Abstract
Emerging data suggest that illicit methylphenidate abuse is a growing problem. Although abuse of the drug typically occurs by the intranasal route, oral (per os; p.o.) methylphenidate also has abuse potential. The present study compared the effects of p.o. and intraperitoneal (i.p.) methylphenidate in rats using the conditioned place preference (CPP) procedure. Young adult male Sprague-Dawley rats were trained to consume oyster crackers injected initially with saline. Next, rats were randomly assigned to receive p.o. or i.p. methylphenidate (3 or 10 mg/kg) or saline immediately or 30 min prior to 30-min conditioning trials. Methylphenidate or saline were each paired 4 times with an end compartment; preference for the methylphenidate-paired compartment was then assessed on a drug-free session. When given immediately prior to conditioning, significant CPP was obtained with both 3 and 10 mg/kg of i.p. methylphenidate, but only with 10 mg/kg of p.o. methylphenidate. When given 30 min prior to conditioning, there was no evidence of CPP for any dose of i.p. or p.o. methylphenidate. These findings are the first demonstration that p.o. methylphenidate has rewarding effects, although i.p. methylphenidate is obtained at a 3 mg/kg dose which did not establish CPP with p.o. administration. The lack of CPP following 30 min pretreatment also suggests that conditioning may require the CS to be associated with a US of ascending, rather than descending, brain levels of methylphenidate. These results are consistent with clinical evidence of the reduced abuse liability of p.o. methylphenidate relative to methylphenidate taken by other (e.g., intranasal) routes.
Keywords: methylphenidate, conditioned place preference, rat, oral administration
1. Introduction
The use of stimulant medications (e.g., methylphenidate; MPH) for the treatment of attention-deficit/hyperactivity disorder (ADHD) is a well-established practice in psychiatric medicine. Unfortunately, evidence of illicit MPH abuse also appears to have increased dramatically over the past several years (Bogle and Smith, 2009; DeSantis et al., 2008; Dupont et al., 2008; Setlik et al., 2009).
One of the factors involved in the misuse of methylphenidate is the method of administration. Typically, methylphenidate is prescribed for oral (per os; p.o.) use in therapeutic settings, which is not associated with high rates of abuse (Swanson and Volkow, 2008). However, p.o. methylphenidate has been shown to function as a reinforcer and increase positive ratings on self-report measures of abuse liability in laboratory settings (e.g., Chait, 1994; Jasinski, 2000; Rush and Baker, 2001), and survey data suggest that p.o. methylphenidate is abused (Teter et al., 2006). In an effort to reduce abuse liability and the need for multiple daily dosings, several extended-release methylphenidate formulations (e.g., Concerta®, Metadate®, Ritalin LA®) have been developed to avoid problems associated with immediate-release formulations. The validity of this approach is supported by several studies showing an attenuated response to extended-release methylphenidate compared to immediate-release methylphenidate on subjective measures of positive drug effects (Kollins et al., 1998; Parasrampuria et al., 2007; Spencer et al., 2006). Unfortunately, the development of extended-release methylphenidate has not eliminated abuse of the drug, since users commonly crush the tablets in order to subvert the slow onset of effects following oral ingestion (Bright, 2008). By pulverizing tablets for insufflation, formulation differences in release are negated, and a rapid onset of drug action is achieved; this may be a primary contributor to the greater frequency of intranasal methylphenidate abuse (Dupont et al., 2008; Volkow and Swanson, 2003). However, the development of a transdermal delivery system may prove useful in this regard (Sane and McGough, 2002).
