Abstract
Parkinson's disease (PD) involves both motor and non-motor disturbances. Non-motor features include alterations in sensory olfactory function which may constitute a viable biomarker for the disorder. It is not clear what causes olfactory dysfunction but it appears to coincide with the development of synucleopathy within the olfactory bulb (OB). Elevation in alpha-synuclein is indeed a risk factor for development of the sporadic disorder. The multifactorial nature of the idiopathic disease combined with variability in its presentation suggests that it is likely to be influenced by several factors and that in vivo models that explore the synergistic effect of alpha-synuclein elevation with other potential contributing factors are likely to be of importance in understanding the disease etiology. Using a dual transgenic mouse model of dopaminergic alpha-synuclein overexpression coupled with doxycycline (Dox)-inducible glutathione depletion in these same cells, we demonstrate an age-related loss in behavioral olfactory function coupled with a significant neurodegeneration of glomerular dopaminergic neurons. This is accompanied by increases in alpha-synuclein levels in non-dopaminergic cells in the granule cell layer. In addition, isolated olfactory bulb synaptosomes from dual transgenic lines with Dox consistently showed a slight but significant reduction in maximum mitochondrial respiration compared to controls. These results suggest that in the presence of increased oxidative stress, increased alpha-synuclein expression within dopaminergic OB neurons results in neurodegeneration in the glomerular layer and increased alpha-synuclein levels in the granular cell layer which coincide with olfactory dysfunction.
Keywords: Parkinson's disease, olfactory deficits, smell test, oxidative stress, neurodegeneration, mitochondrial dysfunction
INTRODUCTION
Parkinson's disease (PD) is a disorder classically associated with well-characterized motor disturbances including bradykinesia, rigidity, resting tremor, and postural instability as a consequence of loss of dopaminergic neurons in the substantia nigra (SN). Recently it has been appreciated that PD is actually a multifactorial condition characterized by several additional non-motor symptoms including reduced or lost olfactory function (hyposmia or anosmia, Simuni and Sethi, 2008; Doty, 2009). Indeed 75-90% of patients diagnosed with sporadic PD display some degree of olfactory dysfunction including variable degrees of deficits in odor detection, although less than 15% of patients are aware of their deficits until odor test (Ward et al., 1983; Berendse et al., 2001; Sobel et al., 2001). Psychophysical or electrophysiological tests are currently available for assessing odor identification and detection, including the University of Pennsylvania Smell Identification Test (UPSIT) (Doty, 2009). Relatives of PD patients who themselves have olfactory deficits are also at higher risk for the disease (Berendse et al., 2001; Doty, 2007; Simuni and Sethi, 2008). This suggests that olfactory dysfunction may be a viable biomarker for the disease.
Loss of olfactory function has been suggested to be a consequence of the development of synucleopathy within the olfactory bulb (OB) (Braak et al., 2003). Lewy bodies and neurites associated with PD are believed to initiate in the anterior olfactory nucleus and bulb before presentation in the brainstem and subsequent cortical regions (Braak et al., 2003; Lerner and Bagic, 2008). Presence of these OB inclusions has been believed to contribute to olfactory dysfunction. The presence of synucleopathy in the olfactory bulb has indeed been demonstrated to be 90-95% predictive of the presence of PD (Attems et al., 2005). Elevation in alpha-synuclein is a risk factor for development of the sporadic disorder.
Sporadic PD appears to be influenced by several different factors which may explain both its multifactorial nature and the variability in its presentation (Doty, 2007). In vivo models that explore the synergistic effect of these factors are therefore likely to be of importance in understanding the disease etiology. Using a dual transgenic mouse model expressing elevated dopaminergic alpha-synuclein levels in the presence of induction of increased oxidative stress as a consequence of dopaminergic glutathione depletion, we report an age-related loss in odor identification and detection coupled with significant dopaminergic neurodegeneration in the olfactory bulb. This is accompanied by increases in alpha-synuclein levels in non-dopaminergic cells in the granule cell layer. A general reduction in presynaptic OB mitochondrial function was also noted. These results suggest that in the presence of increased oxidative stress, increased alpha-synuclein expression within dopaminergic OB neurons results in their age-related neurodegeneration and increased alpha-synuclein levels in non-dopaminergic cells in the granule cell layer coinciding with olfactory dysfunction.
EXPERIMENTAL PROCEDURES
Generation of dual dopaminergic anti-GSH/alpha-synuclein transgenic lines
All animal protocols were conducted in accordance with the United States Public Health Service Guide for the Care and Use of Laboratory Animals and all procedures were approved by the Buck Institute Animal Care and Use Committee. All efforts were made to minimize the number of animals used and their suffering in this study. To generate animals for the described studies, transgenic mice displaying doxycycline (Dox)-inducible depletion in dopaminergic glutathione (anti-GSH, Chinta et al., 2007) were crossed with tyrosine hydroxylase (TH) promoter regulated alpha-synuclein A53T expressing transgenic mice (gift of the DiMonte laboratory, Parkinson's institute, Sunnyvale, CA). Doxycycline was fed to animals to elicit dopaminergic GSH suppression from 1 month of age onward and maintained throughout subsequent behavioural and neuropathological analyses. Animals were maintained under a reverse light cycle (12 h dark/light) one month prior to initiation of behavioral tests.
