came at the expense of separation behavior (discrimination). Older adults successfully labeled 33% (SD = 5) of the lure trials “similar”, whereas young adults did so on 59% (SD = 5) of the lure trials. Critically, performance on other trial types did not differ significantly across groups. When a separation bias (SB) metric was calculated [SB = p(“Similar”∣Lure) – p(“Similar”∣Foil)], older adults demonstrated a significant impairment in pattern separation, t(25) = 4.28, P(two-tailed) < 0.001.
Figure 2.
Experiment 1 behavioral data. Panel (A) shows response proportions on different trial types in young and old participants. Performance on repeated and new items was similar in both groups, while performance on lure items was not. Young adults tended to perform better (59% correctly identified as similar) than older adults (33% correctly identified as similar). Panel (B) shows the difference between young and old separation bias. Older adults had a significantly lower bias score than young adults. Data plotted as mean ± s.e.m.
Bold fMRI Results
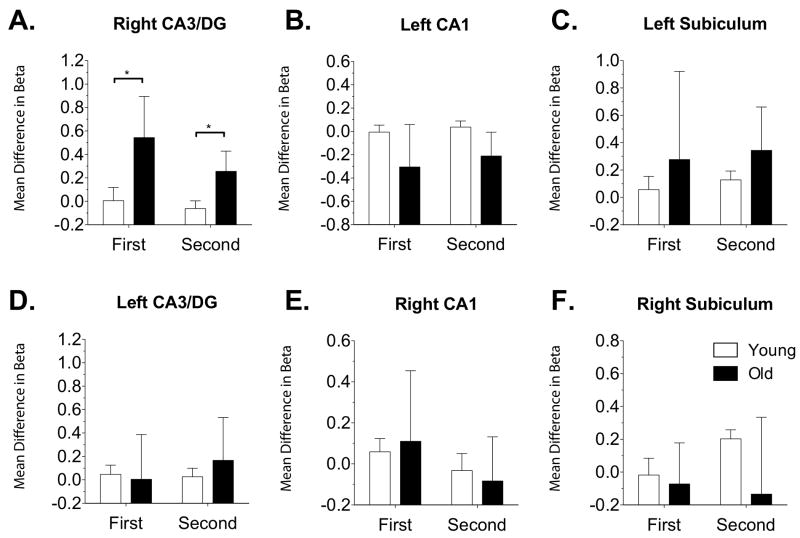
Task-related region of interest activity was isolated using an ANOVA with the overall F statistical map masked with anatomical segmentations of hippocampal subfields. To examine group differences in separation-related activity, we compared young vs. old activity during our critical contrast (lures called “similar” minus lures called “old”) during both the initial presentation and subsequent presentation. The first contrast was based on the first presentations of items that were subsequently tested with a lure (essentially a subsequent memory contrast), whereas the second contrast was based on the actual lure presentation. There was a significant increase in signal in the right CA3/DG region of the hippocampus (Figure 3A) during first presentation, t(25) = 2.19, P(two-tailed) < 0.05, and during second presentation, t(25) = 2.47, P(two-tailed) < 0.05. No other regions showed differences between young and old in these separation-related contrasts (Figure 3B-F). Region x Group ANOVAs were conducted to see if this effect was truly selective for the right CA3/DG. The first ANOVA was conducted on the first presentation contrast [subsequent lure correct rejections (i.e. called similar) minus subsequent lure false alarms (i.e. called old)] in the right CA1 vs. right CA3/DG and revealed a significant interaction, F(1,25) = 5.541, P(two-tailed) < 0.05. The second ANOVA was conducted on the second presentation contrast [lure correct rejections (i.e. called similar) minus lure false alarms (i.e. called old)] in the right CA1 vs. right CA3/DG and also revealed an interaction approaching significance, F(1,25) = 3.159, P(two-tailed) = 0.08. The above ANOVAs were also conducted on the left hemisphere subfields and neither revealed a significant interaction (1st presentation F(1,25) = 1.0, n.s.; 2nd presentation F(1,25) = 0.9, n.s.).
Figure 3.
Hippocampal subfield BOLD activity on the critical lure contrasts based on hybrid anatomical / functional ROIs. The x-axis represents (1) activity during the first presentation of an item that was subsequently tested with a lure (first) and (2) the presentation of the lure itself (second). The y-axis mean difference in beta is the difference between lures called “similar” and lures called “old”. There were no significant differences between groups in any of the subregions or the contrasts except in the right CA3/DG region. This region showed a larger difference between correct rejections and false alarms during first and subsequent presentations. Data plotted as mean ± s.e.m.
It is important to note that the contrasts used in the above analyses are not relative to baseline but rather relative to a zero difference between false alarms and correct rejections. Thus, an increase here should not be interpreted as increased activity compared to the novel foil baseline, but rather as an increase in the difference between correct rejections and false alarms.
Correlations between separation performance and fMRI activity
Significant negative correlations between the extent of activity in the right CA3/DG during correct rejection of lures and behavioral separation bias scores were found during both first presentation, Pearson's r = -0.45, t(25) = -2.52, P(two-tailed) < 0.05, and subsequent presentation, Pearson's r = 0.46, t(25) = -2.12, P(two-tailed) < 0.05. None of the within-group correlations were statistically reliable, likely due to the relatively small sample size. Figure 4 depicts these correlations by group, and illustrates the distribution of scores.
