Abstract
Background
Indications for implantable cardioverter-defibrillator (ICD) implantation in children have expanded, yet pediatric population based data on ICD implantation are lacking.
Objective
We characterized trends in pediatric ICD use in the United States from 1997-2006.
Methods
We examined national hospital administrative data from the 1997, 2000, 2003, and 2006 Kids' Inpatient Database (KID) for new ICD implants in patients younger than 18 years of age and characterized patients, hospitals, and hospitalization-related outcomes.
Results
The number of pediatric ICD implants per year increased three-fold (from 130 in 1997 to 396 in 2006, p= 0.003). Implants with a concomitant diagnosis of life-threatening arrhythmia decreased from 77% to 45% (p=0.001). The average age decreased from 13.6 to 12.2 years (p=0.01), and the percentage of patients younger than 5 years of age tended to increase (up to 10%, p=0.09). In 2006, the number of implants per center ranged from 1 to 24 (median= 3). Over time, the complication rate tended to decrease (from 16 to 10%, p=0.07). Complication rate was not related to a diagnosis of congenital heart disease, age, or implant volume.
Conclusions
ICD use increased dramatically in children from 1997 to 2006, although implantation declined in patients with a concomitant diagnosis of life-threatening arrhythmia (those likely to be implanted for secondary prevention). The complication rate tended to decrease overall. Each center implants relatively few ICDs per year, which may have implications for competency and training.
Keywords: Implantable Cardioverter-Defibrillator, Pediatric, Congenital Heart Disease, Administrative Data, Population Based Data
Introduction
Less than 1% of implantable cardioverter-defibrillators (ICDs) are placed in children and patients with congenital heart disease.(1) As a result, there is a dearth of prospective data on pediatric patients regarding survival benefit and outcomes following ICD placement. Previous reports have shown a high rate of appropriate shocks in pediatric patients, suggesting a potential benefit in children.(2,3) The low incidence of sudden cardiac death in children relative to adults further limits the ability to perform randomized clinical trials to evaluate risk stratification or primary prevention strategies.(4) The majority of existing studies on ICDs in children are retrospective, containing small numbers of patients at single institutions. The largest pediatric ICD study to date involved a registry of four centers and a total of 443 patients over 12 years.(2) Therefore, the data that provides the basis for development of indications for pediatric ICD implantation is primarily extrapolated from adult studies.
Indications for ICD implantation in adults have evolved over time, with the focus expanding from secondary prevention of sudden cardiac death to primary prevention in patients known to be at higher risk of sudden cardiac death.(5,6) Correspondingly, use of ICDs in adults has increased steadily over the last decade. Zhan et al. demonstrated that, at a population level, ICD implantation increased by 60% in adults from 1997-2004.(7) To date, no such population based data have been analyzed for the pediatric population. Recognizing that such analysis may provide insight into implantation trends, changes in underlying diagnoses leading to implantation, and pediatric-specific complications, we sought to attain population based data on ICD use in children.
In order to understand the landscape of pediatric ICD utilization in the United States, we used the Kids' Inpatient Database (KID), a national, all-payer, hospital administrative database, to examine trends in ICD use in pediatric patients from 1997 to 2006 at a population level.
Methods
The KID is the only all-payer, inpatient care database for children in the United States. Released every three years, the KID is a sample of discharges from all community, non-rehabilitation hospitals in states participating in the Healthcare Cost and Utilization Project (HCUP) of the Agency for Healthcare Research and Quality (AHRQ). After 1997, the KID includes patients aged 20 or less at admission. The KID contains information typically found in a discharge abstract, with safeguards to protect the privacy of hospitals, physicians, and patients. The data can be weighted to produce national estimates. The KID for 2006 includes 3,739 hospitals (which represent 73% of all American Hospital Association designated hospitals) from 38 states, whereas the 2003 KID includes 3,438 hospitals (71%) from 36 states. The KID for 2000 includes 2,784 hospitals (58%) from 27 states, and the 1997 KID includes 2,521 hospitals (49%) from 22 states.(8)
We queried the KID from 1997, 2000, 2003, and 2006 for discharges involving patients younger than 18 years of age with International Classification of Diseases, Ninth Revision (ICD-9) procedure codes for new ICD implantation (37.94, 00.51, and 37.95+37.96).(9) We excluded hospitalizations in which the procedure was solely a generator change. Each discharge was examined for the following information: patient characteristics (age, gender, and underlying diagnoses), hospital characteristics (type of hospital and implant volume), and hospitalization-related outcome data (mortality, length of stay, and complications).
