Abstract
Objectives
Auditory cortical N100s were examined in ten auditory neuropathy (AN) subjects as objective measures of impaired hearing.
Methods
Latency and amplitudes of N100 in AN to increases of frequency (4–50%) or intensity (4–8 dB) of low (250 Hz) or high (4000 Hz) frequency tones were compared with results from normal-hearing controls. The sites of auditory nerve dysfunction were pre-synaptic (n=3) due to otoferlin mutations causing temperature sensitive deafness, post-synaptic (n=4) affecting other cranial and/or peripheral neuropathies, and undefined (n=3).
Results
AN consistently had N100s only to the largest changes of frequency or intensity whereas controls consistently had N100s to all but the smallest frequency and intensity changes. N100 latency in AN was significantly delayed compared to controls, more so for 250 than for 4000 Hz and more so for changes of intensity compared to frequency. N100 amplitudes to frequency change were significantly reduced in ANs compared to controls, except for pre-synaptic AN in whom amplitudes were greater than controls. N100 latency to frequency change of 250 but not of 4000 Hz was significantly related to speech perception scores.
Conclusions
As a group, AN subjects’ N100 potentials were abnormally delayed and smaller, particularly for low frequency. The extent of these abnormalities differed between pre- and post-synaptic forms of the disorder.
Significance
Abnormalities of auditory cortical N100 in AN reflect disorders of both temporal processing (low frequency) and neural adaptation (high frequency). Auditory N100 latency to the low frequency provides an objective measure of the degree of impaired speech perception in AN.
Keywords: frequency change, low and high frequency tones, intensity change, otoferlin, temperature sensitive deafness, evoked potentials, speech perception, adaptation, temporal processing
INTRODUCTION
Auditory neuropathy (AN) is a hearing disorder affecting the encoding and processing of auditory temporal cues that are essential for understanding speech (Rance, et al., 2008, Zeng, et al., 2005, Zeng and Liu, 2006). The temporal processing deficits can be quantified by abnormal performance on psychoacoustic tasks such as thresholds for detecting brief silent intervals in continuous sounds (gap detection), and thresholds for detecting rapid changes of sound intensity (temporal modulation transfer functions; Zeng et al., 2005). The diagnosis of AN is based on objective physiological tests (Starr et al., 1996) showing normal function of cochlear hair cells (otoacoustic emissions - OAEs; and/or cochlear microphonics - CMs), but abnormal function of auditory nerve and auditory brainstem pathways (auditory brainstem responses - ABRs; Berlin, et al., 2003, Rance, 2005, Starr, et al., 1996). The physiological bases for auditory perceptual disorders accompanying AN are thought to reflect both altered neural synchrony and reduced magnitude of auditory nerve responses (Starr, et al., 2008). Some of the sites of dysfunction causing AN are (1) pre-synaptic, affecting neurotransmitter release accompanying otoferlin (OTOF) mutations (Marlin, et al., 2010, Rodriguez-Ballesteros, et al., 2008, Santarelli, et al., 2009, Varga, et al., 2006, Yasunaga, et al., 2000); (2) post-synaptic, affecting initiation of synchronous activity in nerve terminals, accompanying OPA1 mutations due to altered mitochondrial functions (Carelli, et al., 2004, Huang, et al., 2009); (3) post-synaptic affecting conduction along auditory nerves accompanying mutations causing hereditary motor and sensory neuropathies (e.g., Charcot-Marie-Tooth disease, Butinar, et al., 1999; Friedrich’s Ataxia Rance, et al., 2008). In all subtypes of AN, the perceptual consequences of AN are similar but may vary in severity across individuals (Michalewski et al., 2009). Temporal bones from patients with AN show both loss and demyelination of auditory and vestibular nerve fibers and preserved cochlear inner and outer hair cells, findings that correspond to the physiological findings (Bahmad, et al., 2007, Hallpike, et al., 1980, Spoendlin, 1974, Starr, et al., 2003).
Despite the absence of auditory brainstem responses (ABRs), auditory N100 cortical potentials can still be recorded in AN subjects and show promise as an objective measure of auditory perceptual deficits such as threshold for gap detection (Michalewski, et al., 2005) and speech perception (Kraus, et al., 2000, Kumar and Jayaram, 2005, Michalewski, et al., 2009, Narne and Vanaja, 2008, Rance, et al., 2002). The study of Michalewski, et al. (2009) showed that N100 latency to 1000 Hz brief tone bursts was significantly correlated with both speech perception scores and thresholds for gap detection (Michalewski, et al., 2009). In the study to be presented below we test whether N100 measures of auditory cortical potentials to changes of ongoing tones, (“acoustic change potentials” or “C-complexes”; Jones and Perez, 2002, Martin and Boothroyd, 2000) were also related to psychoacoustic deficits in AN since acoustic changes of pitch and or intensity are common during natural speech. We chose changes of frequency or intensity of continuous low (250 Hz) or high ( 4000 Hz) tones (Dimitrijevic, et al., 2009, Dimitrijevic, et al., 2008) since AN subjects’ thresholds for detecting frequency change are abnormally elevated for low but are normal for high frequencies (Zeng et al., 2005). Low frequencies are encoded by temporal phase-locked neural auditory nerve and brainstem responses to each period in the stimulus waveforms (Rose, et al., 1967) whereas high frequencies are coded primarily by the locus of activation along the basilar membrane. Temporal coding is abnormal in AN (Starr et al, 2008) whereas place coding is normal (Abdala, et al., 2000, Vinay and Moore, 2007).
We hypothesized that measures of auditory cortical N100 potentials in AN subjects are: (1) correlated with psychoacoustic measures of auditory temporal processes (e.g., speech comprehension and threshold for gaps); (2) abnormal to changes of 250 Hz but not of 4000 Hz; (3) different in pre- and post-synaptic forms of AN.
METHODS
Subjects
Controls
Twenty (8 males, 12 females) subjects (mean age: 21 years, all self-reported right-handed) with normal pure tone thresholds (500 to 8000 Hz) and no history of neurological illnesses participated in the frequency change experiment. A separate group of twelve (6 males, 6 females) subjects (mean age: 24, 11 self-reported “right-handed”) participated as controls in the intensity change study. All subjects gave their informed consent prior to testing. The normative data for controls for frequency and intensity change were previously published (Dimitrijevic et al., 2008; Dimitrijevic et al., 2009).
Auditory Neuropathy
Ten AN subjects were studied (see Table 1). The diagnosis was based on physiological criteria for AN (see Starr et al., 1996) of (1) absent (seven subjects) or abnormal (three subjects) ABRs beyond what is expected for the degree of hearing loss; and (2) preserved activities of outer hair cell function (cochlear microphonics and/or otoacoustic emissions). Table 1 contains details of their demographics: audiological tests (pure tone hearing thresholds, gap detection threshold, speech perception), clinical findings (gene mutations, presence of other cranial or peripheral nerve disorders), used to classify the site of auditory nerve dysfunction as being pre-synaptic (presence of mutation of OTOF), post-synaptic (presence of abnormal cranial and/or peripheral nerve functions, or undefined (absence of relevant gene mutations and normal neurological examinations). We tested the “better” ear except in subjects S4 and S9 who had a unilateral cochlear implant in the untested ear.
Table 1.
Summary description of all AN subjects.
| AN# | Age | Ear tested |
ABR | Pure tone thresholds (dB HL) | Speech (%) |
Gap detection (ms) |
Other clinical | Site of lesion | ||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 250 | 500 | 1000 | 2000 | 4000 | 6000 | |||||||||
| S1 | 30 | 23 | R | 7.0 | 15 | 15 | 0 | 5 | −5 | 5 | 98 | 6 | OTOF | Pre-synaptic |
| S2 | 32 | 17 | L | 7.4 | 30 | 30 | 20 | 25 | 15 | 25 | 100 | 12 | OTOF | Pre-synaptic |
| S3 | 33 | 13 | L | 7.9 | 25 | 15 | 10 | 10 | 0 | 20 | 88 | 11 | OTOF | Pre-synaptic |
| S4* | 38 | 19 | L | NR | 65 | 70 | 25 | 25 | 30 | 35 | 10 | 10 | Optic NP | Post-synaptic |
| S5 | 36 | 55 | R | NR | 60 | 60 | 20 | 25 | 25 | 35 | 36 | 10 | Vestibular NP | Post-synaptic |
| S6 | 40 | 13 | R | NR | 70 | 70 | 45 | 35 | 35 | 35 | 18 | 11 | Optic NP | Post-synaptic |
| S7* | 13 | 35 | L | NR | 60 | 80 | 40 | 25 | 10 | 10 | 50 | 11 | Peripheral NP | Post-synaptic |
| S8 | 10 | 28 | L | NR | 65 | 60 | 30 | 20 | 10 | 5 | 70 | 5 | undefined | |
| S9 | 7 | 37 | R | NR | 65 | 65 | 50 | 10 | 30 | 25 | 64 | 8 | undefined | |
| S10 | 27 | 19 | L | NR | 10 | 15 | 20 | 80 | 80 | 90 | 80 | 14 | undefined | |
| mean | 47 | 48 | 26 | 26 | 23 | 29 | 61 | 10 | ||||||
=cochlear implant (non-test ear); OTOF= hetereozygous otoferlin mutation; NP=neuropathy. NR=no response. The second column refers to AN subject numbers that were used in previously published manuscripts by our group (see Michalewski et al., 2009).
