Abstract
Rats restrained for 3 hours/day for 3 days (RR) lose weight and do not return to the weight of non-restrained controls once restraint has ended. This study tested the importance of restraint-induced corticosterone release in mediating the change in body weight by injecting ADX rats with 2.0 mg corticosterone/kg before each restraint to replicate the restraint-induced surge in circulating corticosterone. Restrained adrenalectomized (ADX) rats injected with corticosterone had the same initial weight loss as intact restrained rats, whereas corticosterone injection in non-restrained ADX rats and restraint of ADX rats injected with saline each produced only half as much initial weight loss. Sustained weight loss, measured for 14 days after the end of RR, was the same for restrained intact rats and restrained ADX rats injected with corticosterone whereas restrained ADX rats injected with saline achieved the same weight gain as their controls. Corticosterone injections had no effect on weight gain of non-restrained intact rats. In situ hybridization showed that corticotropin releasing factor (CRF) mRNA expression in the paraventricular nucleus of the hypothalamus (PVN) was increased by the same degree in ADX rats and restrained intact rats and was not modified by corticosterone injections. There was no significant effect of restraint, ADX or corticosterone injection on PVN arginine vasopressin (AVP) mRNA expression. These data indicate that a surge in corticosterone causes sustained weight loss in ADX rats through a mechanism that can be compensated for in intact rats and is independent of changes in PVN CRF or AVP mRNA expression.
Keywords: Adrenalectomy, in situ hybridization, corticotrophin releasing factor, arginine vasopressin, paraventricular nucleus
INTRODUCTION
Rats exposed to restraint for 3 hours on 3 consecutive days (repeated restraint: RR) lose weight on the days of restraint and do not return to the weight of their controls for at least 80 days (1). Initial weight loss can be attributed to a decreased food intake and an increased energy expenditure on the days of restraint, but both intake and expenditure return to the same levels as found in controls once restraint has ended. The restrained rats maintain a body weight below that of their non-stressed controls because there is no compensation for the energy deficit or weight loss that occurs on the days of restraint (1). This is unusual in that rats that lose weight due to interventions such as food restriction over-eat and rapidly return to the weight of their controls once the restriction on feeding is lifted (2–3) and suggests that restraint causes a chronic disruption of unidentified regulatory mechanisms that normally control body weight. The long-term inhibition of body weight is not specific to restraint and can be induced by other types of stress such as social defeat (4) and immobilization (5), however, restraint provides a model in which a relatively large number of animals can be stressed uniformly and at the same time.
One of the hormonal responses to restraint is an activation of the hypothalamic-pituitary-adrenal (HPA) axis resulting in release of corticotropin-releasing factor (CRF) and arginine vasopression (AVP) from neurosecretory cells in the paraventricular nucleus of the hypothalamus (PVN) into the hypophyseal portal system (6). This ultimately leads to a stimulation of synthesis and release of glucocorticoids from the adrenal glands. Circulating concentrations of the primary glucocorticoid in rodents, corticosterone, peak between 30 and 60 min after HPA axis activation (7). The elevated circulating concentrations of corticosterone produce a negative feedback which inhibits the release of CRF in the hypothalamus and ACTH from the pituitary gland (8–9). Previous studies have shown that restraint increases CRF mRNA in the PVN (1, 10–11), whereas peripheral or central corticosterone inhibits this increase in expression (12–13). AVP mRNA expression does not change in response to acute restraint or immobilization stress (14), but AVP mRNA increases proportionally more than CRF mRNA in the PVN of rats subjected to chronic restraint, suggesting a more important role for AVP in regulating the HPA axis under conditions of chronically elevated corticosterone (15).
When ADX rats are restrained they show an exaggerated increase in hypothalamic CRF mRNA expression compared with adrenal-intact animals due to the lack of normal glucocorticoid negative feedback (16–17). It has previously been reported that ADX rats implanted with corticosterone pellets that clamp circulating corticosterone concentrations at a basal level experience the same weight loss during restraint stress as their controls (18), but there was no information related to the long-term effect of restraint on the body weight of these animals. In addition, there was no attempt to examine the effects of the acute surge in corticosterone that stress initiates in an intact rat. The objective of the studies described here was to further investigate the role of corticosterone in mediating the sustained reduction in body weight of RR rats. We tested whether the large, but transient, increase in corticosterone that occurs during restraint was necessary for initial and sustained weight loss in both intact and ADX rats. We measured CRF and AVP mRNA expression in the PVN to determine hypothalamic differences in response to restraint in intact rats, in ADX rats and in ADX rats given corticosterone to replicate the surge induced by restraint.
METHODS
All rats used in studies described here were male Sprague Dawley rats (Harlan Sprague Dawley, Indianapolis, IN) that weighed approximately 300 g at the start of a study. They were individually housed in hanging wire cages with free access to water and chow (LabDiet 5012, PMI Nutrition International, Brentwood, MO), unless stated otherwise, in a room maintained at 23°C and approximately 55% humidity. Lights were on for 12 hours/day from 7.00 a.m.. All animal procedures were approved by the Institutional Animal Care and Use Committee of the University of Georgia.
Pilot study: Accurate replication of RR-induced corticosterone peak with a corticosterone injection
The amount of corticosterone required to accurately replicate restraint-induced corticosterone levels in RR rats was determined in a pilot study. Initially rats (n=3) were given a subcutaneous injection of 1.0 mg corticosterone/kg dissolved in propylene glycol (19) and tail blood samples were collected after 0, 15, 30, 60, 90 and 120 minutes of restraint. This dose produced a peak circulating concentration of corticosterone of only 200 ng/mL, which was substantially lower than that induced by 3 hours of restraint (Figure 1). When the dose was doubled, serum corticosterone reached a maximum concentration of 400 ng/mL which was similar to the peak concentration previously recorded in RR rats (20). Figure 4 illustrates the similarity in changes of serum corticosterone in rats injected with 2.0 mg/kg corticosterone and those subjected to RR in Experiment 1.
Figure 1.
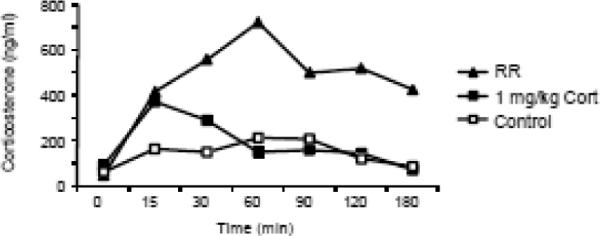
Serum corticosterone of rats (n=3) injected with 1.0 mg/kg corticosterone or subjected to 3 hours of restraint in the pilot study. This dose of corticosterone was doubled in order to replicate the release of corticosterone in rats restrained for 3 hours (see Figure 4 for results from Experiment 1).