Olfactory behavioral analyses
Olfactory tests were performed at 8 and 12 months of age in dual transgenic versus alpha-synuclein or anti-GSH only lines ± Dox (n=5-9 per group). These included: (1) the buried pellet test based on latency in finding food, (2) the nose poke test based on recognition of food smell from a target hole, (3) the wooden block test based on odor discrimination between self and non-self odors (Fleming et al., 2008; Taylor et al., 2009). Prior to olfactory tests, food restriction was applied for 2 weeks to motivate animals to look for food (resulting in ~10% body weight reduction). For the buried pellet test of odor detection/identification, mice were habituated in a clean home cage one hour prior to testing (Fleming et al., 2008). A treat (cereal, Honey Nut Cheerios®, General Mills inc., Minneapolis, MN) was buried approximately 0.5 cm under the bedding in 6 different clock-wise positions in the cage. A mouse was placed in the center of the cage and time to locate and eat the treat was measured using an installed digital camera (recording time: 5 minutes maximum). This test was performed the same time of the day for 6 days (6 trials) with different genotypes of sibling mice (dual transgenic and alpha-synuclein A53T or anti-GSH only ± Dox). The nose poke test was carried out using the Coulbourn TruScan system (Coulbourn Instruments, Whitehall, PA) as an alternative test of odor detection/identification. Prior to placing mouse test subjects on the 4×4 nose poke floor, 15 filter papers (1 cm diameter) wetted with a drop of water were placed in the first 15 holes and a filter paper with a drop of olive oil in the 16th hole. The number of nose pokes was automatically counted by installed sensors and recorded in the TruScan 99 program according to the manufacturer's recommendations. This test was performed for 5 minutes under dim light to minimize visual discrimination of water-wetted papers versus oil wetted ones. In order to avoid memory effects based on hole location, the 4×4 hole plate was rotated 90 degrees for each test (at 4 different locations for 4 test days). Mice were fed olive oil-dipped cereal for two weeks prior to the test in order to correlate the oil smell with food (cereal) under restricted food conditions as described above. The wooden block odor discrimination test was modified from previously published studies (Fleming et al., 2008; Taylor et al., 2009). Four numbered wooden blocks (diameter and height: 2.5 cm each) were placed in the bedding of each test mouse overnight. Each block was placed approximately 2 cm apart on the bedding. Prior to use, blocks were autoclaved to sterilize and to reduce their own odor. One of the 4 blocks was removed from the cage for 30 seconds and returned to the same cage; this process was repeated five times. At the 6th trial, a wooden block from a foreign animal cage (of a same sex mouse) was placed in the cage and time spent sniffing (nose < 1 cm) the replaced block was measured for 5 minutes. This test was performed at four different block positions for 4 days. The investigatory time represents the seconds of time an animal spends sniffing the block scented with a foreign animal's bedding minus the seconds of time spent sniffing the block scented with their own bedding (Fleming et al., 2008; Taylor et al., 2009). Results from male and female mice were combined because we found no sex differences in this test using a block from a same sex cage as described by Taylor et al., (2009).
Immunohistochemistry and stereological dopaminergic (DA) cell count analyses
Prior to sectioning, all brains were perfused in 4% paraformaldehyde and embedded in OCT (Tissue Tek, Sakura Finetek USA, Torrance, CA) and kept at -80 °C until use. Using a cryostat, 40 μm of OB tissue was sectioned from each experimental group on the same set of slides to control for slide variability. Tyrosine hydroxylase (TH: 1:500 dilution, polyclonal rabbit anti-TH antibody, Chemicon, Temecula, CA), NeuN (1:400 dilution, monoclonal mouse anti-NeuN, Millipore), or human specific a-synuclein (1:500 diluted mouse anti-alpha synuclein monoclonal, MAB 5320, Chemicon) antibodies were incubated at 4 °C overnight and secondary antibodies for TH (1:500 diluted biotinylated anti-rabbit IgG, Novus Biologicals, Littleton, CO), for NeuN (1:400 diluted biotinylated anti-mouse IgG, Chemicon), and for alpha-synuclein (1:200 diluted biotinylated anti-mouse IgG, Chemicon) were exposed for 90 minutes at room temperature. The label intensity was boosted using a 1:100 dilution of the Vectastain ABC kit (Vector Laboratories inc., Burlingame, CA) for 60 minutes followed by development in diaminobenzidine (DAB, Chemicon). Dopaminergic OB cell numbers from dual versus single transgenic lines +/- Dox at 8 and 12 months of age were stereologically analyzed in the glomerular layer using the optical fractionator method (Stereo Investigator, MBF Bioscience), as previously described (Chinta et al., 2007). All stereological cell counting in the glomerular layer was performed by a rater who was blind to genotypes and presented as mean TH+ cell number ± SEM and mean cell number/mm2 ± SEM for statistical analyses (Paired t-test, n=7 each group).
Mitochondrial respiration in presynaptic nerve terminals
Whole OB tissue was dissected from dual transgenics + Dox versus – Dox siblings at 12 months (n=7 each). Presynaptic nerve terminals (synaptosomes) were pooled from OB tissues isolated from 2-3 animals for analysis. Basal respiratory rate, coupling efficiency, maximum respiration, and glycolysis were measured using the Seahorse XF24 and synaptosomal viability verified by fluorescent monitoring of mitochondrial membrane potential and calcium levels as previously described (Seahorse Bioscience, North Billerica, MA) (Choi et al., 2009).
Statistical analyses
Dual transgenics were divided into Dox-fed versus no-Dox fed groups and alpha-synuclein Dox versus no Dox mice were included as controls in behavioral studies at both 8 and 12 months of age (n=6-10 per group). Buried pellet and nose poke tests were analyzed using nonparametric statistics and various cohorts compared using the Mann-Whitney U-test. For the wooden block test, time sniffing around foreign minus familiar blocks at different ages was analyzed by the Wilcoxon Signed Rank test. For stereological analyses, we used the paired t-test to compare dual Tg-Dox with α-synuclein-Dox group; results are presented as mean ± SEM. For respiratory studies, 4 μM FCCP-stimulated maximum respiration was evaluated (paired t-test, n=3). For all studies, p<0.05 was considered statistically significant using GraphPad Prism 5 software.
