Abstract
Although a major effect of bisphosphonates on bone is inhibition of resorption resulting from their ability to interfere with osteoclast function, these agents also prevent osteoblast and osteocyte apoptosis in vitro and in vivo. However, the contribution of the latter property to the overall beneficial effects of the drugs on bone remains unknown. We compared herein the action on glucocorticoid-induced bone disease of the classical bisphosphonate alendronate with that of IG9402, a bisphosphonate analog that preserves osteoblast and osteocyte viability but does not induce osteoclast apoptosis in vitro. The bisphosphonates were injected daily (2.3 μmol/kg) to 5-month-old Swiss Webster mice (6–11 per group), starting three days prior to implantation of pellets releasing the glucocorticoid prednisolone (2.1 mg/kg/d). IG9402 did not affect levels of circulating C-telopeptide or osteocalcin, markers of resorption and formation, respectively, nor did it decrease mRNA levels of osteocalcin or collagen1A1 in bone. On the other hand, alendronate decreased all these parameters. Moreover, IG9402 did not reduce cancellous mineralizing surface, mineral apposition rate or bone formation rate, whereas alendronate induced a decrease in each of these bone formation measures. These findings demonstrate that in contrast to alendronate, IG9402 does not inhibit bone turnover. Both alendronate and IG9402, on the other hand, activated survival kinase signaling in vivo, as evidenced by induction of ERK phosphorylation in bone. Furthermore, both bisphosphonates prevented the increase in osteoblast and osteocyte apoptosis as well as the decrease in vertebral bone mass and strength induced by glucocorticoids. We conclude that a bisphosphonate that does not affect osteoclasts prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids in mice.
Keywords: bisphosphonate, apoptosis, osteocyte, osteoblast, glucocorticoid, bone strength
INTRODUCTION
Extensive evidence indicates that apoptosis of bone cells contributes to regulate the growth and maintenance of the skeleton (reviewed in [1]). Whereas all osteoclasts die by apoptosis, 80 % of osteoblasts also die by apoptosis with the remainders becoming lining cells or osteocytes. Osteocytes also can die prematurely and this might have important implications for bone strength. Indeed, increased osteoblast and osteocyte apoptosis is associated with conditions with increased bone fragility, including glucocorticoid excess, sex steroid deficiency, immobilization, and aging. Conversely, stimuli that maintain bone strength, such as parathyroid hormone (PTH), sex steroids, and mechanical forces, preserve osteoblast and osteocyte viability.
Traditionally, the bone protective effects of bisphosphonates have been exclusively attributed to interference with osteoclastic resorption [2]. Nonetheless, over the last several years, we and others have provided evidence that bisphosphonates also act directly on osteoblasts and osteocytes to prolong their lifespan ([3–6] and reviewed in this issue [REFERENCE]). The survival effect of bisphosphonates is exerted via a mechanism different from inhibition of the mevalonate pathway or conversion into toxic metabolites, recognized events by which bisphosphonates act on osteoclasts [2]. Instead, bisphosphonates activate a novel signal transduction pathway in osteoblastic cells strictly dependent on the expression of connexin (Cx) 43 and on the activation of the extracellular signal-regulated kinases (ERKs) [3, 7, 8]. Consistent with different mechanisms of action being responsible for the effects of bisphosphonates in osteoclasts versus osteoblasts/osteocytes, anti-apoptosis of osteoblastic cells is observed in vitro at concentrations about 3 orders of magnitude lower than those required to promote osteoclast apoptosis [3, 5, 9, 10], and it is exerted by compounds such as IG9402 and NE11809 that do not affect osteoclasts [3, 5, 10].
