Abstract
Previous studies in Parkinson's disease (PD) models suggest that early events along the path to neurodegeneration involve activation of the ubiquitin-proteasome system (UPS), endoplasmic reticulum-associated degradation (ERAD), and the unfolded protein response (UPR) pathways, in both the sporadic and familial forms of the disease, and thus ER stress may be a common feature. Furthermore, impairments in protein degradation have been linked to oxidative stress as well as pathways associated with ER stress. We hypothesize that oxidative stress is a primary initiator in a multi-factorial cascade driving dopaminergic (DA) neurons towards death in the early stages of the disease. We now report results from proteomic analysis of a rotenone-induced oxidative stress model of PD in the human neuroblastoma cell line, SH-SY5Y. Cells were exposed to sub-micromolar concentrations of rotenone for 48 hours prior to whole cell protein extraction and shotgun proteomic analysis. Evidence for activation of the UPR comes from our observation of up-regulated Binding immunoglobulin Protein (BiP), heat shock proteins, and foldases. We also observed up-regulation of proteins that contribute to the degradation of misfolded or unfolded proteins controlled by the UPS and ERAD pathways. Activation of the UPR may allow neurons to maintain protein homeostasis in the cytosol and ER despite an increase in reactive oxygen species due to oxidative stress, and activation of the UPS and ERAD may further augment clean-up and quality control in the cell.
Keywords: Oxidative stress, Parkinson’s disease, Rotenone, Proteomics, Unfolded protein response
Introduction
Parkinson’s disease (PD) is a complex, progressive neurodegenerative movement disease that results primarily from the death of dopaminergic (DA) neurons in the substantia nigra pars compacta. Although the cause of sporadic PD is unknown, epidemiological studies suggest cooperation with environmental toxins, notably mitochondrial complex I inhibitors and gene mutations [18]. Oxidative stress is a leading theory of the pathogenesis of PD, supported by analysis of postmortem PD brains [33], and by recapitulation of the oxidative damage seen in PD by low-grade, chronic inhibition of complex 1 with rotenone, both in vivo and in vitro [2, 10]. The common feature associated with oxidative stress and subsequent neurodegeneration is endoplasmic reticulum (ER) stress: a disturbance in the ability of the ER to process and/or fold proteins. The resulting accumulation of misfolded proteins will elicit the Unfolded Protein Response (UPR), leading to transcription of chaperones, foldases, ER-associated degradation (ERAD) machinery and antioxidants. Acute activation of the UPR is beneficial in responding to transient stress. However, if ER stress is chronic, or if protective measures are incapable of maintaining ER homeostasis, the UPR activates apoptotic pathways to avoid damage to neighboring cells [32]. Our goal in this study was to produce an early-stage model of PD by using very small doses of the oxidizing toxin rotenone and report on the changes in the whole cell proteome.
Materials & Methods
Cell Culture
Human neuroblastoma cells (SH-SY5Y) were obtained from the American Type Culture Collection (Rockville, MD).
Cell viability
Cell viability was determined by a MTT (3-(4, 5-dimethylthiazol)-2,5diphenyltetrazolium bromide) assay following the manufacture’s guidelines (ATCC catalog # 30-1010K).
Proteasome Activity Assay - Chymotrypsin-like activity
Control and rotenone treated cells were incubated with Succinyl-Leu-Leu-Val-Tyr-7-amino-4-methylcoumarin (LLVY-MCA) (obtained from Dr. M. Gaczynska, Univ. of Texas Health Science Center, San Antonio) at 37°C for 1 hour. Fluorescence of released MCA was measured at 460 nm (with 380 nm excitation).
ssDNA Apoptosis
Control and rotenone-treated cells were analyzed for apoptosis using a Millipore ssDNA Apoptosis ELISA kit (APT225) in accordance with the manufacture’s guidelines.
Caspase-3 Activity Assay
Caspase-3 activity was quantified using Ac-DEVD-AMC Caspase -3 fluorogenic substrate (BD Pharmingen) in accordance with the manufacture’s guidelines.
Sample Preparation
Cells were prepared for protein identification by incubation for 48 hours at 37°C with 5nM, 10nM, 20nM, 50nM, and 100nM rotenone, or as a control, vehicle only. Proteins from cell culture monolayers were extracted by adding 500µL buffer (25mM Tris-HCl pH 7.6, 150mM NaCl, 1% NP-40, 1% sodium deoxycholate, 0.1% SDS) and 10µL Protease Inhibitor Cocktail (Sigma P8340) to 106 cells. After 30 min lysis at 4°C, cells were centrifuged and the supernatant was stored at −20°C. Total protein was quantified using the Micro BCA kit (Pierce) following manufacture’s protocol. Proteins were carbamidomethylated and precipitated with acetone [37] prior to trypsin digestion (Promega Gold, mass spectrometry grade) (1:20 protein:enzyme). Capillary liquid chromatography-tandem mass spectrometry (LC/MS/MS) was performed with a splitless nanoLC-2D pump (Eksigent), a 50 µm-i.d. column packed with 10 cm of 5 µm-i.d. C18 particles, and a linear ion trap tandem mass spectrometer (LTQ-XLS; ThermoFisher, San Jose, CA), where the top 7 eluting ions were fragmented by collision-induced dissociation (CID). The capillary LC gradient was 2 to 98% 0.1% formic acid/acetonitrile over 60 min at a flow rate of 300 nL/min. Probability-based and error-tolerant protein database searching of MS/MS spectra against the NCBI non-redundant human protein database was performed with a 10-node MASCOT cluster (ver. 2.1). Search criteria included: peak picking with Xcalibur (ver. 2.0.6; ThermoFisher); 1000 ppm precursor ion mass tolerance, 0.8 Da product ion mass tolerance, 3 missed cleavages, trypsin, carbamidomethyl cysteines and oxidized methionines as variable modifications, and an ion score threshold of 20.