Figure 4.
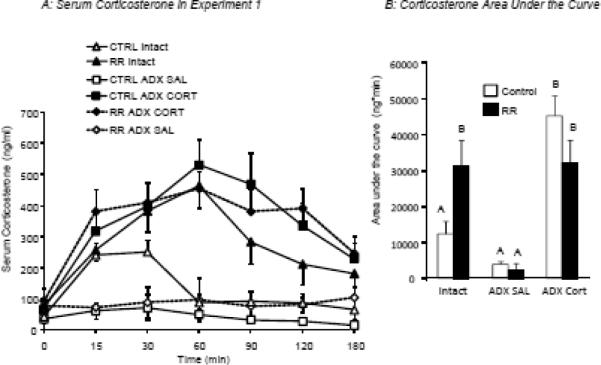
Serum corticosterone levels measured on Day 2 of RR in Experiment 1 are shown in Panel A and average area under the curve for each group is shown in Panel B. Data are means ± sem for groups of 9 or 10 rats and values that do not share a common superscript are significantly different.
Experiment 1: The effect of restraint-associated levels of corticosterone injections in ADX rats
The objective of this study was to test whether a surge in corticosterone during restraint was required for the induction of sustained weight loss in RR rats. Seventy-two rats were housed as described above. After one week of adaptation to housing and handling, 48 of the rats were anesthetized with isoflurane and bilaterally adrenalectomized via a dorsal approach. A subcutaneous injection of analgesic (2 mg/kg ketofen) was given immediately before surgery and again on the day following surgery. ADX rats had free access to a solution of 25μg corticosterone/mL in 0.1% NaCl in place of water until the end of the experiment. This results in rats having low/normal basal circulating concentrations of corticosterone that show circadian variation because water intake increases with food intake during the dark phase of the light cycle, but the rats cannot increase circulating corticosterone concentrations in response to restraint or any other stressor (21).
After eight days of baseline measurement of body weight and food intake corrected for spillage, the rats were weight-matched into six groups. Intact rats were separated into two groups: control (CTRL Intact) and repeated restraint (RR Intact). ADX rats were divided into 4 groups: CTRL ADX SAL, CTRL ADX CORT, RR ADX SAL and RR ADX CORT. All rats were moved to an experimental room during the periods of restraint. RR rats were placed in Perspex restraining tubes (21.6 cm long, 6.4 cm diameter; Plas Labs, Lansing, MI) for 3 hours. Non-restrained CTRL rats were placed in shoebox cages in the same room without food or water for the same duration. Before placing rats in restraining tubes or shoebox cages, CORT rats were injected subcutaneously with 2.0 mg corticosterone/kg in propylene glycol and all other rats were given equivalent injections of sterile saline. At the end of the 3 hours of restraint, all rats were returned to their home cages with free access to food and water or water containing corticosterone and NaCl (ADX rats). The same procedure was repeated on the two subsequent days. Daily body weights and food intakes were measured for an additional 14 days after the end of RR.
On the second day of RR, blood samples (50 μL) were collected by tail bleeding for measurement of corticosterone (Corticosterone Double Antibody – 125I RIA Kit Rats & Mice, MP Biomedicals, Solon, OH) immediately before injecting the rats (time point 0) and at 15, 30, 60, 90, 120 and 180 minutes after the onset of RR. Blood was collected on Day 2 of restraint to reduce the number of novel stressors introduced to the control rats on Day 1 of restraint. For each sample, blood was collected within 2 minutes of handling the rat.
Experiment 2: The effect of restraint-associated levels of corticosterone on non-restrained intact rats
The previous study indicated that corticosterone injections that replicated the pattern of corticosterone release during restraint produced weight loss in both control and restrained ADX rats, therefore the objective of this study was to test whether similar injections of corticosterone would cause weight loss in non-restrained intact rats. Thirty male rats were adapted to housing and handling for one week. Body weights and food intakes were measured daily and after 7 days of baseline measurements they were divided into three weight-matched groups: CTRL, RR and CORT rats. RR rats were restrained for 3 hours on 3 consecutive days, while CTRL and CORT rats were placed in shoebox cages in the same room. RR and CTRL rats received saline injections and CORT rats were injected with 2 mg corticosterone/kg on each day of restraint immediately before the start of the restraint period. Blood was collected by tail bleeding on the second day of RR for measurement of serum corticosterone concentrations. Food intakes and body weights were measured for 14 days after the end of RR.
Experiment 3: Measurement of PVN CRF mRNA and AVP mRNA
The objective of this study was to determine whether restraint-induced levels of corticosterone altered CRF or AVP mRNA expression in the PVN of CTRL and RR rats and whether this correlated with weight loss in the rats. Hypothalamic CRF and AVP mRNA expression was measured immediately after the end of 3 hours of restraint on the second day of restraint. Day 2 was selected in order to reduce the non-specific stress experienced by the Controls, which would be highest on Day 1 of the repeated restraint period. Although restrained rats continue to lose weight after the third restraint the rate of weight loss tends to be less on Day 3 than on Days 1 and 2 (see Figure 2), which may be interpreted as the start of habituation to restraint. Although total weight loss due to restraint is optimized with three days of restraint (22) we have previously reported that a single 3 hour restraint causes a sustained reduction in body weight (23). Therefore, we are confident that the changes in CRF and AVP mRNA expression measured on Day 2 of restraint are representative of those that are associated with the initiation of a sustained reduction in body weight.
Figure 2.
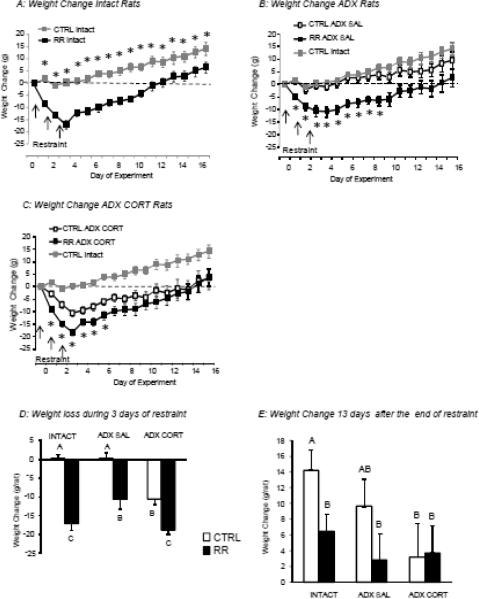
Body weight change of rats in Experiment 1. Data are means ± sem for groups of 9 or 10 rats. Asterisks in Panels A to C indicate significant differences between CTRL and RR rats within each treatment group. Panel D shows the average weight change of each group of rats during the three days of restraint and Panel E shows the average change in weight for each group from the morning immediately before the first restraint to 13 days after the end of restraint, the last day of the experiment. Data in panels D or E that do not share a common superscript are significantly different at P<0.05.
