Abstract
Background
Primary mucinous adenocarcinomas of the ovary are uncommon and their biologic behavior uncertain. Retrospective studies suggest that many mucinous carcinomas diagnosed as primary to the ovary were actually metastatic from another site. A prospective randomized trial provided an opportunity to estimate the frequency of mucinous tumors, diagnostic reproducibility, and clinical outcomes.
Methods
A phase III trial enrolled 4000 women with stage III or IV ovarian carcinoma, treated by surgical staging and debulking, with randomization to one of five chemotherapeutic arms. Slides and pathology reports classified as primary mucinous carcinoma were reviewed independently by three pathologists. Cases were re-classified as primary or metastatic to the ovary according to two methods. Overall survival (OS) of reclassified groups was compared with each other and with that of patients with serous carcinomas.
Results
Forty-four cases were classified as mucinous adenocarcinoma at review. Using either method, only about one third were interpreted by the three reviewers as primary mucinous carcinomas. Reproducibility of interpretations among the reviewers was high with unanimity of opinion in 30 of the 44 (68%) cases. The median survival (MS) did not differ significantly between the groups interpreted as primary or metastatic, but the OS was significantly less than that for women with serous carcinoma (14 vs 42 months, p<0.001).
Conclusion
Advanced stage mucinous carcinoma of the ovary is very rare and is associated with poor OS. Many mucinous adenocarcinomas that are diagnosed as primary ovarian neoplasms appear to be metastatic to the ovary.
Keywords: ovary, carcinoma, mucinous, advanced stage
INTRODUCTION
Mucinous tumors of the ovary remain problematic to the pathologist and treating physician. Unresolved issues include the determination of the minimal criteria for the distinction of an invasive mucinous carcinoma from a borderline tumor, the criteria that distinguish metastatic from primary mucinous carcinoma, and behavior of mucinous carcinoma.
In 1971, Norris and Hart proposed that a mucinous tumor be considered invasive in those cases in which the depth of epithelial stratification exceeded three cell layers, or in the presence of stromal invasion in the form of either "destructive" growth as irregular cords or nest of cells haphazardly scattered in the stroma or as large sheets of glands with no intervening stroma.(1) They found a 59% survival for these patients with mucinous ovarian carcinoma with a median followup of eight years.
During the past decade, several investigations have concluded that many cases previously diagnosed as primary mucinous carcinoma were actually metastatic to the ovary. (2,3) Mucinous tumors may arise from a variety of sites, particularly within the gastrointestinal tract, including colon, appendix and pancreas, and less often stomach and biliary tract, as well as from the endometrium and endocervix. (2–16) Immunohistochemistry may be of some assistance in making these distinctions, but determination of the site of origin for mucinous carcinomas involving the ovary remains a significant diagnostic challenge. (2,7,11,13,17–22) Consequently, no gold standard exists to distinguish primary from metastatic mucinous adenocarcinomas of the ovary. Yemelyanova et al using modified criteria of Seidman et al (3), and Lee and Young have independently proposed sets of pathologic characteristics that might help the pathologist better distinguish between primary and metastatic mucinous tumors that involve the ovary. (23,9)
The behavior of mucinous carcinoma of the ovary has not been clearly defined, in part due to the diagnostic problems described previously. When stage is not considered, mucinous carcinomas overall seem to have a better prognosis than serous carcinomas. For example, Kikkawa et al recently reported a five-year overall survival (OS) rate of about 75% in a group of 169 women with mucinous carcinomas of the ovary, and a survival rate of about 28% for the subset with Stage III disease. (24) Chaitin et al reviewed 70 cases of mucinous tumors of the ovary, and they reported a seven-year OS of 72% for stage I mucinous carcinoma but only 8% for patients of stage II or higher. (25) The apparently poor survival for advanced stage patients in some studies could reflect the intrinsic aggressiveness of the tumors, a resistance of mucinous carcinoma to agents that are effective for serous carcinoma of the ovary or the misclassification of mucinous tumors as primary to that site when they are actually metastatic to the ovary.
The goals of the study were three-fold as follows: 1) to estimate the frequency of reclassification of mucinous carcinoma of the ovary as metastatic mucinous ovarian carcinoma, using 2 simple gross and microscopic algorithms; 2) to estimate whether the survival of women with advanced stage primary mucinous carcinoma of the ovary differs significantly from that of women with mucinous carcinoma metastatic to the ovary based on those classifications; and 3) to determine whether the survival of women with advanced stage primary mucinous carcinomas treated with chemotherapy is significantly different from that of serous epithelial ovarian carcinoma.
MATERIAL AND METHODS
In collaboration with the Gynecologic Cancer Intergroup, the Gynecologic Oncology Group (GOG) initiated a phase III clinical trial involving women with newly diagnosed International Federation of Gynecology and Obstetrics (FIGO) Stage III or IV epithelial ovarian or primary peritoneal carcinoma, with either optimal or sub-optimal residual tumor after primary cytoreductive surgery. The protocol received local IRB review and all patients signed informed consent. The primary endpoints of that study were OS and progression-free survival (PFS). The protocol was open to patient entry between January 2001 and September 2004. Submission of surgical pathology reports and slides documenting the primary tumor and highest stage of disease were required for central pathology review. The GOG enrolled 3435 of the 3698 patients entered from US cooperative groups on this protocol, of which 54 (1.5%) were diagnosed as mucinous adenocarcinoma. Immunohistochemical stains were not required for entry as mucinous adenocarcinoma on this protocol. Following a preliminary review (by RJZ), 10 of these cases had either insufficient pathology material for the review or were judged not to be of mucinous histologic type.
The surgical pathology report and slides for each of the 44 cases were independently reviewed by each of the three pathologists (RJZ, SL, HM) at her/his institution without access to any of the clinical outcome data. Each reviewer performed a separate assessment and recording of 12 pathologic characteristics for each primary tumor based upon examination of the hematoxylin and eosin stained slides and accompanying surgical pathology report as follows: maximum primary ovarian tumor diameter, presence or absence of bilateral ovarian involvement, surface involvement of ovary, neoplastic cells in the hilum, a nodular growth pattern in the ovary, an infiltrative pattern of invasion, small neoplastic glands or tubules, single neoplastic cells, signet ring cells, necrotic luminal debris, an expansile pattern of invasion and complex papillae. The determination of some characteristics, such as bilaterality and maximum tumor dimension could not be made in a few cases in which the surgical pathology reports did not clearly indicate the information. In a few cases, the surgical pathology report also indicated the results of immunohistochemical staining for various cytokeratins, but the results of these stains were not used in the assessment of primary site for this study.