Criteria for Protein Identification
Scaffold (version scaffold_2_06_00, Proteome Software Inc., Portland, OR) was used to validate MS/MS based peptide and protein identifications. Peptide identifications were accepted if they could be established at greater than 90.0% probability (Peptide Prophet algorithm [15]). Protein identifications were accepted if they could be established at a greater than 95.0% probability (Protein Prophet algorithm [26]) and contained at least 1 identified peptide.
Results & Discussion
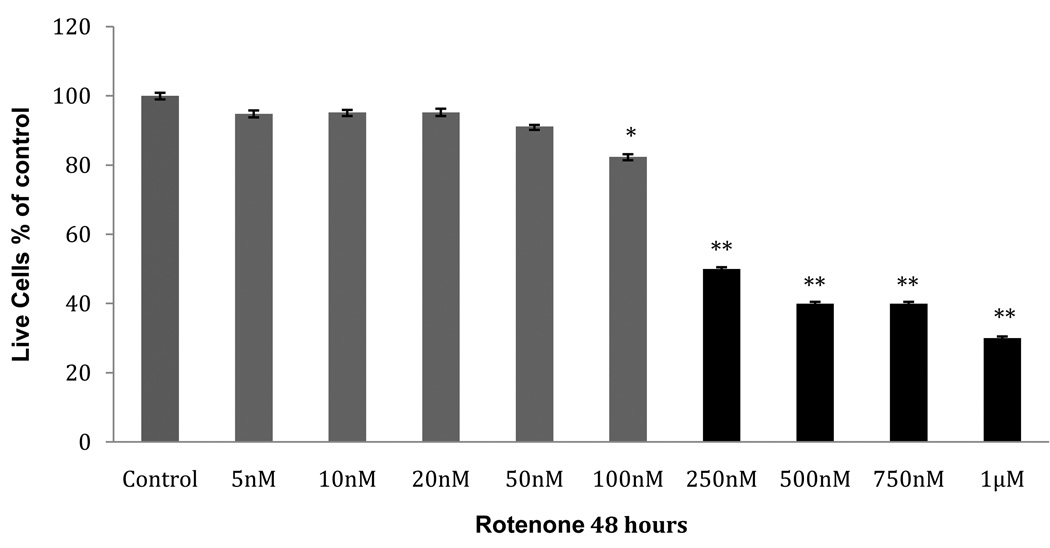
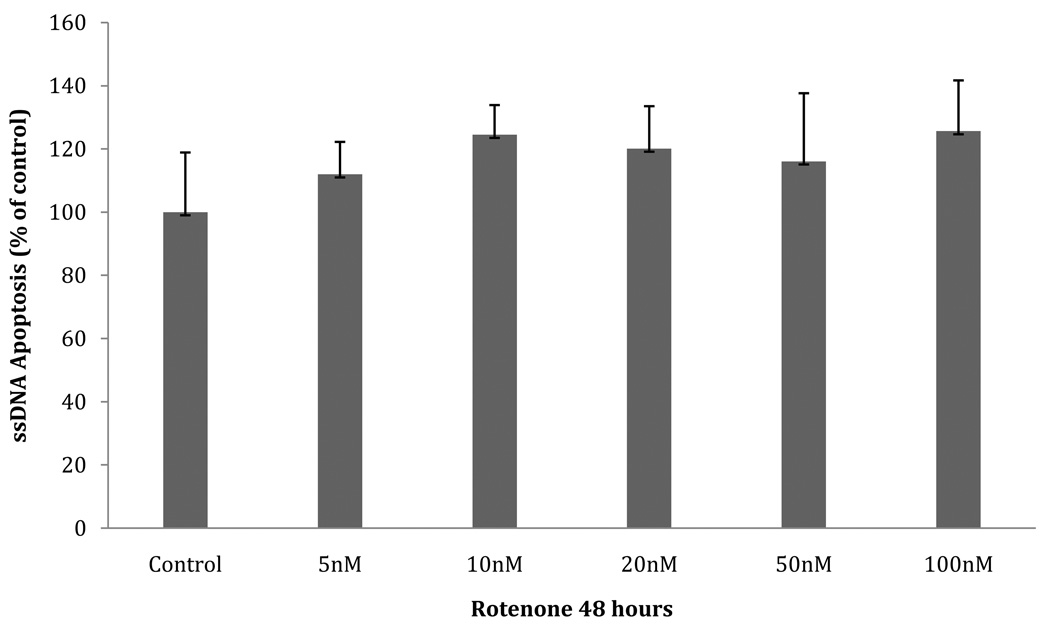
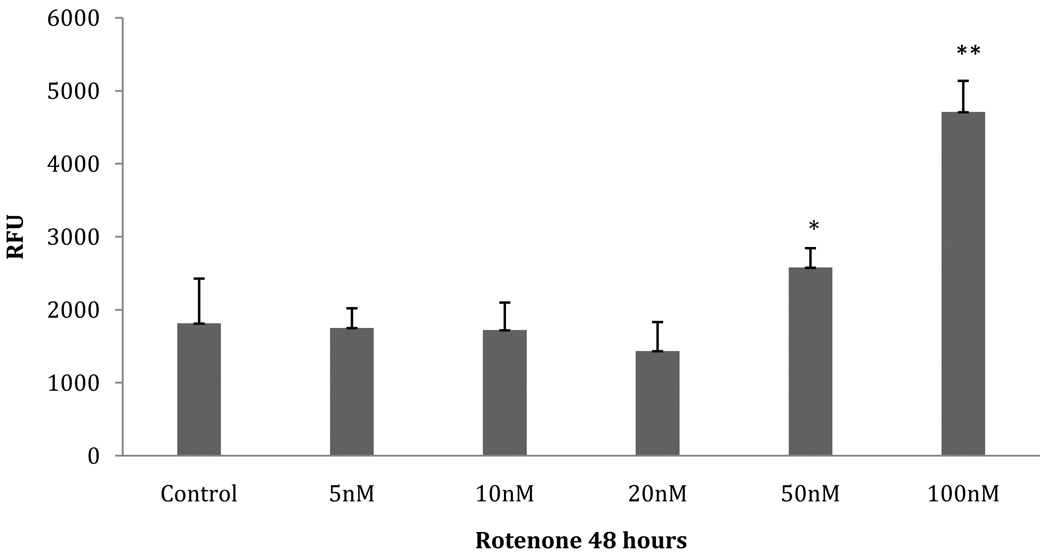
In order to analyze the proteome of early-stage PD, we sought to identify a concentration range of rotenone in which cultured neuroblastoma cells are viable and are not apoptotic after 48 hours. We evaluated the results of three different measurements: cellular viability/mitochondrial activity, apoptosis activity assessed by single-stranded DNA (ssDNA), and apoptosis activity assessed by caspase-3 activity. SH-SY5Y cells were incubated with submicromolar concentrations of rotenone for 48 hours. Mitochondrial activity (MTT assay, Fig.1) was unaffected at a rotenone concentration ≤ 50 nM (p≤ 0.05). However, when the concentration of rotenone reached ≥100 nM, a significant decrease in cell viability was seen (100nM: p=.0001; 100nM-1µM: p=.0006). The ssDNA apoptosis assay showed no significant increase in apoptosis (Fig.2). Furthermore, the caspase-3 activity showed a significant increase at 50 nM and 100 nM (p= .043 and p= .014, respectively) (Fig. 3). Approximately a 2.5 fold increase in activity was seen, which we estimate may only represent 25% of the maximal caspase-3 activation [21]. The increase in caspase-3 activity at 50 nM and 100 nM rotenone does not correlate with an increase in ssDNA apoptosis at the same concentrations, suggesting that compensatory factors were released by the cell to counteract the apoptotic cascade, potentially through UPR activation and subsequent production of anti-apoptotic proteins. Therefore, we chose the unaffected range (5nM, 10nM, 20nM, and 50nM) of rotenone concentrations to look for the initial response of the cells to oxidative stress. This time point and concentration range may represent the earliest phase in the progressive development of Parkinson’s-like neurodegeneration. We also used exposure to 100nM rotenone to examine the proteome in cells displaying decreased viability.
Fig. 1. MTT viability assay.
