Abstract
Background
Patients with abdominal aortic aneurysms (AAA) are predisposed to cardiovascular events and often experience continual expansion of their aneurysm. Cardiovascular events and expansion rates are positively correlated with aneurysm size. AAA is usually associated with intraluminal thrombus, which has previously been implicated in AAA pathogenesis.
Objectives
The aims of this study were to prospectively assess the association of infra-renal abdominal aortic thrombus volume with cardiovascular events and AAA growth.
Methods
98 patients with AAAs underwent computed tomography angiography (CTA). The volume of infra-renal aorta thrombus was measured by a previously validated technique. Patients were followed prospectively for a median of 3 (inter-quartile range 2.0–3.6) years and cardiovascular events (non-fatal stroke, non-fatal myocardial infarction, coronary revascularization, amputation and cardiovascular death) recorded. 39 of the original patients underwent repeat CTA a median of 1.5 (inter-quartile range, 1.1–3.3) years after entry to the study. Kaplan-Meier and Cox-proportional analysis were used to examine the association of aortic thrombus with cardiovascular events and average weighted AAA growth.
Results
A total of 28 cardiovascular event occurred during follow-up. The incidence of cardiovascular events was 23.4 and 49.2% for patients with small (<median) and large (≥median) volumes of aortic thrombus, respectively, at 4 years (p=0.040). AAA thrombus volume ≥median was associated with increased cardiovascular events (RR 2.8, 95% CI 1.01– 5.24) independent of other risk factors including initial AAA diameter, but only of borderline significance when patients were censored at the time of AAA repair (RR 2.35, 95% CI 0.98–5.63). In the sub-set of patients with CTA follow-up median annual increase in AAA volume was 5.1cm3 (inter-quartile range 0.8–10.3). Annual AAA volume increase was positively correlated with initial AAA diameter (r=0.44, p=0.006) and thrombus volume (r=0.50, p=0.001). Aortic thrombus volume ≥median was associated with rapid AAA volume increase (≥5cm/year), independent of initial aortic diameter (OR 15.0, 95% CI 1.9–115.7, p=0.009).
Conclusion
In this small cohort infra-renal aortic thrombus volume was associated with the incidence of cardiovascular events and AAA progression. These results need to be confirmed and mechanisms underlying the associations clarified in large further studies.
Introduction
Abdominal aortic aneurysms (AAA) are a common pathology affecting approximately 7% of males and 1% of females aged ≥65 years (1). Patients who have AAAs have two principal concerns. Firstly AAAs are associated with an excess risk of mortality and cardiovascular complications, such as myocardial infarction and ischemic stroke (2, 3), distinct from the risk of AAA rupture. Secondly AAA tend to expand over time to a size often requiring surgery and where AAA rupture is more prevalent (4). Previous studies indicate that ~60% of small AAAs measuring ≥40mm enlarge to a diameter requiring surgery within 5 years (5, 6). The determinants of AAA progression and cardiovascular events in these patients are currently poorly defined. The most consistent prognostic factor for cardiovascular events and AAA progression is initial AAA diameter (2, 3–14). AAA is usually associated with intra-luminal thrombus which has been shown to contain leukocytes, pro-inflammatory cytokines and proteolytic enzymes, and implicated in AAA development, progression and rupture (15–23). AAA thrombus products are also released into the circulation where they have potential to stimulate leukocytes and other changes which might promote atherosclerotic plaque activation and acute coronary and cerebrovascular events (24–28). No previous study has examined the association of AAA thrombus with subsequent cardiovascular events. The first aim of this study was to examine the association of AAA thrombus volume with future cardiovascular events. The second aim was to assess the association of AAA thrombus with AAA growth.
Methods
Patients and clinical definitions
Patients were prospectively recruited from the vascular surgery clinic at The Townsville Hospital, Queensland, Australia between May 2003 and July 2008. Inclusion criteria included: 1) verbal and written informed consent; 2) the treating physician required a computed tomographic angiogram (CTA) to further assess the patients. The indications for CTA included aneurysm morphology assessment prior to surgery; inadequate ultrasound assessment; requirement for more detailed AAA diameter assessment; and analysis of concurrent athero-thrombosis; 3) initial maximal axial infra-renal aortic diameter of ≥30mm measured on CTA. Exclusion criteria included: 1) refusal to participate; 2) previous surgical repair of the abdominal aorta; 3) contra-indication to CTA, such as abnormal serum creatinine and contrast allergy. Numbers and demographics of patients excluded were not recorded.
Intermittent claudication was diagnosed by a consultant vascular physician based on an appropriate history along with clinical signs of lower limb ischemia and CTA evidence of occlusive or stenotic peripheral artery disease. Hypertension and diabetes were defined by previous history or treatment for these conditions. Cigarette smoking classification was based on smoking history and defined ultimately as ever or never smoked. Coronary heart disease (CHD) was defined by a history of myocardial infarction, angina or coronary revascularisation. Body mass index (BMI) was calculated by weight (kg)/height (m)2. Ethics approval for this study was provided by Human Research Ethics Committees of the Townsville Health Service District and James Cook University.
CT Angiography
Contrast enhanced CT images were obtained using a 64-slice multiscanner (Philips, North Ryde, NSW), under a set acquisition protocol. The images were recorded at 3mm intervals, with a slice thickness of 3mm, in order to construct 3mm contiguous image slices for analysis. 100ml of contrast agent (Ultravist 300), delivered by an automatic CT injection driver system (MEDRAD) was given intra-venously. A low dose preliminary CT locater was set above the renal arteries, which triggered the CTA when the Hounsfield Unit (HU) at the center of the aorta reached 130 after the delivery of the contrast agent.
