Abstract
The objective of this study was to evaluate the effects of aging on the performance of specific memory-related tasks in rats as well as to determine the levels of several nerve growth factor (NGF)-related proteins in relevant brain regions. The results indicated age-related impairments in spatial learning in a water maze task as well as deficits in recognition memory in a Spontaneous Novel Object Recognition task. In the prefrontal cortex and hippocampus, aged rats (compared to young controls) had elevated levels of the proneurotrophin, proNGF (+1.8–1.9 fold), p75NTR receptors (+1.6–1.8 fold) and sortilin (+1.8–2.1 fold), and decreased levels of mature NGF (−36–44%), and phospho-TrkA receptors (−45–49%). The results of this study support the argument that NGF signaling is altered in the aging brain, and that such alterations may contribute to an age-related decline in cognitive function. These results may also help to identify specific components of the NGF-signaling pathway that could serve as targets for novel drug discovery and development for age-related disorders of cognition (e.g., Alzheimer’s disease).
Keywords: Aging, Alzheimer’s disease, cognition, memory, NGF, proNGF, neurotrophin
1. Introduction
The continual improvement in life expectancy and the consequent increases in elderly populations in developed countries have, unfortunately, led to a significant rise in the incidence of a variety of age-related illnesses. Among these illnesses, the prevalence of devastating disorders of cognition such as Alzheimer’s disease (AD) could be considered epidemic given the alarming recent estimate of 24 million victims worldwide (a number that is expected to double by the year 2020, [1]). Even in the absence of frank AD, there is clear evidence that older individuals with less pronounced levels of cognitive impairment (now commonly referred to as “Mild Cognitive Impairment” or MCI) constitute a high-risk population for developing dementia [2]. Accordingly, there is a critical need for the identification of therapeutic targets that could be exploited in order to prevent the progression of age-related cognitive decline. Given their established roles in neuronal plasticity (i.e., both synaptic and morphological plasticity, [3]) the family of proteins known as the “neurotrophins” and their receptors have been viewed as potential targets for dementia-related drug discovery and development for several years. Of the various neurotrophins, nerve growth factor (NGF) may be especially important given evidence of its decrease in the brain with age particularly in memory-related areas such as the hippocampus [4–6]. NGF is now viewed as especially important for the survival of forebrain cholinergic neurons [7] which are well documented to be involved in cognitive function, to degenerate with age, and to be markedly diminished in AD brains [8]. Additional support for the importance of NGF as a potential therapeutic target is evident in the results of experiments which suggested that deficits in NGF release and subsequent signaling (i.e., tyrosine receptor kinase phosphorylation) contribute to age-related deficits in long-term potentiation [9], a form of neuronal plasticity that is widely believed to facilitate learning and memory [10]. Moreover, impaired performance of aged rats in a water maze spatial learning task was correlated with decreased levels of NGF [11], while chronic intraventricular administration of NGF was shown to reverse age-related deficits in long-term potentiation [12] and spatial learning [13].
Notwithstanding the experimental results described above which support the validity of NGF as a therapeutic target for age-related disorders of cognition, there are some limitations to the studies that should be considered. Much of the earlier work where NGF protein and NGF mRNA levels were quantified relied on commercial ELISAs and polymerase chain reaction (PCR) methods that were not designed to discriminate between the proneurotrophin, proNGF and mature NGF (mNGF) (a discrimination that is now known to be of considerable importance, see below). In addition, very few studies have comprehensively evaluated the full complement of NGF-related proteins (including receptors) that are involved in the neutrotrophin response in the mammalian brain especially as it ages. Under normal conditions, mNGF binding to its high affinity receptor, TrkA promotes TrkA autophosphorylation which activates pathways that enhance cholinergic neuron survival [7]. Conversely, proNGF, the uncleaved precursor form of NGF, binds to the p75NTR receptor with higher affinity than mNGF and it is more selective for the p75NTR receptor relative to TrkA [14]. Notably, the p75NTR receptor is well-known for its role in mediating neuronal cell death [15]. There is also increasing evidence that proNGF forms a heterotrimeric complex with the p75NTR receptor and the neurotensin receptor, sortilin, to activate apoptotic cascades [16–18] and that this series of events may become more predominant in the setting of advanced age and neuropathological conditions such as AD.
The objective of the experiments described here was, therefore, to evaluate the effects of aging on the levels of NGF-related proteins in the rodent brain including, proNGF, mNGF, the neurotrophin receptors, TrkA, phospho-TrkA (i.e., the activated form of TrkA), p75NTR, and sortillin. The brain regions analyzed (hippocampus, prefrontal cortex) were selected based on their well-established functional roles in human cognition (and disorders of cognition including AD). The test subjects were initially evaluated in behavioral tasks (i.e. the Morris water maze and spontaneous novel object recognition) that are known to rely on these same brain regions for optimal performance (see Discussion for further details).
2. Materials and Methods
2.1. Test Subjects
Six month old, male, Long Evans (retired breeder) rats were obtained from Harlan Sprague-Dawley, Inc. (Indianapolis, IN) and then maintained at the Small Animal Behavior Core at the Medical College of Georgia until they were 19–22 months of age. During this time they were handled at least once per week. Young male, Long Evans rats (3–4 months old) were obtained for the age comparisons. All test subjects were housed individually after arrival in a temperature controlled room (25°C), maintained on a 12-hour light/dark cycle and allowed free access to food (Teklad Rodent Diet 8604 pellets, Harlan, Madison, WI) and water. All procedures employed during this study were reviewed and approved by the Medical College of Georgia Institutional Animal Care and Use Committee and are consistent with AAALAC guidelines. Measures were taken to minimize pain or discomfort in accordance with the National Institute of Health Guide for the Care and Use of Laboratory Animals (NIH Publications No. 80–23) revised 1996. Significant efforts were also made to minimize the total number of animals used while maintaining statistically valid group numbers.
2.2. Behavioral Experiments
Test subjects were handled beginning the day after arrival, and received a minimum of two weeks of daily handling prior to the initiation of behavioral testing. Behavioral experiments were conducted in rooms equipped with white noise generators (San Diego Instruments, San Diego, CA) set to provide a constant background level of 70 dB and ambient lighting of approximately 25–30 Lux (lumen/m2). Test subjects were transferred (in their home cages) to the behavioral testing rooms each morning approximately 30 min before the beginning of experiments. Separate cohorts of young and aged subjects (N=8–12) were used in the water maze experiments and the spontaneous novel object recognition tests (see below).
Water Maze
Test Apparatus
Water maze experiments were performed in a circular pool (diameter: 180 cm, height: 76 cm) made of black plastic and filled to a depth of 35 cm of water (maintained at 25.0±1.0°C). The pool was located in a large room with a number of extra-maze visual cues including geometric images (squares, triangles, circles etc.) hung on the wall, and black curtains used to hide the experimenter (visually) and the resting test subjects. Swimming activity of each rat was monitored via a television camera mounted overhead, which relayed information including latency to find the platform, total distance traveled, time and distance spent in each quadrant etc. to a video tracking system (Noldus EthoVision® Pro 3.1).).
Hidden Platform Task
For these experiments, an invisible (black) 10 cm × 10 cm square platform was submerged approximately 1.0 cm below the surface of the water and placed in the center of a quadrant (one-fourth of the total pool area defined via the tracking software). For each test session rats were given 2 trials per day for 10 consecutive days to locate and climb on to the hidden platform. A trial was initiated by placing the rat in the water directly facing the pool wall (i.e., nose approximately 2 cm from the wall) in one of the 4 quadrants. The daily order of entry into individual quadrants was pseudo-randomized such that all 4 quadrants were used once every two training days. For each trial, the rat was allowed to swim a maximum of 90 sec, in order to find the platform. When successful the rat was allowed a 30-sec rest period on the platform. If unsuccessful within the allotted time period, the rat was given a score of 90 sec and then physically placed on the platform and also allowed the 30-sec rest period. In either case the rat was given the next trial after an additional 1.5 min rest period (i.e., intertrial interval =2.0 min).
Probe Trials (Transfer Tests)
Twenty-four hours following the last hidden platform trial, probe trials (90 sec in length) were conducted in which the platform was removed from the pool and the time spent in each quadrant as well as the number of crossings over the previous platform location were measured. The mean number of seconds (± SEM) spent in each quadrant divided into 3–30 second blocks was compared to examine spatial bias for the previous platform location as well as potential differences in extinction.
Visible Platform Task
Approximately 10 minutes after probe trials were completed in all the test subjects, a visible platform test was performed in order to determine whether age-related (gross) impairments in visual acuity or abnormal search/escape behaviors might have contributed to alterations in hidden platform test performance or probe trials. To accomplish this task, a highly visible (white) cover fitted with a small white flag was attached to the platform which raised the surface approximately 1.0 cm above the surface of the water. Each rat was gently lowered into the water in the quadrant diametrically opposite to the platform quadrant and given one or more trials with a 90 sec time limit to locate and climb on to the platform. When a rat was successful (on its own accord without assistance) it was then given a series of 4 additional trials (with a 1.0 min intertrial interval) and the latency (in sec) to locate the platform was recorded. The platform was moved on each trial to a different quadrant (the subject was always entered from the opposite quadrant) until the test was conducted once in all 4 quadrants.
