Abstract
We previously reported that some main olfactory bulb (MOB) mitral/tufted (M/T) cells send a direct projection to the ‘vomeronasal’ amygdala in female mice and selectively respond to volatile male mouse urinary odors. We asked whether MOB M/T cells that project to the vomeronasal amygdala exist in male mice and whether there is a sexually dimorphic response of these neurons to volatile male urinary pheromones. Gonadectomized male and female mice received bilateral injections of the retrograde tracer, Cholera toxin-B (CTb) into the medial amygdala (Me), which is part of the vomeronasal amygdala. All subjects were then treated with estradiol benzoate and progesterone before being exposed to volatile male urinary odors whereupon they were sacrificed 90 min later. Sections of the MOB were immunostained for Fos protein and/or CTb. Male mice, like females, displayed a small population of MOB M/T cells that project to the Me. While the general localization of these cells was similar in the two sexes, there were statistically significant sex differences in the percentage of MOB M/T cells in the anterior and posterior medial segments of the MOB that were retrogradely labeled by CTb. Male urinary volatiles stimulated equivalent, significant increases in Fos expression by MOB M/T neurons projecting to the Me in the two sexes. By contrast, in the same mice exposure to male urinary volatiles stimulated a significant increase in Fos expression by mitral cells in the accessory olfactory bulb (AOB) only in female subjects. Thus any sexually dimorphic behavioral or neuroendocrine responses to male urinary volatiles likely depend on the differential processing of these odor inputs in the AOB and/or other downstream forebrain structures after their detection by the main olfactory system.
Keywords: pheromone, accessory olfactory bulb, Fos protein, estradiol, progeste
Two primary components of the mouse chemosensory system are the main and accessory olfactory pathways (Brennan and Zufall, 2006). The accessory olfactory system includes sensory neurons in the vomeronasal organ (VNO) and their axonal projections to the accessory olfactory bulb (AOB), along with secondary connections of AOB mitral cells to targets in the ‘vomeronasal’ amygdala, including the medial amygdala (Me) and posterior medial amygdala (PMCo) (Winans and Scalia, 1970, Scalia and Winans, 1975, Lehman and Winans, 1982). The Me in turn projects to brain regions controlling many aspects of reproductive behavior, including the medial preoptic area (MPOA), the anterior hypothalamus (AH), the bed nucleus of the stria terminalis (BNST) and the ventromedial nucleus of the hypothalamus (VMH) (Kevetter and Winans, 1981a, Coolen and Wood, 1998, Choi et al., 2005).
One view holds that the accessory olfactory system is solely responsible for the processing of pheromones, which are chemical signals emitted by an individual that elicit specific instinctive behaviors and/or neuroendocrine responses in conspecifics (Karlson and Luscher, 1959, Brennan and Zufall, 2006). For example, lesioning or genetic disabling of the VNO can have profound consequences on sexual/reproductive behaviors in mice. In the absence of a functional VNO, females show impaired lordosis behavior (Keller et al., 2006b, Martel and Baum, 2009a) and are not able to show a Bruce effect (pregnancy block) in response to male pheromones (Lloyd-Thomas and Keverne, 1982, Ma et al., 2002). However, functional inactivation of the VNO, due to either surgical removal (Pankevich et al., 2004) or a mutation in the TRPC2 cation channel (Leypold et al., 2002, Stowers et al., 2002), does not prevent normal mating behavior in male mice, supporting the notion that mating performance in males relies on the main olfactory system (Mandiyan et al., 2005). In mice of both sexes the motivation to remain in proximity to non-volatile urinary pheromones of opposite-sex conspecifics appears to rely on an intact VNO (Pankevich et al., 2004, Keller et al., 2006b, Martel and Baum, 2009a).
The main olfactory system includes sensory neurons in the main olfactory epithelium (MOE) whose axons target MOB glomeruli innervated by mitral and tufted (M/T) cells. Axons of M/T cells, in turn, project via the lateral olfactory tract (LOT) to the olfactory tubercle (OT), piriform cortex (Pir), lateral entorhinal cortex (LEnt), and the ‘olfactory’ amygdala which includes the anterior cortical (ACo) and posterolateral cortical (PLCo) amygdaloid nuclei (Scalia and Winans, 1975, Kevetter and Winans, 1981b, Shipley and Adamek, 1984). Several lines of evidence suggest that the main olfactory system plays a role in pheromone detection in many mammalian species (Kelliher and Baum, 2001, Brennan and Zufall, 2006).
An early study using rabbits (Scalia and Winans, 1975) provided anatomical evidence that, like AOB projection neurons, some MOB M/T cells send axons directly to the Me. More recently, the existence of a direct MOB-Me projection has been demonstrated in the sheep (Jansen et al., 1998), rat (Pro-Sistiaga et al., 2007), and mouse (Kang et al., 2009). Although the function of these Me-projecting MOB M/T cells has not been fully determined, exposure to volatile male urinary odors induced significantly more Fos protein immunoreactivity (Fos-IR; an index of neuronal activation) in Me-projecting MOB M/T cells of estrous female mice than did exposure to female or cat urinary odors (Kang et al., 2009). In order to characterize the distribution of Me-projecting MOB M/T cells in male mice and to identify a possible sex difference in the activation of these cells by volatile male urinary odors, we injected the retrograde tracer, CTb, into the Me to label M/T cell afferents from the MOB in gonadectomized male and female mice treated with the same ovarian sex hormones, estradiol benzoate (EB) and progesterone (P). We later compared Fos-IR in these retrogradely labeled MOB M/T cells as well as in the AOB of female vs. male subjects after exposure to either volatile male urinary odors or clean air.
EXPERIMENTAL PROCEDURES
Subjects and urine collection
Nineteen female and twenty four male Swiss Webster mice (7–12 weeks of age) were purchased (Taconic Farms, Germantown, NY) and maintained on a 12h:12h light/dark cycle with food and water available at all times. All subjects were ovariectomized or castrated under 1.5% isoflurane anesthesia 2 weeks before a CTb injection was administered. Same-sex mice were group housed (4 per cage) until being singly housed 24 hours before odor exposure (see below). All mice received subcutaneous injections of 20 μg EB 3 and 1 days prior to the administration of 500 μg P for two cycles. Odor presentation was carried out 4 hours after the last P injection. Urine was collected from an additional 12 gonadally intact adult male mice housed sequentially in a metabolic cage. Urine from all 12 males was pooled, aliquotted, and frozen at −80º C until use. All procedures involving live animals were approved by the Boston University Charles River Campus Institutional Animal Care and Use Committee.
Reagents
Cholera toxin B (CTb, #104) and goat anti-CTb antibody, #703, were purchased from List Biological Laboratories (Campbell, CA); rabbit anti-cFos antibody, SC-52, was purchased from Santa Cruz Biotechnology (Santa Cruz, CA); biotinylated donkey anti-rabbit antibody was purchased from Jackson ImmunoResearch Laborarories (West Grove, PA); and biotinylated rabbit anti-goat, ABC Elite reagent and diaminobenzidine (DAB) with nickel enhancement were obtained from Vector Laboratories (Burlingame, CA).