We prospectively identified four major categories of underlying diagnosis: congenital heart disease (CHD), cardiomyopathy (CM), Long QT Syndrome (LQTS) and “Other.”
Hospital type is specified within the KID, and is based on information provided by the National Association of Children's Hospitals and Related Institutions (NACHRI).(8) We evaluated three categories of NACHRI hospitals: “not children's hospital”, “children's unit in a general hospital”, and “children's hospital” (the latter is a combination of “children's specialty hospital” and “children's general hospital”).
We described implant volume as the number of implants per center per year. Based on the clinical competency statement on training pathways for implantation of ICDs in pediatric and congenital heart patients, we deemed centers implanting fewer than ten ICDs per year low-volume centers for the purpose of this study.(10)
Length of stay in the KID is determined by calendar dates. Patients who were admitted and discharged on the same calendar day are reported as having a length of stay of zero days. Patients who were admitted on one calendar day and discharged the next day are counted as having a length of stay of one day, even if the duration of hospitalization was fewer than 24 hours.
We identified complications as a composite of the following ICD-9 codes: mechanical complications caused by ICDs (996.04, 996.72, and E878.1) and infection and inflammatory reaction due to cardiac device, implant and graft (996.61).(2,7,9,11) AHRQ's Pediatric Quality Indicators provided the basis for other complications. These indicators are a set of measures that can be used with hospital inpatient discharge data to identify iatrogenic events and potentially preventable complications in pediatric inpatients.(7) The following Pediatric Quality Indicators were felt to be the most relevant to ICD implantation and were therefore also included in the composite of complications: iatrogenic pneumothorax (512.1), accidental puncture or laceration (E870.0, E870.6, 998.2), and postoperative hemorrhage or hematoma (998.11, 998.12).(9,12)
Because of difficulty differentiating those ICDs implanted for primary versus secondary prevention using this particular database, we presumed that cases with codes for both ICD implantation and a life-threatening arrhythmia were more likely performed for secondary prevention. We therefore identified discharges with a code for both ICD implantation as well as one of the following ICD-9 diagnosis codes for life-threatening arrhythmia: paroxysmal ventricular tachycardia (427.1), ventricular fibrillation (427.41), ventricular flutter (427.42), and cardiac arrest (427.5).(9,13)
Statistical analysis
Data in the KID are stratified by geographic region, location/teaching status, bed size category, ownership, and whether the hospital is a freestanding children's hospital. Discharges are also stratified by uncomplicated in-hospital births, complicated in-hospital births, and non-newborn pediatric discharges. Discharge weights are created for each stratum in proportion to the number of known American Hospital Association discharges nationally. Using the discharge weights, individual observations (discharges) are then extrapolated to produce national estimates. (14)
The domain for analysis of this study consisted of 597 observations over the 4 sample years with an ICD diagnostic code and that met the criteria of age ≤ 17 years and length of stay ≤ 365 days.(15) The primary sampling unit is the hospital. With the exception of hospital data, all data presented are weighted national estimates, not actual observations. Data regarding the number of hospitals reflect only the hospitals within the KID hospital sample; therefore hospital data are not weighted for national estimates. Cells based on ten or fewer observations were suppressed in the tables due to the HCUP data use agreement, but we included those values in the analysis.(16)
We used survey analysis methods for stratified, two-stage cluster samples. Trends in continuous variables across years were estimated by linear regression for discharges (implants) and ANOVA for age and length of stay.(17,18) We used t-tests to compare pair-wise differences between means of continuous variables. Trends in categorical variables across years were estimated by logistic regression. In order to test for interaction between categorical variables in contingency tables, we used Rao-Scott chi-square tests.(19) Three missing values of hospital category for a single hospital identifier in 2003 were imputed from the value of the same identifier in 2006. The remaining 21 missing values of hospital category for seven other hospital identifiers and four missing values of gender in the domain were excluded from calculations.
To determine predictors of complications, univariate analysis was performed using composite data from all four years. We compared the complication rates of children's hospital types individually against the “not children's hospital” group.
Calculations were performed with SAS for Windows 9.2 (SAS Institute Inc., Cary NC).