Spontaneous Novel Object Recognition Test (NOR)
NOR tests were conducted as described in detail previously [19]. Briefly, habituation to the test apparatus consisted of two daily 10-min sessions in which the animals were allowed to freely explore the open field box. NOR testing was conducted and video recorded on the third day. Each test day began with a 3-minute training session (i.e., the A/A session with identical objects) followed by a six hour delay period and a subsequent 3-minute test session (i.e., the A/B session with dissimilar objects). The objects discriminated were made of glass, ceramic, clay, or plastic. The total exploration time that the subjects spent investigating each object was recorded. A discrimination index (d2) was calculated on each A/B trial and was defined as the difference in time spent exploring the novel and familiar objects divided by the total exploration time for both objects: d2 index = (novel-familiar)/(novel + familiar). This measure is considered an index of recognition memory and takes into account individual differences in the total amount of object exploration time.
2.3. Neurochemistry Experiments
Immediately after behavioral testing (i.e., after the visible platform test in the water maze studies and after the retention-A/B session in the NOR experiments), the test subjects were anesthetized with KetaVed™ (ketamine hydrochloride injection; Vedco, Inc., St. Joseph, MO), intracardially-perfused with phosphate buffered saline (PBS, pH 7.4), and then decapitated. Brains were quickly harvested, immediately frozen in dry ice-cooled 2-methylbutane (isopentane), and stored at −70 °C until dissected. Detailed methods for the dissection of brain regions and the preparation of brain lysates have been published previously [20]. Protein concentrations were determined by the bicinchoninic acid method (BCA Protein Assay Kit, Sigma, USA). The brains of representative test subjects from the cohorts evaluated in water maze and NOR experiments were used in the neurochemistry experiments.
Western Blot Analyses
Equal amounts of protein were resolved in SDS-polyacrylamide gels (ranging from 7–12%) and transferred electrophoretically onto a nitrocellulose membrane (Bio-Rad). Membranes were blocked for 1 h in PBST (3.2 mM Na2HPO4,. 0.5 mM KH2PO4, 1.3 mM KCl, 135 mM NaCl, 0.05% Tween-20) and 5% non-fat milk (or BSA as per the manufacturers recommendations) and incubated overnight with the indicated antibodies. The primary antibodies used were anti-NGF (1: 250; #AN-240; Almone Labs, Jerusalem, Israel), anti-TrkA (1:300; #sc-118; Santa Cruz Biotech, CA, USA), anti-pTrkA/B (1:300; #4621; Cell Signaling, MA, USA), anti-p75NTR (1:300; #sc-5634; Santa Cruz Biotech), anti-sortilin (1:1000; #ab16640; Abcam, Cambridge, MA), or anti-β-actin (1:1500; #A-5441; Sigma). After washing with PBST, the membranes were incubated for 1 h with horseradish peroxidase-conjugated anti-rabbit or anti-mouse anti-sera in PBST and 3% non-fat milk. The membranes were washed again with PBST, and proteins were visualized by enhanced chemiluminescence. The optical density of the immunoreactive bands was measured using NIH ImageJ software. The densitometric values for the proteins of interest were corrected for protein loading using β-actin.
Immunoprecipitation
Since the available anti-pTrkA antibodies also recognize phospho-TrkB and to confirm the age-related differences observed using the anti-pTrkA/B antibody (see Results), a second series of experiments was conducted in which TrkA was immunoprecipitated with a selective anti-TrkA antibody and subsequent Western Blot Analyses were conducted using a phosphotyrosine antibody, anti-pY20 (1:500–1000; Santa Cruz Biotech). To precipitate TrkA, samples (~500 µg of protein) were incubated with anti-TrkA (Santa Cruz Biotech, Santa Cruz, CA) at 4°C for 1 hr with constant rocking in 1 ml of the modified RIPA buffer (50 mM Tris-HCl, pH 7.4, 150 mM NaCl, 1% NP-40, 0.25% sodium deoxycholate, 1 mM PMSF, 1 mM EDTA, 1 µg/ml aprotinin, leupeptin, and pepstatin protease inhibitors). Protein A- agarose beads (50 µl) were added and incubated at 4 °C for 1hr. After washing, bound proteins were resolved by SDS Polyacrylamide Gel Electrophoresis (SDS-PAGE).
2.4 Statistical Analyses, Areas Under the Distance Curves
All statistical analyses were performed using SigmaPlot Version 11 (SPSS Inc., Chicago, IL) or JMP™ version 5 (Cary, NC). One, two- or three-way analysis of variance (with repeated measures when indicated) and two-tailed student’s t-tests were used for group comparisons. Student Newman Keuls multiple comparison procedures (SigmaPlot) and orthogonal t-tests (JMP) corrected for multiple comparisons (via the method of Bonferroni) were used to examine post hoc differences when indicated. Statistical significance was assessed using an alpha level of 0.05
3. Results
3.1. Behavioral Experiments
Water Maze Testing
Hidden Platform Test
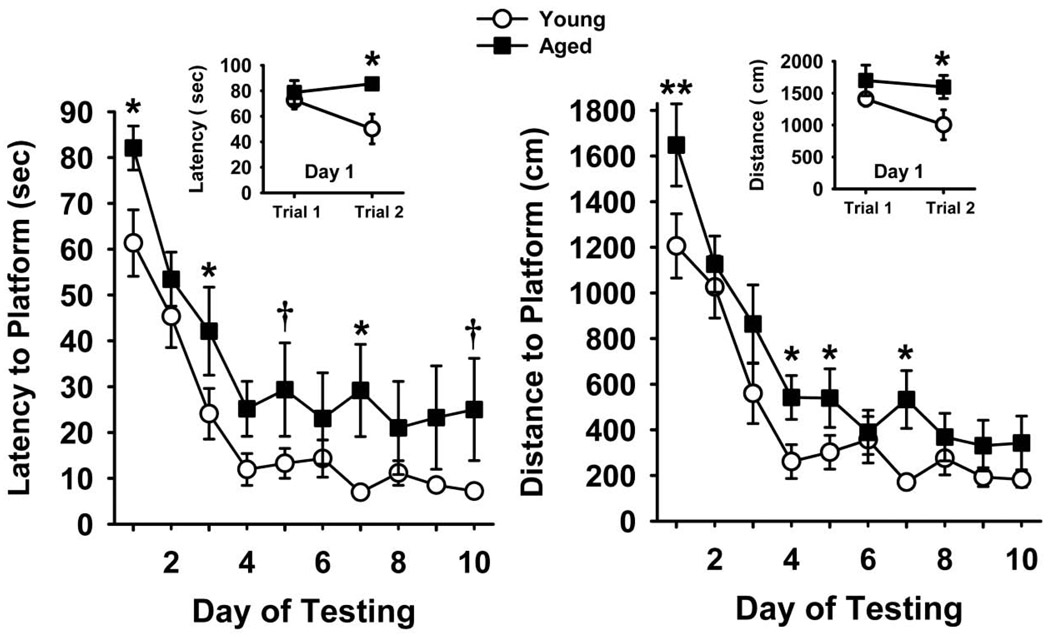
Fig 1 illustrates the efficiency (mean latencies and distances swam ± SEM) of each experimental group to locate a hidden platform in the water maze task over 10 consecutive days of testing. The rats in each experimental group learned to locate the hidden platform with increasing levels of efficiency over the course of the 10 days as evident by the decreasing slope of the acquisition curves. Statistical analyses provided the following results: latency comparison: main effect of group (F1,17 =6.2, p=0.02), session (F9,152 =25.0, p<0.001), group × session interaction (F9,152 =0.4, p=0.92); distance comparison: main effect for group (F1,17 =13.2, p=0.002), session (F9,152 =27.4, p<0.001), group × session interaction (F9,152 =0.8, p=0.64). Post hoc analysis indicated that the aged rats were significantly (p<0.05) or nearly significantly (p<0.08) impaired in this test compared to young rats on several individual days of testing. In order to further evaluate group differences observed on day 1 of hidden platform testing, we compared the first two individual trials statistically. As indicated in the insets to Fig 1, significant learning between trials 1 and 2 was observed in the young rats (as indicated by the lower latencies and shorter distances swam), an effect that was not observed in the aged rats: latency, main effect of group, (F1,17 =4.8, p=0.04), distance (F1,17 =4.7, p=0.04). Post hoc analyses indicated significant differences (p<0.05) between trials 1 and 2 in the young, but not the aged subjects.
Fig 1.
Performance of a water maze spatial learning task by young and aged rats. Daily task acquisition (average of two trials per day) is indicated by the latency (in seconds) and distance swam (in centimeters) for each experimental group to locate a hidden platform over 10 consecutive days of testing. The insets in each figure indicate performance in the first two individual trials (i.e., on day 1 of testing). Data points represent the mean ± S.E.M for each experimental group, N=8–11. Post hoc differences are indicated as follows: * = significant (p<0.05), ** (p<0.01), or nearly significant † (p<0.08) performance deficit when compared to young adult controls.