The three pre-synaptic subjects (S1–3) had a mutation of OTOF (Varga, et al., 2006), expressed as a temperature sensitive transient deafness with loss of both ABR and ability to understand speech (Starr, et al., 1998). Two of the three pre-synaptic subjects (S2 and S3) are siblings. The recognition of hearing impairment in pre-synaptic subjects was delayed until speech was developing around 3 years of age when “hearing was lost” during febrile episodes. Other subjects developed hearing complaints as teenagers or adults. The three OTOF subjects were tested when afebrile and showed a delayed Wave V of the ABR without earlier components, normal OAEs, normal or mildly elevated pure tone audiograms, elevated gap detection thresholds, and normal speech perception (88–100%).
The four post-synaptic subjects (S4–7) had absent ABRs, normal OAEs, mild to moderate elevated pure tone audiograms, abnormal gap thresholds, and severely abnormal speech perception (10–50%). All had neuropathies of other cranial and/or peripheral nerves. The three remaining subjects (S8–10) did not have evidence of other cranial or peripheral nerve involvement and were classified as “undefined”. They had absent ABRs, normal OAEs, moderate to severely elevated pure tone audiograms, abnormal gap thresholds, and severely abnormal speech perception (64–80%). The site of the auditory nerve dysfunction in this group is unknown.
Stimuli
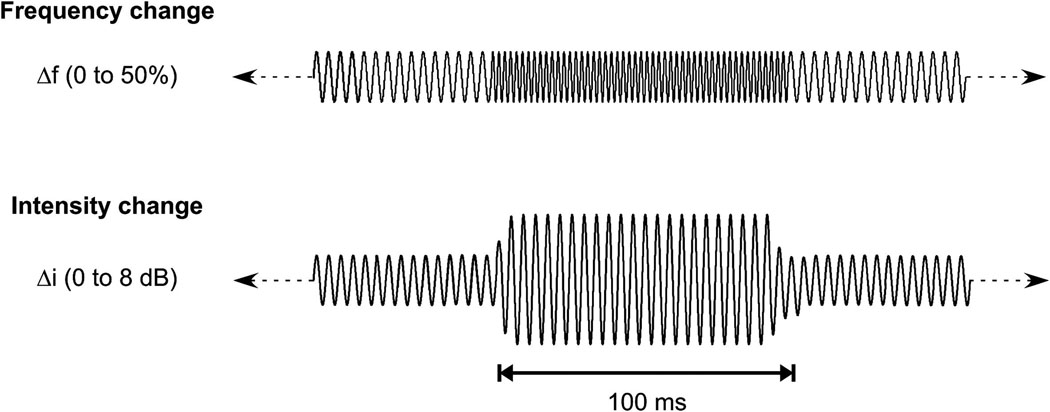
Details of the stimuli have been previously described (Dimitrijevic et al., 2008; Dimitrijevic et al., 2009) for frequency and intensity change respectively and are briefly described here. The ongoing stimuli were tones whose baseline frequency was either 250 or 4000 Hz presented at 80 dB SPL This intensity was 15 dB above threshold or greater. Instantaneous rapid frequency increases and then decreases back to baseline frequency were 0, 2, 4, 10, 25, and 50% above the baseline frequency, and occurred in a random sequence every 1.4 seconds. The change lasted for approximately 100 ms and had an integer number of cycles to minimize the audibility of transients. The intensity change stimulus was presented at the same baseline intensity and frequencies. Random changes in intensity occurred every 1.4 seconds, lasted 100 ms and were windowed using a 5 ms Blackman window. Intensity changes were of 0, 2, 4, 6 and 8 dB. A schematic illustration of the stimuli used is shown in Figure 1. Some of the AN subjects described that during testing the continuous tone would “disappear” or become “muffled” while the changes were still perceived as “beeps”.
Figure 1.
Schematic of continuous tonal stimuli. The first row illustrates the frequency change stimulus (only one change shown) and the second row shows intensity change stimulus (only one change shown).
Recordings
A 64-channel Neuroscan Synamps2 recording system was used to collect electrophysiological data. Electrode placements included the standard 10–20 locations and at intermediate sites. Impedances were kept below 10 kΩ. Lateral and vertical eye movements were monitored using two bipolar electrodes above and below the right eye and two bipolar electrodes on the left and right outer canthi for defining the electro-oculogram (EOG). Signals were digitized at 1000 Hz, amplified by a factor of 2010, and band-pass filtered (cutoffs at 0.05 and 200 Hz). Epochs were extracted using a −200 to 1900 ms window relative to the start time of the frequency/intensity increase. Offline analysis included re-referencing the recordings to an average reference (excluding the EOGs). Eye movement effects on scalp potentials were removed offline in the continuous recording in each subject using a singular value decomposition-based spatial filter utilizing principal component analysis of averaged eye blinks for each subject (Ille, et al., 2002).
Procedure
Both the controls and AN subjects were recorded while seated in a comfortable reclining chair and watching a silent close-captioned movie of their choice. Stimuli were presented monaurally to either the left or right ear and the associated electroencephalogram was recorded. Each stimulus condition (i.e., 100 trials for each frequency or intensity change) lasted approximately 15 minutes. All subjects could hear the 80 dB SPL continuous tones which were at least 15 dB SL. Ongoing EEG was continuously monitored for evidence of sleep or drowsiness and if these occurred, subjects were roused before continuing.
In our previous studies (Dimitrijevic et al., 2008, 2009), we found that in normal-hearing subjects, potentials accompanying frequency or intensity changes consisted of an N100 followed by a slow negativity lasting up to 600 ms whereas in ANs, the slow negativity and the P200 were not consistently identified. We therefore chose to measure only the N100 component.
The N100 was defined as the most negative peak in the time window of 80 to 180 ms at electrode FCz. Confirmation that the chosen peak was N100 was verified by topography with maximum amplitudes at fronto-central sites and polarity inversion (i.e., positive peak) at posterior electrodes. If amplitudes were approximate to those during the pre-stimulus baseline we tested whether the selected peak was “noise” using the method developed by Schimmel (1967); half of the individual trials comprising the average were randomly selected and then subtracted from an average of the other half of the trials. This procedure creates a residual noise waveform which was then used in for comparison with the averaged waveform of all trials. Peaks occurring at the same latency as N100 in the residual noise and in the averaged evoked potential waveforms were considered “noise” and not included in the analysis. Using this method we identified that one of the AN subjects’ “N100” potentials was residual noise.
Psychophysical data: Thresholds for gap detection and speech perception were measured using previously published methods (Zeng, et al., 2005, Zeng and Liu, 2006). Briefly, gap detection was performed in a 3-interval, 3-alternative forced-choice (2-down, 1-up) adaptive procedure to quantify performance (70.7% correct response). Broadband noise bursts lasting 500 ms were presented monaurally at a most comfortable loudness level. The subject identified which of the three stimulus segment contained a discontinuous sound (i.e., “gap”). Speech recognition was measured by BKB sentence materials (Bench, et al., 1979) presented through a speaker at the most comfortable loudness level. A total of 10 sentences were used for each condition and each sentence contained 3–5 key words. Percent correct scores were calculated based on the number of words correctly identified.
Statistical Analysis
Repeated measures analysis of variance procedures (ANOVA) were used to examine the N100 data. Post-hoc comparisons were made using Tukey Honestly Significant Difference test. All control data are presented as their 95% confidence range. These were calculated using our previously published normative data Dimitrijevic et al. (2008 and 2009). Fisher’s exact test statistic was used to compare incidence of detecting N100 (Zar, 1984, p.390–397).
Repeated measures ANOVAs were used to compare the AN data to controls whenever possible. However, when ANOVA was precluded by missing data, differences between controls and AN were examined using Student’s t-tests. Additionally, the data from individual AN subjects were compared to the 95% confidence limits of normal controls.
Correlations were computed between N100 latencies/amplitudes to frequency change and psychophysical measures (word recognition, and gap detection thresholds) in AN subjects. Correlations were only computed when an N100 was clearly present. In those subjects who did not have a N100 we designated them as having a missing value rather than assigning a value of “0”. The latter does not discriminate N100 absence as being due to methodological limitations or to brain unresponsiveness. No correlation analyses for intensity changes were performed as the number of subjects with N100 was less than five and was deemed too small for a reliable conclusion.