Results
The total number of pediatric ICD implants per year increased from 130 ± 27 in 1997 to 396 ± 50 in 2006, a three-fold increase (p=0.003). Table 1 provides the number of total ICD implantations by year, as well as associated patient data. The mean age of patients decreased from 13.6 ± 0.4 to 12.2 ± 0.3 years (p=0.01), and the percentage of patients under 5 years of age receiving an ICD showed a trend toward increasing (up to 10% in 2006, p= 0.09). Figure 1 displays the proportions of implants per year in patients with each major diagnostic category. Long QT Syndrome as an underlying diagnosis did not appear in our data set until 2006, reflecting the fact that an ICD-9 code for the Long QT syndrome was first introduced in 2005.(20)
Table 1. Patient Data.
| 1997 | 2000 | 2003 | 2006 | p-value | |
|---|---|---|---|---|---|
| Total Implants/Year: N ± Std Error Total Implants/4 Years: 1083 ± 156 |
130 ± 27 | 228 ± 38 | 329 ± 41 | 396 ± 50 | 0.003 |
| Age in Years: Mean ± Std Error | 13.6 ± 0.4 | 12.8 ± 0.5 | 12.6 ± 0.3 | 12.2 ± 0.3 | 0.01 |
| Age ≤ 5 Years: N (%) | --* | --* | 24 (7) | 39 (10) | 0.09 |
| Gender - Female: % | 33 | 39 | 40 | 40 | 0.37 |
| Diagnosis: N (%) | |||||
| Congenital Heart Disease | 28 (22) | 65 (29) | 137 (42) | 81 (21) | |
| Cardiomyopathy | 39 (30) | 89 (39) | 105 (32) | 143 (36) | |
| Long QT Syndrome | --† | --† | --† | 107 (27) | |
| Other | 63 (48) | 74 (32) | 87 (26) | 65 (16) | |
| Concomitant Diagnosis of Life-Threatening Arrhythmia: % | 77 | 49 | 55 | 45 | 0.001 |
Cells based on ten or fewer observations were suppressed due to the HCUP data use agreement.
The ICD-9 code for Long QT Syndrome was first introduced in 2005. We suspect that, prior to 2006; patients with LQTS were coded as “abnormal electrocardiogram,” which falls in the “Other” category.
Figure 1. Implants by Underlying Diagnosis by Year.
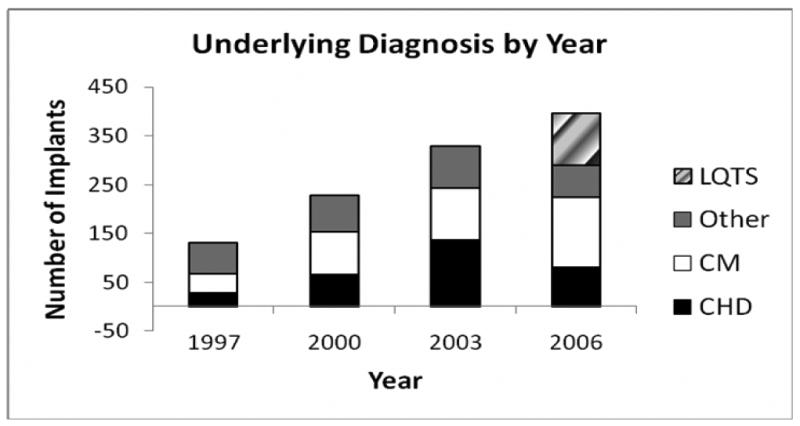
The number of implants is depicted for each of the four major categories of underlying diagnosis: congenital heart disease (CHD), cardiomyopathy (CM), Other, and Long QT syndrome (LQTS). The ICD-9 code for LQTS was first introduced in 2005; therefore implants performed in patients with a diagnosis of LQTS did not appear in the KID until 2006.
During the study period, the percentage of patients who received an ICD who also had a diagnosis of life-threatening arrhythmia decreased by 32% (p= 0.001), (Table 1, Figure 2). Restricting this analysis to those with a concomitant diagnosis of ventricular fibrillation or cardiac arrest (34% of those with a life-threatening arrhythmia) revealed a trend towards decreasing number of implants over the study period (from 25% in 1997 to 15% in 2006, p=0.06).
Figure 2. Life-Threatening Arrhythmia by Year.
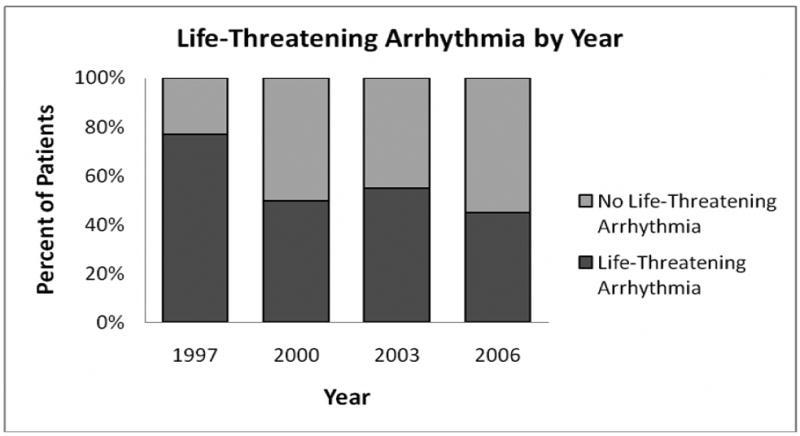
Implants with an associated diagnosis of life-threatening arrhythmia (dark gray bars) are presented as a percentage of the total implants per year.