Increased survival of osteoblasts leading to positive balance within each remodeling unit is consistent with the increased wall width observed in dogs, humans, and non-human primates upon long term bisphosphonate administration [11–14]. In addition, inhibition of osteocyte apoptosis contributing to preservation of the osteocyte network would allow a normal response of the skeleton to mechanical, hormonal, and pharmacotherapeutic cues [1]. However, the potent anti-resorptive action of bisphosphonates may mask the consequences of the anti-apoptotic effect in osteoblasts and osteocytes. Indeed, although the bisphosphonate alendronate did not prevent osteoblast and osteocyte apoptosis induced by glucocorticoids in Cx43 deficient mice, it was still able to abolish the bone loss induced by the steroids [6]. To single out the role of the survival effect of bisphosphonates on bone, we examined herein the actions in mice of IG9402, one of the bisphosphonates that preserves osteoblast and osteocyte viability in vitro, but does not affect osteoclasts [3, 10]. IG9402, an analog of the bisphosphonate olpadronate that has an amino instead of hydroxyl group in the R1 position, was originally developed to study the role of the R1 residue of bisphosphonates in the ability of the drugs to bind to mineral and to inhibit resorption [15]. IG9402 binds to mineral with similar affinity than olpadronate, but, unlike the latter, it does not inhibit 45calcium release from fetal mouse metacarpals in vitro [15] or increase ash weight in the tibia when administered to growing mice [16]. The lack of anti-resorptive activity of the IG9402 compound is due to its inability to inhibit enzymes of the mevalonate pathway [17], the recognized mechanism for blocking resorption by amino-bisphosphonates. We found that, unlike alendronate, IG9402 did not reduce bone turnover under basal conditions, but it was as effective as alendronate in activating pro-survival kinase signaling and preventing osteoblast and osteocyte apoptosis and the loss of bone mass and strength induced by glucocorticoids. Therefore, preservation of osteoblast and osteocyte viability without affecting bone remodeling is sufficient to ameliorate the deleterious effects of glucocorticoids in bone.
MATERIALS AND METHODS
Mice
Female 5-month-old Swiss Webster mice (n=6–11 per group) were implanted subcutaneously with pellets containing placebo or pharmacologic doses of prednisolone, releasing 2.1 mg/kg/day (Innovative Research of America, Sarasota, FL). Mice were administered daily subcutaneous injections of 2.3 μmol/kg/day of alendronate (0.75 mg/kg/day), IG9402 (0.60 mg/kg/day) or the equivalent volume of saline starting three days prior to pellet implantation, as previously published [3]. Ten days after pellet implantation, blood samples were obtained, mice were sacrifice and tissues were collected. The bisphosphonates used in this study were provided by Gador S.A. (Buenos Aires, Argentina).
Markers of bone turnover
Plasma osteocalcin and C-telopeptide were measured using an enzyme radiometric assay (Biomedical Technologies, Soughton, MA) and an enzyme linked immunosorbant assay (RatLaps, Immunodiagnostic Systems Inc., Fountain Hills, AZ), respectively [18].
Bone mass
BMD was determined by scanning the plastic embedded lumbar spine (L1–L4) by DEXA using a PIXImus densitometer (G.E. Medical Systems, Lunar Division, Madison, WI) [6].
Bone histomorphometry and apoptosis
L1 to L4 were fixed in 4°C Millonig's phosphate-buffered 10% formalin, pH 7.4, and embedded undecalcified in methyl methacrylate as previously described [3]. Static histomorphometry was performed in 3-μm thick longitudinal sections stained with 1% toluidine blue (pH 2.8). Osteoclasts were quantified on sections stained for tartrate-resistant acid phosphatase (TRAP) [19]. An osteoclast was defined as a multinucleated cell (>2 nuclei), with one side attached to the bone tissue. Dynamic histomorphometry was performed in 7-μm unstained longitudinal sections by epifluorescence microscopy. For this purpose, tetracycline HCl (Sigma Chemical Co., St. Louis, MO) (30 mg/kg body weight) was intra-peritoneally injected 7 and 3 days before sacrifice. The histomorphometric examination was performed with a computer and digitizer tablet (OsteoMetrics, Decatur, GA) interfaced to a Zeiss Axioscope with a drawing tube attachment, as previously indicated [20]. All measurements were made at X400 magnification (plan-neofluar objective, numerical aperture 0.75). The terminology used was that recommended by the Histomorphometry Nomenclature Committee of the American Society of Bone and Mineral Research [21]. Apoptosis of osteoblasts and osteocytes was detected by in situ nick-end labeling (ISEL) using the DNA fragmentation Klenow enzyme (Oncogene Research Products, Cambridge, MA) in vertebral sections counterstained with 2% methyl green as previously described [6]. The prevalence of apoptotic osteoblasts and osteocytes was calculated by enumerating the total number and the ISEL-positive cells exhibiting condensed chromatin, nuclear fragmentation or cell shrinkage.
Biomechanical testing
The load bearing properties of the sixth lumbar vertebrae (L6) were measured using a single column material testing machine and a calibrated tension/compression load cell (Model 5542, Instron Corp., Norwood, MA), as previously described [22]. The length, width, and depth of bones were measured with a digital caliper at a resolution of 0.01 mm (Mitutoyo no.500–196, Ace Tools, Wantagh, NY).