The size and laterality algorithm classifies all bilateral mucinous carcinomas as metastatic, unilateral mucinous carcinomas less than 13 cm in maximum diameter as metastatic, and unilateral mucinous carcinomas equal to or larger than 13 cm in diameter as primary. (3) Since this algorithm is based entirely on two pieces of data extracted from the surgical pathology report, it was performed only once (by RJZ).
Each of the pathologists separately recorded her/his interpretation of each case as primary or metastatic, employing a constellation of 12 (of the 16 discriminatory) criteria provided by Lee and Young (Table 1). (9) This included nine criteria that favored a metastasis and three criteria that favored a primary mucinous tumor of the ovary. Admixtures of features were frequent, and some tumors displayed more than one pattern of invasion (e.g. expansile and infiltrative). Each feature present was recorded in these cases. Since a mixture of features favoring both primary and metastatic tumor was often present in an individual case, each pathologist applied the criteria according to her/his judgment. Each pathologist was provided a copy of each of the relevant publication for review prior to assessment and interpretation of the cases. (9)
The collection of followup data included identification of any subsequent primary tumors. Fisher’s exact test was used to assess each of the hypotheses that a specific histologic feature is independent of the interpreted origin of cancer conditioned on the observed marginal frequencies. (27) A logrank procedure was used to assess the hypotheses that the death rates are independent of specific histologic features. (28)
The p-values are two-sided and not adjusted for multiple testing. The Kaplan-Meier procedure was used to estimate the survival distribution. (29)
Table 1.
Mucinous carcinomas involving the ovary – distribution of pathologic features*
| Pathologic feature | N with feature | Assessable cases | % cases |
|---|---|---|---|
| Diameter <13 cm | 16 | 44 | 36 |
| Bilaterality | 24 | 40 | 60 |
| Nodularity | 12 | 44 | 27 |
| Surface involvement | 22 | 44 | 50 |
| Infiltrative invasion | 34 | 44 | 77 |
| Single neoplastic cells | 28 | 44 | 64 |
| Signet ring cells | 9 | 44 | 20 |
| Small glands/tubules | 34 | 44 | 77 |
| Hilar involvement | 5 | 44 | 11 |
| Necrotic luminal debris | 8 | 44 | 18 |
| Expansile growth pattern | 17 | 44 | 39 |
| Complex papillae | 16 | 44 | 36 |
Number and % of cases with feature present as interpreted by reviewer #1. Pathologic feature listed above the solid line have been associated with mucinous carcinoma metastatic to the ovary, while those below the line with primary mucinous ovarian carcinoma; from Lee and Young.(9)
RESULTS
Of the 3435 patients enrolled by the GOG onto an international five-arm randomized trial, 54 (1.5%) had submitting diagnoses of primary mucinous adenocarcinoma. The mean number of slides submitted per case for review was six, with a range of 2 to 35 slides. At review (by reviewer #1), 10 of these cases had either inadequate pathology material (five cases) or were judged not to be of mucinous histologic type (five cases), leaving 44 cases for the current study.
Of the 41 cases for which the data were complete, with the application of the size and laterality algorithm (23), 12 (29%) of the tumors would be considered to be primary to the ovary, while 29 (71%) would be reclassified as metastatic. Specifically, 16 tumors (40%) were unilateral, while 24 (60%) involved both ovaries (one 12 cm tumor without laterality specified). There were 16 (39%) tumors less than 13 cm in maximum diameter, while 25 (61%) were equal to, or more than, 13 cm in diameter. Nine cases had typical histologic features of pseudomyxoma peritonei in non-ovarian foci of disease, and all of these cases were interpreted as representing carcinomas of non-ovarian origin.
The constellation of pathologic criteria (Table 1) formed the basis for the three pathologists' individual interpretation of cases as primary or metastatic. (9) The distribution frequency with which each of the 12 potentially discriminatory pathologic features was identified by reviewer #1 varied between 11% and 77% of cases and is presented in Table 1. The combined frequency of features pertaining to some related categories, such as infiltrative and expansile patterns of invasion, exceeded 100% of cases, reflecting the observation that some tumors displayed different patterns of invasion in different areas. The relationship between each pathologic characteristic (assigned by reviewer #1) and the interpretation of case as primary or metastatic is displayed in Table 2. The presence of an expansile growth pattern (88% vs 7%; p<0.001) and complex papillae (88 vs 4%; p<0.001) were each strongly associated with the retention of classification as a primary mucinous carcinoma of the ovary. The presence of other features was associated (p < 0.05) with an interpretation of carcinoma metastatic to the ovary. Reviewer #1 judged all cases to be metastatic in the presence of hilar involvement or a nodular growth pattern, but these features were present in only a minority of all patients (11% and 27%, respectively).
Table 2.
Mucinous carcinomas involving the ovary- pathologic features associated with reviewer interpretation as primary ovarian mucinous carcinoma
| Pathologic feature | N with feature | % cases with feature diagnosed as primary* |
|---|---|---|
| Diameter <13 cm | 16 | -- |
| Bilaterality | 24 | 29 |
| Nodularity | 12 | 0 |
| Surface involvement | 22 | 23 |
| Infiltrative invasion | 34 | 23 |
| Single neoplastic cells | 28 | 21 |
| Signet ring cells | 9 | 33 |
| Small glands/tubules | 34 | 26 |
| Hilar involvement | 5 | 0 |
| Necrotic luminal debris | 8 | 0 |
| Expansile growth pattern | 17 | 88 |
| Complex papillae | 16 | 94 |
Number and % of cases with feature present interpreted by reviewer #1 as primary mucinous carcinoma.
Pathologic feature listed above the solid line have been associated with mucinous carcinoma metastatic to the ovary, while those below the line with primary mucinous ovarian carcinoma; from Lee and Young.(9)
Overall, only 16 (36%), 17 (39%), and 18 (41%) cases were individually judged by the three reviewers to be primary to the ovary, while 57%–63% were interpreted as metastatic to the ovary. There was unanimity of opinion by the 3 reviewers in 30 of the 44 (68%) cases, with complete agreement on 45% of the total as representing metastatic carcinoma, and 23% of the total as primary mucinous ovarian carcinoma. The specific features for which the three reviewers had the greatest interpretative concordance were the maximum diameter of the tumor, the presence of bilaterality, and an infiltrative pattern of invasion (Table 3).