RESULTS
Frequency changes in AN: Detection of N100
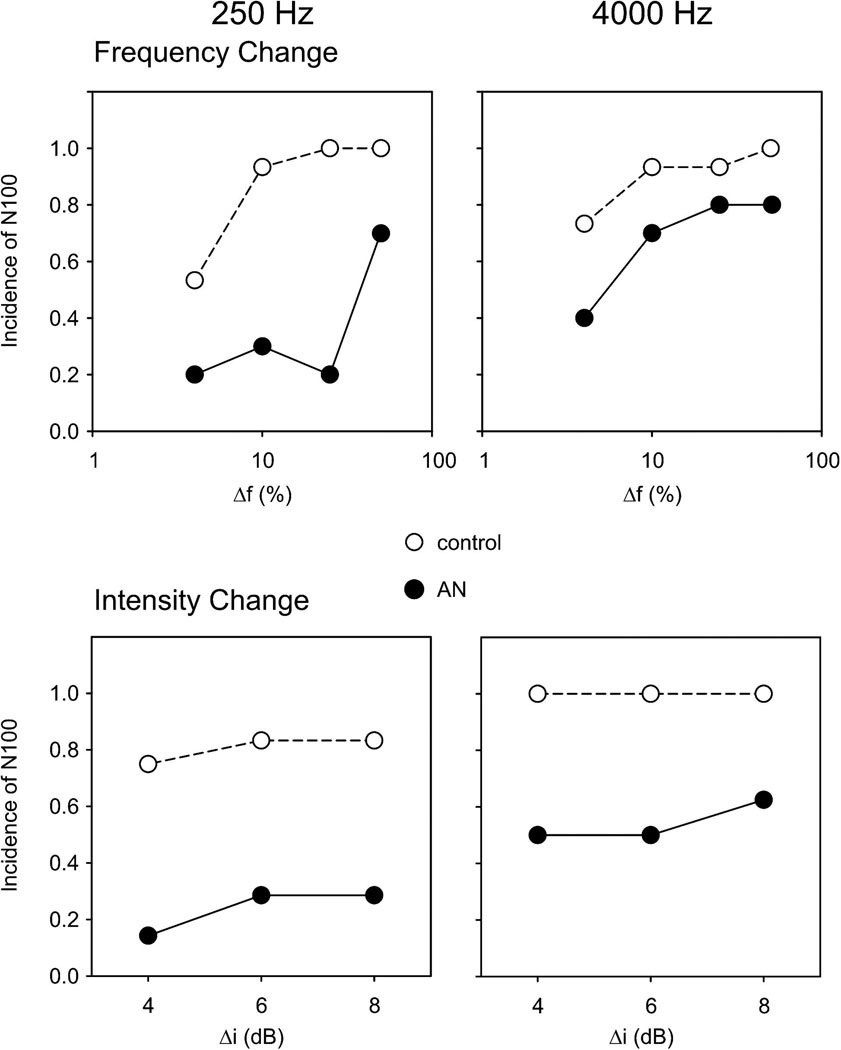
N100 to changes in frequency of both 250 and 4000 Hz were identified consistently (>90%) in controls to all (50, 25, 10%) but the smallest changes (4%). In contrast, N100 potentials in AN subjects were identified at high rates (70–80%) only for 50% frequency changes for both 250 and 4000 Hz and at significantly reduced incidence to 250 Hz for both 25% (25%; p=0.045) and 10% (30%; p=0.032) frequency changes (top half Figure 2; also in Table 2 in the column “detection” ). For 4000 Hz, the only detection rate that differed between controls and ANs was for 10% change. The frequency change that consistently yielded the highest incidence of N100 was 50% for both AN and controls. Therefore, the remainder of the analysis was on 50% changes.
Figure 2.
Proportion of subjects that had an N100 response (at FCz). Left column indicates 250 Hz and the right column shows 4000 Hz. Frequency change (logarithmic scale) is in the top row while intensity change is in the bottom row. Controls and AN subjects are shown with the closed and open symbols, respectively.
Table 2.
Frequency change N100 to 250 and 4000 Hz showing detection rate, mean and standard deviation (SD) latency (ms) and amplitude (µV). Not all AN subjects were tested with a 25 and 4% condition. Significance of t-tests and Fisher’s exact test comparing AN and controls are indicated by *.
| N100 | |||||||
|---|---|---|---|---|---|---|---|
| 250 Hz | 4000 Hz | ||||||
| condition | subjects | detection | ms | µV | detection | ms | µV |
| 50% | Controls | 15/15 | 105 (8) | −4.4 (1.6) | 15/15 | 101 (8) | −3.2 (1.3) |
| AN | 7/10 | 135 (29)** | −2.8 (1.7)* | 8/10 | 115 (13)** | −3.8 (1.6) | |
| 25% | Controls | 15/15 | 115 (12) | −3.9 (1.5) | 14/15 | 108(11) | −3.5 (1.7) |
| AN | 1/5** | 120 | −5.4 | 4/5 | 132 (29)* | −3.3 (0.9) | |
| 10% | Controls | 14/15 | 134 (20) | −3.2 (1.0) | 14/15 | 109 (11) | −3.0 (1.2) |
| AN | 3/10** | 154 (11) | −1.0 (1.7)* | 5/10* | 128 (19)* | −1.7 (0.9) | |
| 4% | Controls | 9/15 | 165(18) | −2.6 (0.7) | 11/15 | 112(9) | −2.8 (1.7) |
| AN | 1/5 | 165 | −2.2 | 2/5 | 133 (16) | −1.5 (0.2) | |
p<0.05;
p<0.01;
p<0.001
Frequency changes in AN: N100 measures
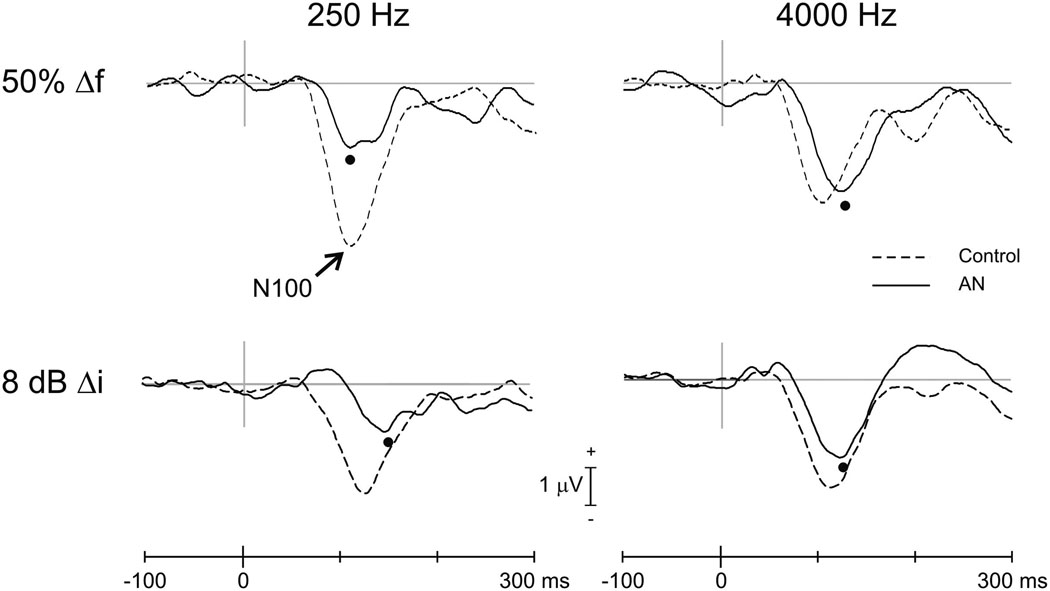
N100 latencies to 50% frequency change were delayed in AN compared to controls for both 250 and 4000 Hz (Table 2). Figure 3 (top) contains the grand averaged waveforms for AN and controls to 50% frequency changes showing AN N100 peak delays (black circle) both for 250 Hz (AN=135 ms; controls=105 ms; [t(20)=4.0; p<0.001]) and 4000 Hz, (AN=115 ms; controls = 101 ms; [t(21)=3.1; p=0.005]). N100 amplitudes were significantly smaller in AN compared to controls to 250 Hz (AN=−2.8 µV; controls=−4.4 µV; [t(20)=2.13;p=0.045]) but not to 4000 Hz (AN=−3.8 µV; controls=−3.2 µV; [t(21)=0.95;p=0.354).
Figure 3.