A number of preclinical animal studies have also shown that methylphenidate produces abuse-related effects in several different models, although most studies have focused on intravenous (i.v.) and intraperitoneal (i.p.) administration. It has been shown that i.v. methylphenidate functions as a reinforcer in the self-administration procedure in rats (Botly et al., 2008; Collins et al., 1984; Hiranita et al., 2009; Marusich and Bardo, 2009; Marusich et al., 2010; Nielsen et al., 1984) and monkeys (Johanson and Schuster, 1975; Lile et al., 2003; Wee and Woolverton, 2004; Gasior et al., 2005). In the conditioned place preference (CPP) procedure, both i.v. and i.p. methylphenidate have been shown to establish place preference (Martin-Iverson et al., 1985; Mithani et al., 1996; Gatley et al., 1996; Meririnee et al., 2001; Sellings et al., 2006). There has been some concern that treatment with methylphenidate for ADHD may lead to a greater propensity to substance abuse. Indeed, evidence from preclinical studies suggests that methylphenidate injections can increase subsequent cocaine self-administration (Brandon et al., 2001; Griggs et al., 2010), although one study using p.o. methylphenidate found the opposite effect (Thanos et al., 2007). Discrepant findings have also been reported in clinical literature, as methylphenidate treatment of ADHD has been reported to decrease the risk for substance abuse in this vulnerable population (Kollins, 2008), yet other evidence suggests methylphenidate-exposed individuals report greater ‘liking’ and ‘wanting’ of initial cocaine experiences (Lambert et al., 2006). While much of the discrepancy in the literature remains to be reconciled, additional preclinical studies of p.o. methylphenidate are warranted since this is the route used for treatment of ADHD.
The abuse-related effects of p.o. methylphenidate have not been studied widely in animal models. One study using rats found that i.p. methylphenidate produced significantly greater hyperactivity and extracellular dopamine efflux in nucleus accumbens compared to equivalent doses of p.o. methylphenidate (Gerasimov et al., 2000). The present investigation was undertaken to explore the rewarding effects of p.o. and i.p. methylphenidate in rats using the CPP model of drug reward.
2. Materials and methods
2.1. Animals
A total of 60 male Sprague-Dawley rats (Harlan Industries, Indianapolis, IN, USA) initially weighing 250–275 g were used. Rats were 60 days old at the start of the experiment. Rats were individually housed in standard plastic cages in a temperature- and humidity-controlled facility set to a 14:10 hr light/dark cycle (lights on at 0600 hr), and were handled and acclimated to the colony for 1 week prior to the start of the experiment. Experimental sessions were conducted during the light phase between 1400 and 1600 hr. Rats were initially given ad libitum access to food and water while in the home cage, but were restricted to 13 g of food per day once the experiment began. Experimental protocols were in accordance with the 1996 NIH Guide for the Care and Use of Laboratory Animals and were approved by the Institutional Animal Care and Use Committee at the University of Kentucky.
2.2. Apparatus
Experiments were conducted with eight automated CPP chambers (ENV-013, Med Associates, St. Albans, VT, USA). Each chamber measured 21 × 21 × 68 cm and consisted of three distinct compartments. Two 28 cm-long side compartments (one black compartment with a steel rod floor and one white compartment with a steel mesh floor) were separated by a 12-cm long central gray compartment with a smooth PVC floor. A guillotine door separated both end compartments, such that the apparatus could be configured to either confine rats to one end compartment or to allow free access to all compartments. Six photo beams spaced 1.25 cm from the end wall and 5 cm apart were located inside each end compartment, and three photo beams spaced 4.75 cm apart were located in the central compartment. Each chamber was interfaced to a personal computer running MED-PC IV (Med Associates) software.
2.3. Experimental procedure
After the initial 7-day colony acclimation period was completed, rats were restricted to 13 g of food per day and trained to consume oyster crackers (~2 g each; Nabisco brand, East Hanover, NJ, USA) that were injected with saline only (to avoid conditioned taste aversion); each rat received one cracker per day. Rats rapidly (i.e., within ~5 sessions) learned to consume the cracker within 2–3 minutes. The experiment began once it was verified that each rat learned to eat the cracker. At that point, rats were allowed to freely explore the CPP apparatus during 2 consecutive, daily 15-min sessions. The first day served as an apparatus habituation session, and the second day served as the pre-conditioning test session. For the pre-conditioning test, the amount of time the rat spent in the white and black end compartments was monitored. Over the next 8 days, rats were confined to each end compartment for 30 min on alternating days. One end compartment (counter-balanced) was paired with either p.o. or i.p. methylphenidate, and the other end compartment was paired with saline. Rats received methylphenidate (3 or 10 mg/kg) immediately or 30 min prior to the start of the drug conditioning session. For rats assigned to receive p.o. methylphenidate, methylphenidate was injected into the oyster cracker, and the i.p. injection was saline. For rats assigned to receive i.p. methylphenidate, saline was injected into the oyster cracker and the i.p. injection was methylphenidate. Rats used in the control groups received saline in both the cracker and i.p. injection. Thus, all rats were given both oyster crackers and i.p. injections. Prior to each session, it was verified that rats consumed the entire cracker, ensuring administration of the correct dose. Within all groups, an equal number of rats were conditioned with methylphenidate in the white and black end compartments.