MTT cell viability assay performed on SH-SY5Y cultures pre-incubated with various concentrations of rotenone for 48 hours. Gray bars indicate cells extracted for proteomic analysis (Table 1). Data (mean ± SEM) are expressed as % of control (no rotenone). Asterisk (*) indicates p<0.0001 by ANOVA: 100nM rotenone-treated compared to 50nM; double asterisk (**) indicates p<0.0006 by ANOVA: 250nM-1µM rotenone-treated compared to 5nM–50nM group.
Fig. 2. ssDNA apoptotic assay (ELISA).
SH-SY5Y cultures pre-incubated with various concentrations of rotenone for 48 hours. Data (mean ± SEM) expressed as % of control (no rotenone).
Fig. 3. Caspase-3 activity assay.
Caspase-3 activity measured on SH-SY5Y cells pre-incubated with various concentrations of rotenone for 48 hours. Data expressed as relative fluorescence units (RFU). Asterisk (*) indicates p=0.043 by ANOVA: 50nM rotenone-treated compared to 5nM–20nM; double asterisk (**) indicates p=0.014 by ANOVA: 100nM rotenone-treated compared to 5nM–50nM rotenone group.
In control SH-SY5Y cells, we identified tryptic peptides from 242 proteins with high probability, including proteins that are related to the UPR and ER stress pathways that are listed in Table 1. Three heat-shock proteins were identified: HSP90 (both the cytosolic HSP90α and ER HSP90β forms), GRP75, and HSP60. GRP75, in concert with HSP60, is thought to participate in the refolding of proteins translocated into the mitochondria [17, 20]. Additionally, some components involved in the UPS system were identified: the ubiquitin carboxyl-terminal hydrolase enzyme, and a ubiquitin E1 activating enzyme. Both proteins are vital to the function of the UPS and both have been found to be dysfunctional in moderate to late stages of PD [23]. Finally, glutathione S-transferase (GST), a major player in the detoxification and protection against oxidizing toxins, was identified in the control group [6]. This constitutive expression of stress-related proteins in dopaminergic neurons suggests that these neurons have adapted to the endogenous stress associated with dopamine metabolism and subsequent production of reactive oxygen species (ROS) [1]. In other words, neurons may rely on their constitutive levels of HSPs as a preventative mechanism of defense against protein misfolding induced by stressful factors or those that are associated with neurodegenerative diseases [4].
Table 1. Identified proteins related to UPR/ER stress pathways following treatment with rotenone.
Proteins listed with International Protein Index (IPI) [16], MASCOT score, and function. Protein not described in text listed with references to PD and neurodegeneration.