Workstation protocols
AAA thrombus volume was measured using a previously validated protocols with an inter-observer coefficient of variation of ~5% (29). Images from the origin of the lowest renal artery (excluding accessory arteries) to the bifurcation of the aorta were transferred to Philips MxView Visualization Workstation software for analysis. Thrombus was thresholded by utilizing previously defined Hounsfield units (HU) (Center level 0 HU and Window width 140 HU). Using the volume of interest tool an encircling line was drawn around the aorta to form a region of interest (ROI). The ROI was individually drawn for each slice to ensure that only the aorta was included. The selected images were then saved onto the workstation and re-loaded into the 3D mode. The software program computed the volume of thrombus in cm3. Maximal axial infra-renal aortic diameter was assessed using the “CTA viewer function” on the Philips Workstation. The region was scouted to find the area of maximal diameter, taking many measurements with electronic calipers. Maximal diameter was recorded in millimeters (to the nearest 0.1mm).
Clinical data
The following information was collected by a vascular physician at entry into the study: sex, age, height, weight, smoking status, diabetes mellitus, hypertension, CHD and medication history. Following the initial consultation a CTA was arranged and the patients were subsequently followed up according to the AAA diameter. Patients with small AAAs measuring 30–39 mm were followed up yearly and those with initial AAA diameter ≥40 mm every 6 months. The primary outcome recorded during follow-up was admission for cardiovascular events (non-fatal stroke, non-fatal myocardial infarction, coronary revascularization, amputations and cardiovascular death) in order to assess aim 1. Outcome data was recorded during follow-up visits and subsequently checked via review of patients charts by two independent researchers (for all patients) and by additional phone calls to selected patients (no chart entry in previous 2 years; primary residence outside The Townsville Hospital catchment area). While the majority of patients had their AAA monitored using ultrasound some patients underwent repeat CTA based on concern that the AAA had expanded to a size requiring surgery, difficulty in imaging by ultrasound or concern over the accuracy of the ultrasound. Only patients undergoing repeat CTA were included in order to assess aim 2 due to the lack of comparability between ultrasound and CT diameters (30).
Growth measurements
Initial and follow-up CTAs were assessed for maximal axial infra-renal aortic diameter and total infra-renal aortic volume, using previously validated techniques (29). Using these measurements weighted annual change in AAA volume was calculated. We utilized this assessment method since we have previously found volume to be more sensitive to change than diameter (31).
Statistical analysis
Data was prospectively entered into a spreadsheet (Microsoft Excel) and later transferred to SPSS (Version 17.0) for Windows for further analysis. To assess aim 1 the total cohort of patients was included. Median initial aortic thrombus in this group was 29.6cm3 (inter-quartile range, 13.6–54.5). Aortic thrombus was defined as small (thrombus volume <25.0cm3); and large (thrombus volume ≥25.0cm3) based on rounding down thrombus median to the nearest 5cm3. To assess aim 2 the 39 patients with repeat CTA assessment were included. Median initial aortic thrombus in this group was 16.4cm3 (inter-quartile range, 10.6–32.0). Aortic thrombus was defined as small (thrombus volume <15.0cm3); and large (thrombus volume ≥15.0cm3) based on rounding down thrombus median to the nearest 5cm3. Initially continuous variables were assessed using Kolmogorov-Smirnov test, histograms and normal quantile-quantile plots, which demonstrated that they were not normally distributed. Continuous variables were compared with Mann Whitney U test and nominal variables with Fischer s exact test. Kaplan-Meier analysis was used to determine freedom from cardiovascular events. In the primary analysis patients were censored at loss to follow-up or death from causes other than cardiovascular events. In a secondary analysis patients were also censored at the time of AAA repair in order to exclude confounding effects of surgical intervention. Cox-proportional analysis was employed to assess the effects of known risk factors on cardiovascular events. Categorical variables were dummy-coded. Known risk factors, including age, gender, smoking status, diabetes mellitus, hypertension, dyslipidaemia, CHD, maximal axial infra-renal aortic diameter, intermittent claudication and use of calcium channel blockers, statins, warfarin, aspirin, angiotensin converting enzyme inhibitor and beta-blockers were examined for inclusion in the final model. Association of risk factors with cardiovascular events was examined using a stepwise selection procedure (backward and forward likelihood ratios). Variables not in the stable model were then included individually in the model. Risk factors which led to a ≥10% change in the coefficient of the model were retained in the final model as a potential confounder. Variables not included in the final model did not significantly impact on the results. Determinants of rapid AAA growth were assessed using logistic regression.
Results
Characteristics of patients at recruitment
The risk factors of the 98 patients in relation to the volume of infra-renal AAA thrombus are shown in Table 1. Median infra-renal axial AAA diameter was 47.2mm. 19 patients had an AAA with axial diameter >55mm at entry to the study. Thrombus volume was greater in large AAAs, males and patients who did not have intermittent claudication (Table 1). Thrombus volume was not associated with warfarin or aspirin use.
Table 1.
