Abstract
Background
Only a minority of patients receiving implantable cardioverter-defibrillators (ICDs) for the primary prevention of sudden death receive appropriate shocks, yet almost as many are subjected to inappropriate shocks and device complications. Identifying and quantifying myocardial scar, which forms the substrate for ventricular tachyarrhythmias, may improve risk-stratification.
Objective
To determine if the absence of myocardial scar detected by novel 12-lead ECG Selvester QRS-scoring criteria identifies patients with low risk for appropriate ICD shocks.
Methods
We applied QRS-scoring to 797 patients from the ICD arm of the Sudden Cardiac Death in Heart Failure Trial. Patients were followed for a median of 45.5 months for ventricular tachycardia/fibrillation treated by the ICD or sudden tachyarrhythmic death (combined group referred to as VT/VF).
Results
Increasing QRS-score scar size predicted higher rates of VT/VF. Patients with no scar (QRS-score=0) represented a particularly low-risk cohort with 48% fewer VT/VF events than the rest of the population (absolute difference 11%; hazard ratio 0.52, 95% CI=0.31–0.88). QRS-score scar absence vs. presence remained a significant prognostic factor after controlling for 10 clinically-relevant variables. Combining QRS-score (scar absence vs. presence) with ejection fraction (≥25% vs. <25%) distinguished low-, middle-, and high-risk subgroups with 73% fewer VT/VF events in the low- vs. high-risk group (absolute difference 22%; hazard ratio=0.27, 95% CI=0.12–0.62).
Conclusions
Patients with no scar by QRS-scoring have significantly fewer VT/VF events. This inexpensive 12-lead ECG tool provides unique, incremental prognostic information and should be considered in risk-stratifying algorithms for selecting patients for ICDs.
Introduction
The Sudden Cardiac Death in Heart Failure Trial (SCD-HeFT) and the Multicenter Automatic Defibrillator Implantation Trial II (MADIT-II) demonstrated that implantation of a cardioverter-defibrillator (ICD) for primary prevention of SCD significantly decreased mortality in patients with reduced left ventricular ejection fraction (LVEF) (1,2). However, only a minority of patients receive appropriate ICD shocks for ventricular tachyarrhythmias, while almost as many patients receive inappropriate shocks, which are associated with increased mortality (3,4). Furthermore, patients are subjected to device related complications (5). Finally, under current implantation guidelines the extent to which ICDs are cost-effective is controversial (6). Better risk stratification of potential ICD candidates could improve the cost-effectiveness of this therapy if device placement is avoided in those unlikely to benefit and instead, specifically targeted to those in whom maximal benefit is expected (6–8). For this purpose, a widely available, inexpensive, noninvasive diagnostic tool that facilitates mass screening would be most ideal.
Ventricular tachycardia and fibrillation (VT/VF) leading to SCD result from the interaction of abnormal myocardial anatomic/functional substrate and electrophysiologic triggering events (9). Although reduced LVEF is a risk factor for arrhythmogenesis, it is not synonymous with the structural myocardial damage needed to support arrhythmic circuits (9,10). Regions of prior myocardial infarction are potentially arrhythmogenic, irrespective of LVEF, as are myocardial scars in nonischemic cardiomyopathies (11,12). Characterization of the myocardial substrate (scar) as a risk predictor has not been investigated in published, large randomized, ICD clinical trials. Recent observational studies have used contrast-enhanced magnetic resonance imaging (MRI) to identify and quantify myocardial scar. MRI scar presence and characteristics predict inducibility of arrhythmias by programmed stimulation (13–15) and prognosis (16–19). While MRI analysis of myocardial scar is a promising risk-stratifying tool for arrhythmias, it is costly and not commonly available. In contrast, the 12-lead ECG is inexpensive and universally available, and can be readily used to perform Selvester QRS-scoring, which estimates infarct/scar size by quantifying changes in Q-, R- and S-wave durations, amplitudes and morphologies (20).
The Selvester QRS-score consists of 32 total possible points with each point reflecting myocardial infarction involving 3% of the left ventricle (LV) (20). With training, the score can be performed in 2–5 minutes and multiple automated versions have been developed with further implementation for widespread use underway (20–24). Prior studies with QRS scoring excluded ECG confounding factors such as the presence of ventricular hypertrophy and bundle branch blocks which were thought to preclude accurate electrocardiographic infarct diagnosis. However, modified QRS-scoring systems for use in the presence of confounders were created based on computer simulation and recently validated in comparison to MRI scar size and shown to predict the substrate for arrhythmias defined by inducibility of monomorphic VT during programmed stimulation (25).
The current study was performed to test the hypothesis that the absence of scar by 12-lead ECG QRS-scoring identifies patients with a low risk of ICD shocks for sustained VT/VF or sudden tachyarrhythmic death in SCD-HeFT.
Methods
Study patients
The main SCD-HeFT study enrolled patients with New York Heart Association (NYHA) class II or III heart failure with LVEF ≤35% (1). The study was approved by an institutional review committee and all subjects gave informed consent. Notably, heart failure etiology was defined as ischemic if patients had ≥75% narrowing in ≥1 major coronary artery or a history of infarction. Patients without these criteria were defined as nonischemic. Patients were randomly assigned to one of three arms: single-chamber ICD (n=829), amiodarone (n=845) or placebo (n=847). We retrospectively performed QRS-scoring on the baseline, pre-device 12-lead ECGs of the patients randomized to ICD. Of these patients, 18 of 829 (2%) did not receive an ICD and were excluded from analysis.
