Abstract
Background
Exercise capacity as measured by VO2peak (peak oxygen uptake) is low in hemodialysis patients. The current study assesses the determinants of VO2peak in patients with chronic kidney failure who changed kidney replacement modality to either frequent hemodialysis or received a kidney transplant.
Study design
Cohort study with assessment at baseline and 6-months following modality change.
Setting & Participants
Participants included non-diabetic individuals receiving conventional hemodialysis who a) remained on conventional hemodialysis (n=13), b) changed to short daily hemodialysis (n=10), or c) received a transplant (n=5), and d) individuals who received pre-emptive transplant (n=15). Additionally, 34 healthy controls were assessed at baseline only.
Predictor
Modality change
Measurement & Outcomes
Exercise capacity, assessed from the physiologic components of the Fick equation (VO2 = cardiac output x a-vO2dif, where a-vO2dif is arterial to venous oxygen difference) was determined by measurement of VO2peak and cardiac output during symptom-limited exercise testing. Analysis of covariance was used to compare the differences in changes in VO2peak, cardiac output, heart rate, stroke volume, and a-vO2dif, at peak exercise between those who remained on hemodialysis and those who underwent transplant.
Results
Transplant was the only modality change that was associated with a significant change in VO2peak, occurring as a result of increased peak cardiac output and reflecting increased heart rate without change in peak a-vO2dif, despite increased hemoglobin levels. There were no differences in those who changed to daily hemodialysis compared to those who remained on conventional hemodialysis.
Limitations
Small, non-randomized study.
Conclusions
VO2peak increases significantly following kidney transplant but not with daily hemodialysis; this improvement reflects increased peak cardiac output via increased peak heart rate. Despite statistical significance, the increase in VO2peak was not clinically significant, suggesting the need for interventions such as exercise training to increase VO2peak in all patients, regardless of treatment modality.
Index Words: Exercise Capacity, oxygen uptake, Fick Equation, cardiac output, ESRD, frequent hemodialysis, kidney transplantation
It is well documented that exercise capacity as measured by peak oxygen uptake (VO2peak) is low in patients with end stage renal disease (ESRD) treated with dialysis1–5. There are many factors prevalent in dialysis patients that may contribute to low VO2peak in this patient group, including anemia, cardiac dysfunction (reduced contractility, increased preload and afterload), vascular dysfunction (limiting ability to divert cardiac output to skeletal muscle), skeletal muscle abnormalities (reduced fiber size, capillary density, mitochondrial density/function, increased diffusion distance) and/or metabolic abnormalities and autonomic dysfunction6–8,9, 10,11,12, 13. A 2002 study by Sietsema et al10 had sufficient power to perform regression analysis to identify clinical and demographic predictors of VO2peak; in 193 hemodialysis patients, older age, lower hemoglobin and diabetes were the only significant clinical or demographic predictors of lower VO2peak. There has been only one published study that measured the determinants of VO2peak in hemodialysis patients during peak exercise (e.g. cardiac output and arterial to venous oxygen difference (a-vO2dif). This study by Moore, et al 1 found that both cardiac output and a-vO2dif were similar to normal reported values at rest; however, at peak exercise, both the cardiac output (primarily reflecting a blunted heart rate response in the setting of a normal stroke volume) and a-vO2dif (reflecting reduced arterial oxygen content) were reduced, resulting in reduced oxygen supply to the working muscles.
The suggestion that factors associated with uremia contribute to systemic limitations in VO2peak is supported by data published on the effects of kidney transplants on VO2peak. Painter et al15 reported that shortly following successful transplant, VO2peak increased 27% without exercise training (from 1.68 ± 0.35 to 2.13 ± 0.42 L/min). Peak heart rate increased from 81% of age-predicted levels to 93% of age-predicted levels after transplant, suggesting that this blunted heart rate could contribute to impaired cardiac output at peak exercise. In addition, Chan et al16 reported increases in VO2peak following increases in frequency of hemodialysis from 4 hours thrice weekly to 8–10 hours 5–6 nights/week, although peak heart rates remained below expected normal levels. In these studies, normal age-predicted levels of VO2peak were not achieved after either of these modality changes.
The aim of the current study was to identify physiologic mechanisms whereby uremia limits exercise capacity and the extent to which they change with a kidney transplant or more frequent dialysis. The hypothesis was that the physiological responses to exercise and determinants of VO2peak would improve in patients who changed to short daily hemodialysis (HD) compared to those who remained on conventional hemodialysis and would be similar to those who received a transplant.
METHODS
Study Design
Four groups of patients with ESRD were studied in a pre-post design 6 months apart (baseline and visit 2): group 1 included patients who were treated with conventional hemodialysis (3–4 hr, 3x/week) and did not change modality; group 2 changed from conventional hemodialysis to short daily hemodialysis (3 hours, 5–6 days/week); group 3 changed from conventional hemodialysis to receipt of a kidney transplant; and group 4 included patients who received a preemptive kidney transplant. Transplant recipients were tested within 2 weeks of living donor transplant and again 6 months after the procedure. Those changing to short daily dialysis were tested within a week of starting training for daily dialysis and again 6 months after beginning daily treatments. A fifth group comprised of sedentary individuals was recruited from a list of kidney donors and served as controls; these individuals were tested only once.
Subjects
The study was designed to evaluate the effects of reduction of uremia. Thus patients with other comorbidities/conditions that would have independent effects on exercise capacity (i.e. diabetes mellitus, pulmonary disease, cardiac disease, etc) were excluded. Inclusion criteria were: SRD requiring kidney replacement therapy; either stable on conventional hemodialysis for at least 3 months or scheduled for transplant; able to provide informed consent; over 18 years of age; and English speaking. Other exclusion criteria were: orthopedic or musculoskeletal factors that limit or could be exacerbated by exercise; hematocrit <33%; recent (within 1 year) cardiovascular event; pulmonary disease; peripheral vascular disease or progressive degenerative muscular disease; diabetes; and contraindications to maximal exercise testing as defined by the American College of Sports Medicine 17. Demographic and clinical information was obtained from the medical record or dialysis chart or from self-report.