RESULTS
Buried pellet test
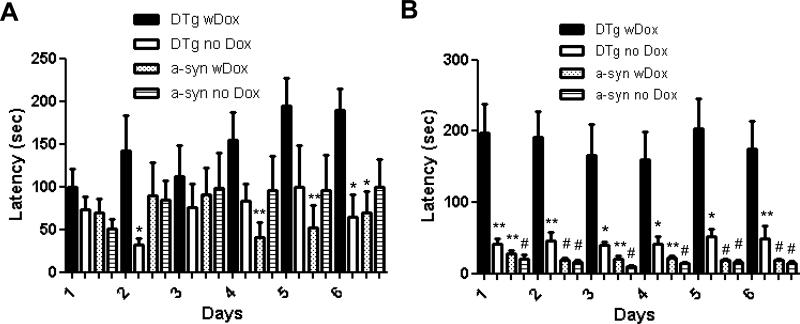
Dual transgenics with Dox at 12 months of age were analyzed and found to display difficulties in finding buried food within the allotted 5 minute period. In contrast, both dual transgenics without Dox and single α-synuclein transgenics with and without Dox were able to locate the treat within 40 seconds in most tests (Fig. 1B). Alpha-synuclein with Dox (n=8, p<0.01 or p<0.001) and alpha-synuclein without Dox (n=9, p<0.001) as well as dual transgenic without Dox (n=8, p<0.05 or p<0.01) showed significantly faster times in finding a treat compared with dual transgenic with Dox (n=9, Mann-Whitney U-test). Control single anti-GSH mice +/- Dox displayed no differences at either 8 or 12 months of age (n=4 each, data not shown) compared to the other controls in any of the behavioral tests performed. These pellet test results suggest that olfactory detection in dual transgenics with Dox was substantially affected compared with age-matched single transgenic and dual transgenic without Dox siblings. At the age of 8 months, although the latency in finding a treat in the dual transgenic with Dox (n=8) was not consistently higher than in the control groups, a similar trend was noted (p>0.05, Mann-Whitney U-test, Fig. 1A).
Fig. 1.
Buried pellet odor detection test at 8 months (A) and at 12 months of age (B) over a 6 day repeat trial period. The latency to find and eat a pellet was measured and reported as mean in seconds (s) ± SEM. Comparisons between DTg + Dox (black filled bar) and three control groups (DTg – Dox and a-syn +/- Dox) are reported (*: p<0.05, **: p<0.01, and #: p<0.001, Mann-Whitney U-test).
Nose-poke test
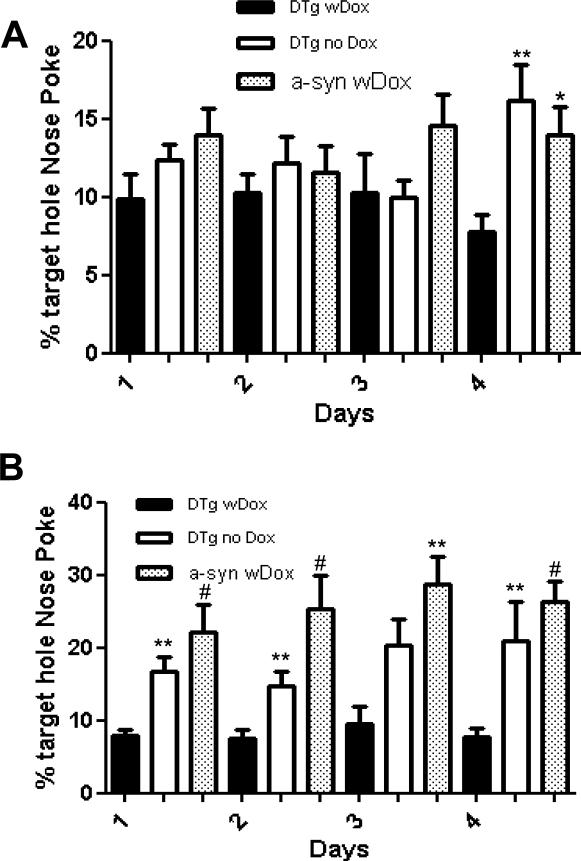
In the nose-poke test, 12 month old controls displayed high frequencies of nose-poking into the correct target hole (percent mean ± SEM, Mann-Whitney U-test), while dual transgenics with Dox-induced GSH depletion (n=8) showed values within the random range (random chance: 6.25%). The distribution of nose pokes demonstrated that dual transgenics with Dox (8.33 ± 1.41%, p>0.05) failed to recognize the food odor from the target hole in contrast to alpha-synuclein with Dox (22.43 ± 3.69, p<0.01) and dual transgenic mice without Dox (17.05 ± 3.43, p<0.05) controls (Fig. 2B). In contrast, no significant differences among these groups were noted by 8 months of age (p>0.05, Mann-Whitney U-test, Fig. 2A).
Fig 2.
Nose poke odor detection/identification test at 8 months (A) and at 12 months of age (B) over a 4 day repeat trial period. The percentage of nose pokes in the target versus other holes was calculated and reported as mean ± SEM. 6.25% represents a random nose-poke (1/16 chance). DTg + Dox (black filled bar) as compared to DTg - Dox and a-syn + Dox. (*: p<0.05, **: p<0.01, and #: p<0.001, Mann-Whitney U test).