Grand averaged evoked potentials (FCz electrode) for frequency and intensity change. The top row shows the control (interrupted line) and AN (continuous line) potentials for 50% frequency changes (Δf). The bottom row shows waveforms for 8 dB intensity changes (Δi). The left and right columns contain averaged potentials for changes of 250 and 4000 Hz, respectively. N100 peaks for AN are indicated by the filled circles. In the case of 250 Hz, 50% frequency change a double peak is seen reflecting the averaging across different N100 latencies in the AN subtypes (see Figure 4).
A double peak in the N100 to 250 Hz, 50 % frequency change is seen in Figure 3. The double peaks occur because pre-synaptic ANs have earlier N100’s compared to both post-synaptic and undefined ANs (see below). Therefore, the lumping of subgroups in the average reveals multiple peaks that are not seen in any individual subject.
Intensity changes in AN: Detection of N100
Figure 2 (bottom) and Table 3 (“detection” column) show the incidence of N100 for intensity changes. In controls, N100 potentials to intensity changes of both 250 and 4000 Hz were identified consistently (>80% average) to changes of 4, 6, 8 dB. In contrast, N100 potentials in AN subjects were identified at reduced rates (< 30% average to 4, 6, 8 dB changes) for 250 Hz and between 50% (4 and 6 dB change) and 63% (8 dB) for 4000 Hz. The intensity increase that consistently yielded the highest incidence of N100 was 8 dB for both AN and controls. Therefore, differences of N100 latency and amplitude will be reported only for 8 dB changes.
Table 3.
Intensity change N100 to 250 and 4000 Hz showing detection rate, mean and standard deviation (SD) latency (ms) and amplitude (µV). Significance of t-tests and Fisher’s exact test comparing AN and controls are indicated by *.
| 250 Hz | 4000 Hz | ||||||
|---|---|---|---|---|---|---|---|
| N100 | N100 | ||||||
| detection | ms | µV | detection | ms | µV | ||
| 8 dB | Controls | 10/12 | 132 (4) | −3.3 (0.3) | 12/12 | 114 (3) | −3.5 (0.4) |
| AN | 2/7* | 151 (1) | −2.2(0.4) | 5/8* | 124 (6) | −2.1 (0.4) | |
| 6 dB | Controls | 10/12 | 141 (4) | −2.5 (0.3) | 12/12 | 124 (6) | −2.4 (0.5) |
| AN | 2/7* | 178 (4)* | −1.9 (0.4) | 4/8* | 122 (13) | −1.3 (0.8) | |
| 4 dB | Controls | 9/12 | 147 (6) | −2.1 (0.3) | 12/12 | 127 (9) | −2.5 (0.3) |
| AN | 1/7* | 176 | −1.5 | 4/8* | 143 (17) | −1.5 (1.3) | |
p<0.05;
p<0.01;
p<0.001
Intensity changes in AN: N100 measures
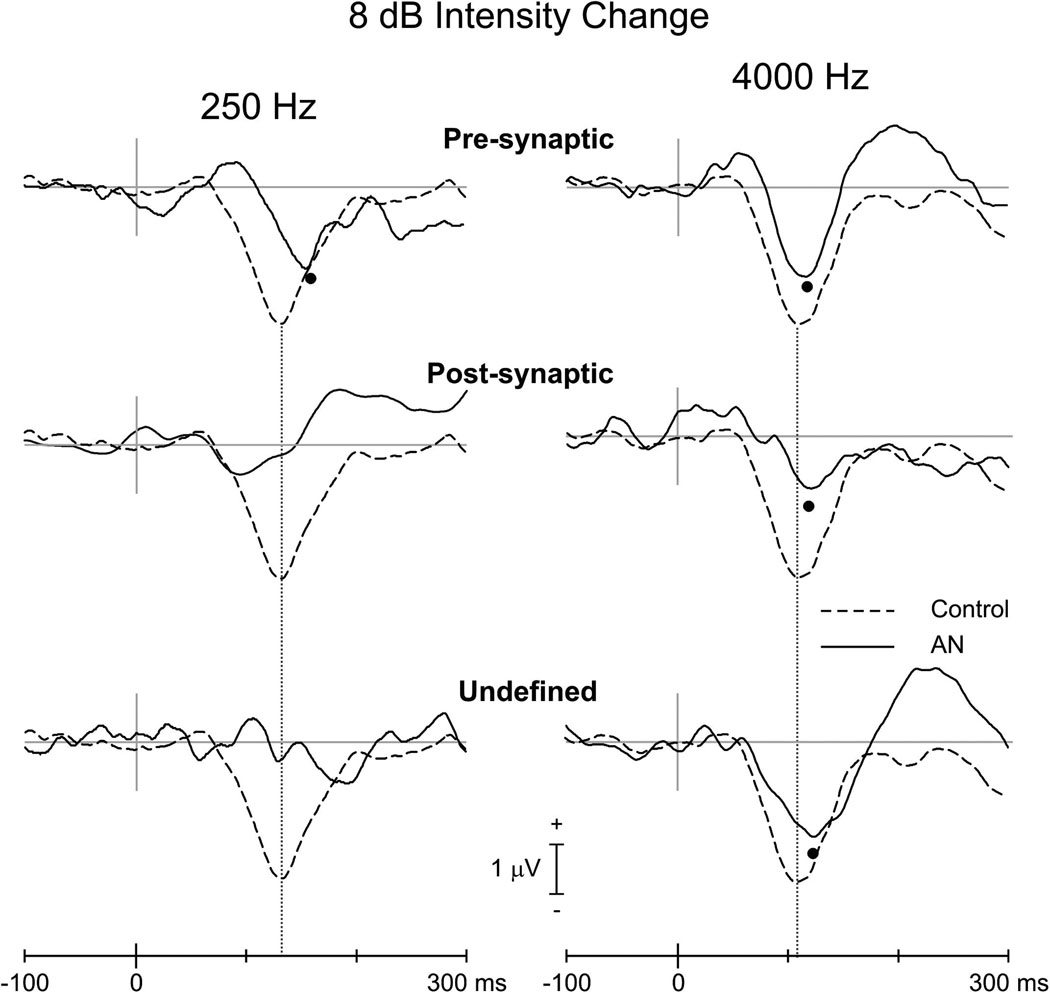
Only two AN subjects had an N100 response to intensity change for 250 Hz. Both responses were within the control limits for latency but were reduced (S2) below the range of normal amplitudes (see subjects S1 and S2 in Table 4). For intensity change at 4000 Hz, AN subjects had N100 latencies and amplitudes that did not differ from controls (Table 3). Figure 3 (bottom) contains the grand mean averaged waveforms for AN and controls to 8 dB intensity for 250 and 4000 Hz. No N100 differences were seen at 4000 Hz: latency (AN=124 ms; controls=114 ms; [t(15)=1.9; p=0.074]), amplitude (AN=−2.1 µV; controls = −3.5 µV; [t(15)=1.7; p=0.109]).
Table 4.
N100 amplitude and latency to 50% frequency change (Δf) and 8 dB intensity change (Δi) for AN individuals and their subtypes.