Conventional hemodialysis patients were recruited from five centers, CHD-SDD from 3 centers and transplant recipients from the University of California San Francisco (UCSF) and the University of Minnesota.
Dialysis patients responded to posted fliers or were referred by dialysis staff; preemptive transplant recipients gave permission to nurse coordinators or physicians to be referred. Sedentary healthy control subjects were recruited from kidney donors (>1 year post donation with normal kidney function as indicated by estimated GFR) at the University of Minnesota. A letter was sent to 420 kidney donors within a 100-mile radius of the medical center, from which 180 interested individuals responded. Study subjects were selected in an attempt to match the group of control subjects to the patient group by percentage of women, general physical activity levels and age decades. All subjects provided informed consent, and the study was approved by the Committee on Human Subjects at UCSF and the Institutional Review Board at the University of Minnesota.
Testing
All testing was performed in the General Clinical Research Center at UCSF or the University of Minnesota. Testing was performed on a mid-week non-dialysis day, at least 15 hours after completion of the dialysis treatment; most patients were tested between 20 and 24 hours following completion of their dialysis treatment. All blood pressure medications were held after the dialysis session; since most patients did not take antihypertensive medication prior to dialysis, most were off medications for over 24 hours, including beta-blocking agents. As beta-blockers can affect exercise heart rate, it is important to note that the longest half-life of beta blocking agents is up to 24 hours in patients with kidney failure. Thus, patients were tested when the medication was reduced to at least one-half its peak levels. Non-dihydropyridine calcium channel blockers may also lower heart rates and have a half-life of 12 hours. Patients were fasted upon arrival for blood sampling. Exercise testing was performed one hour after a small meal.
Exercise Testing
Exercise testing used a branching treadmill protocol that started at a brisk walking speed with gradual increases in grade every 2 minutes to assure that at least 4 measurements were obtained between rest and peak exercise. Patients were instructed to exert themselves to maximal levels. When the subject was unable to keep pace with the treadmill or requested to stop, cardiac output and blood pressure were measured before stopping. Electrocardiogram (heart rate) was measured continuously, and blood pressure, oxygen uptake (VO2 in L/min), and cardiac output were measured at each stage. Peak values were those measured at peak exercise.
Oxygen uptake was measured by analysis of expired oxygen, carbon dioxide and volume using the Quinton QMC metabolic measurement system (www.cardiacscience.com) at UCSF or the Beck Physiological Measurement System at UMN. The Beck system uses a mass spectrometer for analysis of oxygen and CO2, and a pneumotachograph for expired air volumes. Measurement systems were calibrated with known calibration gases prior to each test. Cardiac output (L/min) was measured noninvasively using the Acetylene Open Circuit (OpCirc) inert gas wash-in method, and analyzed by Beck Integrated Physiological System software. The patient breathed into a non-rebreathing valve which is connected to a pneumatic valve that allowed switching the inspired gas from room air to a test gas mixture (0.7%C2H2, 21% O2, 9% He, balance N2). The patient was switched to the test gas during an expiration, so that the following inspiration was test gas. Eight to ten breaths were sampled using a mass spectrometer (Perkin-Elmer 1100; www.matechservices.com). This method of cardiac output measurement was previously tested against direct Fick measurements by Johnson, et al 18 and was reported to be highly correlated with direct Fick at rest and during exercise (R2=0.90) with coefficient of variation at moderate to high exercise intensities determined to be 3–5%. These investigators concluded that the OpCirc method provides reproducible and reliable measurements of cardiac output during all levels of exercise. The coefficient of variation for this measurement at maximal exercise in our lab is 2.9%, with test-retest correlations during exercise of r=0.93.
Calculations/Definitions
The Fick equation was used to calculate arterial-venous oxygen difference (a-vO2dif=cardiac output/VO2). Also calculated were: stroke volume (which is equal to cardiac output divided by hear rate); arterial oxygen content (equal to 1.36*[hemoglobin]*0.97)+0.3); mixed venous oxygen content (equal to difference between arterial oxygen content and a-vO2dif [assuming 97% saturation and PaO2 of 95 mmHg with no hemoconcentration with exercise]); and age predicted heart rate (or 220-age). Predicted VO2 was calculated using the prediction equations referenced by Bruce et al 19.
Laboratory Measures
Fasting blood samples were used to assess blood chemistries and hematology. The most recent value for Kt/V was obtained from the dialysis chart to assess dialysis adequacy. For the daily dialysis group, the single pool Kt/V value reported in the monthly labs was multiplied by the number of days dialyzed per week.
Statistical Analysis
Basic descriptive data was calculated for all groups. Patient values were compared to sedentary controls at baseline and at visit 2 using analysis of variance. A change score was calculated for each variable (visit 2–baseline). Significance of change from baseline to visit 2 was determined by pooling the data and performing analysis of covariance with baseline values and group as covariates with the change score as the outcome variable. Post hoc analysis compared each of the 3 groups that changed modalities to the reference group that remained on conventional HD.
Because of the small sample sizes in the patient groups and because the two groups that remained on hemodialysis and received a transplant were similar, we combined those who remained on dialysis (those who began on conventional HD and either remained on that modality or changed to short daily HD) and also combined those who received transplants (both those who started on conventional HD and those who had a pre-emptive transplant). Change from baseline to visit 2 was determined for all outcome variables by pooling the data and performing analysis of covariance with baseline values and group (dialysis versus transplant) as covariates. Significance for all tests was set at p=0.05. Statistical analysis was performed using SPSS v17 (SPSS, www.spss.com).
