Abstract
Purpose
Adequate velopharyngeal control is essential for speech, but may be impaired in Parkinson’s disease (PD). Bilateral subthalamic nucleus deep brain stimulation (STN DBS) improves limb function in PD, but the effects on velopharyngeal control remain unknown. We tested whether STN DBS would change aerodynamic measures of velopharyngeal control, and whether these changes were correlated with limb function and stimulation settings.
Methods
Seventeen PD participants with bilateral STN DBS were tested within a morning session after a minimum of 12 h since their most recent dose of anti-PD medication. Testing occurred when STN DBS was on, and again 1 h after STN DBS was turned off, and included aerodynamic measures during syllable production, and standard neurological ratings of limb function.
Results
We found that PD participants exhibited changes with STN DBS, primarily consistent with increased intraoral pressure (n = 7) and increased velopharyngeal closure (n = 5). These changes were modestly correlated with measures of limb function, and were correlated with stimulation frequency.
Conclusion
Our findings suggest that STN DBS may change velopharyngeal control during syllable production in PD, with greater benefit associated with low frequency stimulation. However, DBS demonstrates a more subtle influence on speech-related velopharyngeal control than limb motor control. This distinction and its underlying mechanisms are important to consider when assessing the impact of STN DBS on PD.
Keywords: Aerodynamic, Air flow, Air pressure, Frequency, Speech, Velopharyngeal area
1. Background
Parkinson’s disease (PD) affects more than 1 million individuals in the United States, resulting in impairment of limb motor control, including deficits in force recruitment, velocity, and acceleration of limb movements (DeLong, 1990, 2000; Desmurget et al., 2004; Pfann, Buchman, Comella, & Corcos, 2001; Pfann et al., 2004; Robichaud, Pfann, Vaillancourt, Comella, & Corcos, 2005). Most individuals with PD will develop impairment of speech motor control, including abnormal respiratory, laryngeal, and supralaryngeal control for breathing and speech (Baker, Ramig, Luschei, & Smith, 1998; Barlow, Iacono, Paseman, Biswas, & D’Antonio, 1998; Gallena, Smith, Zeffiro, & Ludlow, 2001; Hunker, Abbs, & Barlow, 1982; Luschei, Ramig, Baker, & Smith, 1999). The Unified Parkinson’s Disease Rating Scale (UPDRS, Fahn & Elton, 1987) is a commonly employed assessment tool for PD that correlates well with more specific measures of limb motor function (Robichaud et al., 2005; Vaillancourt et al., 2006). However, the UPDRS is primarily a measure of limb-related function and only includes two speech-related items that correlate weakly with specific measures of speech function (Richards, Marder, Cote, & Mayeaux, 1994). Therefore, in assessing disease severity and the potential influence of intervention on PD, it would be valuable to efficiently sample and index specific speech-related physiology.
Adequate intraoral air pressure is essential for speech, and is often substantially decreased in PD (Bunton, 2005; Solomon & Hixon, 1993; Tamaki, Matsuo, Yanagihara, & Abe, 2000; Tzelepis, McCool, Friedman, & Hoppin, 1988). Velopharyngeal function may be less commonly recognized as a problem in PD. However, several reports described significant velopharyngeal impairment in PD including decreased velopharyngeal closure and increased transnasal air flow (Darley, Aronson, & Brown, 1975; Hirose, Kiritani, Ushijima, Yoshioka, & Sawashima, 1981; Hoodin & Gilbert, 1989a, 1989b; Logemann, Fisher, Boshes, & Blonsky, 1978; Robbins, Logemann, & Kirschner, 1986). Intraoral air pressure deficits and velopharyngeal impairments in PD may contribute to reduced speech audibility, linguistic confusion, and communicative impairment. Because these cranial systems are negatively impacted by PD, it is important to examine their response to interventions such as deep brain stimulation (DBS). However, the effects of DBS on intraoral pressure and velopharyngeal control are unknown.
In order to achieve appropriate intraoral pressure and oral resonance during production of a simple syllable such as 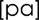 , air flow should be directed through the mouth, and air flow through the nasal cavity should be negligible. Conservative estimates suggest that adequate approximation of the soft palate (velum) with the lateral and posterior pharyngeal wall should result in a velopharyngeal orifice area of less than 5 mm2, and nasal air flow of less than 125 cc/s (Baken & Orlikoff, 2000; Dalston & Warren, 1986; Dalston, Warren & Smith, 1990; Thompson & Hixon, 1979;Warren, 1964, 1967a, 1967b;Warren & DuBois, 1964; Zajac & Mayo, 1996). Air pressure during speech may be assessed directly through a polyethylene tube in the oral cavity during simple speech production such as in the syllable
, air flow should be directed through the mouth, and air flow through the nasal cavity should be negligible. Conservative estimates suggest that adequate approximation of the soft palate (velum) with the lateral and posterior pharyngeal wall should result in a velopharyngeal orifice area of less than 5 mm2, and nasal air flow of less than 125 cc/s (Baken & Orlikoff, 2000; Dalston & Warren, 1986; Dalston, Warren & Smith, 1990; Thompson & Hixon, 1979;Warren, 1964, 1967a, 1967b;Warren & DuBois, 1964; Zajac & Mayo, 1996). Air pressure during speech may be assessed directly through a polyethylene tube in the oral cavity during simple speech production such as in the syllable 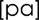 (Smitheran & Hixon, 1981). Air flow during speech may be measured directly through a nasal mask placed over the nose during syllable production. The nasal air flow signal provides information regarding velopharyngeal closure. Intraoral pressure and nasal air flow can be used together to estimate velopharyngeal area (Lotz, Shaughnessy, & Netsell, 1981; Netsell, Lotz, & Shaughnessy, 1982;Warren & DuBois, 1964). Therefore, measuring air pressure and air flow during syllable production provides a simple, non-invasive assessment to examine changes in velopharyngeal control with DBS. However, the degree to which these aerodynamic measures of velopharyngeal function are affected by STN DBS in PD is unknown.
(Smitheran & Hixon, 1981). Air flow during speech may be measured directly through a nasal mask placed over the nose during syllable production. The nasal air flow signal provides information regarding velopharyngeal closure. Intraoral pressure and nasal air flow can be used together to estimate velopharyngeal area (Lotz, Shaughnessy, & Netsell, 1981; Netsell, Lotz, & Shaughnessy, 1982;Warren & DuBois, 1964). Therefore, measuring air pressure and air flow during syllable production provides a simple, non-invasive assessment to examine changes in velopharyngeal control with DBS. However, the degree to which these aerodynamic measures of velopharyngeal function are affected by STN DBS in PD is unknown.