Risk factors of patients at recruitment in relation to AAA thrombus volumes.
| Characteristic | Total | Thrombus <25.0cm3 | Thrombus ≥25.0cm3 | p-value |
|---|---|---|---|---|
| Patients | 98 | 43 | 55 | |
| Age, years | 73 (67–77) | 72 (67–75) | 74 (68–78) | 0.245 |
| Male | 75 (76.5) | 28 (65.1) | 47 (85.5) | 0.029 |
| Follow-up, years | 3.0 (2.0–3.6) | 3.2 (2.0–3.7) | 2.8 (1.9–3.4) | 0.358 |
| Maximum AAA axial diameter, mm | 47.2 (34.9–54.6) | 34.4 (32.2–42.6) | 52.8 (49.5–58.1) | <0.001 |
| Body Mass Index, weight(kg)/height(m)2 | 28.2 (25.0–30.8) | 28.7 (25.6–32.0) | 27.8 (24.5–30.3) | 0.150 |
| Intermittent claudication | 29 (29.6) | 18 (41.9) | 11 (20.0) | 0.026 |
| Diabetes mellitus | 20 (20.4) | 8 (18.6) | 12 (21.8) | 0.803 |
| Ever smoked | 86 (87.8) | 36 (83.7) | 50 (90.9) | 0.357 |
| Hypertension | 75 (76.5) | 33 (76.7) | 42 (76.4) | 1.000 |
| Coronary heart disease | 59 (60.2) | 25 (58.1) | 34 (61.8) | 0.836 |
| Warfarin | 13 (13.3) | 7 (16.3) | 6 (10.9) | 0.552 |
| Aspirin | 62 (63.3) | 27 (62.8) | 35 (63.6) | 1.0 |
| Calcium channel blocker | 24 (24.5) | 13 (30.2) | 11 (20.0) | 0.344 |
| ACE inhibitor | 42 (42.9) | 22 (51.2) | 20 (36.4) | 0.156 |
| Statin | 65 (66.3) | 24 (55.8) | 41 (74.5) | 0.057 |
| Beta blocker | 35 (35.7) | 18 (41.9) | 17 (30.9) | 0.293 |
Continuous results are presented as medians (inter-quartile range) and compared by Mann Whitney U; nominal results are presented as number (%) and are compared by Fisher’s exact test (2-tailed significance).
Cardiovascular events
Patients were followed for a median of 3.0 (inter-quartile range, 2.0–3.6) years. The following cardiovascular events occurred during follow-up: Non-fatal myocardial infarction (n=13), coronary revascularization (n=4), stroke (n=1), below knee amputation (n=1), and cardiovascular death (n=9). Patients with a large volume of AAA thrombus had a higher incidence of cardiovascular events (Fig. 1). The incidence of cardiovascular events was 23.4% [95% CI, 16.0–30.8] and 49.2% [95% CI, 39.8–58.6] for patients with small and large AAA thrombus volumes, respectively, at 4 years (p=0.040). The incidence of cardiovascular events was 24.5% [95% CI, 16.7–32.3] and 46.6% [95% CI, 37.9–55.3] for patients with small and large maximal axial AAA diameters, respectively, at 4 years (p=0.074) (Fig. 2). AAA thrombus volume ≥25.0cm3 was associated with increased incidence of new cardiovascular independent of other risk factors by Cox analysis (OR 2.3, 95% CI 1.01–5.24, Table 2). Sex was identified as a confounding factor. All other variables, including initial AAA diameter, did not significantly impact on the results.
Figure 1.
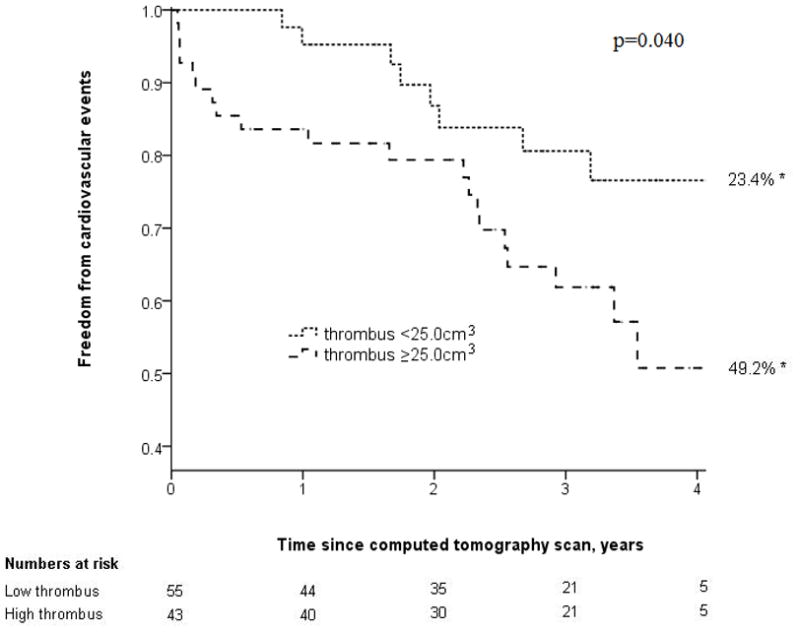
Kaplan Meier analysis showing freedom from cardiovascular events in patients with small (<26.0cm3) and large (>26.0cm3) AAA thrombus volumes. * = cardiovascular event rate at 4 years.
Figure 2.