The day after the final conditioning session, rats were again allowed to explore freely each compartment of the apparatus during a 15-min session. The dependent measure was the difference in time spent in the methylphenidate-paired compartment on the post-conditioning test minus the time spent on the pre-conditioning test.
2.4. Drug
Methylphenidate HCl was obtained from Mallinckrodt (St. Louis, MO) and prepared in 0.9% NaCl (saline). Drug was administered in a volume of 1 ml/kg of body weight by both p.o. and i.p. routes. Doses reflect the salt weight.
2.5. Data analysis
Data represent the mean (±S.E.M.) change (Δ) in time spent in the methylphenidate-paired compartment on the post-conditioning test relative to the pre-conditioning test. Data were analyzed with an overall analysis of variance (ANOVA) with route of administration, dose and pretreatment interval as between-subjects factors. Following detection of significant main effects or interactions, post-hoc tests were conducted to compare the effects to saline controls, or to compare the effects of methylphenidate doses given by different routes of administration. Significance was declared at P<0.05.
3. Results
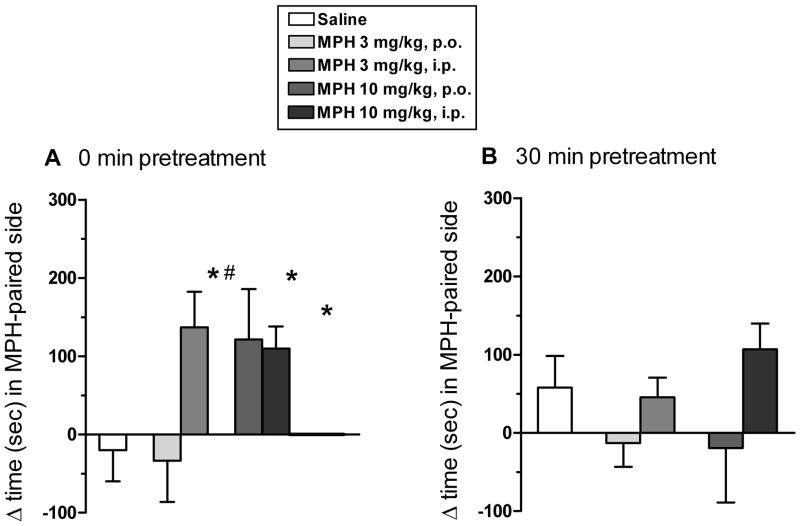
Fig. 1 illustrates the expression of CPP for p.o. and i.p. methylphenidate (3 or 10 mg/kg) given immediately (Fig. 1A) or 30 min (Fig. 1B) prior to conditioning sessions. The overall ANOVA revealed a significant main effect of route of administration (F(1,50)=7.19; P< 0.01) and a significant route of administration × dose × pretreatment interval interaction (F(1,50)=3.82; P< 0.05). With immediate treatment, the 10 mg/kg dose of p.o. methylphenidate, and both doses of i.p. methylphenidate, produced significant CPP relative to saline (Fig. 1A). With 30 min pretreatment, there was no evidence of CPP for either dose of p.o. or i.p. methylphenidate (Fig. 1B).
Fig. 1.
CPP with oral (per os, p.o.) or intraperitoneal (i.p.) methylphenidate (MPH) relative to saline controls. Bars represent the mean (±S.E.M.) change in the amount of time (sec) spent in the MPH-paired compartment on the post-conditioning test minus the amount of time spent in the MPH-paired compartment on the pre-conditioning test. During conditioning, MPH (3 or 10 mg/kg) was given p.o. or i.p. immediately (A) or 30 min (B) prior to daily conditioning sessions. Symbol (*) indicates a significant difference relative to saline controls (P<0.05). Symbol (#) indicates a significant difference relative to the corresponding dose of p.o. MPH (P<0.05). N=6 rats per group.