During the past decade, deep brain stimulation (DBS) of the subthalamic nucleus (STN) has rapidly emerged as a treatment for advanced idiopathic Parkinson’s disease (PD) (Breit, Schulz, & Benabid, 2004; Volkmann, 2004;Weaver et al., 2009). STN DBS involves stereotactic neurosurgical placement (typically bilateral) of a quadripolar stimulating electrode into the sensorimotor region of the subthalamic nucleus, and surgical placement of an implantable pulse generator (IPG) subcutaneously in the pectoral region of the chest. Following surgery, the clinician may program the IPG to select the stimulation frequency, pulse width, and voltage, and the combination of active contacts on the quadripolar electrode. The primary goal of STN DBS is to achieve optimal clinical benefit for limb-related function, as typically evaluated using the UPDRS. DBS has been associated with marked improvements in limb motor function and overall quality of life (Vaillancourt et al., 2006; Vaillancourt, Prodoehl, Metman, Bakay, & Crocos, 2004). However, earlier speech-related studies of STN DBS revealed different outcomes (D’Alatri et al., 2008; Dromey, Kumar, Lang, & Lozano, 2000; Farrell, Theodoros, Ward, Hall, & Silburn, 2005; Gentil, Chauvin, Pinto, Pollak, & Benabid, 2001; Gentil, Garcia-Ruiz, Pollak, & Benabid, 1999; Hoffman-Ruddy, Schulz, Vitek, & Evatt, 2001; Klostermann et al., 2008; Pinto, Gentil, Fraix, Benabid, & Pollak, 2003; Pinto et al., 2004, 2005;Rousseaux et al., 2004; Santens, De Letter, Van Borsel, De Reuck, & Caemaert, 2003; Wang, Metman, Bakay, Arzbaecher, & Bernard, 2003; Wang et al., 2006) (see reviews: Barlow & Hammer, 2009; Jones, Kendall, Sudhyadhom, & Rosenbek, 2007; Schulz & Grant, 2000;Trail et al., 2005). For example, some groups reported significant improvements in speech following bilateral STN DBS (Gentil et al., 2001), some reported modest improvements that did not reach functional significance (Dromey et al., 2000; Farrell et al., 2005), and others reported significant degradation of speech function (Klostermann et al., 2008). Non-speech studies demonstrated improvements in force recruitment and precision of upper lip, lower lip, and tongue movement in PD participants with STN DBS(Gentil et al., 1999; Pinto et al., 2003, 2004). Extended improvements up to 5 years following the initiation of STN DBS were described for isometric lip and tongue force control, but were also accompanied by increasingly severe dysarthria (Pinto et al., 2003).
The reasons for the different responses of speech, non-speech, and limb function to STN DBS may be due to the different functional requirements. High frequency STN DBS is optimized for limb-related function, as typically determined by the UPDRS, and the localization and programming of the DBS electrodes are optimized for limb-related somatotopy. However, improving the ability to generate larger magnitudes of force, as reported in the limb and orofacial force studies, may be of less benefit for the complex, low-level forces that are required for speech and voice (Barlow & Muller, 1991; Bunton & Weismer, 1994; Montgomery, 2007; Tornqvist, Schalen, & Rehncrona, 2005). Moreover, these differences are further reflected by the observation that speech may benefit more from low frequency stimulation than high frequency stimulation (Montgomery, 2007; Tornqvist et al., 2005). However, no studies known to these authors have examined changes in aerodynamic measures of speech-related velopharyngeal control in PD with STN DBS, or whether these changes may be associated with stimulation parameters (e.g., frequency, pulse width, voltage) or measures of limb function (e.g., UPDRS).
The first objective of this study was to examine the effects of bilateral STN DBS on aerodynamic measures of velopharyngeal control in PD participants. Based on the heterogeneity of participant responses reported in previous studies, we expected to observe individual increases and decreases for each of the three aerodynamic measures with DBS. However, we also wanted to examine if the heterogeneity of responses might be related to DBS simulation parameters, as suggested by earlier reports (Montgomery, 2007; Tornqvist et al., 2005), and if speech-related velopharyngeal control was related to limb function. Accordingly, our second objective was to test whether there was a significant association between stimulation parameters (e.g., frequency, pulse width, voltage) and standard neurological ratings of limb function (e.g., UPDRS) with changes in the aerodynamic measures of velopharyngeal function.
2. Methods
2.1. Participants
This investigation was conducted in accordance with NIH regulations for the ethical treatment of human subjects. The protocol in this investigation was approved by the local institutional ethics committee for the safety of human subjects. Participants were informed of the general purposes of the study and written informed consent was obtained prior to enrolling any participants in the study. Data were collected from 17 individuals (14 men and 3 women) with advanced idiopathic PD, an average of 12 years since diagnosis, a minimum of 3 months since bilateral STN DBS surgery (Table 1), with a mean age of 59 years. Each individual also participated in a separate experiment focused on respiratory and laryngeal control (Hammer, Barlow, Lyons, & Pahwa, 2010).
Table 1.
Individual neurological measures organized by participant. Unified Parkinson’s Disease Rating Scale (UPDRS) UPDRSMOTOR: 0 = no symptoms; 108 = maximum score. Hoehn and Yahr (H&Y) Staging: 1 = mild; 5 = maximum score. Schwab and England (S&E) Activities of Daily Living Scale: 100 = completely independent.
| Participant | Sex | Age | Months | Speech item only | PD motor measures | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Post Surgery | UPDRS ADL | UPDRS MOTOR | UPDRS MOTOR | H&Y | S&E | ||||||||
| Pre | Post | Pre | Post | Pre | Post | Pre | Post | Pre | Post | ||||
| 1 | M | 76 | 4 | 2 | 2 | 2 | 2 | 25 | 24 | 2 | 2 | 80 | 80 |
| 2 | F | 38 | 32 | 2 | 1 | 2 | 2 | 39 | 48 | 3 | 4 | 60 | 60 |
| 3 | M | 64 | 6 | 2 | 1 | 2 | 1 | 42 | 13 | 3 | 3 | 60 | 90 |
| 4 | M | 55 | 24 | 2 | 2 | 2 | 2 | 44 | 35 | 4 | 3 | 60 | 90 |
| 5 | F | 73 | 9 | 2 | 3 | 2 | 3 | 49 | 38 | 3 | 4 | 80 | 80 |
| 6 | M | 55 | 3 | 1 | 1 | 2 | 1 | 30 | 14 | 2 | 2 | 70 | 70 |
| 7 | M | 68 | 4 | 2 | 2 | 2 | 2 | 42 | 22 | 3 | 2 | 60 | 90 |
| 8 | M | 48 | 25 | 2 | 3 | 2 | 2 | 38 | 32 | 3 | 2 | 80 | 90 |
| 9 | M | 74 | 13 | 2 | 3 | 2 | 2 | 34 | 24 | 3 | 2 | 70 | 70 |
| 10 | M | 44 | 27 | 3 | 2 | 2 | 1 | 28 | 28 | 3 | 2 | 60 | 90 |
| 11 | M | 75 | 3 | 3 | 2 | 2 | 2 | 43 | 27 | 3 | 2 | 70 | 80 |
| 12 | F | 68 | 22 | 2 | 2 | 2 | 1 | 49 | 19 | 3 | 2 | 80 | 90 |
| 13 | M | 36 | 3 | 3 | 2 | 2 | 2 | 46 | 30 | 3 | 2 | 70 | 90 |
| 14 | M | 72 | 3 | 2 | 2 | 2 | 1 | 49 | 7 | 2 | 2 | 70 | 100 |
| 15 | M | 65 | 6 | 2 | 2 | 2 | 2 | 45 | 15 | 3 | 3 | 70 | 80 |
| 16 | M | 42 | 6 | 4 | 3 | 4 | 3 | 36 | 20 | 2 | 2 | 70 | 90 |
| 17 | M | 53 | 12 | 2 | 1 | 2 | 1 | 43 | 17 | 3 | 2 | 70 | 90 |
ADL SPEECH ITEM: 0 = normal; 1 = mildly affected, no difficulty being understood; 2 = moderately affected, sometimes asked to repeat statements; 3 = severely affected, frequently asked to repeat statements; 4 = Unintelligible most of the time. UPDRS MOTOR SPEECH ITEM: 0 = normal; 1 = slight loss of expression, diction and/or volume; 2 = monotone, slurred but understandable, moderately impaired; 3 = marked impairment, difficult to understand; 4 = unintelligible. Scores are provided prior to surgical placement (Pre) and following activation of the bilateral STN DBS (post). Individuals were tested a minimum of 12 h since their last dose of anti-PD medication.