Table 2 reveals the characteristics of implanting hospitals during the study period. The majority of implanting centers were designated as “children's unit in a general hospital.” The percentage of implants being performed at “children's hospitals” ranged from 34-51%, with a similar percentage of implants being performed in hospitals designated as “children's unit in a general hospital” (28-46%). The percentage of implants in “not children's hospitals” tended to decrease over time (from 23% in 1997 to 8% in 2006). The median number of implants per center was low (2-3), and although the percentage of centers implanting more than 10 ICDs per year increased over the study period, the overall percentage of high-volume centers remained low at 10% in 2006. Implants by age and hospital type are depicted in Figure 3. The majority of implants in “not children's hospitals” were performed in older patients.
Table 2. Hospital Data*.
| 1997 | 2000 | 2003 | 2006 | ||
|---|---|---|---|---|---|
| Hospital Type: N (%) | |||||
| Not Children's Hospital | 15 (39) | 20 (35) | 23 (28) | 19 (22) | |
| Children's Unit in General Hospital | 17 (45) | 22 (39) | 39 (48) | 48 (55) | |
| Children's Hospital | 6 (16) | 15 (26) | 20 (24) | 21 (24) | |
| Implants by Hospital Type: N (%) | |||||
| Not Children's Hospital | 30 (23) | 46 (20) | 50 (15) | 32 (8) | |
| Children's Unit in General Hospital | 54 (42) | 65 (28) | 143 (44) | 180 (46) | |
| Children's Hospital | 46 (35) | 116 (51) | 113 (34) | 166 (42) | |
| Implants per Center: Median (Range) | 2 (1-18) | 2 (1-20) | 2 (1-14) | 3 (1-24) | |
| Hospitals With Implant Volume: N (%) | |||||
| ≥10 implants/year | 2 (5) | 3 (5) | 5 (6) | 9 (10) | |
| 5-9 implants/year | 4 (11) | 11 (19) | 18 (21) | 14 (15) | |
| <5 implants/year | 32 (84) | 44 (76) | 63 (73) | 70 (75) | |
Hospital data are based on actual observations, not weighted estimates.
Figure 3. Implants by Age and Hospital Type.
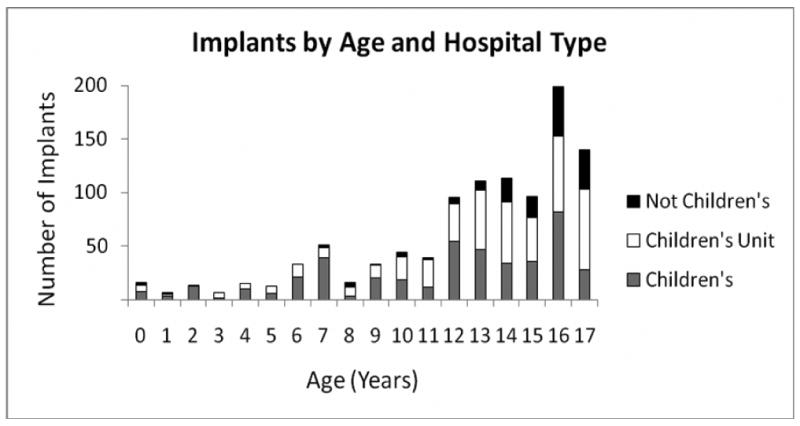
ICDs implants are displayed by age and type of hospital. ICDs were placed predominantly in teenage patients. The majority of implants in non-children's hospitals were performed in patients over age 14 years of age.
Hospitalization-related outcome data are displayed in Table 3. Mortality during hospitalization for ICD implantation was rare. Length of stay was not significantly changed over time (median: 10 days in 1997 to 4 days in 2006, p=0.86), though the range in length of stay was highly variable. The percentage of ICD implantations with associated complications tended to decrease during the study period (from 16 in 2000 to 10% in 2006, p= 0.07).