Stereotaxic Injections of Tracers
Mice were given bilateral injections of the retrograde tracer, CTb, aimed at layer Ia of the Me. All stereotaxic injections were made with pulled glass micropipettes (tip inner diameter = 10–12 μm) and a CS3 high voltage precision current source (Midgard, Newton, MA). Subjects were anaesthetized using 1–2% isoflurane vapor, and the head was fixed in a stereotaxic apparatus (David Kopf Instruments, Tujunga, CA) using ear bars. The skull was exposed, and lambda and bregma were placed in the same horizontal plane by adjusting the incisor bar. A small hole was drilled above the injection target, and a pipette was carefully lowered into the brain. The retrograde tracer CTb (0.5% in 0.1 M PB, pH 6.0) was injected iontophoretically into the Me. In an attempt to increase the chance of successfully targeting layer 1 of the Me, CTb was delivered at 3 depths for each injection site. The coordinates were 1.2 mm posterior to bregma, 2.0 mm lateral to the midline, and 4.3, 4.45, and 4.6 mm below dura, respectively. A +2 μA alternating (7 sec on/7 second off) current was given for 7 minutes at each depth to deliver CTb. The pipette was left in place for 5 minutes after termination of each injection and was withdrawn from the brain under a −2 μA current. A piece of sterile aluminum foil was glued to the skull using cyanoacryl ester adhesive to seal the hole, and the skin was sutured to close the wound. Between 10 and 14 days after surgery animals were exposed to clean air or male urinary odors, whereupon subjects were sacrificed and the brains were removed for later immunohistochemical processing.
Odor exposure
Mice were placed individually into a narrow plastic box (5 × 10 × 25 cm) equipped with a suspended metal floor with rows of holes so that subjects’ own urine and feces fell below them. Forced air (1.5 L/min) was blown into the chamber from one end and exhausted into a fume hood via an opening on the opposite end. Clean air filtered with activated charcoal was passed through the chamber in the first hour. Over the following 30 min, animals either continued to receive clean air (n=7 and n=8 for females and males, respectively) or male urine (n=12 and n=16 for females and males, respectively). Volatile male urinary vapors were generated by passing clean air over 5 ml of 20% male urine diluted in water, as in previous studies (Schaefer et al., 2001, Martel and Baum, 2007, Kang et al., 2009). Odor delivery consisted of six 3-min exposures, each separated by 2 min of clean air. Clean air was also pulsed off and then on again at these same intervals. One hour after receiving the final odor stimulus, animals were sacrificed and olfactory bulbs were isolated, cryosectioned in the sagittal plane, and processed by immunohistochemistry. Alternate sections were used for 1) double-labeling to visualize CTb and Fos in the MOB, and 2) single-labeling to compare the effects of male mouse urinary volatiles on Fos expression in the AOB (Martel and Baum, 2007, 2009b). Several subjects in which CTb injections missed the Me were included in the latter analysis. Forebrain sections were also collected and single-labeled with CTb antibody to confirm the location of the Me injections in every subject.
Immunohistochemistry
Mice were deeply anesthetized with sodium pentobarbital (150 mg/kg) and perfused transcardially with 100 ml 0.1 M phosphate-buffered saline (PBS) followed by 50 ml 4% paraformaldehyde. Brains were removed and post-fixed in the same fixative for two hours before soaking in 30% sucrose overnight at 4 C. Free-floating sagittal (olfactory bulb) and coronal (forebrain) sections (40 μm) were cut on a freezing microtome and washed in 0.1% Triton-X 100 in PBS (PBST). For single-labeling of CTb or Fos, peroxidases were first inactivated by incubating sections in 40% methanol and 1% H2O2 in PBS for 10 min. Sections were then incubated in blocking solution (PBST containing 5% rabbit or goat serum) for one hour at RT, followed by an overnight incubation at 4 C in one of the following primary antibodies in blocking solution: goat anti-CTb (1:40,000), or rabbit anti-cFos (1:1000). After washes in PBST, sections were transferred to biotinylated secondary antibodies—either biotinylated rabbit anti-goat (1:400) or biotinylated donkey anti-rabbit (1:600)—in blocking solution for two hours at RT. After PBST washes, sections were incubated with ABC (1:200), rinsed in PBST and 0.05 M Tris-Cl buffer (pH=7.6), and visualized in DAB with nickel enhancement. Double-labeling for Fos and CTb was accomplished by first immunostaining for Fos, then re-fixing sections in 4% paraformaldehyde for 10 min and washing in PBST before a second immunostaining for CTb. 5% rabbit serum and 2% donkey serum were added to the secondary antibody solution. Two different chromogens (DAB-nickel, black; and DAB-only, brown) were used for Fos and CTb staining, respectively. After labeling, sections were rinsed in water, mounted and dried in air overnight. Some sections were counter-stained with 1% neutral red before coverslipping.
Image Processing
Light images were captured with an Olympus (BH2) microscope equipped with a Nikon DXM1200 digital camera. Drawings/tracings were made using a Nikon E600 microscope (60X objective, bright field) and a Neurolucida® tracing system (Micro Brightfield, Williston, VT) generously made available by Dr. Douglas Rosene of the Boston University Medical School. Functions (cropping, arranging, merging and labeling) in Adobe Photoshop CS (version 8.0) were used to prepare all figures.
Mapping of CTb- and Fos- labeled cells in the MOB
Although all mice received bilateral CTb injections targeting the Me, only MOB tissues from hemispheres that received successful Me injections of tracer were included in the grouped data analysis. Exclusion criteria included: an injection of CTb outside of the Me or deeper than layer Ia of the Me, or no apparent AOB mitral cell retrograde CTb labeling. Note that in the mouse MOB M/T cells have been reported to project only to targets in the ipsilateral hemisphere (Shipley and Adamek, 1984, Recio et al., 2007). We also found in the present study (more details in Results) that in 14 mice that received successful Me injections of CTb in only one hemisphere, retrograde CTb labeling of M/T cells was seen only in the ipsilateral MOB. No CTb labeling was seen in the contralateral hemisphere of these 14 mice. In an additional 6 subjects that received successful Me injections bilaterally, CTb-labeled MOB cell counts and ratios were averaged from the two hemispheres to yield a single measure for each subject. These values were combined with the appropriate values from mice that had received successful unilateral Me injections of CTb.