| 250 Hz latency (ms) | 4000 Hz latency (ms) | 250 Hz amplitude (µV) | 4000 Hz amplitude (µV) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Δf | Δi | diff | Δf | Δi | diff | Δf | Δi | diff | Δf | Δi | diff | |||||
| S1 | Pre-synaptic | 108 | 150 | 42 | 111 | 113 | 2 | −3.3 | −2.5 | 0.7 | −6.1* | −2.0 | 4.1 | |||
| S2 | Pre-synaptic | 102 | 152 | 50 | 103 | 127 | 24 | −5.7 | −1.9 | 3.8 | −5.7* | −2.2 | 3.5 | |||
| S3 | Pre-synaptic | 112 | NR | 110 | 124 | 14 | −4.2 | NR | −4.7 | −1.6 | 3.1 | |||||
| S4 | Post-synaptic | NR | DNT | NR | NR | NR | DNT | NR | NR | |||||||
| S5 | Post-synaptic | NR | NR | 145 | NR | NR | NR | −3.8 | NR | |||||||
| S6 | Post-synaptic | 170 | NR | 111 | NR | −2.6 | NR | −2.2 | NR | |||||||
| S7 | Post-synaptic | 147 | DNT | 118 | 129 | 11 | −1.5 | DNT | −2.0 | −2.8 | −0.7 | |||||
| S8 | Undefined | NR | NR | 116 | 125 | 9 | NR | NR | −4.1 | −2.1 | 2.0 | |||||
| S9 | Undefined | 170 | DNT | 107 | DNT | −0.5 | DNT | −2.0 | DNT | |||||||
| S10 | Undefined | 139 | NR | NR | DNT | −2.1 | DNT | NR | DNT | |||||||
| AN mean | 135(29) | 151(1) | 49 | 115(13) | 124(6) | 12 | −2.8(1.7) | −2.2(0.4) | 1.6 | −3.8(1.6) | −2.1(0.4) | 2.4 | ||||
| Pre-synaptic mean | 107(5) | 151(1) | 108(4) | 121(7) | −4.4(1.2) | −2.2(0.4) | −5.5(0.7) | −1.9(0.3) | ||||||||
| Post-synaptic mean | 159(16) | -- | 125(18) | -- | −2.1(0.8) | -- | −2.7(1.0) | -- | ||||||||
| Undefined mean | 155(22) | -- | 112(6) | 125 | −1.3(1.1) | -- | −3.1(1.5) | −2.1 | ||||||||
| Control mean | 105(8) | 132(4) | 27 | 101(8) | 114(3) | 13 | −4.4(1.6) | −3.3(0.3) | 1.1 | −3.2(1.3) | −3.5(0.4) | −0.2 | ||||
NR=no response; DNT = did not test; italic=values below the 95% control range (i.e., smaller N100 amplitude or later N100 latency);
=above the 95% control range (i.e., larger N100 amplitude)
Pre- and Post-synaptic AN
Frequency changes and AN subtypes
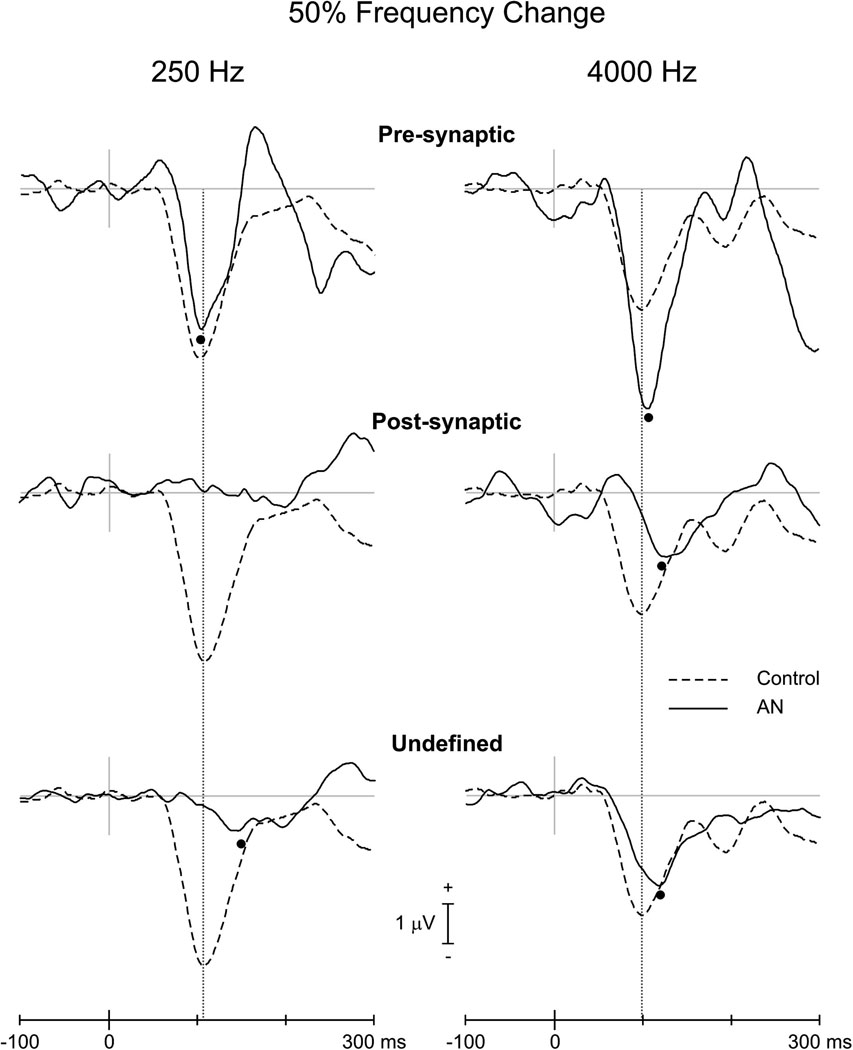
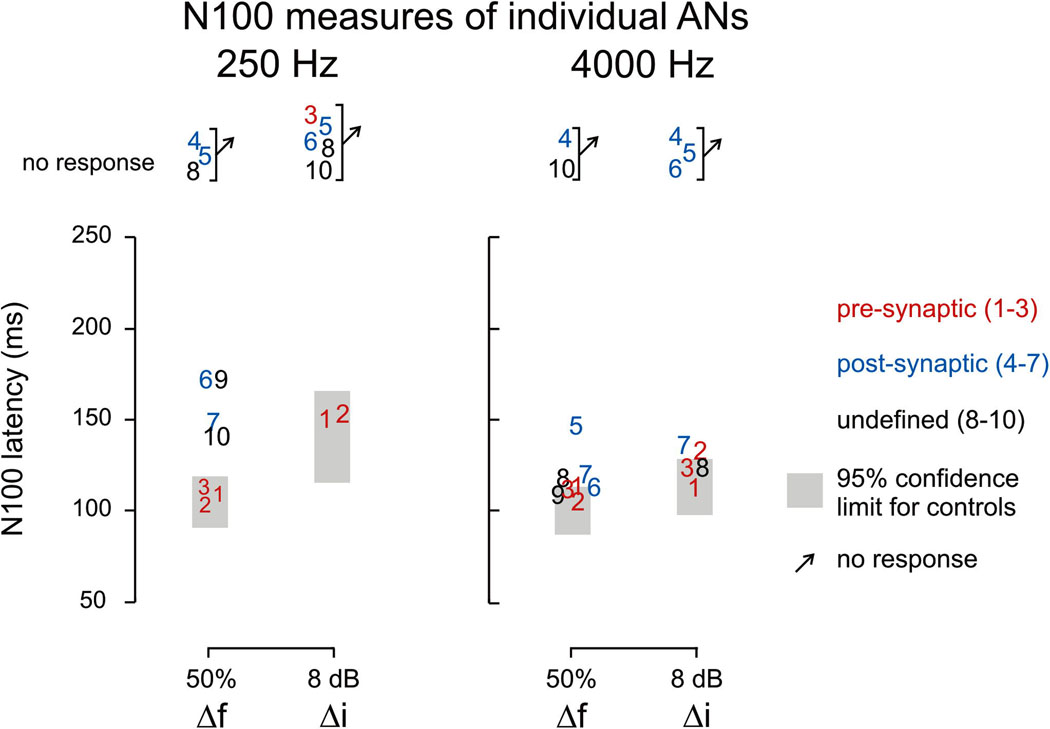
The three presynaptic subjects had N100 responses to 250 and 4000 Hz changes. Two of the four post-synaptic ANs had N100s to 250 Hz and three of the four had responses to 4000 Hz. The amplitudes of the N100 potentials in pre-synaptic ANs were larger than those obtained from both post-synaptic and undefined for both 250 and 4000 Hz (Table 4 and Figure 4). Moreover, pre-synaptic ANs had N100 amplitudes that were larger than the upper limits of controls for 4000 Hz (Table 4 and Figure 4). N100 latencies for frequency change were normal for pre-synaptic and abnormally delayed for post-synaptic. (Table 4, Figure 4, Figure 5)
Figure 4.
Grand averaged evoked potentials (FCz electrode) to frequency change of 50% for controls (interrupted line) and AN subtypes (continuous line). The left and right columns show the responses for 250 and 4000 Hz, respectively. All subjects were included in averages. N100 peaks are indicated by the filled circles. In the waveform of the post-synaptic ANs (250 Hz), the latencies of two subjects that did have a response were separated by 20 ms resulting in a non-identifiable N100.
Figure 5.
Scatter plots of N100 latency to frequency and intensity change. Individual AN subjects are represented by digits (corresponding to Table 1) and are color-coded in the figure according to the site of lesion. The 95% confidence limits of the controls are indicated by grey rectangles.
Intensity changes and AN subtypes
There was a striking difference in the occurrence of N100 to intensity changes between pre- and post-synaptic ANs. Two of the three pre-synaptic subjects had N100 responses to 250 Hz and all three had N100 responses to 4000 Hz. In contrast none of the post-synaptic ANs had N100 to intensity change for 250 Hz and only a single subject of the four had a response to 4000 Hz (see Figure 6 and Table 4).
Figure 6.
Grand averaged evoked potentials (FCz electrode) to intensity change of 8 dB for controls (interrupted line) and AN subtypes (continuous line). The left and right columns show the responses for 250 and 4000 Hz, respectively. All subjects were included in averages. N100 peaks are indicated by the filled circles. In the waveform of the undefined ANs (250 Hz), the negativity at 200 ms was beyond our upper limit of N100 latency.