RESULTS
Subjects
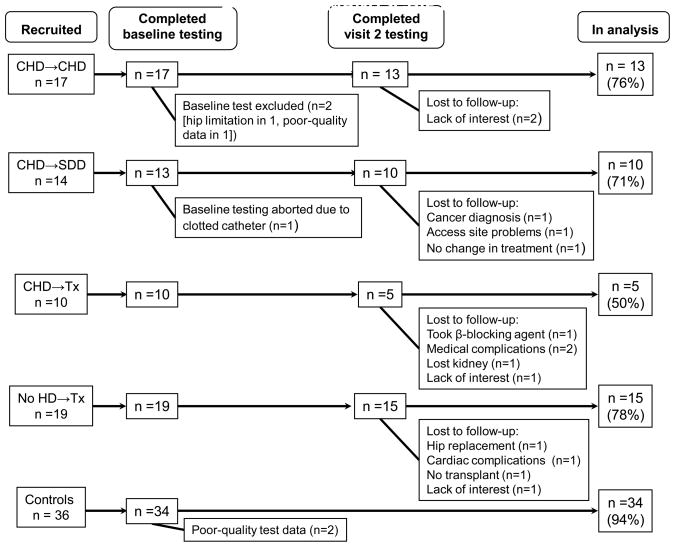
A total of 61 patients and 36 sedentary controls were recruited into the study and tested. Of the total recruited (n=97), 77 subjects are included in the analyses (43 patients and 34 controls). Figure 1 shows the reasons for loss to analysis in all groups.
Figure 1.
Recruitment and loss to analysis. Abbreviations: CHD->CHD, remained on conventional hemodialysis; CHD->SDD, changed from conventional hemodialysis to short daily hemodialysis; CHD->Tx; changed from conventional hemodialysis to transplant; No HD-TX, received a preemptive transplant
The patient groups consisted of 18% women (15% in the dialysis group and 22% in the transplant group), and 17% of the controls were women (Table 1). The age within patient groups was similar; however control subjects were significantly older than the patient groups. There was no difference among groups in BMI. Those in the group that changed from conventional HD to transplant were younger than controls, and participants in the combined transplant group were on dialysis less time than those who remained on hemodialysis (p<.001). There was no difference in the total number of antihypertensive medications among patient groups. Six patients (all in the preemptive transplant group) were taking non-dihydropyridine calcium channel blockers at baseline. Most patients treated with dialysis were taking erythropoietin stimulating agents at baseline. All but four patients receiving hemodialysis at baseline had arteriovenous fistulas; the four dialyzing with catheters were in the group whose members changed from conventional HD to short daily HD.
Table 1.
Demographic and Clinical Characteristics at Baseline
| Individual Groups | Composite Groups | ||||||
|---|---|---|---|---|---|---|---|
| Variable | CHD->CHD (n=13) | CHD->SDD (n=10) | CHD->Tx (n=5) | No HD->Tx (n=15) | Controls (n=34) | Remained on HD* (n=23) | Tx recipient** (n=20) |
| Sex (M/F) | 11/2 | 9/1 | 5/0 | 13/2 | 28/6 | 20/3 | 17/3 |
| Age | 45.5 ± 10.4 | 42.6 ± 12.4 | 34.1 ± 11.1 * | 44.2 ±10.4 | 47.7 ± 8.5* | 41.0 ± 11.3 | 43.5 ± 10.9 |
| BMI | 27.4 ± 4.0 | 28.3 ± 4.4 | 29.2 ± 4.1 | 28.2 ± 4.6 | 27.1 ± 4.7 | 27.1 ± 4.4 | 28.1 ± 5.0 |
| Time on dialysis (mo) | 28.5 ± 21.2 | 33.8 ± 44.3 | 16.6 ± 21.1 | 0 | - | 38.3 ± 32.5 | 5.3 ± 13.7 |
| Ethnicity (%) | |||||||
| White | 52.9% | 50.0% | 83.3% | 93.3% | 100% | 51.6% | 93.1% |
| African American | 29.4% | 35.7% | - | 6.7% | 32.3% | 3.4% | |
| Hispanic | 11.8% | 7.1% | 16.6% | - | 9.7% | 3.4% | |
| Asian | - | - | 3.2% | - | |||
| Native American | 5.9% | 7.1% | - | 3.2% | - | ||
| Cause of Kidney Failure | - | ||||||
| Hypertension | 23.5% | 21.4% | - | 13.3% | 22.6% | 6.9% | |
| GN/FSGN | 17.6% | 7.1% | 16.6% | 20.0% | 12.9% | 17.2% | |
| IgA Nephropathy | 11.8% | - | 33.3% | 26.7% | 6.5% | 24.1% | |
| PKD | 5.9% | 14.3% | 16.6% | 26.7% | 9.7% | 24.1% | |
| Other/Unknown | 41.4% | 58.1% | 33.3% | 13.3% | 48.4% | 38.4% | |
| Baseline medication use | |||||||
| No. of antihypertensive medications | 1.8 ± 1.0 | 1.4 ± 1.3 | 1.0 ± 1.4 | 2.3 ± 0.9 | 2 | 1.6 ± 1.1 | 1.8 ± 1.1 |
| # of patients on beta blockers | 9 | 4 | 3 | 6 | 1 | 13 | 9 |
| Receiving ESA | 82.4% | 78.6% | 66% | 26.6% | - | 80.5% | 41.4% |
| SCr (mg/dL) | 8.9 ± 1.9† | 10.2 ± 2.2† | 8.3 ± 2.4† | 6.1 ± 2.2† | 1.2 ± 0.2 | 9.7 ± 2.2† | 6.8 ± 2.4† |
| SUN (mg/dL) | 36.2 ± 9.2† | 45.1 ± 23.8† | 38,2 ± 14.8† | 68.4 ± 23.2† | 17.9 ± 5.1 | 40.3 ± 17.1† | 58.3 ± 25.0† |
| Hemoglobin (g/dL | 12.6 ± 0.9† | 12.2 ± 1.4† | 13.7 ± 0.5 | 11.9 ± 1.4† | 13.4 ± 1.4 | 12.6 ± 0.9† | 12.5 ± 1.4† |
Note: Continuous variables are given as mean +/− SD; categorical variables are given as percentage.
Abbreviations and definitions: CHD->CHD, remaining on conventional hemodialysis; CHD->SDD, conventional hemodialysis to short daily hemodialysis; CHD->Tx; conventional hemodialysis to transplant; No HD->Tx, received a preemptive transplant; GN/FSGN: Glomerulonephritis/Focal Sclerosing Glomerulonephritis; PKD: polycystic Kidney Disease; IgA; immunoglobulin A; ESA; erthyopoiesis-stimulating agents; SUN, serum urea nitrogen; SCr, serum creatinine; BMI, body mass index
combines CHD->CHD and CHD->SDD
combines CHD->TX and no HD->Tx
p<.03 values compared to controls
Conversion factors for units: serum creatinine in mg/dL to μmol/L, x 88.4; urea nitrogen in mg/dL to mmol/L, x 0.357.