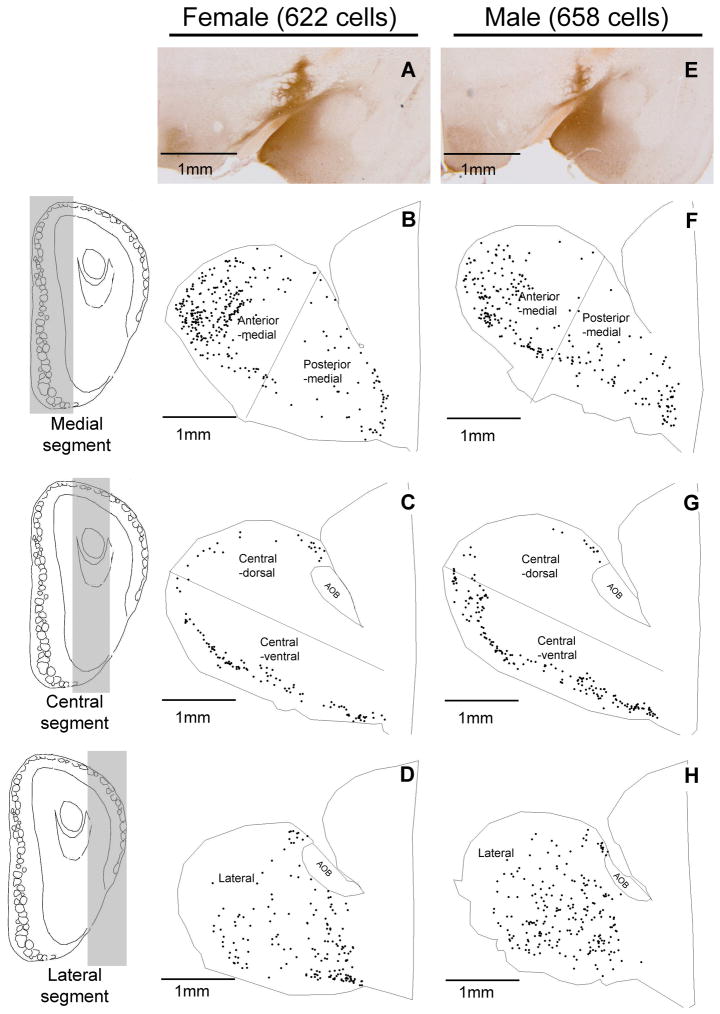
To visualize the distribution of CTb-labeled cells in the MOB, cell positions were traced from sagittal sections using a Neurolucida system. MOB sections were categorized as lateral (>0.92 mm), central (≤0.92 and ≤ 0.64 mm), or medial (<0.64 mm), respectively, based on their estimated distance from the midline (Fig 2). Cells traced from sections in the same category were collapsed to generate maps representing lateral, central and medial segments of the MOB. Sections assigned to the central segment were further divided into dorsal and ventral parts by a virtual line drawn parallel to the ventral surface of the MOB and across the most rostral point of the arch of the MOB mitral cell layer, and sections assigned to the medial segment were further divided into anterior and posterior halves by a virtual line drawn perpendicular to the ventral surface of the olfactory bulb. This categorization yielded 5 segments for analysis (anterior-medial, posterior-medial, central-dorsal, central-ventral, and lateral (see Fig 2). Similar subdividing of the MOB for the analysis of Me-projecting MOB M/T cells was used in our previous paper (Kang et al., 2009) that involved only female mice.
Figure 2.
Collapsed stacks of sagittal Neurolucida drawings from one representative female and one representative male mouse show the distribution of cholera toxin B (CTb)-labeled cells (black dots) in the medial (panels B and F), central (panels C and G), and lateral (panels D and H) segments of the MOB (shown in the sagittal plane; the extent of each segment on the rostral surface of the MOB is shown on the left side of the figure, in coronal sections) following an injection of CTb into the medial amygdala (Me) (Panels A and E). Medial segments (Panels B and F) were further subdivided into anterior and posterior halves by a virtual line drawn perpendicular to the ventral surface of the olfactory bulb. The central segments (Panels C and G) of the MOB were further subdivided into dorsal and ventral regions by an imaginary line drawn parallel to the ventral surface of the olfactory bulb and across the arch of the mitral cell layer at the most rostral position. The shaded areas in the drawings of a coronal section of the olfactory bulb (three panels on left side) are used to illustrate the three MOB segments. CTb-labeled AOB mitral cells were not plotted. The total number of CTb-labeled cells (shown in parentheses) and the general distribution pattern of CTb-labeled cells were similar in these two representative examples of the male and female mouse MOB. However, quantitative analysis (Fig. 3) showed that female mice had significantly more and significantly fewer cells than males in the anterior medial and posterior medial MOB segments, respectively.
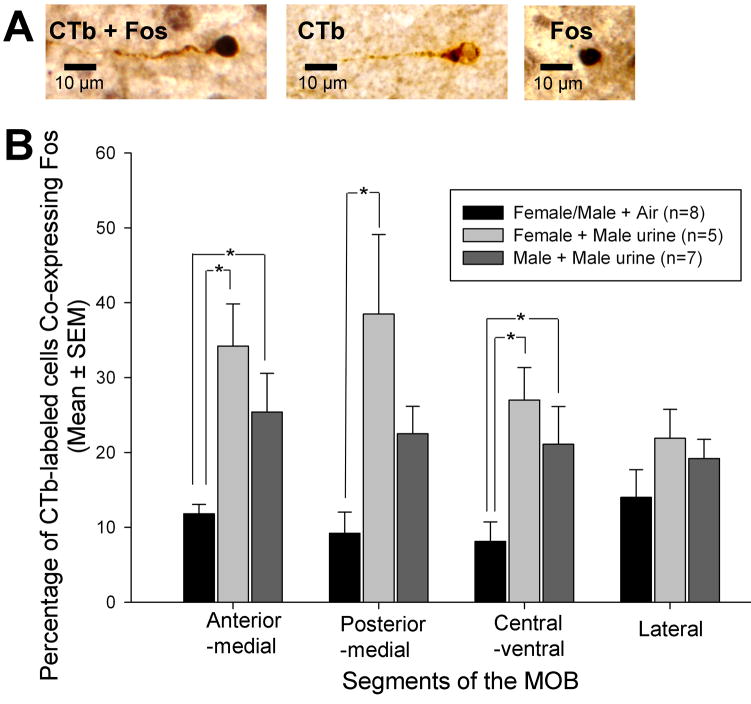
In order to determine whether a sex difference existed in the distribution of Me-projecting MOB cells, CTb-labeled cells in each of the five MOB segments were counted, and the percentage of CTb-labeled cells in each MOB segment was calculated. For this analysis, retrogradely labeled MOB M/T cells that either expressed or did not express Fos protein were identified based on differences in color and cellular localization of staining (Fos: black, nucleus; CTb: brown, cytoplasm, see Fig 4, panel A) under a 40X objective. Using these criteria, cells in following categories were identified using the Neurolucida system without the investigator having any knowledge of sex/odor exposure group: 1) Me-projecting but not activated by odor (CTb-labeled only), and 2) both Me-projecting and activated by odor (CTb/Fos double–labeled). In order to assess the distribution of Me-projecting cells in the MOB, the percentage of CTb-labeled cells (including CTb-labeled only and CTb/Fos double–labeled cells) in each MOB segment was calculated for 5 different segments using the following formula: number of CTb-labeled cells in each segment/number of CTb-labeled cells in the entire MOB X 100. Two-way ANOVAs were used to detect differences in the percentage of CTb-labeled cells for MOB with Sex and Segment as factors. In order to investigate the response of Me-projecting cells to male urinary odors, the percentage of CTb-immunoreactive (IR) cells that co-expressed Fos was calculated for each MOB segment. In the absence of a significant difference between male and female subjects that were exposed to clean air, these two groups were combined into a single clean air control group for comparison with separate groups of males and females that were exposed to male urinary vapors prior to sacrifice. One-way ANOVAs were then used to compare group differences in each segment in the percentage of cells that were co-labeled with Fos; following a significant overall group effect, post hoc comparisons of pairs of means were carried out using Student Newman Keuls tests.