Gene expression
Total RNA was purified from fifth lumbar vertebrae (L5) using Ultraspec reagent (Biotecx Laboratories, Houston, TX), according to the manufacturer's instructions. Taqman quantitative RT-PCR was performed as previously described [6] using primer probe sets from Applied Biosystems (Foster City, CA). Relative mRNA expression levels were normalized to the house-keeping gene ribosomal protein S2 using the ΔCt method [23].
ERK activation
Female 5-month-old Swiss Webster mice (n=4 per group) were administered a single injection of 2.3 μmol/kg/day of alendronate, IG9402 or the equivalent volume of saline. Twenty four hours after injection, mice were sacrificed and protein lysates were obtained from the 6th lumbar vertebrae, as previously published [24]. Fifty μg protein were used to determine phosphorylated and total ERK1/2 by Western blot analysis using rabbit anti-phospho Tyr204-ERK and anti-total ERK (Santa Cruz Biotechnology, Santa Cruz, CA).
Statistical analysis
Data was analyzed using SigmaStat (SPSS Science, Chicago, IL). All values are reported as the mean ± S.D. Differences in group means were analyzed by one-way or two-way analysis of variance, and t-test was used to estimate the level of significance of differences between means.
RESULTS
The aminobisposphonate IG9402 does not reduce bone remodeling
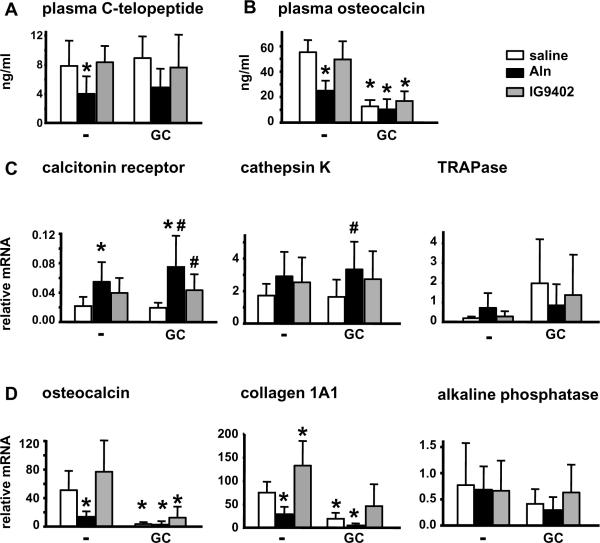
Daily administration of IG9402 for 13 days did not affect circulating levels of the bone resorption marker C-telopeptide, whereas alendronate significantly decreased it (Figure 1A). Plasma levels of osteocalcin were also unchanged in mice treated with IG9402, whereas were significantly lower in mice treated with alendronate (Figure 1B). Quantitative RT-PCR analysis demonstrated that mRNA levels of the osteoclast-specific genes calcitonin receptor, cathepsin K, and TRAP were not changed in vertebral bone of animals receiving IG9402 (Figure 1C). Bones from mice treated with alendronate showed increased mRNA levels for calcitonin receptor and no significant changes in the levels of cathepsin K and TRAP mRNAs. Whereas alendronate decreased the levels of both osteocalcin and collagen IaI, IG9402 did not reduce osteocalcin expression and even increased collagen IaI mRNA expression (Figure 1D). None of the drugs induced any change in alkaline phosphatase expression. Taken together, these results indicate that whereas alendronate induced the expected decrease in bone remodeling and the expression of osteoblastic genes, IG9402 did not reduce bone remodeling or osteoblastic gene expression.
Figure 1. IG9402 does not decrease bone turnover in mice.
Mice were treated with daily injections of alendronate or IG9402, starting 3 days prior to pellet implantation. Ten days after pellet implantation, mice were sacrificed and plasma and lumbar vertebrae were collected. Levels of circulating C-telopeptide (A), circulating osteocalcin (B) and mRNA for osteoclastic (C) and osteoblastic (D) genes were determined as indicated in Methods. Aln: alendronate. *: p<0.05 versus saline-placebo and #: p<0.05 versus saline-GC by two-way anova, n= 6–11 mice/group.
Administration of prednisolone did not affect either circulating C-telopeptide or the expression of osteoclast markers (Figure 1A and C). In contrast, the glucocorticoid reduced dramatically circulating levels of osteocalcin, as well as the expression of osteocalcin and collagen 1a1 mRNA (Figure 1B and D). Reduction of osteocalcin protein and mRNA was not prevented by either bisphosphonate, suggesting that these compounds are not able to prevent the effect of glucocorticoids on the transcription of osteocalcin gene [25].