| Rotenone 48 hrs | Function | Protein | Accession Numbers | Mascot Score |
|---|---|---|---|---|
| Control | chaperone/foldase | HSP90-beta [5,14] | IPI00414676 | 422 |
| chaperone/foldase | HSP90-alpha isoform 2 [5,14] | IPI00382470 | 288 | |
| chaperone/foldase | GRP75 | IPI00007765 | 90 | |
| chaperone/foldase | HSP60, mitochondrial | IPI00784154 | 151 | |
| chaperone/foldase | TCP1 T-complex subunit 3 isoform b [38] | IPI00290770 | 67 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit alpha [38] | IPI00290566 | 75 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit beta [38] | IPI00297779 | 202 | |
| chaperone/foldase | Peptidyl-prolyl cis-trans isomerase A | IPI00419585 | 40 | |
| UPS system | Ubiquitin carboxyl-terminal hydrolase isozyme | IPI00018352 | 192 | |
| UPS system | Ubiquitin-like modifier-activating enzyme 1 | IPI00645078 | 127 | |
| antioxidant | Glutathione S-transferase | IPI00219757 | 132 | |
| 5nM | chaperone/foldase | HSP90-beta [6,15] | IP00I414676 | 155 |
| chaperone/foldase | HSP60, mitochondria | IPI00784154 | 101 | |
| chaperone/foldase | HSP75, mitochondria | IPI0030275 | 65 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit zeta [38] | IPI00027626 | 53 | |
| chaperone/foldase | Peptidyl-prolyl cis-trans isomerase A | IPI00419585 | 63 | |
| chaperone/foldase | Prefoldin subunit 5 | IPI00015361 | 93 | |
| UPS system | Ubiquitin-like modifier activating enzyme 1 | IPI00552452 | 33 | |
| UPS system | Proteasome subunit beta type 3 | IPI00028004 | 88 | |
| antioxidant | Glutathione S-Transferase | IPI00219757 | 34 | |
| antioxidant | Peroxiredoxin-2 [9] | IPI00027350 | 31 | |
| mitochondrial maintenance | Prohibitin-2 [22] | IPI00027252 | 59 | |
| modulates oxidative stress | Protein DJ-1 | IPI00298547 | 40 | |
| 10nM | chaperone/foldase | HSP90-beta [5,14] | IPI00414676 | 241 |
| chaperone/foldase | HSP90AA1 isoform 1 [5,14] | IPI00784295 | 77 | |
| chaperone/foldase | HSP90AB4P [5,14] | IPI00555565 | 49 | |
| chaperone/foldase | BiP | IPI00003362 | 97 | |
| chaperone/foldase | HSP70 protein 6 [25] | IPI00339269 | 45 | |
| chaperone/foldase | HSC71 isoform 1[25] | IPI00003865 | 130 | |
| chaperone/foldase | GRP75 | IPI00007765 | 53 | |
| chaperone/foldase | HSP60, mitochondrial | IPI00784154 | 29 | |
| chaperone/foldase | HSP90 co-chaperone Cdc37 | IPI00013122 | 70 | |
| chaperone/foldase | HSP70/HSP90 organizing protein [4] | IPI00013894 | 29 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit beta [38] | IPI00297779 | 116 | |
| chaperone/foldase | Peptidyl-prolyl cis-trans isomerase A [36] | IPI00419585 | 44 | |
| chaperone/foldase | Protein disulfide-isomerase A6 isoform 2 [36] | IPI00299571 | 89 | |
| chaperone/foldase | Protein disulfide-isomerase A3 [36] | IPI00025252 | 38 | |
| chaperone/foldase | Prefoldin subunit 6 | IPI00005657 | 46 | |
| UPS system | Ubiquitin carboxyl-terminal hydrolase isozyme | IPI00018352 | 51 | |
| UPS system | Ubiquitin-like modifier-activating enzyme 1 | IPI00645078 | 46 | |
| UPS system | Ubiquitin-conjugating enzyme E2 | IPI00003949 | 57 | |
| UPS system | 26S proteasome subunit S10B protease | IPI0021926 | 43 | |
| UPS system | 26S proteasome subunit B5 isoform 3 | IPI00383971 | 29 | |
| antioxidant | Glutathione S-transferase | IPI00219757 | 152 | |
| antioxidant | Superoxide dismutase | IPI00218733 | 58 | |
| modulates oxidative stress | Protein DJ-1 | IPI00298547 | 62 | |
| 20nM | chaperone/foldase | HSP90-beta [5,14] | IPI00414676 | 347 |
| chaperone/foldase | HSP90-alpha isoform 1 [5,14] | IPI00784295 | 107 | |
| chaperone/foldase | HSP90B1 endoplasmin/GRP94 [5,14] | IPI00027230 | 73 | |
| chaperone/foldase | BiP | IPI00003362 | 77 | |
| chaperone/foldase | HSC71 isoform 1 [25] | IPI00003865 | 329 | |
| chaperone/foldase | HSP70 protein 7 [25] | IPI00011134 | 109 | |
| chaperone/foldase | GRP75 | IPI00007765 | 58 | |
| chaperone/foldase | HSP60, mitochondrial | IPI00784154 | 258 | |
| chaperone/foldase | HSP27 [24,28] | IPI00025512 | 31 | |
| chaperone/foldase | TCP1 T-complex subunit 3 isoform b [38] | IPI00290770 | 63 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit alpha [38] | IPI00290566 | 91 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit beta [38] | IPI00297779 | 52 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit delta [38] | IPI00302927 | 72 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit epsilon [38] | IPI00010720 | 110 | |
| chaperone/foldase | Peptidyl-prolyl cis-trans isomerase A | IPI00419585 | 109 | |
| chaperone/foldase | Peptidyl-prolyl cis-trans isomerase B | IPI00646304 | 60 | |
| chaperone/foldase | Protein disulfide-isomerase A6 isoform 2 [36] | IPI00299571 | 73 | |
| chaperone/foldase | Protein disulfide-isomerase A3 [36] | IPI00025252 | 70 | |
| chaperone/foldase | Calreticulin | IPI00020599 | 38 | |
| UPS system | Ubiquitin-conjugating enzyme E2 | IPI00003949 | 69 | |
| UPS system | Proteasome subunit alpha type-1 | IPI00016832 | 49 | |
| UPS system | Proteasome subunit alpha type-7 | IPI00024175 | 40 | |
| antioxidant | Glutathione S-transferase | IPI00246975 | 41 | |
| antioxidant | Superoxide dismutase | IPI00218733 | 92 | |
| antioxidant | Peroxiredoxin-1 [9] | IPI00000874 | 48 | |
| antioxidant | Peroxiredoxin-6 [9] | IPI00220301 | 122 | |
| mitochondrial maintenance | Prohibitin-1 [22] | IPI00017334 | 59 | |
| mitochondrial maintenance | Prohibitin-2 [22] | IPI00027252 | 78 | |
| microtubule remodeling | Stathmin | IP00479997 | 104 | |