Table 3.
Mucinous carcinomas involving the ovary - frequency of cases with unanimous reviewer agreement on the presence or absence of individual pathologic features
| Pathologic feature | N with feature | (N, %)* | Unanimous agreement | (N, %)** |
|---|---|---|---|---|
| Diameter <13 cm | 16 | 36 | 39 | 80 |
| Bilaterality | 24 | 60 | 27 | 61 |
| Nodularity | 12 | 27 | 12 | 27 |
| Surface involvement | 22 | 50 | 15 | 34 |
| Infiltrative invasion | 34 | 77 | 27 | 63 |
| Signet ring cells | 9 | 20 | 26 | 59 |
| Small glands/tubules | 34 | 77 | 20 | 45 |
| Hilar involvement | 5 | 11 | 25 | 27 |
| Necrotic luminal debris | 8 | 18 | 24 | 55 |
| Expansile growth pattern | 17 | 39 | 21 | 48 |
| Complex papillae | 16 | 36 | 17 | 39 |
Number and % of cases with feature present as interpreted by reviewer #1
Number and percentage of cases in which each of the three reviewers agreed on the presence or absence of the specified pathologic feature
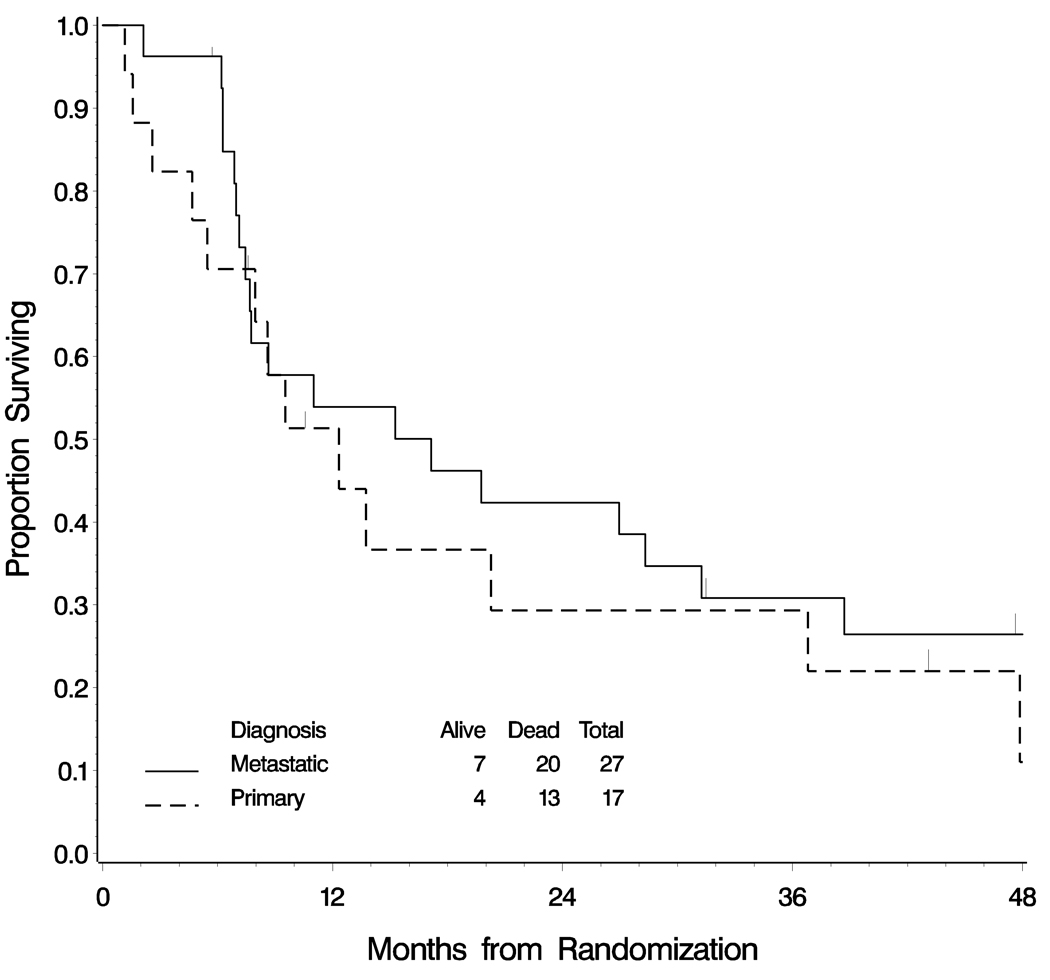
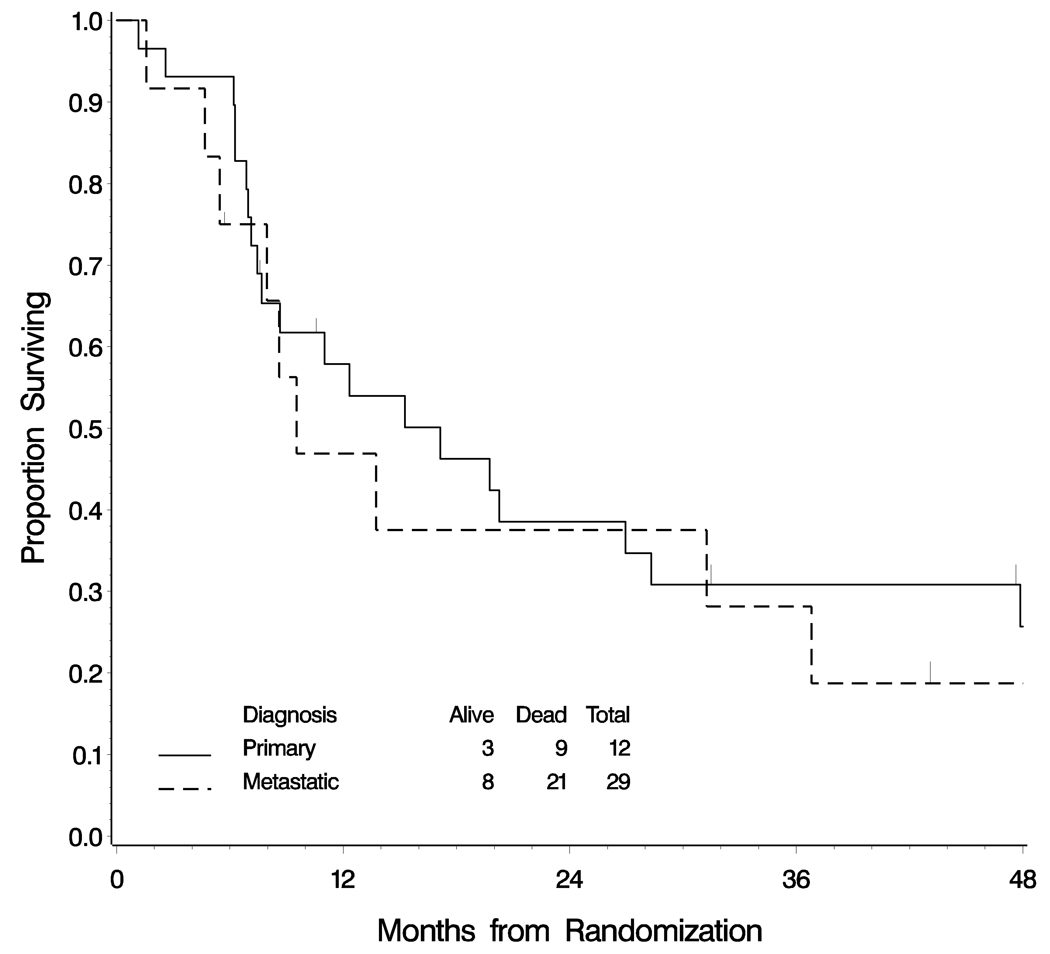
Using either classification system, similar numbers of cases were judged to be primary to the ovary (between 12 and 18 cases). However, diagnostic concordance was observed in only 30 of 41 cases (73%) when cases diagnosed as primary or metastatic using the algorithm were compared with the interpretation by reviewer #1 using the constellation of criteria. (9) Since the diagnosis differed in 27% of cases, survival curves for those women diagnosed as primary and metastatic mucinous carcinomas were examined by each of the two methods of interpretation (Figures 1 and 2). By the logrank test, no significant difference in survival was detected between women with primary or metastatic mucinous carcinomas using either method of classification.
Figure 1.
Overall survival of women with mucinous carcinomas involving the ovary classified as primary or metastatic according to the size and laterality algorithm. This definition classifies all tumors with a maximum size < 13 cms as metastatic. Tumors that are ≥ 13 cms are also considered metastatic if they are bilateral. There is no significant difference in survival between the two groups using this classification system (logrank test p=0.639)
Figure 2.
Overall survival of women with mucinous carcinomas involving the ovary classified as primary or metastatic according to reviewer #1 applying a constellation of criteria suggested by Lee and Young. There is no significant difference in survival between the two groups using this classification system (logrank test p=0.468)