Table 3. Hospitalization-Related Outcome Data.
| 1997 | 2000 | 2003 | 2006 | p-value | |
|---|---|---|---|---|---|
| Survival to Hospital Discharge: % | 100 | 100 | 99 | 99.6 | |
| Length of Stay - Days: Median | 10 | 3 | 4 | 4 | 0.86 |
| Mean ± Standard Error | 11.4 ± 1.2 | 6.3 ± 0.8 | 8 ± 1 | 8.1 ± 1.1 | |
| Range | 2-37 | 1-61 | 1-91 | 1-179 | |
| Complication rate: % | --* | 16 | 11 | 10 | 0.07 |
Cells based on ten or fewer observations were suppressed due to the HCUP data use agreement.
Results of univariate analysis for predicting complications in the pediatric population undergoing ICD implantation are shown in Table 4. The rate of complications was not related to an underlying diagnosis of congenital heart disease, age, or hospital implant volume. Compared to centers designated as “not children's hospitals,” the complication rate was lower for implants performed in “children's units in a general hospital” (p=0.05).
Table 4. Univariate Analysis of Predictors of Complications.
| Predictor (Denominator) | Complications: N (%) | p-value |
|---|---|---|
| Congenital Heart Disease | 0.12 | |
| Yes (310) | 28 (9) | |
| No (773) | 104 (13) | |
| Age | 0.57 | |
| ≤ 5 years (80) | --* | |
| >5 years (1,003) | 124 (12) | |
| Hospital Implant Volume | 0.7 | |
| <5 implants/year (382) | 45 (12) | |
| 5-9 implants/year (373) | 51 (14) | |
| ≥10 implants/year (329) | 35 (11) | |
| Hospital Type | ||
| Not Children's Hospital (157) | 31 (20) | |
| Children's Unit in General Hospital (447) | 44 (10) | 0.05† |
| Children's Hospital (441) | 52 (12) | 0.12† |
Cells based on ten or fewer observations were suppressed due to the HCUP data use agreement.
Compared to Not Children's Hospital
Discussion
These results, based on weighted national estimates from a validated inpatient database, reflect trends in ICD implantation nationwide, incorporating data from centers of various sizes, hospital types, and degrees of experience with ICD implantation in children. These data provide a real-world snapshot of pediatric ICD use from 1997-2006.
Our results illustrate that the total number of ICD implants per year among children increased dramatically from 1997 to 2006. This 3-fold increase in implantation volume in our study is comparable to the increase reported by Walsh from a single center over the same time period.(4) This increase also mirrors the growth rate of ICD utilization in adult patients as the indications for adult ICD implantation have expanded over time.(7) Pediatric guidelines for ICD implantation for secondary prevention parallel the adult guidelines, and recommendations regarding pediatric ICD implantation for primary prevention are extrapolated from data from adult studies.(1,21,22) The rate of increase in pediatric ICD implantation may be the result of expanding indications in children. Likewise, ICD implantation in children may still be in the “early adoption phase”, and the dramatic increase may be reflective of a change in a small denominator of implants overall.
Although the majority of pediatric implants were performed in adolescents, age at implantation decreased significantly throughout the study period, and the percentage of patients younger than 5 years of age showed a trend toward increasing as well. This may reflect improvements over time in technology and technique (e.g. epicardial patches and subcutaneous arrays), allowing for implantation in younger, smaller patients.(23)
In a registry of 443 pediatric and congenital heart disease patients with ICDs, Berul et al. reported a total of 46% of patients with an underlying diagnosis of congenital heart disease and 23% with cardiomyopathy.(2) Our data include fewer patients with congenital heart disease (22-42%) and more with cardiomyopathy (30-39%). This difference likely reflects ascertainment bias between registry data obtained from large children's hospitals with robust congenital heart disease programs and administrative data from a cross-section of hospitals nationwide.
As indications for implantation have been refined over the last decade, there has been a shift in focus towards implantation of ICDs for primary prevention in patients at high risk of sudden cardiac death. Berul et al. reported an increase in primary prevention indications in the registry population during the more recent era (2000-2004).(2) Unfortunately, the type of data available in the KID database limits our ability to determine whether implants were performed for primary or secondary prevention of sudden cardiac death. We surmised that patients with a concomitant diagnosis of life-threatening arrhythmia during hospitalization for ICD implantation were likely undergoing placement for secondary prevention. This percentage decreased during the study period, suggesting that the incidence of implantation for primary prevention may have increased over time.
Despite the overall increase in number of implants at a national level, the median number of implants per center remains low, as does the percentage of centers implanting more than 10 ICDs per year. Current recommendations for maintenance certification criteria for experienced pediatric electrophysiologists for ICD implantation suggest 10 primary implants, revisions or replacements per year.(10) Our study excluded patients older than 17 years of age and generator replacements; nevertheless, the data suggest that few centers implant an adequate volume in children to meet this criterion. Of note, the data also did not show a statistically significant association between implant volume and complication rate.