Cortical N100 to frequency versus intensity changes
In both controls and ANs, N100 latencies to intensity change were more delayed than to frequency change. These differences were comparable (12–13 ms) at 4000 Hz for controls and ANs whereas differences were larger in ANs than controls (47 ms vs. 27 ms respectively) at 250 Hz (Table 4). Moreover in AN subjects (n=4) who had N100 to only one type of change, the response was always to frequency change.
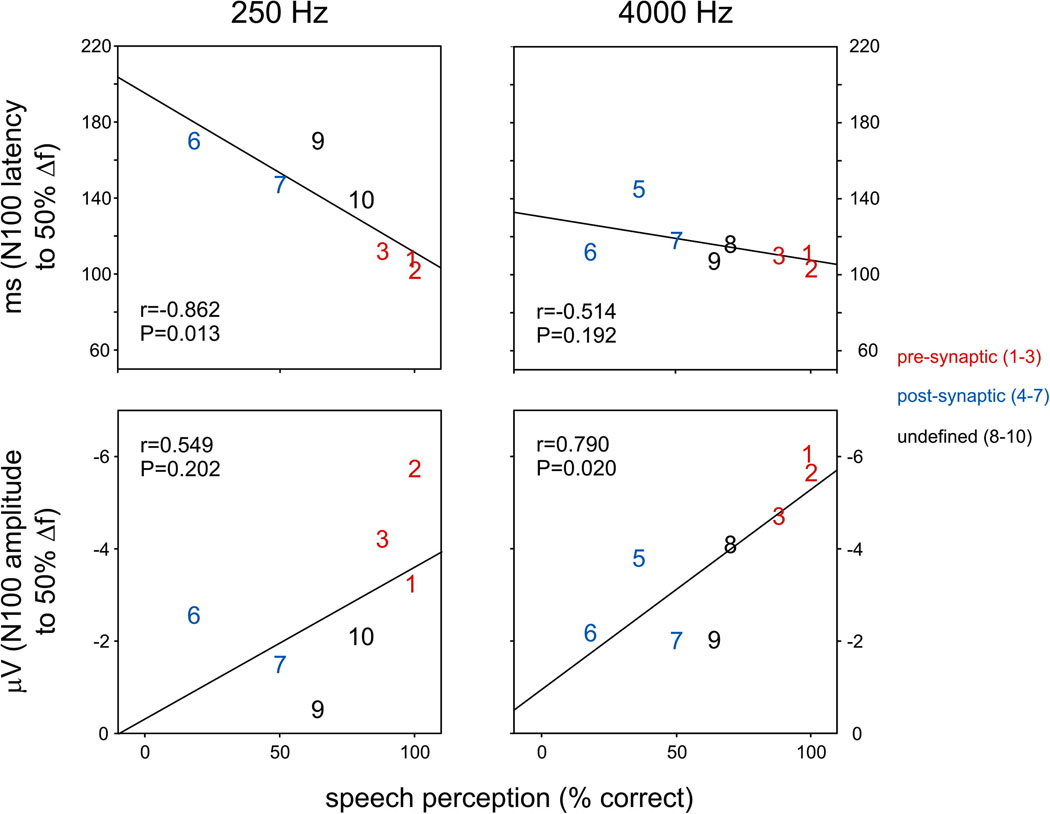
Correlations of N100 and psychoacoustics
Significant correlations were found between speech scores and N100 latency to frequency change at 250 Hz (Fig 7, r=−0.862; p=0.013) as well as for N100 amplitude to frequency changes at 4000 Hz (Figure 7; r=−0.790; p=0.020). No significant correlations were found between N100 latency or amplitudes and gap detection for frequency changes of either 250 or 4000Hz. There were too few subjects (five or less, Table 4) with N100 potentials to intensity changes to test for correlations. Correlations of N100 latency/amplitude with audibility at 250 Hz were not considered reliable since AN subjects’ thresholds were clustered into two widely separate groups: six subjects with both elevated pure tone averages of 250 and 500 Hz (50–70 dB) and speech perception scores that varied widely from 10% to 70%; and a second group of four subjects with normal or minimally elevated low tone PTAs and normal speech scores.
Figure 7.
Relationship between N100 latency elicited by frequency (50%) and speech perception as measured by word identification. A significant relationship is seen with 250 Hz N100 latency and 4000 Hz amplitude. Individual AN subjects are shown as digits (see Table 1) and color-coded depending on the site of lesion.
DISCUSSION
There are three new findings in the present study of auditory cortical “change” N100 potentials in AN subjects to changes of frequency or intensity of ongoing tones: (1) N100 latency and amplitude measures were correlated with speech perception; (2) N100 latency and amplitudes were more affected to changes of low (250 Hz) compared to high (4000 Hz) frequencies; and (3) N100 cortical activity amplitudes differed between pre- and post-synaptic subtypes of AN.
N100 measures and speech perception
The finding in the present study that N100 measures in AN to brief changes of frequency of ongoing tones were significantly related to behavioral measures of speech perception supports an earlier finding by Michalewski et al. (2009) that auditory cortical N100 activity to relatively simple tonal stimuli, such as tone bursts may provide objective measures of speech perception. Significant correlations between N100 latency and speech perception were obtained with low (250 Hz, present study, 1000 Hz, Michalewski et al., 2009) but not high (4000 Hz, present study) tones in AN. These spectral differences are consistent with impaired auditory temporal processing in AN for encoding low but not high tonal frequencies.
One of the earliest recognitions that auditory cortical auditory N100 latency to acoustic stimuli is sensitive to temporal cues at tone onset was made by Davis and colleagues (Onishi and Davis, 1968). Onishi and Davis identified that N100 latency to tone bursts becomes delayed as the onset rise time increases and that changes of stimulus intensity had a minimal effect on N100 latency. The processing of temporal cues is abnormal in AN and is one of the major sources of their speech perception deficits (Zeng et al, 1999). For instance, small temporal differences of voice onset times (VOTs) are critical for distinguishing stop consonants (e.g., /ta/ vs /da/). Rance and colleagues (2008) showed that AN subjects with Friedreich’s ataxia are impaired in discriminating stop consonants whereas the distinction between fricatives (e.g., /s/ vs. /f/), based on high frequency spectral differences, is relatively normal. In contrast, individuals with sensorineural hearing loss are typically able to distinguish stop consonants whereas discrimination of fricatives is impaired. Amplification of speech certainly benefits the discrimination of fricatives in sensorineural loss whereas amplification is typically without benefit in AN for enhancing discrimination of stop consonants.
There have been four studies of AN examining the correlation between auditory cortical measures and speech perception. The degree to which speech perception can be inferred from evoked potentials varied with the type of stimulus used (clicks, tone bursts) and the cortical potential examined. The first by Rance and colleagues (2002) showed in children with AN, that the presence or absence of auditory cortical potentials using both speech tokens and tone bursts predicted the subsequent development of speech comprehension: those with cortical potentials developed speech while those without cortical potentials did not develop speech. The study by Michalewski et al (2009) in adult ANs, showed that the extent of N100 latency delays to tone bursts were significantly correlated with speech comprehension scores. The third study by Kumar and Jayaram (2005) used the latency of mismatch negativity (MMN) in AN subjects to consonants with two different VOTs and found no correlation between MMN latency and degree of impairment of speech comprehension. The MMN is a slow potential of gradual onset making the measurement of peak latency difficult to define. Narne and Vanaja (2008) used click stimuli to evoke cortical potentials in AN and did not find significant correlations between N100 latency and speech perception. Clicks contain predominantly high frequencies (2–4 kHz) and their results are in line with our findings that N100 latency to 4000 Hz change was not correlated with speech perception scores. In both the present study (using high frequency tones, 4000Hz) and that of Name and Vanje (2009), using clicks, N100 amplitude did correlate with speech perception scores. However, amplitude measures are normally variable and would probably not be generally useful in clinical applications. Further studies are needed to define whether auditory cortical potential measures can be a reliable predictor of speech comprehension especially in children. In children the P100 would be measured since N100 does not develop until approximately 10 years of age.
N100 to changes of frequency of low versus high frequencies
The present study showed that AN subjects have relatively normal cortical measures to changes of frequency of high (4000 Hz) but not of low (250 Hz) frequencies. This finding is consistent with behavioral data (Starr et al, 1991; Zeng et al 2005) showing that difference limens in AN are normal for high (4000 Hz and above) but not low frequencies. Tuning curves in AN defined both by otoacoustic emissions (Abdala, et al., 2000) and by noise masking (Vinay and Moore, 2007) are normal for both high and low tonal frequencies, consistent with normal cochlear basilar mechanics. In contrast, the identification of psychophysical abnormalities of processing low frequencies is consistent with abnormal neural temporal encoding of low frequencies.