Swim Speeds
Swim speeds (i.e., the distance swam in cm divided by the latency to find the platform in sec) during hidden platform testing were also compared. Swim speeds ranged (on average) from approximately 23 to 26 cm/sec in both test groups over the 10 days of hidden platform testing. Speeds were somewhat faster during the later sessions of the study, but were not statistically different between the test groups: main effect of group (F1,17 =0.21, p=0.65), session (F9,152 =3.6, p<0.001), group × session interaction (F9,152 =1.5, p=0.15).
Probe Trials
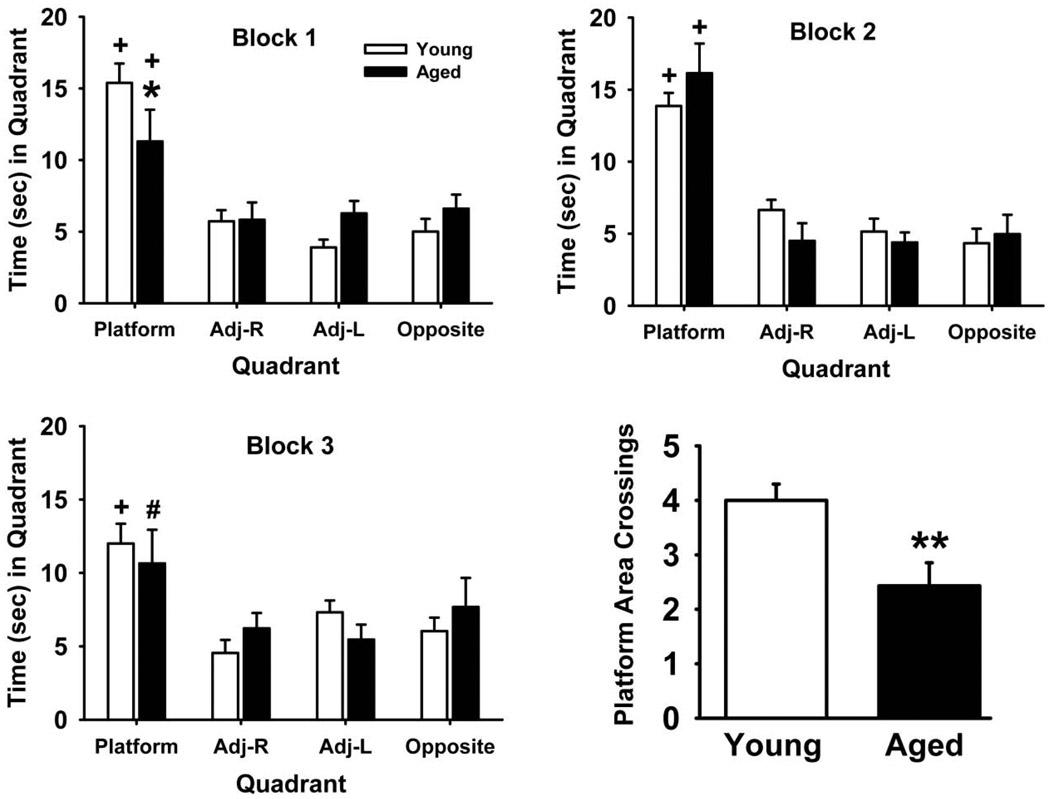
Fig 2 illustrates the performance of 90 second probe trials by the two test groups. The mean number of seconds (± SEM) spent in each quadrant divided into three-30 second blocks was compared to examine spatial bias for the previous platform location as well as potential differences in extinction. The total number of crossings over the previous 10 cm × 10 cm target area for the 90 second period (in the correct quadrant) was also compared (see lower right Fig). For the time comparisons, all factors including group, quadrant, block, and all interactions between these factors were analyzed. Of these analyses, the following comparisons were statistically significant: quadrant (F3,165 =64.0, p<0.001), quadrant × block (F6,165 =2.49, p=0.02), group × quadrant × block (F6,165 =2.22, p=0.04). Post hoc analyses revealed that there was a modest (but statistically significant) deficit in performance (indicated by the lower time spent in the platform quadrant) by the aged rats in block 1. However, there was a clear spatial bias for the previous platform quadrant (compared to the other 3 quadrants) observed in both test groups during all 3 test blocks. While the times spent in the target quadrant were slightly lower in block 3 compared to blocks 1 and 2 (for both test groups) the differences were not statistically significant. Moreover, there were no age-related differences in this comparison. Collectively, these analyses indicate that there were no apparent differences in extinction rates between the test groups. In the more definitive platform area crossing analysis, aged rats were significantly (p<0.01) impaired compared to young rats, indicative of a less accurate spatial bias for the previous platform location.
Fig 2.
Performance of water maze probe trials by young and aged rats. A single (90 second) probe trial was administered to each test subject and the time spent in each quadrant of the pool is presented in 30 second blocks. The figure at the bottom right indicates the total number of crossings (mean ± S.E.M.) over the previous 10 × 10 cm platform area for the 90 second trial. Post hoc differences are indicated as follows: * = significantly (p<0.05), ** (p<0.01) inferior performance when compared to young adult controls. + = significant difference (p<0.05) between the time spent in the target quadrant versus each of the other 3 quadrants, # = significant difference (p<0.05) between time spent in the target quadrant versus all but the opposite quadrant. N=8–11
Visible Platform Test
The average times (± SEM) required to reach a highly visible (reflective) platform in the water maze were 9.3±1.2 sec for the young rats and 6.82±0.6 sec for the aged rats and were not significantly different (p>0.05). This result indicated that group differences in performance of the previous hidden platform tests or probe trials were unlikely to be a result of gross impairments in visual acuity or altered search behaviors associated with old age.
Spontaneous Novel Object Recognition (NOR)
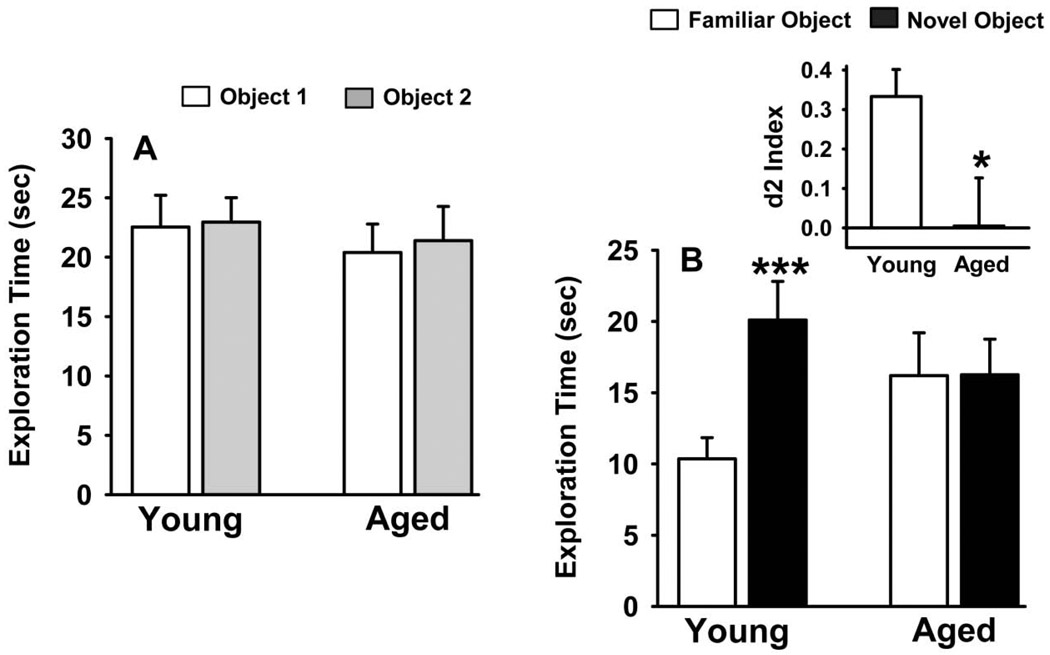
Fig 3 illustrates the effects of age on the performance of an NOR task with six hour delays. As expected, in the training (A/A) session (see Fig 3A), the rats in both age groups spent similar times investigating each of the identical objects and there were no differences in total exploration times between the test groups. The results of statistical comparisons of performance in the testing (A/B) session (see Fig 3B) were: main effect of group, (F1,22=0.14, p=0.71), object type (i.e., novel versus familiar), (F1,22=4.72, p=0.04), group × object type interaction, (F1,22=4.60, p=0.04). Post hoc analysis indicated that there was a significant (p<0.001) preference for the novel object in the young rats that was not present in the aged rats. This preference was further exemplified (p<0.03) in the d2 ratio analysis (see Fig 3B inset).
Fig 3.