| microtubule remodeling | Stathmin-2 | IPI00218667 | 49 | |
| microtubule remodeling | Stathmin-4 isoform 1 | IPI00006575 | 41 | |
| export misfolded proteins | Transitional ER ATPase | IPI00022774 | 38 | |
| antinflammatory | Cytosolic Phosolipase A2 | IPI00384577 | 54 | |
| 50nM | chaperone/foldase | HSP90-beta [5,14] | IPI00414676 | 266 |
| chaperone/foldase | HSP90-alpha isoform 2 [5,14] | IPI00382470 | 173 | |
| chaperone/foldase | HSP90B1 endoplasmin/GRP94 [5,14] | IPI00027230 | 32 | |
| chaperone/foldase | BiP | IPI00003362 | 71 | |
| chaperone/foldase | HSP75, mitochondrial | IPI00030275 | 77 | |
| chaperone/foldase | HSP70 protein 1 [25] | IPI00304925 | 30 | |
| chaperone/foldase | HSP70 protein 6 [25] | IPI00339269 | 23 | |
| chaperone/foldase | HSC71 isoform 1 [25] | IPI00003865 | 103 | |
| chaperone/foldase | HSP60, mitochondrial | IPI00784154 | 182 | |
| chaperone/foldase | HSP10, mitochondrial [11] | IPI00220362 | 78 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit alpha [38] | IPI00290566 | 45 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit 3 isoform c [38] | IPI00552715 | 54 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit zeta [38] | IPI00027626 | 34 | |
| chaperone/foldase | Peptidyl-prolyl cis-trans isomerase A | IPI00419585 | 36 | |
| chaperone/foldase | Peptidyl-prolyl cis-trans isomerase B | IPI00646304 | 52 | |
| chaperone/foldase | ERp57 [7] | IPI0025252 | 51 | |
| chaperone/foldase | Protein disulfide isomerase A5 [36] | IPI0031479 | 51 | |
| UPS system | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | IPI00018352 | 62 | |
| UPS system | E3 Ubiquitin ligase UBR5 | IPI00026320 | 35 | |
| UPS system | E3 Ubiquitin ligase MARCH6 | IPI00105518 | 29 | |
| UPS system | Proteasome subunit alpha type-5 | IPI00291922 | 23 | |
| antioxidant | Glutathione S-transferase | IPI00219757 | 60 | |
| antioxidant | Thioredoxin | IPI00216298 | 71 | |
| mitochondrial maintenance | Prohibitin-1 [22] | IPI00017334 | 38 | |
| mitochondrial maintenance | Prohibitin-2 [22] | IPI00027252 | 54 | |
| microtubule remodeling | Stathmin | IP00479997 | 37 | |
| neurite growth | Neuron Navigator 2 isoform 1 | IPI00217052 | 27 | |
| antinflammatory | Cytosolic Phosolipase A2 | IPI00384577 | 62 | |
| mitochondrial apoptosis | Porin/VDAC1 | IPI00216308 | 48 | |
| mitochondrial apoptosis | Porin/VDAC2 | IPI00241145 | 54 | |
| 100nM | chaperone/foldase | HSP90-beta [5,14] | IPI00414676 | 170 |
| chaperone/foldase | HSP90-alpha isoform 2 [5,14] | IPI00382470 | 52 | |
| chaperone/foldase | HSP90AB2P [5,14] | IPI00455599 | 49 | |
| chaperone/foldase | HSP90B1 endoplasmin/GRP94 [5,14] | IPI00027230 | 59 | |
| chaperone/foldase | HSP90 ATPase activator [5,14] | IPI00030706 | 53 | |
| chaperone/foldase | BiP | IPI00003362 | 101 | |
| chaperone/foldase | HSP70 protein 1 [25] | IPI00304925 | 93 | |
| chaperone/foldase | HSP70 protein 4 [25] | IPI00002966 | 53 | |
| chaperone/foldase | HSP70 protein 6 [25] | IPI00339269 | 93 | |
| chaperone/foldase | HSC71 isoform 1 [25] | IPI00003865 | 236 | |
| chaperone/foldase | GRP75 | IPI00007765 | 76 | |
| chaperone/foldase | HSP60 | IPI00917575 | 235 | |
| chaperone/foldase | HSP70/HSP90 organizing protein [3] | IPI00013894 | 33 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit beta [38] | IPI00297779 | 94 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit delta [38] | IPI00302927 | 67 | |
| chaperone/foldase | TCP1 T-complex protein 1 subunit epsilon [38] | IPI00010720 | 109 | |
| chaperone/foldase | Peptidyl-prolyl cis-trans isomerase B | IPI00646304 | 72 | |
| chaperone/foldase | Protein disulfide-isomerase A3 [36] | IPI00025252 | 59 | |
| chaperone/foldase | Protein disulfide-isomerase A6 isoform 2 [36] | IPI00299571 | 107 | |
| chaperone/foldase | Calnexin | IPI00020984 | 52 | |
| UPS system | Ubiquitin carboxyl-terminal hydrolase isozyme L1 | IPI00018352 | 56 | |
| UPS system | Ubiquitin-like modifier-activating enzyme 1 | IPI00645078 | 38 | |
| UPS system | Proteasome subunit beta type-1 | IPI00025019 | 30 | |
| UPS system | Proteasome subunit beta type-6 | IPI00000811 | 44 | |
| antioxidant | Glutathione S-transferase | IPI00219757 | 80 | |
| antioxidant | Thioredoxin | IPI00216298 | 57 | |
| microtubule remodeling | Stathmin | IP00479997 | 77 | |
| microtubule remodeling | Stathmin-2 | IPI00218667 | 65 | |
| mitochondrial maintenance | Prohibitin-1 [22] | IPI00017334 | 60 | |
| mitochondrial maintenance | Prohibitin-2 [22] | IPI00027252 | 70 | |
| export misfolded proteins | Transitional ER ATPase | IPI00022774 | 46 | |
| suppress apoptosis | Apoptosis Inhibitor 5 isoform 2 | IPI00554742 | 32 | |
| pro-apoptosis | Cytochrome c | IPI00465315 | 34 |
At 5nM, 10nM, 20nM, and 50nM concentrations of rotenone we identified tryptic peptides from 545 proteins with high probability, including proteins that are related to survival, growth, and protection (Table 1). Of great interest, at 5nM and 10nM, DJ-1 was identified. DJ-1 is classically associated with PD by deletion and point mutations shown to be responsible for the onset of familial PD [34]. We also detected BiP, starting at 10nM and continuing through 100nM. The expression of BiP indicates the activation of the UPR [32]. BiP can act to protect cells from oxidative stress. This stress can cause partial unfolding or aggregation of proteins. However, BiP can temporarily bind to hydrophobic residues exposed by ROS, thereby allowing the protein to potentially refold and/or preventing protein aggregation [25].