N100 to changes of intensity
We postulated that cortical responses to intensity change would be relatively unaffected in AN since difference limens of intensity change to 1000 Hz, (Zeng, et al., 2005) did not show significant differences between AN and controls. We found the converse: N100 latency and amplitudes were more affected by intensity than by frequency changes: (Table 4). A clue as to mechanisms that may be involved with abnormalities of processing of both intensity and frequency changes is suggested by reports of some AN subjects during the testing that their perception of the ongoing tones changed. Some reported that the continuous tone “disappeared” or slowly changed to that of an “underwater muffled” sound. In these subjects, the stimulus changes were reported as “beeps”. These descriptions are consistent with abnormal loudness adaptation typically seen in retrocochlear lesions (Owens, 1964). Physiologically, loudness adaptation is thought to be due to abnormally decreased rates of neural discharges during continuous stimulation (Javel, 1996). We suggest that the failure to detect of N100 to intensity change is consistent with abnormal neural adaptation in AN.
N100 and subtypes of AN
The characterization of subtypes of AN depends on clinical evidence (Starr, et al., 2008). Individuals with pre-synaptic AN have impaired auditory nerve function due to altered transmitter release by inner hair cells while ribbon synapses in other neural systems appear to be unaffected (Rodriguez-Ballesteros, et al., 2008; Yasunaga et al., 2000). In post-synaptic AN, inner hair cells appear to be normal whereas auditory nerve as well as other peripheral and cranial nerves function abnormally. AN associated with Charcot-Marie-Tooth syndrome and Friedrich’s ataxia, show abnormalities of peripheral and other cranial nerves in addition to the auditory nerve (Butinar et al., 2008; Rance et al., 2008; Starr et al., 2003).
Electrocochleography (ECochG) has promise for distinguishing between pre- and post-synaptic AN. McMahon et al. (2008) reported that pre-synaptic ANs have a normal latency summating potential followed by a broad negativity whereas post-synaptic ANs have a prolonged summating potential. Pre-synaptic AN in contrast, are characterized by presence of normal latency summating potential followed by a prolonged neural depolarization potential without a distinct compound action potential (Huang, et al., 2009, Santarelli, et al., 2009). Post-synaptic ANs are characterized by the presence of normal latency summating potential and both a delayed and attenuated compound action potential.
The pre-synaptic ANs of the present study have a mutation of OTOF affecting a temperature sensitive deafness (Varga et al., 2006) likely due to abnormal temperature-dependent functions of the ribbon synapses of inner hair cells. ABRs are present when patients are afebrile but are absent when patients become febrile (Starr, et al., 1998).
In the present study we found electrophysiological evidence of differences of cortical processing between pre-synaptic disorder compared both to controls and to post-synaptic subjects. N100 amplitudes to changes of frequency of 4000 Hz were abnormally increased in pre-synaptic ANs (Figure 4, Table 4) even though audibility at 4000 Hz was comparable to that of controls. The finding of enhanced amplitudes in subjects with hearing impairments is unexpected. We suggest that abnormal adaptation of neural responses to the high frequency continuous tone (4000 Hz baseline frequency) in pre-synaptic ANs is due to abnormal reduction of both neurotransmitter release and excitation of their auditory nerve fibers. In the adapted state the presentation of frequency change (i.e., 6000 Hz for 50% change) activates a new pool of non-adapted neurons and the enhanced responses are as if a new stimulus were presented. These neurons, although adapted, are still capable of responding to increases of intensity of the ongoing stimulus (intensity change). This role for adaptation is supported by the finding that in normal subjects the amplitude of N100 to tones without a preceding acoustic background is approximately three times larger than the N100 to a frequency change in an on-going tone (Pratt, et al., 2009). We suggest that abnormal adaptation in presynaptic AN to high frequency tones is a consequence of altered function of inner hair cell ribbon synapses.
Conclusions
The results of this study confirmed the first hypothesis that the extent of cortical N100 delays would be related to speech perception and suggest that cortical N100 latency to low tonal frequencies may provide an objective measure of speech perception in AN. The second hypothesis that cortical N100 potentials in AN subjects would be delayed in latency to changes of low compared to high frequencies was also confirmed consistent with psychoacoustic studies showing that AN subjects have abnormally elevated thresholds for detecting changes of low compared to high frequency signals (Zeng et al., 2005). The third hypothesis that cortical N100 potentials would differ between pre- and post-synaptic subtypes was also confirmed by the finding of enhanced N100 amplitudes in pre-synaptic but not post-synaptic AN, consistent with abnormal adaptation to continuous tones in the pre-synaptic group.
ACKNOWLEDGEMENTS
This study was supported by Grant DC 02618 from the National Institutes of Health and partially supported by the U.S.-Israel Binational Science Foundation, and by the Rappaport Family Institute for Research in the Medical Sciences. The authors would also like to thank Dr. Lenny Kitzes for helpful discussions relating to auditory neurophysiology.
REFERENCES
- Abdala C, Sininger YS, Starr A. Distortion product otoacoustic emission suppression in subjects with auditory neuropathy. Ear Hear. 2000;21(6):542–553. doi: 10.1097/00003446-200012000-00002. [DOI] [PubMed] [Google Scholar]
- Bahmad F, Jr, Merchant SN, Nadol JB, Jr, Tranebjaerg L. Otopathology in Mohr-Tranebjaerg syndrome. Laryngoscope. 2007;117(7):1202–1208. doi: 10.1097/MLG.0b013e3180581944. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bench J, Kowal A, Bamford J. The BKB (Bamford-Kowal-Bench) sentence lists for partially-hearing children. Br J Audiol. 1979;13(3):108–112. doi: 10.3109/03005367909078884. [DOI] [PubMed] [Google Scholar]
- Berlin CI, Morlet T, Hood LJ. Auditory neuropathy/dyssynchrony: its diagnosis and management. Pediatr Clin North Am. 2003;50(2):331–340. doi: 10.1016/s0031-3955(03)00031-2. vii–viii. [DOI] [PubMed] [Google Scholar]
- Butinar D, Zidar J, Leonardis L, Popovic M, Kalaydjieva L, Angelicheva D, Sininger Y, Keats B, Starr A. Hereditary auditory, vestibular, motor, and sensory neuropathy in a Slovenian Roma (Gypsy) kindred. Ann Neurol. 1999;46(1):36–44. doi: 10.1002/1531-8249(199907)46:1<36::aid-ana7>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- Carelli V, Ross-Cisneros FN, Sadun AA. Mitochondrial dysfunction as a cause of optic neuropathies. Prog Retin Eye Res. 2004;23(1):53–89. doi: 10.1016/j.preteyeres.2003.10.003. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, Lolli B, Michalewski HJ, Pratt H, Zeng FG, Starr A. Intensity changes in a continuous tone: auditory cortical potentials comparison with frequency changes. Clin Neurophysiol. 2009;120(2):374–383. doi: 10.1016/j.clinph.2008.11.009. [DOI] [PubMed] [Google Scholar]
- Dimitrijevic A, Michalewski HJ, Zeng FG, Pratt H, Starr A. Frequency changes in a continuous tone: auditory cortical potentials. Clin Neurophysiol. 2008;119(9):2111–2124. doi: 10.1016/j.clinph.2008.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hallpike CS, Harriman DG, Wells CE. A case of afferent neuropathy and deafness. J Laryngol Otol. 1980;94(8):945–964. doi: 10.1017/s0022215100089696. [DOI] [PubMed] [Google Scholar]
- Huang T, Santarelli R, Starr A. Mutation of OPA1 gene causes deafness by affecting function of auditory nerve terminals. Brain Res. 2009;1300:97–104. doi: 10.1016/j.brainres.2009.08.083. [DOI] [PubMed] [Google Scholar]
- Ille N, Berg P, Scherg M. Artifact correction of the ongoing EEG using spatial filters based on artifact and brain signal topographies. J Clin Neurophysiol. 2002;19(2):113–124. doi: 10.1097/00004691-200203000-00002. [DOI] [PubMed] [Google Scholar]