Performance of a spontaneous novel object recognition task by young and aged rats. A. The training (A/A) session; B. The test (A/B/) session conducted 6 hours after the training session. The inset to Fig 2B illustrates the effects of age on the “Discrimination Index (d2)” which refers to the proportion of the total exploration time the animal spent investigating the novel object (see Methods); * = significantly (p<0.05), *** (p<0.001) inferior performance when compared to young adult controls, Young adult controls. Data are expressed as the mean ± S.E.M. N=12.
3.2. Neurochemical measurements
ProNGF and mNGF protein levels (Fig 4)
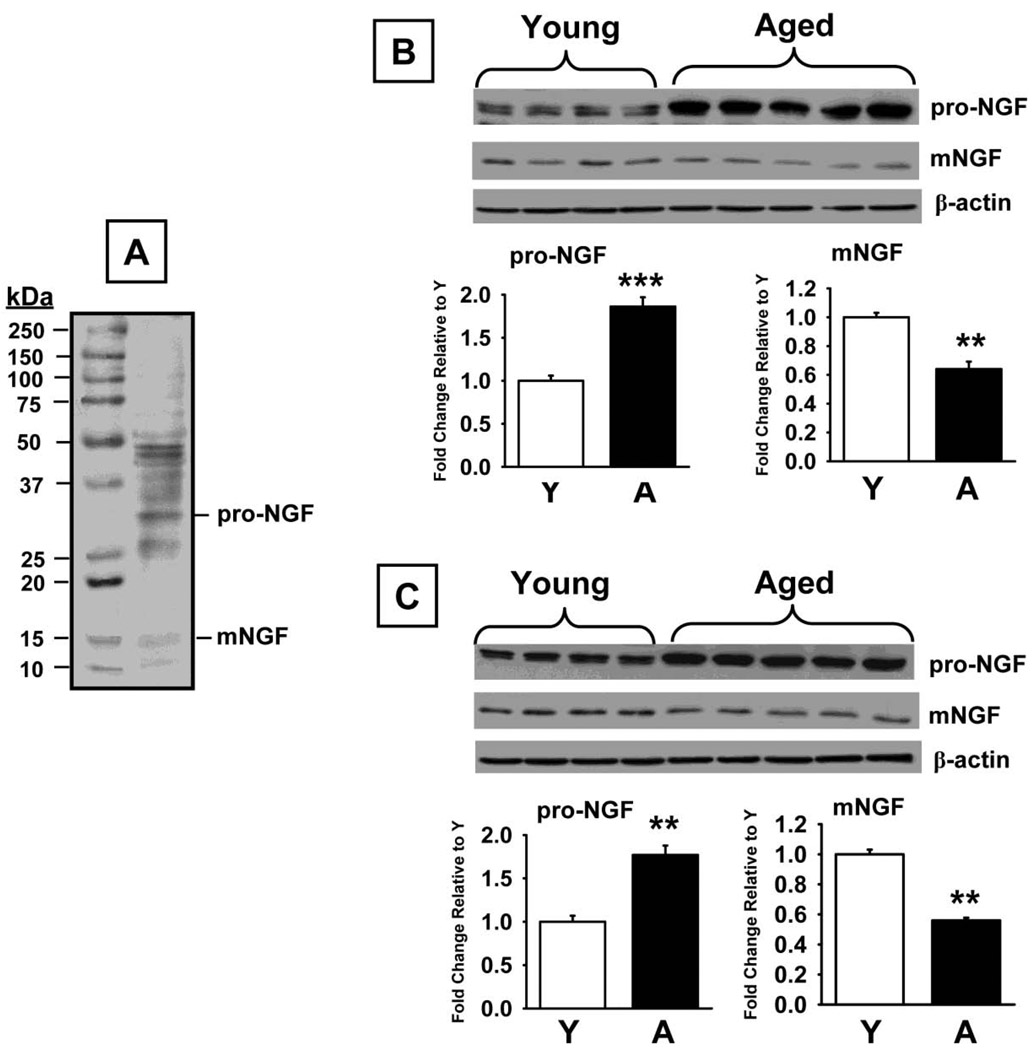
Fig 4.
Pro-NGF and mature NGF levels measured by Western Blot in prefrontal cortex and hippocampus of young and aged rats. A. Representative blot illustrating a molecular weight marker, proNGF (~32 kDa), and NGF (~14 kDa). B. Blots illustrating proNGF, NGF, and β-actin (~38–40 kDa) in the same samples from the prefrontal cortex. C. Blots illustrating proNGF, NGF, and β-actin (~38–40 kDa) in the same samples from the hippocampus. Bar graphs representing fold change in optical density (OD) are illustrated in the lower portion of each figure. Data are expressed as mean ± SEM. ** and *** indicates significant difference compared to young control rats, p<0.001, p<0.0001 (respectively), two-tailed Student's t-tests. N = 4–5. Y=young adult rats; A = aged rats.
Western blot analysis using an antibody that recognizes proNGF and mNGF (~32 kDa and ~14 kDa immunoreactive bands, respectively) indicated that proNGF levels were increased in both brain regions analyzed in aged rats compared to young adult rats (prefrontal cortex, t=6.89, p<0.0001; hippocampus, t=5.83 p<0.001, Fig 4B and 4C, respectively). In contrast, NGF levels were decreased in aged rats compared to young adult rats in both brain regions (prefrontal cortex, t=4.22, p<0.002; hippocampus, t=4.11 p=0.002, Fig 4B and 3C, respectively)