Wooden block test
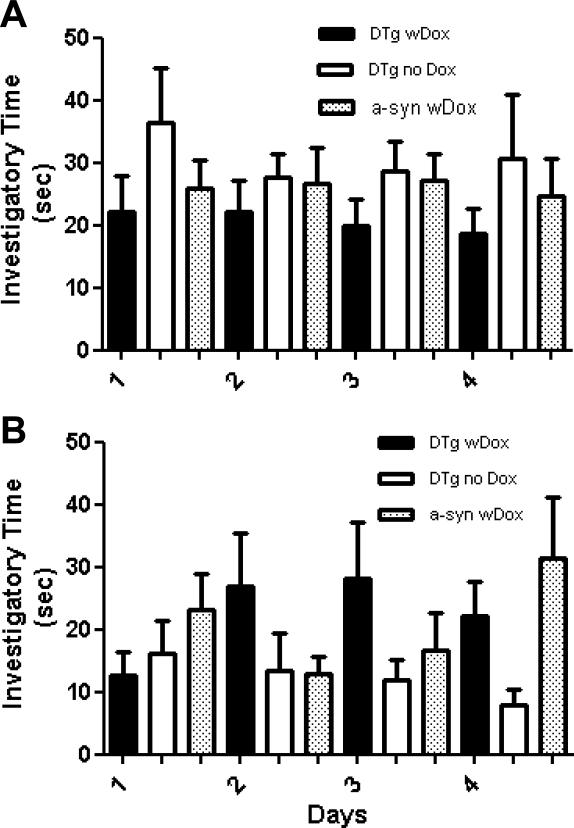
The same cohort of dual transgenic mice with Dox (n=8) who showed olfactory dysfunction in the buried pellet and nose-poke test were still able to distinguish non-self blocks from self-blocks at 12 months of age, similarly to dual Tg without Dox (n=5) and a-syn with Dox (n=9) controls (Fig. 3B). These results suggest selectivity in the maintenance of olfactory capabilities in the dual transgenics with Dox depends on the type of stimuli or the level of intensity. Although our results at 8 months of age showed a weak trend, no significant differences were found among any of the groups in 4 days of testing (Fig. 3A). We found no significant differences among the groups at either 8 or 12 months of age (p>0.05, mean ± SEM, Wilcoxon Signed Rank test).
Fig. 3.
Wooden block test to assess odor discrimination at 8 months (A) and at 12 months of age (B). Bars represent mean investigatory time (time spent sniffing block scented with a foreign animal's scent minus the time spent sniffing self-odored blocks) ± SEM. DTg + Dox (black filled bar) was compared and analyzed with DTg - Dox and a-syn + Dox (Wilcoxon signed rank test).
Stereological analysis of TH+ and NeuN+ cells in the glomerular layer
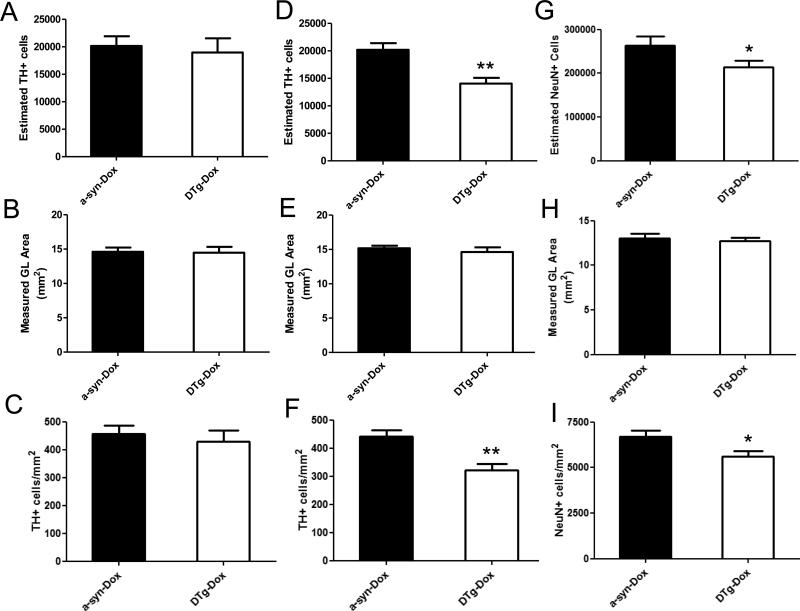
No differences were found in volume of the glomerular cell layer between dual Tg + Dox and alpha-synuclein + Dox sections in which this region was delineated at 8 and 12 months of age (Figs. 4B and E, respectively). However, the total TH+ cell number by 12 months was found to be significantly reduced (~30%) in the dual transgenic + Dox compared with α-synuclein + Dox controls (n=7 each, Paired t-test: p<0.01, Fig. 4D). Accordingly, the TH+ cell density/mm2 in the glomerular layer was also reduced in the dual transgenic + Dox compared to the α-synuclein + Dox control (mean ± SEM, Paired t-test: p<0.01, Fig. 4F). To assure that loss of TH-immunopositive staining was not merely a consequence of reduced TH expression as opposed to cell loss, NeuN labeled total neuron numbers were also assessed at 12 months of age and were found to be correspondingly reduced in the dual Tg + Dox compared to alpha-synuclein + Dox (mean ± SEM, Paired t-test: p<0.05, Fig. 4I). In contrast, no statistically significant differences were noted in total TH+ cell number or the density of TH-labeled cells between alpha-synuclein + Dox (n=7) and anti-GSH + Dox controls at 12 months of age (n=4, data not shown) nor in any of the groups at 8 months of age (n=7 for each, Paired t-test: p>0.05, Figs. 4A-C).