2.2. Background testing
Testing was coordinated with the participant’s scheduled neurological evaluation. Each participant was tested during a single morning session a minimum of 12 h since their most recent dose of anti-PD medication. During the scheduled evaluation, each participant underwent post-operative neurological assessment (DBS ON only) by a movement disorder specialist (R.P.) including the motor scale of the UPDRS, Hoehn and Yahr (H&Y) staging, and the Schwab and England Activities of Daily Living Scale (S&E) (Fahn & Elton, 1987; Hoehn & Yahr, 1967; Schwab & England, 1968). This post-operative neurological assessment (DBS ON, no medication) was compared with the most recent pre-operative assessment (no medication). Participants exhibited significant pre- vs. post-operative improvement in standard neurological ratings of limb function with STN DBS (UPDRS, t = −5.05, p < .001; H&Y, t = −2.38, p < .04; S&E, t = 4.93, p < .001). Individual neurological assessment scores are in Table 1. DBS stimulation parameters (e.g., frequency, pulse width, voltage) are provided in Table 2
Table 2.
Individual deep brain stimulation (DBS) parameters and settings for each participant including stimulation frequency (Hz), voltage (V), and pulse width (µs) for left and right internalized pulse generator (IPG), and electrode configuration used for DBS.
| Participant | Frequency (Hz) | Voltage (V) | Pulse width (µs) | Electrode configuration (“0” = off, “+” = anode, “−” = cathode) |
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Left active contacts | Right active contacts | |||||||||||||||
| Left IPG | Right IPG | Left IPG | Right IPG | Left IPG | Right IPG | 0 | 1 | 2 | 3 | IPG case | 0 | 1 | 2 | 3 | IPG case | |
| 1 | 145 | 145 | 2.8 | 2.9 | 60 | 60 | 0 | − | − | 0 | + | 0 | 0 | − | − | + |
| 2 | 145 | 145 | 1.5 | 1.8 | 60 | 60 | 0 | 0 | 0 | − | + | 0 | 0 | 0 | − | + |
| 3 | 145 | 145 | 2.4 | 2.3 | 60 | 60 | 0 | − | 0 | 0 | + | 0 | 0 | − | 0 | + |
| 4 | 170 | 160 | 3.7 | 3.9 | 90 | 90 | + | 0 | 0 | − | 0 | 0 | + | 0 | − | 0 |
| 5 | 170 | 170 | 3 | 3.6 | 90 | 90 | 0 | 0 | − | − | + | 0 | 0 | − | − | + |
| 6 | 145 | 145 | 3.2 | 1.6 | 60 | 60 | 0 | − | 0 | 0 | + | 0 | − | 0 | 0 | + |
| 7 | 145 | 160 | 2.3 | 2.9 | 60 | 90 | 0 | 0 | − | − | + | 0 | + | 0 | 0 | + |
| 8 | 160 | 185 | 3.2 | 4.1 | 90 | 90 | 0 | 0 | − | − | + | − | − | + | 0 | 0 |
| 9 | 160 | 160 | 3.6 | 2.8 | 60 | 60 | − | + | − | 0 | 0 | 0 | 0 | − | 0 | + |
| 10 | 185 | 185 | 2.8 | 2.4 | 90 | 60 | 0 | − | 0 | 0 | + | 0 | 0 | − | 0 | + |
| 11 | 185 | 170 | 2.9 | 2.3 | 60 | 90 | 0 | − | − | 0 | + | 0 | − | 0 | 0 | + |
| 12 | 145 | 145 | 2.5 | 1.8 | 90 | 90 | 0 | + | − | 0 | 0 | 0 | + | − | 0 | 0 |
| 13 | 145 | 145 | 3.5 | 1.5 | 90 | 60 | + | 0 | − | 0 | 0 | 0 | 0 | − | 0 | + |
| 14 | 160 | 185 | 3 | 4 | 60 | 60 | 0 | 0 | − | 0 | + | 0 | 0 | − | 0 | + |
| 15 | 145 | 160 | 2.1 | 3 | 60 | 60 | 0 | 0 | − | 0 | + | 0 | 0 | − | 0 | + |
| 16 | 145 | 185 | 2.4 | 3.8 | 60 | 90 | 0 | 0 | − | 0 | + | + | 0 | 0 | − | 0 |
| 17 | 170 | 170 | 3.8 | 4.5 | 60 | 90 | 0 | 0 | − | 0 | + | 0 | − | 0 | 0 | + |
After carefully considering our participants and the limited availability for testing during their scheduled post-operative neurological evaluations, we chose to assess velopharyngeal function by sampling intraoral pressure and transnasal airflow with DBS on, and again 1 h after DBS was turned off. We examined whether changes in the standard neurological ratings of limb function (e.g., UPDRS, pre- vs. post-operative) were associated with changes in measures of velopharyngeal function (DBS ON vs. DBS OFF), and whether changes in velopharyngeal function were associated with DBS stimulation parameters (e.g., frequency).
2.3. Air pressure and air flow
Participants were comfortably seated in an exam chair and instructed to repeat the syllable 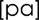 at a rate of 2 syllables per second at a comfortable pitch and loudness. Intraoral air pressure was measured directly using a polyethylene catheter (Intramedic PE 260, 1.77 mm ID, 7 cm length) placed in the mouth near the oral angle and oriented perpendicular to the breath stream during speech (Smitheran & Hixon, 1981). The catheter was coupled to a pressure transducer (Honeywell model 164PC01D37). A 10 cm H2O pressure source (U-tube manometer) was used to calibrate the air pressure transducer. Air flow was measured using a Hans Rudolph nasal respiratory mask (model CR1650) and pneumotachometer (model R4719) instrumented with a pressure transducer (model 163PC01D36). A 500 cc/s air flow source (Glottal Enterprises model MCU-2) was used to calibrate the air flow transducer. Both air pressure and air flow signals were conditioned by bridge amplifiers (Biocommunications Electronics, model 201, LP −3 dB @ 50 Hz, Butterworth 3-pole). Lip closure was carefully monitored for each participant. Only syllables with complete seal of the lips during consonant production were included in analysis. Signals were digitized at 3.3 kHz using a custom designed data acquisition and analysis program to compute intraoral pressure (PO) and nasal air flow (Barlow, Suing, & Andreatta, 1999). We used the pressure and air flow values to compute velopharyngeal (VP) area (Baken & Orlikoff, 2000; Lotz et al., 1981; Netsell et al., 1982; Warren & DuBois, 1964).
at a rate of 2 syllables per second at a comfortable pitch and loudness. Intraoral air pressure was measured directly using a polyethylene catheter (Intramedic PE 260, 1.77 mm ID, 7 cm length) placed in the mouth near the oral angle and oriented perpendicular to the breath stream during speech (Smitheran & Hixon, 1981). The catheter was coupled to a pressure transducer (Honeywell model 164PC01D37). A 10 cm H2O pressure source (U-tube manometer) was used to calibrate the air pressure transducer. Air flow was measured using a Hans Rudolph nasal respiratory mask (model CR1650) and pneumotachometer (model R4719) instrumented with a pressure transducer (model 163PC01D36). A 500 cc/s air flow source (Glottal Enterprises model MCU-2) was used to calibrate the air flow transducer. Both air pressure and air flow signals were conditioned by bridge amplifiers (Biocommunications Electronics, model 201, LP −3 dB @ 50 Hz, Butterworth 3-pole). Lip closure was carefully monitored for each participant. Only syllables with complete seal of the lips during consonant production were included in analysis. Signals were digitized at 3.3 kHz using a custom designed data acquisition and analysis program to compute intraoral pressure (PO) and nasal air flow (Barlow, Suing, & Andreatta, 1999). We used the pressure and air flow values to compute velopharyngeal (VP) area (Baken & Orlikoff, 2000; Lotz et al., 1981; Netsell et al., 1982; Warren & DuBois, 1964).