A 2008 survey of 49 pediatric electrophysiology programs reported a median implant volume of 7 ICDs per center per year (range 1-30).(10) Our lower median implant volume of 2-3 ICDs per center per year (range in 2006: 1-24) likely reflects the breadth of the KID data from a nationwide sample that includes adult hospitals as well. The same survey included eleven programs that identify as having a specialized pediatric electrophysiology training program, with a median implant volume of 12 ICDs per center per year (range 1-30).(10) Our data from 2006 are comparable, including nine hospitals with an implant volume of greater than or equal to 10 implants per year, and a maximum implant volume of 24 ICDs per year.
Device implantation is known to be more technically challenging in the pediatric population, due not only to patient size, but also to complex anatomy related to congenital heart disease. Therefore, complication rates in pediatric ICD implantation are likely to be higher than in adults. In fact, children have been shown in prior studies to have a relatively higher incidence of infections and lead malfunctions than adults with ICDs.(24) The adult complication rate for ICD implantation from 1997-2003 was reported by Zhan to be 2.3-3.4%.(7) The complication rate in our study showed a trend towards decreasing over time (16% in 2000 to 10% in 2006, p=0.07), and is comparable to the 13 % perioperative complication rate reported by Silka and the 14% acute complication rate reported by Alexander.(3,25) Complication rate in our study was not related to a diagnosis of congenital heart disease or age. The rate of complications was also not related to hospital implant volume, a reassuring result given the overall low volume of implants at each center.
Study limitations
Administrative, population based databases have the benefit of including large groups of patients and providing a cross-section of national data. For rare diseases, they can be particularly helpful in garnering larger sample sizes than would be available from single or even multi-institution studies. Yet, administrative databases are limited by a lack of clinical detail and are susceptible to coding errors.(7,26) Because of the lack of clinical information available in the KID, we were unable to ascertain the exact indication for ICD implantation (primary versus secondary prevention), a question of particular interest as ICD indications evolve over time.
Data in the KID are limited to one particular hospitalization (in our case, the hospitalization in which the ICD was implanted). As a result, complications occurring after discharge were not captured in our analysis. In children, common complications such as lead fracture would be missed.
For the same reason, analysis of this particular database did not allow for determination of whether patients received appropriate or inappropriate shocks, an important source of morbidity in pediatric patients with ICDs. Children with congenital heart disease and ICDs, in particular, have been found to receive more inappropriate shocks than adults with congenital heart disease, due to lead failure, sinus or atrial tachycardias and oversensing.(2)
Implant volume in our study refers to the number of pediatric ICD implantations in a given hospital per year. Since the KID is limited to patients younger than 18 years of age, it does not include data on the total number of ICD implants (adult and pediatric combined) per center per year. As a result, we were unable to determine whether a low volume center in our sample is actually a high volume adult electrophysiology group implanting an ICD in an occasional pediatric patient.
Finally, one of the strengths of the KID database is its ability to produce national estimates. This has been validated in comparison with both administrative and clinical databases. Compared with the National Hospital Discharge Survey, a geographically representative administrative database of 445 hospitals from all 50 states and the District of Columbia, the KID estimates of length of stay and discharge counts were accurate and precise. Estimates of mortality were generally lower in the KID.(27) Relative to clinical databases (the Congenital Heart Surgeons Society database and a Society of Thoracic Surgeons cohort), Welke showed that the KID had slightly higher mortality rates, although our data revealed very few procedural mortalities.(28) Yet pediatric ICD implantation, a highly specialized and relatively uncommon procedure, is not necessarily evenly distributed geographically. How this affects our ability to extrapolate national estimates from the KID is uncertain.
Conclusions
ICD use has increased dramatically in the pediatric population from 1997 to 2006, paralleling the increase in the adult population and reflecting expansion of indications for ICD implantation. Despite the technical challenges of a physically smaller population with potentially more complex anatomy, implantation among younger patients increased as well. Moreover, younger patients and those with more complex anatomy related to congenital heart disease did not have an increased rate of complications. Although the overall implant volume increased from 1997 to 2006, each center implants relatively few ICDs per year, which may impact competency and training requirements.
Acknowledgments
No external sources of funding were used, and there are no relationships with industry to disclose. The views expressed are those of the authors and do not necessarily reflect official NHLBI positions.