Fig. 4.
Stereological analyses of TH+ or NeuN+ cells in the glomerular layer (GL) at 8 months (A-C) and at 12 months of age (D-F for TH and G-I for NeuN). Absolute number (A, D, and G), measured GL area (B, E, and H), and the density (C, F, and I) of TH+ or NeuN+ cells in the GL area were analyzed in dual Tg + Dox versus a-syn + Dox (n=7 each, Mean ± SEM, **: p< 0.01, *: p< 0.05, Paired t-test).
Immunohistochemistry
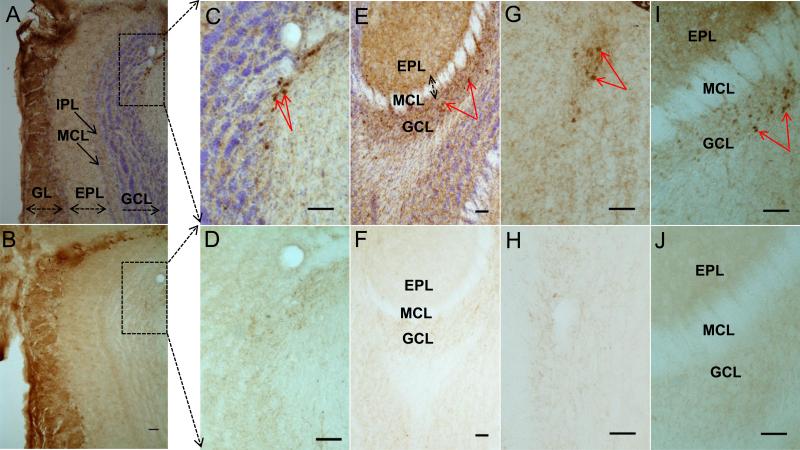
Along with TH-positive staining in glomerular OB neurons, we also assessed the localization of transgenic human alpha-synuclein in adjacent sections to those used for TH staining in the 12 month old dual transgenic OB. As expected, human alpha-synuclein was detected within TH-positive dopaminergic neurons in the glomerular layer (Figs. 5A and B). Interestingly, we also consistently found clearly labeled human-specific alpha-synuclein labeling within non-TH positive cells outside of the glomerular layer within the granule cell layer at both 8 and 12 months of age (Figs. 5A, C, and E). These alpha-synuclein labeled cells were not positive for TH-immunostaining (Figs. 5B, D, F, H, and J).
Fig. 5.
Immunohistochemistry of alpha-synuclein (A, C, E, G, and I) and TH (B, D, F, H, and J) labeled OB cells. Boxes in A and B were shown enlarged in C and D, respectively. Top row and bottom row are neighboring sections at the same scale. Red arrows in C, E, G, and I represent alpha-synuclein labeled cells in the granule cell layer. Alpha-synuclein was detected in the GCL at 8 months (G and I) and 12 months (A, C, and E). Abb: GL: glomerular layer, EPL:external plexiform layer, GCL:granule cell layer, MCL: mitral cell layer, IPL:internal plexiform layer. Size bar: 50 μm.
Mitochondrial respiration in presynaptic OB terminals
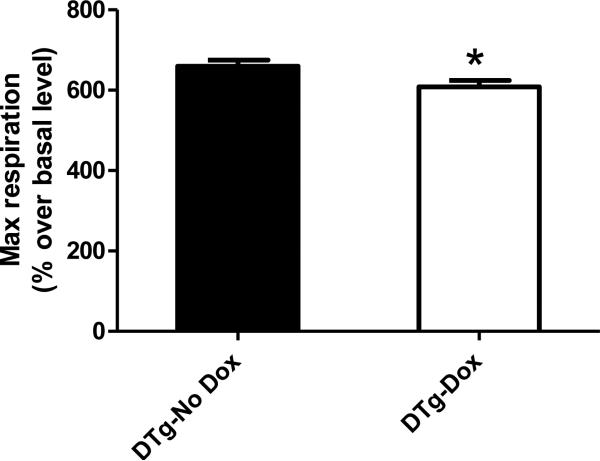
Presynaptic nerve terminals isolated from entire OB tissue of dual transgenics at 12 month old were analyzed for basal mitochondrial respiration, coupling efficiency, and maximum respiration; synaptosomal viability was verified via fluorescent staining for mitochondrial membrane potential and calcium levels as previously described (Choi et al., 2009). In three independent experiments (n=7), maximal respiration (with 4 μM FCCP stimulus) in dual Tg + Dox was slightly but significantly reduced (~10%) compared with control (dual Tg – Dox) (*: p<0.05, paird t-test, Fig. 6). No other changes were noted among all other mitochondrial activities.
Fig. 6.
FCCP-stimulated maximal respiration (4 μM) in presynaptic nerve terminals (synaptosomes) isolated from the entire olfactory bulb. Rates were calculated relative to basal respiration level in medium containing glucose but not pyruvate (n=7 each, Mean ± SEM, *: p< 0.05, Paired t-test). Maximum respiration was presented as percent basal level. Three independently executed results were analyzed for statistical analysis (n=3, Paired t-test).
