Abstract
Implicit motor learning is preserved after stroke, but how the brain compensates for damage to facilitate learning is unclear. We used a random effects analysis to determine how stroke alters patterns of brain activity during implicit sequence‐specific motor learning as compared to general improvements in motor control. Nine healthy participants and nine individuals with chronic, right focal subcortical stroke performed a continuous joystick‐based tracking task during an initial functional magnetic resonance images (fMRI) session, over 5 days of practice, and a retention test during a separate fMRI session. Sequence‐specific implicit motor learning was differentiated from general improvements in motor control by comparing tracking performance on a novel, repeated tracking sequence during early practice and again at the retention test. Both groups demonstrated implicit sequence‐specific motor learning at the retention test, yet substantial differences were apparent. At retention, healthy control participants demonstrated increased blood oxygenation level dependent (BOLD) response in left dorsal premotor cortex (PMd; BA 6) but decreased BOLD response left dorsolateral prefrontal cortex (DLPFC; BA 9) during repeated sequence tracking. In contrast, at retention individuals with stroke did not show this reduction in DLPFC during repeated tracking. Instead implicit sequence‐specific motor learning and general improvements in motor control were associated with increased BOLD response in the left middle frontal gyrus BA 8, regardless of sequence type after stroke. These data emphasize the potential importance of a prefrontal‐based attentional network for implicit motor learning after stroke. This study is the first to highlight the importance of the prefrontal cortex for implicit sequence‐specific motor learning after stroke. Hum Brain Mapp, 2011. © 2010 Wiley‐Liss, Inc.
Keywords: implicit learning, sequence‐specific, retention, dorsolateral prefrontal cortex, premotor cortex
INTRODUCTION
How the brain compensates for damage after stroke to promote motor learning is unclear. Despite shifts in cortical representations that are present even after subcortical stroke [Calautti et al., 2003], motor learning is preserved [Boyd et al., 2007a; Boyd and Winstein 2001, 2004b]. However, the dynamic evolution of neural activity associated with motor learning after stroke is not well understood. Experience dependent cortical plasticity may explain motor learning after stroke [Nudo et al., 1996], yet no clear pattern of motor‐related brain activation has emerged that explains how the brain compensates for stroke‐related damage during motor learning.
Practice induces activity‐dependent adaptations within the distributed neural networks needed for skilled movement and changes in cortical representations [Karni et al., 1995, 1998]. Yet, after stroke the pattern of brain activity associated with movement of the hemiparetic upper extremity (UE) clearly differs from that seen in control subjects and in individuals with stroke moving their unaffected limb [Johansen‐Berg et al., 2002a; Marshall et al., 2000]. Despite differences in the composition of the neural network activated, it is clear that stroke does not abolish capacity for implicit motor learning [Boyd and Winstein, 2004b, 2006; Boyd et al., 2007a; Vidoni and Boyd, 2009]. The hallmark of implicit motor learning is the capacity to acquire skill through physical practice without conscious recollection of what elements of performance improved [Squire, 1987]. The maintenance of the ability to learn new implicit motor skills despite stroke has been attributed to the distributed nature of the neuroanatomic regions that support this form of learning [Poldrack and Packard, 2003; Poldrack et al., 2005; Reber et al., 1998; Squire, 1987, 1992]. These include the cerebellum, basal ganglia, primary motor cortex, supplementary motor area, premotor and prefrontal cortices [Squire, 1987]. Disruption of one portion of the neural network that supports implicit motor learning is not without penalty [Boyd et al., 2007a; Boyd and Winstein, 2003, 2004a, b], but the ability to acquire new implicit motor skills is rarely abolished by brain damage.
To date, no neuroimaging work has illustrated the neural network associated with implicit motor learning after stroke. The generalizability of past work considering patterns of brain activation during motor task performance after stroke is limited. For example, past work has commonly relied on individuals who could successfully participate in motor tasks using the hemiparetic hand or fingers and thus, excluded the majority of individuals with stroke who have poor residual function [Carey et al., 2002; Cramer and Bastings, 2000; Jueptner et al., 1995; Weiller et al., 1993]. In addition, the network of brain regions subserving motor learning engages differentially according to the level of performance. Thus, networks that are activated in association with initial performance are not necessarily those that underpin motor learning [Butefisch et al., 2003; Fridman et al., 2004; Zemke et al., 2003]. Critically, nearly all past neuroimaging work considering motor learning after stroke has employed a region of interest (ROI) approach to evaluate brain regions identified a priori [Askim et al., 2009; Carey et al., 2002; Dong et al., 2006], and thus has not elucidated the impact of stroke across brain networks. Because previous work has either (1) examined single points in time [Nelles et al., 1999; Pineiro et al., 2002; Weiller et al., 1993], (2) followed patients longitudinally without controlling behavior [Johansen‐Berg et al., 2002a; Ward et al., 2003], or (3) limited the view of the brain to a group of discrete ROIs [Carey et al., 2002; Dong et al., 2006], the direct relationship between changes in patterns of brain activation across the whole brain and implicit motor learning remain unclear. For instance, reductions in cortical activation have been noted with increasing time after stroke [Johansen‐Berg et al., 2002a; Ward et al., 2003]. However, these effects do not directly relate to motor learning per se as no specific skill was acquired, but rather describe generalized improvements in gross motor control. Although previous findings are important, they do not explain the relationship between implicit motor learning and neural activity. Therefore, the purpose of the current study was to directly address the differential recruitment of cortical areas associated with improved motor control and implicit sequence‐specific motor learning in healthy individuals as compared to those employed by individuals with stroke. By limiting our participants to individuals with focal lesions in the right subcortex, we were able to consider changes associated with implicit sequence‐specific motor learning across the whole brain rather than be limited by predefined regions of interest based upon prior hypotheses.
In this study, functional magnetic resonance images (fMRI) were collected during early practice of a continuous tracking task and after 5 days of practice during a separate retention test in the scanner. We defined motor learning as positive behavioral change (i.e., less tracking error) from initial performance at baseline (Day 1) to a delayed retention test (on Day 7) during performance of our novel motor task [Boyd et al., 2007a; Boyd and Winstein 2003, 2004a, b; Schmidt and Lee, 2005]. In our operational definition of motor learning, the focus is on change in behavior (i.e., improved tracking accuracy) from baseline regardless of initial accuracy of performance [Boyd et al., 2007a; Boyd and Winstein, 2003, 2004a, b; Schmidt and Lee, 2005; Wulf and Schmidt, 1997].
Because practice of a motor task can lead to improved performance of both generalized movements and also of movements that are specific to the task, we required participants to practice both random and repeated sequences. Behaviorally, change in random sequence performance reflects improvements in generalized motor control, whereas behavioral change during repeated sequences illustrates implicit sequence‐specific motor learning. Importantly, we included tests of explicit knowledge at the conclusion of the study to verify that explicit awareness for the repeating sequence was not gained.
For our fMRI analyses, direct comparison between patterns of brain activity for individuals with stroke with healthy controls revealed which areas showed differential compensatory activation during random sequence performance at the delayed retention test and were considered to be associated with motor control; regions showing compensatory activation during repeated sequences at the retention test were defined as being associated with implicit sequence‐specific motor learning.
We hypothesized that individuals with stroke would show higher tracking error across random and repeated sequences as compared to healthy controls. Further, we predicted that during early practice at fMRI Session 1 both individuals with stroke and healthy control participants would show activity in a frontal‐parietal sensorimotor network previously demonstrated during similar visuomotor tracking tasks [Chouinard and Goodale, 2009; Goodale and Milner, 1992; Meehan and Staines, 2009]. However, we expected that individuals with stroke would demonstrate increased cortical activity in this network as compared to healthy control participants which is indicative of compensation [Calautti et al., 2007].