Figure 4.
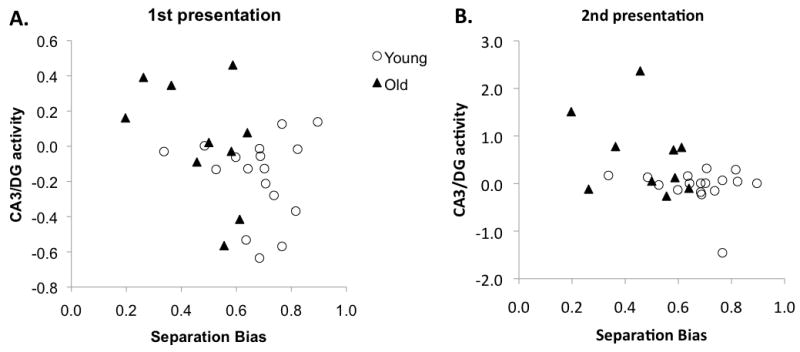
Correlations between CA3/DG activity during the critical 1st and 2nd presentations of lures labeled as “similar” versus lures labeled as “old” and behavioral separation bias scores.
Experiment 2
Mnemonic Similarity
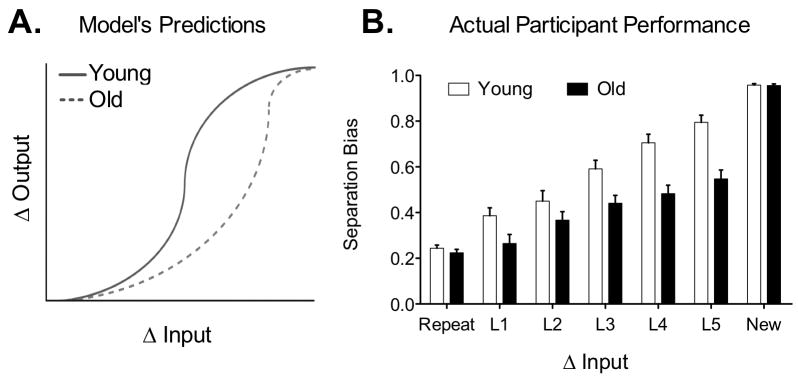
Data from 96 young participants tested in a variant of this task were used to create a mnemonic similarity metric for each pair of items. These data yielded a “similarity metric” for each stimulus-pair showing the degree of change in input among “old” items and their “lures”. This permitted us to use the Wilson et al. model to investigate our behavioral data. Figure 5B shows the data for a separate sample of 18 young adults (not part of the sample used for the “similarity metric” procedure), and a sample of 18 older participants, with change in input increasing along the x-axis and change in output increasing along the y-axis. A two-way ANOVA with condition (L1 through L5) and group (young vs. old) entered as fixed factors revealed a significant effect of group (F(1,170) =138.2, P(two-tailed)<0.0001) accounting for 18.6% of the total variance and a significant effect of condition (F(4,170) =101.38, P(two-tailed)<0.0001) accounting for 54.88% of the total variance. There was also a significant interaction (F(4,170)=6.75, P(two-tailed)<0.0001) accounting for 3.64% of the total variance. This suggests that, consistent with the model's predictions (Figure 5A), larger amounts of change in input were required for separation to occur in older adults. With little or no change in input (repetitions), or with very large changes in input (new items), older participants behaved exactly like the young. As we increased the change in input, however, the behavior diverged and it took larger changes (greater dissimilarity) for older participants to behaviorally separate and call the lure item “similar” rather than “old”. Critically, the significant interaction illustrates that this is not merely an effect of task difficulty, since the most difficult items would be the ones most similar, and those were the items with the smallest group difference. Response bias-corrected versions of this plot are shown in Figure 6. The corrections illustrate that the effect of response bias is quite modest and does not change the overall pattern of the results.
Figure 5.
Parametric analysis of behavioral data based on binned mnemonic similarity ratings in Experiment 2. (A) Model predictions for young and old separation and completion behavior. (B) As predicted, older adults performance diverges from the young such that larger changes in input are required for them to successfully separate (label lure items as “similar” instead of “old”). Data plotted as mean ± s.e.m.
One pertinent question is whether this effect is predominantly due to mnemonic processing or perhaps a side effect of group differences in perceptual processing. In order to address this possibility, we also ran a perceptual similarity/working memory version of the task on a subset of our young (n = 16, mean age = 23) and older adults (n = 13, mean age = 75) in which lure pairs, repeated pairs, and novel foil pairs were sequentially presented, separated by a white noise mask to eliminate potential contributions of sensory memory. We found no significant differences in performance across groups suggesting that the effect reported in this study is mnemonic and not perceptual in nature (See Figure 7A). When we binned the stimuli according to mnemonic similarity as we did before, we found that both young and old participants struggled only on the most similar lures (L1). However, there was no interaction between age and similarity during this version of the perceptual task (Figure 7B), once again supporting the notion that our findings are likely the result of differential mnemonic and not perceptual processing.
Figure 7.
Results of the perceptual similarity / working memory task. Panel A shows results in the same format as Figure 2, illustrating the lack of age group differences on any of the task conditions. Panel B shows the breakdown by lure bins according to our mnemonic similarity ratings. There is clearly no group difference on any of the lure bins, suggesting that our aging effect is primarily driven by differences in mnemonic and not perceptual processing.