IG9402 prevents glucocorticoid-induced bone loss
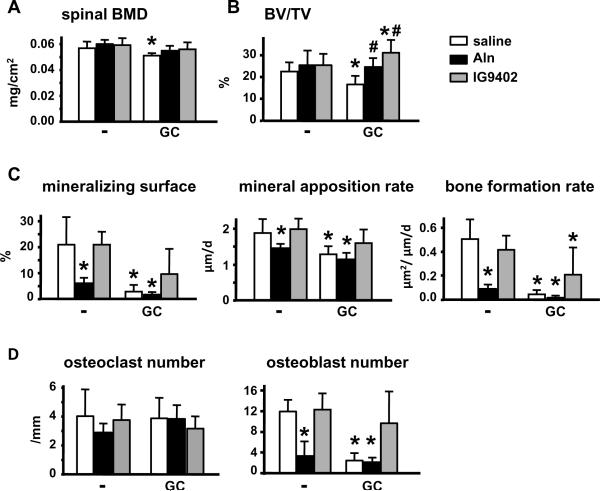
We next investigated the effect of the bisphosphonates on vertebral bone mass and histomorphometric indexes. Neither alendronate nor IG9402 altered BMD or bone volume (BV/TV) in the lumbar spine under basal conditions (Figure 2A and B). On the other hand, and consistent with the lack of anti-remodeling effect of IG9402, only alendronate reduced mineralizing surface, mineral apposition rate, and bone formation rate (Figure 2C), and osteoblast numbers without decreasing significantly osteoclast numbers (Figure 2D).
Figure 2. IG9402 prevents the decrease in vertebral bone mass induced by glucocorticoids.
(A) Bone mineral density in lumbar vertebrae was determined by DEXA. (B–D) Histomorphometric measurements were determined in lumbar vertebral sections stained with toluidine blue (B and D) or unstained (C). Osteoclast numbers were scored in sections stained for TRAP. GC: prednisolone, Aln: alendronate. *: p<0.05 versus saline-placebo and #: p<0.05 versus saline-GC by two-way anova, n= 6–11 mice/group.
Administration of prednisolone caused a significant reduction in BMD and BV/TV, as well as mineralizing surface, mineral apposition rate and bone formation rate (Figure 2A–C). In addition, the glucocorticoid did not alter osteoclast number but reduced dramatically osteoblast number (Figure 2D). Administration of alendronate or IG9402 prevented prednisolone-induced decrease in BMD and BV/TV. However, alendronate was not able to reverse the decrease in mineralizing surface, mineral apposition rate, bone formation rate, or osteoblast number induced by prednisolone. IG9402, on the other hand, prevented this decrease in mineralizing surface and mineral apposition rate (Figure 2C). However, bone formation rate in animals treated with IG9402 and the glucocorticoid was still lower than that of the control group treated with saline and placebo. Nevertheless, the decrease in osteoblast numbers induced by glucocorticoids was prevented by IG9402. These results suggest that alendronate and IG9402 prevent the loss of bone mass induced by glucocorticoids via different mechanism of action. Thus, whereas the main effect of alendronate is to stop bone resorption preventing glucocorticoid-induced bone loss, IG9402 preserves osteoblast activity resulting in maintenance of bone mass.
IG9402 induces ERK activation and abolishes glucocorticoid-induced osteoblast and osteocyte apoptosis in vivo
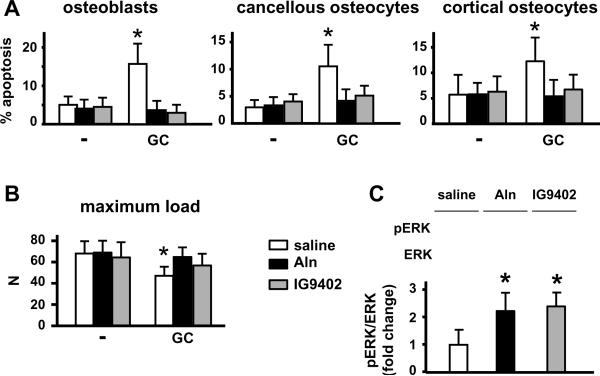
Administration of alendronate or IG9402 did not alter the already low levels of osteoblast and osteocyte apoptosis in the vertebrae of placebo-treated mice (Figure 3A). On the other hand, prednisolone increased osteoblast, and cancellous and cortical osteocyte apoptosis, as previously shown [3, 6, 26, 27], and daily injections of either alendronate or IG9402 prevented this effect. In addition, both alendronate and IG9402 were able to prevent glucocorticoid-induced decrease in bone strength as measured by the load required to break the 6th lumbar vertebra (Figure 3B). These findings suggest that preservation of osteoblast and osteocyte viability in the absence of anti-resorptive effect is sufficient to maintain bone strength.