The relative death rate was examined for each of the pathologic characteristics. There is some evidence that the death rate is higher among patients with signet ring cells (hazard ratio (HR): 2.44, 95% confidence interval (CI): 1.11–5.31, p=0.021). The precision of this estimate is limited, since there were only nine patients with this feature identified. Though not statistically significant, the absence of an infiltrative pattern of invasion (HR=2.01; 95% CI: 0.825–4.90, p=0.12), and small neoplastic glands HR=2.04; 95% CI: 0.826–5.02, p=0.12) were each associated with a lower death rate. Table 4 summarizes the estimated probability of surviving three years for patients with and without each of the pathologic characteristics. No second primary solid malignant neoplasm was identified in any of the 44 patients in followup.
Table 4.
Estimated Probability of Surviving more than 3 years
| Prob of surviving more than 3 years |
Univariate hazard ratio (95% CI) |
|||
|---|---|---|---|---|
| Tumor Characteristic | Absent | Present | ||
| Bilateral tumor | 0.27 | 0.33 | 0.76 | (0.363–1.61) |
| Microscopic surf involv. | 0.24 | 0.37 | 0.72 | (0.362–1.45) |
| Hilar involvement | 0.29 | 0.40 | 0.84 | (0.254–2.75) |
| Nodular growth pattern | 0.25 | 0.42 | 0.72 | (0.334–1.56) |
| Infiltrative invasive pattern | 0.57 | 0.22 | 2.01 | (0.825–4.90) |
| Small neoplastic glands | 0.50 | 0.24 | 2.04 | (0.826–5.02) |
| Single neoplastic cells | 0.45 | 0.23 | 1.64 | (0.775–3.58) |
| Signet ring cells | 0.35 | 0.11 | 2.44 | (1.11–5.31) |
| Expansile invasive pattern | 0.27 | 0.36 | 0.75 | (0.361–1.55) |
| Complex papillae | 0.30 | 0.31 | 0.94 | (0.454–1.94) |
| Necrotic luminal debris | 0.37 | 0.0 | 1.45 | (0.618–3.42) |
| Tumor size ≥ 13 cms | 0.39 | 0.26 | 1.35 | (0.640–2.83) |
| Primary vs Metastatic | 0.29 | 0.31 | 0.77 | (0.383–1.56) |
| Residuum (macroscopic) | 0.42 | 0.20 | 1.87 | (0.921–3.79) |
Probability of surviving more than 3 years is based on the Kaplan-Meier procedure.
(Absent versus present refers to those patients without the tumor characteristics versus those with the characteristic). The hazard ratio is the death rate of those with the tumor characteristic relative to those without the characteristic. For primary vs metastatic disease the reference group consists of those with metastatic disease.