12-lead ECG Protocol
All patients received baseline 12-lead ECGs, before randomization (1). Standard 12-lead ECGs (10 mm/mV and 25 mm/sec) were sent to the ECG QRS-scoring core laboratory for analysis. There were no uniform ECG filter settings. Analysis was performed blinded to all clinical and ICD data except for age and gender, which are considered when performing QRS-scoring. A single investigator analyzed all ECGs using a standardized protocol with quality control by two additional investigators. The QRS-score analysis protocol has been reported previously (20,25) (Appendix). Briefly, ECGs were first classified by primary ventricular conduction/hypertrophy type: left bundle branch block (LBBB), left anterior fascicular block (LAFB), left ventricular hypertrophy, right bundle branch block (RBBB), RBBB+LAFB or no confounders. The QRS-scoring system for the appropriate conduction/hypertrophy type was then applied, which involves measurements of Q-, R-, and S-wave amplitudes, durations, amplitude ratios and notches in 10 of the 12 standard ECG leads (excluding leads III and aVR). No patients had ventricular pacing or preexcitation, which would preclude QRS-scoring.
ICD Protocol, Electrogram Classification and Death Classification
The ICD implantation protocol, device settings and electrogram core laboratory protocol have been reported previously in detail (3). By design, the ICDs in SCD-HeFT were restricted to single-lead devices with a detection rate of ≥188 beats/min and no anti-tachycardia pacing programmed (1,3). Bradycardia pacing was set to 50 beats/min with a hysteresis of 34 beats/min. Recorded ICD data were sent to the SCD-HeFT ICD electrogram core laboratory for review at 3-month follow-up visits, after known ICD therapy and, when possible, after patient deaths (3). Two members of the committee, blinded to all patient data, classified each rhythm before and after each shock episode according to pre-specified criteria which included onset characteristics, electrograms recorded before detection of the arrhythmia and after delivery of the shock, and plots of RR intervals (3). Differences were adjudicated by the full committee.
The death classification protocol has been reported in detail previously (26). Each patient’s cause of death was classified by an events committee that was blinded to the patient’s randomized therapy (i.e. ICD, amiodarone or placebo). Thus the committee did not receive ICD interrogation data, but did receive ECG rhythm strips when available (26). Of note, in the main SCD-HeFT study, sudden tachyarrhythmic death occurred in 4.5% of patients assigned to ICD therapy and 11.2% of patients in the placebo arm.
For the current QRS-score SCD-HeFT substudy, the primary endpoint consisted of first occurrence of an appropriate shock for sustained VT/VF or sudden tachyarrhythmic death. The secondary endpoint of first occurrence of an appropriate shock or mortality of any cause was also included to account for any limitations in classifying the cause of death. The additional secondary outcomes of first-occurrence of inappropriate shock and all-cause mortality were also examined and are presented in the Online Data Supplement.
Statistical Analysis
Baseline patient data are presented with standard descriptive statistics with Wilcoxon rank-sum and chi-square statistic for comparisons. Kappa analysis (κ) was used to assess intra- and inter-observer variability of QRS-scoring in a subset of 70 randomly selected patients that included all conduction types. Spline transformations were applied to confirm the linearity between continuous QRS-score and outcome. The QRS-score was then assessed using dichotomous cutpoints. The relationship of QRS-score to events was assessed with univariate Cox proportional-hazards models for all patients stratified by cardiomyopathy etiology and using subgroup analysis of the ischemic and nonischemic cohorts. Event-free survival was depicted using the Kaplan-Meier method.
Adjusted hazard ratios were calculated with multivariable Cox models controlling for 10 clinical and ECG characteristics commonly associated with increased arrhythmic risk, including variables found to be significant in a model developed from MADIT-II (excluding blood urea nitrogen, which was not available in SCD-HeFT; however creatinine was included) (27). Dichotomous cutpoints were selected based on the prior MADIT-II multivariable model: age >70 years, NYHA Class >II, nonischemic vs. ischemic heart failure etiology, creatinine >1.3 mg/dl, diabetes presence, heart rate ≥80 bpm, QRS duration >120 ms, LBBB presence and atrial fibrillation/flutter presence. Although the MADIT-II model used an LVEF cutpoint of ≥20%, we used ≥25% as it represented the median LVEF for the SCD-HeFT cohort. All p-values are two-sided with p-values <0.05 considered statistically significant.
Results
Study Population
Of the 811 patients receiving ICDs, 797 (98%) had technically adequate 12-lead ECGs. Table 1 shows the population baseline characteristics, which were similar to those of the overall SCD-HeFT population. Patients with no ECG-estimated scar (QRS-score=0) differed from those with scar (QRS-score ≥ 1) in ischemic vs. nonischemic heart failure etiology, LVEF, QRS duration and ECG conduction type (Table 1). Of note, patients without scar had similar median LVEF than patients with scar (25% vs. 24%) and there was no overall correlation between QRS-score scar size and LVEF (r = −0.018, p=0.61). After a 45.5 month median follow-up, 177 patients (22.2%) had at least one appropriate ICD shock for VT/VF (101 VT and 76 VF). Eighteen additional patients (2.3%) had sudden tachyarrhythmic death and 83 additional patients (10.4%) died from non-tachyarrhythmic causes without any known appropriate shock prior to death. Thus, 24.5% of patients met the primary endpoint (appropriate ICD shock or sudden tachyarrhythmic death) and 34.9% of patients met the secondary endpoint (appropriate ICD shock or mortality of any cause).
Table 1.