Discussion
This study provides evidence that there is a behavioral memory deficit with aging that can be characterized as a shift in bias from pattern separation to pattern completion. This behavioral deficit may not be detected in typical recognition tasks that do not tax pattern separation, as evidenced by our participants' unimpaired performance on novel and repeated items. A recent report by Toner and colleagues also observed similar behavioral results (increased probability of calling lure items “old”) in older adults using the same task (Toner et al., 2009). Consistent with the computational models and the animal studies, older adults are more biased towards pattern completion at the expense of pattern separation. The additional behavioral analysis afforded by the normative similarity ratings in Experiment 2 further suggests that the pattern of impairment accurately maps onto the predictions of the computational model. We found that older adults require a larger degree of dissimilarity in the input before separation can occur (the inflection point in the tuning curve is shifted to the right compared to the young). Critically, this was not simply due to an effect of task difficulty, since the significant interaction across conditions L1 through L5 suggests that the age group difference was smaller in the most similar lures (L1) than the most dissimilar lures (L5). We also found no such age differences in a perceptual similarity/working memory version of the same task. Here, it is important to note that although performance on lures was not as high as performance on targets and foils, there was no difference among groups across any of the lure bins. This strongly suggests that although perceptual similarity does seem to have a general modulatory effect on performance on this task, the aging effect is not due to perceptual processing deficits but rather due to deficits in mnemonic abilities.
One pertinent question is how much change in input is required for older adults to separate in comparison to young adults. In order to address this question several factors must be considered. First, our experiment used simple everyday objects. Separation bias is quite likely different for different stimulus classes, e.g. scenes or faces or abstract figures. Although we hypothesize that the pattern of the group difference will overlap across stimulus classes, the exact amounts of change in input will likely vary. Additional experiments are currently being conducted in our laboratory to more directly address this question. Second, the relative emotionality of the stimuli is an important factor in how vividly they are remembered at least in part due to the amygdala's influence on hippocampal encoding (c.f. McGaugh, 2004 for review) and thus may interact with participants' bias to separate. This was not assessed in the current study but is an important future research direction. Finally, in order to derive a quantitative measure of relative or absolute change required for older adults to separate, we would require several variants (morphs) of each stimulus to derive this value for specific stimuli. This analysis was not feasible in the current study, as it would require a large number of new stimuli and hundreds of additional participants. Nevertheless, we can reasonably infer based on the current results that older adults require larger amounts of change in input across a wide range of stimulus similarity in order to successfully separate.
Previous studies have observed increased levels of activity in the CA3 of the aged rats that also show impairments in pattern separation (Wilson et al., 2003; Wilson et al., 2005a; Wilson et al., 2005b; Wilson et al., 2006). Here, we provide converging evidence from the human for a link between dysfunctional CA3 hyperactivity and the behavioral deficit. Overall, on the trials in which older adults were able to behaviorally separate and call lure items “similar”, they exhibited greater activation in the CA3/DG region of the hippocampus (relative to when they called lure items “old”), consistent with findings in the rodent. One notable difference, however, was that hyperactivity in the rodent CA3 was noted during free exploration of novel and familiar environments and was not linked to a specific task or condition. Since functional MRI is contrastive in nature, the direct analog of place cell recordings was not feasible. Instead, we selected a contrast that is heavily weighted towards separation to assess CA3's BOLD activity.
On a network level, hyperactivity is likely the result of diminished inhibitory input into the CA3 (Geinisman et al., 1992), which normally regulates the recurrent auto-associative collaterals, an excitatory input that comprises the majority of synapses onto the CA3 pyramidal neurons. For example, even a modest reduction in cortical input to CA3 and the dentate gyrus could alter the balance of excitation and inhibition in the CA3 region (Smith et al., 2000; Burke and Barnes, 2006). Additionally, cholinergic modulation by the medial septum, which is believed to be involved in the switching between recall and storage modes in the hippocampus (Hasselmo et al., 1995; Hasselmo, 2006), is decreased with aging (Sugaya et al., 1998; Nicolle et al., 1999). Overall, CA3's disinhibition and subsequent elevated activity could be the underlying mechanism by which computational bias shifts from separation to completion. Although one cannot assess this with any degree of certainty using BOLD fMRI, the results from animal and human work are consistent with this account.
A recent study by Miller et al. (2008a) in healthy older adults demonstrated that low-performing older adults activated their right hippocampus to a greater extent than young or high-performing older adults. This activity was found on successful encoding trials (relative to unsuccessful encoding trials), suggesting that hippocampal hyperactivity may be necessary for those individuals to successfully perform the task, perhaps as part of a compensatory mechanism. While such a “compensatory” account may at first appear to be in conflict with the “dysfunctional” account, these ideas are not mutually exclusive. The Miller et al. results are actually quite consistent with ours and suggest that although there may be an attempt to compensate, individuals showing these hyperactive patterns do not perform as well on the task overall. According to this view, hyperactivity is an index of network dysfunction that does not provide effective compensation. This would be consistent with the rodent studies and with the current computational model of aging. It is also worth nothing that negative correlations between the extent of CA3/DG activity during the separation condition and participants' behavioral separation bias were observed in our study, further suggesting that increased CA3/DG activity is an index of impairment. However, since within-group correlations were not quite significant we hesitate to make strong conclusions on the basis of these correlations. Further investigation with larger samples is needed to fully elucidate this brain-behavior relationship.