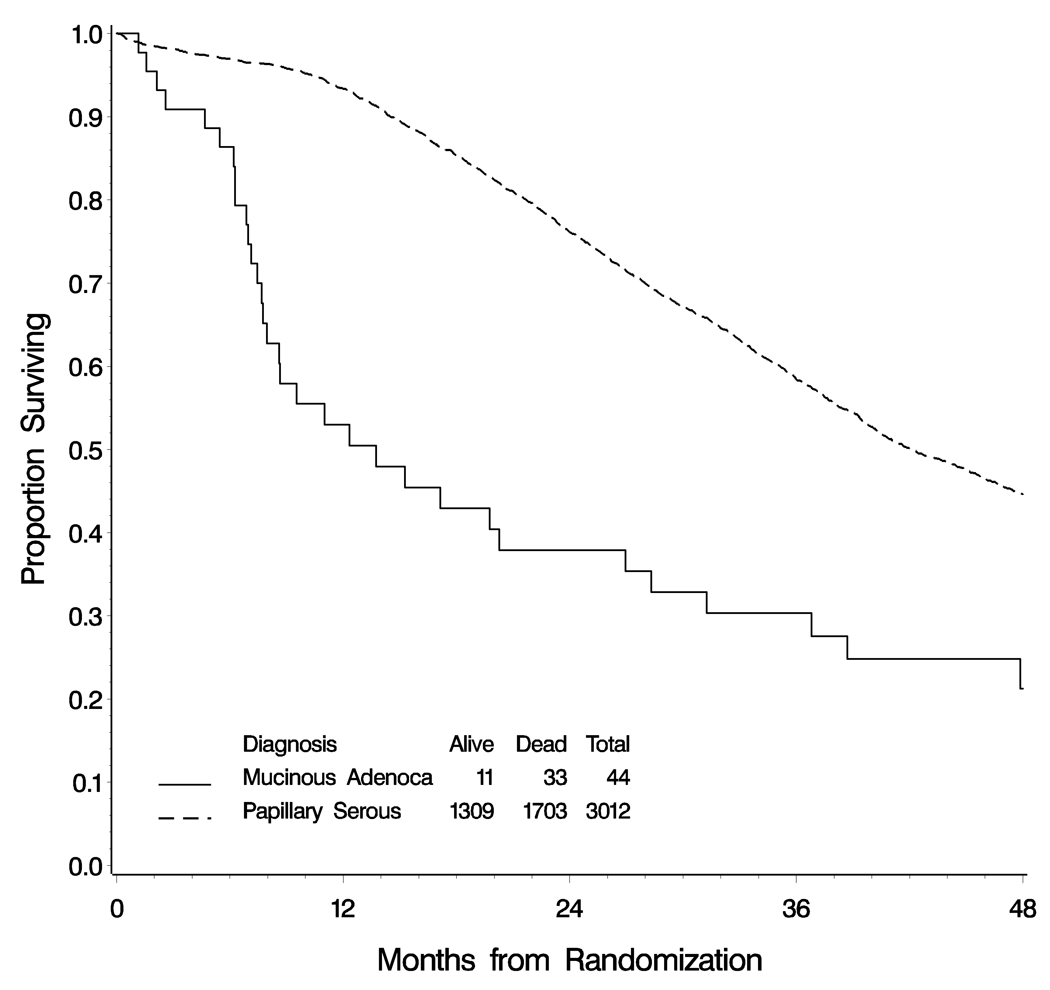
The OS for the combined groups of women with advanced stage mucinous carcinomas was significantly shorter than that of women with advanced stage serous carcinoma (Figure 3), with an estimated median survival of only of 14 months versus 42 months, (logrank test p<0.001).
Figure 3.
Overall survival of women with serous carcinoma and mucinous carcinoma of the ovary. Median overall survival for women with papillary serous carcinoma is 42 months, but for women with mucinous adenocarcinoma it is only 14 months (log-rank test p<0.001).
DISCUSSION
In this subset analysis of advanced stage primary epithelial carcinoma of the ovary, the reviewers reclassified the majority (57% to 63%) of the mucinous carcinomas as metastatic to the ovary rather than primary to the ovary. These results are disturbing but not surprising. Many, if not most, of the carcinomas described and reported in some of the classic papers on mucinous ovarian carcinoma are now suspected to have represented occult metastatic mucinous tumors. However, even at present, no gold standard exists by which primary mucinous adenocarcinomas can be distinguished from those that are metastatic to the ovary. (2–4,6–12,14–22,30) Immunohistochemistry with a variety of antibodies, including CDX2, villin, beta catenin, CEA, MUC2, MUC5, Dpc4, and the presence and distribution of CK7 and CK 20, have been employed to assist in the distinction of primary from metastatic mucinous carcinomas, and this has reduced but not eliminated misclassification of these tumors. (2,3,6–8,11,13,15,17–20,22,31) The addition of an immunohistochemical panel (with antibodies such as CK 7, CK 20, and CDX-2) for all of the mucinous adenocarcinomas on this protocol would have increased the ability to correctly distinguish primary mucinous ovarian carcinomas from those metastatic to that site, but it was not mandated in the protocol, and it was performed in only a few of the cases. Interestingly, in several of these cases, the immunohistochemical results supported the interpretation of the lesions as metastatic from the colon, but the women were still enrolled on this protocol that was limited to women with advanced stage primary ovarian carcinoma.
Ulbright et al, and Lee and Young, have identified partially overlapping sets of gross and microscopic characteristics that are more often associated with primary or metastatic carcinomas involving the ovary. (14,9) Interpretative judgment is still required since no single feature or group of features provides high sensitivity coupled with high specificity. In this study, the three review pathologists independently classified the tumors based on an assessment of 12 of the criteria of Lee and Young. (9) While each of the reviewers disagreed with the submitting interpretation in about 60% of cases, there was a high degree of agreement among the reviewers independent classification, with unanimity of classification in 68% of cases.
Using the laterality and size algorithm that all bilateral mucinous carcinomas of the ovary and all unilateral carcinomas less than 13 cm in diameter are metastatic, 29 of 41 (70%) tumors were reclassified as metastatic, similar to the result obtained from using the constellation of criteria. (23) This rule is reported to correctly classify almost 90% of mucinous ovarian neoplasms as primary or metastatic. However, it should be noted that the rule was derived from a set of mucinous tumors obtained by a retrospective review of cases at the Johns Hopkins Hospital.(23) In that study, twenty-one tumors were considered to be primary mucinous adenocarcinoma of the ovary. The distribution by stage was not provided, but in a previous study including the same institution, the majority were of early stage. (3) In another study of 220 primary ovarian carcinomas from the Washington Hospital Center, six were mucinous carcinoma and only one of these was of advanced stage. (26) Bilateral involvement of the ovaries is very uncommon in stage I mucinous carcinoma. However, bilateral involvement is typical in advanced stage serous carcinoma, and it might also be common in advanced stage mucinous carcinoma. Since most mucinous carcinomas of the ovary are stage I and advanced tumors were so uncommon in those prior studies, this would have influenced the creation of the classification criteria. There is little existing data on the pattern of spread and distribution of tumor specifically for advanced stage mucinous carcinoma of the ovary. Most publications have not described in detail the presence of bilateral involvement or its location in the contralateral ovary in cases of advanced stage mucinous carcinoma.(3,32–34) Consequently, the size and laterality algorithm may correctly classify most mucinous ovarian tumors, but it may not do so for those few primary mucinous carcinomas that are of advanced stage. (It may be appropriate to consider modification of the portion of the rule that classifies all bilateral tumors as metastatic, in order to permit small foci of surface involvement of the contralateral ovary in the presence of other extra-ovarian disease.)