Figure 4.
Effect of volatile male urinary odor exposure on the co-expression of Fos in MOB M/T cells of female and male mice that were retrogradely labeled by a prior injection of cholera toxin B (CTb) into the medial amygdala (Me). Panel A: Three possible types of labeling occurred in MOB M/T cells following Me CTb injections and urinary odor exposure: CTb/Fos double-labeled (CTb + Fos), CTb-single labeled (CTb), and Fos-single labeled (Fos) M/T cells. Panel B: The percentage of CTb-labeled (Me-projecting) M/T cells that co-expressed Fos across 4 segments of the MOB following exposure to volatile male urinary odors or to clean air. Note that male and female subjects exposed to clean air were combined into a single control group. * P<0.05, Student-Newman-Keuls post hoc comparisons between pairs of treatment groups following a significant overall ANOVA.
Counting Fos-IR cells in the AOB
Every fourth sagittal tissue section from the left olfactory bulb of all mice use in this study (all mice had received CTb tracer injections intended to target the left Me) was stained with Fos antibody. Two anatomically matched sagittal sections (which contained the full length of the AOB and were 160μm apart from each other) were analyzed to determine the number of Fos-IR mitral as well as granule cells in the AOB. Digital images were taken via a 40X microscope objective for each of four standard counting areas (circle of 0.28 mm2) that included the anterior and posterior mitral cell and granule cell layers in the two separate olfactory bulb sections. The number of Fos-IR cells in the anterior and posterior mitral and granule cell layers in each section was counted manually by an investigator who had no knowledge of the sex/odor condition of the subjects involved. The values for the anterior and posterior mitral as well as granule cell layers were summed in each section. These values for the two sections for each subject were averaged and later used to compute group mean numbers of Fos-IR cells in the mitral and granule cell layers of the AOB. Again, since the number of Fos-IR cells in the mitral and granule cell layers of the AOB from males and females exposed to clean air did not differ, these data were combined and later compared with values obtained in the AOB of females as well as males that were killed after being exposed to male urinary vapors. One-way ANOVAs were used to compare group differences in the number of Fos-IR AOB mitral and granule cells. Because of prior results (Martel and Baum, 2007) indicating that Fos expression in AOB mitral and granule cells of female, but not male mice, was significantly stimulated by exposure to male urinary volatiles, planned 1-tailed t-test comparisons were used in the present study to compare 1) male-urine exposed female subjects to the clean air control group, and 2) male-urine exposed male subjects to the clean air control group.
RESULTS
Distribution of Me-projecting MOB M/T cells in female and male mice
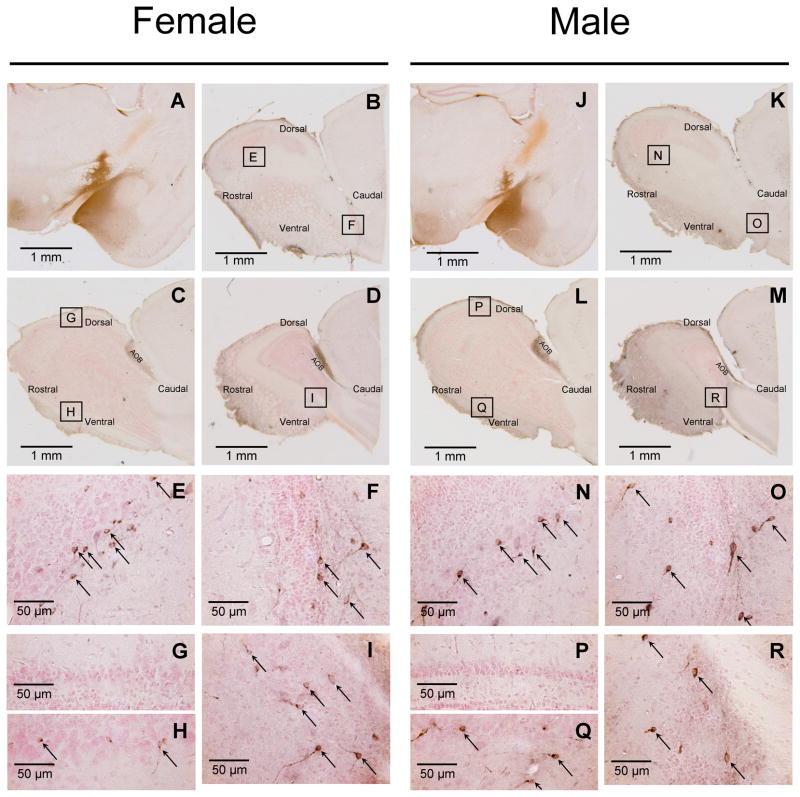
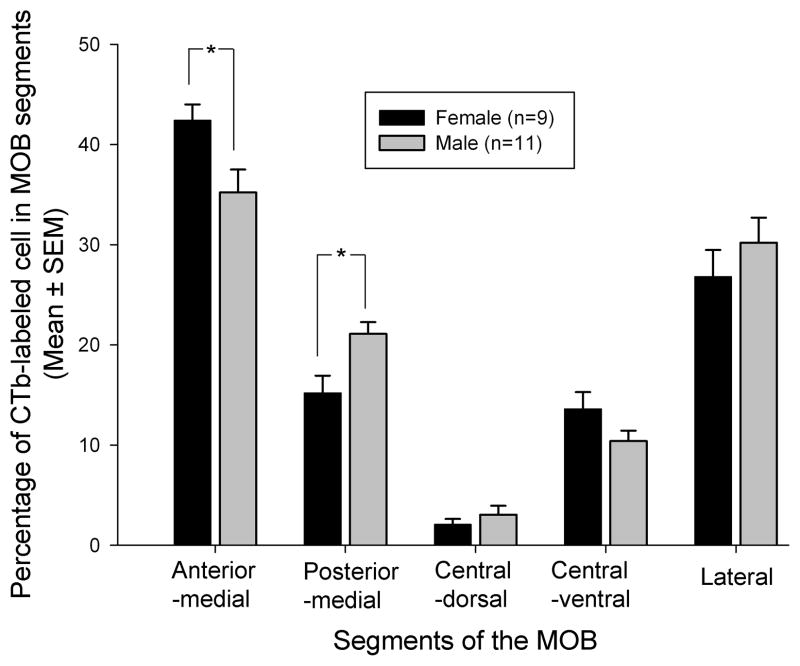
Following injections of CTb into the Me in both female and male mice (Fig 1; panels A and J, respectively), retrogradely labeled cells were found in the mitral cell layer and occasionally in the adjacent external plexiform layer of the MOB as well as in the AOB in both sexes (Fig 1). As was previously found (Kang et al., 2009), CTb injections that targeted the Me, but which missed layer Ia, produced no labeling in MOB M/T cells in either sex (data not shown). Successful retrograde labeling of ipsilateral MOB M/T cells after Me injections of CTb was seen in 30% of injected hemispheres (26 successful injections out of 86 injected hemispheres from 43 animals). Fourteen mice (8 males and 6 females) had retrogradely labeled M/T cells in one (ipsilateral) hemisphere. An additional six mice (3 males and 3 females) had retrogradely labeled M/T cells in both hemispheres. The mean (± SEM) number of CTb labeled cells plotted in male (n=11) and female (n=9) subjects was 90 ± 8 and 101 ± 12, respectively (t(18) = 0.74; p = 0.466). Successful Me injections of CTb resulted in strong retrograde labeling of M/T cells that were concentrated in 4 of the 5 designated MOB segments. These segments included the anterior part of the medial wall (Fig 1, panels B, K, E and N), the posterior part of the medial wall (Fig 1, panels B, K, F and O), a central-ventral region (Fig 1, panels C, L, H and Q), and a region located caudally in the lateral MOB (Fig 1, panels D, M, I and R). The central-dorsal segment contained very few CTb-labeled cells in either sex (Fig 1, panels C, L, G and P). CTb-labeled cells in the 5 MOB segments were mapped in the medial, central, and lateral planes of the MOB for one representative female and one representative male (Fig 2). Although the general pattern of CTb labeling of the MOB was similar in the two sexes, a two-way ANOVA revealed that there was a statistically significant interaction between MOB segment and sex in the percentage of CTb-labeled cells (F (4, 90) = 4.3, P = 0.003). Subsequent post hoc (Student Newman Keuls) tests show that females had a significantly higher percentage of CTb labeled cells in the anterior medial segment than males (P<0.05), whereas males had a significantly higher percentage of CTb labeled cells in the posterior medial segment than females (P<0.05) (Fig 3).