Our results are also generally consistent with other age-related mnemonic findings in the literature. For example, older adults typically do not perform as well as young adults on tasks that require the formation of new episodic memories (Craik and Simon, 1980; Small et al., 1999; Hedden and Gabrieli, 2004; Hedden and Gabrieli, 2005), spatial memory and navigation (Newman and Kaszniak, 2000), and contextual source memory (Henkel et al., 1998). Norman and O'Reilly (O'Reilly and Norman, 2002; Norman and O'Reilly, 2003) argue that rich contextual processing such as that required for the above tasks places larger demands on pattern separation, and thus separation-related impairments can lead to the differences in behavioral performance observed. Another pertinent finding in the literature is a decrease in recollection and a concomitant increase in familiarity when resolving recognition tasks (Jennings and Jacoby, 1997). Failure to recollect and relying instead on familiarity or “gist” representations can be thought of us as a combination of poor pattern separation during encoding and a shift to pattern completion during retrieval. Concepts such as separation and completion are not orthogonal to traditional ways to think about memory and related dissociations (e.g. recollection vs. familiarity, source vs. item memory), but serve to further specify them and are largely consistent with their predictions.
The current investigation has several advantages over previous studies of the MTL in aging. First, by using high-resolution functional MRI, we are capable of detecting activity in hippocampal subfields with a high degree of specificity. For example, both our study and the Miller et al. study found hyperactivity in the right hippocampus, however our high-resolution methods are able to further localize this activity with greater precision in the CA3/DG region of the hippocampus. Second, our cross-participant alignment technique focuses its power on our particular regions of interest and can achieve over 90% overlap across participants in many cases. Traditional fMRI studies that use a standard alignment technique such as SPM's normalization, which we have recently shown only results in 40-50% overlap in the hippocampus (Yassa and Stark, 2009), will underestimate or in some cases completely miss hippocampal activity (Stark and Okado, 2003; Miller et al., 2005; Kirwan et al., 2007). Third, the experimental manipulation taps into a specific function of the hippocampus and shows a clear behavioral deficit in older adults. For example, if the task were a typical Yes/No recognition task (as is often the case with fMRI recognition tests), no behavioral deficit would have been observed. Fourth, the contrast selected for examination in this study (the one showing hyperactivity) is immune from baseline differences between groups which could easily contribute to increases or decreases in BOLD response. A number of studies (Small et al., 2004; Ances et al., 2009; Fleischer et al. 2008) have now shown that increases or decreases in BOLD response could easily arise in comparisons across groups and regions as an artifact of basal state factors such as metabolic rate and neurovascular coupling. In our study, we chose a contrast comparing two types of responses to the same stimuli (i.e. lures called “similar” minus lures called “old) in order to investigate differences that are free from baseline effects. Although this is a notable strength of our design, it is possible that basal factors may interact with task conditions in a way that would induce artificial hyperactivity on such a condition, however the latter possibility is an unlikely alternative. Future research using calibrated functional MRI may shed light on this issue (Davis et al., 1998).”
We quantified stimulus similarity based on actual participants' memory performance. We feel this is a much more objective way to assess the relevant similarity in question – mnemonic similarity, than asking participants to rate stimulus pairs on a Likert scale for example. One potential question that arises is why we did not use these ratings to sort fMRI trials post hoc and examine the BOLD activity the same way we did behavior. We argue that in other designs it would be informative to sort the fMRI trials this way, but it was not ideal in the current study. In an overt recognition memory task, such as the one used here, separation and completion can both occur across many different trial types. For example, when a participant is asked to judge whether an item was seen before or if it was a similar item that was actually seen before, recall of the previous item's presentation may be used to correctly reject the item by saying “similar” and not “old” (a “recall to reject” strategy). Both “old” and “similar” responses to lures likely involve some amount of pattern completion. However, the load on separation is higher in the “similar” trials than in the “old” trials (partly due to explicit demands in the task). Although each of these trial types likely reflects a mixture of processes, the contrast between “similar” and “old” responses to lures should be selective for pattern separation. Although this is not a process-pure approach, we opted to use it in order to assay memory performance in the same task. For one to independently assess the effect of mnemonic similarity on hippocampal activity and be able to detect the sharp transition associated with separation, the task would be better administered as an incidental encoding task similar to our previous study (Bakker et al., 2008), so that contamination from incidental recall during rejection of lure items is avoided. However, assessing performance during the incidental task would not have been possible (and, given the automatic nature of encoding and retrieval, even the incidental approach is not entirely process-pure).