Figure 3. IG9402 prevents osteoblast and osteocyte apoptosis and the loss of bone strength induced by glucocorticoids and activates ERK survival signaling.
(A) The prevalence of apoptosis of osteoblasts, and cancellous and cortical osteocytes was determined by ISEL in lumbar vertebrae. (B) Maximum load of 6th lumbar vertebrae was measured by vertebral compression strength. GC: prednisolone, Aln: alendronate. * p<0.05 versus saline-placebo by two-way anova, n= 6–11 mice/group. (C) Mice (4/group) were given a single injection of saline, or 2.3 μM/kg/day alendronate or IG9402. Twenty four hours later mice were sacrificed and the 6th lumbar vertebrae were collected. Levels of phosphorylated and total ERK were determined by Western blotting and are expressed as fold change in phosphor-ERK/ERK over saline-treated mice. Each line corresponds to an individual animal. * p<0.05 versus saline by one-way anova.
We have shown that the anti-apoptotic effect of bisphosphonates in vitro requires activation of ERKs [3]. To determine whether this pathway was also activated in vivo, mice received a single injection of saline, alendronate, or IG9402 and were sacrificed 24 h later. ERK activation was measured by Western blotting in vertebral bone lysates. As previously found in vitro, both bisphosphonates increased the fraction of active (phosphorylated) ERKs (Figure 3C).
DISCUSSION
In this report, we have dissected the contribution of preservation of osteoblast and osteocyte viability to the overall actions of bisphosphonates in bone by examining the effects on the mouse skeleton of administering the bisphosphonate IG9402 that activates exclusively survival signaling in osteoblastic cells without affecting osteoclasts. We show that whereas IG9402 did not alter bone turnover, it was still effective in preventing osteoblast and osteocyte apoptosis induced by glucocorticoids, resulting in maintenance of bone mass and strength. Furthermore, IG9402 significantly increased collagen 1a1 levels in vertebral bone and prevented the decrease in the expression of this gene induced by glucocorticoids.
Alendronate was also effective in preventing apoptosis of osteoblastic cells and the loss of bone mass and strength induced by glucocorticoids. However, this effect was accompanied by a decrease in bone turnover, as evidenced by decreased circulating levels of markers of resorption and formation, as well as osteoblast numbers. Nonetheless, osteoclast numbers and osteoclast-specific genes were not decreased by alendronate. This is consistent with the accumulation of inactive large TRAP positive osteoclasts in humans treated with bisphosphonates [28, 29] and with findings demonstrating that alendronate inhibits resorption in vitro without affecting calcitonin receptor expression [30].
The reduction in bone turnover induced by traditional bisphosphonates may explain why prolongation of osteoblast lifespan is not translated into a considerable increase in BMD, likely due to the reduction in the extent of osteoblast-covered surfaces. However, previous evidence has shown that where osteoblasts are present, more bone is made, as indicated by an increase in wall thickness in cancellous bone of animals and humans under long-term treatment with bisphosphonates [11–14]. In the current study, inhibition of glucocorticoid-induced osteoblast apoptosis by IG9402, in the absence of a reduction in bone turnover, was sufficient to abolish the decrease in osteoblast number induced by the steroid. Moreover, although bone formation rate in animals treated with steroids and IG9402 was still reduced, the decrease in mineralizing surface and mineral apposition rate induced by the steroid was prevented. This indicates that the percent of bone surface covered by osteoblasts, as well as the synthetic activity of individual osteoblasts, was preserved by IG9402, even when glucocorticoids were present. Moreover, IG9402 prevented the decrease in spinal BMD and in cancellous bone volume induced by prednisolone. These findings support a role for activation of survival signaling by bisphosphonates in conditions in which the prevalence of osteoblast apoptosis is augmented. Nonetheless, not detectable effects of IG9402 on BMD, bone volume, or indexes of bone formation were observed under basal conditions. This could be due to the short-term duration of the current study and/or to the unchanged levels of osteoblast apoptosis in placebo-treated animals receiving IG9402. In support of this possibility, the increase in osteoblast number and bone formation rate induced by PTH or PTH-related peptide is associated with decreased basal levels of osteoblast apoptosis, at least in murine cancellous bone [18, 31, 32]. Future experiments will be required to examine whether osteoblast apoptosis under basal conditions is reduced by long-term treatment with IG9402, thereby inducing an anabolic response.