Figure 1.
Immunostaining for cholera toxin B (CTb) shows retrograde labeling of the accessory olfactory bulb (AOB) and the main olfactory bulb (MOB) after injection of CTb into the medial amygdala (Me) of one representative mouse of each sex. Panels A and J: 14 days after tracer injection, CTb injection sites (black staining) was centered in layer I of the Me. Panels B-D and K-M: 14 days after injection of CTb into the Me, CTb-labeled cells (black staining) were seen in the AOB and in the medial (panels B and K), central (panels C and L), and lateral (panels D and M) segments of the MOB. Panels E-I and N-R: high magnification images show that the anterior medial (panels E and N), posterior medial (panels F and O), central ventral (panels H and Q), and lateral (panels I and R) portions of MOB contained CTb-labeled cells (black arrows). Panels G and P: high magnification images show that CTb-labeled cells were absent in the central dorsal portion of the MOB. Boxes shown in Panels B-D and K-M show the exact locations of the MOB regions depicted at higher magnification in Panels E-I and Panels N-R. Tissue was counterstained with neutral red.
Figure 3.
Quantitative sex comparison of medial amygdala (Me)-projecting MOB M/T cells across segments of the MOB. The figure shows the percentage of Me-projecting M/T cells from the entire MOB identified in each of five segments of the MOB following CTb tracer injections into the Me. * P<0.05, Student-Newman-Keuls post hoc comparisons between groups following a significant overall 2-way ANOVA.
Fos expression in response to volatile male urinary odors in Me-projecting MOB M/T cells
In previous mouse studies (Halem et al., 1999, Bodo and Rissman, 2007, Martel and Baum, 2007) forebrain regions of females showed a Fos response to male urinary odors whereas forebrain regions of males showed a lower Fos response to these odors. To determine whether the direct MOB-to-Me pathway responds differently in the two sexes to the same (male) social odors, gonadectomized female and male subjects that previously received CTb tracer into the Me were treated with ovarian hormones and later exposed to volatile urinary odors from adult males. One-way ANOVAs revealed that there were significantly higher percentages of MOB M/T cells that co-expressed Fos in animals exposed to volatile male urinary odors than after exposure to clean air in the anterior-medial (F(2,17) = 7.4, P = 0.004), posterior-medial (F(2,17) = 7.0, P = 0.006), and central-ventral MOB segments (F(2,17) = 5.8, P = 0.012). Following these significant overall group effects, post hoc tests confirmed that male urinary vapor induced a significantly higher percentage of CTb-Fos co-labeling in anterior-medial and central-ventral MOB segments of both male and female subjects compared with the clean air control condition (Fig. 4). Male urinary vapor exposure induced a significantly higher percentage of CTb-Fos co-labeling in the posterior-medial MOB segment only in female subjects (Fig. 4). No significant group differences in the percentage of CTb-Fos co-labeled cells were seen in the lateral MOB segment; the dorsal MOB segment was not analyzed because very few retrogradely labeled M/T cells were seen in this sub-region. In summary, the results suggest that Me-projecting MOB M/T cells were activated by male mouse urinary volatiles in both sexes.
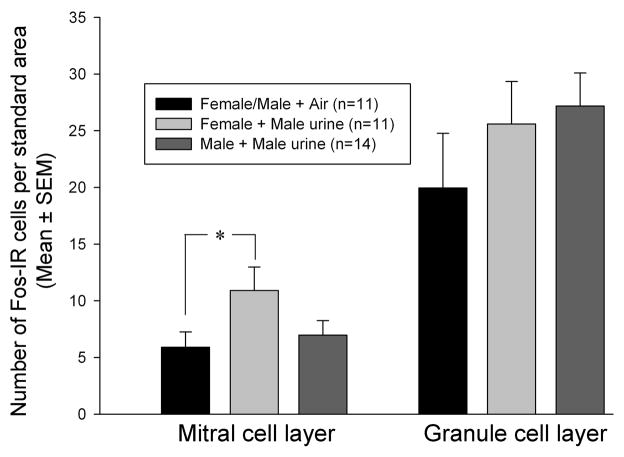
AOB Fos responses to volatile urinary odors
A one-way ANOVA revealed that there was non-significant trend for male urinary vapors to stimulate Fos expression in the AOB mitral cell layer of female subjects (F(2,33) = 2.7, P = 0.085) (Fig 5). Subsequently, a planned comparison using a 1-tailed t test showed that the number of AOB Fos-IR mitral cells was greater in females exposed to male urinary odors than in the control mice exposed to clean air (t(20) = 2.03, p = 0.0275). By contrast, no Fos induction was seen in AOB mitral cells of male subjects exposed to male urinary vapors (t(23) = 0.566, p > 0.05). There were no significant effects of male urinary vapors on the number of Fos-IR AOB granule cells in either male or female subjects (Fig 5).
Figure 5.
Effect of volatile male urinary odor exposure on the expression of Fos in AOB mitral and granule cell layers of female vs male mice. Note that male and female subjects exposed to clean air were combined into a single control group. * p = 0.0275 by a planned one-tailed t-test comparison.