One notable limitation of our study is the inability to functionally distinguish between the CA3 and the dentate gyrus, even in our high-resolution protocol. Although both regions are thought to play a role in pattern separation, recent evidence from rodent studies suggests that the mechanisms are different (Leutgeb et al., 2007) and that the dentate is the primary source of separation signals when changes in the input are small. Work by the Tonegawa lab (McHugh et al., 2007) has also shown that NMDA receptor knockout in dentate granule cells results in selective deficits in dissociating similar contexts (stressing pattern separation) but not in contextual fear conditioning, providing further evidence that the dentate is the source of the separation signal in the hippocampus. In the context of the aging brain, a large literature has shown that dentate granule cells are particularly vulnerable to age-related impairment (Gazzaley et al., 1996; Moreno et al., 2007; Penner et al. 2010; Small et al. 2002, 2004; West, 1993; see also recent review by Burke and Barnes, 2010). These studies stress the important distinctions between the dentate gyrus and the CA3 region, especially in the context of the aging brain. Indeed, the rodent model we test in this study makes different predictions for the two regions (namely a reduction in activity in the dentate, and an increase in the CA3). Dissociating the two regions using functional MRI may be difficult at this time, however it is possible that future studies with high-resolution imaging may be able to distinguish BOLD signals from these two regions and find evidence consistent with their hypothesized computations. At the moment, resolution limitations have forced us to group the two and we are unably to functionally dissociate them.
It is also worth mentioning that since our older adult sample had a mean age of 75, some participants could be characterized as “old-old”. One important question that remains to be answered concerns the nature of these age-related changes, their time of onset, and their progression. Although the current investigation does not allow us to directly answer this question, future investigation using a complete lifespan approach will be necessary to fully understand the nature of age-related change.
In conclusion, we present evidence that older adults show impairments in behavioral pattern separation, and tend to shift into using pattern completion to resolve a memory task. This shift can be further characterized as an increased requirement for dissimilarity before separation can successfully occur. We also show that hippocampal circuitry is altered in the course of aging, such that the CA3 and dentate regions exhibit increased activity during trials loaded on pattern separation. This is consistent with recent work in the rodent as well as computational models of neurocognitive aging. It also may also be a feature that can dissociate normal aging from disease states such as Alzheimer's disease. For example, recent work suggests that CA1 place field physiology is affected in AD transgenic mice (Cacucci et al., 2008), in contrast to the CA3 place field changes observed in aged rodents. Future studies should focus on the CA3/dentate region as a potential locus of change that may underlie age-related memory deficits, and attempt to understand how this is different in the course of dementia.
Acknowledgments
We would like to thank the staff of the F.M. Kirby Center for Functional Brain Imaging for their assistance in data collection, Dr. Barry Gordon for assistance with recruitment and neurological screening, and Dr. C. Brock Kirwan for valuable contributions to study design. Additionally, we thank Ms. Samantha Rutledge, Ms. Megan Deaton, Mr. Greg Sanchez and Mr. Marlo Asis for their assistance with data collection.
Grant sponsor: NIA Grant numbers: P50 AG05146 and 2 P01 AG09973
Grant sponsor: NSF Grant number: BCS-0544959
Grant sponsor: Glenn Foundation Award.
References
- Ances BM, Liang CL, Leontiev O, Perthen JE, Fleisher AS, Lansing AE, Buxton RB. Effects of aging on cerebral blood flow, oxygen metabolism, and blood oxygenation level dependent responses to visual stimulation. Human Brain Mapping. 2009;30(4):1120–1132. doi: 10.1002/hbm.20574. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bakker A, Kirwan CB, Miller NI, Stark CEL. Pattern separation in the human hippocampal CA3 and dentate gyrus. Science. 2008;319:1640–1642. doi: 10.1126/science.1152882. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bassett SS, Yousem DM, Cristinzio C, Kusevic I, Yassa MA, Caffo BS, Zeger SL. Familial risk for Alzheimer's disease alters fMRI activation patterns. Brain. 2006;129:1229–1239. doi: 10.1093/brain/awl089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bookheimer SY, Strojwas MH, Cohen MS, Saunders AM, Pericak-Vance MA, Mazziotta JC, Small GW. Patterns of brain activation in people at risk for Alzheimer's disease. N Engl J Med. 2000;343:450–456. doi: 10.1056/NEJM200008173430701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brandt J, Spencer M, Folstein M. The Telephone Interview for Cognitive Status. Neuropsychiatry, Neuropsychol Behav Neurol. 1988;1:111–117. [Google Scholar]