4. Discussion
The present study is the first demonstration that p.o. methylphenidate has a rewarding effect in rats. The CPP procedure was chosen because the results obtained with stimulant drugs are typically similar to results obtained with the self-administration procedure, and because it is sensitive to pharmacokinetic factors (Bardo and Bevins, 2000). Although evidence of CPP with both p.o. and i.p. methylphenidate was obtained with immediate pretreatment, only the high dose of p.o. methylphenidate was effective, whereas each dose of i.p. methylphenidate produced CPP. However, no CPP was evident following the longer 30 min pretreatment interval.
It is interesting to note the attenuated response to 3 mg/kg of p.o. methylphenidate since CPP was obtained with 3 mg/kg of i.p. methylphenidate. Previous work has shown that doses of i.p. methylphenidate ranging from 1.25–20 mg/kg can elicit CPP (Kankaanpaa et al., 2002; Martin-Iverson et al., 1985; Meririnne et al., 2001). One potential explanation for the present finding is that the brain bioavailability of 3 mg/kg of p.o. methylphenidate is less than that of a lower dose (e.g., 1.25 mg/kg) of i.p. methylphenidate. Although levels of methylphenidate in brain and plasma were not measured in the present report, the absolute bioavailability of p.o. methylphenidate has been reported to be ~0.19 in at least one report (Wargin et al., 1983). Thus, it is possible that the absolute bioavailability of 3 mg/kg of p.o. methylphenidate in brain may have been lower than that following administration of 1.25 mg/kg of i.p. methylphenidate reported by Merirrine et al. (2001) to be the minimum threshold dose for establishing CPP in rats. It will be important in future work to determine more precisely the mechanism(s) mediating the differential effects of i.p. and p.o. methylphenidate in the CPP procedure.
One potential mechanism underlying the differential effects of 3 mg/kg of p.o. and i.p. methylphenidate could be that the rise in dopamine content produced by p.o. administration was insufficient to elicit reward. Accordingly, a comparable dose of 2 mg/kg of p.o. methylphenidate did not affect extracellular nucleus accumbens dopamine levels measured with in vivo microdialysis, although the same dose of i.p. methylphenidate was effective in this regard (Gerasimov et al., 2000). In addition, those authors found corresponding decreases in brain and plasma drug levels with p.o. methylphenidate compared to i.p. methylphenidate. Collectively, these results suggest that lower bioavailability of 3 mg/kg of p.o. methylphenidate, and the corresponding blunting in dopamine release, may contribute to the differential effect of this dose following p.o. and i.p. routes of administration.
In contrast to the low dose, 10 mg/kg of methylphenidate produced significant CPP with both p.o. and i.p. administration. This finding suggests that differences in bioavailability underlying the differential effects of 3 mg/kg of methylphenidate are overcome by administration of higher doses. These findings are in accord with clinical work showing that even though different formulations of methylphenidate are more effective than others in producing reward (e.g., immediate release methylphenidate > extended release methylphenidate; intranasal methylphenidate > p.o. methylphenidate; Kollins et al., 1998, Spencer et al., 2006; Stoops et al., 2003), it is possible to achieve rewarding effects with any formulation, providing sufficiently high doses are used (Martin et al., 1971; Stoops et al., 2005)
When drug administration preceded conditioning trials by a 30 min pretreatment, no dose of p.o. or i.p. methylphenidate supported conditioning. One possible explanation for this finding is that peak brain uptake of methylphenidate may have occurred during the 30 min pretreatment interval prior to the start of the conditioning trial. In this case, the drug US onset may have preceded the contextual CS presentation, thus setting up a backward conditioning trial that failed to establish CPP (see Bardo and Bevins, 2000). Since the time to maximal concentration is 10–30 min in the rat (Wargin et al., 1984), this would suggest that the contextual CS was associated with falling drug levels, which may have produced neutral or aversive effects. Similar work using cocaine has shown that cocaine CPP is robust when drug is administered immediately prior to the start of conditioning sessions, but not when drug is given 15 min prior to the session (Ettenberg et al., 1999; Ettenberg and Ranardi, 2007). However, to determine conclusively if backward conditioning offers a viable explanation for the failure to obtain CPP when methylphenidate was given 30 min prior to session, it would be important to reverse the onset of the drug US pharmacologically prior to exposing the rat to the contextual CS (see Bardo and Neisewander, 1984). Alternatively, it is possible that the relatively small number of rats in each test group (i.e., n=6) may have yielded a type 2 (false negative) error. That is, significant CPP may have been observed with one or more MPH doses, particularly with i.p. administration, if a greater number of rats had been used in each group.