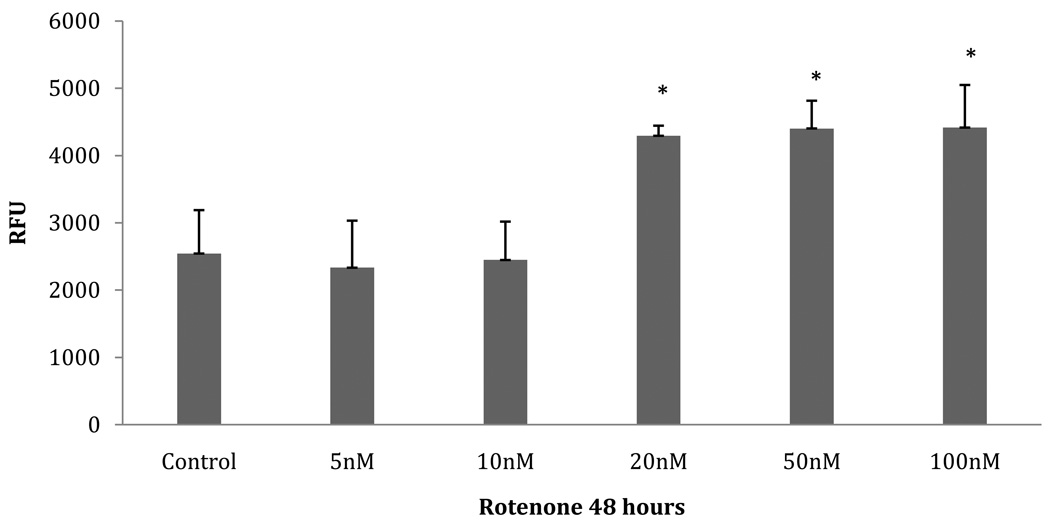
UPR activation has been shown to increase the expression of multiple chaperones, foldases, and components of the UPS and ERAD system [32]. This increase is clearly evident in Table 1. A recent immunohistochemistry study showed an increase in ER-resident chaperones in PD brain [35], suggesting that our results are relevant to human pathology. To validate an increase in UPS and ERAD components, we assayed the chymotrypsin-like activity of the 20S proteasome (Figure 4). From 20nM to 100nM rotenone, a significant increase in the activity of the chymotrypsin-like activity is seen (p= .000012) compared to the lower concentrations of rotenone and control. This suggests that the UPR has increased the expression of proteasome components necessary to handle the increased burden of misfolded/unfolded proteins due to the increase in oxidative stress. Also, of interest is the identification of the transitional ER ATPase at the 20nM and 100nM group. This protein regulates E3 ligase activity and may be required for export of misfolded proteins from the ER to be degraded by the proteasome. Of further importance, we identified two, components of the mitochondrial permeability transition pore (mPTP): Voltage-Dependant Anion Channel (VADC) 1 and 2. It has been shown recently that VDAC1 acts as a mitochondrial target of Parkin-mediated poly-ubiquitin chains and is therefore necessary for PINK1/Parkin-directed autophagy of damaged mitochondria [8]. Specifically it has been shown that with a decrease in mitochondrial membrane potential, Parkin translocates to the mitochondria in response to ROS. So with VDAC1 acting as a mitochondrial target of Parkin-mediated poly-ubiquitin chains, a Parkin-dependent mitophagic clearance could arrest the release of pro-apoptotic factors from damaged mitochondria [8]. So an increase in VDAC1 can aid in the removal of damaged mitochondria by assisting damaged mitochondria into forming autophagosomes.
Fig. 4. Proteasome activity Assay.
Fluorogenic substrate measured chymotrypsin-like activity in 20S proteasomes from SH-SY5Y cells exposed to various concentrations of rotenone for 48 hours. Data expressed as relative fluorescence units (RFU). Asterisk (*) indicates p=0.000012 by ANOVA: 20nM–100nM rotenone-treated group compared to control-10nM group.
At higher rotenone concentrations, we found several proteins that are involved in cytoskeletal remodeling and development of neurites (Stathmin1, Stathmin2, Neuron Navigator 2), as well as maintaining mitochondrial homeostasis (Prohibitin1, Prohibitin2) [27, 19, 30].
The UPR activation and its related products are able to keep the neurons healthy enough to sustain viability and overcome the induced stress up to a point. It has been clearly shown in past studies that with sustained UPR activation, apoptosis occurs [32, 13, 18]. Previous studies showed activation of the UPR after oxidative stress was induced in neuronal cells [12, 31] using high toxin concentrations that resulted in 50% cell death within 24-hours. We observed similar effects with 100nM rotenone, which significantly decreased cell viability (Fig. 1), increased caspase-3 activity (Fig. 3), and increased cytochrome c (Table 1). We believe this marks a threshold of defense that the neurons are capable of producing.
Our results indicate that SH-SY5Y cells activate the UPR acutely at rotenone doses that the cells can survive. As the concentration of rotenone increased so did the expression of proteins necessary to handle the accumulation of misfolded or unfolded proteins (Table 1). Much information exists about neurodegenerative disorders in the moderate to late stages of the diseases, where most of the outward symptoms manifest themselves. However, an understanding of the early stages of the progression towards apoptosis in PD can greatly assist research toward preventative therapies.