DISCUSSION
Damage affecting olfactory function may be due to either direct cellular insults to the olfactory bulb itself and/or to central pathways involved in odor information processing (Arnold et al., 1998; Luzzi et al., 2007; Lafreniere and Mann, 2009). Olfactory perception is initiated in the olfactory epithelia in the dorsal aspect of the nasal cavity when odorants bind to receptors and odor information is relayed to the olfactory bulb. The glomerular layer in the OB is a key structure, made up of globular tangles of axons from olfactory receptor neurons in the olfactory epithelium, dendrites from the mitral cells, tufted cells, and other cell types (Doty, 2009). Glomeruli are critical gateways in the pathway from the nasal epithelia to the olfactory cortex. Each glomerulus receives input from olfactory receptor neurons expressing only one type of olfactory receptor (Reed et al., 1992; Lafreniere and Mann, 2009). The largest concentration of dopaminergic cells in the OB reside in the glomeruli (97% of TH-positive cells), although only a small portion of all periglomerular neurons are dopaminergic neurons (Cave and Baker, 2009). Here we demonstrate a significant cell loss in dopaminergic (TH+) cells in the GL in dual transgenics compared with alpha-synuclein only siblings. This does not appear to be attributable to a reduction in TH+ expression alone as it coincides with an actual neuronal cell loss. We report that dopaminergic alpha-synuclein expression while on its own having no impact on survival of these cells with age results in their neurodegeneration in the presence of increased oxidative stress in the form of glutathione depletion. This suggests a synergistic interaction between alpha-synuclein and oxidative stress. This was accompanied by detection of alpha-synuclein within non-DA cells in the granule cell layer and a reduction in odor detection.
Olfactory deficits in human PD do not appear to involve frank loss of dopaminergic neurons in the OB (Huisman et al., 2008, Ubeda-Banon et al., 2010). The discrepancy between what is observed pathologically in human PD patients could be derived from the higher cellular insults of alpha-synuclein over-expression and oxidative stress combined in TH-positive neurons in our mouse model. Dopamine has, however, been reported to modulate olfactory signal processing in the olfactory bulb and altered dopamine homeostasis in olfaction-related brain regions results in olfactory deficits (Cave and Baker, 2009). Recently both dopamine transporter knockout (DAT -/-) and dopamine receptor 2 knockout (D2 -/-) mice have been reported to display an olfactory discrimination deficit, although their odor habituation, olfactory sensitivity or odor recognition memory were intact (Tillerson et al., 2006). In this current study, our dual transgenics still display the capability to distinguish non-self odor from self via the wooden block test. As smell deficit depends on the site of the lesion, we can postulate that either glomeruli were not sufficiently damaged in the odor discrimination pathway and other brain regions to obliterate dopamine-dependent signaling as observed in the dopamine-signaling knockout models or simply that the odor stimulus was beyond the threshold for odor discrimination even in the damaged transgenic mice.
The observed cell loss in our mouse model is unlikely to be the only factor responsible for the observed olfactory deficits as a trend towards olfactory disturbances is already observable by 8 months of age. By this earlier time point, we already observe presence of alpha-synuclein within non-DA neurons in the GCL (Figs. 5G and I). It is formally possible that the observed human alpha-synuclein protein in the granule cell layer (GCL) is due to its local synthesis. However, no TH-positive cells exist in the vicinity of noted GCL expression and therefore this would require its aberrant expression as the human alpha-synuclein antibody used in our experiments is highly specific for the human protein. Presence of transgenic protein within these cells may be due to its release from dopaminergic cells and subsequent uptake by non-DA cells in the granule cell layer likely via transmission through neighboring astrocytes, oligodendrocytes or other neurons such as those within the mitral cell layer which connects these two populations, perhaps via an endocytotic mechanism (Desplats et al., 2009; Lu et al., 2009). Interestingly, recent analyses of PD patients have suggested the presence of alpha-synuclein within both mitral and GCL neurons as well as occasionally within dopaminergic glomerular cells (Ubeda-Banon et al., 2010). It is important to bear in mind that these human studies were performed on autopsy from end-stage disease (3-5 on the Braak rating scale) and therefore do not provide information about synucleopathy origination or progression in the OB. It is possible that it may originate in DA glomerular neurons and be progressively taken up into mitral and then non-DA cells in the GCL.
Mitral cells are critical for the information transport to the entorhinal and pyriform cortices and the amygdala and disruption of these connections results in olfactory deficits (Kovacs, 2004; Lafreniere and Mann, 2009; Wattendorf et al., 2009). Oxidative stress in our model may act as a trigger to allow release from DA neurons and alpha-synuclein uptake by cells observed to contain the protein in the human disease. Further studies are required to verify the precise mechanisms involved. Increased levels of alpha-synuclein may in turn account for increased mitochondrial damage noted in presynaptic OB nerve terminals which may in turn impact on function. Increased alpha-synuclein expression has been demonstrated to coincide with mitochondrial deficits (Hsu et al., 2000). Alpha-synuclein is reported to be physically localized within mitochondria and as a consequence can result in mitochondrial dysfunction (Liu et al., 2009). The small difference we obtained likely reflects the fact that these analyses were performed in whole OB synaptosomes rather than those populations selectively impacted.
CONCLUSIONS
We report an age-related olfactory dysfunction in odor identification and detection in a dual transgenic model displaying combined dopaminergic alpha-synuclein elevation and increased oxidative stress level as a consequence of inducible glutathione reduction in these cells. This is accompanied by dopaminergic neurodegeneration and increases alpha-synuclein levels in non-dopaminergic cells in the GCL, the latter emulating what is observed in the human condition. These results suggest that in the presence of increased oxidative stress, increased alpha-synuclein expression within dopaminergic OB neurons results in their age-related neurodegeneration and increased alpha-synuclein levels in non-dopaminergic cells in the olfactory bulb coinciding with olfactory dysfunction.