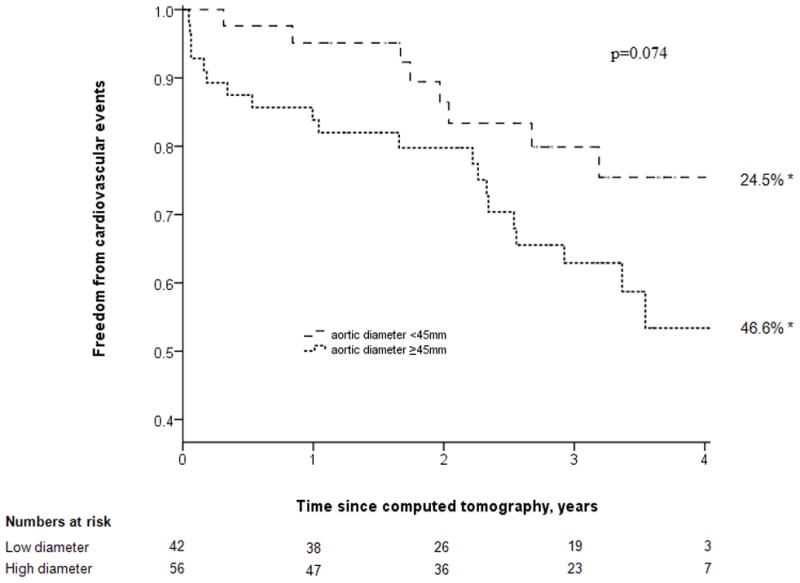
Kaplan Meier analysis showing freedom from cardiovascular events in patients with small (<45.0mm) and large (≥45.0mm) diameter AAAs. * = cardiovascular event rate at 4 years.
Table 2.
Cox proportional analysis examining prognostic factors for cardiovascular events.
| Prognostic factor | Sample size [n=98] | Number of cardiovascular events [n=28] | Relative risk | 95% CI | p-value |
|---|---|---|---|---|---|
| Thrombus volume | |||||
| <25.0cm3 | 43 | 8 | 1 | reference | 0.046 |
| ≥25.0cm3 | 55 | 20 | 2.30 | 1.01– 5.24 | |
| Sex* | |||||
| Male | 75 | 19 | 1 | reference | 0.115 |
| Female | 23 | 9 | 1.95 | 0.85–4.46 | |
| Maximal axial aortic diameter# | |||||
| <45.0mm | 42 | 8 | 1 | reference | 0.857 |
| ≥45.0mm | 56 | 20 | 1.1 | 0.27–4.72 | |
CI= confidence interval;
identified as a confounder (i.e. <10% change in thrombus coefficient);
not identified as a confounder but included to illustrate the impact of diameter when thrombus included in analysis.
AAA surgery
During follow-up 37 patients underwent AAA repair; 12 by open and 25 by endovascular repair. No patients died within 30 days of surgery. In a secondary analysis of cardiovascular events the association of AAA thrombus was examined censoring these patients at AAA repair. The findings of this analysis were similar to the primary analysis. The incidence of cardiovascular events was 26.9% [95% CI, 18.3–35.5] and 48.9% [95% CI, 40.6–59.5] for patients with small and large AAA thrombus volumes, respectively, at 4 years (p=0.049). The incidence of cardiovascular events was 26.1% [95% CI, 17.5–34.7] and 48.6% [95% CI, 38.1–59.1] for patients with small and large maximal axial AAA diameters, respectively, at 4 years (p=0.065). Patients with AAA thrombus volume ≥25.0cm3 had an increased incidence of cardiovascular events (OR 2.35, 95% CI 0.98–5.63) after adjusting for other risk factors although the association was no longer significant, p=0.056.
AAA growth
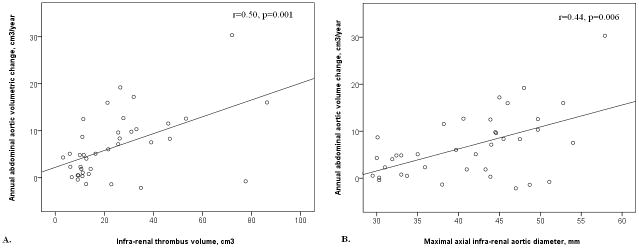
A total of 39 patients had repeat CT scans of their AAAs a median of 1.5 years (inter-quartile range, 1.1–3.3) after their initial imaging and prior to any surgery. These 39 include 15 patients selected for AAA repair and another 24 patients in who the physician treating the patients decided to obtain a further CT. The risk factors of the 39 patients who underwent repeat CTA in relation to slow (<5cm3) and rapid (≥5cm3) annual abdominal aortic volume expansion are shown in Table 3. Rapid expansion was associated with larger initial AAA diameter and thrombus volume (p≤0.001). Annual median (inter-quartile range) AAA volume and diameter increase were 5.1cm3 (0.8–10.3) and 1.3mm (−0.2–2.7), respectively. Annual abdominal aortic volume change was positively correlated with maximal axial initial AAA diameter (r=0.44, p=0.006) and thrombus volume (r=0.50, p=0.001) (Fig. 3). Initial AAA thrombus volume ≥15.0cm3 was associated with rapid volumetric growth (≥5cm2/year) (OR 15.0, 95% CI 1.9–115.7, p=0.009). Initial AAA diameter, smoking and diabetes mellitus were not significantly associated with rapid growth after adjusting for thrombus volume.
Table 3.