Fifty-six rats were adapted to housing and handling for 7 days. Thirty-two rats were adrenalectomized as described above and were given free access to 25μg corticosterone/mL in 0.1% NaCl. The animals were divided into 7 groups of 8 rats each. Intact rats were separated into 3 weight-matched groups: RR Intact SAL, CTRL Intact SAL and CTRL Intact CORT. ADX rats were divided into 4 weight-matched groups: CTRL ADX SAL, CTRL ADX CORT, RR ADX SAL, and RR ADX CORT and were treated as described above except that they were killed at the end of 3 hours of restraint on Day 2 of RR. We did not include a group of rats that were intact, restrained and injected with corticosterone (RR Intact CORT) because corticosterone concentrations would have been raised well above the concentrations experienced with restraint and it would have been difficult to interpret the relevance of responses in these animals. Tail blood was collected at times 0 and 30 min on Day 2 of RR for measurement of serum corticosterone concentrations. Rats were decapitated and brains were rapidly dissected, frozen in dry ice and stored at −80°C. Using coordinates in the Paxinos and Watson rat brain atlas (24), 15 μm sections of the PVN were collected, mounted on gelatin and chromium potassium sulfate coated slides and analyzed for CRF and AVP mRNA expression by in situ hybridization. Every tenth section was stained with 0.1% thionin to confirm the neuroanatomical location.
Slides were treated with 4% formaldehyde in 0.12 M sodium phosphate-buffered saline (PBS) for 5 min, rinsed with PBS three times, and treated with 0.25% acetic anhydride in 0.1 M triethanolamine in 0.9% saline for 10 min. Slides were then treated with 70%, 80%, 95% and 100% ethanol washes, soaked in chloroform for 5 min, rinsed in 100% ethanol and rinsed in 95% ethanol before air drying. The CRF and AVP oligonucleotides (Table 1) were synthesized by Integrated DNA Technologies (Coralville, IA). Terminal deoxynucleotidyl transferase (Roche, Indianapolis, IN), 35S-dATP and tailing buffer were used to label the oligodeoxynucleotides at the 3' end and labeled probes were filtered through Stratagene Nuctrap Columns.
Table 1.
Sequence of oligonucleotide probes used for in situ hybridization in Experiment 3.
Slides were exposed to radiolabeled probes in a buffer of 50% formamide, 600 mM NaCl, 80 mM Tris-HCl, 4 mM EDTA, 0.1% sodium pyrophosphate, 0.2% SDS, 0.2 mg/mL heparin sulfate and 10% dextran sulfate for 24 hours. Slides were rinsed three times with 1×SSC and washed three times for 20 min each in 2×SSC/50% formamide at 40°C, followed by two washes in 2×SSC/50% formamide at 22°C. After one 30 min wash in 1×SSC, a brief dip in deionized water and then 70% ethanol, the slides were left to dry. Slides were exposed to autoradiographic film (Amersham Hyperfilm MP, GE Healthcare,) for 3 weeks. Film was developed, scanned with a high resolution scanner and density of the PVN mRNA expression in the lateral and medial parvocellular subdivisions (pPVN) was measured by tracing the areas and quantifying the density using the NIH computer image analysis program ImageJ. Measurements were corrected for background density.
Statistical Analysis
All body weight, food intake and corticosterone data were analyzed by two-way ANOVA, adjusted for repeated measures (Statistica, StatSoft, Tulsa, OK). Significant differences between specific groups on particular days were determined by post hoc Duncan's multiple range test. In situ hybridization and single time point measures were compared by oneway ANOVA. Significant differences between groups were determined by post hoc Duncan's multiple range test. P ≤ 0.05 was considered significant.
RESULTS
Experiment 1
The objective of this study was to test the importance of the surge in corticosterone that occurs during restraint stress on body weight of rats. We compared rats that were Intact with those that were ADX and injected with saline and those that were ADX and injected with corticosterone to replicate the change in corticosterone that was induced by restraint. Intact rats weighed more than ADX rats at the start of the experimental period (Intact: 371 ± 4 g, ADX: 321 ± 3 g, P<0.0001). Because of this difference we compared the change in weight from baseline, which was the weight measured in the morning of Day 0, immediately before the first restraint. There were significant effects of both restraint and treatment group on the weight change of the rats during and after RR (RR: P<0.0001, Trt: P< 0.01, Day: P< 0.0001, RR X Day: P<0.0001, Trt X Day: P<0.0003). All RR and all CORT rats lost weight on the three days of restraint (Figure 2A–D). Restrained ADX rats injected with saline and control ADX rats injected with corticosterone lost about half as much weight as restrained Intact rats or restrained ADX rats injected with corticosterone, but this difference was significant only on Day 3 of restraint (Figure 2D). For Intact rats, weight gain from baseline was significantly greater in CTRL than RR rats for all days of the experiment (Figure 2A: RR: P<0.0001, Day: P<0.0001, RR × Day: P<0.0001).
In ADX rats injected with saline, the weight gain of RR rats was less than that of their controls for only 6 days after the end of restraint (Figure 2B: RR: P<0.02, Day: P<0.0001, RR × Day: NS) and the shorter duration of inhibition of weight gain was primarily due to the controls failing to gain weight, rather than restrained rats gaining weight at a faster rate than RR Intact rats (Fig 2E). For ADX rats injected with corticosterone, weight gain of control and restrained rats, compared with their respective baseline weights, was significantly different for only 3 days after the end of restraint (Figure 2C: RR: NS, Day: P<0.0001, RR × Day: NS). This was because although the CTRL ADX CORT rats lost less weight on the days that they were injected, they recovered less of this weight after restraint ended than did the RR rats. When the change in weight from baseline was compared for all CTRL groups 13 days after the end of restraint, ADX rats injected with corticosterone gained less weight than CTRL Intact rats (Figs 2C, 2E). The only significant difference in weight change of the RR groups was that the ADX rats injected with saline lost less weight on the days of restraint than did the two other groups of RR rats (Fig 2D). There was no difference in weight change by the end of the experiment (Fig 2E).