We also predicted that both the healthy controls and participants with stroke would demonstrate implicit sequence‐specific motor learning, defined as less error during repeated sequence tracking as compared to random sequence tracking at the retention test without explicit awareness of the repeating sequence. Further, we hypothesized that implicit sequence‐specific motor learning observed at the retention test would be associated with increased activity in premotor cortex and decreased activity in dorsolateral prefrontal cortex in the control participants during the retention test. In contrast, we hypothesized that for individuals with stroke increases and decreases in cortical activity associated with implicit sequence‐specific learning observed at the retention test would be distributed across the sensorimotor network.
METHODS
Participants
To avoid the possibility that altered brain activation with practice reflects neural processes associated with acute physiologic recovery, only individuals with chronic stroke (at least 12 months poststroke onset [Jorgensen et al., 1995]) were studied. Nine individuals with first time, right sided, ischemic stroke (ST) confined to the subcortex (Fig. 1) and nine age‐ and sex‐matched healthy controls (HC) participated (Table I). Poststroke participants' physical impairment level was determined via the Fugl‐Meyer upper extremity motor scale [Fugl‐Meyer et al., 1975] (Table I).
Figure 1.
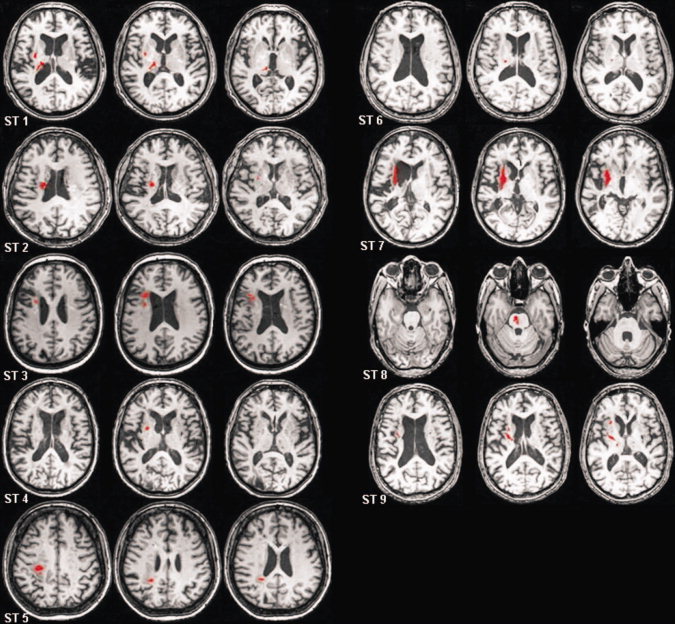
Lesion location (outlined in red) for the nine participants of the ST group.
Table I.
Participant characteristics
| Participants | Sex | Age | MMSEa | Poststroke durationb | Fugl–Meyer motor UEc | Digits backwards |
|---|---|---|---|---|---|---|
| Stroke | ||||||
| ST 1 | 65 | M | 28 | 20 | 51 | 5 |
| ST 2 | 72 | M | 29 | 169 | 32 | 2 |
| ST 3 | 59 | F | 30 | 42 | 61 | 6 |
| ST 4 | 74 | M | 29 | 65 | 36 | 8 |
| ST 5 | 55 | F | 30 | 19 | 51 | 9 |
| ST 6 | 64 | M | 30 | 29 | 66 | 6 |
| ST 7 | 65 | F | 29 | 90 | 60 | 9 |
| ST 8 | 58 | M | 30 | 17 | 66 | 10 |
| ST 9 | 63 | M | 29 | 28 | 66 | 7 |
| Healthy control | ||||||
| HC 1 | 54 | F | 29 | — | — | 6 |
| HC 2 | 64 | F | 30 | — | — | 11 |
| HC 3 | 72 | F | 30 | — | — | 7 |
| HC 4 | 67 | F | 30 | — | — | 5 |
| HC 5 | 63 | M | 30 | — | — | 12 |
| HC 6 | 60 | F | 30 | — | — | 10 |
| HC 7 | 51 | M | 30 | — | — | 5 |
| HC 8 | 68 | M | 29 | — | — | 10 |
| HC 9 | 69 | M | 29 | — | — | 11 |
MMSE, Mini‐Mental Status Exam (range 0–30).
Poststroke duration is in months.
UE, upper extremity (range for Fugl–Meyer UE motor test 0–66; lower scores denote less hemiparetic arm function).
Participants were not enrolled if they: (1) scored below the 25th percentile on the Mini‐Mental Status Exam using age adjusted norms [Crum et al., 1993], (2) were left handed [Oldfield, 1971], (3) were in the HC group and exhibited any frank or clinically evident signs of neurological impairment or disease [Lundy‐Ekman, 1998], (4) had any orthopedic condition or color blindness that would impair response ability, or (5) had any contraindications to magnetic resonance imaging (MRI). Participants were recruited from the University of British Columbia, the local community, and the Brain Behavior Lab database. Each participant's consent was obtained according to the Declaration of Helsinki; the research ethics boards at the University of British Columbia approved all aspects of this work.
Procedure
Because the neural structures that implement action change with motor skill acquisition [Doyon et al., 2003; Karni et al., 1998; Ungerleider et al., 2002], single time‐point views of brain can be misleading. Therefore, we assessed changes in brain activation at early practice and at a separate retention test using fMRI. Retention is classically defined as testing performance of a motor task following an interval of practice to assess learning [Schmidt and Lee, 2005]. For this study, we a priori identified our retention test as the second fMRI session. Based on our previous work [Boyd et al., 2007a, b, 2009; Boyd and Winstein, 2004b] participants completed six additional practice sessions on different days. To obtain brain images across motor skill acquisition, early practice (Day 1) and retention tests (Day 7) took place inside a 3‐Tesla MRI scanner. Days 2–6 consisted of task practice in the lab. The same procedures were repeated for each participant.
Behavioral Task
During the practice days (Days 2–6) participants were seated in front of a computer monitor and engaged in continuous tracking of a target moving in a sine‐cosine waveform by manipulating a non‐ferrous joystick (Current Designs, Philadelphia, PA) using their hemiparetic/left arm [Boyd and Winstein, 2004a, b; Vidoni and Boyd, 2008; Wulf and Schmidt, 1997]. The target appeared as a white circle and participant movements were represented as a red dot (Fig. 2A). Participants performed five blocks (10 sequence repetitions = 1 block) of practice on each day for a total of 250 repetitions of the random and repeated sequences 3.
Figure 2.
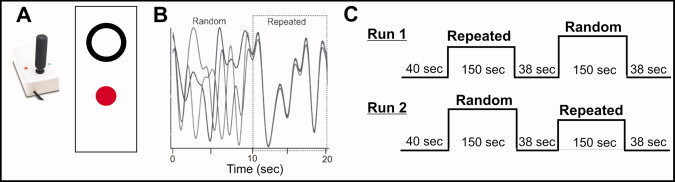
(A) Depiction of the target and participants' position representations as well as the joystick used by the participant during the tracking task (see methods for details). (B) Examples of the random and repeated tracking sequences that comprised the 20 s blocks during Days 2–6, the different shaded lines represents a separate block. (C) An example of the time course of the behavioral task during fMRI acquisition on Days 1 and 7. For the early practice and retention testing the random and repeated sequences were presented in separate 150 s blocks with the order of presentation counterbalanced across fMRI acquisition scans. [Color figure can be viewed in the online issue, which is available at wileyonlinelibrary.com.]