The present work should have relevance to future studies testing potential alterations in reactivity to cocaine or other drugs of abuse. Specifically, the discrepant findings of prior research (e.g., Brandon et al., 2001; Griggs et al., 2010; Thanos et al., 2007) are likely attributable to differences in the dose and route of methylphenidate administration. Thus, use of p.o. doses > 3 mg/kg should be avoided if attempting to model clinical usage, as p.o. administration of therapeutic doses typically does not lead to abuse (Swanson and Volkow, 2009). It will be of interest to determine if administration of low p.o. doses alters subsequent cocaine self-administration.
In sum, the present results provide preliminary evidence suggesting that p.o. methylphenidate has abuse potential as determined in the CPP procedure. While different results may have been obtained with an increase in statistical power, these data indicate that higher doses are required in order for p.o. methylphenidate to function as a rewarding stimulus than required for i.p. methylphenidate. These results support the notion that the abuse potential of methylphenidate may be reduced by formulations that prevent tampering and subsequent intranasal use in humans. Our results showing that 10 mg/kg of p.o. methylphenidate establishes CPP are similar to clinical data showing that extended release methylphenidate produces abuse-related subjective and reinforcing effects at high doses (Spencer et al., 2006). The present findings are important because they demonstrate that the rewarding properties of p.o. methylphenidate can be studied with preclinical models, which provides concordance between animal and human clinical research. Future studies should be able to use this procedure to examine the neurobiology, as well as possible long-term consequences, of administration of rewarding vs. non-rewarding doses of p.o. methylphenidate.
Acknowledgments
This work was funded by NIH grants P50 DA05312, R01 DA13519 and F31 DA023853.
Footnotes
The authors have no financial disclosures to report.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Bardo MT, Neisewander JL. Single-trial conditioned place preference using intravenous morphine. Pharmacol Biochem Behav. 1984;25:1101–1105. doi: 10.1016/0091-3057(86)90092-4. [DOI] [PubMed] [Google Scholar]
- Bardo MT, Bevins RA. Conditioned place preference: what does it add to our preclinical understanding of drug reward? Psychopharmacology. 2000;153:31–43. doi: 10.1007/s002130000569. [DOI] [PubMed] [Google Scholar]
- Bogle KE, Smith BH. Illicit methylphenidate use: a review of prevalence, availability, pharmacology, and consequences. Curr Drug Abuse Rev. 2009;2:157–176. doi: 10.2174/1874473710902020157. [DOI] [PubMed] [Google Scholar]
- Botly LC, Burton CL, Rizos Z, Fletcher PJ. Characterization of methylphenidate self-administration and reinstatement in the rat. Psychopharmacology. 2008;199:55–66. doi: 10.1007/s00213-008-1093-z. [DOI] [PubMed] [Google Scholar]
- Brandon CL, Marinelli M, Baker LK, White FJ. Enhanced reactivity and vulenerability to cocaine following methylphenidate treatment in adolescent rats. Neuropsychopharmacology. 2001;25:651–61. doi: 10.1016/S0893-133X(01)00281-0. [DOI] [PubMed] [Google Scholar]
- Bright GM. Abuse of medications employed for the treatment of ADHD: results from a large-scale community survey. Medscape J Med. 2008;10:111. [PMC free article] [PubMed] [Google Scholar]
- Chait LD. Reinforcing and subjective effects of methylphenidate in humans. Behav Pharmacol. 1994;5:281–288. doi: 10.1097/00008877-199406000-00005. [DOI] [PubMed] [Google Scholar]