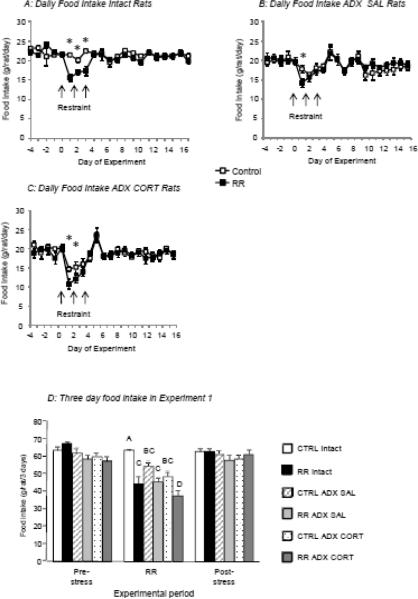
Food intake was inhibited by RR in all treatment groups (Figure 3A–C), but the pattern of response on the three days of restraint was different between groups. Intact RR rats ate less than their controls on all days of restraint (Figure 3A), whereas RR ADX CORT rats ate less than their controls on the first two days of restraint (Figure 3C) and RR ADX SAL rats ate less than their controls on only the first day of restraint (Figure 3B). Daily injections of either saline or corticosterone inhibited food intake of ADX Control rats (Figure 3B and C). When food intake was summed for 3 days before, during and after restraint (Figure 3D) there were no differences between groups immediately before or immediately following restraint. CTRL Intact rats ate more than any other group on the 3 days of restraint. RR inhibited food intake of all rats although this did not reach significance in the ADX SAL rats. The intake of RR ADX CORT rats was lower than that of any other group on the days of restraint.
Figure 3.
Food intake of rats in Experiment 1. Data are means ± sem for groups of 9 or 10 rats. Asterisks in Panels A to C indicate significant differences between CTRL and RR rats within each treatment group. Total three day food intake (Panel D) of each group of rats before, during and after restraint. Values for 3 day intake on the days of restraint that do not share a common superscript are significantly different at P<0.05.
On Day 2 of RR there were no differences in basal serum corticosterone concentrations (Figure 4A). All of the saline-injected ADX rats maintained low levels of serum corticosterone throughout the test period, confirming the efficacy of the ADX surgery. Corticosterone of CTRL Intact rats increased to a maximum of 251 ng/mL at time 30 min, but returned to basal levels by 60 min. This increase in corticosterone was probably due to the stress associated with handling, tail bleeding and injecting the rats at the start of the period of restraint. Serum corticosterone of all of the corticosterone-injected rats was the same as for restrained Intact rats and peaked between 450 and 550 ng/mL at time 60 min (Figure 4A). Area under the curve for corticosterone (Figure 4B: Trt:P<0.0001, RR: NS, Trt x RR:P<0.006) was the same for both Control and RR ADX rats injected with saline as for Intact Controls but was significantly higher for Intact RR rats and for both Control and RR ADX rats injected with corticosterone.
In summary, the corticosterone injection accurately replicated corticosterone release during restraint of Intact rats. Independently, corticosterone injection and restraint stress caused half as much weight loss as was produced by the combination of restraint and a surge in corticosterone that was experienced by Intact RR rats and by CORT ADX RR rats. Food intake was not an accurate predictor of weight loss in the rats.
Experiment 2
This study tested the effect of corticosterone injection on body weight in Intact rats to determine whether corticosterone alone would produce the same weight loss in Intact rats as it did in ADX rats in Experiment 1. RR rats lost weight on the days of restraint and maintained a reduced body weight compared with CTRL rats until the end of the experimental period (Figure 5A). By contrast, the rats that were injected with corticosterone did not lose weight and, like CTRL rats, gained weight steadily until the end of the experiment (Figure 5A).
Figure 5.
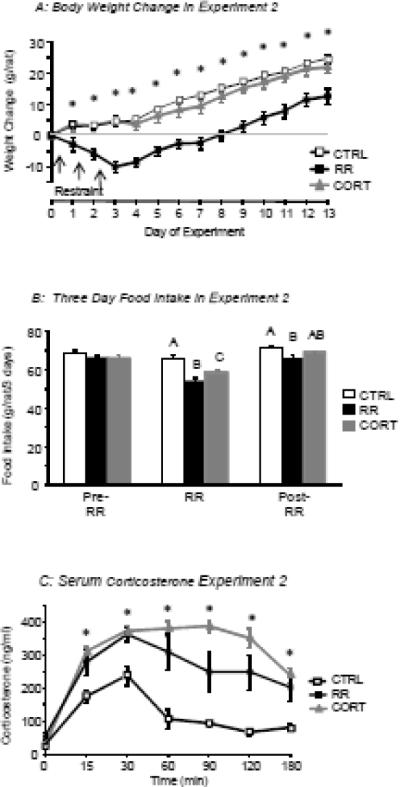
Body weight change of rats in Experiment 2 (Panel A). Asterisks indicate significant differences between RR rats and the two other groups of rats. Data are means ± sem for groups of 10 rats. Panel B shows the total three day food intake of the different groups of rats before, during and after restraint. Values for intake on the days of restraint that do not share a common superscript are significantly different at P<0.05. Panel C shows serum corticosterone levels measured on Day 2 of RR in Experiment 2, asterisks indicate a significant difference between the CTRL and the RR and CORT groups.
There were no differences in total 3 day food intake of the groups during the 3 days before restraint (Figure 5B), but food intake was reduced on the 3 days of restraint in both restrained and corticosterone-injected groups compared with controls. The intake of RR rats remained lower than that of controls during the 3 days after the end of restraint (Figure 5B: RR: P<0.002, Time: P<0.0001, RR × Time: P<0.0001).
On the second day of RR, serum corticosterone levels of CTRL rats reached approximately 200 ng/ml 30 min after the start of restraint, but returned to basal levels by 60 min (Figure 5C). Serum corticosterone of RR and CORT rats peaked at approximately 400 ng/ml 60 min after the start of restraint and remained above baseline at the end of the 3 hour restraint (Figure 5C). Therefore, the results of this study indicate that a surge in corticosterone alone does not induce weight loss or a reduction in food intake of rats that have an intact HPA axis.
Experiment 3
The objective of this study was to test whether weight loss in response to restraint and/or corticosterone injection could be attributed to differential changes in hypothalamic CRF or AVP mRNA expression. As in Experiment 1, Intact rats were significantly heavier than ADX rats at the start of this study (Intact: 373 ± 4 g, ADX: 296 ± 7 g). Serum corticosterone on Day 2 of RR was the same for all groups of rats at time 0 and remained low in all of the saline-injected ADX rats 30 min after the start of restraint (Table 2). Serum corticosterone concentrations were similar for RR Intact SAL rats and all corticosterone-injected rats at 30 min after the start of restraint (Table 2).
Table 2.