Risk factors of patients at recruitment in relation to AAA expansion ≥ or < median.
| Characteristic | Total | Annual AAA volume increase <5.0cm3/year | Annual AAA volume increase ≥5.0cm3/year | p-value |
|---|---|---|---|---|
| Patients | 39 | 19 | 20 | |
| Age, years | 73 (69–77) | 74 (69–77) | 71 (68–75) | 0.518 |
| Male | 27 (69.2) | 13 (68.4) | 14 (70.0) | 0.981 |
| Follow-up, years | 1.5 (1.1–3.3) | 1.4 (0.9–3.3) | 1.6 (1.1–3.3) | 0.771 |
| Maximum AAA diameter, mm | 42.1 (33.0–47.0) | 22.0 (30.3–43.3) | 44.8 (41.0–49.3) | 0.001 |
| Total AAA volume, cm3 | 73.6 (46.6–95.2) | 49.6 (40.4–60.0) | 86.0 (75.2–107.3) | <0.001 |
| Thrombus volume | 16.4 (10.6–32.0) | 11.1 (9.2–13.7) | 27.1 (21.4–44.3) | <0.001 |
| Warfarin | 2 (5.1) | 1 (5.3) | 1 (5.0) | 1.0 |
| Intermittent claudication | 15 (38.5) | 10 (52.6) | 5 (25.0) | 0.105 |
| Diabetes Mellitus | 9 (23.1) | 6 (31.6) | 3 (15.0) | 0.237 |
| Ever smoked | 32 (82.1) | 15 (78.9) | 17 (85.0) | 0.695 |
| Hypertension | 29 (74.4) | 16 (84.2) | 13 (65.0) | 0.273 |
| Coronary heart disease | 22 (56.4) | 14 (73.7) | 8 (40.0) | 0.054 |
Continuous results are presented as medians (inter-quartile range) and compared by Mann Whitney U; nominal results are presented as number (%) and are compared by Fisher’s exact test (2-tailed significance).
Figure 3.
Scatterplots displaying the association of annual abdominal aortic volume change with thrombus volume (a.) and maximal axial abdominal aortic diameter (b.).
Discussion
The main finding of this study was that the volume of AAA thrombus was associated with subsequent cardiovascular events and rapid AAA growth. This association was independent of initial AAA diameter at least in our primary analysis. There are few previous studies examining the association of thrombus quantity with AAA growth (18, 19) and we are unaware of any previous investigation examining the relationship of thrombus volume and cardiovascular outcomes.
Large AAA diameter has been independently associated with increased risk of perioperative cardiovascular complications (32) and 3-year cardiovascular death (2, 33) post-endoluminal aneurysm repair. A similar association is found in patients would have small AAAs treated conservatively (2, 3, 7–9). Five large studies have previously reported that initial abdominal aortic diameter predicts future cardiovascular events (7–9) all-cause mortality (3, 7, 8) and cardiovascular mortality (2, 3, 7) even when not in the aneurysmal range (3). Thus there is convincing data of the association between aortic diameter and cardiovascular events. The reason for this association is however currently unknown.
A recent study by our group found thrombus volume was closely correlated with maximum axial aortic diameter (r=0.74, p<0.0001) in patients with AAA or aortic ectasia (29). This association between AAA diameter and thrombus are in keeping with the findings of previous smaller studies (34, 35). Currently the factors determining thrombus deposition are not know and are not examined by this study. AAA thrombus products have been demonstrated within the systemic circulation and have potential to influence pathways implicated in athero-thrombosis (24–28). We therefore postulated that AAA thrombus products could be one of a number of causal links between aortic diameter and cardiovascular events. Our findings support this hypothesis and suggest the association between AAA thrombus and outcome warrants further assessment in larger studies.
Initial AAA diameter is recognized as the most powerful and consistent predictor of AAA growth (4, 5, 11, 13, 36–41). Two previous studies have examined the association between AAA thrombus and growth (18, 19). Wolf et al. reviewed CT scans of 80 patients who underwent repeat CT scans greater than 6 months apart (19). On the section with the largest cross sectional diameter the researchers measured maximal diameter, aneurysm area, lumen area, thrombus thickness and arc of aortic wall covered by thrombus using hardcopy images. From these measurements they calculated AAA volume, thrombus volume and thrombus percentage. The authors reported that large thrombus arc, thrombus percent and thrombus area were highly significantly associated with increased AAA expansion (p<0.001 in all variables). There was no report of the reproducibility of the measurements techniques used and the association was not adjusted for initial AAA diameter. A recent longitudinal, prospective study (18) examined 195 AAA patients (40–49mm) with 2 CT scans greater than 6 months apart. The authors assessed continuous (increased growth rates) versus discontinuous growth. Aneurysms with no thrombus or concentric thrombus were associated exclusively with discontinuous and slower growth rates (p=0.05). These two previous studies along with the current investigation provide support for a role of thrombus in AAA progression.
AAA thrombus has been demonstrated to contain large numbers of polymorphonuclear leukocytes and high concentrations of matrix metalloproteinases, elastase and plasmin (16, 17, 42). Elastase has been suggested to inhibit mesenchymal cells binding to fibrin and thereby impair repair of aortic injury (43). Plasmin activation of matrix metalloproteinases at the thrombus and aortic wall interface has been postulated to enhance proteolysis (16). These mechanisms provide a potential link between AAA thrombus and progression. In keeping with these findings a recent study reported an association between reduced small AAA progression and aspirin prescription and two pre-clinical studies have suggested that anti-platelet interventions inhibit AAA progression (44–46).
In our primary assessment of risk factors for cardiovascular events we did not censor patients at the time of AAA repair since these events are still common after surgery. In a secondary analysis we censored patients who required AAA repair at the time of surgery. This analysis showed similar findings to our primary assessment except that the association of thrombus volume with cardiovascular events adjusted for other risk factors was only of borderline significance.