DISCUSSION
In previous work on female mice, anterograde and retrograde tracing techniques were used to show that a population of MOB M/T cells project to sub-nuclei of the Me (Kang et al., 2009). In the present study the retrograde tracer, CTb, was used to confirm that the same direct MOB-Me projection exists in male mice. Another recent study (Kang et al., 2010) also used anterograde tracers to confirm the existence of this MOB-Me projection in male mice. In the present experiment maps of retrogradely labeled Me-projecting M/T cells revealed that these cells were clustered in several MOB segments, particularly the lateral and anterior-medial regions. Overall, the mean number of CTb-labeled MOB M/T cells was similar in males and females. Nevertheless, there were some small, but significant sex differences in the distribution of these cells across MOB segments. The latter anatomical sex differences were not predictive of a functional sex difference in the ability of male urinary vapors to stimulate Fos expression in Me-projecting MOB M/T cells. Thus male urinary vapors stimulated equivalent, significant increments in both female and male subjects in the percentage of CTb-labeled cells in the anterior-medial MOB segment that co-expressed Fos even though there were significantly more CTb-labeled (Me-projecting) M/T cells in this MOB segment in female compared to male subjects. Also, male urinary vapors stimulated significant increments in the percentage of CTb-labeled cells in the posterior-medial MOB of female, but not male, subjects that co-expressed Fos despite the fact that the number of CTb-labeled M/T cells in this segment of the MOB was significantly higher in male than in female subjects. Finally, male urinary vapors stimulated equivalent, significant increments in the percentage of CTb-labeled cells in the central-ventral MOB of both sexes that co-expressed Fos, and there were similar numbers of CTb-labeled M/T cells in this segment of the MOB for both sexes. The absence of consistent sex differences in functional (Fos) responses of Me-projecting MOB M/T cells in the present experiment corresponds to the absence of consistent sex differences in the ability of male vs female urinary vapors to activate MOB glomeruli (indexed by increased Fos expression in periglomerular cells) in gonadectomized male and female mice (Martel and Baum, 2007).
The absence of any consistent functional sex dimorphism in the ability of male urinary vapors to activate Me-projecting MOB M/T cells likely implies that these odors are biologically significant to both sexes following their detection by the main olfactory system. In the case of female mice these male odors signal the presence of a potential reproductive partner. Indeed, destruction of the main olfactory epithelium disrupted mate recognition (Ma et al., 2002, Keller et al., 2006a) as well as the expression of lordosis behavior in female mice (Keller et al., 2006a). In the case of male mice, male urinary vapors signal the presence of a potential competitor. Indeed, genetic disruption of main olfactory signaling eliminated aggressive behavior in male mice (Mandiyan et al., 2005). In our previous study using only female mice (Kang et al., 2009) MOB M/T cells projecting to the Me showed significant Fos co-expression after exposure to male, but not female, urinary vapors. It will be important in future studies to further explore the ability of volatile female urinary odors to activate MOB M/T cells in male subjects and to confirm that these female odors fail to activate this population of MOB M/T cells in female subjects. It may be that same-sex urinary vapors lack biological significance in female mice whereas such odors retain considerable significance in males, which are territorial and aggressive.
Whereas the ability of male urinary vapors to stimulate Fos in Me-projecting M/T cells of the MOB was very similar in the two sexes, volatile male odors stimulated Fos expression in AOB mitral cells (the majority of which project to the Me) of only female subjects. This result corroborates a previous study (Martel and Baum, 2007) showing that only urinary vapors from opposite-sex mice stimulated significant AOB mitral cell Fos responses in both male and female subjects. In that previous study AOB Fos responses were eliminated by zinc sulfate lesioning of the main olfactory epithelium, suggesting that the ability of urinary vapors to stimulate AOB Fos responses depended on their detection and initial processing by the main olfactory system. Two previous tract tracing studies using mice (Barber, 1982, Fan and Luo, 2009) showed that a population of Me neurons extend centrifugal axons to the AOB. In another recent study (Martel and Baum, 2009b), exposure of female subjects to urinary vapors from male, but not from female conspecifics, stimulated Fos expression in this population of AOB-projecting Me neurons. These latter data together with the present results suggest that the Me of female mice processes inputs received from the main olfactory system so that only information about opposite-sex conspecifics is conveyed to the AOB. It should be noted that in another recent study using rats (Larriva-Sahd, 2008) a direct MOB projection to the AOB was documented. It is not known whether a similar projection exists in mice and whether it conveys MOB inputs directly to the AOB in this species. In our previous study (Martel and Baum, 2007) exposure to opposite sex urinary vapors stimulated significant Fos responses in the AOB granule cell layer (in addition to the AOB mitral cell layer) in mice of both sexes. No such stimulation of AOB granule cell Fos expression was seen in the present study even in female subjects, perhaps due to a distortion of the centrifugal Me inputs to the AOB due to a degree of Me destruction following the bilateral infusion of CTb targeting the Me.
The absence of any consistent sex difference in the ability of volatile male urinary odors to stimulate Fos responses in Me-projecting MOB M/T cells contrasts with results of previous studies pointing to a functional sex difference in the responsiveness of VNO sensory neurons as well as AOB mitral cells to non-volatile components of male mouse urine and/or soiled bedding. Thus non-volatile odors from soiled male bedding stimulated significantly more Fos expression in basal VNO sensory neurons of gonadectomized, EB-treated female vs male mice (Halem et al., 1999, Halem et al., 2001), and these functional sex differences correspond with reports (Herrada and Dulac, 1997, Alekseyenko et al., 2006) of sex differences in the expression of receptor genes in the mouse VNO. AOB mitral cells project to targets in the Me as well as other forebrain sites including the bed nucleus of the stria terminalis (Scalia and Winans, 1975, Kang et al., 2010). We (Kang et al., 2006) previously found that direct nasal application of male urine stimulated Fos expression in significantly more Me-projecting AOB mitral cells of female vs male mice. This result corresponds to the present observation that volatile male urinary vapors stimulated Fos expression more readily in AOB mitral cells of female vs male subjects. These observed sex differences in AOB function correlate with a larger literature pointing to anatomical sex differences in the volume of several nuclei along the central projection of the VNO-AOB inputs to the amygdala and hypothalamus (Caminero et al., 1991, Guillamon and Segovia, 1997, Cooke et al., 2003) and in the ability of male pheromones to stimulate Fos expression in hypothalamic targets of this pathway in mice (Halem et al., 1999, Bodo and Rissman, 2007), rats (Bressler and Baum, 1996), and ferrets (Kelliher et al., 1998).