In conclusion, our findings support the notion that preservation of osteoblast and osteocyte viability is an additional mechanism by which bisphosphonates maintain skeletal mass and strength. Moreover, although both alendronate and IG9402 prevent the decrease in bone mass induced by glucocorticoids, they do so by different mechanisms. Thus, while alendronate stops bone resorption and therefore halts the erosion of the bone that occurs in the presence of the corticoisteroids, IG9402 prevents osteoblast apoptosis and the decrease in osteoblast numbers, leading to the preservation of the bone forming function of the osteoblasts. Bisphosphonate analogs, like IG9402, which prevent osteoblast and osteocyte apoptosis without affecting bone remodeling, might represent a valuable tool in the treatment of bone fragility in conditions exhibiting decreased osteoblast performance, such as idiopathic juvenile osteoporosis [33], or when a decrease in bone remodeling is not desirable.
ACKNOWLEDGEMENTS
The authors thank Kanan Vyas, Joseph Goellner, Keith Condon, and Jeffrey Benson for technical assistance; the histomorphometry core at UAMS for processing the bone samples; and Stavros C. Manolagas, Robert S. Weinstein, A. Michael Parfitt, and other members of the UAMS Center for Osteoporosis and Metabolic Bone Diseases, and David Burr and Matt Allen from the Anatomy and Cell Biology Department at IUSM, for advice and insightful discussions. This research was supported by the National Institutes of Health (R01-AR053643, KO2-AR02127, R03 TW006919, R01-DK076007, and P01-AG13918).
Abbreviations
- BMD
bone mineral density
- PTH
parathyroid hormone
- Cx
connexin
- TRAP
tartrate-resistant acid phosphatase
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- [1].Jilka RL, Bellido T, Almeida M, Plotkin LI, O'Brien CA, Weinstein RS, Manolagas SC. Apoptosis in bone cells. In: Bilezikian JP, Raisz LG, Martin TJ, editors. Principles of Bone Biology. Academic Press; San Diego, San Francisco, New York, London, Sydney, Tokyo: 2008. pp. 237–61. [Google Scholar]
- [2].Rogers MJ. From molds and macrophages to mevalonate: a decade of progress in understanding the molecular mode of action of bisphosphonates. Calcif. Tissue Int. 2004;75:451–61. doi: 10.1007/s00223-004-0024-1. [DOI] [PubMed] [Google Scholar]
- [3].Plotkin LI, Weinstein RS, Parfitt AM, Roberson PK, Manolagas SC, Bellido T. Prevention of osteocyte and osteoblast apoptosis by bisphosphonates and calcitonin. J. Clin. Invest. 1999;104:1363–74. doi: 10.1172/JCI6800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Follet H, Li J, Phipps RJ, Hui S, Condon K, Burr DB. Risedronate and alendronate suppress osteocyte apoptosis following cyclic fatigue loading. Bone. 2007;40:1172–7. doi: 10.1016/j.bone.2006.12.052. [DOI] [PubMed] [Google Scholar]
- [5].Kogianni G, Mann V, Ebetino F, Nuttall M, Nijweide P, Simpson H, Noble B. Fas/CD95 is associated with glucocorticoid-induced osteocyte apoptosis. Life Sci. 2004;75:2879–95. doi: 10.1016/j.lfs.2004.04.048. [DOI] [PubMed] [Google Scholar]
- [6].Plotkin LI, Lezcano V, Thostenson J, Weinstein RS, Manolagas SC, Bellido T. Connexin 43 is required for the anti-apoptotic effect of bisphosphonates on osteocytes and osteoblasts in vivo. J. Bone Miner. Res. 2008;23:1712–21. doi: 10.1359/JBMR.080617. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [7].Plotkin LI, Manolagas SC, Bellido T. Transduction of cell survival signals by connexin-43 hemichannels. J. Biol. Chem. 2002;277:8648–57. doi: 10.1074/jbc.M108625200. [DOI] [PubMed] [Google Scholar]