The current study has a number of limitations. Firstly, small sample size made analysis difficult. Interaction terms were not able to be employed to assess the relationship between thrombus and AAA diameter in relation to cardiovascular events. The results may be confounded by the relationship between thrombus and AAA diameter, in addition to other variables. A larger study is needed to adequately assess this aspect before any conclusions can be reached. Secondly, follow up periods were short. Larger studies with longer term follow-up are required to confirm the associations we report. Thirdly, a large portion of patients in the high thrombus group had an AAA repair, potentially confounding results. Fourthly, volumetric analysis of thrombus may have certain limitations. As the length of the infra-renal aorta varies between individuals this method may overestimate the thrombus burden in patients with greater length. However, as volumetric analysis assesses thrombus quantity this should provide an estimation of the thrombus available for potential thrombus-derived product production and release into the circulation. Finally the findings of this study are based on patients with AAA who underwent CT assessment only. Whether the findings can be related to all small AAAs remains to be established.
In conclusion this is the first study to demonstrate an association between AAA thrombus volume and subsequent cardiovascular events. This association could reflect cardiovascular risk factors we have not been able to adjust for in this study or may result from the systemic effects of circulating thrombus products. Further studies are required to assess the clinical importance of the association.
Footnotes
Financial disclosure and conflict of interest
Funding from the National Health and Medical Research Council (540404) Australia supported this work. JG holds a Practitioner Fellowships from the National Health and Medical Research Council, Australia (431503).
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Norman PE, Jamrozik K, Lawrence-Brown MM, et al. Population based randomised controlled trial on impact of screening on mortality from abdominal aortic aneurysm. BMJ. 2004 November 27;329(7477):1259. doi: 10.1136/bmj.38272.478438.55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Brady AR, Fowkes FGR, Thompson SG, et al. Aortic Aneurysm Diameter and Risk of Cardiovascular Mortality. Arterioscler Thromb Vasc Biol. 2001 July 1;21(7):1203–7. doi: 10.1161/hq0701.091999. [DOI] [PubMed] [Google Scholar]
- 3.Norman P, Le M, Pearce C, et al. Infrarenal Aortic Diameter Predicts All-Cause Mortality. Arterioscler Thromb Vasc Biol. 2004 July 1;24(7):1278–82. doi: 10.1161/01.ATV.0000131261.12051.7f. [DOI] [PubMed] [Google Scholar]
- 4.Brady AR, Thompson SG, Fowkes FGR, et al. Abdominal Aortic Aneurysm Expansion: Risk Factors and Time Intervals for Surveillance. Circulation. 2004 July 6;110(1):16–21. doi: 10.1161/01.CIR.0000133279.07468.9F. [DOI] [PubMed] [Google Scholar]
- 5.Vega de Ceniga M, Gomez R, Estallo L, et al. Growth Rate and Associated Factors in Small Abdominal Aortic Aneurysms. European Journal of Vascular and Endovascular Surgery. 2006;31(3):231–6. doi: 10.1016/j.ejvs.2005.10.007. [DOI] [PubMed] [Google Scholar]
- 6.Lederle FA, Wilson SE, Johnson GR, et al. Immediate Repair Compared with Surveillance of Small Abdominal Aortic Aneurysms. N Engl J Med. 2002 May 9;346(19):1437–44. doi: 10.1056/NEJMoa012573. [DOI] [PubMed] [Google Scholar]
- 7.Newman AB, Arnold AM, Burke GL, et al. Cardiovascular disease and mortality in older adults with small abdominal aortic aneurysms detected by ultrasonography: the cardiovascular health study. Annals of Internal Medicine. 2001;134(3):182–90. doi: 10.7326/0003-4819-134-3-200102060-00008. [DOI] [PubMed] [Google Scholar]
- 8.Freiberg MS, Arnold AM, Newman AB, et al. Abdominal aortic aneurysms, increasing infrarenal aortic diameter, and risk of total mortality and incident cardiovascular disease events: 10-year follow-up data from the Cardiovascular Health Study. Circulation. 2008;117(8):1010–7. doi: 10.1161/CIRCULATIONAHA.107.720219. [DOI] [PubMed] [Google Scholar]
- 9.Galland R, Whiteley M, Magee T. The fate of patients undergoing surveillance of small abdominal aortic aneurysms. European Journal of Vascular and Endovascular Surgery. 1998;16(2):104–9. doi: 10.1016/s1078-5884(98)80150-0. [DOI] [PubMed] [Google Scholar]
- 10.Brown PM, Pattenden R, Gutelius JR. The selective management of small abdominal aortic aneurysms: the Kingston study. Journal of Vascular Surgery. 1992;15(1):21–5. doi: 10.1067/mva.1992.33840. [DOI] [PubMed] [Google Scholar]