2.4. Statistical analyses
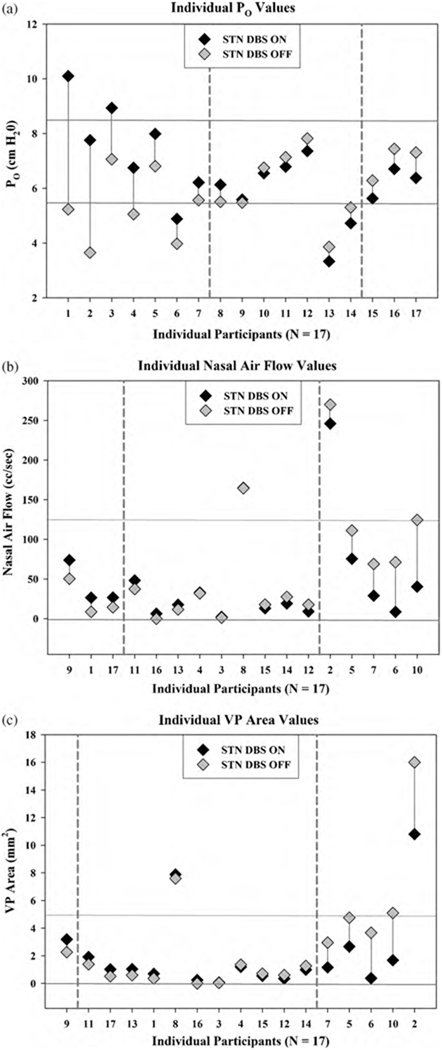
Based on the heterogeneity of participant responses reported in previous studies, we expected to observe individual increases and decreases for each of the three aerodynamic measures with DBS such that traditional group analysis (paired t-tests DBS ON vs. DBS OFF, α = .05/3 measures = .017) would not reflect significant changes. We expected that individual PD participants would exhibit significant absolute changes in each measure, and used the individual change data (Table 3) to compute the group mean magnitude of change and the group standard error for each measure. We then examined whether individuals exhibited an absolute magnitude of change that exceeded 2 times the group standard error, and used this as our criterion for significance (Fig. 1).
Table 3.
Individual mean value, standard error (se), and Δ (ON–OFF) for each speech measure with deep brain stimulation (DBS ON vs. DBS OFF) including intraoral air pressure (PO), nasal air flow, and velopharyngeal (VP) area.
| Participant | Po (cm H20) | Nasal air flow (cc/s) | VP area (mm2) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| MEAN (se) | Δ | Mean (se) | Δ | Mean (se) | Δ | ||||
| DBS ON | DBS OFF | DBS ON | DBS OFF | DBS ON | DBS OFF | ||||
| 1 | 10.10 (0.39) | 5.23 (0.26) | 4.87 | 26.50 (9.80) | 8.69 (2.00) | 17.81 | 0.70 (0.26) | 0.36 (0.09) | 0.34 |
| 2 | 7.76 (0.57) | 3.65 (0.83) | 4.11 | 246.00 (40.00) | 270.00 (47.00) | −24.00 | 10.80 (2.60) | 16.00 (3.70) | −5.20 |
| 3 | 8.94 (0.46) | 7.06 (0.39) | 1.88 | 2.13 (0.80) | 1.22 (0.46) | 0.91 | 0.07 (0.03) | 0.05 (0.02) | 0.02 |
| 4 | 6.75 (0.32) | 5.06 (0.26) | 1.69 | 33.00 (7.80) | 31.90 (18.00) | 1.10 | 1.20 (0.27) | 1.36 (0.78) | −0.16 |
| 5 | 7.99 (0.37) | 6.81 (0.43) | 1.18 | 75.50 (8.90) | 111.30 (15.00) | −35.80 | 2.67 (0.34) | 4.76 (0.94) | −2.09 |
| 6 | 4.88 (0.12) | 3.98 (0.15) | 0.91 | 8.53 (1.70) | 71.30 (8.50) | −62.77 | 0.39 (0.08) | 3.68 (0.50) | −3.30 |
| 7 | 6.21 (0.12) | 5.57 (0.22) | 0.65 | 29.00 (5.00) | 68.90 (5.60) | −39.90 | 1.17 (0.21) | 2.96 (0.29) | −1.79 |
| 8 | 6.13 (0.47) | 5.51 (0.36) | 0.62 | 165.00 (29.00) | 164.40 (16.00) | 0.60 | 7.89 (1.80) | 7.60 (1.20) | 0.29 |
| 9 | 5.59 (0.24) | 5.48 (0.29) | 0.11 | 73.90 (5.80) | 50.40 (4.30) | 23.50 | 3.20 (0.29) | 2.27 (0.22) | 0.93 |
| 10 | 6.55 (0.37) | 6.75 (0.36) | −0.20 | 40.40 (3.90) | 124.60 (12.00) | −84.20 | 1.69 (0.18) | 5.10 (0.59) | −3.41 |
| 11 | 6.79 (0.31) | 7.14 (0.24) | −0.35 | 48.20 (7.00) | 37.40 (11.00) | 10.80 | 1.92 (0.31) | 1.39 (0.40) | 0.53 |
| 12 | 7.36 (0.56) | 7.82 (0.33) | −0.46 | 9.19 (1.10) | 17.60 (7.30) | −8.41 | 0.35 (0.06) | 0.61 (0.26) | −0.26 |
| 13 | 3.33 (0.20) | 3.86 (0.19) | −0.53 | 17.70 (2.80) | 11.65 (2.30) | 6.05 | 1.04 (0.19) | 0.60 (0.12) | 0.44 |
| 14 | 4.73 (0.27) | 5.30 (0.13) | −0.58 | 19.10 (7.30) | 27.50 (4.80) | −8.40 | 0.99 (0.41) | 1.27 (0.25) | −0.28 |
| 15 | 5.63 (0.18) | 6.28 (0.12) | −0.65 | 13.15 (1.80) | 17.80 (2.80) | −4.65 | 0.56 (0.08) | 0.72 (0.12) | −0.16 |
| 16 | 6.71 (0.32) | 7.44 (0.42) | −0.73 | 6.29 (1.6) | 0.00 (0.00) | 6.29 | 0.25 (0.07) | 0.00 (0.00) | 0.25 |
| 17 | 6.38 (0.33) | 7.31 (0.26) | −0.93 | 26.70 (4.70) | 14.40 (2.00) | 12.30 | 1.03 (0.18) | 0.52 (0.07) | 0.51 |
| Group mean, standard error (se), minimum (MIN), and maximum (MAX) magnitude of change for each measure. | |||||||||
| MEAN (se) | 1.20 (0.32) | 20.44 (5.67) | 1.17 (0.36) | ||||||
| MIN | 0.11 | 0.60 | 0.02 | ||||||
| MAX | 4.87 | 84.20 | 5.20 | ||||||
Fig. 1.
Individual values for each participant with DBS ON (black symbols) and DBS OFF (gray symbols). (a) Intraoral pressure (PO), (b) nasal air flow, and (c) velopharyngeal (VP) area. In each graph, dashed vertical reference lines demarcate boundaries between significant increases (left), non-significant changes (middle), and significant decreases (right). Solid horizontal reference lines demarcate a reasonably conservative typical operating range.
Next, we compared our data with typical operating ranges reported in the literature for each of the three aerodynamic measures (Baken & Orlikoff, 2000; Dalston & Warren, 1986; Dalston et al., 1990; Netsell & Hixon, 1978; Smitheran & Hixon, 1981; Thompson & Hixon, 1979; Warren, 1964, 1967a, 1967b; Warren & DuBois, 1964; Zajac & Mayo, 1996). We examined the individual baselines, responses to DBS, and examined whether individual values approached, departed from, or stayed within the typical operating range with DBS (Table 4, Fig. 1).
Table 4.