All of the subjects used in the present study had been gonadectomized and treated with EB sequenced with P (as opposed to being left gonad-intact) prior to being exposed to different urinary vapors. We designed our study in this manner so that any anatomical and/or functional (Fos expression) differences obtained would likely reflect hard-wired sex differences as opposed simply to sex differences in circulating gonadal hormones at the time the experiment was conducted (Becker et al., 2005). We chose to administer this regimen of ovarian hormones in the present study because our prior experiments (Martel and Baum, 2007, Kang et al., 2009, Martel and Baum, 2009b) suggested that in response to male urinary vapors, more robust Fos expression in several different forebrain regions was obtained in ovariectomized female mice given these ovarian hormones as opposed to no sex steroids. It remains possible that future studies could reveal a sex difference in either the anatomy of Me-projecting MOB M/T cells and/or in the ability of urinary vapors from estrous females to stimulate Fos expression in these MOB M/T cells of gonadectomized males and females that are treated with either no hormone or with testosterone as opposed to ovarian hormones.
CONCLUSIONS
The present data confirm the previous report (Kang et al., 2009) that a direct pathway from the MOB to the Me exists and is activated by male urinary odors in female mice; our new data extend these findings to the male mouse. Although there is a subtle, significant, sex difference in the MOB distribution of Me-projecting M/T cells, there was no evidence that these M/T cells respond differently in the two sexes to male urinary volatiles. More work will be needed to compare the ability of other social odors (e.g., estrous female urinary volatiles) as well as either food or predator odors to activate this MOB-Me pathway in the two sexes.
Acknowledgments
This work was supported by NIH grant DC 008962 to JAC. We appreciate the help of Dr. Douglas Rosene and Dr. Eustathis Lela Giannaris with Neurolucida cell tracing.
List of Abbreviations
- ACo
anterior cortical amygdala
- AH
anterior hypothalamus
- AOB
accessory olfactory bulb
- BNST
bed nucleus of the stria terminalis
- CTb
Cholera toxin-B
- EB
estradiol benzoate
- Fos-IR
Fos protein immunoreactivity
- LEnt
lateral entorhinal cortex
- LOT
lateral olfactory tract
- Me
medial amygdala
- MOE
main olfactory epithelium
- MPOA
medial preoptic area
- M/T
mitral and tufted cell
- Pir
piriform cortex
- PLCo
posterolateral cortical amygdala,
- PMCo
posteromedial cortical amygdla
- VMH
ventromedial nucleus of the hypothalamus
- VNO
vomeronasal organ
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alekseyenko OV, Baum MJ, Cherry JA. Sex and gonadal steroid modulation of pheromone receptor gene expression in the mouse vomeronasal organ. Neuroscience. 2006;140:1349–1357. doi: 10.1016/j.neuroscience.2006.03.001. [DOI] [PubMed] [Google Scholar]
- Barber PC. Adjacent laminar terminations of two centrifugal afferent pathways to the accessory olfactory bulb in the mouse. Brain Res. 1982;245:215–221. doi: 10.1016/0006-8993(82)90803-4. [DOI] [PubMed] [Google Scholar]
- Becker JB, Arnold AP, Berkley KJ, Blaustein JD, Eckel LA, Hampson E, Herman JP, Marts S, Sadee W, Steiner M, Taylor J, Young E. Strategies and methods for research on sex differences in brain and behavior. Endocrinology. 2005;146:1650–1673. doi: 10.1210/en.2004-1142. [DOI] [PubMed] [Google Scholar]
- Bodo C, Rissman EF. Androgen receptor is essential for sexual differentiation of responses to olfactory cues in mice. Eur J Neurosci. 2007;25:2182–2190. doi: 10.1111/j.1460-9568.2007.05484.x. [DOI] [PubMed] [Google Scholar]
- Brennan PA, Zufall F. Pheromonal communication in vertebrates. Nature. 2006;444:308–315. doi: 10.1038/nature05404. [DOI] [PubMed] [Google Scholar]
- Bressler SC, Baum MJ. Sex comparison of neuronal Fos immunoreactivity in the rat vomeronasal projection circuit after chemosensory stimulation. Neuroscience. 1996;71:1063–1072. doi: 10.1016/0306-4522(95)00493-9. [DOI] [PubMed] [Google Scholar]
- Caminero AA, Segovia S, Guillamon A. Sexual dimorphism in accessory olfactory bulb mitral cells: a quantitative Golgi study. Neuroscience. 1991;45:663–670. doi: 10.1016/0306-4522(91)90279-w. [DOI] [PubMed] [Google Scholar]
- Choi GB, Dong HW, Murphy AJ, Valenzuela DM, Yancopoulos GD, Swanson LW, Anderson DJ. Lhx6 delineates a pathway mediating innate reproductive behaviors from the amygdala to the hypothalamus. Neuron. 2005;46:647–660. doi: 10.1016/j.neuron.2005.04.011. [DOI] [PubMed] [Google Scholar]
- Cooke BM, Breedlove SM, Jordan CL. Both estrogen receptors and androgen receptors contribute to testosterone-induced changes in the morphology of the medial amygdala and sexual arousal in male rats. Horm Behav. 2003;43:336–346. doi: 10.1016/s0018-506x(02)00047-8. [DOI] [PubMed] [Google Scholar]
- Coolen LM, Wood RI. Bidirectional connections of the medial amygdaloid nucleus in the Syrian hamster brain: simultaneous anterograde and retrograde tract tracing. J Comp Neurol. 1998;399:189–209. doi: 10.1002/(sici)1096-9861(19980921)399:2<189::aid-cne4>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- Fan S, Luo M. The organization of feedback projections in a pathway important for processing pheromonal signals. Neuroscience. 2009;161:489–500. doi: 10.1016/j.neuroscience.2009.03.065. [DOI] [PubMed] [Google Scholar]
- Guillamon A, Segovia S. Sex differences in the vomeronasal system. Brain Res Bull. 1997;44:377–382. doi: 10.1016/s0361-9230(97)00217-7. [DOI] [PubMed] [Google Scholar]
- Halem HA, Baum MJ, Cherry JA. Sex difference and steroid modulation of pheromone-induced immediate early genes in the two zones of the mouse accessory olfactory system. J Neurosci. 2001;21:2474–2480. doi: 10.1523/JNEUROSCI.21-07-02474.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halem HA, Cherry JA, Baum MJ. Vomeronasal neuroepithelium and forebrain Fos responses to male pheromones in male and female mice. J Neurobiol. 1999;39:249–263. [PubMed] [Google Scholar]
- Herrada G, Dulac C. A novel family of putative pheromone receptors in mammals with a topographically organized and sexually dimorphic distribution. Cell. 1997;90:763–773. doi: 10.1016/s0092-8674(00)80536-x. [DOI] [PubMed] [Google Scholar]