- 11.Santilli SM, Littooy FN, Cambria RA, et al. Expansion rates and outcomes for the 3.0-cm to the 3.9-cm infrarenal abdominal aortic aneurysm. Journal of Vascular Surgery. 2002;35(4):666–71. doi: 10.1067/mva.2002.121572. [DOI] [PubMed] [Google Scholar]
- 12.Norman P, Spencer CA, Lawrence-Brown MM, et al. C-reactive protein levels and the expansion of screen-detected abdominal aortic aneurysms in men. Circulation. 2004;110(7):862–6. doi: 10.1161/01.CIR.0000138746.14425.00. [DOI] [PubMed] [Google Scholar]
- 13.Stonebridge P, Draper T, Kelman J. Growth rate of infrarenal aortic aneurysms. European Journal of Vascular and Endovascular Surgery. 1996;11(1):70–3. doi: 10.1016/s1078-5884(96)80137-7. [DOI] [PubMed] [Google Scholar]
- 14.Schouten O, van Laanen JH, Boersma E, et al. Statins are associated with a reduced infrarenal abdominal aortic aneurysm growth. European Journal of Vascular & Endovascular Surgery. 2006;32(1):21–6. doi: 10.1016/j.ejvs.2005.12.024. [DOI] [PubMed] [Google Scholar]
- 15.Ouriel K, Donayre C, Shortell CK, et al. The hemodynamics of thrombus formation in arteries. Journal of Vascular Surgery. 1991;14(6):757–62. doi: 10.1067/mva.1991.33157. [DOI] [PubMed] [Google Scholar]
- 16.Carrell TW, Burnand KG, Booth NA, et al. Intraluminal thrombus enhances proteolysis in abdominal aortic aneurysms. Vascular. 2006;14(1):9–16. doi: 10.2310/6670.2006.00008. [DOI] [PubMed] [Google Scholar]
- 17.Panek B, Gacko M, Palka J. Metalloproteinases, insulin-like growth factor-I and its binding proteins in aortic aneurysm. International Journal of Experimental Pathology. 2004;85(3):159–64. doi: 10.1111/j.0959-9673.2004.00386.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Vega de Ceniga M, Gomez R, Estallo L, et al. Analysis of Expansion Patterns in 4-4.9 cm Abdominal Aortic Aneurysms. Annals of Vascular Surgery. 2008;22(1):37–44. doi: 10.1016/j.avsg.2007.07.036. [DOI] [PubMed] [Google Scholar]
- 19.Wolf YG, Thomas WS, Brennan FJ, et al. Computed tomography scanning findings associated with rapid expansion of abdominal aortic aneurysms. Journal of Vascular Surgery. 1994;20(4):529–35. doi: 10.1016/0741-5214(94)90277-1. [DOI] [PubMed] [Google Scholar]
- 20.Satta J, Laara E, Juvonen T. Intraluminal thrombus predicts rupture of an abdominal aortic aneurysm. Journal of Vascular Surgery. 1996;23(4):737–9. doi: 10.1016/s0741-5214(96)80062-0. [DOI] [PubMed] [Google Scholar]
- 21.Hans SS, Jareunpoon O, Balasubramaniam M, et al. Size and location of thrombus in intact and ruptured abdominal aortic aneurysms. Journal of Vascular Surgery. 2005;41(4):584–8. doi: 10.1016/j.jvs.2005.01.004. [DOI] [PubMed] [Google Scholar]
- 22.Houard X, Rouzet F, Touat Z, et al. Topology of the fibrinolytic system within the mural thrombus of human abdominal aortic aneurysms. The Journal of Pathology. 2007;212(1):20–8. doi: 10.1002/path.2148. [DOI] [PubMed] [Google Scholar]
- 23.Houard X, Ollivier V, Louedec L, et al. Differential inflammatory activity across human abdominal aortic aneurysms reveals neutrophil-derived leukotriene B4 as a major chemotactic factor released from the intraluminal thrombus. FASEB J. 2009 May;23(5):1376–83. doi: 10.1096/fj.08-116202. [DOI] [PubMed] [Google Scholar]
- 24.Takagi H, Manabe H, Kawai N, et al. Plasma Fibrinogen and D-dimer Concentrations are Associated with the Presence of Abdominal Aortic Aneurysm: A Systematic Review and Meta-analysis. European Journal of Vascular and Endovascular Surgery. 2009;38(3):273–7. doi: 10.1016/j.ejvs.2009.05.013. [DOI] [PubMed] [Google Scholar]
- 25.Parry D, Al-Barjas H, Chappell L, et al. Haemostatic and fibrinolytic factors in men with a small abdominal aortic aneurysm. British Journal of Surgery. 2009;96(8):870–7. doi: 10.1002/bjs.6632. [DOI] [PubMed] [Google Scholar]
- 26.Danesh J, Whincup P, Walker M, et al. Fibrin D-Dimer and Coronary Heart Disease: Prospective Study and Meta-Analysis. Circulation. 2001 May 15;103(19):2323–7. doi: 10.1161/01.cir.103.19.2323. [DOI] [PubMed] [Google Scholar]
- 27.Morange PE, Bickel C, Nicaud V, et al. Haemostatic Factors and the Risk of Cardiovascular Death in Patients With Coronary Artery Disease: The AtheroGene Study. Arterioscler Thromb Vasc Biol. 2006 December 1;26(12):2793–9. doi: 10.1161/01.ATV.0000249406.92992.0d. [DOI] [PubMed] [Google Scholar]
- 28.Smith A, Patterson C, Yarnell J, et al. Which Hemostatic Markers Add to the Predictive Value of Conventional Risk Factors for Coronary Heart Disease and Ischemic Stroke? The Caerphilly Study Circulation. 2005 November 15;112(20):3080–7. doi: 10.1161/CIRCULATIONAHA.105.557132. [DOI] [PubMed] [Google Scholar]
- 29.Golledge J, Wolanski P, Parr A, et al. Measurement and determinants of infrarenal aortic thrombus volume. European Radiology. 2008;18(9):1987–94. doi: 10.1007/s00330-008-0956-3. [DOI] [PubMed] [Google Scholar]