Direction of change for each speech measure with deep brain stimulation (on minus off) including intraoral air pressure, nasal air flow, and velopharyngeal (VP) area. Total indicates the number and percentage of participants who exhibited a significant change (increase or decrease) with deep brain stimulation. Approach indicates changes toward a typical operating range. Depart indicates changes away from a typical operating range. Stay indicates a change in which the measure was within a typical operating range during both test conditions (on and off).
| Variable | Number of increases | Number of decreases | ||||||
|---|---|---|---|---|---|---|---|---|
| Approach | Depart | Stay | Total | Approach | Depart | Stay | Total | |
| Intraoral pressure (cm H20) | 4* | 1 | 2 | 7 (41%) | 0 | 0 | 3 | 3 (18%) |
| Nasal air flow (cc/s) | – | 0 | 3 | 3 (18%) | 1 | – | 4 | 5 (29%) |
| VP area (mm2) | – | 0 | 1 | 1 (6%) | 2 | – | 3 | 5 (29%) |
Includes approaches that overshot typical range.
To test whether the heterogeneity of speech-related responses was related to DBS simulation parameters (e.g., frequency) and standard neurological ratings of limb function (e.g., UPDRS), we calculated Pearson product moment correlation coefficients (α = .05).
3. Results
Individual values and corresponding change for each aerodynamic measure of velopharyngeal control are shown in Fig. 1, and are summarized in Table 3. As expected, we observed individual increases and decreases for each of the three aerodynamic measures with DBS such that traditional group analysis (paired t-tests DBS ON vs. DBS OFF, all p-values >.05) did not reflect significant changes. However, individual PD participants exhibited changes in each measure (Table 4 and Fig. 1): PO (n = 10), nasal air flow (n = 8), and VP area (n = 6). Of these participants, most exhibited beneficial increases in PO (n = 7), with decreases in nasal airflow (n = 5) and VP area (n = 5) (Table 4). These changes, though relatively modest, were primarily consistent with increased intraoral pressure and increased velopharyngeal closure. In addition, PD participants exhibited distinct individual responses to STN DBS. For example, of the 10 PD participants who exhibited changes in PO with STN DBS, 7 manifested increased PO while 3 exhibited decreased PO.
Next, we compared our data with typical operating ranges reported in the literature for each of the three aerodynamic measures (Fig. 1) (Baken & Orlikoff, 2000; Dalston & Warren, 1986; Dalston et al., 1990; Netsell & Hixon, 1978; Smitheran & Hixon, 1981; Thompson & Hixon, 1979; Warren, 1964, 1967a, 1967b;Warren & DuBois, 1964; Zajac & Mayo, 1996) to examine the individual baselines, responses to DBS, and whether individual values approached, departed from, or stayed within a typical operating range with DBS (Table 4). For example, one PD participant (1) that exhibited PO below the typical range (OFF) approached but overshot the typical range with DBS. Very few PD participants exhibited values beyond the typical range with DBS OFF, and most exhibited values within the typical range for each measure with DBS on. Therefore, most PD participants exhibited modest changes and most exhibited measures within the typical range with STN DBS.
Finally, we examined the degree to which changes in each aerodynamic measure was associated with stimulation parameters (e.g., frequency) and changes in standard neurological ratings of limb function (e.g., UPDRS, H&Y). We found moderate correlations (all p-values <.05) between stimulation frequency of the right STN with PO (r = −.50) and between stimulation frequency of the left STN with VP area (r = .49). We also found that PO was moderately correlated with the UPDRS (r = .60) and H&Y (r = .50), and VP area was moderately correlated with the H&Y (r = −.50) (Pearson product–moment correlation coefficients, all p-values <.05). Therefore, changes in aerodynamic measures of PO and VP area were related to DBS stimulation frequency and modestly paralleled standard neurological ratings of limb function.
4. Discussion
This study presents the first data examining the effects of bilateral STN DBS on velopharyngeal control in PD, and how these effects relate to changes in ratings of limb function (e.g., UPDRS) and DBS stimulation parameters (e.g., frequency). We observed relatively subtle changes in aerodynamic measures of speech velopharyngeal control with STN DBS. As expected, we observed individual increases and decreases for each of the three aerodynamic measures with DBS, consistent with the heterogeneity of speech-related responses described previously. However, most of the changes we observed in these PD participants were generally consistent with increased PO and increased velopharyngeal closure.
We also examined if the heterogeneity of changes in speech-related velopharyngeal control might be related to DBS simulation parameters, as suggested by earlier reports (Montgomery, 2007; Tornqvist et al., 2005) and found that the speech-related responses were related to DBS simulation frequency. We observed a moderate negative correlation between stimulation frequency of the right STN with change in PO and a moderate positive correlation between stimulation frequency of the left STN with change in VP area, indicating that lower frequency stimulation may be associated with greater PO and smaller VP area. These correlations are consistent with previous observations that the complex, fine forces employed during speech may benefit more from low frequency stimulation than high frequency stimulation (Barlow & Muller, 1991; Bunton & Weismer, 1994; Montgomery, 2007; Tornqvist et al., 2005), and further suggest a differential effect of STN DBS for speech-related velopharyngeal control and limb-related control. The oscillatory patterns of the basal ganglia-thalamocortical network that influence speech, including velopharyngeal control, may operate at lower resonant frequencies, and therefore respond more positively to low frequency DBS (Montgomery, 2007). An earlier report also noted a differential effect for pulse width and voltage (Tripoliti et al., 2008). We did not observe this effect in the present study. Finally, the correlations observed in the present study may also confirm previously reported hemispheric effects (Santens et al., 2003;Wang et al., 2003, 2006). It is possible that stimulation of the right STN may have more influence on PO and stimulation of the left STN may exert more influence on VP closure.
Changes in speech-related aerodynamic measures of velopharyngeal function with STN DBS may reflect changes in central scaling of speech-related regulation of intraoral pressure and velopharyngeal control. When we observed improvements, it may be that abnormally altered patterns of neural firing presumed to occur throughout the basal ganglia-thalamocortical network may approximate a more normal pattern of oscillation with DBS. It is also possible that these abnormal patterns may be more amenable to beneficial compensation by remaining healthy brain tissue in the presence of DBS. However, the changes we observed were relatively subtle. In fact, most of the aerodynamic changes were within normal limits. These relatively modest changes may relate to the fact that these participants received high frequency DBS over a fairly narrow range of stimulation frequencies (145–185 Hz). In addition to stimulation frequency, several other factors may account for the modest changes and correlations we observed including variability in localization of the active DBS electrodes, stimulation fields and potential current spread beyond the STN target (e.g., internal capsule), individual variability in somatotopic organization of STN, hemispheric interactions (Santens et al., 2003;Wang et al., 2003, 2006), and differences in pre-treatment speech severity (Dromey et al., 2000; Farrell et al., 2005).
We observed that changes in the speech-related measures modestly paralleled limb-related changes (e.g., UPDRS), indicating that lower levels of PD limb severity may be associated with increased PO and decreased VP area. The modest strength of these correlations is not surprising given that, for example, the UPDRS is weighted almost entirely for limb function, and lacks the detail to accurately and reliably assess the speech-related subsystems (Miller et al., 2007; Miller, Noble, Jones, Allcock, & Burn, 2008; Richards et al., 1994). Because of the limitations of standard neurological ratings of limb-related function to identify change in speech-related function, we would suggest a need to further integrate speech physiology measures within neurological assessments to accurately assess even subtle speech related changes in participants with neurological diseases such as PD. Aerodynamic measures of velopharyngeal control may be useful within a more comprehensive battery of speech-related measures to index potential changes in motor control associated with STN DBS in PD. Clinical neurological assessment scales focus almost exclusively on limb motor function and do not offer specific indices to detect subtle changes in speech-related motor control that may signal the onset and progression of a disease, and its potential response to treatment. Therefore, the development, refinement, and employment of clinically relevant measures of speech-related physiology, including aerodynamic assessment of velopharyngeal control, may be useful during neurological assessment of individuals with PD.