- DeSantis AD, Webb EM, Noar SM. Illicit use of prescription ADHD medications on a college campus: a multimethodological approach. J Am Coll Health. 2008;57:315–324. doi: 10.3200/JACH.57.3.315-324. [DOI] [PubMed] [Google Scholar]
- Dupont RL, Coleman JJ, Bucher RH, Wilford BB. Characteristics and motives of college students who engage in nonmedical use of methylphenidate. Am J Addict. 2008;17:167–71. doi: 10.1080/10550490802019642. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Raven MA, Danluck DA, Necessary BD. Evidence of opponent-process actions of intravenous cocaine. Pharmacol Biochem Behav. 1999;64:507–12. doi: 10.1016/s0091-3057(99)00109-4. [DOI] [PubMed] [Google Scholar]
- Ettenberg A, Bernardi RE. Effects of buspirone on the immediate positive and delayed negative properties of intravenous cocaine as measured in the conditioned place preference test. Pharmacol Biochem Behav. 2007;87:171–178. doi: 10.1016/j.pbb.2007.04.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gatley SJ, Meehan SM, Chen R, Pan DF, Schechter MD, Dewey SL. Place preference conditioning and microdialysis studies with two derivatives of methylphenidate. Life Sci. 1996;58:345–352. doi: 10.1016/0024-3205(96)00222-6. [DOI] [PubMed] [Google Scholar]
- Gerasimov MR, Franceschi M, Volkow ND, Gifford A, Gatley SJ, Marsteller D, Molina PE, Dewey SL. Comparison between intraperitoneal and oral methylphenidate administration: A microdialysis and locomotor activity study. J Pharmacol Exp Ther. 2007;295:51–57. [PubMed] [Google Scholar]
- Griggs R, Weir C, Wayman W, Koeltzow TE. Intermittent methylphenidate during adolescent development produces locomotor hyperactivity and an enhanced response to cocaine compared to continuous treatment in rats. Pharmacol Biochem Behav. 2010;96:166–74. doi: 10.1016/j.pbb.2010.04.026. [DOI] [PubMed] [Google Scholar]
- Jasinski DR. An evaluation of the abuse potential of modafinil using methylphenidate as a reference. J Psychopharmacol. 2000;14:53–60. doi: 10.1177/026988110001400107. [DOI] [PubMed] [Google Scholar]
- Kollins SH, Rush CR, Pazzaglia PJ, Ali JA. Comparison of acute behavioral effects of sustained-release and immediate-release methylphenidate. Exp Clin Psychopharmacol. 1998;6:367–374. doi: 10.1037//1064-1297.6.4.367. [DOI] [PubMed] [Google Scholar]
- Kollins SH. A qualitative review of issues arising in the use of psycho-stimulant medications in patients with ADHD and co-morbid substance use disorders. Curr Med Res Opin. 2008;24:1345–57. doi: 10.1185/030079908x280707. [DOI] [PubMed] [Google Scholar]
- Kuczenski R, Segal DS. Stimulant actions in rodents: implications for attention- deficit/hyperactivity disorder treatment and potential substance abuse. Biol Psychiatry. 2005;57:1391–1396. doi: 10.1016/j.biopsych.2004.12.036. [DOI] [PubMed] [Google Scholar]
- Lambert NM, McLeod M, Schenk S. Subjective responses to initial experience with cocaine: an exploration of the incentive-sensitization theory of drug abuse. Addiction. 2006;101:713–25. doi: 10.1111/j.1360-0443.2006.01408.x. [DOI] [PubMed] [Google Scholar]
- Marusich JA, Bardo MT. Differences in impulsivity on a delay-discounting task predict self-administration of a low unit dose of methylphenidate in rats. Behav Pharmacol. 2009;20:447–454. doi: 10.1097/FBP.0b013e328330ad6d. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin WR, Sloan JW, Sapira JD, Jasinski DR. Physiological, subjective, and behavioral effects of amphetamine, methamphetamine, ephedrine, phenmetrazine, and methylphenidate in man. Clin Pharmacol Ther. 1971;12:245–258. doi: 10.1002/cpt1971122part1245. [DOI] [PubMed] [Google Scholar]
- Meririnne E, Kankaanpaa A, Seppala T. Rewarding properties of methylphenidate: sensitization by prior exposure to the drug and effects of dopamine D1- and D2-receptor antagonists. J Pharmacol Exp Ther. 201;298:539–550. [PubMed] [Google Scholar]