Following transplant there were significant improvements in serum BUN (to 21.6 ± 5.1 mg/dL) and creatinine (to 1.7 ± 0.5 mg/dL). Hemoglobin increased slightly (to 13.2± 1.9 g/L; p = 0.05); however, the hemoglobin remained low in the patient groups compared to the controls. Although serum creatinine and BUN were normalized following transplant, the eGFR was significantly lower than that of the control subjects (49.2 ± 2.4 vs. 69.1 ± 13.1 ml/min/1.73m2; p=0.01). Immunosuppression was primarily tacrolimus, sirolimus and mycophenolate mofetil; 12 patients were taking prednisone. Following transplant, 10 were taking beta-blocking agents and 2 remained on non-dihydropyridine calcium channel blockers. Following transplant, 3 of the 5 patients in the conventional HD to transplant group had functioning arteriovenous fistulas.
Patients in the conventional to short daily HD group changed to the NxStage® dialysis machine (www.nxstage.com), increasing the frequency of treatments from 3 days/week (3.4 ± 0.3 hours/session) to an average of 5.6 ± 0.5 days/week (2.8 ± 0.2 hours/session), which resulted in a change in a weekly Kt/V from 1.39 ± 0.34 to 2.77 ± 0.48. Those who remained on conventional hemodialysis dialyzed 3 days/week for 3.4 ± 0.5 hours/session and Kt/V averaged 1.44 ± 0.21; this did not change at visit 2. At visit 2, only on dialysis patient in the group that changed to short daily HD had a catheter and three patients in this group had discontinued beta-blocking agents. There were no changes in blood pressure medications in the group that remained on conventional HD.
Exercise capacity
Physiologic variables at baseline and visit 2 for all groups are found in table 2. At baseline the patients that remained on dialysis had significantly lower VO2peak than controls, while patients who were scheduled for transplant were similar in VO2peak to controls. All patients at baseline had similar peak cardiac output as controls; however patients achieved peak cardiac output through significantly higher peak stroke volumes because peak heart rates were lower in all the patients at baseline compared to controls. The a-vO2dif was also lower in all patients at baseline compared to controls (table 2).
Table 2.
Physiologic Variables at Peak Exercise
| Individual Groups | Composite Groups | ||||||
|---|---|---|---|---|---|---|---|
| Variable | CHD->CHD (n=13) | CHD->SDD (n=10) | CHD->Tx (n=5) | No HD->Tx(n=15) | Controls (n=34) | Remained on HD** (n=23) | Tx recipient*** (n=20) |
| VO2 (L/min) | |||||||
| Baseline | 2.17 ± 0.43† | 1.97 ± 0.55† | 2. 31± 0.62 | 2.27 ± 0.56 | 2.40 ± 0.54 | 2.08 ± 0.48† | 2.28 ± 0.57 |
| Visit 2 | 2.08 ± 0.48¶ | 1.91 ± 0.48¶ | 2.53 ± 0.74 | 2.56 ± 0.52 | 2.01 ± 047¶ | 2.55 ± 0.57 | |
| Adjusted mean change* | −0.08 (−0.32, 0.15) | −0.15 (−0.43, 0.13) | 0.11 (−0.25, −0.46) | 0.29§ (0.06, 0.53) | −0.10 (−0.27, 0.06) | 0.28@ (0.10, 0.47) | |
| Cardiac Output (L/min) | |||||||
| Baseline | 17.46 ± 3.94 | 14.12 ± 3.64 | 18.42 ± 3.91 | 17.01 ± 3.20✝ | 15.28 ± 3.76 | 16.01 ± 4.09 | 17.46 ± 3.41 |
| Visit 2 | 16.37 ± 3.49 | 13.68 ± 2.85 | 20.51 ± 4.08 | 18.17 ± 3.45¶ | 15.21 ± 3.36 | 18.88 ± 3.71¶ | |
| Adjusted mean change* | −0.86 (−0.22, 0.48) | −0.15 (−2.91, 0.34) | 2.82 (0.83, 4.80) | 0.96§ (−0.33, 2.25) | −1.03 (−2.05, −0.01) | 1.45@ (0.39, 2.59) | |
| Stroke Volume (ml/beat) | |||||||
| Baseline | 117.0 ± 29.3† | 101.4 ± 28.4† | 122.3 ± 37.7 | 114.9 ± 25.5† | 89.2 ± 23.7 | 110.2 ± 29.4† | 117.3 ± 29.2† |
| Visit 2 | 115.4 ± 28.8¶ | 93.7 ± 18.7 | 135.3 ± 38.1 | 112.9 ± 21.1¶ | 105.9 ± 26.8¶ | 119.7 ± 28.5¶ | |
| Adjusted mean change* | −0.71 (−9.62, 8.19) | −11.28 (−21.6, 0.92) | 18.19 (5.08, 31.31) | −3.45 (−12.05, 5.13) | −5.21 −12.56, 2.15) | 2.92 (−4.97, 10.80) | |
| Heart Rate (beats/min) | |||||||
| Baseline | 150± 18† | 141 ± 21† | 155 ± 23 | 151 ± 24† | 173 ± 15 | 147 ± 19† | 152 ± 23† |
| Visit 2 | 145 ± 21¶ | 147 ± 23¶ | 158 ± 21 | 162 ± 23¶ | 146 ± 23¶ | 161 ± 22¶ | |
| Adjusted mean change* | −5.06 (−12.91, 2.78) | 4.08 (−5.02, 13.2) | −1.11 (−8.80, 10.58) | 11.32§ (3.75, 18.91) | −1.22 (−7.41, 4.98) | 7.75@ (1.11, 14.39) | |
| a-vO2diff (ml/100ml) | |||||||
| Baseline | 12.72 ± 2.75† | 14.45 ± 4.97† | 12.91 ± 2.83 | 13.55 ± 3.36† | 15.99 ± 3.15 | 13.47 ± 3.87† | 13.35 ± 3.15† |
| Visit 2 | 12.95 ± 2.54¶ | 14.17 ± 3.08¶ | 11.78 ± 2.53¶ | 14.22 ± 1.97¶ | 13.48 ± 2.79¶ | 13.48 ± 2.38¶ | |