NGF receptors and Sortilin
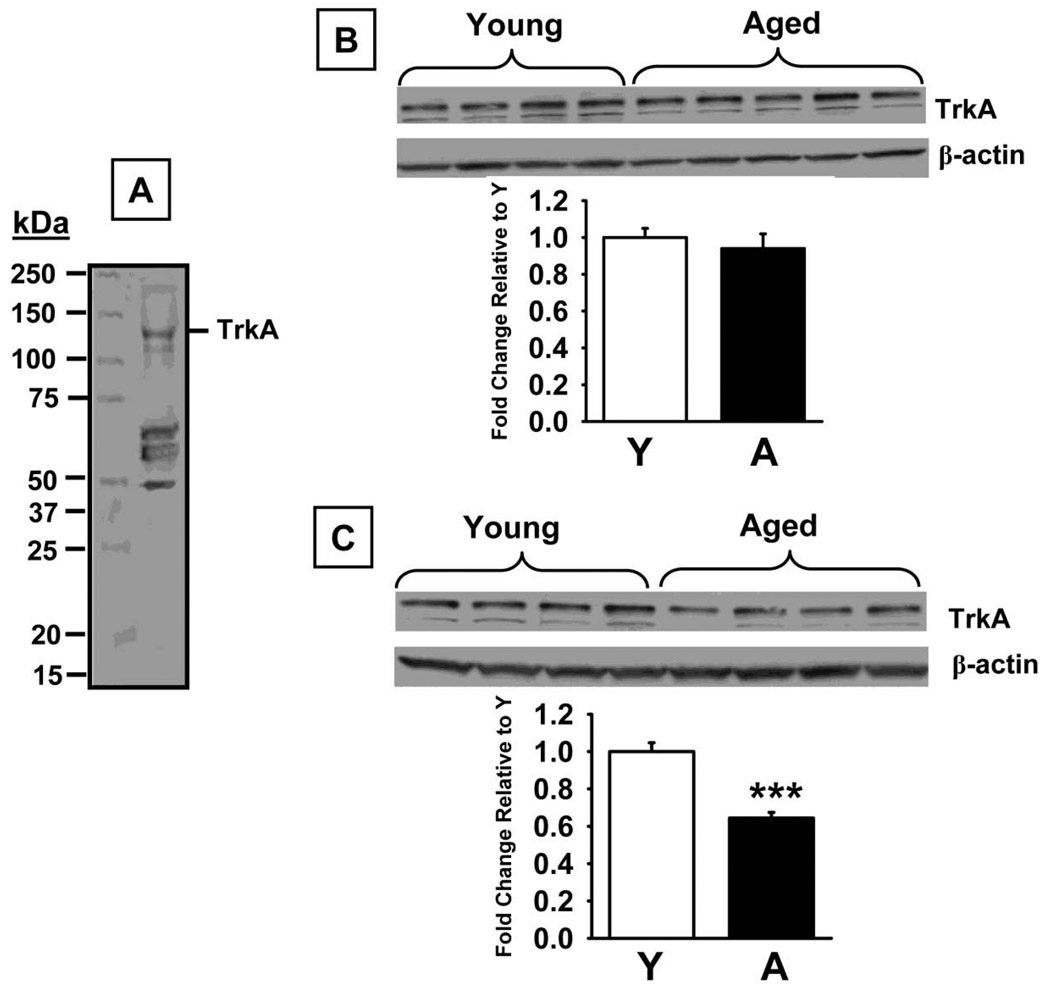
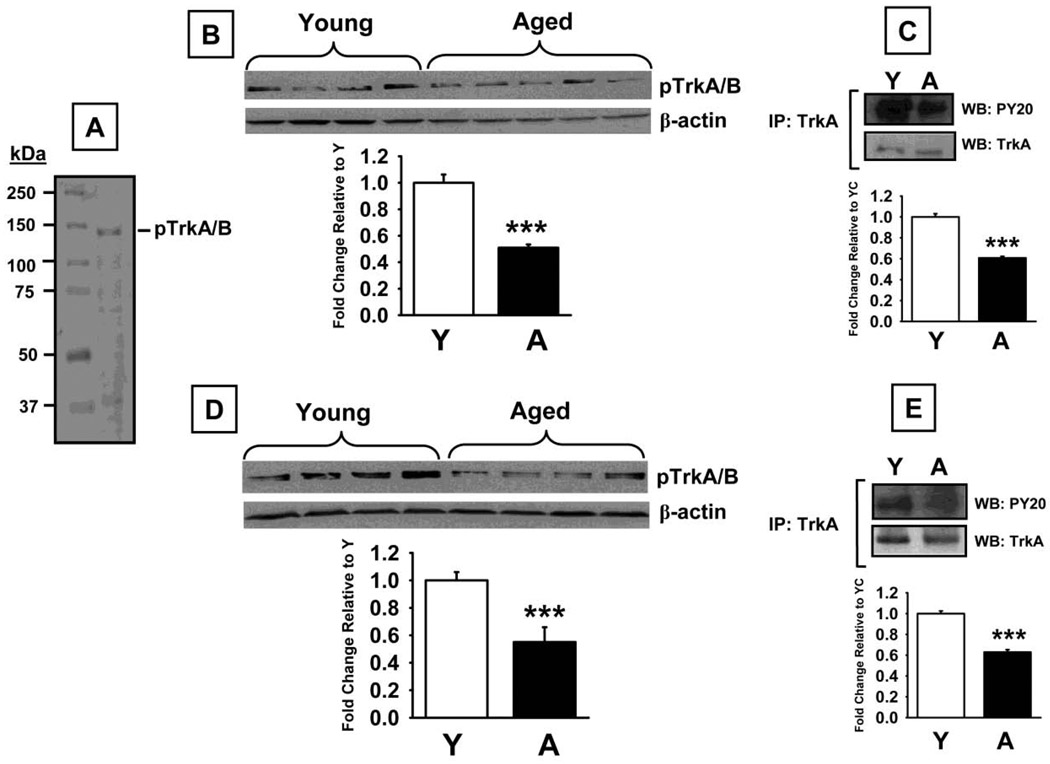
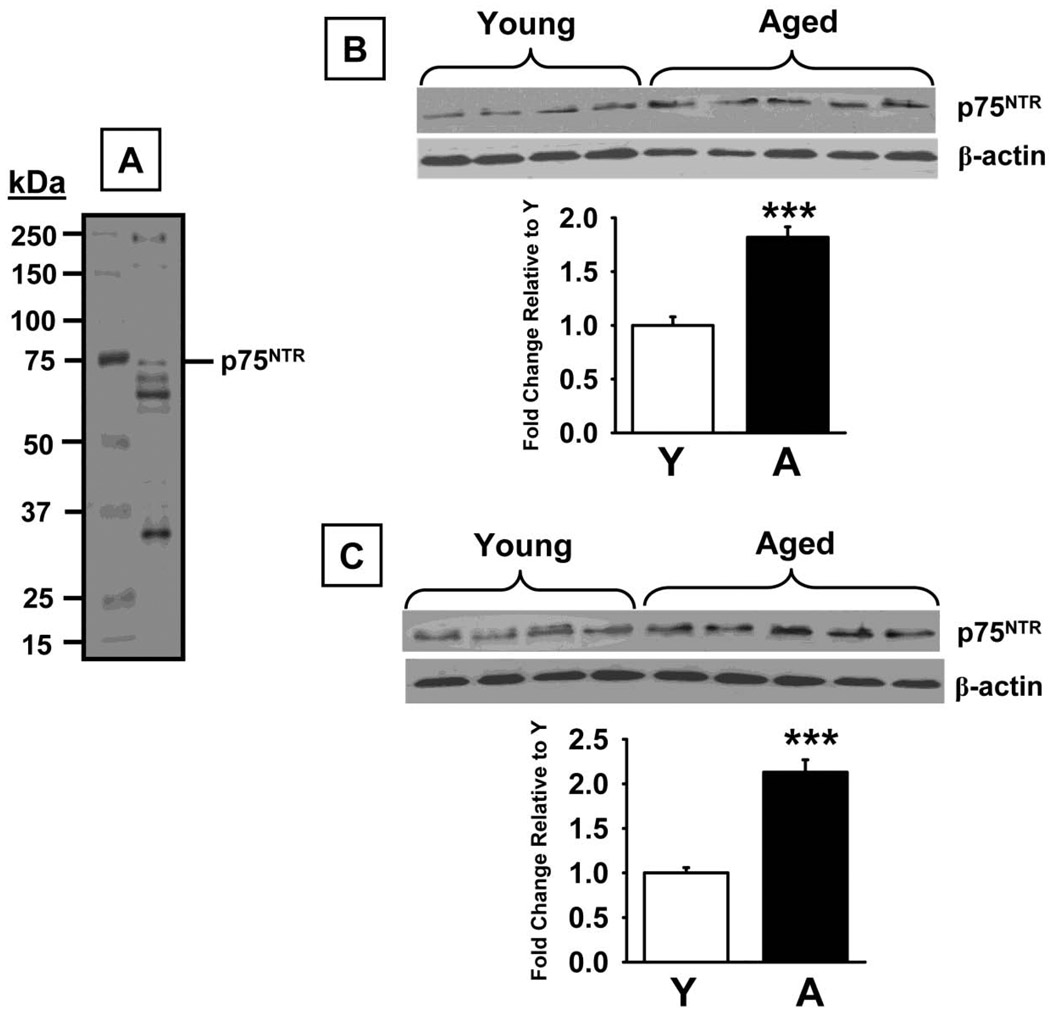
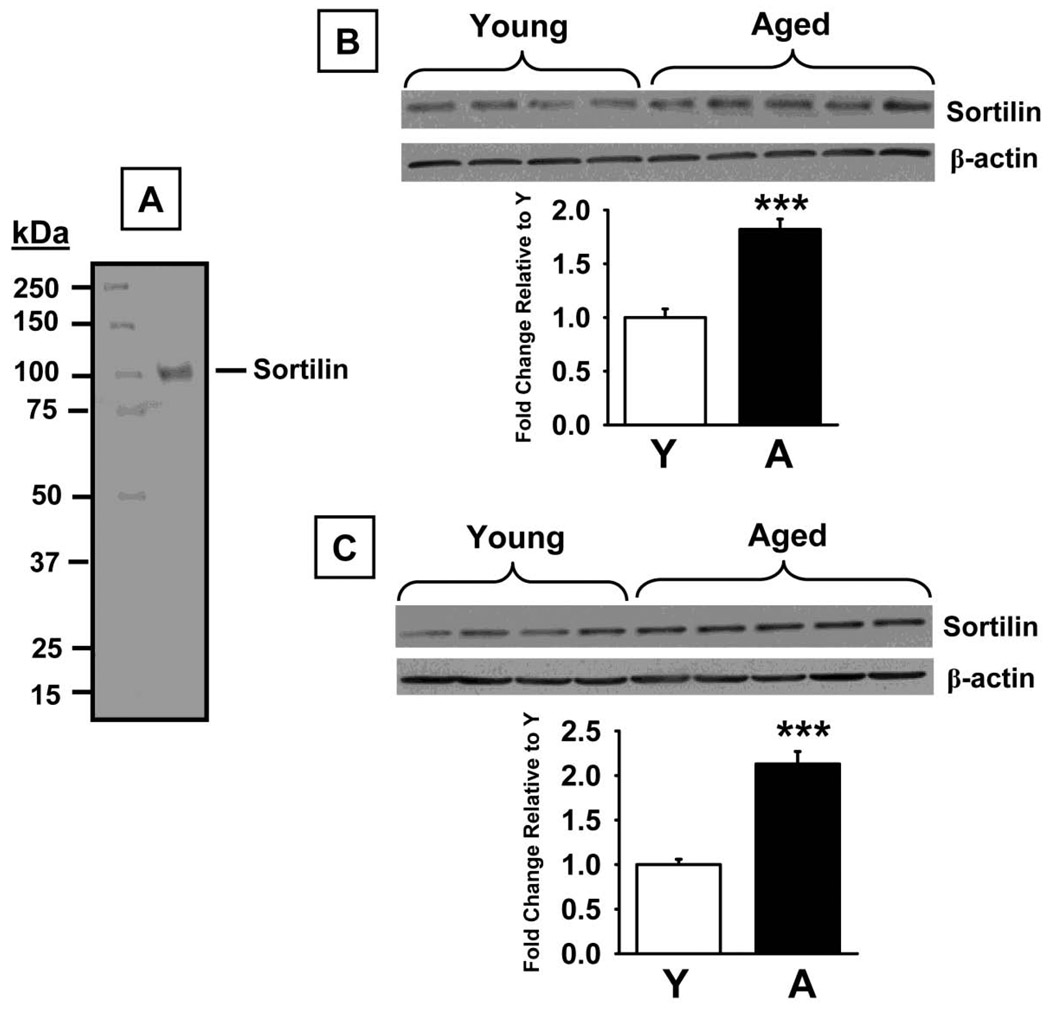
Western blot analysis using a specific antibody against TrkA (~140 kDa band) revealed no significant differences in protein levels between the experimental groups (Fig 5B) in the prefrontal cortex (t=0.61, p=0.56). Conversely, a significant decrease in TrkA was detected in aged versus young rats in the hippocampus (Fig 5C, t=6.41, p<0.0001). Phospho-TrkA/B (~140 kDa band) levels were also lower in both the prefrontal cortex (t=7.46, p<0.0001), and hippocampus (t=6.00, p<0.0001) in aged rats compared to young adult rats (Fig 6B and D, respectively). This aged-related decrease in phospo-TrK receptors was confirmed (and that phospho-TrkA levels, specifically, were lower) in the subsequent experiments in which TrkA was immunoprecipitated with a selective anti-TrkA antibody and subsequent Western Blot Analyses were conducted using an anti-phosphotyrosine antibody (Fig 6C and E for the prefrontal cortex and hippocampus, respectively). We also detected significant increases in p75NTR protein (75 kDa band) levels in prefrontal cortex (t=7.68, p<0.0001), and hippocampus (t=4.49, p=0.001, Fig 7B and C, respectively), of aged rats compared to young adult rats. Finally, sortilin (~95 kDa) levels were significantly higher in prefrontal cortex (t=6.45, p<0.0001), and hippocampus (t=7.39, p<0.0001), of aged rats compared to young adult rats (Fig 8B and C, respectively).