- Jansen HT, Iwamoto GA, Jackson GL. Central connections of the ovine olfactory bulb formation identified using wheat germ agglutinin-conjugated horseradish peroxidase. Brain Res Bull. 1998;45:27–39. doi: 10.1016/s0361-9230(97)00279-7. [DOI] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. A direct main olfactory bulb projection to the ‘vomeronasal’ amygdala in female mice selectively responds to volatile pheromones from males. Eur J Neurosci. 2009;29:624–634. doi: 10.1111/j.1460-9568.2009.06638.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Baum MJ, Cherry JA. Different profiles of main and accessory olfactory bulb mitral/tufted cell projections revealed in mice using an anterograde tracer and a whole mount, flattened cortex preparation. Chem Senses. 2010 doi: 10.1093/chemse/bjq120. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang N, Janes A, Baum MJ, Cherry JA. Sex difference in Fos induced by male urine in medial amygdala-projecting accessory olfactory bulb mitral cells of mice. Neurosci Lett. 2006;398:59–62. doi: 10.1016/j.neulet.2005.12.062. [DOI] [PubMed] [Google Scholar]
- Karlson P, Luscher M. Pheromones’: a new term for a class of biologically active substances. Nature. 1959;183:55–56. doi: 10.1038/183055a0. [DOI] [PubMed] [Google Scholar]
- Keller M, Douhard Q, Baum MJ, Bakker J. Destruction of the main olfactory epithelium reduces female sexual behavior and olfactory investigation in female mice. Chem Senses. 2006a;31:315–323. doi: 10.1093/chemse/bjj035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keller M, Pierman S, Douhard Q, Baum MJ, Bakker J. The vomeronasal organ is required for the expression of lordosis behaviour, but not sex discrimination in female mice. Eur J Neurosci. 2006b;23:521–530. doi: 10.1111/j.1460-9568.2005.04589.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher KR, Baum MJ. Nares occlusion eliminates heterosexual partner selection without disrupting coitus in ferrets of both sexes. J Neurosci. 2001;21:5832–5840. doi: 10.1523/JNEUROSCI.21-15-05832.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelliher KR, Chang YM, Wersinger SR, Baum MJ. Sex difference and testosterone modulation of pheromone-induced NeuronalFos in the Ferret’s main olfactory bulb and hypothalamus. Biol Reprod. 1998;59:1454–1463. doi: 10.1095/biolreprod59.6.1454. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. I. Efferents of the “vomeronasal amygdala”. J Comp Neurol. 1981a;197:81–98. doi: 10.1002/cne.901970107. [DOI] [PubMed] [Google Scholar]
- Kevetter GA, Winans SS. Connections of the corticomedial amygdala in the golden hamster. II. Efferents of the “olfactory amygdala”. J Comp Neurol. 1981b;197:99–111. doi: 10.1002/cne.901970108. [DOI] [PubMed] [Google Scholar]
- Larriva-Sahd J. The accessory olfactory bulb in the adult rat: a cytological study of its cell types, neuropil, neuronal modules, and interactions with the main olfactory system. J Comp Neurol. 2008;510:309–350. doi: 10.1002/cne.21790. [DOI] [PubMed] [Google Scholar]
- Lehman MN, Winans SS. Vomeronasal and olfactory pathways to the amygdala controlling male hamster sexual behavior: autoradiographic and behavioral analyses. Brain Res. 1982;240:27–41. doi: 10.1016/0006-8993(82)90641-2. [DOI] [PubMed] [Google Scholar]
- Leypold BG, Yu CR, Leinders-Zufall T, Kim MM, Zufall F, Axel R. Altered sexual and social behaviors in trp2 mutant mice. Proc Natl Acad Sci U S A. 2002;99:6376–6381. doi: 10.1073/pnas.082127599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd-Thomas A, Keverne EB. Role of the brain and accessory olfactory system in the block to pregnancy in mice. Neuroscience. 1982;7:907–913. doi: 10.1016/0306-4522(82)90051-3. [DOI] [PubMed] [Google Scholar]
- Ma D, Allen ND, Van Bergen YC, Jones CM, Baum MJ, Keverne EB, Brennan PA. Selective ablation of olfactory receptor neurons without functional impairment of vomeronasal receptor neurons in OMP-ntr transgenic mice. Eur J Neurosci. 2002;16:2317–2323. doi: 10.1046/j.1460-9568.2002.02303.x. [DOI] [PubMed] [Google Scholar]
- Mandiyan VS, Coats JK, Shah NM. Deficits in sexual and aggressive behaviors in Cnga2 mutant mice. Nat Neurosci. 2005;8:1660–1662. doi: 10.1038/nn1589. [DOI] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Sexually dimorphic activation of the accessory, but not the main, olfactory bulb in mice by urinary volatiles. Eur J Neurosci. 2007;26:463–475. doi: 10.1111/j.1460-9568.2007.05651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. Adult testosterone treatment but not surgical disruption of vomeronasal function augments male-typical sexual behavior in female mice. J Neurosci. 2009a;29:7658–7666. doi: 10.1523/JNEUROSCI.1311-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martel KL, Baum MJ. A centrifugal pathway to the mouse accessory olfactory bulb from the medial amygdala conveys gender-specific volatile pheromonal signals. Eur J Neurosci. 2009b;29:368–376. doi: 10.1111/j.1460-9568.2008.06564.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pankevich DE, Baum MJ, Cherry JA. Olfactory sex discrimination persists, whereas the preference for urinary odorants from estrous females disappears in male mice after vomeronasal organ removal. J Neurosci. 2004;24:9451–9457. doi: 10.1523/JNEUROSCI.2376-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pro-Sistiaga P, Mohedano-Moriano A, Ubeda-Banon I, Del Mar Arroyo-Jimenez M, Marcos P, Artacho-Perula E, Crespo C, Insausti R, Martinez-Marcos A. Convergence of olfactory and vomeronasal projections in the rat basal telencephalon. J Comp Neurol. 2007;504:346–362. doi: 10.1002/cne.21455. [DOI] [PubMed] [Google Scholar]
- Recio JS, Weruaga E, Gomez C, Valero J, Brinon JG, Alonso JR. Changes in the connections of the main olfactory bulb after mitral cell selective neurodegeneration. J Neurosci Res. 2007;85:2407–2421. doi: 10.1002/jnr.21387. [DOI] [PubMed] [Google Scholar]
- Scalia F, Winans SS. The differential projections of the olfactory bulb and accessory olfactory bulb in mammals. J Comp Neurol. 1975;161:31–55. doi: 10.1002/cne.901610105. [DOI] [PubMed] [Google Scholar]
- Schaefer ML, Young DA, Restrepo D. Olfactory fingerprints for major histocompatibility complex-determined body odors. J Neurosci. 2001;21:2481–2487. doi: 10.1523/JNEUROSCI.21-07-02481.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shipley MT, Adamek GD. The connections of the mouse olfactory bulb: a study using orthograde and retrograde transport of wheat germ agglutinin conjugated to horseradish peroxidase. Brain Res Bull. 1984;12:669–688. doi: 10.1016/0361-9230(84)90148-5. [DOI] [PubMed] [Google Scholar]
- Stowers L, Holy TE, Meister M, Dulac C, Koentges G. Loss of sex discrimination and male-male aggression in mice deficient for TRP2. Science. 2002;295:1493–1500. doi: 10.1126/science.1069259. [DOI] [PubMed] [Google Scholar]
- Winans SS, Scalia F. Amygdaloid nucleus: new afferent input from the vomeronasal organ. Science. 1970;170:330–332. doi: 10.1126/science.170.3955.330. [DOI] [PubMed] [Google Scholar]