- Nielsen JA, Duda NJ, Mokler DJ, Moore KE. Self-administration of central stimulants by rats: a comparison of the effects of d-amphetamine, methylphenidate and McNeil 4612. Pharmacol Biochem Behav. 1984;20:227–232. doi: 10.1016/0091-3057(84)90247-8. [DOI] [PubMed] [Google Scholar]
- Parasrampuria DA, Schoedel KA, Schuller R, Silber SA, Ciccione PE, Gu J, Sellers EM. Do formulation differences alter abuse liability of methylphenidate? A placebo-controlled, randomized, double-blind, crossover study in recreational drug users. J Clin Psychopharmacol. 2007;27:459–467. doi: 10.1097/jcp.0b013e3181515205. [DOI] [PubMed] [Google Scholar]
- Rush CR, Baker RW. Behavioral pharmacological similarities between methylphenidate and cocaine in cocaine abusers. Exp Clin Psychopharmacol. 2001;9:59–73. doi: 10.1037/1064-1297.9.1.59. [DOI] [PubMed] [Google Scholar]
- Sane N, McGough JJ. MethyPatch Noven. Curr Opin Investig Drugs. 2002;3:1222–1224. [PubMed] [Google Scholar]
- Sellings LH, McQuade LE, Clarke PB. Characterization of dopamine-dependent rewarding and locomotor stimulant effects of intravenously-administered methylphenidate in rats. Neuroscience. 2006;141:1457–1468. doi: 10.1016/j.neuroscience.2006.04.040. [DOI] [PubMed] [Google Scholar]
- Setlik J, Bond GR, Ho M. Adolescent prescription ADHD medication abuse is rising along with prescriptions for these medications. Pediatrics. 2009 doi: 10.1542/peds.2008-0931. [DOI] [PubMed] [Google Scholar]
- Spencer TJ, Biederman J, Ciccone PE, Madras BK, Dougherty DD, Bonab AA, Livni E, Parasrampuria DA, Fischman AJ. PET study examining pharmacokinetics, detection and likeability, and dopamine transporter receptor occupancy of short- and long-acting oral methylphenidate. Am J Psychiatry. 2006;163:387–395. doi: 10.1176/appi.ajp.163.3.387. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Glaser PE, Rush CR. Reinforcing, subject-rated, and physiological effects of intranasal methylphenidate in humans: a dose-response analysis. Drug Alc Depend. 2003;71:179–186. doi: 10.1016/s0376-8716(03)00131-5. [DOI] [PubMed] [Google Scholar]
- Stoops WW, Lile JA, Fillmore MT, Glaser PE, Rush CR. Reinforcing effects of methylphenidate: influence of dose and behavioral demands following drug administration. Psychopharmacology. 2005;177:349–355. doi: 10.1007/s00213-004-1946-z. [DOI] [PubMed] [Google Scholar]
- Swanson JM, Volkow ND. Psychopharmacology: concepts and opinions about the use of stimulant medications. J Child Psychol Psychiatry. 2009;50:180–193. doi: 10.1111/j.1469-7610.2008.02062.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teter CJ, McCabe SE, LaGrange K, Cranford JA, Boyd CJ. Illicit use of specific prescription stimulants among college students: prevalence, motives, and routes of administration. Pharmacotherapy. 2006;26:1501–1510. doi: 10.1592/phco.26.10.1501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanos PK, Michaelides M, Benveniste H, Wang GJ, Volkow ND. Effects of chronic oral methylphenidate on cocaine self-administration and striatal dopamine D2 receptors in rodents. Pharmacol Biochem Behav. 2007;87:426–33. doi: 10.1016/j.pbb.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Volkow ND, Wang G, Fowler JS, Logan J, Gerasimov M, Maynard L, Ding Y, Gatley SJ, Gifford SJ, Franceschi D. Therapeutic doses of oral methylphenidate significantly increase extracellular dopamine in the human brain. J Neurosci. 2001;21:RC121. doi: 10.1523/JNEUROSCI.21-02-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Volkow ND, Swanson JM. Variables that affect the clinical use and abuse of methylphenidate in the treatment of ADHD. Am J Psychiatry. 2003;160:1909–1918. doi: 10.1176/appi.ajp.160.11.1909. [DOI] [PubMed] [Google Scholar]