- [8].Plotkin LI, Aguirre JI, Kousteni S, Manolagas SC, Bellido T. Bisphosphonates and estrogens inhibit osteocyte apoptosis via distinct molecular mechanisms downstream of ERK activation. J. Biol. Chem. 2005;280:7317–25. doi: 10.1074/jbc.M412817200. [DOI] [PubMed] [Google Scholar]
- [9].Hughes DE, Wright KR, Uy HL, Sasaki A, Yoneda T, Roodman GD, Mundy GR, Boyce BF. Bisphosphonates promote apoptosis in murine osteoclasts in vitro and in vivo. J. Bone Min. Res. 1995;10:1478–87. doi: 10.1002/jbmr.5650101008. [DOI] [PubMed] [Google Scholar]
- [10].Plotkin LI, Manolagas SC, Bellido T. Dissociation of the pro-apoptotic effects of bisphosphonates on osteoclasts from their anti-apoptotic effects on osteoblasts/osteocytes with novel analogs. Bone. 2006;39:443–52. doi: 10.1016/j.bone.2006.02.060. [DOI] [PubMed] [Google Scholar]
- [11].Chavassieux PM, Arlot ME, Reda C, Wei L, Yates AJ, Meunier PJ. Histomorphometric assessment of the long-term effects of alendronate on bone quality and remodeling in patients with osteoporosis. J. Clin. Invest. 1997;100:1475–80. doi: 10.1172/JCI119668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [12].Storm T, Steiniche T, Thamsborg G, Melsen F. Changes in bone histomorphometry after long-term treatment with intermittent, cyclic etidronate for postmenopausal osteoporosis. J. Bone Min. Res. 1993;8:199–208. doi: 10.1002/jbmr.5650080211. [DOI] [PubMed] [Google Scholar]
- [13].Balena R, Toolan BC, Shea M, Markatos A, Myers ER, Lee SC, Opas EE, Seedor JG, Klein H, Frankenfield D. The effects of 2-year treatment with the aminobisphosphonate alendronate on bone metabolism, bone histomorphometry, and bone strength in ovariectomized nonhuman primates. J. Clin. Invest. 1993;92:2577–86. doi: 10.1172/JCI116872. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Boyce RW, Paddock CL, Gleason JR, Sletsema WK, Eriksen EF. The effects of risedronate on canine cancellous bone remodeling: three-dimensional kinetic reconstruction of the remodeling site. J. Bone Min. Res. 1995;10:211–21. doi: 10.1002/jbmr.5650100207. [DOI] [PubMed] [Google Scholar]
- [15].Van Beek E, Lowik C, Que I, Papapoulos S. Dissociation of binding and antiresorptive properties of hydroxybisphosphonates by substitution of the hydroxyl with an amino group. J. Bone Min. Res. 1996;11:1492–7. doi: 10.1002/jbmr.5650111016. [DOI] [PubMed] [Google Scholar]
- [16].Brown RJ, Van Beek E, Watts DJ, Lowik CW, Papapoulos SE. Differential effects of aminosubstituted analogs of hydroxy bisphosphonates on the growth of Dictyostelium discoideum. J. Bone Min. Res. 1998;13:253–8. doi: 10.1359/jbmr.1998.13.2.253. [DOI] [PubMed] [Google Scholar]
- [17].Sanders JM, Ghosh S, Chan JM, Meints G, Wang H, Raker AM, Song Y, Colantino A, Burzynska A, Kafarski P, Morita CT, Oldfield E. Quantitative Structure-Activity Relationships for gammadelta T Cell Activation by Bisphosphonates. J Med. Chem. 2004;47:375–84. doi: 10.1021/jm0303709. [DOI] [PubMed] [Google Scholar]
- [18].Bellido T, Ali AA, Plotkin LI, Fu Q, Gubrij I, Roberson PK, Weinstein RS, O'Brien CA, Manolagas SC, Jilka RL. Proteasomal degradation of Runx2 shortens parathyroid hormone-induced anti-apoptotic signaling in osteoblasts. A putative explanation for why intermittent administration is needed for bone anabolism. J. Biol. Chem. 2003;278:50259–72. doi: 10.1074/jbc.M307444200. [DOI] [PubMed] [Google Scholar]
- [19].Erlebacher A, Derynck R. Increased expression of TGF-beta 2 in osteoblasts results in an osteoporosis-like phenotype. J. Cell Biol. 1996;132:195–210. doi: 10.1083/jcb.132.1.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [20].Aguirre JI, Plotkin LI, Stewart SA, Weinstein RS, Parfitt AM, Manolagas SC, Bellido T. Osteocyte apoptosis is induced by weightlessness in mice and precedes osteoclast recruitment and bone loss. J. Bone Min. Res. 2006;21:605–15. doi: 10.1359/jbmr.060107. [DOI] [PubMed] [Google Scholar]