Serum corticosterone (ng/ml) on Day 2 of RR in Experiment 3
| Time 0 | Time 30 min | |
|---|---|---|
| CTRL Intact | 34 ± 5 | 215 ± 19A |
| RR Intact | 45 ± 13 | 323 ± 24B |
| CORT Intact | 35 ± 7 | 339 ± 21B |
| CTRL ADX SAL | 68 ± 13 | 29 ± 5D |
| RR ADX SAL | 70 ± 20 | 59 ± 14C |
| CTRL ADX CORT | 31 ± 7 | 281 ± 59AB |
| RR ADX CORT | 76 ± 20 | 288 ± 34AB |
Data are means ± sem for groups of 8 rats. Serum corticosterone was measured immediately before injection of saline or corticosterone and the start of restraint (Time 0). A second sample was collected 30 minutes after the start of restraint. There were no differences in corticosterone at Time 0. Values for corticosterone at 30 minutes that do not share a common superscript are significantly different at P<0.05.
Both ADX and RR caused a significant increase in CRF mRNA expression when compared with CTRL Intact rats injected with saline (Figure 6A). By contrast, corticosterone injections had no significant effect on CRF mRNA expression in either Intact or ADX rats. Neither ADX nor RR had a significant effect on AVP mRNA expression in the pPVN and the only significant difference in expression was between RR Intact and RR ADX SAL rats (Figure 6B).
Figure 6.
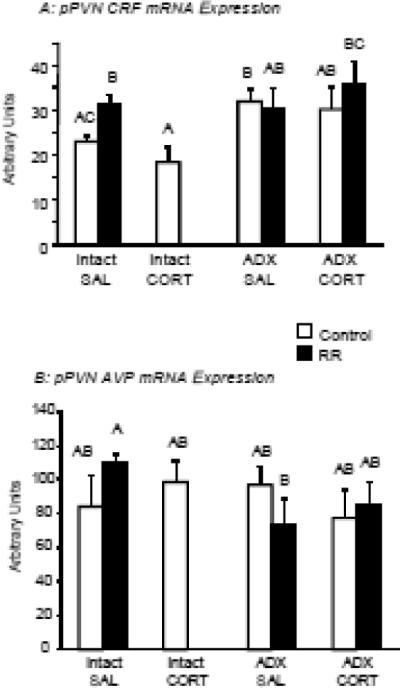
Panel A shows CRF mRNA expression and Panel B shows AVP mRNA expression in the PVN in Experiment 3. Superscript letters indicate significant differences in density of expression between groups (P<0.05).
DISCUSSION
We have previously demonstrated that rats subjected to three hours of restraint on each of three consecutive days (RR) do not compensate for the weight loss that is experienced on the days of restraint. Therefore, RR rats weigh less than their non-restrained controls during the post-restraint period and this difference in body weight is maintained for at least 80 days after the end of restraint (1). A single three hour restraint also produces a long-term down regulation of body weight (23), but the effect is optimized in the RR paradigm (22). Restraint is both a physical and psychological stress. The rats cannot escape from the restraint tubes, but they do not struggle once placed inside the tube and appear calm although measurements of serum corticosterone (25) and energy expenditure (1) indicate that they are experiencing a significant stress. One of the endocrine responses to restraint is release of corticosterone from the adrenal cortex which then down-regulates HPA activation (26), inhibiting both PVN CRF mRNA expression and pituitary ACTH release (27–28).
The experiments described here tested the importance of restraint-induced levels of corticosterone in initiating long-term changes in body weight of RR rats and we found that corticosterone caused weight loss in both control and restrained ADX rats, but not in non-stressed Intact rats. Experiment 1 did not test whether the corticosterone was acting peripherally or centrally to cause weight loss in ADX rats, but it is well established that high levels of corticosterone have catabolic effects in muscle tissue (29), therefore it is likely that direct effects of corticosterone on muscle contributed to the weight loss. Previously we have shown that glucose uptake is inhibited in adipose, but not muscle tissue one day after the end of restraint and that adipocytes β-adrenergic receptor number is increased (30). Five days after restraint, however, the composition of the weight difference between control and restrained rats is a combination of fat and lean tissue and adipocyte glucose metabolism is the same as in controls (30–31).
In Experiment 1 the surge in corticosterone during restraint accounted for approximately half of the weight loss experienced by restrained ADX rats injected with corticosterone on the days of restraint, indicating the presence of additional, undefined aspects of restraint that influence body weight. We have previously shown that RR causes a significant increase in energy expenditure during the three hours that the rats are in the restraining tubes (1), which may represent the undefined response to restraint, but because energy expenditure was not measured in this study we need to confirm that restraint-induced thermogenesis is inhibited in ADX rats.
Despite the clear effect of corticosterone injections on body weight in ADX rats, we found no effect on body weight of Intact rats in Experiment 2. This suggests that Intact rats are able to compensate for the effects of corticosterone on body weight and that this compensatory mechanism is disrupted in ADX rats. Alternatively, because ADX increases the number of glucocorticoid receptors present in both peripheral (32) and brain tissue (33) the endocrine phenotype of ADX rats may have allowed the acute corticosterone injection to activate mineralocorticoid and/or glucocorticoid receptors that were not available in Intact rats.
Due to the slow rate of weight gain in non-restrained ADX rats the results from Experiment 1 are more difficult to interpret in terms of the effect of restraint-induced corticosterone on long-term regulation of body weight. By the end of the experiment weight change from baseline was higher in Intact controls than for ADX controls that had been injected with corticosterone, but was not different from that for ADX control rats that had been injected with saline, suggesting that the surge in corticosterone during restraint does contribute to the initiation of events that produce a sustained reduction of body weight in RR rats. At the end of the experiment there was not, however, any difference in the weight change of any of the groups of restrained rats, suggesting that restraint, even in the absence of a surge of corticosterone, activates the mechanisms that produce a long-term down-regulation of body weight. Because the weight change of non-restrained ADX rats injected with corticosterone was identical to that of the restrained rats it is possible that the surge in corticosterone during restraint and some other undefined aspect of restraint activate the same, or different, but redundant, pathways that modify body weight regulation. It is well established that the activity of the HPA axis is determined by the integration of information from multiple sites influencing the activity of PVN CRF-containing neurons (27, 34), which in turn control pituitary function. The bed nucleus stria terminalis receives input from other limbic structures such as the hippocampus and amygdala and has projections to the PVH (27) where they could potentially modulate energy balance in addition to HPA activity (35). Therefore, these nuclei represent alternate candidate sites for RR-induced changes in body weight.