Patient Baseline Characteristics by QRS-score and for All Patients
| Variable | Scar Absent (QRS- score=0) (N=101) | Scar Present (QRS- score≥1) (N=696) | p-value | All Patients (N=797) |
|---|---|---|---|---|
| Age, median [IQR], y | 58 [49–67] | 60 [52–69] | 0.09 | 60 [52–69] |
| Male, No. (%) | 76 (75.3) | 537 (77.2) | 0.67 | 613 (76.9) |
| Caucasian, No. (%) | 79 (78.2) | 535 (76.9) | 0.76 | 614 (77.0) |
| NYHA Heart Failure Class III, No. (%) | 26 (25.7) | 227 (32.6) | 0.16 | 253 (31.7) |
| Ischemic cause of heart failure, No. (%) | 28 (27.7) | 382 (54.9) | <0.001 | 410 (51.4) |
| Ejection Fraction, median [IQR] | 25 [20–30] | 24 [19–30] | 0.03 | 24 [19–30] |
| Creatinine, median [IQR], mg/dl | 1.1 [0.9–1.3] | 1.1 [0.9–1.4] | 0.12 | 1.1 [0.9–1.3] |
| Diabetes, No. (%) | 24 (23.8) | 220 (31.6) | 0.10 | 244 (30.6) |
| Hypertension, No. (%) | 56 (55.5) | 376 (54.0) | 0.79 | 432 (54.2) |
| Hyperlipidemia, No. (%) | 45 (44.6) | 366 (52.7) | 0.13 | 411 (51.6) |
| Heart rate, median [IQR], beats/min | 75 [64–80] | 74 [65–84] | 0.67 | 74 [65–84] |
| QRS Duration, median [IQR], ms | 100 [92–110] | 114 [98–130] | <0.001 | 112 [96–130] |
| Atrial fibrillation or flutter, No. (%) | 4 (3.9) | 60 (8.6) | 0.08 | 64 (8.0) |
| Conduction type, No. (%) | <0.001 | |||
| No Confounders | 58 (57.4) | 307 (44.1) | 365 (45.8) | |
| LVH | 32 (31.7) | 127 (18.3) | 159 (20.0) | |
| LAFB | 3 (3.0) | 58 (8.3) | 61 (7.7) | |
| LBBB | 7 (6.9) | 164 (23.6) | 171 (21.5) | |
| RBBB | 1 (1.0) | 11 (1.6) | 12 (1.5) | |
| RBBB+LAFB | 0 (0.0) | 29 (4.2) | 29 (3.6) | |
| ACE-inhibitors or ARB, No. (%) | 97 (96.0) | 657 (94.4) | 0.48 | 754 (94.6) |
| Beta-blockers, No. (%) | 75 (74.3) | 481 (69.1) | 0.29 | 556 (69.8) |
| Diuretic therapy, No. (%) | 88 (87.1) | 602 (86.5) | 0.86 | 690 (86.6) |
| Digitalis therapy, No. (%) | 64 (63.4) | 471 (67.7) | 0.39 | 535 (67.1) |
ACE = angiotensin converting enzyme; ARB = angiotensin receptor blocker; IQR = interquartile range; LAFB = left anterior fascicular block; LBBB = left bundle branch block; LVH = left ventricular hypertrophy; No. = number; NYHA = New York Heart Association; RBBB = right bundle branch block.
QRS-Scoring Analysis
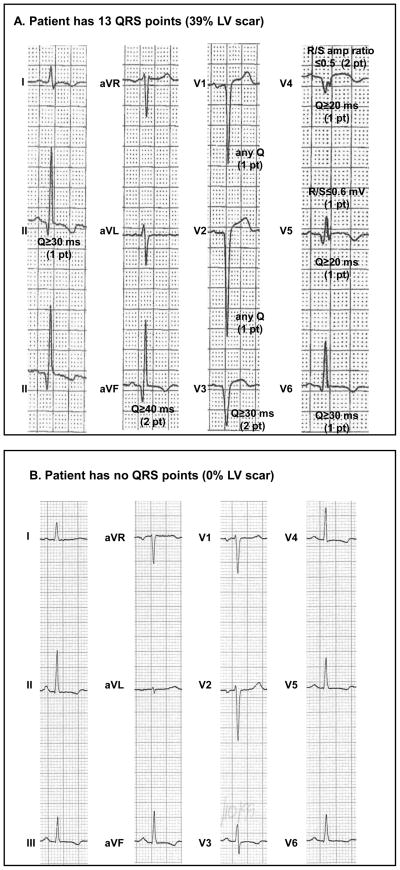
The distribution of ECG conduction types was 171 LBBB (21.5%), 12 RBBB (1.5%), 61 LAFB (7.7%), 29 RBBB and LAFB (3.6%), 159 left ventricular hypertrophy (20.0%) and 365 no confounders (45.8%). For the entire cohort, the median QRS-score was 5 QRS points (15% of LV infarcted/scarred; interquartile range 2–7 points). In the ischemic patients, the median QRS-score was 6 points (range 0–20) while in the nonischemics, it was 3 QRS points (range 0–20). Twenty-eight ischemic and 73 nonischemic patients had a QRS-score of 0 (13% of all patients) suggesting no localized LV scar. The QRS-score criteria obtained from the ECG in Figure 1A are indicated, totaling 13 points (39% of the LV). In contrast, the ECG in Figure 1B received a QRS score of 0 (no scar).
Figure 1. ECG Examples.
(A) No confounder ECG with QRS points indicated. The patient receives 13 QRS points (39% LV infarct/scar) in inferior (leads II, III) and anterior-apical (leads V1-V6) regions. (B) No confounder ECG that receives no QRS points, suggesting no LV scar. See Appendix and prior publications (20,25) for complete QRS-scoring criteria.
The intra- and interobserver agreements for exactly matched QRS-scores were 64% (κ=0.61) and 63% (κ =0.59), respectively, in the randomly selected cohort. However, the average absolute values of the intra- and interobserver differences were only 0.4 and 0.6 QRS points (equivalent to 1.2% and 1.8% of the total LV). Using a cutoff of ±1 QRS point for agreement, the intra- and interobserver agreement improved to κ =0.92 and κ =0.86. There was 100% intra- and 95% inter-observer agreement in the classification of ≥1 vs. 0 QRS points.
QRS-Score Association with Ventricular Tachycardia/Fibrillation
QRS-score as a continuous variable (per 3 point increase) stratified by heart failure etiology was significantly related to VT/VF events (hazard ratio [HR]=1.14; 95% confidence interval [CI], 1.02–1.28; p=0.02). Three of 10 other clinically relevant variables were also significantly associated with outcome: LVEF, atrial fibrillation and NYHA class. After multivariable adjustment with the 10 other clinically relevant variables, QRS-score remained significant (adjusted HR=1.13; 95% CI 1.003–1.27; p=0.044) along with LVEF, atrial fibrillation and NYHA class.