Figure 3.
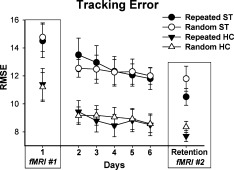
Tracking performance (average RMSE) for both the HC and ST groups during early practice (fMRI No. 1), learning (Days 2–6) and at retention (fMRI No. 2) for the random and repeated sequences. Bars indicate standard error.
Use of the joystick‐based task enabled more severe participants to be studied (i.e., fractionated/individual finger movement was not a prerequisite). In addition, use of a joystick enabled us to consider implicit motor learning of a complex, continuous task with two levels of motor tracking (random and repeated sequences). Contrasts between performance of random and repeated sequences allowed the consideration of implicit sequence‐specific motor learning (see definition in the Introduction). Joystick position sampling and all stimuli were presented at 40 Hz using custom software developed on the LabView platform (v. 7.1; National Instruments, Austin, TX).
The pattern of target movement was predefined according to a method modified from Wulf and Schmidt [Vidoni and Boyd, 2008; Wulf and Schmidt, 1997]. One block consisted of 10 and 20 s trials (Fig. 2B). Each unique 20 s trial was constructed from two 10 s sine‐cosine segments. Unknown to the participants, one segment of each tracking trial was repeated and identical across practice and retention, the other tracking segment followed a random path. The order in which the repeated and random segments appeared within each trial was random. The sine‐cosine pattern was constructed using the polynomial equation as described by Wulf and Schmidt [1997] with the following general form:
The repeated segment was constructed using the same coefficients for every trial (b o = 2.0, a 1 = −4.0, b 1 = 3.0, a 2 = −4.9, b 2 = −3.6, a 3 = 3.9, b 3 = 4.5, a 4 = 0.0, b 4 = 1.0, a 5 = −3.8, b 5 = −0.5, a 6 = 1.0, and b 6 = 2.5). The random segments of the tracking pattern were generated randomly using coefficients ranging from 5.0 to −5.0. A different random sequence was used for every trial (Fig. 2B); however, to ensure uniformity across participants the same set of trials were practiced by all of the participants so that on any given trial the random segments were the same for each participant. In each segment of the tracking pattern there were 10 separate reversals in the direction. The trajectories of the target and participants' movements did not leave a trail and thus, participants could not visualize the overall target pattern.
The tracking task was similar during fMRI acquisition (Days 1 and 7); however, the presentation of the tracking target and the organization of the blocks varied. During fMRI acquisition, the visual display was back projected (Panasonic LCD projector, model PT‐L75OU) onto an opaque screen located above the participants head and visible via a reflecting mirror located in the radio frequency head coil. Before the first fMRI session, all participants practiced one 60 s run of random sequence tracking to familiarize them with the motor task. EMG measurements from both arms were taken during the first practice session to ensure mirror movements did not take place during fMRI task performance [Cramer and Bastings, 2000]. The random and repeated target sequences were presented in a block design (40 s rest/150 s stimulation/40 s rest/150 s stimulation/40 s rest) such that each block of stimulation consisted of either random or repeated sequences with the order of presentation within a scan counterbalanced across scans (Fig. 2C).
Participants were not explicitly informed of the existence of the repeating sequence but instructed daily to track the target as accurately as possible by controlling the position of the cursor with the joystick. The same behavioral task was practiced in the fMRI (Days 1 and 7) and also across the 5 days of practice.
MRI Data Acquisition
Functional and anatomical imaging was performed at the UBC MRI Research Centre on a Philips Achieva 3.0 T whole body MRI scanner (Phillips Healthcare, Andover, MD) using a sensitivity encoding head coil (SENSE). Blood oxygenation level dependent (BOLD) images were acquired axially using echo‐planar images (EPI) with a single‐shot readout (TR = 2,000 ms, TE = 30 ms, flip angle θ = 90°, FOV = 240 mm, 36 slices, 3 mm thickness with a 1‐mm gap). Prior to acquisition of the functional data, participants underwent a high‐resolution anatomical scan (TR = 12.4 ms, TE = 5.4 ms, flip angle θ = 8°, FOV = 256 mm, 170 slices, 1 mm thickness) for later co‐registration with the functional maps. Total scan time was ∼60 min.
Explicit Awareness of the Repeated Sequence
On Day 7 following the retention test in the fMRI, participants were shown 10, 10 s blocks of continuous target movement and asked to decide if they recognized any as the repeated pattern that they practiced. Three of the 10 were “true” repeating sequences, i.e., the same as the repeated practice pattern; 7 were foils. Individuals who identified the repeated sequence at a better than chance rate, i.e., 2 of 3 repeated sequences identified correctly as being recognized and 4 of 7 novel, random epochs identified correctly as never having been seen before, were considered to have gained explicit awareness of the repeating sequence [Boyd et al., 2009; Vidoni and Boyd, 2008].
Behavioral Outcome Measures
Motor performance was evaluated during early practice (Day 1), across practice (Days 2–6), and retention (Day 7). Our analysis considered changes in root mean squared error (RMSE), which reflects overall tracking error in the kinematic pattern and is the average difference between the target pattern and participant movements. This score was calculated separately for random and repeating sequences on every tracking trial and averaged by block (every 10 trials) [Boyd et al., 2007a; Boyd and Winstein, 2004a, b].
fMRI Data Analyses
Neuroimaging data were analyzed using BrainVoyager QX 1.10 software (Brain Innovation, Masstricht, The Netherlands). First, functional data were 3D motion and slice time corrected. Estimated translation and rotation measures were visually inspected and never exceeded 4 mm and 3°, respectively. The time courses were then high‐pass filtered to remove linear trends. Spatial smoothing of the functional data was not employed.
Following preprocessing, the functional data were transformed into Talairach space [Talairach and Tournoux, 1988] through co‐registration with spatially transformed 3D anatomical data sets for each individual subject. The resulting volume time courses were then filtered using an 8‐mm Gaussian kernel at full width half maximum.
To evaluate the differences in the magnitude of the hemodynamic response across conditions, a random effects general linear model was employed. The model consisted of four predictors that corresponded to the four experimental conditions performed during separate scans: (1) random sequence tracking, Day 1, (2) repeated sequence tracking, Day 1, (3) random sequence tracking, Day 7, and (4) repeated sequence tracking, Day 7. Six additional predictors of no interest were included to account for translational and rotational motion in the x, y, and z planes. The predictors were the same for both the healthy and stroke participants.
To investigate changes in the network associated with motor skill learning, we chose a voxel‐based approach rather than an a priori approach based upon anatomically defined ROIs. We were able to take this approach due to the relatively focal, homogenous nature of the subcortical lesions in our stroke group.