Corticosterone injections also caused a significant inhibition of food intake in both control and restrained ADX rats, which might be expected to have contributed to weight loss. The results from Experiment 2 in which Intact rats were injected with corticosterone suggest, however, that inhibition of food intake and weight loss during restraint are not tightly associated because corticosterone-injected Intact rats had a reduced food intake, but did not lose weight. We have previously reported that third ventricle infusion of a CRF Type 2 receptor antagonist immediately before each restraint prevents a drop in food intake of RR rats, but does not prevent either the acute or sustained weight loss of the rats. Conversely third ventricle infusion of a CRF Type I receptor (CRFR1) antagonist before restraint does not block an inhibition of food intake or acute weight loss in RR rats, but does prevent the sustained down-regulation of body weight. These studies indicate a dissociation between food intake on the days of restraint and weight loss and imply an important role for stress-induced changes in energy expenditure. The results suggest that Intact rats are able to make a compensatory adjustment of energy expenditure to counteract the inhibition of food intake in the hours between the three corticosterone injections. From this study we cannot determine whether this compensation is disrupted by removal of the adrenal gland and negative energy balance contributes to weight loss in the ADX rats, or whether the catabolic effects of corticosterone mask a potential compensatory adjustment in energy expenditure.
As mentioned above we did not determine whether the corticosterone was acting peripherally or centrally in the studies described here. We have previously shown that although activation of CRFR1 in areas surrounding the third ventricle is not required for weight loss during restraint, it is required for RR rats to maintain their reduced body weight in the post-restraint period (20). Therefore, it would be reasonable to assume that the long-term effects of corticosterone on body weight in ADX rats also are mediated by receptors in the brain rather than peripheral tissue. The exact location of the CRFR1 responsible for the initiation of a long-term down regulation of body weight has not been determined, but CRFR1 are expressed in hypothalamic areas associated with control of energy intake and expenditure, including the paraventricular, dorsomedial, arcuate and lateral nuclei of the hypothalamus (36). CRFR1 mRNA expression in the PVN is increased by restraint (36), which would be consistent with our observation that antagonism of these receptors only during the period of restraint was adequate for inhibition of a long-term effect of restraint on body weight regulation (20).
Measurement of CRF mRNA in the PVN of rats in Experiment 3 confirmed previous observations that both ADX and RR increase CRF mRNA expression in the parvocellular areas of the PVN (1, 10–11, 37–38). There was no additive effect of the two treatments even though the ADX effect may have been attenuated by the rats having access to corticosterone in their drinking water. In addition, the acute injection of corticosterone before the start of restraint did not inhibit CRF mRNA expression in the ADX rats. Others have reported that a single injection of corticosterone inhibits PVN expression of CRF hnRNA (39–40) and chronic, continuous delivery of corticosterone has been shown to down-regulate PVN CRF mRNA expression in a dose-dependent manner (13, 40). The ADX rats in the studies described here consumed corticosterone with their drinking water, which produced low/normal circulating concentrations of corticosterone. These levels of corticosterone obviously were not adequate to normalize PVN CRF mRNA expression, but may have blunted the effect of ADX on CRF mRNA. Restraint had no significant effect on AVP mRNA in the PVN, confirming observations from this laboratory and others (23, 38, 41–42) and supporting the suggestion that AVP mRNA expression changes only after extended exposure to repeated restraint or chronic restraint (38, 43). The similarity of AVP PVN mRNA expression in control saline-injected Intact and ADX rats is consistent with reports that low levels of corticosterone normalize AVP mRNA expression in the PVN of adrenalectomized rats (42, 44) because all of the ADX rats in this study had free access to corticosterone in their drinking water. The similarity in AVP mRNA expression in the hypothalamus of all rats in Experiment 3 also shows that AVP is not directly involved in determining weight loss of stressed rats which is consistent with a previous report that AVP-deplete Brattleboro rats lost weight in a manner similar to controls when they were exposed to 60 minutes of restraint on each of 15 days (45).
In summary, these experiments demonstrate that a surge in corticosterone contributes to the initial weight loss in restrained ADX rats, but that corticosterone alone does not produce a similar weight loss in Intact rats, possibly due to an increased sensitivity to the catabolic effects of glucocorticoids in ADX rats. The results also show that there are other aspects of restraint that contribute to weight loss because corticosterone injection in non-restrained ADX rats and restraint of ADX rats injected with saline each produced only half as much weight loss as was observed in Intact restrained rats. Corticosterone also may contribute to the initiation of a sustained weight loss in RR rats because non-restrained ADX rats injected with corticosterone showed the same rate of weight gain in the post-restraint period as Intact restrained rats. We confirmed that both RR and ADX produce significant increases in CRF mRNA expression in the PVN, but do not change AVP mRNA, however, the mechanism by which acute surges of corticosterone cause weight loss in ADX rats does not appear to involve a change in PVN CRF mRNA expression.
Research Highlights.
Restraint stress induces both acute and sustained weight loss in rats.
Adrenalectomy attenuates acute stress-induced weight loss
Stress-induced surges in corticosterone cause acute and sustained weight loss in ADX rats
Stress-induced surges in corticosterone do not influence CRF or AVP mRNA in the hypothalamus
Stress-induced weight loss is partially dependent on a surge in corticosterone
ACKNOWLEDGEMENTS