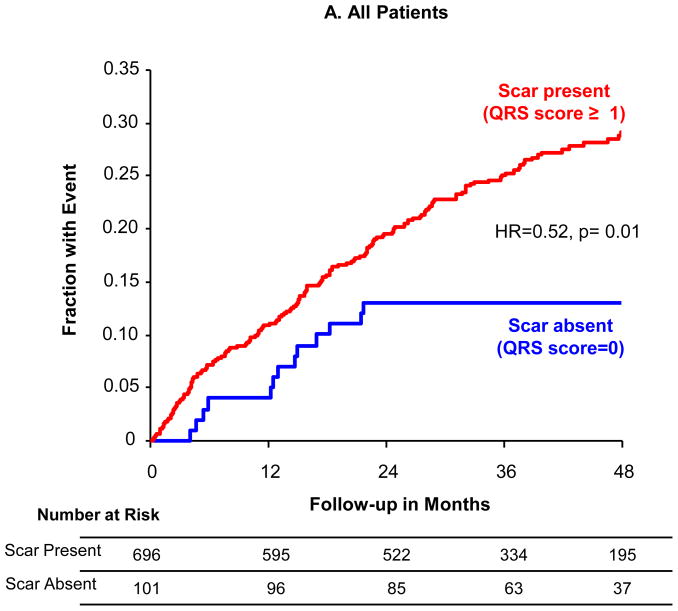
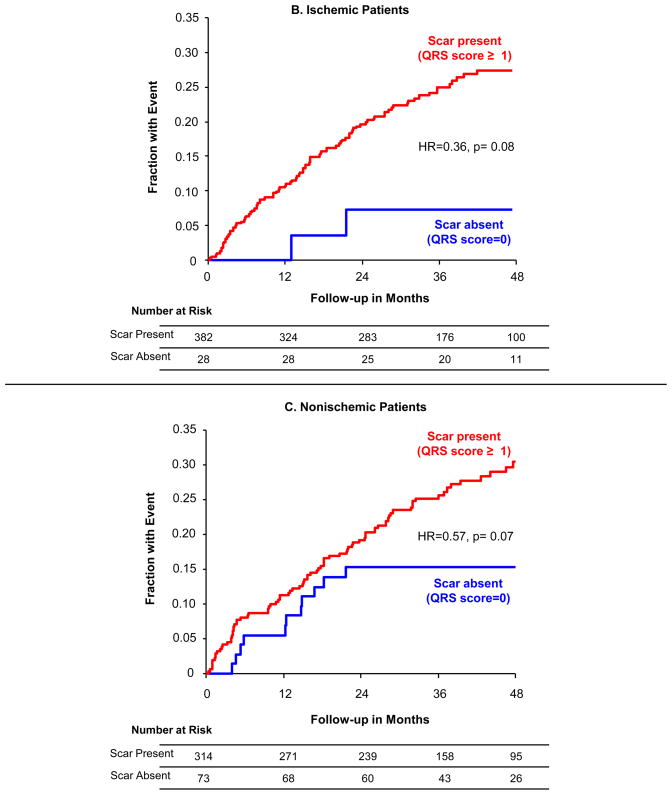
While QRS-score had a continuous linear relationship with outcome, the lowest-risk cohort could be identified when using a dichotomous cutpoint of scar absence vs. presence (QRS-score < vs. ≥1) (Figure 2a) with 48% fewer events in the patients with no scar (HR=0.52, 95% CI=0.31–0.88, p=0.01). At four years of follow-up, the absolute event rate in patients with no scar was 13% versus 29% in patients with scar. At a cutoff of 2 QRS points the difference was not statistically significant, although cutoffs at very-high QRS points identified a subgroup with significantly increased risk. When using the QRS-score scar absence vs. presence, the performance within ischemic (Figure 2b) and nonischemic (Figure 2c) subgroups was similar, though not reaching statistical significance given the relatively small numbers of patients in each subgroup. As with the whole population, significantly higher-risk populations with scar could be identified within ischemic and nonischemic cohorts.
Figure 2. Event Rates by QRS Score.
Kaplan-Meier event rates over 48 months for time-to-first ICD shock for VT/VF or sudden tachyarrhythmic death for patients without scar (QRS-score =0) vs. with scar (QRS-score ≥1): (A) all patients, (B) ischemic patients only and (C) nonischemic patients only.
Table 2 shows univariate and adjusted HR for QRS-score and the 10 other clinically-relevant variables using dichotomous cutpoints from prior risk-stratification studies. In univariate analysis, QRS-score scar absence had the strongest ability to identify a low-risk group, followed by LVEF, atrial fibrillation and NYHA class. Of note, none of the other ECG variables such as QRS duration ≤120 ms, absence of LBBB, or other ECG ventricular conduction types identified a low-risk group. After multivariable adjustment, three variables remained significant in identifying a low-risk group: the absence of scar by QRS-score, LVEF ≥25%, and the absence of atrial fibrillation/flutter.
Table 2.