In conclusion, our whole cell proteomic analysis identified proteins that are involved in the protective UPR and ER stress pathways in dopaminergic neurons subjected to oxidative stress. Our results clearly show that these cells have the ability to overcome low-level oxidative damage, which might resemble the initial biochemical events of PD. However, with higher levels of stress, or prolonged stress, the protective pathways are insufficient to prevent the activation of apoptosis. The question remains whether the UPR and ER pathways can be harnessed and manipulated to provide a new early intervention strategy for treatment of PD and other neurodegenerative diseases.
Acknowledgments
We thank the UTSA Proteomics Core (supported by NIH G12 RR013646), and Dr. Maria Gaczynska for assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berg D. Redox imbalance. In: Qureshi G, Parvez S, editors. Oxidative stress and neurodegenerative diseases. Elsevier; 2005. pp. 183–191. [Google Scholar]
- 2.Cannon J, Tapias V, Na H, Honick A, Drolet R, Greenamyre JT. A highly reproducible rotenone model of Parkinson’s disease. Neurobiology of Disease. 2009;34:279–290. doi: 10.1016/j.nbd.2009.01.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen S, Smith D. Hop as an adaptor in the heat shock protein 70 (HSP70) and HSP90 chaperone machinery. J. Biol. Chem. 1998;273:35194–35200. doi: 10.1074/jbc.273.52.35194. [DOI] [PubMed] [Google Scholar]
- 4.Cheng S, Brown I. Neuronal expression of constitutive heat shock proteins: implications for neurodegenerative disorders. Cell Stress & Chaperones. 2007;12:51–58. doi: 10.1379/CSC-236R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Csermely P, Schainder T, Sȍti C, Prohászka Z, Nardai G. The 90-kDa molecula chaperone family: structure, function, and clinical applications. A comprehensive review, Pharmacol. Ther. 1998;79:129–168. doi: 10.1016/s0163-7258(98)00013-8. [DOI] [PubMed] [Google Scholar]
- 6.Douglas KT. Mechanisms of action of glutathione-dependent enzymes. Adv. Enzymol. 1987;59:103–167. doi: 10.1002/9780470123058.ch3. [DOI] [PubMed] [Google Scholar]
- 7.Frickel EM, Frei P, Bouvier M, Stafford W, Helenius A, Clockshuber R, Ellgaard L. ERp57 is a multifunctional thiol-disulfide oxioreductase. J. Biol. Chem. 2004;279:18277–18287. doi: 10.1074/jbc.M314089200. [DOI] [PubMed] [Google Scholar]
- 8.Geisler S, Holmstrom KM, Skujat D, Fiesel FC, Rothfuss OC, Kahle PJ, Springer W. PINK1/PARKIN-mediated mitophagy is dependent on VDAC1 and p62/SQSTM1. Nature Cell Biology. 2009;12:119–131. doi: 10.1038/ncb2012. [DOI] [PubMed] [Google Scholar]
- 9.Graves J, Metukuri M, Scott D, Rothermund K, Prochownik E. Regulation of reactive oxygen species homeostasis by peroxiredoxin and c-Myc. J. Biol. Chem. 2009;284:6520–6529. doi: 10.1074/jbc.M807564200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Greenamyer JT, Betarbet R, Sherer TB. The rotenone model of Parkinson’s disease: genes, environment and mitochondria. Parkinsonism and Related Disorders. 2003;9:59–64. doi: 10.1016/s1353-8020(03)00023-3. [DOI] [PubMed] [Google Scholar]
- 11.Hohfeld J, Hartl FU. Role of the chaperonin cofactor HSP10 in protein folding and sorting in yeast mitochondria. J. Cell. Biol. 1994;126:305–315. doi: 10.1083/jcb.126.2.305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Holtz WA, Turetzky JM, Jong YI, O’Malley KL. Oxidative stress-triggered unfolded protein response is upstream of intrinsic cell death evoked by parkinsonian mimetics. J. Neurochem. 2006;99:54–69. doi: 10.1111/j.1471-4159.2006.04025.x. [DOI] [PubMed] [Google Scholar]
- 13.Hoozemans JJM, van Haastert ES, Eikelenboom P, de Vas RAI, Rozemuller JM, Sheper W. Activation of the unfolded protein response in Parkinson’s disease. B.B.R.C. 2007;354:707–711. doi: 10.1016/j.bbrc.2007.01.043. [DOI] [PubMed] [Google Scholar]
- 14.Imai J, Maruya M, Yashiroda H, Yahara I, Tanaka K. The molecular chaperone HSP90 plays a role in the assembly and maintenance of the 26S proteasome. EMBO J. 2003;22:3557–3567. doi: 10.1093/emboj/cdg349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Keller A, Nesvizhskii AI, Kolker E, Aebersold R. Emperical statistical model to estimate the accuracy of peptide identification made by MS/MS and database search. Anal. Chem. 2002;74:5383–5392. doi: 10.1021/ac025747h. [DOI] [PubMed] [Google Scholar]
- 16.Kersey PJ, Duarte J, Williams A, Karavidopoulou Y, Birney E, Apweiler R. The International Protein Index: An integrated database for proteomics experiments. Proteomics. 2004;4:1985–1988. doi: 10.1002/pmic.200300721. [DOI] [PubMed] [Google Scholar]
- 17.Koll H, Guiard B, Rassow J, Ostermann J, Horwich A, Neupert W, Hartl FU. Antifolding activity of HSP60 couples protein import into the mitochondrial matrix with export to the intermembrane space. Cell. 1992;68:1163–1175. doi: 10.1016/0092-8674(92)90086-r. [DOI] [PubMed] [Google Scholar]
- 18.Lin CY, Kaufman RJ. The unfolded protein response. Journal of Cell Science. 2003;116:1861–1862. doi: 10.1242/jcs.00408. [DOI] [PubMed] [Google Scholar]