This work was supported by NIH grant MH068281 awarded to RBSH. The authors thank Samantha Haring, Rachel Doyle and Hayden Kramer for their assistance with this project.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- 1.Harris RB, Palmondon J, Leshin S, Flatt WP, Richard D. Chronic disruption of body weight but not of stress peptides or receptors in rats exposed to repeated restraint stress. Horm Behav. 2006;49:615–625. doi: 10.1016/j.yhbeh.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 2.Harris RB, Martin RJ. Recovery of body weight from below “set point” in mature female rats. J Nutr. 1984;114:1143–1150. doi: 10.1093/jn/114.6.1143. [DOI] [PubMed] [Google Scholar]
- 3.Harris RB, Kasser TR, Martin RJ. Dynamics of recovery of body composition after overfeeding, food restriction or starvation of mature female rats. J Nutr. 1986;116:2536–2546. doi: 10.1093/jn/116.12.2536. [DOI] [PubMed] [Google Scholar]
- 4.Meerlo P, De Boer SF, Koolhaas JM, Daan S, Van den Hoofdakker RH. Changes in daily rhythms of body temperature and activity after a single social defeat in rats. Physiol Behav. 1996;59:735–739. doi: 10.1016/0031-9384(95)02182-5. [DOI] [PubMed] [Google Scholar]
- 5.Valles A, Marti O, Garcia A, Armario A. Single exposure to stressors causes long-lasting, stress-dependent reduction of food intake in rats. Am J Physiol Regul Integr Comp Physiol. 2000;279:R1138–1144. doi: 10.1152/ajpregu.2000.279.3.R1138. [DOI] [PubMed] [Google Scholar]
- 6.Sawchenko PE, Imaki T, Potter E, Kovacs K, Imaki J, Vale W. The functional neuroanatomy of corticotropin-releasing factor. Ciba Found Symp. 1993;172:5–21. doi: 10.1002/9780470514368.ch2. [DOI] [PubMed] [Google Scholar]
- 7.Garcia A, Marti O, Valles A, Dal-Zotto S, Armario A. Recovery of the hypothalamic-pituitary-adrenal response to stress. Effect of stress intensity, stress duration and previous stress exposure. Neuroendocrinology. 2000;72:114–125. doi: 10.1159/000054578. [DOI] [PubMed] [Google Scholar]
- 8.Oitzl MS, van Haarst AD, Sutanto W, de Kloet ER. Corticosterone, brain mineralocorticoid receptors (MRs) and the activity of the hypothalamic-pituitary-adrenal (HPA) axis: the Lewis rat as an example of increased central MR capacity and a hyporesponsive HPA axis. Psychoneuroendocrinology. 1995;20:655–675. doi: 10.1016/0306-4530(95)00003-7. [DOI] [PubMed] [Google Scholar]
- 9.Bale TL, Vale WW. CRF and CRF receptors: role in stress responsivity and other behaviors. Annu Rev Pharmacol Toxicol. 2004;44:525–557. doi: 10.1146/annurev.pharmtox.44.101802.121410. [DOI] [PubMed] [Google Scholar]
- 10.Kalin NH, Takahashi LK, Chen FL. Restraint stress increases corticotropin-releasing hormone mRNA content in the amygdala and paraventricular nucleus. Brain Res. 1994;656:182–186. doi: 10.1016/0006-8993(94)91382-x. [DOI] [PubMed] [Google Scholar]
- 11.Hsu DT, Lombardo KA, Herringa RJ, Bakshi VP, Roseboom PH, Kalin NH. Corticotropin-releasing hormone messenger RNA distribution and stress-induced activation in the thalamus. Neurosci. 2001;105:911–921. doi: 10.1016/s0306-4522(01)00239-1. [DOI] [PubMed] [Google Scholar]
- 12.Laugero KD, Gomez F, Manalo S, Dallman MF. Corticosterone infused intracerebroventricularly inhibits energy storage and stimulates the hypothalamo-pituitary axis in adrenalectomized rats drinking sucrose. Endocrinology. 2002;143:4552–4562. doi: 10.1210/en.2002-220613. [DOI] [PubMed] [Google Scholar]
- 13.Schwartz MW, Strack AM, Dallman MF. Evidence that elevated plasma corticosterone levels are the cause of reduced hypothalamic corticotrophin-releasing hormone gene expression in diabetes. Regul Pept. 1997;72:105–112. doi: 10.1016/s0167-0115(97)01043-4. [DOI] [PubMed] [Google Scholar]
- 14.Makara GB, Mergl Z, Zelena D. The role of vasopressin in hypothalamo-pituitary-adrenal axis activation during stress: an assessment of the evidence. Ann N Y Acad Sci. 2004;1018:151–161. doi: 10.1196/annals.1296.018. [DOI] [PubMed] [Google Scholar]
- 15.Ma XM, Lightman SL, Aguilera G. Vasopressin and corticotropin-releasing hormone gene responses to novel stress in rats adapted to repeated restraint. Endocrinology. 1999;140:3623–3632. doi: 10.1210/endo.140.8.6943. [DOI] [PubMed] [Google Scholar]
- 16.Marti O, Harbuz MS, Andres R, Lightman SL, Armario A. Activation of the hypothalamic-pituitary axis in adrenalectomised rats: potentiation by chronic stress. Brain Res. 1999;821:1–7. doi: 10.1016/s0006-8993(98)01212-8. [DOI] [PubMed] [Google Scholar]
- 17.Makino S, Schulkin J, Smith MA, Pacak K, Palkovits M, Gold PW. Regulation of corticotropin-releasing hormone receptor messenger ribonucleic acid in the rat brain and pituitary by glucocorticoids and stress. Endocrinology. 1995;136:4517–4525. doi: 10.1210/endo.136.10.7664672. [DOI] [PubMed] [Google Scholar]
- 18.Gomez F, Houshyar H, Dallman MF. Marked regulatory shifts in gonadal, adrenal, and metabolic system responses to repeated restraint stress occur within a 3-week period in pubertal male rats. Endocrinology. 2002;143:2852–2862. doi: 10.1210/endo.143.8.8929. [DOI] [PubMed] [Google Scholar]
- 19.Fleshner M, Deak T, Nguyen KT, Watkins LR, Maier SF. Endogenous glucocorticoids play a positive regulatory role in the anti-keyhole limpet hemocyanin in vivo antibody response. J Immunol. 2001;166:3813–3819. doi: 10.4049/jimmunol.166.6.3813. [DOI] [PubMed] [Google Scholar]
- 20.Chotiwat C, Harris RB. Antagonism of specific corticotropin-releasing factor receptor subtypes selectively modifies weight loss in restrained rats. Am J Physiol Regul Integr Comp Physiol. 2008;295:R1762–1773. doi: 10.1152/ajpregu.00196.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobson L, Akana SF, Cascio CS, Shinsako J, Dallman MF. Circadian variations in plasma corticosterone permit normal termination of adrenocorticotropin responses to stress. Endocrinology. 1988;122:1343–1348. doi: 10.1210/endo-122-4-1343. [DOI] [PubMed] [Google Scholar]
- 22.Harris RB, Mitchell TD, Simpson J, Redmann SM, Jr., Youngblood BD, Ryan DH. Weight loss in rats exposed to repeated acute restraint stress is independent of energy or leptin status. Am J Physiol Regul Integr Comp Physiol. 2002;282:R77–88. doi: 10.1152/ajpregu.2002.282.1.R77. [DOI] [PubMed] [Google Scholar]
- 23.Rybkin II, Zhou Y, Volaufova J, Smagin GN, Ryan DH, Harris RB. Effect of restraint stress on food intake and body weight is determined by time of day. American Journal of Physiology. 1997;273:R1612–1622. doi: 10.1152/ajpregu.1997.273.5.R1612. [DOI] [PubMed] [Google Scholar]