The OS for women with a reviewer diagnosis of advanced stage primary mucinous carcinoma of the ovary was not significantly different from that of the women with tumors interpreted as metastatic, using either the size and laterality algorithm or the constellation of criteria. An examination of the estimated relative death rates for each of the pathologic characteristics revealed that possibly the absence of signet ring cells, an infiltrative pattern of invasion, and small neoplastic glands, were each associated with longer survival. The later two features were among the five statistically best discriminators of primary from metastatic mucinous carcinomas of the ovary in the study by Lee and Young.(9) However, in contrast to their study, we did not find that bilaterality or an expansile pattern of invasion discriminated effectively those likely to survive from those likely to die of tumor.
There are several limitations to this study that are shared with most retrospective analyses of large international clinical trials, including the inability to obtain or perform immunohistochemical stains on each case, and the availability of only a limited number of slides from most cases. Confirmation of the pathologic interpretation of many of these tumors as metastatic to the ovary would be helpful, but is lacking. While the protocol required notification of the identification of subsequent primary tumors, there may have been under-diagnosis of such. Evaluation for a second primary tumor may not be as aggressive for women with advanced widespread adenocarcinomas, especially since gastrointestinal involvement is so common in advanced stage ovarian carcinoma and the mean overall survival was so short. Nevertheless, in the absence of identification of any second primary site of neoplasm in the followup of any of these 44 women, it is possible, although highly unlikely, that all of the tumors in this study represent advanced stage mucinous adenocarcinomas of the ovary. The strengths of this study include the large number of total cases of advanced ovarian carcinoma, the large number of cases submitted as mucinous carcinoma (greatly exceeding that of any prior study), the uniform staging requirements, standardized chemotherapy, and ability to track survival data.
Most importantly, we found that the OS for women with advanced stage mucinous carcinomas (whether arising in the ovary or carcinoma metastatic to that site) is significantly less than that for women with advanced stage serous carcinoma (median survival of 14 months versus 42 months, respectively). This could reflect either an intrinsic aggressive behavior of mucinous ovarian carcinoma or its resistance to agents that are effective in the treatment of serous carcinoma. These results are very similar to those reported by Hess. et al. (35)
who identified 27 cases of FIGO Stage III or IV mucinous carcinoma of the ovary over an eight year interval They found an OS of 12 months for patients with mucinous ovarian carcinoma vs 37 months for controls with ovarian carcinoma of non-mucinous histology. We support their recommendation that consideration should be given to future investigation of therapy of advanced mucinous carcinoma of the ovary with agents that are effective in treating carcinomas of the gastrointestinal tract. From a pragmatic perspective, given our limitations in diagnostic classification, at the current time it may not be as critical therapeutically or prognostically to distinguish advanced stage primary ovarian from metastatic mucinous adenocarcinomas of the ovary, since the behavior of the tumors is so poor for both subsets of patients. However, efforts should continue to find methods to correctly classify these tumors since effective chemotherapy in the future might be limited to one subset based on the site of origin.
In conclusion, we found that advanced stage primary mucinous carcinoma of the ovary is very rare (0.5–1.5% of advanced ovarian epithelial invasive neoplasms), and that 61% (27/44) of the patients enrolled on this protocol as primary mucinous carcinomas were probably metastatic carcinoma to the ovary. This conclusion rests on the assumption that the published and generally accepted criteria applied by the reviewers to make this distinction are accurate, since no further investigation into identification of non-ovarian sites of origin was possible in this international study. While it would be ideal to have immunohistochemical or molecular assays that are highly specific for gastrointestinal or müllerian mucinous tumors, none have been identified to date. Surprisingly, there was no significant difference in survival between the groups of women with ovarian and non-ovarian mucinous carcinomas. However, we found that advanced stage primary mucinous ovarian carcinoma is highly lethal, with a significantly shorter survival than that of women with serous carcinoma. It is reasonable to consider the use of chemotherapeutic agents that are effective in treating carcinomas of the gastrointestinal tract in future trials of this tumor.
Acknowledgments
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA27469) and the Gynecologic Oncology Group Statistical and Data Center (CA37517).
Footnotes
The following Gynecologic Oncology Group member institutions participated in this study: Roswell Park Cancer Institute, University of Alabama at Birmingham, Duke University Medical Center, Abington Memorial Hospital, Walter Reed Army Medical Center, University of Minnesota Medical School, University of Mississippi Medical Center, Colorado Gynecologic Oncology Group P.C., University of California at Los Angeles, University of Washington, University of Pennsylvania Cancer Center, Milton S. Hershey Medical Center, University of Cincinnati, University of North Carolina School of Medicine, University of Iowa Hospitals and Clinics, University of Texas Southwestern Medical Center at Dallas, Indiana University School of Medicine, Wake Forest University School of Medicine, University of California Medical Center at Irvine, Tufts-New England Medical Center, Rush-Presbyterian-St. Luke's Medical Center, Magee Women's Hospital, SUNY Downstate Medical Center, University of Kentucky, University of New Mexico, The Cleveland Clinic Foundation, State University of New York at Stony Brook, Southwestern Oncology Group, Washington University School of Medicine, Cooper Hospital/University Medical Center, Columbus Cancer Council, MD Anderson Cancer Center, University of Massachusetts Medical School, Fox Chase Cancer Center, Women's Cancer Center, University of Virginia Health Sciences Center, University of Chicago, Tacoma General Hospital, Thomas Jefferson University Hospital, Mayo Clinic, Case Western Reserve University, Tampa Bay Cancer Consortium, Gynecologic Oncology Network, Ellis Fischel Cancer Center, Fletcher Allen Health Care, Australian New Zealand Gynaecological Oncology, Yale University, University of Wisconsin Hospital, Cancer Trials Support Unit, University of Texas-Galveston, Women and Infants Hospital, Community Clinical Oncology Program, Australian New Zealand Gynaecological Oncology Group/NHMRC Clinical Trial Centre.