One limitation of the present study was that we only included post-operative speech testing (DBS ON vs. DBS OFF) using syllables, and correlated these speech changes with pre- vs. post-operative changes in limb function. Using these different time points for comparison may be misleading. Therefore, we would suggest inclusion of pre- and postoperative testing for comparison, to examine the potential influence of pre-treatment speech severity, and inclusion of assessments of phrases and sentences. We would also suggest that future studies consider repeating the standard neurological ratings of limb function with STN DBS turned off, to directly compare limb function DBS ON vs. DBS OFF, and include electrode coordinates with stimulation parameters to correlate with speech and limb function. A second limitation of this study was that we did not include other instrumental or perceptual measures of resonance or intelligibility. Therefore, future study designs should consider integration of speech aerodynamics within a more comprehensive instrumental assessment (e.g., laryngoscopy, nasometry, and perceptual ratings).
5. Conclusion
The effects of STN DBS on speech-related velopharyngeal control in PD were previously unknown. In our study, we applied non-invasive aerodynamic techniques to examine speech-related velopharyngeal function. We found that bilateral STN DBS may influence velopharyngeal control during syllable production in PD. However, bilateral STN DBS demonstrates a more subtle effect on speech-related velopharyngeal control than for limb motor control. The effects on velopharyngeal control are not uniform across participants and only modestly correlate with changes in limb-related function. Our findings suggest that speech-related velopharyngeal control may benefit more from low frequency stimulation than high frequency stimulation. The distinctions between speech and limb-related function, and between high vs. low frequency stimulation, are important to consider when assessing the impact of STN DBS on PD, and may be valuable to guide clinicians in the optimization of DBS stimulation parameters for individuals with PD.
Acknowledgments
Dr. Hammer’s research is supported through NIH grants DC007260, RR025012, and RR023268. Dr. Barlow’s research is supported through NIH grants DC003311, DC005803, and HD02528. Drs. Pahwa and Lyons are consultants for and have received honoraria from Medtronic and St. Jude Medical (Advanced Neuromodulation Systems).
Appendix A. Continuing education
-
The Unified Parkinson’s Disease Rating Scale (UPDRS) is
A commonly employed assessment tool for Parkinson’s disease
Correlates well with specific measures of limb motor function
Includes only two speech-related items
Correlates weakly with specific measures of speech function
All of the above
-
Deep brain stimulation has been associated with
Marked improvements in limb function
Consistent improvements in speech and voice
Improved quality of life
All of the above
a and c
-
The article demonstrated
A significant group effect of deep brain stimulation on velopharyngeal control
No significant group effect of deep brain stimulation on velopharyngeal control
Significant individual effects of deep brain stimulation on velopharyngeal control
The relatively subtle effects of deep brain stimulation on velopharyngeal control
The influence of heterogeneity of outcomes on group design
a, c, d, and e
b, c, d, and e
-
The article demonstrated that intraoral air pressure and velopharyngeal area correlated with
Stimulation frequency
Stimulation inter-stimulus interval
Stimulation pulse width
Stimulation rise time
Stimulation voltage
-
Possible reasons for reported differences in limb outcomes compared with speech function with deep brain stimulation of the subthalamic nucleus include
Variability in localization of the active electrodes
Potential current spread beyond the subthalamic nucleus
Individual variability in somatotopic organization of the subthalamic nucleus
Differences in stimulation effects on each hemisphere
Differences in pre-treatment speech severity
Optimization of DBS settings and localization for limb rather than speech function
All of the above
Footnotes
Learning outcomes: As a result of this activity, the participant will be able to (1) describe the effects of deep brain stimulation on limb and speech function; (2) describe the effects of deep brain stimulation on velopharyngeal control; and (3) discuss the possible reasons for differences in limb outcomes compared with speech function with deep brain stimulation of the subthalamic nucleus.
References
- Baken RJ, Orlikoff RF. Clinical measurement of speech and voice. San Diego: Singular Publishing Group Inc.; 2000. [Google Scholar]
- Baker KK, Ramig LO, Luschei ES, Smith ME. Thyroarytenoid muscle activity associated with hypophonia in Parkinson disease and aging. Neurology. 1998;51:1592–1598. doi: 10.1212/wnl.51.6.1592. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Hammer MJ. Pallidotomy and deep brain stimulation in Parkinson’s disease: Effects on speech and voice. In: McNeil M, editor. The clinical management of sensorimotor speech disorders. New York: Thieme Medical Publishers; 2009. pp. 362–364. [Google Scholar]
- Barlow SM, Iacono RP, Paseman LA, Biswas A, D’Antonio LD. The effects of experimental posteroventral pallidotomy on force and speech aerodynamics in Parkinson’s disease. In: Cannito MP, Yorkston KM, Beukelman DR, editors. Speech motor control. Baltimore: Paul H. Brookes Publishing Company; 1998. pp. 117–156. [Google Scholar]
- Barlow SM, Muller EM. The relation between interangle span and in vivo resultant force in the perioral musculature. Journal of Speech and Hearing Research. 1991;34:252–259. doi: 10.1044/jshr.3402.252. [DOI] [PubMed] [Google Scholar]
- Barlow SM, Suing G, Andreatta RD. Speech aerodynamics using AEROWIN. In: Barlow SM, editor. Handbook of clinical speech physiology. San Diego: Singular Publishing Group Inc.; 1999. pp. 165–190. [Google Scholar]
- Breit S, Schulz JB, Benabid A-L. Deep brain stimulation. Cell and Tissue Research. 2004;318:275–288. doi: 10.1007/s00441-004-0936-0. [DOI] [PubMed] [Google Scholar]
- Bunton K. Patterns of lung volume use during an extemporaneous speech task in persons with Parkinson disease. Journal of Communication Disorders. 2005;38:331–348. doi: 10.1016/j.jcomdis.2005.01.001. [DOI] [PubMed] [Google Scholar]
- Bunton K, Weismer G. Evaluation of a reiterant force-impulse task in the tongue. Journal of Speech and Hearing Research. 1994;37:1020–1031. doi: 10.1044/jshr.3705.1020. [DOI] [PubMed] [Google Scholar]
- D’Alatri L, Paludetti G, Contarino MF, Galla S, Marchese MR, Bentivoglio AR. Effects of bilateral subthalamic nucleus stimulation and medication on parkinsonian speech impairment. Journal of Voice. 2008;22:365–372. doi: 10.1016/j.jvoice.2006.10.010. [DOI] [PubMed] [Google Scholar]
- Dalston RM, Warren DW. Comparison of Tonar II, pressure-flow, and listener judgments of hypernasality in the assessment of velopharyngeal function. Cleft Palate Journal. 1986;23:108–115. [PubMed] [Google Scholar]