- Burke SN, Barnes CA. Neural plasticity in the ageing brain. Nat Rev Neurosci. 2006;7:30–40. doi: 10.1038/nrn1809. [DOI] [PubMed] [Google Scholar]
- Burke SN, Barnes CA. Senescent synapses and hippocampal circuit dynamics. Trends in Neuroscience. 2010 doi: 10.1016/j.tins.2009.12.003. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buxton RB. Introduction to Functional Magnetic Resonance Imaging: Principles and Techniques. Cambridge, UK: Cambridge University Press; 2001. [Google Scholar]
- Cacucci F, Yi M, Wills TJ, Chapman P, O'Keefe J. Place cell firing correlates with memory deficits and amyloid plaque burden in Tg2576 Alzheimer mouse model. Proc Natl Acad Sci U S A. 2008;105(22):7863–8. doi: 10.1073/pnas.0802908105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cabeza R, Daselaar SM, Dolcos F, Prince SE, Budde M, Nyberg L. Task-independent and task-specific age effects on brain activity during working memory, visual attention and episodic retrieval. Cereb Cortex. 2004;14:364–375. doi: 10.1093/cercor/bhg133. [DOI] [PubMed] [Google Scholar]
- Celone KA, Calhoun VD, Dickerson BC, Atri A, Chua EF, Miller SL, DePeau K, Rentz DM, Selkoe DJ, Blacker D, Albert MS, Sperling RA. Alterations in memory networks in mild cognitive impairment and Alzheimer's disease: an independent component analysis. J Neurosci. 2006;26:10222–10231. doi: 10.1523/JNEUROSCI.2250-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chee MW, Goh JO, Venkatraman V, Tan JC, Gutchess A, Sutton B, Hebrank A, Leshikar E, Park D. Age-related changes in object processing and contextual binding revealed using fMR adaptation. J Cogn Neurosci. 2006;18:495–507. doi: 10.1162/jocn.2006.18.4.495. [DOI] [PubMed] [Google Scholar]
- Craik FIM, Simon D. In: Age differences in memory: the roles of attention and depth of processing. Poon LW, Fozard JL, Cermak LS, Arenberg D, editors. Hillsdale, NJ: Lawrence Erlbaum Associates; 1980. [Google Scholar]
- Daselaar SM, Fleck MS, Dobbins IG, Madden DJ, Cabeza R. Effects of healthy aging on hippocampal and rhinal memory functions: an event-related fMRI study. Cereb Cortex. 2006;16:1771–1782. doi: 10.1093/cercor/bhj112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Davis TL, Kwong KK, Weisskoff RM, Rosen BR. Calibrated functional MRI: mapping the dynamics of oxidative metabolism. Proc Natl Acad Sci U S A. 1998;95(4):1834–9. doi: 10.1073/pnas.95.4.1834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dennis NA, Hayes SM, Prince SE, Madden DJ, Huettel SA, Cabeza R. Effects of aging on the neural correlates of successful item and source memory encoding. J Exp Psychol Learn Mem Cogn. 2008;34:791–808. doi: 10.1037/0278-7393.34.4.791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Bates JF, Atiya M, Killiany RJ, Greve DN, Dale AM, Stern CE, Blacker D, Albert MS, Sperling RA. Medial temporal lobe function and structure in mild cognitive impairment. Annuals of Neurology. 2004;56:27–35. doi: 10.1002/ana.20163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Salat DH, Greve DN, Chua EF, Rand-Giovannetti E, Rentz DM, Bertram L, Mullin K, Tanzi RE, Blacker D, Albert MS, Sperling RA. Increased hippocampal activation in mild cognitive impairment compared to normal aging and AD. Neurology. 2005;65:404–411. doi: 10.1212/01.wnl.0000171450.97464.49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duverne S, Motamedinia S, Rugg MD. Effects of Age on the Neural Correlates of Retrieval Cue Processing are Modulated by Task Demands. J Cogn Neurosci. 2008;21:1–17. doi: 10.1162/jocn.2009.21001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fleisher AS, Podraza KM, Bangen KJ, Taylor C, Sherzai A, Sidhar K, Liu TT, Dale AM, Buxton RB. Cerebral perfusion and oxygenation differences in Alzheimer's disease risk. Neurobiology of Aging. 2008;30(11):1737–48. doi: 10.1016/j.neurobiolaging.2008.01.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley AH, Siegel SJ, Kordower JH, Mufson EJ, Morrison JH. Circuit-specific alterations of N-methyl-D-aspartate receptor subunit 1 in the dentate gyrus of aged monkeys. Proc Natl Acad Sci U S A. 1996;93(7):3121–3125. doi: 10.1073/pnas.93.7.3121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geinisman Y, deToledo-Morrell L, Morrell F, Persina IS, Rossi M. Age-related loss of axospinous synapses formed by two afferent systems in the rat dentate gyrus as revealed by the unbiased stereological dissector technique. Hippocampus. 1992;2:437–444. doi: 10.1002/hipo.450020411. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. The role of acetylcholine in learning and memory. Curr Opin Neurobiol. 2006;16:710–715. doi: 10.1016/j.conb.2006.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasselmo ME, Schnell E, Barkai E. Dynamics of learning and recall at excitatory recurrent synapses and cholinergic modulation in rat hippocampal region CA3. J Neurosci. 1995;15:5249–5262. doi: 10.1523/JNEUROSCI.15-07-05249.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Healthy and pathological processes in adult development: new evidence from neuroimaging of the aging brain. Curr Opin Neurol. 2005;18:740–747. doi: 10.1097/01.wco.0000189875.29852.48. [DOI] [PubMed] [Google Scholar]