ACKNOWLEDGEMENTS
We thank Dr. Sheila Fleming at the University of Cincinnati for help in designing pellet and wooden block tests and for statistical consultation. This study was supported by NIH grant RL1 NS062415 (Andersen) NIH/NCRR Interdisciplinary Research Consortium (U54).
Abbreviations
- PD
Parkinson's disease
- GSH
Glutathione
- Dox
doxycycline
- OB
Olfactory bulb
- a-syn
alpha-synuclein
- TH
tyrosine hydroxylase
- GL
glomerular layer
- EPL
external plexiform layer
- GCL
granule cell layer
- MCL
mitral cell layer
- IPL
internal plexiform layer
- Tg
transgenic mice
- DTg
dual Transgenic
- DA
dopaminergic cells
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Arnold SE, Smutzer GS, Trojanowski JQ, Moberg PJ. Cellular and molecular neuropathology of the olfactory epithelium and central olfactory pathways in Alzheimer's disease and schizophrenia. Ann N Y Acad Sci. 1998;30855:762–775. doi: 10.1111/j.1749-6632.1998.tb10656.x. [DOI] [PubMed] [Google Scholar]
- Attems J, Lintner F, Jellinger KA. Olfactory involvement in aging and Alzheimer's disease: an autopsy study. J Alzheimers Dis. 2005;7(2):149–57. doi: 10.3233/jad-2005-7208. discussion 173-180. [DOI] [PubMed] [Google Scholar]
- Beach TG, White CL, 3rd, Hladik CL, Sabbagh MN, Connor DJ, Shill HA, Sue LI, Sasse J, Bachalakuri J, Henry-Watson J, et al. Olfactory bulb alpha-synucleinopathy has high specificity and sensitivity for Lewy body disorders. Acta Neuropathol. 2009;117(2):169–174. doi: 10.1007/s00401-008-0450-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berendse HW, Booij J, Francot CM, Bergmans PL, Hijman R, Stoof JC, Wolters EC. Subclinical dopaminergic dysfunction in asymptomatic Parkinson's disease patients’ relatives with a decreased sense of smell. Ann Neurol. 2001;50(1):34–41. doi: 10.1002/ana.1049. [DOI] [PubMed] [Google Scholar]
- Braak H, Del Tredici K, Rub U, de Vos RA, Jansen Steur EN, Braak E. Staging of brain pathology related to sporadic Parkinson's disease. Neurobiol Aging. 2003;24(2):197–211. doi: 10.1016/s0197-4580(02)00065-9. [DOI] [PubMed] [Google Scholar]
- Cave JW, Baker H. Dopamine systems in the forebrain. Adv Exp Med Biol. 2009;651:15–35. doi: 10.1007/978-1-4419-0322-8_2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chinta SJ, Kumar MJ, Hsu M, Rajagopalan S, Kaur D, Rane A, Nicholls DG, Choi J, Andersen JK. Inducible alterations of glutathione levels in adult dopaminergic midbrain neurons result in nigrostriatal degeneration. J Neurosci. 2007;27(51):13997–14006. doi: 10.1523/JNEUROSCI.3885-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi SW, Gerencser AA, Nicholls D. Bioenergetic analysis of isolated cerebrocortical nerve terminals on a microgram scale: spare respiratory capacity and stochastic mitochondrial failure. J Neurochem. 2009;109(4):1179–1191. doi: 10.1111/j.1471-4159.2009.06055.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desplats P, Lee HJ, Bae EJ, Patrick C, Rockenstein E, Crews L, Spencer B, Masliah E, Lee SJ. Inclusion formation and neuronal cell death through neuron-to-neuron transmission of alpha-synuclein. Proc Natl Acad Sci U S A. 2009;106(31):13010–13015. doi: 10.1073/pnas.0903691106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty RL. The olfactory system and its disorders. Semin Neurol. 2009 Feb;29(1):74–81. doi: 10.1055/s-0028-1124025. [DOI] [PubMed] [Google Scholar]
- Doty RL. Olfaction in Parkinson's disease. Parkinsonism Relat Disord. 2007;13(Suppl 3):S225–8. doi: 10.1016/S1353-8020(08)70006-3. [DOI] [PubMed] [Google Scholar]
- Fleming SM, Tetreault NA, Mulligan CK, Hutson CB, Masliah E, Chesselet MF. Olfactory deficits in mice overexpressing human wildtype alpha-synuclein. Eur J Neurosci. 2008;28(2):247–256. doi: 10.1111/j.1460-9568.2008.06346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu LJ, Sagara Y, Arroyo A, Rockenstein E, Sisk A, Mallory M, Wong J, Takenouchi T, Hashimoto M, Masliah E. alpha-synuclein promotes mitochondrial deficit and oxidative stress. Am J Pathol. 2000;157(2):401–410. doi: 10.1016/s0002-9440(10)64553-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu M, Srinivas B, Kumar J, Subramanian R, Andersen J. Glutathione depletion resulting in selective mitochondrial complex I inhibition in dopaminergic cells is via an NO-mediated pathway not involving peroxynitrite: implications for Parkinson's disease. J Neurochem. 2005;92(5):1091–1103. doi: 10.1111/j.1471-4159.2004.02929.x. [DOI] [PubMed] [Google Scholar]
- Huisman E, Uylings HB, Hoogland PV. Gender-related changes in increase of dopaminergic neurons in the olfactory bulb of Parkinson's disease patients. Mov Disord. 2008;30;23(10):1407–1413. doi: 10.1002/mds.22009. [DOI] [PubMed] [Google Scholar]