- Dalston RM, Warren DW, Smith LR. The aerodynamic characteristics of speech produced by normal speakers and cleft palate speakers with adequate velopharyngeal function. Cleft Palate Journal. 1990;27:393–401. doi: 10.1597/1545-1569(1990)027<0393:tacosp>2.3.co;2. [DOI] [PubMed] [Google Scholar]
- Darley F, Aronson A, Brown J. Motor speech disorders. Philadelphia: Singular Publishing Group Inc.; 1975. [Google Scholar]
- DeLong MR. Primate models of movement disorders of basal ganglia origin. Trends in Neuroscience. 1990;13:281–285. doi: 10.1016/0166-2236(90)90110-v. [DOI] [PubMed] [Google Scholar]
- DeLong MR. Basal ganglia. In: Kandel E, Schwartz J, Jessel T, editors. Principles of neural science. New York: McGraw-Hill; 2000. pp. 853–867. [Google Scholar]
- Desmurget M, Gaveau V, Vindras P, Turner RS, Broussolle E, Thobois S. On-line motor control in patients with Parkinson’s disease. Brain. 2004;127:1755–1773. doi: 10.1093/brain/awh206. [DOI] [PubMed] [Google Scholar]
- Dromey C, Kumar R, Lang AC, Lozano AM. An investigation of the effects of subthalamic nucleus stimulation on acoustic measures of voice. Movement Disorders. 2000;15:1132–1138. doi: 10.1002/1531-8257(200011)15:6<1132::aid-mds1011>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- Fahn S, Elton R. Members of the UPDRS development committee. In: Fahn S, Marsden CD, Calne DB, Goldstein M, editors. Recent developments in Parkinson’s disease. Florham Park, NJ: Macmillian Health Care Information; 1987. pp. 153–163.pp. 293–304. [Google Scholar]
- Farrell A, Theodoros D, Ward E, Hall B, Silburn P. Effects of neurosurgical management of Parkinson’s disease on speech characteristics and oromotor function. Journal of Speech Language Hearing Research. 2005;48:5–20. doi: 10.1044/1092-4388(2005/002). [DOI] [PubMed] [Google Scholar]
- Gallena S, Smith PJ, Zeffiro T, Ludlow CL. Effects of levodopa on laryngeal muscle activity for voice onset and offset in Parkinson disease. Journal of Speech Language Hearing Research. 2001;44:1284–1299. doi: 10.1044/1092-4388(2001/100). [DOI] [PubMed] [Google Scholar]
- Gentil M, Chauvin P, Pinto S, Pollak P, Benabid AL. Effect of bilateral stimulation of the subthalamic nucleus on parkinsonian voice. Brain and Language. 2001;78:233–240. doi: 10.1006/brln.2001.2466. [DOI] [PubMed] [Google Scholar]
- Gentil M, Garcia-Ruiz P, Pollak P, Benabid AL. Effect of stimulation of the subthalamic nucleus on oral control of patients with parkinsonism. Journal of Neurology Neurosurgery and Psychiatry. 1999;67:329–333. doi: 10.1136/jnnp.67.3.329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hammer MJ, Barlow SM, Lyons KE, Pahwa R. Subthalamic nucleus deep brain stimulation changes speech respiratory and laryngeal control in Parkinson’s disease. Journal of Neurology. 2010 doi: 10.1007/s00415-010-5605-5. doi:10.1007/s00415-010-5605-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirose H, Kiritani S, Ushijima T, Yoshioka H, Sawashima M. Patterns of dysarthric movements in patients with parkinsonism. Folia Phoniatrica et Logopedia. 1981;33:204–215. doi: 10.1159/000265595. [DOI] [PubMed] [Google Scholar]
- Hoehn MM, Yahr MD. Parkinsonism: Onset, progression and mortality. Neurology. 1967;17:427–442. doi: 10.1212/wnl.17.5.427. [DOI] [PubMed] [Google Scholar]
- Hoffman-Ruddy B, Schulz G, Vitek J, Evatt M. A preliminary study of the effects of subthalamic nucleus (STN) deep brain stimulation (DBS) on voice and speech characteristics in Parkinson’s disease (PD) Clinical Linguistics and Phonetics. 2001;15:97–101. doi: 10.3109/02699200109167638. [DOI] [PubMed] [Google Scholar]
- Hoodin RB, Gilbert HR. Nasal airflows in parkinsonian speakers. Journal of Communication Disorders. 1989a;22:169–180. doi: 10.1016/0021-9924(89)90014-2. [DOI] [PubMed] [Google Scholar]
- Hoodin RB, Gilbert HR. Parkinsonian dysarthria: An aerodynamic and perceptual description of velopharyngeal closure for speech. Folia Phoniatrica et Logopedia. 1989b;41:249–258. doi: 10.1159/000265976. [DOI] [PubMed] [Google Scholar]
- Hunker CJ, Abbs JH, Barlow SM. The relationship between Parkinsonian rigidity and hypokinesia in the orofacial system: A quantitative analysis. Neurology. 1982;32:755–761. doi: 10.1212/wnl.32.7.749. [DOI] [PubMed] [Google Scholar]
- Jones HN, Kendall DL, Sudhyadhom A, Rosenbek JC. The effects of lesion therapy and deep brain stimulation on speech function in patients with Parkinson’s disease. Communicative Disorders Review. 2007;1:133–173. [Google Scholar]
- Klostermann F, Ehlen F, Vesper J, Nubel K, Gross M, Marzinzik F, et al. Effects of subthalamic deep brain stimulation in dysarthrophonia in Parkinson’s disease. Journal of Neurology Neurosurgery and Psychiatry. 2008;79:522–529. doi: 10.1136/jnnp.2007.123323. [DOI] [PubMed] [Google Scholar]
- Logemann JA, Fisher HB, Boshes B, Blonsky ER. Frequency and cooccurrence of vocal tract dysfunctions in the speech of a large sample of Parkinson patients. Journal of Speech and Hearing Disorders. 1978;43:47–57. doi: 10.1044/jshd.4301.47. [DOI] [PubMed] [Google Scholar]
- Lotz WK, Shaughnessy AL, Netsell R. Velopharyngeal and nasal cavity resistance during speech production. Abstracts from the American Speech Language hearing association annual convention. 1981 [Google Scholar]
- Luschei ES, Ramig LO, Baker KL, Smith ME. Discharge characteristics of laryngeal single motor units during phonation in young and older adults and in persons with Parkinson disease. Journal of Neurophysiology. 1999;81:2131–2139. doi: 10.1152/jn.1999.81.5.2131. [DOI] [PubMed] [Google Scholar]
- Miller N, Allcock L, Jones D, Noble E, Hildreth AJ, Burn DJ. Prevalence and pattern of perceived intelligibility changes in Parkinson’s disease. Journal of Neurology Neurosurgery and Psychiatry. 2007;78:1188–1190. doi: 10.1136/jnnp.2006.110171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller N, Noble E, Jones D, Allcock L, Burn DJ. How do I sound to me? Perceived changes in communication in Parkinson’s disease. Clinical Rehabilitation. 2008;22:14–22. doi: 10.1177/0269215507079096. [DOI] [PubMed] [Google Scholar]
- Montgomery EB. Deep brain stimulation and speech: A new model of speech function and dysfunction in Parkinson’s disease. Journal of Medical Speech Language Pathology. 2007;15:9–25. [Google Scholar]
- Netsell R, Hixon TJ. A noninvasive method for clinically estimating subglottal pressure. Journal of Speech and Hearing Disorders. 1978;43:326–330. doi: 10.1044/jshd.4303.326. [DOI] [PubMed] [Google Scholar]
- Netsell R, Lotz WK, Shaughnessy AL. Abstracts from the association for research in otolaryngology. 1982. Nasal cavity resistance estimates during vocalization. [Google Scholar]
- Pfann KD, Buchman AS, Comella CL, Corcos DM. Control of movement distance in Parkinson’s disease. Movement Disorders. 2001;16:1048–1065. doi: 10.1002/mds.1220. [DOI] [PubMed] [Google Scholar]
- Pfann KD, Robichaud JA, Gottlieb GL, Comella CL, Brandabur M, Corcos DM. Muscle activation patterns in point-to-point and reversal movements in healthy, older subjects and in subjects with Parkinson’s disease. Experimental Brain Research. 2004;157:67–78. doi: 10.1007/s00221-003-1821-x. [DOI] [PubMed] [Google Scholar]