- Hedden T, Gabrieli JD. Insights into the ageing mind: a view from cognitive neuroscience. Nat Rev Neurosci. 2004;5:87–96. doi: 10.1038/nrn1323. [DOI] [PubMed] [Google Scholar]
- Henkel LA, Johnson MK, De Leonardis DM. Aging and source monitoring: cognitive processes and neuropsychological correlates. J Exp Psychol Gen. 1998;127:251–268. doi: 10.1037//0096-3445.127.3.251. [DOI] [PubMed] [Google Scholar]
- Jennings JM, Jacoby LL. An opposition procedure for detecting age-related deficits in recollection: telling effects of repetition. Psychol Aging. 1997;12:352–361. doi: 10.1037//0882-7974.12.2.352. [DOI] [PubMed] [Google Scholar]
- Kirwan CB, Jones C, Miller MI, Stark CEL. High-resolution fMRI investigation of the medial temporal lobe. Human Brain Mapping. 2007;28:959–966. doi: 10.1002/hbm.20331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirwan CB, Stark CEL. Overcoming interference: An fMRI investigation of pattern separation in the medial temporal lobe. Learning and Memory. 2007;14:625–633. doi: 10.1101/lm.663507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee I, Rao G, Knierim JJ. A double dissociation between hippocampal subfields: differential time course of CA3 and CA1 place cells for processing changed environments. Neuron. 2004;1042(5):803–15. doi: 10.1016/j.neuron.2004.05.010. [DOI] [PubMed] [Google Scholar]
- Leutgeb JK, Leutgeb S, Moser MS, Moser EI. Pattern separation in the dentate gyrus and CA3 of the hippocampus. Science. 2007;315(5814):961–6. doi: 10.1126/science.1135801. [DOI] [PubMed] [Google Scholar]
- Leutgeb S, Leutgeb JK, Treves A, Moser MB, Moser EI. Distinct ensemble codes in hippocampal areas CA3 and CA1. Science. 2004;305(5688):1295–8. doi: 10.1126/science.1100265. [DOI] [PubMed] [Google Scholar]
- McClelland JL, McNaughton BL, O'Reilly RC. Why there are complementary learning systems in the hippocampus and neocortex: insights from the successes and failures of connectionist models of learning and memory. Psychol Rev. 1995;102:419–457. doi: 10.1037/0033-295X.102.3.419. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McHugh TJ, Jones MW, Quinn JJ, Balthasar N, Coppari R, Elmquist JK, Lowell BB, Fanselow MS, Wilson MA, Tonegawa S. Dentate gyrus NMDA receptors mediate rapid pattern separation in the hippocampal network. Science. 2007;317(5834):94–9. doi: 10.1126/science.1140263. [DOI] [PubMed] [Google Scholar]
- Miller MI, Beg MF, Ceritoglu C, Stark C. Increasing the power of functional maps of the medial temporal lobe by using large deformation diffeomorphic metric mapping. Proc Natl Acad Sci U S A. 2005;102:9685–9690. doi: 10.1073/pnas.0503892102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Celone K, DePeau K, Diamond E, Dickerson BC, Rentz D, Pihlajamaki M, Sperling RA. Age-related memory impairment associated with loss of parietal deactivation but preserved hippocampal activation. Proc Natl Acad Sci U S A. 2008a;105:2181–2186. doi: 10.1073/pnas.0706818105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller SL, Fenstermacher E, Bates J, Blacker D, Sperling RA, Dickerson BC. Hippocampal activation in adults with mild cognitive impairment predicts subsequent cognitive decline. J Neurol Neurosurg Psychiatry. 2008b;79:630–635. doi: 10.1136/jnnp.2007.124149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milner B, Squire LR, Kandel ER. Cognitive neuroscience and the study of memory. Neuron. 1998;20:445–468. doi: 10.1016/s0896-6273(00)80987-3. [DOI] [PubMed] [Google Scholar]
- Moreno H, Wu WE, Lee T, Brickman A, Mayeux R, Brown TR, Small SA. Imaging the Abeta-related neurotoxicity of Alzheimer disease. Archives of Neurology. 2007;64(10):1467–77. doi: 10.1001/archneur.64.10.1467. [DOI] [PubMed] [Google Scholar]
- Murty VP, Sambataro F, Das S, Tan HY, Callicott JH, Goldberg TE, Meyer-Lindenberg A, Weinberger DR, Mattay VS. Age-related Alterations in Simple Declarative Memory and the Effect of Negative Stimulus Valence. J Cogn Neurosci. 2008;21:1920–33. doi: 10.1162/jocn.2009.21130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Newman MC, Kaszniak AW. Spatial memory and aging: Performance on a human analog of the Morris Water Maze Task. Aging, Neuropsychology, and Cognition. 2000;7:86–93. [Google Scholar]
- Newson RS, Kemps EB. The nature of subjective cognitive complaints of older adults. Int J Aging Hum Dev. 2006;63:139–151. doi: 10.2190/1EAP-FE20-PDWY-M6P1. [DOI] [PubMed] [Google Scholar]
- Nicolle MM, Colombo PJ, Gallagher M, McKinney M. Metabotropic glutamate receptor-mediated hippocampal phosphoinositide turnover is blunted in spatial learning-impaired aged rats. J Neurosci. 1999;19:9604–9610. doi: 10.1523/JNEUROSCI.19-21-09604.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Norman KA, O'Reilly RC. Modeling hippocampal and neocortical contributions to recognition memory: a complementary-learning-systems approach. Psychol Rev. 2003;110:611–646. doi: 10.1037/0033-295X.110.4.611. [DOI] [PubMed] [Google Scholar]