Risk of ICD Shock for VT/VF or Sudden Arrhythmic Death (Primary Endpoint)
| Variable | Univariate Hazard Ratio (95% CI) | p-value | Adjusted Hazard Ratio (95% CI) | p-value |
|---|---|---|---|---|
| Scar Absence (QRS-score < vs. 1 point) | 0.52 (0.31–0.88) | 0.01 | 0.58 (0.34–0.997) | 0.049 |
| High LVEF (≥vs. < 25%) | 0.53 (0.40–0.71) | <0.001 | 0.55 (0.41–0.74) | <0.001 |
| No A fib/flutter (No vs. Yes) | 0.55 (0.36–0.84) | 0.006 | 0.61 (0.39–0.96) | 0.03 |
| NYHA HF Class II (II vs. III) | 0.68 (0.50–0.90) | 0.008 | 0.74 (0.55–1.003) | 0.053 |
| Low Creatinine (≤vs. >1.3 mg/dl) | 0.73 (0.54–1.002) | 0.052 | 0.76 (0.55–1.05) | 0.10 |
| Narrow QRS duration (≤vs. >120 ms) | 0.87 (0.65–1.16) | 0.34 | 0.95 (0.63–1.43) | 0.82 |
| Low Heart rate (< vs. ≥80 beats/min) | 0.92 (0.69–1.23) | 0.57 | 0.99 (0.73–1.33) | 0.95 |
| No LBBB (No vs. Yes) | 0.93 (0.66–1.30) | 0.66 | 1.05 (0.65–1.68) | 0.85 |
| Young Age (≤vs. >70 yrs) | 0.95 (0.68–1.34) | 0.78 | 1.04 (0.73–1.50) | 0.82 |
| No Diabetes (No vs. Yes) | 0.96 (0.70–1.30) | 0.78 | 0.93 (0.67–1.27) | 0.63 |
| Nonischemic HF (Nonischemic vs. Ischemic) | 1.01 (0.76–1.33) | 0.96 | 1.03 (0.77–1.39) | 0.83 |
HF = heart failure; LBBB = left bundle branch block; LVEF = left ventricular ejection fraction; NYHA = New York Heart Association; VT/VF = ventricular tachycardia or fibrillation.
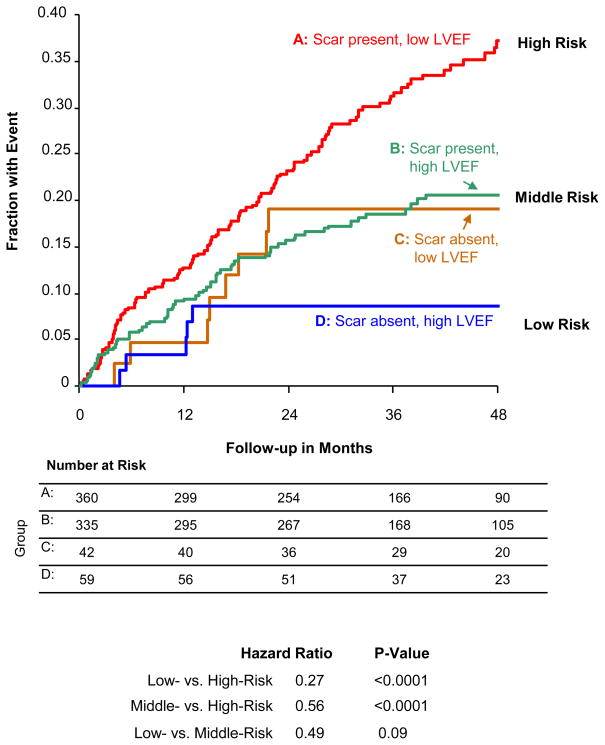
Figure 3 demonstrates how a simplified risk-score combining QRS-score (scar absence vs. presence) with ejection fraction (≥25% vs. <25%) can divide patients into low-, middle-, and high-risk subgroups. The lowest risk occurs when there is no scar AND high LVEF with an event rate of 9% at four years. There is intermediate risk when there is scar OR low LVEF (20% event rate at four years). There is high risk when there is scar AND low LVEF (37% event rate at four years). Thus, the middle-risk group has ~50% fewer events than the high risk-risk group and the low-risk group has ~50% fewer events than the middle risk group. This demonstrates the complementary value of considering both LVEF and QRS-score scar size in predicting the risk for VT/VF.
Figure 3. Event Rates by QRS Score and LVEF.
Kaplan-Meier event rates for time-to-first ICD shock for VT/VF or sudden tachyarrhythmic death for the four combinations of scar and LVEF. The lowest risk occurs when there is no scar AND high LVEF. There is intermediate risk when there is scar OR low LVEF. There is high risk when there is scar AND low LVEF.
QRS-Score Association with the Secondary Endpoint (VT/VF or All-Cause Mortality)
As with the primary endpoint, when considered as a continuous variable QRS-score was significantly associated with the combined endpoint of time-to-incident VT/VF or all-cause mortality in univariate Cox models stratified by ischemic vs. nonischemic etiology (per 3 QRS point increase, HR=1.13; 95% CI, 1.03–1.24; p=0.01) and as a dichotomous variable (QRS-score ≥1 vs. 0, HR=0.54; 95% CI, 0.35–0.84; p=0.006). Controlling for the 10 other clinically relevant characteristics, QRS-score remained a significant predictor as a continuous variable (adjusted HR=1.11, 95% CI 1.01–1.23, p=0.03) and almost reached significance as a dichotomous variable (adjusted HR, 0.64; 95% CI, 0.41–1.005; p=0.052).
Discussion
This study demonstrates that by applying 12-lead ECG-scoring to quantify myocardial scar in patients with cardiomyopathy and heart failure, those with no scar had a 48% reduced risk of appropriate ICD shocks for VT/VF or sudden tachyarrhythmic death. This is the first time an index of myocardial scar, which provides the substrate for reentrant arrhythmias, has been tested in a large ICD clinical trial. The relationship between QRS-score scar size and risk for malignant arrhythmias was independent of other clinical characteristics known to predict outcome. A simple risk-score incorporating the absence vs. presence of scar and high vs. low LVEF distinguishes low-, middle-, and high-risk groups, with 73% fewer events in the low- compared to high-risk patients.
Current criteria to identify candidates for ICDs for primary prevention of SCD rely exclusively on global LV function (LVEF ≤35%) (1,2). While ICDs unequivocally reduce mortality in patients with heart failure and low LVEF, most patients do not receive appropriate ICD shocks and almost as many receive inappropriate shocks (3). Furthermore, SCD-HeFT patients had a 5% acute rate and 9% chronic rate of device-related complications, which may be even higher in clinical practice (5).