| Adjusted mean change* | −0.21 (−1.42, 0.99) | 0.33 (−1.02, 1.71) | −1.62 (−3.39, 0.15) | −0.81 (−0.35, 1.96) | 0.04 (−0.91, 0.98) | 0.79 (−0.93, 1.09) | |
| Arterial O2 content (ml/100ml) | |||||||
| Baseline | 16.9 ± 1.1† | 15.9 ± 1.1† | 17.8 ± 0.6 | 15.6 ± 1.7† | 18.8 ± 1.1 | 16.5 ± 1.2† | 16.3 ± 1.8† |
| Visit 2 | 16.6 ± 1.6¶ | 15.3 ± 1.7¶ | 17.3 ± 3.0 | 17.1 ± 2.3¶ | 16.0 ± 1.7¶ | 17.2 ± 2.3¶ | |
| Adjusted mean change* | 0.01 (− 1.27,1.29) | −1.02 (−2.42, −038) | 0.69 (−1.11, 2.49) | 0.92 (−0.27, 2.11) | −0.46 (−1.38, 0.45) | 0.85@ (−0.06, 1.76) | |
| Mixed venous O2 content (ml/100ml) | |||||||
| Baseline | 4.3 ± 3.2 | 1.5 ± 5.4 | 4.9 ± 2.5 | 2.0 ± 3.3 | 2.8 ± 2.6 | 3.0 ± 4.5 | 2.0 ± 3.3 |
| Visit 2 | 3.7 ± 1.9 | 1.1 ± 2.4 | 5.9 ± 2.2 | 2.9 ± 1.5 | 2.5 ± 2.5 | 3.8 ± 2.2 | |
| Adjusted mean change* | 0.52 (−0.32, 1.36) | −1.30 (−2.22, 0.38) | 2.49 (1.31, 3.67) | 0.39 (−0.37, 1.17) | −0.32 (−1.03, 0.39) | 0.58 (−0.32, 1.35) | |
Note: unless otherwise indicated, values are given as mean +/− SD.
Abbreviations and definitions: CHD->CHD, remaining on conventional hemodialysis; CHD->SDD, conventional hemodialysis to short daily hemodialysis; CHD->Tx; conventional hemodialysis to transplant; No HD->Tx, received a preemptive transplant; VO2, ***; a-vO2diff, arterial to venous oxygen difference
combines CHD->CHD and CHD->SDD
combines CHD->TX and no HD->Tx
change scores adjusted for baseline values; 95% confidence intervals are given in parentheses
p< 0.03 baseline values compared to controls;
p< 0.05 compared to controls at visit 2.
p < 0.02 for change from baseline to visit 2, compared to those who remained on CHD.
p < 0.04 for change from baseline to visit 2, compared to remained on dialysis;
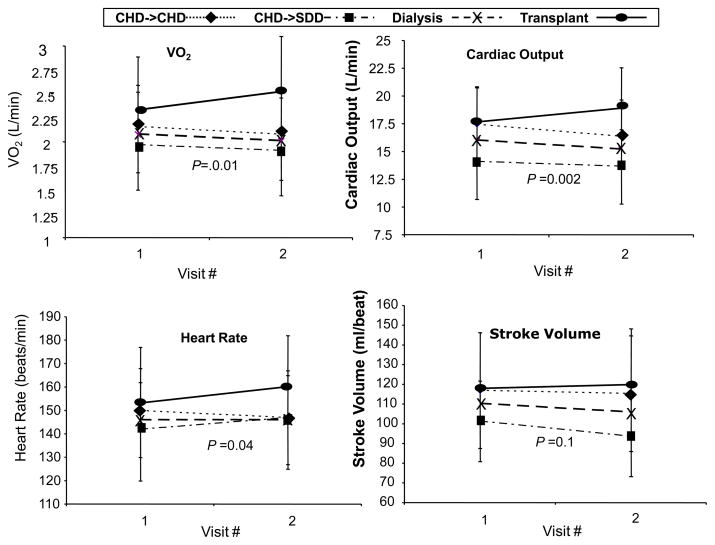
VO2peak did not change from baseline to visit 2 in those who remained on dialysis but increased significantly among the patients who received a transplant (p=0.01) (table 2; figure 2). Significant differences were found between those who remained on dialysis and those who received a transplant in the change in the physiological determinants of VO2peak: the transplant group increased peak cardiac output compared to hemodialysis (p=0.002) as a result of increased peak heart rate (p=0.04). The change in a-vO2dif was not different in those who remained on dialysis versus those who were transplanted (Table 2 and Figure 2) There were no differences in any of these physiological measures between those who stayed on conventional hemodialysis and those who changed to daily hemodialysis (p= 0.9) (Table 2).
Figure 2.
Changes in determine of VO2 at peak exercise. P value pertains to difference in Change between Dialysis and Transplant. Abbreviations: CHD->CHD, remained on conventional hemodialysis; CHD->SDD, changed from conventional hemodialysis to short daily hemodialysis
Despite changes following transplant, patient groups achieved only 68% (hemodialysis) and 79% (transplant) of age-predicted VO2peak. All patient groups remained significantly lower than the controls in peak heart rate with the dialysis patients achieving only 82% of age-predicted heart rate, and transplant patients achieving an average of 90% of age-predicted heart rate at visit 2. Patients also remained lower than controls in a-vO2dif (p=0.03).
Discussion
This is the first study to measure the determinants of VO2peak in ESRD patients treated with different kidney replacement therapies. Patients who remained on dialysis did not change in VO2peak whereas those who were transplanted significantly improved their VO2peak. This change following transplant was the result of increases in peak cardiac output through increases in peak heart rate with no change in stroke volume. There were no changes in a-vO2dif, despite a slight but significant increase in hemoglobin in the transplant group. The change in VO2peak in transplant recipients in this study was less than has been reported previously15, perhaps because the current group had higher VO2peak prior to transplant than in the earlier study (1.68 L/min vs 2.29 L/min). The increase in VO2peak in this study (adjusted mean change of 0.28 L/min), though statistically significant, was minimal in terms of clinical significance. However, given that those remaining on dialysis did not change, increased exercise capacity can be identified as a physiologic benefit of transplants.