- O'Reilly RC, Norman KA. Hippocampal and neocortical contributions to memory: advances in the complementary learning systems framework. Trends Cogn Sci. 2002;6:505–510. doi: 10.1016/s1364-6613(02)02005-3. [DOI] [PubMed] [Google Scholar]
- Penner MR, Roth TL, Chawla MK, Hoang LT, Roth ED, Lubin FD, Sweatt JD, Worley PF, Barnes CA. Age-related changes in Arc transcription and DNA methylation within the hippocampus. Neurobiology of Aging. 2010 doi: 10.1016/j.neurobiolaging.2010.01.009. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rand-Giovannetti E, Chua EF, Driscoll AE, Schacter DL, Albert MS, Sperling RA. Hippocampal and neocortical activation during repetitive encoding in older persons. Neurobiol Aging. 2006;27:173–182. doi: 10.1016/j.neurobiolaging.2004.12.013. [DOI] [PubMed] [Google Scholar]
- Raz N, Lindenberger U, Rodrigue KM, Kennedy KM, Head D, Williamson A, Dahle C, Gerstorf D, Acker JD. Regional brain changes in aging healthy adults: general trends, individual differences and modifiers. Cereb Cortex. 2005;15:1676–1689. doi: 10.1093/cercor/bhi044. [DOI] [PubMed] [Google Scholar]
- Small SA, Perera GM, DeLaPaz R, Mayeux R, Stern Y. Differential regional dysfunction of the hippocampal formation among elderly with memory decline and Alzheimer's disease. Ann Neurol. 1999;45:466–472. doi: 10.1002/1531-8249(199904)45:4<466::aid-ana8>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- Small SA, Chawla MK, Buonocore M, Rapp PR, Barnes CA. Imaging correlates of brain function in monkeys and rats isolates a hippocampal subregion differentially vulnerable to aging. Proc Natl Acad Sci U S A. 2004;101(18):7181–6. doi: 10.1073/pnas.0400285101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Small SA, Tsai WY, DeLaPaz R, Mayeux R, Stern Y. Imaging hippocampal function across the human life span: is memory decline normal or not? Annals of Neurology. 2002;51(3):290–295. doi: 10.1002/ana.10105. [DOI] [PubMed] [Google Scholar]
- Smith TD, Adams MM, Gallagher M, Morrison JH, Rapp PR. Circuit-specific alterations in hippocampal synaptophysin immunoreactivity predict spatial learning impairment in aged rats. J Neurosci. 2000;20:6587–6593. doi: 10.1523/JNEUROSCI.20-17-06587.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sperling R. Functional MRI studies of associative encoding in normal aging, mild cognitive impairment, and Alzheimer's disease. Ann N Y Acad Sci. 2007;1097:146–155. doi: 10.1196/annals.1379.009. [DOI] [PubMed] [Google Scholar]
- Squire LR, Stark CEL, Clark RE. The medial temporal lobe. Ann Rev Neurosci. 2004;27:279–306. doi: 10.1146/annurev.neuro.27.070203.144130. [DOI] [PubMed] [Google Scholar]
- Stark CEL, Okado Y. Making memories without trying: Medial temporal lobe activity associated with incidental memory formation during recognition. J Neurosci. 2003;23:6748–6753. doi: 10.1523/JNEUROSCI.23-17-06748.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugaya K, Greene R, Personett D, Robbins M, Kent C, Bryan D, Skiba E, Gallagher M, McKinney M. Septo-hippocampal cholinergic and neurotrophin markers in age-induced cognitive decline. Neurobiol Aging. 1998;19:351–361. doi: 10.1016/s0197-4580(98)00072-4. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P. A Co-Planar Steres Jotactic Atlas of the Human Brain. Stuttgard, Germany: Thieme; 1988. [Google Scholar]
- Toner CK, Pirogovsky E, Kirwan CB, Gilbert PE. Visual object pattern separation deficits in nondemented older adults. Learn Mem. 2009;16:338–342. doi: 10.1101/lm.1315109. [DOI] [PubMed] [Google Scholar]
- Vazdarjanova A, Guzowski JF. Differences in hippocampal neuronal population responses to modifications of an environmental context: evidence for distinct, yet complementary, functions of CA3 and CA1 ensembles. J Neurosci. 2004;24(29):6489–96. doi: 10.1523/JNEUROSCI.0350-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West MJ. Regionally specific loss of neurons in the aging human hippocampus. Neurobiology of Aging. 1993;14(4):287–293. doi: 10.1016/0197-4580(93)90113-p. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Gallagher M, Eichenbaum H, Tanila H. Neurocognitive aging: prior memories hinder new hippocampal encoding. Trends Neurosci. 2006;29:662–670. doi: 10.1016/j.tins.2006.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gallagher M, Eichenbaum H, Tanila H. Age-associated alterations of hippocampal place cells are subregion specific. J Neurosci. 2005a;25:6877–6886. doi: 10.1523/JNEUROSCI.1744-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, Gurevicius K, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cells of aged rats in two visually identical compartments. Neurobiol Aging. 2005b;26:1099–1106. doi: 10.1016/j.neurobiolaging.2004.09.006. [DOI] [PubMed] [Google Scholar]
- Wilson IA, Ikonen S, McMahan RW, Gallagher M, Eichenbaum H, Tanila H. Place cell rigidity correlates with impaired spatial learning in aged rats. Neurobiol Aging. 2003;24:297–305. doi: 10.1016/s0197-4580(02)00080-5. [DOI] [PubMed] [Google Scholar]
- Yassa MA, Stark CEL. A Quantitative Evaluation of Cross-Participant Registration Techniques for MRI Studies of the Medial Temporal lobe. NeuroImage. 2009;44:319–327. doi: 10.1016/j.neuroimage.2008.09.016. [DOI] [PubMed] [Google Scholar]