Statistical Analyses
Behavioral data
Data from the first fMRI session (Day 1), motor sequence practice in the lab (Days 2–6) and the retention test during the second fMRI session (Day 7) were considered separately. RMSE representing tracking accuracy was the dependent variable for all behavioral analyses. Early changes in tracking accuracy across the two sequence types during the first fMRI session were considered using a repeated measures Group (ST, HC) by Sequence (Repeated, Random) ANOVA. Performance‐related changes were evaluated via practice data collected outside the scanner with a Group (ST, HC) by Day (2–6) by Sequence (Repeated, Random) ANOVA with a repeated measures correction. Finally, to assess implicit sequence‐specific motor learning, retention test data were evaluated with a repeated measures Group (ST, HC) by Sequence (Repeated, Random) ANOVA.
fMRI data
There were three independent variables of interest: Group (HC, ST), Sequence (Random, Repeated) and Session (Pre, Post). The omnibus Group by Sequence by Session mixed ANOVA was decomposed into two separate hypotheses‐guided mixed model ANOVAs to assess differences in the cortical networks between the groups during tracking of the random and repeated sequences during early practice and at retention testing. Separate Group (ST, HC) by Sequence (Repeated, Random) ANOVAs were performed for early practice (Day 1) and the retention test day (Day 7). Voxels were deemed significant if they exceed corrected threshold of P < 0.05. Correction was performed using a spatial cluster threshold of seven contiguous voxels (or 189 mm3 in the original acquisition space), determined using the Cluster Level Statistical Threshold Estimator (BrainVoyager Plugins, Brain Innovation, Masstricht, The Netherlands) with 1,000 Monte Carlo simulations [Forman et al., 1995]. Post‐hoc linear contrasts were used to investigate the changes in the resulting significant clusters. The Talairach coordinates of the center of gravity for each significant cluster were extracted and used to determine the Brodmann Area for each cluster.
To determine the relationship between brain activity and behaviour for implicit sequence‐specific motor learning, we computed two‐tailed spearman correlation coefficients between the percent signal change of post‐hoc selected active brain regions and RMSE scores from the retention test.
RESULTS
Behavioral
Consistent with past work [Boyd et al., 2009, 2007a] at early practice (Day 1) individuals in the ST group made more error during tracking (Main Effect of Group F(1,16) = 4.7, P = 0.046) when compared to the HC group (Fig. 3). However, during early practice the pattern of change across practice was similar for both groups regardless of sequence type (Group by Sequence interaction P = 0.316).
Across acquisition practice (Days 2–6) both groups reduced tracking error (Main Effect of Day F(1,16) = 9.2, P = 0.008); however, more improvements were made for the repeating than the random sequence (Sequence by Day interaction F(1,16) = 3.5, P = 0.038). Between group differences persisted as a result of higher tracking error by participants with stroke (Main Effect of Group F(1,16) = 8.1, P = 0.012).
Although the difference in general tracking performance between the HC and ST groups persisted at retention (Main Effect of Group, F(1,16) = 6.7, P = 0.020), both demonstrated sequence‐specific motor learning as evidenced by more accurate tracking for the repeated than the random sequence at the retention test (Main Effect of sequence (F(1,16) = 14.4, P = 0.002). Equivalent improvement in tracking error was evident for both the HC and ST groups as shown by the absence of a Group by Sequence interaction (P = 0.194).
Explicit Awareness of the Repeated Sequence
Regardless of group, participants failed to gain explicit knowledge during the recognition test. In general, participants correctly recognized sequences at a chance level: ST participants correctly identified 53.9% of sequences; HC participants accurately recognized 53.1% of the presented sequences.
fMRI Data
Although both the ST and HC groups demonstrated similar patterns of tracking error for the random versus repeated sequences during early practice, a number of areas demonstrated a differential BOLD response. Areas demonstrating a significant Group by Sequence interaction on Day 1, their center of gravity, and cluster sizes are shown in Table II. The areas demonstrating a significant interaction are grouped according to the results of the linear contrast.
Table II.
Clusters demonstrating significant group (healthy/patient) × sequence (random/repeated) interaction during early practice on Day 1
| Anatomical area | Brodmann area | X | Y | Z | Cluster size (mm3) |
|---|---|---|---|---|---|
| Contrast: HC random < HC repeated, ST random > ST repeated | |||||
| Rt. postcentral gyrus | BA 2 | 49 | −22 | 42 | 500 |
| Lt. cingulate gyrus | BA 24 | −10 | −3 | 31 | 2,110 |
| Lt. parahippocampal gyrus | BA 34 | −22 | −1 | −10 | 382 |
| Rt. Culmen | — | 10 | −25 | −20 | 222 |
| Rt. cingulate gyrus | BA 32 | 12 | 15 | 23 | 970 |
| Lt. cerebellar tonsil | — | −41 | −47 | −38 | 246 |
| Rt. inferior frontal gyrus | BA 47 | 49 | 32 | −6 | 509 |
| Rt. middle frontal gyrus | BA 9 | 45 | 15 | 38 | 302 |
| Rt. middle frontal gyrus | BA 46 | 43 | 31 | 19 | 291 |
| Contrast: HC random = HC repeated, ST random > ST repeated | |||||
| Rt. Insula | BA 13 | 45 | 5 | −2 | 304 |
| Lt. inferior parietal lobule | BA 40 | −46 | −26 | 24 | 426 |
| Contrast: HC random < HC repeated, ST random = ST repeated | |||||
| Lt. insula | BA 13 | −46 | 8 | 0 | 299 |
Clusters are grouped according to the results of the linear contrasts comparing BOLD response during random and repeated sequence tracking for the HC and ST group. For Example, HC random < HC repeated, ST random > ST repeated, indicates BOLD response was significantly greater during repeated sequence tracking compared to random sequence tracking in the HC group but that BOLD response was greater during random sequence compared to repeated sequence tracking in the ST group.
At the retention test the number of regions that showed a Group by Sequence interaction decreased, indicative of changes in the compensatory mechanisms employed by poststroke individuals after practice. However, despite the reduction in areas demonstrating differential activation, implicit sequence‐specific motor learning in the ST group still resulted in differential activation in several areas at the retention test. These areas are shown in Figure 4 (see Table III for the center of gravity and cluster sizes).
Figure 4.
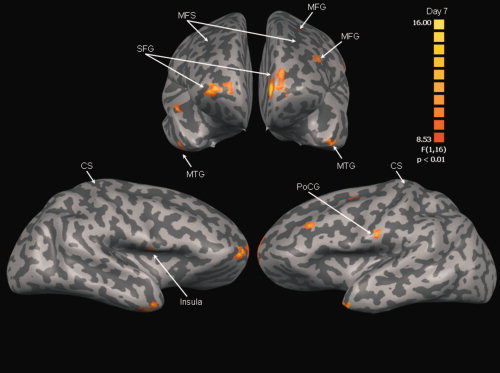
Anterior (top) and lateral (bottom) views of areas demonstrating significant Group × Sequence interaction at retention (Day 7). See Table III for interpretation. The color scale reflects t‐values. MFS, middle frontal sulcus; CS, central sulcus; MFG, middle frontal gyrus; SFG, superior frontal gyrus; MTG, middle temporal gyrus; PoCG, postcentral gyrus.
Table III.
Clusters demonstrating significant Group (healthy/patient) × sequence (random/repeated) interaction at the retention test on Day 7
| Anatomical area | Brodmann area | X | Y | Z | Cluster size (mm3) |
|---|---|---|---|---|---|
| Contrast: HC random > HC repeated, ST random = ST repeated | |||||
| Lt. middle frontal gyrus | BA 9 | −43 | 31 | 36 | 307 |
| Contrast: HC random < HC repeated, ST random = ST repeated | |||||
| Lt. middle frontal gyrus | BA 6 | −25 | −1 | 39 | 470 |
| Contrast: HC random > HC repeated, ST random < HC repeated | |||||
| Rt. Insula | BA 13 | 46 | −4 | 8 | 323 |
| Lt. postcentral gyrus | BA 1 | −64 | −17 | 27 | 295 |
| Bil. superior frontal gyrus | BA 10 | 25/–11 | 64/62 | 15/17 | 1,233/1,504 |
| Bil. middle temporal gyrus | BA 21 | 43/–49 | 0/9 | −24/–23 | 822/214 |
Clusters are grouped according to the results of the linear contrasts comparing BOLD response during random and repeated sequence tracking for the HC and ST group. For example, HC random > HC repeated, ST random = ST repeated, indicates BOLD response was significantly greater during random sequence tracking compared to repeated sequence tracking in the HC group but that there was no difference between the two tracking sequences in the ST group.