Transplant recipients achieved a VO2peak similar to that of the control subjects, however, it should be noted that the control subjects in this study performed worse than expected (84% of age predicted VO2peak). Thus, although VO2peak values improved significantly following transplant, they remained low (79% of their age-predicted VO2peak levels). Kempeneers et al20 reported that VO2peak in kidney transplant recipients remained low compared to normal subjects. It is possible that exercise training may improve VO2peak above that observed with TX alone through improvement in muscle function, which might increase the ability to widen the a-vO2dif. Since we did not observe any change in a-vO2dif with transplant alone, exercise training may be warranted to further improve VO2peak.
It has been suggested that VO2peak in hemodialysis patients could be limited by both central oxygen delivery mechanisms and/or peripheral (skeletal muscle) oxygen utilization factors2, 21, 22. The current study is the first to measure the determinants of VO2peak using the Fick Equation to evaluate physiologic responses to exercise after a change in uremic state. We found that that VO2peak is limited by central factors primarily due to a blunted heart rate response to maximal exercise. In hemodialysis patients, treatment of uremia with successful transplant improves but does not normalize these central responses to exercise and does not change peripheral factors (i.e. ability to widen the a-vO2dif).
Peak heart rates were lower than the controls in the patient groups at both baseline and at visit 2. Beta-blocking agents could have affected the heart rate response to exercise; however, peak heart rate in the transplant group increased at visit 2 despite beta-blocking agents being added to one patient and remained low in the hemodialysis group despite 3 patients discontinuing this class of medication. We attempted to minimize the effects of beta-blocking agents by holding them for at least 15 hours prior to the exercise testing, with most being held from 20–24 hours prior to testing. Painter et al15 analyzed changes in peak heart rate after transplant in only those not on beta-blockers and reported similar changes. The abnormal chronotropic response to exercise in HD patients has been reported elsewhere and could reflect a manifestation of autonomic dysfunction commonly reported in dialysis patients 23–28.
Changes in hemoglobin levels in the transplant group could contribute to changes in VO2peak; however this would have resulted in a widening (or increase) of the a-vO2dif, which was not observed. The earlier study by Painter, et al15 reported that the correlation between change in VO2peak following transplant and change in hematocrit was r2 = 0.13. Also, normalization of hemoglobin with epoetin (from 10.7 g/L to 13.6 g/L) in HD patients did not change VO2peak4. In a recent systematic review and meta-analysis of exercise capacity and ESA treatment in hemodialysis patients, Johansen et al9 concluded that there is no real increase in VO2peak with increasing hematocrit beyond partial correction to 30–33%. Likewise, as reported by Stray-Gundersen et al14 when hemoglobin was increased in hemodialysis patients, the a-vO2dif stayed relatively constant (i.e., with the increase in arterial oxygen content, there was a parallel increase in mixed venous oxygen content 29, suggesting a set limitation in oxygen extraction (that is represented by a-vO2dif). In our transplant recipients, the venous oxygen content did not change, suggesting no change in muscle oxygen extraction/utilization after transplant.
The low a-vO2dif at both testing times in all patient groups suggests a peripheral limitation in these patients. A limitation in a-vO2dif could be due to lower arterial oxygen content, as well as any number of peripheral factors that would affect oxygen extraction by the skeletal muscle (and thus mixed venous oxygen content), including abnormal blood flow to the working muscle30, 31, abnormalities in the skeletal muscle such as reduced capillary density2, 32, increased diffusion distances which reduce oxygen conductance from the capillary to the myofibril30, and abnormal oxidative enzyme levels or activities. Kempeneers et al20 suggested that myopathic abnormalities were responsible for the limitation in VO2peak in kidney transplant recipients.
In patients treated with hemodialysis, the only known way to improve VO2peak is with exercise training. Moore et al2 reported improvements in VO2peak following exercise training in hemodialysis patients that were the result of increases in both cardiac output and a-vO2dif Their study was performed prior to the availability of epoetin, so arterial oxygen content did not change; thus the change in a-vO2dif resulted from changes in oxygen extraction by the skeletal muscle. Although transplants improve VO2peak in ESRD patients, the magnitude of the change observed with a transplant alone is similar to that achieved with exercise training in hemodialysis. Exercise training following transplant improves VO2peak above what is achieved with a transplant alone33. Because transplant recipients may still be limited in cardiac output by a subnormal peak heart rate, optimizing VO2peak following transplant may require exercise training to enhance the skeletal muscle functioning (thus improving the ability to widen the a-vO2dif).
There are limitations to this study, including the fact that patients were not randomized to the various treatments. Although the Frequent Hemodialysis Study is a randomized trial (c comparing remaining on conventional hemodialysis vs. switching to frequent hemodialysis), it is probably not ethical to randomize patients to transplant vs. continued dialysis, especially those who have a living donor readily available. The baseline demographics of the patient groups were similar, and all of the patients who remained on dialysis qualified for transplants (and all except one were on the transplant waiting list). Thus, we believe that the potential bias due to the non-randomized nature of the study was minimized by the strict inclusion criteria. On the other hand, because of our vigorous efforts to include only healthy dialysis patients in order to assure comparable patient groups and isolate the influence of uremia (no diabetes, or cardiovascular disease), this study is not representative of the general ESRD population. We also required significant physical effort over the course of 8 hours, and patients had to be the highest functioning of those on dialysis in order to complete all testing. Thus, we expect that our results overestimate the level of fitness of dialysis patients, but inclusion of sicker dialysis patients would have jeopardized the quality of the exercise tests and run the risk of overestimating the effects of uremia on VO2peak. Additionally, using noninvasive measures often requires assumptions that may not provide a complete picture of what is happening physiologically. The indirect calculation of a-vO2dif with no direct measure of arterial oxygen may not be a true indicator of muscle oxygen use.