Fig 5.
TrkA levels measured by Western Blot in prefrontal cortex and hippocampus of young and aged rats. A. Representative blot illustrating a molecular weight marker and TrkA (~140 kDa). B. Blots illustrating TrkA and β-actin (~38–40 kDa) in the same samples from the prefrontal cortex. C. Blots illustrating TrkA and β-actin (~38–40 kDa) in the same samples from the hippocampus. Bar graphs representing fold change in optical density (OD) are illustrated in the lower portion of each figure. Data are expressed as mean ± SEM. *** indicates significant difference compared to young control rats, p<0.0001, two-tailed Student's t-tests. N = 4–5. Y=young adult rats; A = aged rats.
Fig 6.
Phospho-Trk (pTrk) levels measured by Western Blot in prefrontal cortex and hippocampus of young and aged rats. A. Representative blot illustrating a molecular weight marker and pTrkA/B (~140 kDa). B. Blots illustrating pTrkA/B and β-actin (~38–40 kDa) in the same samples from the prefrontal cortex. C. Results from a confirmatory series of experiments in which samples of prefrontal cortex were protein precipitated with TrkA antibody before immunoblotting with a phosphotyrosine (pY20) antibody. D. Blots illustrating pTrkA/B and β-actin (~38–40 kDa) in the same samples from the hippocampus. E. Results from a confirmatory series of experiments in which samples of hippocampus were protein precipitated with TrkA antibody before immunoblotting with a phosphotyrosine (pY20) antibody. Bar graphs representing fold change in optical density (OD) are illustrated in the lower portion of each figure. Data are expressed as mean ± SEM. *** indicates significant difference compared to young control rats, p<0.0001, two-tailed Student's t-tests. N = 4–5.
Fig 7.
p75NTR levels measured by Western Blot in prefrontal cortex and hippocampus of young and aged rats. A. Representative blot illustrating a molecular weight marker and p75NTR (~75 kDa). B. Blots illustrating p75NTR protein and β-actin (~38–40 kDa) in the same samples from the prefrontal cortex. C. Blots illustrating p75NTR protein and β-actin (~38–40 kDa) in the same samples from the hippocampus. Bar graphs representing fold change in optical density (OD) are illustrated in the lower portion of each figure. Data are expressed as mean ± SEM. *** indicates significant difference compared to young control rats, p<0.0001, two-tailed Student's t-tests. N = 4–5.
Fig 8.
Sortilin levels measured by Western Blot in prefrontal cortex and hippocampus of young and aged rats. A. Representative blot illustrating a molecular weight marker and sortilin (~95–100 kDa). B. Blots illustrating sortilin protein and β-actin (~38–40 kDa) in the same samples from the prefrontal cortex. C. Blots illustrating sortilin protein and β-actin (~38–40 kDa) in the same samples from the hippocampus. Bar graphs representing fold change in optical density (OD) are illustrated in the lower portion of each figure. Data are expressed as mean ± SEM. *** indicates significant difference compared to young control rats, p<0.0001, two-tailed Student's t-tests. N = N = 4–5.
4. Discussion
The results of this study can be summarized as follows: 1) old age was associated with spatial learning deficits that could not be attributed to sensory (i.e., visual acuity) or motor (swim speed) deficits; 2) old age was associated with recognition memory impairments as indicated by deficits in performance of a novel object recognition task; 3) in both of the brain regions that were analyzed (prefrontal cortex, hippocampus), aged rats (compared to young vehicle-treated controls) had elevated levels of pro-NGF, sortilin and p75NTR receptors and decreased levels of NGF and phospho-TrkA receptors. TrkA levels were also reduced in the hippocampus, but not the prefrontal cortex in old rats.
In the behavioral experiments, the water maze procedure was employed since it requires the hippocampus (which is well documented to be adversely affected in aging and AD), important components of human learning and memory such as information acquisition and encoding, consolidation, retention, and retrieval [21,22]. Furthermore, it is relatively well established that water maze task performance declines with increasing age in animals [23], an important issue in this study. It is generally accepted that there are two main (complementary) strategies used by rats to learn the location of a hidden platform in a pool of water: an idiocentric (or egocentric) strategy, based on the subject's body position in space, and an allocentric strategy, which is dependent on the subject's ability to learn spatial cues [24,25]. It has been argued that the second strategy is primarily dependent on the hippocampus, while the first is dependent on the striatum [26,27]. While generally not considered critical for spatial learning, neocortical areas such as the prefrontal cortex are also likely to contribute to such mnemonic processes and to optimal water maze hidden platform task acquisition in naive rats [23,28,29].
The NOR results of this study agree with those of other investigators who have observed age-related deficits in NOR performance [30–32]. NOR [33] is a rodent model of (non-spatial) recognition memory, which by definition consists of two components, a recollective (episodic) component and a familiarity component [34]. Recognition memory is demonstrated in the NOR task when subjects explore a novel object more than a familiar one. There is considerable evidence that the hippocampus is involved in object recognition memory in both rodents [35–37] and humans [38,39] and further, object recognition memory has also been observed to be negatively affected in non-demented aged individuals as well as in patients with Alzheimer's disease [40–42]. While the role of the prefrontal cortex in object recognition memory is somewhat controversial, it has been argued that the hippocampus and prefrontal cortex both play important and complementary roles in the recollection component of recognition memory [43].
In the neurochemical analyses, we detected age-related elevations in neurotrophin proteins that are normally associated with apoptosis and neuronal death (i.e., proNGF and p75NTR) while levels of a neurotrophin receptor normally associated with neuronal survival and plasticity (i.e., TrkA and especially, its activated form, phospho-TrkA) were diminished. The basis for the age-related (and brain region-specific) differences in the expression of TrkA versus phospho-TrkA (i.e. a decrease in TrkA in the hippocampus with no change in the PFC, but a decrease in phospho-TrkA in both brain regions) is unclear. Given that NGF binding to TrkA promotes TrkA phosphorylation, the later observation may be related to the lower NGF levels present in both brain regions of old animals.
The data described here appear to complement a growing body of evidence (including clinical literature) which supports an important role of neurotrophin function in aging and age-related neurodegenerative conditions. For example, it was recently demonstrated that proNGF is increased in the cortex of patients with mild cognitive impairment (MCI) as well as AD [44]. Further, the authors of the study found a negative correlation between proNGF levels and Mini Mental Status Examination (MMSE) scores indicating that elevated levels of proNGF correlated with impairments in cognitive function. Moreover, proNGF purified from the brains of AD patients (i.e., who had significantly elevated levels of proNGF) was found to induce apoptosis in neuronal cell cultures via its interactions with the p75NTR receptor [45]. Interestingly, studies in mice indicate that during normal aging, there is a progressive increase in the level of p75NTR and a parallel decrease in the level of TrkA expression, [46]. Similarly, p75NTR receptor levels were found to be markedly increased in both the cortex [47] and hippocampus [48] of AD patients, while a significant reduction in TrkA receptor levels was found in the cortex of AD patients compared with age-matched controls [49,50].