- 19.Maes T, Barcelo A, Buesa C. Neuron navigator: a human gene family with homology to unc-53, a cell guidance gene from Caenorhabditis elegans. Genomics. 2002;80:21–30. doi: 10.1006/geno.2002.6799. [DOI] [PubMed] [Google Scholar]
- 20.Manning-Krieg UC, Scherer PE, Schatz G. Sequential action of mitochondrial chaperones in protein import into the matrix. The EMBO Journal. 1991;10:3273–3280. doi: 10.1002/j.1460-2075.1991.tb04891.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mashima T, Naito M, Kataoka S, Kawai H, Tsuruo T. Aspartate-based inhibitor of interleukin-1 beta-converting enzyme prevents antitumor agent-induced apoptosis in human myeloid leukemia U937 cell. B.B.R.C. 1995;209:907–915. doi: 10.1006/bbrc.1995.1584. [DOI] [PubMed] [Google Scholar]
- 22.McClung JK, Jupe ER, Liu XT, Dell’Orco RT. Prohibitin: potential role in senescence, development, and tumor suppression. Exp.Gerontol. 1995;30:99–124. doi: 10.1016/0531-5565(94)00069-7. [DOI] [PubMed] [Google Scholar]
- 23.McNaught KSP, Olanow CW. Proteolytic stress: a unifying concept for the etiopathogenesis of parkinson’s disease. Ann Neuro. 2003;53:73–86. doi: 10.1002/ana.10512. [DOI] [PubMed] [Google Scholar]
- 24.Mehlen P, Hickey E, Weber LA, Arrigo AP. Large unphosphorylated aggregates as the active form of HSP27 which controls intracellular reactive oxygen species and glutathione levels and generates a protection against TNFalpha in NIH-3T3-ras cells. Biochem. Biophys. Res. Commun. 1997;241:187–192. doi: 10.1006/bbrc.1997.7635. [DOI] [PubMed] [Google Scholar]
- 25.Morano K. New tricks for an old dog. The evolving world of HSP70. Ann. N.Y. Acad. Sci. 2007;1113:1–14. doi: 10.1196/annals.1391.018. [DOI] [PubMed] [Google Scholar]
- 26.Nesvizhskii AI, Keller A, Kolker E, Aebersold R. A statistical model for identifying proteins by tandem mass spectrometry. Anal. Chem. 2003;75:4646–4685. doi: 10.1021/ac0341261. [DOI] [PubMed] [Google Scholar]
- 27.Ozon S, El Mestikawy S, Sobel A. Differential, regional, and cellular expression of the Stathmin family transcripts in the adult rat brain. J. Neurosci. Res. 1999;56:553–564. doi: 10.1002/(SICI)1097-4547(19990601)56:5<553::AID-JNR11>3.0.CO;2-J. [DOI] [PubMed] [Google Scholar]
- 28.Parcellier A, Schmitt E, Gurbuxani S, Seigneurin-Berny D, Pance A, Chantome A, Plenchette S, Khochbin S, Solary E, Garrido C. HSP27 is a ubiquitin-binding protein involved in I-κBα proteasomal degradation. Mol. Cell. Biol. 2003;23:5790–5802. doi: 10.1128/MCB.23.16.5790-5802.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Picklo M, Amarnath V, McIntyre J, Graham D, Montine T. 4-Hydroxy-2(E)-Nonenal inhibits CNS mitochondrial respiration at multiple sites. J. Neurochem. 1999;72:1617–1624. doi: 10.1046/j.1471-4159.1999.721617.x. [DOI] [PubMed] [Google Scholar]
- 30.Ross J, Nagy Z, Kirken R. The PHB1/2 phosphocomplex is required for mitochondrial homeostasis and survival of human T cells. J. Biol. Chem. 2008;283:4699–4713. doi: 10.1074/jbc.M708232200. [DOI] [PubMed] [Google Scholar]
- 31.Ryu EJ, Harding HP, Angelastro JM, Vitolo OV, Ron D, Greene LA. Endoplasmic reticulum stress and the unfolded protein response in cellular models of Parkinson’s disease. Journal of Neurosci. 2002;22:10690–10698. doi: 10.1523/JNEUROSCI.22-24-10690.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Schröder M, Kaufman R. The mammalian unfolded protein response. Annu. Rev. Biochem. 2005;74:739–789. doi: 10.1146/annurev.biochem.73.011303.074134. [DOI] [PubMed] [Google Scholar]
- 33.Sherer T, Betarbet R, Stout A, Lund S, Baptista M, Panov A, Cookson M, Greenmyre T. An in vitro model of Parkinson’s Disease: linking mitochondrial impairment to altered α-synuclein metabolism and oxidative damage. Journal of Neurosci. 2002;22:7006–7015. doi: 10.1523/JNEUROSCI.22-16-07006.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Taira T, Saito Y, Niki T, Iguchi-Ariga SMM, Takahashi K, Ariga H. DJ-1 has a role in antioxidative stress to prevent cell death. EMBO reports. 2004;5:213–218. doi: 10.1038/sj.embor.7400074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Wilhelmus MMM, Verhaar R, Andringa G, Bol JGJM, Cras P, Shan L, Hoozemans JJM, Drukarch B. Presence of tissue transglutaminase in granular endoplasmic reticulum is characteristic of melanized neurons in Parkinson’s disease brain. Brain Path. 2010 doi: 10.1111/j.1750-3639.2010.00429.x. in press (doi:10.1111/j.1750-3639.2010.00429.x) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Wilkinson B, Gilbert H. Protein disulfide isomerase. Biochimica et biophysica acta. 2004;1699:35–44. doi: 10.1016/j.bbapap.2004.02.017. [DOI] [PubMed] [Google Scholar]
- 37.Wang N, Xu M, Wang P, Li L. Development of mass spectrometry-based shotgun method for proteome analysis of 500 to 5000 cancer cells. Anal. Chem. 2010;82:2262–2271. doi: 10.1021/ac9023022. [DOI] [PubMed] [Google Scholar]
- 38.Yaffe M, Farr GW, Miklos D, Horwich AL, Sternlicht ML, Sternlicht H. TCP1 complex is a molecular chaperone in tubulin biogenesis. Nature. 1992;358:245–248. doi: 10.1038/358245a0. [DOI] [PubMed] [Google Scholar]