Risk Stratification by 12-lead ECG
On the 12-lead ECG, prolonged QRS duration (>120 ms) is commonly thought of as a risk predictor. While QRS duration has been shown to predict heart failure events and total mortality (27,28), this does not necessarily mean it is predictive of VT/VF. In the current study, QRS duration >120 ms and LBBB did not predict VT/VF, which is consistent with a previous study (7). The ECG-based method tested in this study (QRS-scoring) is unique in that it directly identifies and quantifies the myocardial substrate (infarct/scar) that precipitates and supports reentrant ventricular arrhythmias (11). Another 12-lead ECG marker, fragmented QRS, which is thought to represent myocardial scar, was recently shown to predict the combined endpoint of antitachycardia pacing, ICD shock or sudden cardiac death in a population of primary and secondary prevention ICD patients (29). Further study should compare outcomes and look at the potential complementary value of QRS-scoring and fragmented QRS. ECG scar quantification differs from other electrophysiologic-based risk-stratification tools that focus on electrical triggers such as repolarization abnormalities (e.g. T-wave alternans) or autonomic tone imbalances (e.g. heart rate variability) and require an exercise test or prolonged ECG recording.
Previously, QRS-scoring was shown to predict adverse outcomes in patients with suspected coronary artery disease (30) and in cohorts early after first-time myocardial infarction, including the Framingham population (31,32). Most of these findings pre-dated reperfusion therapy and patients had a wide range of LVEFs (30–32). Little was previously known about quantifying scar by QRS-scoring in chronic cardiomyopathy and its relationship to arrhythmic events. Recent cohort studies have extended the applicability of QRS-scoring to ischemic and nonischemic cardiomyopathy for accurately quantifying scar size and demonstrated a relationship with ventricular irritability (11,25). Because the 12-lead ECG is inexpensive and universally available, ECG algorithms could be immediately applied and tested to aid in SCD risk-stratification. In addition to the application of QRS-scoring to potential ICD candidates, other groups have shown that these new QRS-scores predict response to cardiac resynchronization therapy (33).
Complementary Role of Scar and Ejection Fraction
The current work highlights the complementary prognostic information that may be gained from quantifying both LV scar and global function. While prior studies applying a simplified QRS-score early after first-time myocardial infarction (MI) demonstrated a strong inverse correlation with LVEF (20,34), in the current population with chronically reduced LVEF there was no correlation between QRS-score scar size and LVEF. Furthermore, it is interesting that the presence of ECG-estimated scar had similar value in predicting risk in both the ischemic and nonischemic subgroups (Figure 2). This raises questions about the current definitions used to classify cardiomyopathy patients as “ischemic” vs. “nonischemic” that are based solely on having a clinical history of an infarct or ≥75% narrowing of one of the major coronary arteries. Our findings suggest that classification by scar presence is an important additional factor. This is supported by recent contrast-enhanced MRI studies (13–19).
Limitations
Our study is limited by its retrospective approach, as applied to the ICD arm of SCD-HeFT. However, all ECG QRS-scoring was performed blinded to all clinical data. Furthermore, it included 98% of patients who received ICDs from a large, prospective, randomized clinical trial with thoroughly adjudicated outcomes. Nonetheless, the results need to be validated in another cohort including the placebo arm of SCD-HeFT or MADIT-II. While the ICDs were uniformly programmed with a high detection rate of ≥188 beats/min and no antitachycardia pacing, thus minimizing therapy for arrhythmias that might terminate spontaneously, ICD shocks for VT/VF are not a perfect surrogate for sudden cardiac arrest. Another limitation is that the global QRS-score was used, which contains 53 individual criteria. Future work involving separate development and test cohorts could investigate the prognostic value of individual criteria that could potentially further simplify and improve QRS-score methodology. This may thus allow for the identification of a larger number of patients with reduced risk of ICD shocks, as only 101 of 797 patients (13%) in the current study had a QRS-score of 0. However, from 2006 to 2008, 265,090 ICDs were implanted in the U.S. for primary prevention (35), thus even reducing the number of ICDs by 10% (26,000) would be substantial, particularly as the ECG is so widely available, routinely acquired clinically, and inexpensive relative to the cost of a single ICD. Finally, widespread application of QRS-scoring in everyday clinical practice will depend upon the proliferation and implementation of prospectively validated automated versions that will likely improve observer variability, which can be a limitation of manual scoring. Multiple automated versions of QRS scoring have been developed by different investigators (21–24), however until recently, QRS scoring has not been used for risk-stratification decision-making and hence, there was no strong drive to implement it in clinical ECG machines. This could change with the potential to use QRS-scoring to aid in risk-stratification for ICDs and cardiac resynchronization therapy (33), which will need to be prospectively validated using automated methods.
Conclusion
In conclusion, in patients with reduced LVEF and heart failure who receive an ICD for primary prevention, the absence of scar by 12-lead ECG QRS-scoring is associated with a significantly reduced risk for ICD shocks or sudden tachyarrhythmic death. This finding is independent of LVEF and other clinical variables. A simplified risk score combining the absence vs. presence of scar with high vs. low LVEF further separates patients into low-, middle- and high-risk groups. Future studies should exploit the unique information regarding myocardial substrate that can be provided by the inexpensive, widely used 12-lead ECG and further validate the role of QRS-scoring as a predictor in risk-stratifying algorithms to help physicians evaluate the individual patient’s potential benefit from ICDs.