Glossary of Abbreviations
- ICD
implantable cardioverter-defibrillator
- KID
Kids' Inpatient Database
- HCUP
Healthcare Cost and Utilization Project
- AHRQ
Agency for Healthcare Research and Quality
- ICD-9
International Classification of Diseases, Ninth Revision
- NACHRI
National Association of Children's Hospitals and Related Institutions
- CHD
congenital heart disease
- CM
cardiomyopathy
- LQTS
Long QT Syndrome
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Epstein AE, DiMarco JP, Ellenbogen KA, et al. ACC/AHA/HRS 2008 Guidelines for Device-Based Therapy of Cardiac Rhythm Abnormalities: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Writing Committee to Revise the ACC/AHA/NASPE 2002 Guideline Update for Implantation of Cardiac Pacemakers and Antiarrhythmia Devices): developed in collaboration with the American Association for Thoracic Surgery and Society of Thoracic Surgeons. Circulation. 2008;117:e350–408. doi: 10.1161/CIRCUALTIONAHA.108.189742. [DOI] [PubMed] [Google Scholar]
- 2.Berul CI, Van Hare GF, Kertesz NJ, et al. Results of a multicenter retrospective implantable cardioverter-defibrillator registry of pediatric and congenital heart disease patients. J Am Coll Cardiol. 2008;51:1685–91. doi: 10.1016/j.jacc.2008.01.033. [DOI] [PubMed] [Google Scholar]
- 3.Silka MJ, Kron J, Dunnigan A, Dick M., 2nd Sudden cardiac death and the use of implantable cardioverter-defibrillators in pediatric patients. The Pediatric Electrophysiology Society. Circulation. 1993;87:800–7. doi: 10.1161/01.cir.87.3.800. [DOI] [PubMed] [Google Scholar]
- 4.Walsh EP. Practical aspects of implantable defibrillator therapy in patients with congenital heart disease. Pacing Clin Electrophysiol. 2008;31 1:S38–40. doi: 10.1111/j.1540-8159.2008.00954.x. [DOI] [PubMed] [Google Scholar]
- 5.Moss AJ, Zareba W, Hall WJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–83. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 6.Bardy GH, Lee KL, Mark DB, et al. Amiodarone or an implantable cardioverter-defibrillator for congestive heart failure. N Engl J Med. 2005;352:225–37. doi: 10.1056/NEJMoa043399. [DOI] [PubMed] [Google Scholar]
- 7.Zhan C, Baine WB, Sedrakyan A, Steiner C. Cardiac device implantation in the United States from 1997 through 2004: a population-based analysis. J Gen Intern Med. 2008;23 1:13–9. doi: 10.1007/s11606-007-0392-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.HCUP Kids' Inpatient Database (KID) Agency for Healthcare Research and Quality; Rockville, MD: 1997, 2000, 2003, and 2006. [January 4,2010]. Healthcare Cost and Utilization Project (HCUP) Available at: www.hcup-us.ahrq.gov/kidoverview.jsp. [Google Scholar]
- 9.Hyattsville, MD: National Center for Health Statistics; 2009. [November 3, 2009.]. ICD-9-CM: International Classification of Diseases, Ninth Revision, Clinical Modification. Available at: http://icd9cm.chrisendres.com. [Google Scholar]
- 10.Saul JP, Epstein AE, Silka MJ, et al. Heart Rhythm Society/Pediatric and Congenital Electrophysiology Society Clinical Competency Statement: training pathways for implantation of cardioverter-defibrillators and cardiac resynchronization therapy devices in pediatric and congenital heart patients. Heart Rhythm. 2008;5:926–33. doi: 10.1016/j.hrthm.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 11.Shah MJ. Implantable cardioverter defibrillator-related complications in the pediatric population. Pacing Clin Electrophysiol. 2009;32 2:S71–4. doi: 10.1111/j.1540-8159.2009.02389.x. [DOI] [PubMed] [Google Scholar]
- 12.Agency for Healthcare Research and Quality; Rockville, MD: Mar, 2007. [January 4, 2010]. AHRQ Quality Indicators. Available at: http://www.qualityindicators.ahrq.gov/documentation.htm. [PubMed] [Google Scholar]
- 13.Ruskin JN, Camm AJ, Zipes DP, Hallstrom AP, McGrory-Usset ME. Implantable cardioverter defibrillator utilization based on discharge diagnoses from Medicare and managed care patients. J Cardiovasc Electrophysiol. 2002;13:38–43. doi: 10.1046/j.1540-8167.2002.00038.x. [DOI] [PubMed] [Google Scholar]