In particular, the significant interaction in the left dorsolateral prefrontal cortex (DLFPC; BA 9) can be attributed to a significant difference between the positive BOLD response observed during random sequence tracking and the negative BOLD response during repeated sequence tracking in the HC group. In contrast, there was no difference in the positive BOLD response during random and repeated sequence tracking in this area in the ST group (Fig. 5A). Interestingly, the significant interaction in the left dorsal premotor cortex (PMd; BA 6) can be attributed to a significant difference between the positive BOLD response observed during repeated sequence and the small negative BOLD response observed during random sequence tracking in the HC group but no difference between the small positive BOLD responses seen during random and repeated sequence tracking in the ST group (Fig. 5B). It appears that implicit sequence‐specific motor learning in the HC group was associated with reduced activation of DLPFC but increased PMd activation, a pattern not observed across the ST group (Table III).
Figure 5.
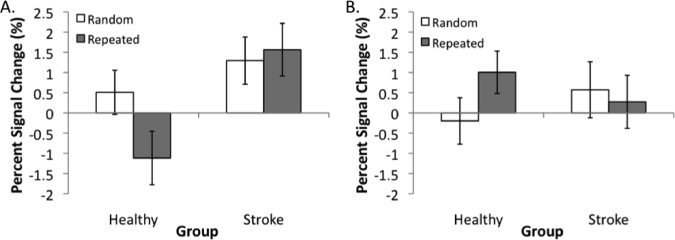
Plot of the mean percent signal change during random and repeated tracking for (A) the area of the DLPFC and (B) the area of the PMd that demonstrated significant Group × Sequence interactions at the retention test (Day 7).
Examination of the relationship between percent signal change in PMd/DLPFC and the magnitude of tracking error revealed a significant relationship (r = –0.717; P = 0.030) between PMd activity and tracking error for individuals in the ST group for repeated sequences at the retention test. Thus, individuals with stroke who showed more activity in PMd during repeated sequence tracking at retention demonstrated less tracking error. No such relationship was evident between repeated sequence tracking error and PMd activity for the HC group. There was not a relationship between DLFPC activity and tracking error for either sequence type (random or repeated) for individuals in the ST or HC group.
In addition to those areas demonstrating a significant Group by Sequence interaction, a number of frontal/prefrontal areas demonstrated a main effect of group during early practice and at retention testing. During early practice, there was a significant difference between the positive BOLD responses observed in the right DLPFC (BA 9), left DLPFC (BA 46 and BA 9, respectively) for the ST group and the negative BOLD response observed during tracking in the HC group regardless of the sequence to be tracked (see Table IV for center of gravity and cluster sizes).
Table IV.
Clusters demonstrating a significant main effect of group during early practice on Day 1 and at the retention test on Day 7
| Anatomical area | Brodmann area | X | Y | Z | Cluster size (mm3) |
|---|---|---|---|---|---|
| Day 1: HC group < ST group | |||||
| Rt. middle frontal gyrus | BA 9 | 25 | 35 | 21 | 349 |
| Lt. precentral gyrus | BA 9 | −34 | 15 | 39 | 375 |
| Lt. inferior frontal gyrus | BA 46 | −42 | 30 | 7 | 627 |
| Day 7: HC group < ST group | |||||
| Lt. middle frontal gyrus | BA 8 | −35 | 30 | 43 | 567 |
| Lt. superior frontal gyrus | BA 8 | −14 | 40 | 54 | 220 |
Clusters are separated according to direction of contrasts for each main effect.
At retention there was a superior shift of the differential activity as the ST group demonstrated a positive BOLD response in the middle frontal (BA 8) and superior frontal gyri (BA 8) (Table IV for center of gravity and cluster size) that was significantly greater than the negative BOLD response observed in the HC in these areas regardless of tracking sequence (Fig. 6). It appears that during early practice participants relied more on areas such as the DLPFC to support task performance, whereas after learning these areas shifted to superior frontal areas with learning.
Figure 6.
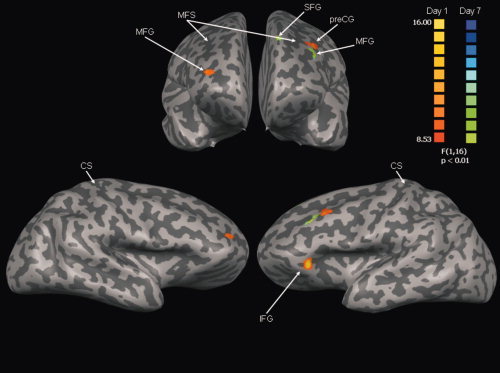
Anterior (top) and lateral (bottom) views of areas demonstrating significant main effect of Group during early practice (Day 1: red/yellow) and at retention (Day 7: blue/green). See Table IV for interpretation. The color scale reflects t‐values. MFS, middle frontal sulcus; CS, central sulcus; MFG, middle frontal gyrus; preCG, precentral gyrus; IFG, inferior frontal gyrus.
DISCUSSION
This study is the first to demonstrate differences in whole brain patterns of activation associated with implicit sequence‐specific motor learning after stroke. Using a novel voxel‐based approach, rather than limiting our analyses to predefined regions of interest, we showed that implicit sequence‐specific motor learning after stroke was associated with increased activity in a distributed sensorimotor network as compared to changes in cortical activity associated with improved motor control. In contrast, healthy control participants demonstrated decreased DLPFC and increased PMd activity with implicit sequence‐specific motor learning. During early practice, individuals with stroke appear to rely upon increased prefrontal areas involved with executive functions such as action selection and attention (BA 9, 46) to perform the tracking task regardless of sequence type (random or repeated). At retention, the gains in motor control and implicit sequence‐specific motor learning are associated with increased activation in prefrontal areas involved with working memory and visuomotor transformations (BA 8).
One of the main strengths of this study is the design that allowed us to draw distinctions between changes associated with motor performance that occur early in practice and longer‐term, stable improvements in motor behaviour that denote motor learning [Salmoni et al., 1984]. Classically defined, motor learning leads to permanent changes in motor behaviour [Schmidt and Lee, 2005]. The inclusion of a retention test on a separate day from training allowed us to assess brain networks associated with sustained improvement in motor performance and distinguish between long‐ and short‐term changes associated with practice. Further, because the neural structures that underpin motor skill change with both learning [Doyon et al., 2002; Karni et al., 1995, 1998; Ungerleider et al., 2002] and after stroke [Carey et al., 2002; Johansen‐Berg et al., 2002b], coupling the retention test with fMRI enabled the dissociation of cortical changes associated with early changes in motor performance from those related to more permanent shifts that underpin motor learning.