The underlying basis for the elevations in proNGF in MCI and AD patients (and similar elevations in our aged rodent subjects as well as concomitant decreases in NGF) is unclear, but could be a result of impaired cleavage of proNGF by proteases such as metalloproteinases (MMPs) and plasmin. In several CNS injury models observations of reduced conversion of proNGF to mature NGF are suggestive of impaired proteolysis of extracellular proNGF or the induction of inhibitors of MMPs and plasmin (reviewed, [51]).
We also detected age-related increases in the neurotensin receptor sortilin in the current study. Sortilin has been associated with intracellular sorting and trafficking of a variety of proteins, while more recently it has also been described as a co-receptor (i.e., with p75NTR) that forms a high affinity binding site for proNGF [16]. Al-Shawi and colleagues [18] concluded (based on extensive rodent experiments) that in old age, increases in the expression of sortilin enhance the vulnerability of neurons to age-related increases in pro-NGF, thus leading to neuronal loss and neurodegeneration.
There are some limitations to the current study that should be discussed. Our neurochemical studies focused on the hippocampus and prefrontal cortex due to their known in roles spatial learning and recognition memory, and the fact that they are highly innervated by cholinergic neurons projecting from the basal forebrain (neurons with well-documented roles in cognitive function). These brain regions also express relatively high levels of NGF-related proteins. However, we did not analyze the basal forebrain itself (the locus of the cell bodies of the cholinergic neurons that innervate the hippocampus and PFC). The basal forebrain also expresses high levels of NGF-related proteins (particularly the p75NTR) and it is implicated in age-related deficits in several aspects of cognition. Since we only evaluated two (memory-related) brain regions, it is also unclear whether the age-related differences observed in our study are limited to these brain regions or if they are widespread across the brain. It is also important to note that all of our neurochemical analyses were conducted in rats that underwent behavioral training. Accordingly, it is unclear if the age-related differences are innate (i.e., in behaviorally naive rats) or if they become more apparent during the process of behavioral testing. Finally, since we only evaluated two age groups (young and old, but not an intermediate age group), it was not possible to determine precisely when the behavioral and neurochemical changes become manifest (e.g., during middle age or old age) or if a clear temporal relationship exists between the biochemical changes and the behavioral changes.
In conclusion, this study further supports the premise that neurotrophin signaling is altered in the aging mammalian brain, and that such alterations may contribute to an age-related decline in cognitive function. Experimental results such as those described here may help to identify specific components of the NGF-signaling pathway that could serve as targets for novel drug discovery and development. For example, pharmacological strategies that enhance TrkA phosphorylation in the aging brain or that attenuate an age- or injury-related induction of proNGF and/or an increase in p75NTR expression might be useful. Likewise, drugs that alter or modify the complex interactions between proNGF, p75, and sortilin might be beneficial as would compounds that facilitate the activation of intracellular or extracellular proteases that specifically cleave proNGF to mature NGF [51].
Acknowledgements
This work was supported by the National Institute of Aging/National Institutes of Health grants AG032140 and AG029617. The authors also wish to thank Ms Samantha Warner and Ms. Kristy Bouchard for technical assistance.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Ferri CP, Prince M, Brayne C, Brodaty H, Fratiglioni L, Ganguli M, Hall K, Hasegawa K, Hendrie H, Huang Y, Jorm A, Mathers C, Menezes PR, Rimmer E, Scazufca M. Alzheimer's Disease International. Global prevalence of dementia: a Delphi consensus study. Lancet. 2005;366:2112–2117. doi: 10.1016/S0140-6736(05)67889-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Luck T, Luppa M, Briel S, Riedel-Heller SG. Incidence of mild cognitive impairment: a systematic review. Dement. Geriatr. Cogn. Disord. 2010;29:164–175. doi: 10.1159/000272424. [DOI] [PubMed] [Google Scholar]
- 3.McAllister AK, Katz LC, Lo DC. Neurotrophins and synaptic plasticity. Annu. Rev. Neurosci. 1999;22:295–318. doi: 10.1146/annurev.neuro.22.1.295. [DOI] [PubMed] [Google Scholar]
- 4.Lärkfors L, Ebendal T, Whittemore SR, Persson H, Hoffer B, Olsen L. Decreased levels of nerve growth factor (NGF) and its messenger RNA in the aged rat brain. Brain Res. 1987;427:55–60. doi: 10.1016/0169-328x(87)90044-1. [DOI] [PubMed] [Google Scholar]
- 5.Gomez-Pinilla F, Cotman CW, Neito-Sampedro M. NGF immunoreactivity in the aged rat brain. Brain. Res. 1989;479:255–262. doi: 10.1016/0006-8993(89)91626-0. [DOI] [PubMed] [Google Scholar]
- 6.Williams BJ, Bimonte-Nelson HA, Granholm-Bentley AC. ERK-mediated NGF signaling in the rat septo-hippocampal pathway diminishes with age. Psychopharmacology (Berlin) 2006;188:605–618. doi: 10.1007/s00213-006-0477-1. [DOI] [PubMed] [Google Scholar]
- 7.Counts SE, Mufson EJ. The role of nerve growth factor receptors in cholinergic basal forebrain degeneration in prodromal Alzheimer disease. J. Neuropathol. Exp. Neurol. 2005;64:263–272. doi: 10.1093/jnen/64.4.263. [DOI] [PubMed] [Google Scholar]
- 8.Bartus RT. On neurodegenerative diseases, models, and treatment strategies: lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000;163:495–529. doi: 10.1006/exnr.2000.7397. [DOI] [PubMed] [Google Scholar]
- 9.Kelly A, Maguire C, Lynch MA. Deficits in nerve growth factor release and tyrosine receptor kinase phosphorylation are associated with age-related impairment in long-term potentiation in the dentate gyrus. Neuroscience. 2000;95:359–365. doi: 10.1016/s0306-4522(99)00460-1. [DOI] [PubMed] [Google Scholar]
- 10.Bliss TV, Collingridge GL. A synaptic model of memory: long-term potentiation in the hippocampus. Nature. 1993;361:31–39. doi: 10.1038/361031a0. [DOI] [PubMed] [Google Scholar]
- 11.Henricksson BG, Söderström S, Gower AJ, Ebendal T, Winblad B, Mohammed AH. Hippocampal nerve growth factor levels are related to spatial learning ability in aged rats. Behav. Brain. Res. 1992;48:15–20. doi: 10.1016/s0166-4328(05)80134-2. [DOI] [PubMed] [Google Scholar]
- 12.Bergado JA, Fernández CI, Gómez-Soria A, González O. Chronic intraventricular infusion with NGF improves LTP in old cognitively-impaired rats. Brain Res. 1997;770:1–9. doi: 10.1016/s0006-8993(97)00610-0. [DOI] [PubMed] [Google Scholar]
- 13.Frick KM, Price DL, Koliatsos VE, Markowska AL. The effects of nerve growth factor on spatial recent memory in aged rats persist after discontinuation of treatment. J. Neurosci. 1997;17:2543–2550. doi: 10.1523/JNEUROSCI.17-07-02543.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Lee R, Kermani P, Teng KK, Hempstead BL. Regulation of cell survival by secreted proneurotrophins. Science. 2001;294:1945–1948. doi: 10.1126/science.1065057. [DOI] [PubMed] [Google Scholar]
- 15.Underwood CK, Coulson EJ. The p75 neurotrophin receptor. Int. J. Biochem. Cell Biol. 2008;40:1664. doi: 10.1016/j.biocel.2007.06.010. 1668. [DOI] [PubMed] [Google Scholar]
- 16.Nykjaer A, Lee R, Teng KK, Jansen P, Madsen P, Nielsen MS, Jacobsen C, Kliemannel M, Schwarz E, Willnow TE, Hempstead BL, Petersen CM. Sortilin is essential for proNGF-induced neuronal cell death. Nature. 2004;427:843–848. doi: 10.1038/nature02319. [DOI] [PubMed] [Google Scholar]
- 17.Arnett MG, Ryals JM, Wright DE. Pro-NGF, sortilin, and p75NTR: potential mediators of injury-induced apoptosis in the mouse dorsal root ganglion. Brain Res. 2007;1183:32–42. doi: 10.1016/j.brainres.2007.09.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Al-Shawi R, Hafner A, Olson J, Chun S, Raza S, Thrasivoulou C, Lovestone S, Killick R, Simons P, Cowen T. Neurotoxic and neurotrophic roles of proNGF and the receptor sortilin in the adult and ageing nervous system. Eur. J. Neurosci. 2008;27:2103–2114. doi: 10.1111/j.1460-9568.2008.06152.x. [DOI] [PubMed] [Google Scholar]