United Kingdom Yeovil, Hull, Aberdeen Royal Infirmary, Airedale General Hospital, Bronglais General Hospital, Broomfield Hospital, Chelsea & Westminster Hospital, Cheltenham General Hospital, Christie Hospital, Churchill Hospital, City Hospital, Birmingham, Derbyshire Royal Infirmary, Derriford Hospital, Essex County Hospital, Guy's and St Thomas' Hospital, Huddersfield Royal Infirmary, Ipswich Hospital NHS Trust, James Cook University Hospital, Lincoln County Hospital, Maidstone Hospital, Manor Hospital Mount Vernon Hospital, New Cross Hospital, Newcastle General Hospital, North Devon District Hospital, Northampton General Hospital, Nottingham City Hospital Trust, Oldchurch Hospital, Pilgrim Hospital, Queen Elizabeth Hospital, Queen Elizabeth the Queen Mother's Hospital, Royal Devon and Exeter Hospital, Russell Hall Hospital, part of the Dudley Group of Hospitals, St Bartholomew's Hospital, St George's Hospital, St James's University Hospital, St Mary's Hospital – Portsmouth, Torbay Hospital UCLH Gynaecological Cancer Centre, Velindre Hospital NHS Trust, Weston Park Hospital, Wexham Park Hospital.
Italy Bari, IEO, Monza “San Gerardo” Pavoda, Palermo “Cervello” Rimini, Thiene, Treviglio, Gallarate.
REFERENCES
- 1.Hart W, Norris H. Borderline and malignant mucinous tumors of the ovary. Cancer. 1973;31:1031–1045. doi: 10.1002/1097-0142(197305)31:5<1031::aid-cncr2820310501>3.0.co;2-7. [DOI] [PubMed] [Google Scholar]
- 2.Hart WR. Diagnostic challenge of secondary (metastatic) ovarian tumors simulating primary endometrioid and mucinous neoplasms. Pathol Int. 2005;55:231–243. doi: 10.1111/j.1440-1827.2005.01819.x. [DOI] [PubMed] [Google Scholar]
- 3.Seidman JD, Kurman RJ, Ronnett BM. Primary and metastatic mucinous adenocarcinomas in the ovaries: incidence in routine practice with a new approach to improve intraoperative diagnosis. Am J Surg Pathol. 2003;27:985–993. doi: 10.1097/00000478-200307000-00014. [DOI] [PubMed] [Google Scholar]
- 4.Daya D, Nazerali L, Frank GL. Metastatic ovarian carcinoma of large intestinal origin simulating primary ovarian carcinoma. A clinicopathologic study of 25 cases. Am J Clin Pathol. 1992;97:751–758. doi: 10.1093/ajcp/97.6.751. [DOI] [PubMed] [Google Scholar]
- 5.Elishaev E, Gilks CB, Miller D, et al. Synchronous and metachronous endocervical and ovarian neoplasms: evidence supporting interpretation of the ovarian neoplasms as metastatic endocervical adenocarcinomas simulating primary ovarian surface epithelial neoplasms. Am J Surg Path. 2005;29:281–294. doi: 10.1097/01.pas.0000152136.81771.12. [see comment] [DOI] [PubMed] [Google Scholar]
- 6.Hart WR. Mucinous tumors of the ovary: a review. Int J Gynecol Pathol. 2005;24:4–5. [PubMed] [Google Scholar]
- 7.Ji H, Isacson C, Seidman JD, et al. Cytokeratins 7 and 20, Dpc4, and MUC5AC in the distinction of metastatic mucinous c arcinomas in the ovary from primary ovarian mucinous tumors: Dpc4 assists in identifying metastatic pancreatic carcinomas. Int J Gynecol Pathol. 2002;21:391–400. doi: 10.1097/00004347-200210000-00009. [DOI] [PubMed] [Google Scholar]
- 8.Lash RH, Hart WR. Intestinal adenocarcinomas metastatic to the ovaries. A clinicopathologic evaluation of 22 cases. Am J Surg Pathol. 1987;11:114–121. doi: 10.1097/00000478-198702000-00005. [DOI] [PubMed] [Google Scholar]
- 9.Lee KR, Young RH. The distinction between primary and metastatic mucinous carcinomas of the ovary: gross and histologic findings in 50 cases. Am J Surg Pathol. 2003;27:281–292. doi: 10.1097/00000478-200303000-00001. [DOI] [PubMed] [Google Scholar]
- 10.Lerwill MF, Young RH. Ovarian metastases of intestinal-type gastric carcinoma: A clinicopathologic study of 4 cases with contrasting features to those of the Krukenberg tumor. Am J Surg Pathol. 2006;30:1382–1388. doi: 10.1097/01.pas.0000213256.75316.4a. [DOI] [PubMed] [Google Scholar]
- 11.Lewis MR, Deavers MT, Silva EG, et al. Ovarian involvement by metastatic colorectal adenocarcinoma: still a diagnostic challenge. Am J Surg Pathol. 2006;30:177–184. doi: 10.1097/01.pas.0000176436.26821.8a. [DOI] [PubMed] [Google Scholar]
- 12.Prat J. Ovarian carcinomas, including secondary tumors: diagnostically challenging areas. Mod Pathol. 2005;18 Suppl 2:S99–S111. doi: 10.1038/modpathol.3800312. [DOI] [PubMed] [Google Scholar]
- 13.Ronnett BM, Shmookler BM, Diener-West M, et al. Immunohistochemical evidence supporting the appendiceal origin of pseudomyxoma peritonei in women. Int J Gynecol Pathol. 1997;16:1–9. doi: 10.1097/00004347-199701000-00001. [DOI] [PubMed] [Google Scholar]
- 14.Ulbright TM, Roth LM, Stehman FB. Secondary ovarian neoplasia. A clinicopathologic study of 35 cases. Cancer. 1984;53:1164–1174. doi: 10.1002/1097-0142(19840301)53:5<1164::aid-cncr2820530523>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 15.Young RH. From Krukenberg to today: the ever present problems posed by metastatic tumors in the ovary: part 1. Historical perspective, general principles, mucinous tumors including the Krukenberg tumor. Adv Anat Pathol. 2006;13:205–227. doi: 10.1097/01.pap.0000213038.85704.e4. [DOI] [PubMed] [Google Scholar]