- Kovács T. Mechanisms of olfactory dysfunction in aging and neurodegenerative disorders. Ageing Res Rev. 2004;3(2):215–232. doi: 10.1016/j.arr.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Lafreniere D, Mann N. Anosmia: loss of smell in the elderly. Otolaryngol Clin North Am. 2009;42(1):123–131. doi: 10.1016/j.otc.2008.09.001. [DOI] [PubMed] [Google Scholar]
- Lang AE, Lozano AM. Parkinson's Disease (First of Two Parts). The New England Journal of Medicine. 1998;339(15):1044–1053. doi: 10.1056/NEJM199810083391506. [DOI] [PubMed] [Google Scholar]
- Lee HJ, Suk JE, Patrick C, Bae EJ, Cho JH, Rho S, Hwang D, Masliah E, Lee SJ. Direct transfer of alpha-synuclein from neuron to astroglia causes inflammatory responses in synucleinopathies. J Biol Chem. 2010;19;285(12):9262–9272. doi: 10.1074/jbc.M109.081125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lerner A, Bagic A. Olfactory pathogenesis of idiopathic Parkinson disease revisited. Mov Disord. 2008;23(8):1076–1084. doi: 10.1002/mds.22066. [DOI] [PubMed] [Google Scholar]
- Liu G, Zhang C, Yin J, Li X, Cheng F, Li Y, Yang H, Uéda K, Chan P, Yu S. alpha-Synuclein is differentially expressed in mitochondria from different rat brain regions and dose-dependently down-regulates complex I activity. Neurosci Lett. 2009;1;454(3):187–192. doi: 10.1016/j.neulet.2009.02.056. [DOI] [PubMed] [Google Scholar]
- Lu XH, Fleming SM, Meurers B, Ackerson LC, Mortazavi F, Lo V, Hernandez D, Sulzer D, Jackson GR, Maidment NT, Chesselet MF, Yang XW. Bacterial artificial chromosome transgenic mice expressing a truncated mutant parkin exhibit age-dependent hypokinetic motor deficits, dopaminergic neuron degeneration, and accumulation of proteinase K-resistant alpha-synuclein. J Neurosci. 2009;18;29(7):1962–1976. doi: 10.1523/JNEUROSCI.5351-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luzzi S, Piccirilli M, Provinciali L. Perception of emotions on happy/sad chimeric faces in Alzheimer disease: relationship with cognitive functions. Alzheimer Dis Assoc Disord. 2007;21(2):130–135. doi: 10.1097/WAD.0b013e318064f445. [DOI] [PubMed] [Google Scholar]
- Reed RR, Bakalyar HA, Cunningham AM, Levy NS. The molecular basis of signal transduction in olfactory sensory neurons. Soc Gen Physiol Ser. 1992;47:53–60. [PubMed] [Google Scholar]
- Sherer TB, Betarbet R, Stout AK, Lund S, Baptista M, Panov AV, Cookson MR, Greenamyre JT. An in vitro model of Parkinson's disease: linking mitochondrial impairment to altered alpha-synuclein metabolism and oxidative damage. J Neurosci. 2002;15;22(16):7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simuni T, Sethi K. Nonmotor manifestations of Parkinson's disease. Ann Neurol. 2008;64(Suppl2):S65–80. doi: 10.1002/ana.21472. [DOI] [PubMed] [Google Scholar]
- Sobel N, Thomason ME, Stappen I, Tanner CM, Tetrud JW, Bower JM, Sullivan EV, Gabrieli JD. An impairment in sniffing contributes to the olfactory impairment in Parkinson's disease. Proc Natl Acad Sci U S A. 2001;98(7):4154–4159. doi: 10.1073/pnas.071061598. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. Alpha-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- Taylor TN, Caudle WM, Shepherd KR, Noorian A, Jackson CR, Iuvone PM, Weinshenker D, Greene JG, Miller GW. Nonmotor symptoms of Parkinson's disease revealed in an animal model with reduced monoamine storage capacity. J Neurosci. 2009;24;29(25):8103–8113. doi: 10.1523/JNEUROSCI.1495-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tillerson JL, Caudle WM, Parent JM, Gong C, Schallert T, Miller GW. Olfactory discrimination deficits in mice lacking the dopamine transporter or the D2 dopamine receptor. Behav Brain Res. 2006 Sep 15;172(1):97–105. doi: 10.1016/j.bbr.2006.04.025. 2006. [DOI] [PubMed] [Google Scholar]
- Ubeda-Bañon I, Saiz-Sanchez D, de la Rosa-Prieto C, Argandoña-Palacios L, Garcia-Muñozguren S, Martinez-Marcos A. alpha-Synucleinopathy in the human olfactory system in Parkinson's disease: involvement of calcium-binding protein- and substance P-positive cells. Acta Neuropathol. 2010;119(6):723–735. doi: 10.1007/s00401-010-0687-9. [DOI] [PubMed] [Google Scholar]
- Ward CD, Hess WA, Calne DB. Olfactory impairment in Parkinson's disease. Neurology. 1983;33(7):943–946. doi: 10.1212/wnl.33.7.943. [DOI] [PubMed] [Google Scholar]
- Wattendorf E, Welge-Lüssen A, Fiedler K, Bilecen D, Wolfensberger M, Fuhr P, Hummel T, Westermann B. Olfactory impairment predicts brain atrophy in Parkinson's disease. J Neurosci. 2009;9;29(49):15410–15413. doi: 10.1523/JNEUROSCI.1909-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]