Past work examining motor learning after stroke has largely utilized a ROI approach and thus has never before demonstrated differences in prefrontal regions associated with motor sequence learning. With our whole brain approach, we were able to demonstrate shifts in brain activity in individuals with stroke that were directly related to learning a novel, implicit, continuous motor skill. In the neurologically intact brain, prefrontal regions that participate in the modulation of attention appeared to be particularly important in early practice [Abe et al., 2007; Debaere et al., 2004]. Recently, it was demonstrated that inhibition of the DLPFC releases activity in premotor cortex suggesting a role in the selection of action [Gangitano et al., 2008]. The increased involvement of DLPFC during early practice in healthy controls may illustrate a role for this region in establishing motor plans during motor sequence learning and indicate a greater reliance upon processing of visual and proprioceptive sensory information to initiate corrective action planning during early practice. In contrast the decreased BOLD response in the DLPFC at the retention test observed in our data suggests a reduced contribution of this region to the motor control network after implicit sequence‐specific learning occurs [Friel et al., 2005; Shmuel et al., 2002; Smith et al., 2000]. This pattern of deactivation of DLPFC in conjunction with increased activation of PMd may be associated with a transition from feedback mechanisms to feedforward memory based control [Abe et al., 2007; Debaere et al., 2004], even in the absence of explicit knowledge.
In contrast, the observation that both DLPFC and PMd show similar magnitude of BOLD response during tracking of random and repeated sequences during early practice and at retention suggests individuals with stroke either (1) rely upon a differential network due to lesion location, or (2) failed to make the transition to feedforward memory‐based control that was shown by the healthy controls. Given the observation that after stroke, there is a relationship between PMd activity and repeated sequence tracking accuracy, it appears that PMd does play a role in implicit sequence‐specific learning poststroke. However, a compensatory network was also evident after stroke that included contralesional primary somatosensory cortex (BA 1), ipsilesional insula (BA 13), and bilateral superior frontal (BA 10) and middle temporal gyri (BA 21).
Sequence‐specific motor learning is associated with increased cerebellar cortical activation during early practice that shifts to the dentate nucleus and a basal ganglia‐premotor network after skill acquisition [Doyon et al., 2003, 2002; Ungerleider et al., 2002]. Although, poststroke and healthy individuals did show differential recruitment of the cerebellar cortex depending upon the nature of the motor sequence during early implicit learning, these differences were not present at retention. However, differences in DLPFC and PMd suggest that the shift to the basal ganglia‐premotor network may have been disrupted in our sub‐cortical stroke group. After stroke, individuals failed to demonstrate the same shift in activation from DLFPC to PMd as was seen in the healthy control group. The inability to shift activity from DLPFC to PMd after stroke may be associated with altered sensorimotor processing that attenuated the formation or implementation of motor plans during early practice. This disruption may be due to changes in input/output to the PMd from sensory and motor cortices, or increased DLPFC involvement associated with an increased need to pay attention to action post‐stroke. Degraded sensorimotor processing may have resulted in increased reliance upon DLPFC and sensorimotor areas. Alternatively, increased DLPFC involvement early in learning may have disrupted implicit extraction of contextual associations [Fogelson et al., 2009]. These two explanations are not mutually exclusive and both may have operated during implicit motor learning in our group of individuals with stroke.
We did not observe any differences in basal ganglia activation between the healthy individuals and the participants with stroke for random sequence compared to repeated sequence tracking at the retention test. The null result in the basal ganglia does not imply that this region was not active, but instead reflects a common level of activation across each group and sequence type. In light of the differences observed in DLFPC, a region that provides modulatory input to the basal ganglia and PMd and a cortical target of inhibitory output from basal ganglia, we speculate that the similar level of activity in the basal ganglia in the two groups may serve different functions [Gangitano et al., 2008]. We suggest that in healthy controls basal ganglia activity during repeated sequence tracking at retention was part of a DLPFC‐basal ganglia‐PMd network that extracts motor plans for execution based upon a learned associations (i.e., contextual cues) [Fogelson et al., 2009]. In contrast, in individuals with stroke, we suggest the basal ganglia served a compensatory role to support implicit sequence‐specific motor learning by exerting influences on the task specific linkage between proprioceptive, temporal, and frontal areas [Downar et al., 2000; Meehan and Staines, 2009]. This explanation is consistent with our observations of differential activation across a distributed cortical network during repeated sequence tracking at the retention test.
To date, little work examining implicit motor learning after stroke has demonstrated the importance of prefrontal areas involved with attention and action selection for skill acquisition. From the current results, the increased BOLD response in the inferior frontal, middle frontal and precentral gyri (BA 9 and 46) suggest that during early practice individuals with stroke may have had to rely upon attentional networks to a greater extent to maintain tracking task performance as compared to the healthy individuals. Interestingly, at retention implicit sequence‐specific motor learning was associated with a shift from areas that regulate attention and action selection to areas in the middle and superior frontal gyri (BA 8) that are largely involved with working memory and visuomotor transformations.
One key element of this study is the presence of a recognition test at the retention test. This element is important as shifts from implicit to explicit memory systems result in differential recruitment of brain networks [Poldrack et al., 2001]. It is possible that this shift may have contributed to the differential effects we observed with implicit sequence‐specific motor learning, particularly if either the healthy controls or individuals with stroke became explicitly aware of the repeated sequence. We do not believe that this was a factor in the differences noted between healthy controls and participants with stroke at the retention test in this work. None of the participants in either group in the present study gained explicit knowledge of the repeating sequence; this is a common finding when continuous tracking tasks are employed [Boyd et al., 2009; Vidoni and Boyd, 2008; Wulf and Schmidt, 1997].
Past work considering motor actions after stroke during motor tasks commonly report bilateral activity in M1 in individuals with stroke [Cao et al., 1998; Cramer et al., 1997; Feydy et al., 2002; Luft et al., 2004; Weiller et al., 1993]. In contrast we did not note any difference in the magnitude of activity in M1. Several factors may account for the difference we noted in patterns of activation in our group of individuals with stroke. First, past work has not considered implicit sequence‐specific motor learning across multiple days and at a delayed retention test. For example, Carey et al. [2002] report reduced contralesional M1 activation following intensive training of a finger tracking task; however, in this work individuals tracked a randomly moving target [Carey et al., 2002]. While not insignificant, Carey et al.'s findings do not directly relate to motor learning, but rather to improvements in finger motor control. Another contributing factor to the absence of significant between group differences in M1 activation in this study likely stems from our experimental approach. We selected a whole brain analysis strategy to probe the neural network that supports implicit sequence‐specific motor learning after stroke and discovered unique activations in the prefrontal cortex that have not been previously described. Differences in findings based on whole brain, random effects analyses of group data versus individual participant ROI approaches have been previously reported [Johansen‐Berg and Matthews, 2002] and largely stem from differences in the numbers of multiple comparisons being run. It is important to note that we did find increased BOLD signal in a secondary motor area (motor cingulate in early practice on Day 1) in our group of individuals with subcortical stroke.
Several issues should be considered during the interpretation of our findings. First, we studied a relatively small (n = 9) group of individuals with stroke. Limited power and our use of a fairly conservative minimal cluster size (based on our monte carlo simulations) may have reduced our ability to detect small cortical and subcortical clusters that demonstrated changes in BOLD signal. However, our conservative management of the data does enable confidence in the regions that were identified as being significantly different between the groups. In addition, the small numbers of participants also may have limited our ability to form robust relationships between brain activity and behavioral change. Finally, we did not design the current experiment to disentangle changes associated with compensation from those that support recovery of function. In fact, our express intent was to identify the network that enables compensatory brain activity associated with implicit sequence‐specific motor learning. We were able to assemble a relatively similar group of individuals with stroke and owing to this factor able to consider compensatory changes across the whole brain that were associated with implicit sequence‐specific motor learning.