- [21].Parfitt AM, Drezner MK, Glorieux FH, Kanis JA, Malluche H, Meunier PJ, Ott SM, Recker RR. Bone histomorphometry: standardization of nomenclature, symbols, and units. J. Bone Min. Res. 1987;2:595–610. doi: 10.1002/jbmr.5650020617. [DOI] [PubMed] [Google Scholar]
- [22].Weinstein RS, Jia D, Powers CC, Stewart SA, Jilka RL, Parfitt AM, Manolagas SC. The skeletal effects of glucocorticoid excess override those of orchidectomy in mice. Endocrinology. 2004;145:1980–7. doi: 10.1210/en.2003-1133. [DOI] [PubMed] [Google Scholar]
- [23].Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods. 2001;25:402–8. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- [24].Kousteni S, Han L, Chen JR, Almeida M, Plotkin LI, Bellido T, Manolagas SC. Kinase-mediated regulation of common transcription factors accounts for the bone-protective effects of sex steroids. J. Clin. Invest. 2003;111:1651–64. doi: 10.1172/JCI17261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Morrison NA, Shine J, Fragonas JC, Verkest V, McMenemy ML, Eisman JA. 1,25-dihydroxyvitamin D-responsive element and glucocorticoid repression in the osteocalcin gene. Science. 1989;246:1158–61. doi: 10.1126/science.2588000. [DOI] [PubMed] [Google Scholar]
- [26].Weinstein RS, Jilka RL, Parfitt AM, Manolagas SC. Inhibition of osteoblastogenesis and promotion of apoptosis of osteoblasts and osteocytes by glucocorticoids: potential mechanisms of their deleterious effects on bone. J. Clin. Invest. 1998;102:274–82. doi: 10.1172/JCI2799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].O'Brien CA, Jia D, Plotkin LI, Bellido T, Powers CC, Stewart SA, Manolagas SC, Weinstein RS. Glucocorticoids act directly on osteoblasts and osteocytes to induce their apoptosis and reduce bone formation and strength. Endocrinology. 2004;145:1835–41. doi: 10.1210/en.2003-0990. [DOI] [PubMed] [Google Scholar]
- [28].Weinstein RS, Roberson PK, Manolagas SC. Giant osteoclast formation and long-term oral bisphosphonate therapy. N. Engl. J. Med. 2009;360:53–62. doi: 10.1056/NEJMoa0802633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Cheung MS, Glorieux FH, Rauch F. Large osteoclasts in pediatric osteogenesis imperfecta patients receiving intravenous pamidronate. J. Bone Miner. Res. 2009;24:669–74. doi: 10.1359/jbmr.081225. [DOI] [PubMed] [Google Scholar]
- [30].Owens JM, Fuller K, Chambers TJ. Osteoclast activation: potent inhibition by the bisphosphonate alendronate through a nonresorptive mechanism. J. Cell Physiol. 1997;172:79–86. doi: 10.1002/(SICI)1097-4652(199707)172:1<79::AID-JCP9>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- [31].Weinstein RS, Jilka RL, Almeida M, Roberson PK, Manolagas SC. Intermittent parathyroid hormone administration counteracts the adverse effects of glucocorticoids on osteoblast and osteocyte viability, bone formation, and strength in mice. Endocrinology. 2010;151:2641–9. doi: 10.1210/en.2009-1488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Miao D, He B, Jiang Y, Kobayashi T, Soroceanu MA, Zhao J, Su H, Tong X, Amizuka N, Gupta A, Genant HK, Kronenberg HM, Goltzman D, Karaplis AC. Osteoblast-derived PTHrP is a potent endogenous bone anabolic agent that modifies the therapeutic efficacy of administered PTH 1–34. J. Clin. Invest. 2005;115:2402–11. doi: 10.1172/JCI24918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [33].Brumsen C, Hamdy NA, Papapoulos SE. Long-term effects of bisphosphonates on the growing skeleton. Studies of young patients with severe osteoporosis. Medicine (Baltimore) 1997;76:266–83. doi: 10.1097/00005792-199707000-00005. [DOI] [PubMed] [Google Scholar]