Supplementary Material
Abbreviations
- ECG
electrocardiogram
- HR
hazard ratio
- ICD
implantable cardioverter defibrillator
- LAFB
left anterior fascicular block
- LBBB
left bundle branch block
- LVEF
left ventricular ejection fraction
- MADIT-II
Multicenter Automatic Defibrillator Implantation Trial II
- MRI
magnetic resonance imaging
- NYHA
New York Heart Association
- RBBB
right bundle branch block
- SCD-HeFT
Sudden Cardiac Death in Heart Failure Trial
- VT/VF
ventricular tachycardia and ventricular fibrillation
Footnotes
This study is registered at http://www.clinicaltrials.gov – identifier NCT00000609
Funding and Disclosures: Dr. Strauss was supported by the Sarnoff Cardiovascular Research Foundation (Great Falls, VA) for work at Johns Hopkins, and has subsequently moved to the U.S. Food and Drug Administration. Views expressed do not represent those of the FDA. Dr. Wu and Dr. Miller were supported by the Donald W. Reynolds Cardiovascular Research Center at Johns Hopkins University (Baltimore, MD) and Dr. Miller was supported by the Doris Duke Charitable Foundation (New York, NY). The main SCD-HeFT study was supported by grants (UO1 HL55766, UO1 HL55297, and UO1 HL55496) from the NHLBI, National Institutes of Health, and by Medtronic, Wyeth Ayerst Laboratories, and Knoll Pharmaceuticals. Dr. Poole has received speaking fees for Medtronic, Boston Scientific and St. Jude Medical, and a research grant from Biotronik. Dr. Wagner has received research support from Physio-Control and Welch-Allyn. Dr. Mark has received research support from Abbott Laboratories, Abbott Vascular Business, Abiomed and Acorn Cardiovascular. Dr. Bardy has received research grants from St. Jude Medical, is a consultant for Phillips Medical and Cardiac Sciences, and is a board member and has equity and intellectual property in Cameron Health Inc.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 2.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 3.Poole JE, Johnson GW, Hellkamp AS, et al. Prognostic importance of defibrillator shocks in patients with heart failure. N Engl J Med. 2008;359:1009–17. doi: 10.1056/NEJMoa071098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Myerburg RJ. Implantable cardioverter-defibrillators after myocardial infarction. N Engl J Med. 2008;359:2245–53. doi: 10.1056/NEJMra0803409. [DOI] [PubMed] [Google Scholar]
- 5.Reynolds MR, Cohen DJ, Kugelmass AD, et al. The frequency and incremental cost of major complications among medicare beneficiaries receiving implantable cardioverter-defibrillators. J Am Coll Cardiol. 2006;47:2493–7. doi: 10.1016/j.jacc.2006.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tung R, Zimetbaum P, Josephson ME. A critical appraisal of implantable cardioverter-defibrillator therapy for the prevention of sudden cardiac death. J Am Coll Cardiol. 2008;52:1111–21. doi: 10.1016/j.jacc.2008.05.058. [DOI] [PubMed] [Google Scholar]
- 7.Buxton AE, Sweeney MO, Wathen MS, et al. QRS duration does not predict occurrence of ventricular tachyarrhythmias in patients with implanted cardioverter-defibrillators. J Am Coll Cardiol. 2005;46:310–6. doi: 10.1016/j.jacc.2005.03.060. [DOI] [PubMed] [Google Scholar]
- 8.Stevenson WG, Epstein LM. Predicting sudden death risk for heart failure patients in the implantable cardioverter-defibrillator age. Circulation. 2003;107:514–6. doi: 10.1161/01.cir.0000053944.35059.fa. [DOI] [PubMed] [Google Scholar]
- 9.Zipes DP, Wellens HJ. Sudden cardiac death. Circulation. 1998;98:2334–51. doi: 10.1161/01.cir.98.21.2334. [DOI] [PubMed] [Google Scholar]
- 10.Dillon SM, Allessie MA, Ursell PC, Wit AL. Influences of anisotropic tissue structure on reentrant circuits in the epicardial border zone of subacute canine infarcts. Circ Res. 1988;63:182–206. doi: 10.1161/01.res.63.1.182. [DOI] [PubMed] [Google Scholar]
- 11.Strauss DG, Wu KC. Imaging myocardial scar and arrhythmic risk prediction--a role for the electrocardiogram? J Electrocardiol. 2009;42:138, e1–8. doi: 10.1016/j.jelectrocard.2008.12.010. [DOI] [PubMed] [Google Scholar]
- 12.Wu KC. MRI with late gadolinium enhancement as a predictor of ventricular arrhythmias. Curr Cardiovasc Imaging Rep. 2009;2:116–23. [Google Scholar]
- 13.Schmidt A, Azevedo CF, Cheng A, et al. Infarct tissue heterogeneity by magnetic resonance imaging identifies enhanced cardiac arrhythmia susceptibility in patients with left ventricular dysfunction. Circulation. 2007;115:2006–14. doi: 10.1161/CIRCULATIONAHA.106.653568. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bello D, Fieno DS, Kim RJ, et al. Infarct morphology identifies patients with substrate for sustained ventricular tachycardia. J Am Coll Cardiol. 2005;45:1104–8. doi: 10.1016/j.jacc.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 15.Nazarian S, Bluemke DA, Lardo AC, et al. Magnetic resonance assessment of the substrate for inducible ventricular tachycardia in nonischemic cardiomyopathy. Circulation. 2005;112:2821–5. doi: 10.1161/CIRCULATIONAHA.105.549659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yan AT, Shayne AJ, Brown KA, et al. Characterization of the peri-infarct zone by contrast-enhanced cardiac magnetic resonance imaging is a powerful predictor of post-myocardial infarction mortality. Circulation. 2006;114:32–9. doi: 10.1161/CIRCULATIONAHA.106.613414. [DOI] [PubMed] [Google Scholar]