- Pinto S, Gentil M, Fraix V, Benabid AL, Pollak P. Bilateral subthalamic stimulation effects on oral force control in Parkinson’s disease. Neurology. 2003;250:179–187. doi: 10.1007/s00415-003-0966-7. [DOI] [PubMed] [Google Scholar]
- Pinto S, Ozsancak C, Tripoliti E, Thobois S, Limousin-Dowsey P, Auzou P. Treatments for dysarthria in Parkinson’s disease. Lancet Neurology. 2004;3:547–556. doi: 10.1016/S1474-4422(04)00854-3. [DOI] [PubMed] [Google Scholar]
- Pinto S, Gentil M, Krack P, Sauleau P, Fraix V, Benabid AL, et al. Changes induced by levodopa and subthalamic nucleus stimulation on parkinsonian speech. Movement Disorders. 2005;20:1507–1515. doi: 10.1002/mds.20601. [DOI] [PubMed] [Google Scholar]
- Richards M, Marder K, Cote L, Mayeaux R. Interrater reliability of the Unified Parkinson’s Disease Rating Scale motor examination. Movement Disorders. 1994;9:89–91. doi: 10.1002/mds.870090114. [DOI] [PubMed] [Google Scholar]
- Robbins JA, Logemann JA, Kirschner HS. Swallow and speech production in Parkinson’s disease. Annals of Neurology. 1986;19:283–287. doi: 10.1002/ana.410190310. [DOI] [PubMed] [Google Scholar]
- Robichaud JA, Pfann KD, Vaillancourt DE, Comella CL, Corcos DM. Force control and disease severity in Parkinson’s disease. Movement Disorders. 2005;20:441–450. doi: 10.1002/mds.20350. [DOI] [PubMed] [Google Scholar]
- Rousseaux M, Krystkowiak P, Kozlowski O, Ozsancak C, Blond S, Destee A. Effects of subthalamic nucleus stimulation on parkinsonian dysarthria and speech intelligibility. Neurology. 2004;251:327–334. doi: 10.1007/s00415-004-0327-1. [DOI] [PubMed] [Google Scholar]
- Santens P, De Letter M, Van Borsel J, De Reuck J, Caemaert J. Lateralized effects of subthalamic nucleus stimulation on different aspects of speech in Parkinson’s disease. Brain and Language. 2003;87:253–258. doi: 10.1016/s0093-934x(03)00142-1. [DOI] [PubMed] [Google Scholar]
- Schulz GM, Grant MK. Effects of speech therapy and pharmacologic and surgical treatments on voice and speech in Parkinson’s disease: A review of the literature. Journal of Communication Disorders. 2000;33:59–88. doi: 10.1016/s0021-9924(99)00025-8. [DOI] [PubMed] [Google Scholar]
- Schwab RS, England AC. Projection technique for evaluating surgery in Parkinson’s disease. In: Gillingham FJ, Donaldson IML, editors. Third symposium on Parkinson’s disease, Royal College of Surgeons in Edinburgh. Edinburgh: E. & S. Livingstone; 1968. pp. 152–157. [Google Scholar]
- Smitheran JR, Hixon TJ. A clinical method for estimating laryngeal airway resistance during vowel production. Journal of Speech and Hearing Disorders. 1981;46:138–146. doi: 10.1044/jshd.4602.138. [DOI] [PubMed] [Google Scholar]
- Solomon NP, Hixon TJ. Speech breathing in Parkinson’s disease. Journal of Speech and Hearing Research. 1993;36:294–310. doi: 10.1044/jshr.3602.294. [DOI] [PubMed] [Google Scholar]
- Tamaki A, Matsuo Y, Yanagihara T, Abe K. Influence of thoracoabdominal movement on pulmonary function in patients with Parkinson’s disease: Comparison with healthy subjects. Neurorehabilitation and Neural Repair. 2000;14:43–47. doi: 10.1177/154596830001400105. [DOI] [PubMed] [Google Scholar]
- Thompson AE, Hixon TJ. Nasal air flow during normal speech production. Cleft Palate Journal. 1979;16:412–420. [PubMed] [Google Scholar]
- Trail M, Fox C, Ramig LO, Sapir S, Howard J, Lai EC. Speech treatment for Parkinson’s disease. Neurorehabilitation. 2005;20:205–221. [PubMed] [Google Scholar]
- Tornqvist AL, Schalen L, Rehncrona S. Effects of different electrical parameter settings on the intelligibility of speech in patients with Parkinson’s disease treated with subthalamic deep brain stimulation. Movement Disorders. 2005;20:416–423. doi: 10.1002/mds.20348. [DOI] [PubMed] [Google Scholar]
- Tripoliti E, Zrinzo L, Martinez-Torres I, Tisch S, Frost E, Borrell E, et al. Effects of contact location and voltage amplitude on speech and movement in bilateral subthalamic nucleus deep brain stimulation. Movement Disorders. 2008;16:2377–2383. doi: 10.1002/mds.22296. [DOI] [PubMed] [Google Scholar]
- Tzelepis GE, McCool DF, Friedman JH, Hoppin FG. Respiratory muscle dysfunction in Parkinson’s disease. American Review of Respiratory Disease. 1988;138:266–271. doi: 10.1164/ajrccm/138.2.266. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Metman LV, Bakay RA, Crocos DM. Effects of deep brain stimulation and medication on bradykinesia and muscle activation in Parkinson’s disease. Brain. 2004;127:491–504. doi: 10.1093/brain/awh057. [DOI] [PubMed] [Google Scholar]
- Vaillancourt DE, Prodoehl J, Sturman MM, Bakay RAE, Verhagen LV, Crocos DM. Effects of deep brain stimulation and medication on strength, bradykinesia, and electromyographic patterns of the ankle joint in Parkinson’s disease. Movement Disorders. 2006;21:50–58. doi: 10.1002/mds.20672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkmann J. Deep brain stimulation for the treatment of Parkinson’s disease. Journal of Clinical Neurophysiology. 2004;21:6–17. doi: 10.1097/00004691-200401000-00003. [DOI] [PubMed] [Google Scholar]
- Wang E, Metman LV, Bakay R, Arzbaecher J, Bernard B. The effect of unilateral electrostimulation of the subthalamic nucleus on respiratory/phonatory subsystems of speech production in Parkinson’s disease – A preliminary report. Clinical Linguistics and Phonetics. 2003;17:283–289. doi: 10.1080/0269920031000080064. [DOI] [PubMed] [Google Scholar]
- Wang EQ, Metman LV, Bakay RAE, Arzbaecher J, Bernard B, Corcos DM. Hemisphere-specific effects of subthalamic nucleus deep brain stimulation on speaking rate and articulatory accuracy of syllable repetitions in Parkinson’s disease. Journal of Medical Speech Language Pathology. 2006;14:323–333. [PMC free article] [PubMed] [Google Scholar]
- Warren DW. Velopharyngeal orifice size and upper pharyngeal pressure-flow patterns in cleft palate speech: A preliminary study. Plastic and Reconstructive Surgery. 1964;34:15–26. doi: 10.1097/00006534-196407000-00003. [DOI] [PubMed] [Google Scholar]
- Warren DW. Nasal emission of air and velopharyngeal function. Cleft Palate Journal. 1967a;4:148–155. [PubMed] [Google Scholar]
- Warren DW. Oral port constriction, nasal resistance, and respiratory aspects of cleft palate speech: An analog study. Cleft Palate Journal. 1967b;4:39–46. [PubMed] [Google Scholar]
- Warren DW, DuBois AB. A pressure-flow technique for measuring velopharyngeal orifice area during continuous speech. Cleft Palate Journal. 1964;16:52–71. [PubMed] [Google Scholar]
- Weaver FM, Follett K, Stern M, Hur K, Harris C, Marks WJ, et al. Bilateral deep brain stimulation vs. best medical therapy for patients with advanced Parkinson disease: A randomized controlled trial. Journal of the American Medical Association. 2009;301:63–73. doi: 10.1001/jama.2008.929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zajac DJ, Mayo R. Aerodynamic and temporal aspects of velopharyngeal function in normal speakers. Journal of Speech and Hearing Research. 1996;39:1199–1207. doi: 10.1044/jshr.3906.1199. [DOI] [PubMed] [Google Scholar]