- 14.Calculating Kids' Inpatient Database (KID) Variances. Agency for Healthcare Research and Quality; Rockville, MD: Dec 16, 2005. [November 3, 2009]. Methods Series Report #2005-5. Healthcare Cost and Utilization Project (HCUP) Available at: http://www.hcupus.ahrq.gov/db/nation/kid/reports/CalculatingKIDVariances.pdf. [Google Scholar]
- 15.Agency for Healthcare Research and Quality; Rockville, MD: [November 3, 2009]. HCUPnet. Healthcare Cost and Utilization Project (HCUP) Available at: http://hcupnet.ahrq.gov/HCUPnet.jsp. [PubMed] [Google Scholar]
- 16.Agency for Healthcare Research and Quality; Rockville, MD: May, 2009. [March 23, 2010]. HCUP Data Use Training. Healthcare Cost and Utilization Project (HCUP) Available at: http://www.hcup-us.ahrq.gov/tech_assist/dua.jsp. [Google Scholar]
- 17.Altman DG. Practical statistics for medical research. 1st. London; New York: Chapman and Hall; 1991. [Google Scholar]
- 18.Emerson PL. Numerical construction of orthogonal polynomials from a general recurrence formula. Biometrics. 1968;24:695–701. [Google Scholar]
- 19.Rao JNK, Scott AJ. On Simple Adjustments to Chi-Square Tests with Sample Survey Data. Annals of Statistics. 1987;15:385–397. [Google Scholar]
- 20.New, revised ICD-9-CM codes to take effect on Oct. 1. [February 3, 2010];AAP News. 2005 September;26(9):38. Available at: http://aapnews.aappublication.org.
- 21.Berul CI. Implantable cardioverter defibrillator criteria for primary and secondary prevention of pediatric sudden cardiac death. Pacing Clin Electrophysiol. 2009;32 2:S67–70. doi: 10.1111/j.1540-8159.2009.02388.x. [DOI] [PubMed] [Google Scholar]
- 22.Gregoratos G, Abrams J, Epstein AE, et al. ACC/AHA/NASPE 2002 guideline update for implantation of cardiac pacemakers and antiarrhythmia devices: summary article: a report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (ACC/AHA/NASPE Committee to Update the 1998 Pacemaker Guidelines) Circulation. 2002;106:2145–61. doi: 10.1161/01.cir.0000035996.46455.09. [DOI] [PubMed] [Google Scholar]
- 23.Stephenson EA, Batra AS, Knilans TK, et al. A multicenter experience with novel implantable cardioverter defibrillator configurations in the pediatric and congenital heart disease population. J Cardiovasc Electrophysiol. 2006;17:41–6. doi: 10.1111/j.1540-8167.2005.00271.x. [DOI] [PubMed] [Google Scholar]
- 24.Link MS, Hill SL, Cliff DL, et al. Comparison of frequency of complications of implantable cardioverter-defibrillators in children versus adults. Am J Cardiol. 1999;83:263–6. A5–6. doi: 10.1016/s0002-9149(98)00834-0. [DOI] [PubMed] [Google Scholar]
- 25.Alexander ME, Cecchin F, Walsh EP, Triedman JK, Bevilacqua LM, Berul CI. Implications of implantable cardioverter defibrillator therapy in congenital heart disease and pediatrics. J Cardiovasc Electrophysiol. 2004;15:72–6. doi: 10.1046/j.1540-8167.2004.03388.x. [DOI] [PubMed] [Google Scholar]
- 26.Saba S, Ravipati LP, Voigt A. Recent trends in utilization of implantable cardioverter-defibrillators in survivors of cardiac arrest in the United States. Pacing Clin Electrophysiol. 2009;32:1444–9. doi: 10.1111/j.1540-8159.2009.02509.x. [DOI] [PubMed] [Google Scholar]
- 27.Whalen D, Houchens R, Elixhauser A. Agency for Healthcare Research and Quality; Rockville, MD: Jun 23, 2006. [July 30, 2010]. 2003 HCUP KIDS' Inpatient Database (KID) Comparison Report. HCUP Methods Series Report # 2006-03. Healthcare Cost and Utilization Project (HCUP) Available at: http://www.hcup-us.ahrq.gov/reports/methods.jsp. [Google Scholar]
- 28.Welke KF, Diggs BS, Karamlou T, Ungerleider RM. Comparison of pediatric cardiac surgical mortality rates from national administrative data to contemporary clinical standards. Ann Thorac Surg. 2009;87:216–22. doi: 10.1016/j.athoracsur.2008.10.032. discussion 222-3. [DOI] [PubMed] [Google Scholar]