- Javel E. Long-term adaptation in cat auditory-nerve fiber responses. J Acoust Soc Am. 1996;99(2):1040–1052. doi: 10.1121/1.414633. [DOI] [PubMed] [Google Scholar]
- Jones SJ, Perez N. The auditory C-process of spectral profile analysis. Clin Neurophysiol. 2002;113(10):1558–1565. doi: 10.1016/s1388-2457(02)00219-5. [DOI] [PubMed] [Google Scholar]
- Kraus N, Bradlow AR, Cheatham MA, Cunnigham J, King CD, Koch DB, Nicol TG, Mcgee TJ, Stein LK, Wright BA. Consequences of neural asynchrony: A case of auditory neuropathy. Journal of the Association for Research in Otolaryngology. 2000;1:33–45. doi: 10.1007/s101620010004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kumar AU, Jayaram M. Auditory processing in individuals with auditory neuropathy. Behav Brain Funct. 2005;1:21. doi: 10.1186/1744-9081-1-21. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marlin S, Feldmann D, Nguyen Y, Rouillon I, Loundon N, Jonard L, Bonnet C, Couderc R, Garabedian EN, Petit C, Denoyelle F. Temperature-sensitive auditory neuropathy associated with an otoferlin mutation: Deafening fever! Biochem Biophys Res Commun. 2010 doi: 10.1016/j.bbrc.2010.03.062. in press. [DOI] [PubMed] [Google Scholar]
- Martin BA, Boothroyd A. Cortical, auditory, evoked potentials in response to changes of spectrum and amplitude. Journal of the Acoustical Society of America. 2000;107(4):2155–2161. doi: 10.1121/1.428556. [DOI] [PubMed] [Google Scholar]
- McMahon CM, Patuzzi RB, Gibson WP, Sanli H. Frequency-specific electrocochleography indicates that presynaptic and postsynaptic mechanisms of auditory neuropathy exist. Ear Hear. 2008;29(3):314–325. doi: 10.1097/AUD.0b013e3181662c2a. [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Starr A, Nguyen TT, Kong YY, Zeng FG. Auditory temporal processes in normal-hearing individuals and in patients with auditory neuropathy. Clin Neurophysiol. 2005;116(3):669–680. doi: 10.1016/j.clinph.2004.09.027. [DOI] [PubMed] [Google Scholar]
- Michalewski HJ, Starr A, Zeng FG, Dimitrijevic A. N100 cortical potentials accompanying disrupted auditory nerve activity in auditory neuropathy (AN): effects of signal intensity and continuous noise. Clin Neurophysiol. 2009;120(7):1352–1363. doi: 10.1016/j.clinph.2009.05.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Narne VK, Vanaja C. Speech identification and cortical potentials in individuals with auditory neuropathy. Behav Brain Funct. 2008;4:15. doi: 10.1186/1744-9081-4-15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Onishi S, Davis H. Effects of duration and rise time of tone bursts on evoked V potentials. Journal of the Acoustical Society of America. 1968;44:582–591. doi: 10.1121/1.1911124. [DOI] [PubMed] [Google Scholar]
- Owens E. Tone Decay in Viiith Nerve and Cochlear Lesions. J Speech Hear Disord. 1964;29:14–22. doi: 10.1044/jshd.2901.14. [DOI] [PubMed] [Google Scholar]
- Pratt H, Starr A, Michalewski HJ, Dimitrijevic A, Bleich N, Mittelman N. Auditory-evoked potentials to frequency increase and decrease of high- and low-frequency tones. Clin Neurophysiol. 2009;120(2):360–373. doi: 10.1016/j.clinph.2008.10.158. [DOI] [PubMed] [Google Scholar]
- Rance G. Auditory neuropathy/dys-synchrony and its perceptual consequences. Trends Amplif. 2005;9(1):1–43. doi: 10.1177/108471380500900102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rance G, Cone-Wesson B, Wunderlich J, Dowell R. Speech perception and cortical event related potentials in children with auditory neuropathy. Ear and Hearing. 2002;23(3):239–253. doi: 10.1097/00003446-200206000-00008. [DOI] [PubMed] [Google Scholar]
- Rance G, Fava R, Baldock H, Chong A, Barker E, Corben L, Delatycki MB. Speech perception ability in individuals with Friedreich ataxia. Brain. 2008;131(Pt 8):2002–2012. doi: 10.1093/brain/awn104. [DOI] [PubMed] [Google Scholar]
- Rodriguez-Ballesteros M, Reynoso R, Olarte M, Villamar M, Morera C, Santarelli R, Arslan E, Meda C, Curet C, Volter C, Sainz-Quevedo M, Castorina P, Ambrosetti U, Berrettini S, Frei K, Tedin S, Smith J, Cruz Tapia M, Cavalle L, Gelvez N, Primignani P, Gomez-Rosas E, Martin M, Moreno-Pelayo MA, Tamayo M, Moreno-Barral J, Moreno F, del Castillo I. A multicenter study on the prevalence and spectrum of mutations in the otoferlin gene (OTOF) in subjects with nonsyndromic hearing impairment and auditory neuropathy. Hum Mutat. 2008;29(6):823–831. doi: 10.1002/humu.20708. [DOI] [PubMed] [Google Scholar]
- Rose JE, Brugge JF, Anderson DJ, Hind JE. Phase-locked response to low-frequency tones in single auditory nerve fibers of the squirrel monkey. J Neurophysiol. 1967;30(4):769–793. doi: 10.1152/jn.1967.30.4.769. [DOI] [PubMed] [Google Scholar]
- Santarelli R, Del Castillo I, Rodriguez-Ballesteros M, Scimemi P, Cama E, Arslan E, Starr A. Abnormal cochlear potentials from deaf patients with mutations in the otoferlin gene. J Assoc Res Otolaryngol. 2009;10(4):545–556. doi: 10.1007/s10162-009-0181-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schimmel H. The (+) reference: accuracy of estimated mean components in average response studies. Science. 1967;157(784):92–94. doi: 10.1126/science.157.3784.92. [DOI] [PubMed] [Google Scholar]
- Sharma A, Marsh CM, Dorman MF. Relationship between N1 evoked potential morphology and the perception of voicing. J Acoust Soc Am. 2000;108(6):3030–3035. doi: 10.1121/1.1320474. [DOI] [PubMed] [Google Scholar]
- Spoendlin H. Optic cochleovestibular degenerations in hereditary ataxias. II. Temporal bone pathology in two cases of Friedreich's ataxia with vestibulo-cochlear disorders. Brain. 1974;97(1):41–48. doi: 10.1093/brain/97.1.41. [DOI] [PubMed] [Google Scholar]
- Starr A, Michalewski HJ, Zeng FG, Fujikawa-Brooks S, Linthicum F, Kim CS, Winnier D, Keats B. Pathology and physiology of auditory neuropathy with a novel mutation in the MPZ gene (Tyr145->Ser) Brain. 2003;126(Pt 7):1604–1619. doi: 10.1093/brain/awg156. [DOI] [PubMed] [Google Scholar]
- Starr A, Picton TW, Sininger Y, Hood LJ, Berlin CI. Auditory neuropathy. Brain. 1996;119(Pt 3):741–753. doi: 10.1093/brain/119.3.741. [DOI] [PubMed] [Google Scholar]
- Starr A, Sininger Y, Winter M, Derebery MJ, Oba S, Michalewski HJ. Transient deafness due to temperature-sensitive auditory neuropathy. Ear Hear. 1998;19(3):169–179. doi: 10.1097/00003446-199806000-00001. [DOI] [PubMed] [Google Scholar]
- Starr A, Zeng FG, Michalewski H, Moser T. Perspectives on auditory neuropathy: disorders of inner hair cell an, and their synapse. (editors): The senses: a comprehensive reference. Amsterdam: Elsevier; 2008. Perspectives on auditory neuropathy: disorders of inner hair cell, auditory nerve, and their synapse; pp. 397–412. [Google Scholar]
- Varga R, Avenarius MR, Kelley PM, Keats BJ, Berlin CI, Hood LJ, Morlet TG, Brashears SM, Starr A, Cohn ES, Smith RJ, Kimberling WJ. OTOF mutations revealed by genetic analysis of hearing loss families including a potential temperature sensitive auditory neuropathy allele. J Med Genet. 2006;43(7):576–581. doi: 10.1136/jmg.2005.038612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinay, Moore BC. Ten(HL)-test results and psychophysical tuning curves for subjects with auditory neuropathy. Int J Audiol. 2007;46(1):39–46. doi: 10.1080/14992020601077992. [DOI] [PubMed] [Google Scholar]
- Yasunaga S, Grati M, Chardenoux S, Smith TN, Friedman TB, Lalwani AK, Wilcox ER, Petit C. OTOF encodes multiple long and short isoforms: genetic evidence that the long ones underlie recessive deafness DFNB9. Am J Hum Genet. 2000;67(3):591–600. doi: 10.1086/303049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zar J. Biostatistical analysis. Englewood Cliffs, N.J: Prentice-Hall; 1984. pp. 390–397. [Google Scholar]
- Zeng FG, Kong YY, Michalewski HJ, Starr A. Perceptual consequences of disrupted auditory nerve activity. J Neurophysiol. 2005;93(6):3050–3063. doi: 10.1152/jn.00985.2004. [DOI] [PubMed] [Google Scholar]
- Zeng FG, Liu S. Speech perception in individuals with auditory neuropathy. J Speech Lang Hear Res. 2006;49(2):367–380. doi: 10.1044/1092-4388(2006/029). [DOI] [PubMed] [Google Scholar]