The small sample size of our study is also a limitation. We were fully dependent on referrals from the transplant and home hemodialysis programs, and referral was much lower than expected despite weekly reminders and a recruitment system that minimized the time required on the part of the referral programs. Among patients who were referred, several could not be included because of late referral or because of time constraints related to employment. The low numbers required us to collapse the groups into two groups for some analyses.
Despite the disappointing referral numbers, patients who were recruited into the study were highly motivated, allowing us to obtain excellent effort on the exercise test as evidenced by average respiratory exchange ratio >1.1 and average subjective ratings of perceived exertion of > 17 (out of 20). Our control subjects were without known disease but were very sedentary and had low levels of fitness (85% of age predicted values). We selected sedentary controls because we felt it was inappropriate to compare the patients to physically active controls, since these patients were basically inactive, and no exercise intervention was prescribed.
In summary, patients with CKD requiring renal replacement therapy have low exercise capacity as measured by VO2peak. Kidney transplant improves VO2peak but daily dialysis does not. The changes in VO2peak following transplant are due to increases in central (oxygen delivery) mechanisms, and the improved oxygen delivery is the result of an increase in peak cardiac output that is due to an increase in peak heart rates rather than changes in peak stroke volume. Thus, a transplant appears to exert its beneficial effects on cardiorespiratory fitness through mitigation of the limitation of peak heart rate observed in hemodialysis patients.
Acknowledgments
The authors would like to acknowledge the following dialysis centers: Satellite Healthcare (in the San Francisco Bay area, CA), Mt Zion/UCSF Outpatient Hemodialysis, DaVita Dialysis in San Francisco and Minneapolis, Clarian Home Dialysis program (Indianapolis, IN) and Barnes Jewish Dialysis Center at Washington University School of Medicine (St. Louis, MO). The authors would like to thank the following individuals for their assistance in helping to make this study happen: Kimberly Topp, Ph.D., Michele Mietus-Snyder, M.D., Deborah Adey, M.D., Connie Manske, M.D., Brett Miller, M.D., Emil Missov, M.D. Patricia Gordon, Ph.D., Kerry Donnelly-Peterson, Ph.D., Erik Sorenson, M.S., Brittney Nelson, M.S., Marilyn Leister, R.N.,Linda Moczkowski, R.N., Margaret Wolverton, Laura Dillon, B.S. Jaume Padilla and Ken Beck.
Support: This study was funded by a grant from the National Institutes of Health National Institute of Nursing Research (NIH/NINR; RO1-NR008286). Dr Painter receives funding from NIH/NINR (Health Trajectories Research) and pilot funding from University of Minnesota.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Moore GE, Brinker KR, Stray-Gundersen J, Mitchell JH. Determinants of VO2peak in patients with end-stage renal disease: on and off dialysis. Med Sci Sports & Exerc. 1993;25:18–23. doi: 10.1249/00005768-199301000-00004. [DOI] [PubMed] [Google Scholar]
- 2.Moore GE, Parsons DB, Painter PL, Stray-Gundersen J, Mitchell J. Uremic Myopathy limits aerobic capacity in hemodialysis patients. Am J Kidney Dis. 1993;22:277–287. doi: 10.1016/s0272-6386(12)70319-0. [DOI] [PubMed] [Google Scholar]
- 3.Painter PL, Messer-Rehak D, Hanson P, Zimmerman S, Glass NR. Exercise capacity in hemodialysis, CAPD and renal transplant patients. Nephron. 1986;42:47–51. doi: 10.1159/000183632. [DOI] [PubMed] [Google Scholar]
- 4.Painter PL, Moore GE, Carlson L, et al. The Effects of Exercise Training plus Normalization of Hematocrit on Exercise Capacity and Health-Related Quality of Life. Am J Kidney Dis. 2002;39:257–265. doi: 10.1053/ajkd.2002.30544. [DOI] [PubMed] [Google Scholar]
- 5.Sietsema KE, Amato A, Adler SG, Brass EP. Exercise Capacity as a Prognostic Indicator among Ambulatory Patients with End Stage Renal Disease. Kidney Int. 2004;65:719–724. doi: 10.1111/j.1523-1755.2004.00411.x. [DOI] [PubMed] [Google Scholar]
- 6.Storer TW. Anabolic Interventions in ESRD. Adv Chron Kidney Dis. 2009;16:511–528. doi: 10.1053/j.ackd.2009.08.008. [DOI] [PubMed] [Google Scholar]
- 7.Painter P. Physical Functioning in End-Stage Renal Disease Patients: update 2005. Hemodialysis Int. 2005;9:218–235. doi: 10.1111/j.1492-7535.2005.01136.x. [DOI] [PubMed] [Google Scholar]
- 8.Johansen KL. Exercise in the End-Stage Renal Disease Population. J Am Soc Nephrol. 2007;18:1845–1854. doi: 10.1681/ASN.2007010009. [DOI] [PubMed] [Google Scholar]
- 9.Johansen KL, Finkelstein FO, Revicki DA, Gitlin M, Evans C, Mayne TJ. Systematic Review and Meta-analysis of Exercise Tolerance and Physical Functioning in Dialysis Patients Treated with Erythropoiesis-Stimulating Agents. Am J Kidney Dis. 2010;55:535–548. doi: 10.1053/j.ajkd.2009.12.018. [DOI] [PubMed] [Google Scholar]
- 10.Sietsema KE, Hiatt WR, Ester A, Adler S, Amato A, Brass EP. Clinical and demographic predictors of exercise capacity in end-stage renal disease. Am J Nephrol. 2002;39:76–85. doi: 10.1053/ajkd.2002.29884. [DOI] [PubMed] [Google Scholar]