- 16.Young RH, Scully RE. Ovarian metastases from carcinoma of the gallbladder and extrahepatic bile ducts simulating primary tumors of the ovary A report of six cases. Int J Gynecol Pathol. 1990;9:60–72. doi: 10.1097/00004347-199001000-00006. [DOI] [PubMed] [Google Scholar]
- 17.Albarracin CT, Jafri J, Montag AG, et al. Differential expression of MUC2 and MUC5AC mucin genes in primary ovarian and metastatic colonic carcinoma. Hum Pathol. 2000;31:672–677. doi: 10.1053/hupa.2000.6799. [DOI] [PubMed] [Google Scholar]
- 18.Chou YY, Jeng YM, Kao HL, et al. Differentiation of ovarian mucinous carcinoma and metastatic colorectal adenocarcinoma by immunostaining with beta-catenin. Histopathology. 2003;43:151–156. doi: 10.1046/j.1365-2559.2003.01687.x. [DOI] [PubMed] [Google Scholar]
- 19.Logani S, Oliva E, Arnell PM, et al. Use of novel immunohistochemical markers expressed in colonic adenocarcinoma to distinguish primary ovarian tumors from metastatic colorectal carcinoma. Mod Pathol. 2005;18:19–25. doi: 10.1038/modpathol.3800260. [DOI] [PubMed] [Google Scholar]
- 20.McCluggage WG, Young RH. Immunohistochemistry as a diagnostic aid in the evaluation of ovarian tumors. Semin Diagn Pathol. 2005;22:3–2. doi: 10.1053/j.semdp.2005.11.002. [DOI] [PubMed] [Google Scholar]
- 21.Riopel MA, Ronnett BM, Kurman RJ. Evaluation of diagnostic criteria and behavior of ovarian intestinal-type mucinous tumors: atypical proliferative (borderline) tumors and intraepithelial, microinvasive, invasive, and metastatic carcinomas. Am J Surg Pathol. 1999;23:617–635. doi: 10.1097/00000478-199906000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Vang R, Gown AM, Barry TS, et al. Cytokeratins 7 and 20 in primary and secondary mucinous tumors of the ovary: analysis of coordinate immunohistochemical expression profiles and staining distribution in 179 cases. Am J Surg Pathol. 2006;30:1130–1139. doi: 10.1097/01.pas.0000213281.43036.bb. [DOI] [PubMed] [Google Scholar]
- 23.Yemelyanova A, Vang R, Judson K, et al. Distinction of primary and metastatic mucinous tumors involving the ovary: analysis of size and laterality by primary site with reevaluation of an algorithm for tumor classification. Am J Surg Pathol. 2008;32:128–138. doi: 10.1097/PAS.0b013e3180690d2d. [DOI] [PubMed] [Google Scholar]
- 24.Kikkawa F, Nawa A, Kajiyama H, et al. Gynecol Oncol. Fletcher Allen Health Care; 2006. Clinical characteristics and prognosis of mucinous tumors of the ovary; pp. 171–175. [DOI] [PubMed] [Google Scholar]
- 25.Chaitin B, Gershenson D, Evans H. Mucinous tumors of the ovary. Cancer. 1985;55:1958–1962. doi: 10.1002/1097-0142(19850501)55:9<1958::aid-cncr2820550921>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- 26.Seidman JD, Horkayne-Szakaly I, Haiba M, et al. The histologic type and stage distribution of ovarian carcinomas of surface epithelial origin. Int J Gynecol Pathol. 2004;23:41–44. doi: 10.1097/01.pgp.0000101080.35393.16. [DOI] [PubMed] [Google Scholar]
- 27.Fisher RA. The Logic of indicative inference (with discussion) J. Royal Statistical Society. 1935;98:39–82. [Google Scholar]
- 28.Mantel N. Evaluation of survival data and two new rank order statistics arising in its consideration. Cancer Chemother Rep. 1966;50:163–170. [PubMed] [Google Scholar]
- 29.Kaplan EL, Meier P. Nonparametric estimation from incomplete observations. J. Amer Stat Assoc. 1958;53:457–481. [Google Scholar]
- 30.Ronnett BM, Kurman RJ, Shmookler BM, et al. The morphologic spectrum of ovarian metastases of appendiceal adenocarcinomas: a clinicopathologic and immunohistochemical analysis of tumors often misinterpreted as primary ovarian tumors or metastatic tumors from other gastrointestinal sites. Am J Surg Pathol. 1997;21:1144–1155. doi: 10.1097/00000478-199710000-00004. [DOI] [PubMed] [Google Scholar]
- 31.Baker P, Oliva E. Immunohistochemistry as a tool in the differential diagnosis of ovarian tumors: an update. Int J Gynecol Pathol. 2004;24:39–55. [PubMed] [Google Scholar]
- 32.Rodriguez I, Prat J. Mucinous tumors of the ovary: a clinicopathologic an alysis of 75 borderline tumors (of intestinal type) and carcinomas. Am J Surg Pathol. 2002;26:139–152. doi: 10.1097/00000478-200202000-00001. [DOI] [PubMed] [Google Scholar]
- 33.Hoerl HD, Hart WR. Primary ovarian mucinous cystadenocarcinomas: a clinicopathologic study of 49 cases with long-term follow-up. Am J Surg Pathol. 1998;22:1449–1462. doi: 10.1097/00000478-199812000-00002. [DOI] [PubMed] [Google Scholar]
- 34.Lee KR, Scully R. Mucinous tumors of the ovary: a clinicopathologic study of 196 borderline tumors (of intestinal type) and carcinomas, including an evaluation of 11 cases with ‘pseudomyxoma peritonei’. Am J Surg Pathol. 2000;24:1447–1464. doi: 10.1097/00000478-200011000-00001. [DOI] [PubMed] [Google Scholar]
- 35.Hess V, A'Hern R, Nasiri N, et al. Mucinous epithelial ovarian cancer: a separate entity requiring specific treatment. J Clin Oncol. 2004;22:1040–1044. doi: 10.1200/JCO.2004.08.078. [DOI] [PubMed] [Google Scholar]