This study is the first to demonstrate the changes in the network supporting implicit sequence‐specific motor learning poststroke. To facilitate neurobiologically based rehabilitation interventions, the network underpinning activity dependent adaptations to motor learning must be understood. Despite the preservation of implicit sequence‐specific motor learning poststroke, increased prefrontal and decreased premotor cortex with learning suggests that these individuals rely upon a compensatory network of different cortical areas to support motor learning. However, it may be that this is simply an intermediary mechanism that may normalize with additional intensive practice. Future work should address the relationship between practice and changes in cortical networks post‐stroke.
REFERENCES
- Abe M, Hanakawa T, Takayama Y, Kuroki C, Ogawa S, Fukuyama H ( 2007): Functional coupling of human prefrontal and premotor areas during cognitive manipulation. J Neurosci 27: 3429–3438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Askim T, Indredavik B, Vangberg T, Haberg A ( 2009): Motor network changes associated with successful motor skill relearning after acute ischemic stroke: A longitudinal functional magnetic resonance imaging study. Neurorehabil Neural Repair 23: 295–304. [DOI] [PubMed] [Google Scholar]
- Boyd L, Winstein C ( 2006): Explicit information interferes with implicit motor learning of both continuous and discrete movement tasks after stroke. J Neurol Phys Ther 30: 46–57; discussion 58–59. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ ( 2001): Implicit motor‐sequence learning in humans following unilateral stroke: The impact of practice and explicit knowledge. Neurosci Lett 298: 65–69. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ ( 2003): Impact of explicit information on implicit motor‐sequence learning following middle cerebral artery stroke. Phys Ther 83: 976–989. [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ ( 2004a): Cerebellar stroke impairs temporal but not spatial accuracy during implicit motor learning. Neurorehabil Neural Repair 18: 134–143. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Winstein CJ ( 2004b): Providing explicit information disrupts implicit motor learning after basal ganglia stroke. Learn Mem 11: 388–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyd LA, Quaney BM, Pohl PS, Winstein CJ ( 2007a): Learning implicitly: Effects of task and severity after stroke. Neurorehabil Neural Repair 21: 444–454. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Vidoni ED, Daly JJ ( 2007b): Answering the call: The influence of neuroimaging and electrophysiological evidence on rehabilitation. Phys Ther 87: 684–703. [DOI] [PubMed] [Google Scholar]
- Boyd LA, Edwards JD, Siengsukon CS, Vidoni ED, Wessel BD, Linsdell MA ( 2009): Motor sequence chunking is impaired by basal ganglia stroke. Neurobiol Learn Mem 92: 35–44. [DOI] [PubMed] [Google Scholar]
- Butefisch CM, Netz J, Wessling M, Seitz RJ, Homberg V ( 2003): Remote changes in cortical excitability after stroke. Brain 126 ( Part 2): 470–481. [DOI] [PubMed] [Google Scholar]
- Calautti C, Leroy F, Guincestre JY, Baron JC ( 2003): Displacement of primary sensorimotor cortex activation after subcortical stroke: A longitudinal PET study with clinical correlation. Neuroimage 19: 1650–1654. [DOI] [PubMed] [Google Scholar]
- Calautti C, Naccarato M, Jones PS, Sharma N, Day DD, Carpenter AT, Bullmore ET, Warburton EA, Baron JC ( 2007): The relationship between motor deficit and hemisphere activation balance after stroke: A 3T fMRI study. Neuroimage 34: 322–331. [DOI] [PubMed] [Google Scholar]
- Cao Y, D'Olhaberriague L, Vikingstad EM, Levine SR, Welch KMA ( 1998): Pilot study of functional MRI to assess cerebral activation of motor function after poststroke hemiparesis. Stroke 29: 112–122. [DOI] [PubMed] [Google Scholar]
- Carey JR, Kimberley TJ, Lewis SM, Auerbach EJ, Dorsey L, Rundquist P, Ugurbil K ( 2002): Analysis of fMRI and finger tracking training in subjects with chronic stroke. Brain 125: 773–788. [DOI] [PubMed] [Google Scholar]
- Chouinard PA, Goodale MA ( 2009): FMRI adaptation during performance of learned arbitrary visuomotor conditional associations. Neuroimage 48: 696–706. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Bastings EP ( 2000): Mapping clinically relevant plasticity after stroke. Neuropharmacology 39: 842–851. [DOI] [PubMed] [Google Scholar]
- Cramer SC, Nelles G, Benson RR, Kaplan JD, Parker RA, Kwong KK, Kennedy DN, Finklestein SP, Rosen BR ( 1997): A functional MRI study of subjects recovered from hemiparetic stroke. Stroke 28: 2518–2527. [DOI] [PubMed] [Google Scholar]
- Crum RM, Anthony JC, Bassett SS, Folstein MF ( 1993): Population‐based norms for the Mini‐Mental State Examination by age and educational level. Jama 269: 2386–2391. [PubMed] [Google Scholar]
- Debaere F, Wenderoth N, Sunaert S, Van Hecke P, Swinnen SP ( 2004): Changes in brain activation during the acquisition of a new bimanual coodination task. Neuropsychologia 42: 855–867. [DOI] [PubMed] [Google Scholar]
- Dong Y, Dobkin BH, Cen SY, Wu AD, Winstein CJ ( 2006): Motor cortex activation during treatment may predict therapeutic gains in paretic hand function after stroke. Stroke 37: 1552–1555. [DOI] [PubMed] [Google Scholar]
- Downar J, Crawley AP, Mikulis DJ, Davis KD ( 2000): A multimodal cortical network for the detection of changes in the sensory environment. Nat Neurosci 3: 277–283. [DOI] [PubMed] [Google Scholar]
- Doyon J, Song AW, Karni A, Lalonde F, Adams MM, Ungerleider LG ( 2002): Experience‐dependent changes in cerebellar contributions to motor sequence learning. Proc Natl Acad Sci USA 99: 1017–1022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doyon J, Penhune V, Ungerleider LG ( 2003): Distinct contribution of the cortico‐striatal and cortico‐cerebellar systems to motor skill learning. Neuropsychologia 41: 252–262. [DOI] [PubMed] [Google Scholar]
- Feydy A, Carlier R, Roby‐Brami A, Bussel B, Cazalis F, Pierot L, Burnod Y, Maier MA ( 2002): Longitudinal study of motor recovery after stroke—Recruitment and focusing of brain activation. Stroke 33: 1610–1617. [DOI] [PubMed] [Google Scholar]
- Fogelson N, Shah M, Scabini D, Knight RT ( 2009): Prefrontal cortex is critical for contextual processing: Evidence from brain lesions. Brain 132( Part 11): 3002–3010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Forman SD, Cohen JD, Fitzgerald M, Eddy WF, Mintun MA, Noll DC ( 1995): Improved assessment of significant activation in functional magnetic resonance imaging (fMRI): Use of a cluster‐size threshold. Magn Reson Med 33: 636–647. [DOI] [PubMed] [Google Scholar]
- Fridman EA, Hanakawa T, Chung M, Hummel F, Leiguarda RC, Cohen LG ( 2004): Reorganization of the human ipsilesional premotor cortex after stroke. Brain 127( Part 4): 747–758. [DOI] [PubMed] [Google Scholar]
- Friel KM, Barbay S, Frost SB, Plautz EJ, Hutchinson DM, Stowe AM, Dancause N, Zoubina EV, Quaney BM, Nudo RJ ( 2005): Dissociation of sensorimotor deficits after rostral versus caudal lesions in the primary motor cortex hand representation. J Neurophysiol 94: 1312–1324. [DOI] [PubMed] [Google Scholar]