- 11.Deligianis A, Kouidid E, Tassoulas E, Gigis P, Tourkantonis A, Coats A. Cardiac Response to Physical Training in Hemodialysis Patinest: An echocardiographic study at rest and during exercise. Int J Cardiol. 1999;70:253–266. doi: 10.1016/s0167-5273(99)00090-x. [DOI] [PubMed] [Google Scholar]
- 12.Petersen AC, Leikis MJ, McMahon LP, Kent AB, McKenna MJ. Effects of endurance training on extrarenal potassium regulation and exercise performance in patients on haemodialysis. Nephrol Dial Transplant. 2009;24:2882–2888. doi: 10.1093/ndt/gfp157. [DOI] [PubMed] [Google Scholar]
- 13.Sangkabutra T, Crankshaw DP, Schneider C, et al. Impaired K+ regulation contributes to exercise limitation in end-stage renal failure. Kidney Int. 2003;63:283–290. doi: 10.1046/j.1523-1755.2003.00739.x. [DOI] [PubMed] [Google Scholar]
- 14.Stray-Gunderson J, Sams B, Goodkind D, Holloway D, Wang C, Thompson J. Improvement in Functional Capacity in Dialysis Patients with Regular Exercise and Correction of Anemia (abstract) J Am Soc Nephrol. 1997;9:212A. [Google Scholar]
- 15.Painter P, Hanson P, Messer-Rehak D, Zimmerman SW, Glass NR. Exercise tolerance changes following renal transplantation. Am J Kidney Dis. 1987;10:452–456. doi: 10.1016/s0272-6386(87)80192-0. [DOI] [PubMed] [Google Scholar]
- 16.Chan CT, Notarius CF, Meriocco AC, Floras JS. Improvement in exercise duration and capacity after conversion to nocturnal home haemodialysis. Nephrol Dial Transplant. 2007;22:3285–3291. doi: 10.1093/ndt/gfm368. [DOI] [PubMed] [Google Scholar]
- 17.American College of Sports Medicine. Guidelines for Exercise Testing and Prescription. 5. Philadelphia: Williams & Wilkins; 1995. [Google Scholar]
- 18.Johnson BD, Beck KC, Proctor DN, Miller J, Dietz NM, Joyner MJ. Cardiac Output during exericse by the open circuit acetylene wash-in method: comparison with direct Fick. J Appl Physiol. 2000;88:1650–1658. doi: 10.1152/jappl.2000.88.5.1650. [DOI] [PubMed] [Google Scholar]
- 19.Bruce RA, Kusumi F, Hosmer D. Maximal oxygen intake and nomographic assessment of functional aerobic impairment in cardiovascular disease. Am Heart J. 1973;85:546–562. doi: 10.1016/0002-8703(73)90502-4. [DOI] [PubMed] [Google Scholar]
- 20.Kempeneers G, Myburgh KH, Wiggins T, Adams B, vanZyl-Smith R, Noakes TD. Skeletal muscle factors limiting exercise tolerance of renal transplant patients: Effects of a graded exercise training program. Am J Kidney Dis. 1990;14:57–65. doi: 10.1016/s0272-6386(12)80786-4. [DOI] [PubMed] [Google Scholar]
- 21.Painter P. Determinants of Exercise Capacity in CKD Patients Treated with Hemodialysis. Adv Chron Kidney Dis. 2009;16:437–448. doi: 10.1053/j.ackd.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 22.Kouidi E, Albani M, Natsis K, et al. The effects of exercise training on muscle atrophy in haemodialysis patients. Nephrol, Dial, Transplant. 1998;13:685–699. doi: 10.1093/ndt/13.3.685. [DOI] [PubMed] [Google Scholar]
- 23.Ewing DJ, Winney R. Autonomic function in patients with chronic renal failure on intermittent haemodialysis. Nephron. 1975;15:424–429. doi: 10.1159/000180525. [DOI] [PubMed] [Google Scholar]
- 24.Cloarec-Blanchard L, Girard A, Houhou S, Grunfeld JP, Elghozi JL. Spectral analysis of Short Term Blood Pressure and Heart Rate Variability in Uremic Patients. Kidney Int. 1992;37:14–18. [PubMed] [Google Scholar]
- 25.Hathaway DK, Cashion AK, Milstead J, et al. Autonomic Dysregulation in Patients Awaiting Kidney Transplantation. Am J Kidney Dis. 1998;32(2):221–229. doi: 10.1053/ajkd.1998.v32.pm9708605. [DOI] [PubMed] [Google Scholar]
- 26.Deligiannis A, Kouidi E, Tourkantonis A. Effects of Physical Training on Heart Rate Variability in Patients on Hemodialysis. Am J Cardiol. 1999;84:197–202. doi: 10.1016/s0002-9149(99)00234-9. [DOI] [PubMed] [Google Scholar]
- 27.Converse RLJ, Jacobsen TN, Toto RD, et al. Sympathetic overactivity in patients with chronic renal failure. N Eng J Med. 1992;327:1012–1918. doi: 10.1056/NEJM199212313272704. [DOI] [PubMed] [Google Scholar]
- 28.Kettner A, Goldberg A, Hagberg J, Delmez J, Harter H. Cardiovascular and metabolic responses to submaximal exercise in hemodialysis patients. Kidney Int. 1984;26:66–71. doi: 10.1038/ki.1984.135. [DOI] [PubMed] [Google Scholar]
- 29.Painter PL, Moore GEM. The impact of r-HuErythropoeitin on Exercise capacity in hemodialysis patients. Adv Renal Repl Ther. 1994;1(1):55–65. doi: 10.1016/s1073-4449(12)80022-7. [DOI] [PubMed] [Google Scholar]
- 30.Marrades R, Roca J, Campistol J, et al. Effects of Erythropoietin on Muscle O2 Transport during Exercise in Patients with Chronic Renal Failure. J Clin Invest. 1996;97:2092–2100. doi: 10.1172/JCI118646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bradley JR, Anderson JR, Evans DB, Cowley AJ. Impaired nutritive skeletal muscle blood flow in patients with chronic renal failure. Clin Sci. 1990;79:239–245. doi: 10.1042/cs0790239. [DOI] [PubMed] [Google Scholar]
- 32.Diesel W, Emms M, Knight BK, et al. Morphologic features of the myopathy associated with chronic renal failure. Am J Kidney Di. 1993;22:677–684. doi: 10.1016/s0272-6386(12)80430-6. [DOI] [PubMed] [Google Scholar]
- 33.Painter PL, Tomlanovich SL, Hector LA, et al. A Randomized Trial of Exercise Training Following Renal Transplantation. Transplantation. 2002;74(12):42–48. doi: 10.1097/00007890-200207150-00008. [DOI] [PubMed] [Google Scholar]