- 19.Terry AV, Jr, Gearhart DA, Warner SE, Zhang G, Bartlett MG, Middlemore ML, Beck WD, Jr, Mahadik SP, Waller JL. Oral haloperidol or risperidone treatment in rats: Temporal effects on nerve growth factor receptors, cholinergic neurons, and memory performance. Neuroscience. 2007;146:1316–1332. doi: 10.1016/j.neuroscience.2007.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gearhart DA, Middlemore ML, Terry AV. ELISA methods to measure cholinergic markers and nerve growth factor receptors in cortex, hippocampus, prefrontal cortex, and basal forebrain from rat brain. J. Neurosci. Methods. 2006;150:159–173. doi: 10.1016/j.jneumeth.2005.06.009. [DOI] [PubMed] [Google Scholar]
- 21.McNamara RK, Skelton RW. The neuropharmacological and neurochemical basis of place learning in the Morris water maze. Brain. Res. Rev. 1993;18:33–49. doi: 10.1016/0165-0173(93)90006-l. [DOI] [PubMed] [Google Scholar]
- 22.McDonald RJ, White NM. Hippocampal and nonhippocampal contributions to place learning in rats. Behav. Neurosci. 1995;109:579–593. doi: 10.1037//0735-7044.109.4.579. [DOI] [PubMed] [Google Scholar]
- 23.Brandeis R, Brandys Y, Yehuda S. The use of the Morris Water Maze in the study of memory and learning. Int. J. Neurosci. 1989;48:29–69. doi: 10.3109/00207458909002151. [DOI] [PubMed] [Google Scholar]
- 24.Long JM, Kesner RP. The effects of dorsal versus ventral hippocampal, total hippocampal, and parietal cortex lesions on memory for allocentric distance in rats. Behav. Neurosci. 1996;110:922–932. doi: 10.1037//0735-7044.110.5.922. [DOI] [PubMed] [Google Scholar]
- 25.Whishaw IQ. Formation of a place learning-set by the rat: a new paradigm for neurobehavioral studies. Physiol. Behav. 1985;35:39–143. doi: 10.1016/0031-9384(85)90186-6. [DOI] [PubMed] [Google Scholar]
- 26.Devan BD, Goad EH, Petri HL. Dissociation of hippocampal and striatal contributions to spatial navigation in the water maze. Neurobiol. Learn. Mem. 1996;66:305–323. doi: 10.1006/nlme.1996.0072. [DOI] [PubMed] [Google Scholar]
- 27.Packard MG, McGaugh JL. Inactivation of hippocampus or caudate nucleus with lidocaine differentially affects expression of place and response learning. Neurobiol. Learn. Mem. 1996;165:65–72. doi: 10.1006/nlme.1996.0007. [DOI] [PubMed] [Google Scholar]
- 28.Kolb B, Pittman K, Sutherland RJ, Whishaw IQ. Dissociation of the contributions of the prefrontal cortex and dorsomedial thalamic nucleus to spatially guided behavior in the rat. Behav. Brain Res. 1982;6:365–378. doi: 10.1016/0166-4328(82)90018-3. [DOI] [PubMed] [Google Scholar]
- 29.D'Hooge R, De Deyn PP. Applications of the Morris water maze in the study of learning and memory. Brain Res. Brain. Res. Rev. 2001;36:60–90. doi: 10.1016/s0165-0173(01)00067-4. [DOI] [PubMed] [Google Scholar]
- 30.Liu P, Smith PF, Appleton I, Darlington CL, Bilkey DK. Potential involvement of NOS and arginase in age-related behavioural impairments. Exp. Gerontol. 2004;39 doi: 10.1016/j.exger.2004.04.008. 1207-1022. [DOI] [PubMed] [Google Scholar]
- 31.de Lima MN, Laranja DC, Caldana F, Bromberg E, Roesler R, Schröder N. Reversal of age-related deficits in object recognition memory in rats with l-deprenyl. Exp. Gerontol. 2005;40:506–511. doi: 10.1016/j.exger.2005.03.004. [DOI] [PubMed] [Google Scholar]
- 32.Pitsikas N, Rigamonti AE, Cella SG, Sakellaridis N, Muller EE. The nitric oxide donor molsidomine antagonizes age-related memory deficits in the rat. Neurobiol. Aging. 2005;26:259–264. doi: 10.1016/j.neurobiolaging.2004.04.003. [DOI] [PubMed] [Google Scholar]
- 33.Ennaceur A, Delacour J. A new one-trial test for neurobiological studies of memory in rats. 1: Behavioral data. Behav. Brain Res. 1988;31:47–59. doi: 10.1016/0166-4328(88)90157-x. [DOI] [PubMed] [Google Scholar]
- 34.Squire LR. Memory systems of the brain: a brief history and current perspective. Neurobiol. Learn. Mem. 2004;82:171–177. doi: 10.1016/j.nlm.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 35.Myhrer T. Exploratory behavior and reaction to novelty in rats with hippocampal perforant path systems disrupted. Behav Neurosci. 1988;102:356–362. doi: 10.1037//0735-7044.102.3.356. [DOI] [PubMed] [Google Scholar]
- 36.Rampon C, Tang YP, Goodhouse J, Shimizu E, Kyin M, Tsien JZ. Enrichment induces structural changes and recovery from nonspatial memory deficits in CA1 NMDAR1-knockout mice. Nat. Neurosci. 2000;3:238–244. doi: 10.1038/72945. [DOI] [PubMed] [Google Scholar]
- 37.Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc. Natl. Acad. Sci. USA. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Reed JM, Squire LR. Impaired recognition memory in patients with lesions limited to the hippocampal formation. Behav. Neurosci. 1997;111:667–675. doi: 10.1037//0735-7044.111.4.667. [DOI] [PubMed] [Google Scholar]
- 39.Squire LR. Memory and the hippocampus: a synthesis from findings with rats, monkeys, and humans. Psychol Rev. 1992;99:195–231. doi: 10.1037/0033-295x.99.2.195. [DOI] [PubMed] [Google Scholar]
- 40.Flicker C, Ferris SH, Crook T, Bartus RT. A visual recognition memory test for the assessment of cognitive function in aging and dementia. Exp. Aging Res. 1987;13:127–132. doi: 10.1080/03610738708259313. [DOI] [PubMed] [Google Scholar]
- 41.Purdy KS, McMullen PA, Freedman M. Changes to the object recognition system in patients with dementia of the Alzheimer's type. Brain Cogn. 2002;49:213–216. [PubMed] [Google Scholar]
- 42.Schiavetto A, Köhler S, Grady CL, Winocur G, Moscovitch M. Neural correlates of memory for object identity and object location: effects of aging. Neuropsychologia. 2002;40:1428–1442. doi: 10.1016/s0028-3932(01)00206-8. [DOI] [PubMed] [Google Scholar]
- 43.DeVito LM, Eichenbaum H. Distinct contributions of the hippocampus and medial prefrontal cortex to the 'what-where-when' components of episodic-like memory in mice. Behav. Brain Res. 2010 doi: 10.1016/j.bbr.2009.09.014. (in press doi:10.1016/j.bbr.2009.09.014) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Peng S, Wuu J, Mufson EJ, Fahnestock M. Increased proNGF levels in subjects with mild cognitive impairment and mild Alzheimer disease. J. Neuropathol. Exp. Neurol. 2004;63:641–649. doi: 10.1093/jnen/63.6.641. [DOI] [PubMed] [Google Scholar]
- 45.Pedraza CE, Podlesniy P, Vidal N, Arévalo JC, Lee R, Hempstead B, Ferrer I, Iglesias M, Espinet C. Pro-NGF isolated from the human brain affected by Alzheimer's disease induces neuronal apoptosis mediated by p75NTR. Am. J. Pathol. 2005;166:533–543. doi: 10.1016/S0002-9440(10)62275-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Costantini C, Weindruch R, Della Valle G, Puglielli L. A TrkA to- p75NTR molecular switch activates amyloid beta-peptide generation during aging. Biochem. J. 2005;391:59–67. doi: 10.1042/BJ20050700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Mufson EJ, Kordower JH. Cortical neurons express nerve growth factor receptors in advanced age and Alzheimer disease. Proc. Natl. Acad. Sci. 1992;89:569–573. doi: 10.1073/pnas.89.2.569. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Hu XY, Zhang HY, Qin S, Xu H, Swaab DF, Zhou JN. Increased p75NTR expression in hippocampal neurons containing hyperphosphorylated tau in Alzheimer patients. Exp. Neurol. 2002;178:104–111. doi: 10.1006/exnr.2002.8018. [DOI] [PubMed] [Google Scholar]
- 49.Hock C, Heese K, Muller-Spahn F, Hulette C, Rosenberg C, Otten U. Decreased trkA neurotrophin receptor expression in the parietal cortex of patients with Alzheimer's disease. Neurosci. Lett. 1998;241:151–154. doi: 10.1016/s0304-3940(98)00019-6. [DOI] [PubMed] [Google Scholar]
- 50.Counts SE, Nadeem M, Wuu J, Ginsberg SD, Saragovi HU, Mufson EJ. Reduction of cortical TrkA but not p75NTR protein in early-stage Alzheimer's disease. Ann. Neurol. 2004;56:520–531. doi: 10.1002/ana.20233. [DOI] [PubMed] [Google Scholar]
- 51.Hempstead B. Regulating proNGF Action: Multiple Targets for Therapeutic Intervention. Neurotox. Res. 2009;16:255–260. doi: 10.1007/s12640-009-9054-9. [DOI] [PMC free article] [PubMed] [Google Scholar]