- 30.Singh K, Jacobsen B, Solberg S, Kumar S, Arnesen E. The difference between ultrasound and computed tomography measurements of aortic diameter increases with aortic diameter: analysis of axial images of abdomial aortic and common iliac artery diameter in normal and aneurysmal aortas. The Tromso Study, 1994–1995. Eur J Vasc Endovasc Surg. 2004 Aug;28(2):158–67. doi: 10.1016/j.ejvs.2004.03.018. [DOI] [PubMed] [Google Scholar]
- 31.Parr A, Jayaratne C, Buttner P, Golledge J. Comparison of volume and diameter measurement in assessing small abdominal aortic aneurysm expansion examined using computed tomographic angiography. Euro J Radiol. 2010 Mar; doi: 10.1016/j.ejrad.2009.12.018. corrected proof. [DOI] [PubMed] [Google Scholar]
- 32.Schouten O, Kok N, Hoedt M, et al. The influence of aneurysm size on preioperative cardiac outcome in elective open infrarenal aortic aneurysm repair. Journal of Vascular Surgery. 2006;44(3):435–41. doi: 10.1016/j.jvs.2006.05.020. [DOI] [PubMed] [Google Scholar]
- 33.Koskas F, Kieffer E. Long-term survival after elective repair of infrarenal abdominal aortic aneurysm: results of a prospective multicentric study. Association for Academic Research in Vascular Surgery (AURC) Annals of Vascular Surgery. 1997;11(5):473–81. doi: 10.1007/s100169900078. [DOI] [PubMed] [Google Scholar]
- 34.Roberts WC, Ko JM, Pearl GJ. Relation of Weights of Intraaneurysmal Thrombi to Maximal Right-to-Left Diameters of Abdominal Aortic Aneurysms. The American Journal of Cardiology. 2006;98(11):1519–24. doi: 10.1016/j.amjcard.2006.06.057. [DOI] [PubMed] [Google Scholar]
- 35.Hans SS, Jareunpoon O, Huang R, et al. Relationship of residual intraluminal to intrathrombotic pressure in a closed aneurysmal sac. Journal of Vascular Surgery. 2003;37(5):949–53. doi: 10.1067/mva.2003.256. [DOI] [PubMed] [Google Scholar]
- 36.Solberg S, Singh K, Wilsgaard T, et al. Increased Growth Rate of Abdominal Aortic Aneurysms in Women. The Tromso Study European Journal of Vascular and Endovascular Surgery. 2005;29(2):145–9. doi: 10.1016/j.ejvs.2004.11.015. [DOI] [PubMed] [Google Scholar]
- 37.Watson C, Walton J, Shaw E, et al. What is the long-term outcome for patients with very small abdominal aortic aneurysms? European Journal of Vascular and Endovascular Surgery. 1997;14(4):299–304. doi: 10.1016/s1078-5884(97)80242-0. [DOI] [PubMed] [Google Scholar]
- 38.Englund R, Hudson P, Hanel K, et al. Expansion rates of small abdominal aortic aneurysms. Australian and New Zealand Journal of Surgery. 1998;68(1):21–4. doi: 10.1111/j.1445-2197.1998.tb04630.x. [DOI] [PubMed] [Google Scholar]
- 39.Nevitt M, Ballard D, Hallett J. Prognosis of abdominal aortic aneurysms. A population-based study. New England Journal of Medicne. 1989;321(15):1009–14. doi: 10.1056/NEJM198910123211504. [DOI] [PubMed] [Google Scholar]
- 40.Vardulaki K, Prevost T, Walker N, et al. Growth rates and risk of rupture of abdominal aortic aneurysms. British Journal of Surgery. 1998;85(12):1674–80. doi: 10.1046/j.1365-2168.1998.00946.x. [DOI] [PubMed] [Google Scholar]
- 41.Guirguis E, Barber G. The natural history of abdominal aortic aneurysms. American journal of surgery. 1991;162(5):481–3. doi: 10.1016/0002-9610(91)90266-g. [DOI] [PubMed] [Google Scholar]
- 42.Fontaine V, Jacob MP, Houard X, et al. Involvement of the mural thrombus as a site of protease release and activat ion in human aortic aneurysms. American Journal of Pathology. 2002;161(5):1701–10. doi: 10.1016/S0002-9440(10)64447-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Fontaine V, Touat Z, Mtairag EM, et al. Role of Leukocyte Elastase in Preventing Cellular ReColonization of the Mural Thrombus. Am J Pathol. 2004 June 1;164(6):2077–87. doi: 10.1016/s0002-9440(10)63766-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Dai J, Louedec L, Philippe M, et al. Effect of blocking platelet activation with AZD6140 on development of abdominal aortic aneurysm in a rat aneurysmal model. Journal of Vascular Surgery. 2009;49(3):719–27. doi: 10.1016/j.jvs.2008.09.057. [DOI] [PubMed] [Google Scholar]
- 45.Lindholt JS, Sorensen HT, Michel JB, Thomsen HF, Henneberg EW. Low-dose aspirin may prevent growth and later surgical repair of medium-sized abdominal aortic aneurysms. Vasc Endovascular Surg. 2008 Aug–Sep;42(4):329–34. doi: 10.1177/1538574408315205. [DOI] [PubMed] [Google Scholar]
- 46.Touat Z, Ollivier V, Dai J, Huisse MG, Bezeaud A, Sebbag U, Palombi T, Rossignol P, Meilhac O, Guillin MC, Michel JB. Renewal of mural thrombus releases plasma markers and is involved in aortic abdominal aneurysm evolution. Am J Pathol. 2006 Mar;168(3):1022–30. doi: 10.2353/ajpath.2006.050868. [DOI] [PMC free article] [PubMed] [Google Scholar]