- Fugl‐Meyer AR JL, Leyman I, Olsson S, Steglind S ( 1975): The post‐stroke hemiplegic patient: A method for evaluation of physical performance. Scand J Rehabil 7: 13–31. [PubMed] [Google Scholar]
- Gangitano M, Mottaghy FM, Pascual‐Leone A ( 2008): Release of premotor activity after repetitive transcranial magnetic stimulation of prefrontal cortex. Soc Neurosci 3: 289–302. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD ( 1992): Separate visual pathways for perception and action. Trends Neurosci 15: 20–25. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Matthews PM ( 2002): Attention to movement modulates activity in sensori‐motor areas, including primary motor cortex. Exp Brain Res 142: 13–24. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Dawes H, Guy C, Smith SM, Wade DT, Matthews PM ( 2002a): Correlation between motor improvements and altered fMRI activity after rehabilitative therapy. Brain 125: 2731–2742. [DOI] [PubMed] [Google Scholar]
- Johansen‐Berg H, Rushworth MFS, Bogdanovic MD, Kischka U, Wimalaratna S, Matthews PM ( 2002b): The role of ipsilateral premotor cortex in hand movement after stroke. Proc Natl Acad Sci USA 99: 14518–14523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorgensen HS, Nakayama H, Raaschou HO, Vivelarsen J, Stoier M, Olsen TS ( 1995): Outcome and time‐course of recovery in stroke. II. Time‐course of recovery—the Copenhagen Stroke Study. Arch Phys Med Rehabil 76: 406–412. [DOI] [PubMed] [Google Scholar]
- Jueptner IH, Rijntes M, Weiller C, Faiss JH, Timmann D, Mueller SP, Diener HC ( 1995): Localization of a cerebellar timing process using PET. Neurology 45: 1540–1545. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Jezzard P, Adams MM, Turner R, Ungerleider LG ( 1995): Functional MRI evidence for adult motor cortex plasticity during motor skill learning. Nature 377: 155–158. [DOI] [PubMed] [Google Scholar]
- Karni A, Meyer G, Rey‐Hipolito C, Jezzard P, Adams MM, Turner R, Ungerleider LG ( 1998): The acquisition of skilled motor performance: Fast and slow experience‐driven changes in primary motor cortex. Proc Natl Acad Sci USA 95: 861–868. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Luft AR, Waller S, Forrester L, Smith GV, Whitall J, Macko RF, Schulz JB, Hanley DF ( 2004): Lesion location alters brain activation in chronically impaired stroke survivors. Neuroimage 21: 924–935. [DOI] [PubMed] [Google Scholar]
- Lundy‐Ekman L, editor ( 1998): Neuroscience: Fundamentals for Rehabilitation. Philadelphia: WB Saunders; pp 198–199. [Google Scholar]
- Marshall RS, Perera GM, Lazar RM, Krakauer JW, Constantine RC, Delapaz RL ( 2000): Evolution of cortical activation during recovery from corticospinal tract infarction. Stroke 31: 656–661. [DOI] [PubMed] [Google Scholar]
- Meehan SK, Staines WR ( 2009): Task‐relevance and temporal synchrony between tactile and visual stimuli modulates cortical activity and motor performance during sensory‐guided movement. Hum Brain Mapp 30: 484–496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelles G, Spiekermann G, Jueptner M, Leonhardt G, Muller S, Gerhard H, Diener HC ( 1999): Reorganization of sensory and motor systems in hemiplegic stroke patients—A positron emission tomography study. Stroke 30: 1510–1516. [DOI] [PubMed] [Google Scholar]
- Nudo RJ, Milliken GW, Jenkins WM, Merzenich MM ( 1996): Use‐dependent alterations of movement representations in primary motor cortex of adult squirrel monkeys. J Neurosci 16: 785–807. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oldfield RC ( 1971): The assessment and analysis of handedness: The Edinburgh Inventory. Neuropsychologia 9: 97–113. [DOI] [PubMed] [Google Scholar]
- Pineiro R, Pendlebury S, Johansen‐Berg H, Matthews PM ( 2002): Altered hemodynamic responses in patients after subcortical stroke measured by functional MRI. Stroke 33: 103–109. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Packard MG ( 2003): Competition among multiple memory systems: Converging evidence from animal and human brain studies. Neuropsychologia 41: 245–251. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Clark J, Pare‐Blagoev EJ, Shohamy D, Moyano JC, Myers C, Gluck MA ( 2001): Interactive memory systems in the human brain. Nature 414: 546–550. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Sabb FW, Foerde K, Tom SM, Asarnow RF, Bookheimer SY, Knowlton BJ ( 2005): The neural correlates of motor skill automaticity. J Neurosci 25: 5356–5364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reber PJ, Stark CE, Squire LR ( 1998): Cortical areas supporting category learning identified using functional MRI. Proc Natl Acad Sci USA 95: 747–750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salmoni AW, Schmidt RA, Walter CB ( 1984): Knowledge of results and motor learning: A review and critical reappraisal. Psychol Bull 95: 355–386. [PubMed] [Google Scholar]
- Schmidt RA, Lee TD ( 2005): Motor Control and Learning: A Behavioral Emphasis. Champaign: Human Kinetics. [Google Scholar]
- Shmuel A, Yacoub E, Pfeuffer J, Van de Moortele PF, Adriany G, Hu X, Ugurbil K ( 2002): Sustained negative BOLD, blood flow and oxygen consumption response and its coupling to the positive response in the human brain. Neuron 36: 1195–1210. [DOI] [PubMed] [Google Scholar]
- Smith AT, Singh KD, Greenlee MW ( 2000): Attentional suppression of activity in the human visual cortex. Neuroreport 11: 271–277. [DOI] [PubMed] [Google Scholar]
- Squire LR ( 1987): Memory and Brain. New York: Oxford University Press. [Google Scholar]
- Squire LR ( 1992): Declarative and nondeclarative memory: Multiple brain systems supporting learning and memory. J Cogn Neurosci 4: 223–243. [DOI] [PubMed] [Google Scholar]
- Talairach J, Tournoux P ( 1988): Co‐Planar Stereotaxic Atlas of the Human Brain. New York: Thieme. [Google Scholar]
- Ungerleider LG, Doyon J, Karni A ( 2002): Imaging brain plasticity during motor skill learning. Neurobiol Learn Mem 78: 553–564. [DOI] [PubMed] [Google Scholar]
- Vidoni ED, Boyd LA ( 2008): Motor sequence learning occurs despite disrupted visual and proprioceptive feedback. Behav Brain Funct 4: 32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vidoni ED, Boyd LA ( 2009): Preserved motor learning after stroke is related to the degree of proprioceptive deficit. Behav Brain Funct 5: 36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ward NS, Brown MM, Thompson AJ, Frackowiak RSJ ( 2003): Neural correlates of motor recovery after stroke: a longitudinal fMRI study. Brain 126: 2476–2496. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiller C, Ramsay SC, Wise RJS, Friston KJ, Frackowiak RSJ ( 1993): Individual patterns of functional reorganization in the human cerebral‐cortex after capsular infarction. Ann Neurol 33: 181–189. [DOI] [PubMed] [Google Scholar]
- Wulf G, Schmidt RA ( 1997): Variability of practice and implicit motor learning. J Exp Psychol: Learn Mem Cognit 23: 987–1006. [Google Scholar]
- Zemke AC, Heagerty PJ, Lee C, Cramer SC ( 2003): Motor cortex organization after stroke is related to side of stroke and level of recovery. Stroke 34: e23–e28. [DOI] [PubMed] [Google Scholar]