- 17.Wu KC, Weiss RG, Thiemann DR, et al. Late gadolinium enhancement by cardiovascular magnetic resonance heralds an adverse prognosis in nonischemic cardiomyopathy. J Am Coll Cardiol. 2008;51:2414–21. doi: 10.1016/j.jacc.2008.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Assomull RG, Prasad SK, Lyne J, et al. Cardiovascular magnetic resonance, fibrosis, and prognosis in dilated cardiomyopathy. J Am Coll Cardiol. 2006;48:1977–85. doi: 10.1016/j.jacc.2006.07.049. [DOI] [PubMed] [Google Scholar]
- 19.Roes SD, Borleffs CJW, van der Geest RJ, et al. Infarct Tissue Heterogeneity Assessed With Contrast-Enhanced MRI Predicts Spontaneous Ventricular Arrhythmia in Patients With Ischemic Cardiomyopathy and Implantable Cardioverter-Defibrillator. Circulation: Cardiovascular Imaging. 2009;2:183–90. doi: 10.1161/CIRCIMAGING.108.826529. [DOI] [PubMed] [Google Scholar]
- 20.Strauss DG, Selvester RH. The QRS complex--a biomarker that “images” the heart: QRS scores to quantify myocardial scar in the presence of normal and abnormal ventricular conduction. J Electrocardiol. 2009;42:85–96. doi: 10.1016/j.jelectrocard.2008.07.011. [DOI] [PubMed] [Google Scholar]
- 21.Haisty WK, Jr, Pahlm O, Wagner NB, Pope JE, Wagner GS. Performance of the automated complete Selvester QRS scoring system in normal subjects and patients with single and multiple myocardial infarctions. J Am Coll Cardiol. 1992;19:341–6. doi: 10.1016/0735-1097(92)90489-a. [DOI] [PubMed] [Google Scholar]
- 22.Horacek BM, Warren JW, Albano A, et al. Development of an automated Selvester Scoring System for estimating the size of myocardial infarction from the electrocardiogram. J Electrocardiol. 2006;39:162–8. doi: 10.1016/j.jelectrocard.2005.08.013. [DOI] [PubMed] [Google Scholar]
- 23.Pope JE, Wagner NB, Dubow D, Edmonds JH, Wagner GS, Haisty WK., Jr Development and validation of an automated method of the Selvester QRS scoring system for myocardial infarct size. Am J Cardiol. 1988;61:734–8. doi: 10.1016/0002-9149(88)91057-0. [DOI] [PubMed] [Google Scholar]
- 24.Andresen A, Dobkin J, Maynard C, et al. Validation of advanced ECG diagnostic software for the detection of prior myocardial infarction by using nuclear cardiac imaging. J Electrocardiol. 2001;34:243–8. doi: 10.1054/jelc.2001.28907. [DOI] [PubMed] [Google Scholar]
- 25.Strauss DG, Selvester RH, Lima JA, et al. ECG quantification of myocardial scar in cardiomyopathy patients with or without conduction defects: correlation with cardiac magnetic resonance and arrhythmogenesis. Circ Arrhythm Electrophysiol. 2008;1:327–36. doi: 10.1161/CIRCEP.108.798660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Packer DL, Prutkin JM, Hellkamp AS, et al. Impact of implantable cardioverter-defibrillator, amiodarone, and placebo on the mode of death in stable patients with heart failure: analysis from the sudden cardiac death in heart failure trial. Circulation. 2009;120:2170–6. doi: 10.1161/CIRCULATIONAHA.109.853689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldenberg I, Vyas AK, Hall WJ, et al. Risk stratification for primary implantation of a cardioverter-defibrillator in patients with ischemic left ventricular dysfunction. J Am Coll Cardiol. 2008;51:288–96. doi: 10.1016/j.jacc.2007.08.058. [DOI] [PubMed] [Google Scholar]
- 28.Wang NC, Maggioni AP, Konstam MA, et al. Clinical implications of QRS duration in patients hospitalized with worsening heart failure and reduced left ventricular ejection fraction. JAMA. 2008;299:2656–66. doi: 10.1001/jama.299.22.2656. [DOI] [PubMed] [Google Scholar]
- 29.Das MK, Maskoun W, Shen C, et al. Fragmented QRS on twelve-lead electrocardiogram predicts arrhythmic events in patients with ischemic and nonischemic cardiomyopathy. Heart Rhythm. 2010;7:74–80. doi: 10.1016/j.hrthm.2009.09.065. [DOI] [PubMed] [Google Scholar]
- 30.Bounous EP, Jr, Califf RM, Harrell FE, Jr, et al. Prognostic value of the simplified Selvester QRS score in patients with coronary artery disease. J Am Coll Cardiol. 1988;11:35–41. doi: 10.1016/0735-1097(88)90163-5. [DOI] [PubMed] [Google Scholar]
- 31.Jones MG, Anderson KM, Wilson PW, Kannel WB, Wagner NB, Wagner GS. Prognostic use of a QRS scoring system after hospital discharge for initial acute myocardial infarction in the Framingham cohort. Am J Cardiol. 1990;66:546–50. doi: 10.1016/0002-9149(90)90479-k. [DOI] [PubMed] [Google Scholar]
- 32.Roubin GS, Shen WF, Kelly DT, Harris PJ. The QRS scoring system for estimating myocardial infarct size: clinical, angiographic and prognostic correlations. J Am Coll Cardiol. 1983;2:38–44. doi: 10.1016/s0735-1097(83)80374-x. [DOI] [PubMed] [Google Scholar]
- 33.Sweeney MO, van Bommel RJ, Schalij MJ, Borleffs CJ, Hellkamp AS, Bax JJ. Analysis of ventricular activation using surface electrocardiography to predict left ventricular reverse volumetric remodeling during cardiac resynchronization therapy. Circulation. 2010;121:626–34. doi: 10.1161/CIRCULATIONAHA.109.894774. [DOI] [PubMed] [Google Scholar]
- 34.Palmeri ST, Harrison DG, Cobb FR, et al. A QRS scoring system for assessing left ventricular function after myocardial infarction. N Engl J Med. 1982;306:4–9. doi: 10.1056/NEJM198201073060102. [DOI] [PubMed] [Google Scholar]
- 35.Hammill SC, Kremers MS, Kadish AH, et al. Review of the ICD Registry’s third year, expansion to include lead data and pediatric ICD procedures, and role for measuring performance. Heart Rhythm. 2009;6:1397–401. doi: 10.1016/j.hrthm.2009.07.015. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.