- 24.Paxinos G, Watson C. The rat brain. Academic Press; New York: 1998. [Google Scholar]
- 25.Harris RB, Zhou J, Youngblood BD, Rybkin II, Smagin GN, Ryan DH. Effect of repeated stress on body weight and body composition of rats fed low- and high-fat diets. Am J Physiol. 1998;275:R1928–1938. doi: 10.1152/ajpregu.1998.275.6.R1928. [DOI] [PubMed] [Google Scholar]
- 26.Keller-Wood ME, Dallman MF. Corticosteroid inhibition of ACTH secretion. Endocr Rev. 1984;5:1–24. doi: 10.1210/edrv-5-1-1. [DOI] [PubMed] [Google Scholar]
- 27.Jankord R, Herman JP. Limbic regulation of hypothalamo-pituitary-adrenocortical function during acute and chronic stress. Ann N Y Acad Sci. 2008;1148:64–73. doi: 10.1196/annals.1410.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Ulrich-Lai YM, Herman JP. Neural regulation of endocrine and autonomic stress responses. Nat Rev Neurosci. 2009;10:397–409. doi: 10.1038/nrn2647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Quan ZY, Walser M. Effect of corticosterone administration at varying levels on leucine oxidation and whole body protein synthesis and breakdown in adrenalectomized rats. Metabolism. 1991;40:1263–1267. doi: 10.1016/0026-0495(91)90026-s. [DOI] [PubMed] [Google Scholar]
- 30.Zhou J, Shi MX, Mitchell TD, Smagin GN, Thomas SR, Ryan DH, Harris RB. Changes in rat adipocyte and liver glucose metabolism following repeated restraint stress. Exp Biol Med. 2001;226:312–319. doi: 10.1177/153537020122600408. [DOI] [PubMed] [Google Scholar]
- 31.Zhou J, Yan X, Ryan DH, Harris RB. Sustained effects of repeated restraint stress on muscle and adipocyte metabolism in high-fat-fed rats. Am J Physiol. 1999;277:R757–R766. doi: 10.1152/ajpregu.1999.277.3.R757. [DOI] [PubMed] [Google Scholar]
- 32.Gregory MC, Duval D, Meyer P. Changes in cardiac and hepatic glucocorticoid receptors after adrenalectomy. Clin Sci Mol Med. 1976;51:487–493. doi: 10.1042/cs0510487. [DOI] [PubMed] [Google Scholar]
- 33.Tornello S, Orti E, De Nicola AF, Rainbow TC, McEwen BS. Regulation of glucocorticoid receptors in brain by corticosterone treatment of adrenalectomized rats. Neuroendocrinology. 1982;35:411–417. doi: 10.1159/000123429. [DOI] [PubMed] [Google Scholar]
- 34.Herman JP, Ostrander MM, Mueller NK, Figueiredo H. Limbic system mechanisms of stress regulation: hypothalamo-pituitary-adrenocortical axis. Prog Neuropsychopharmacol Biol Psychiatry. 2005;29:1201–1213. doi: 10.1016/j.pnpbp.2005.08.006. [DOI] [PubMed] [Google Scholar]
- 35.Dong HW, Swanson LW. Projections from bed nuclei of the stria terminalis, anteromedial area: cerebral hemisphere integration of neuroendocrine, autonomic, and behavioral aspects of energy balance. J Comp Neurol. 2006;494:142–178. doi: 10.1002/cne.20788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Van Pett K, Viau V, Bittencourt JC, Chan RK, Li HY, Arias C, Prins GS, Perrin M, Vale W, Sawchenko PE. Distribution of mRNAs encoding CRF receptors in brain and pituitary of rat and mouse. J Comp Neurol. 2000;428:191–212. doi: 10.1002/1096-9861(20001211)428:2<191::aid-cne1>3.0.co;2-u. [DOI] [PubMed] [Google Scholar]
- 37.Makino S, Gold PW, Schulkin J. Corticosterone effects on corticotropin-releasing hormone mRNA in the central nucleus of the amygdala and the parvocellular region of the paraventricular nucleus of the hypothalamus. Brain Res. 1994;640:105–112. doi: 10.1016/0006-8993(94)91862-7. [DOI] [PubMed] [Google Scholar]
- 38.Makino S, Smith MA, Gold PW. Increased expression of corticotropin-releasing hormone and vasopressin messenger ribonucleic acid (mRNA) in the hypothalamic paraventricular nucleus during repeated stress: association with reduction in glucocorticoid receptor mRNA levels. Endocrinology. 1995;136:3299–3309. doi: 10.1210/endo.136.8.7628364. [DOI] [PubMed] [Google Scholar]
- 39.Ginsberg AB, Campeau S, Day HE, Spencer RL. Acute glucocorticoid pretreatment suppresses stress-induced hypothalamic-pituitary-adrenal axis hormone secretion and expression of corticotropin-releasing hormone hnRNA but does not affect c-fos mRNA or fos protein expression in the paraventricular nucleus of the hypothalamus. J Neuroendocrinol. 2003;15:1075–1083. doi: 10.1046/j.1365-2826.2003.01100.x. [DOI] [PubMed] [Google Scholar]
- 40.Imaki T, Xiao-Quan W, Shibasaki T, Yamada K, Harada S, Chikada N, Naruse M, Demura H. Stress-induced activation of neuronal activity and corticotropin-releasing factor gene expression in the paraventricular nucleus is modulated by glucocorticoids in rats. J Clin Invest. 1995;96:231–238. doi: 10.1172/JCI118026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pinnock SB, Herbert J. Corticosterone differentially modulates expression of corticotropin releasing factor and arginine vasopressin mRNA in the hypothalamic paraventricular nucleus following either acute or repeated restraint stress. Eur J Neurosci. 2001;13:576–584. doi: 10.1046/j.0953-816x.2000.01406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Pace TW, Gaylord RI, Jarvis E, Girotti M, Spencer RL. Differential glucocorticoid effects on stress-induced gene expression in the paraventricular nucleus of the hypothalamus and ACTH secretion in the rat. Stress. 2009;12:400–411. doi: 10.1080/10253890802530730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ma XM, Lightman SL. The arginine vasopressin and corticotrophin-releasing hormone gene transcription responses to varied frequencies of repeated stress in rats. J Physiol. 1998;510(Pt 2):605–614. doi: 10.1111/j.1469-7793.1998.605bk.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Imaki T, Nahan JL, Rivier C, Sawchenko PE, Vale W. Differential regulation of corticotropin-releasing factor mRNA in rat brain regions by glucocorticoids and stress. J Neurosci. 1991;11:585–599. doi: 10.1523/JNEUROSCI.11-03-00585.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zelena D, Foldes A, Mergl Z, Barna I, Kovacs KJ, Makara GB. Effects of repeated restraint stress on hypothalamo-pituitary-adrenocortical function in vasopressin deficient Brattleboro rats. Brain Res Bull. 2004;63:521–530. doi: 10.1016/j.brainresbull.2004.04.007. [DOI] [PubMed] [Google Scholar]
- 46.Jingami H, Matsukura S, Numa S, Imura H. Effects of adrenalectomy and dexamethasone administration on the level of prepro-corticotropin-releasing factor messenger ribonucleic acid (mRNA) in the hypothalamus and adrenocorticotropin/beta-lipotropin precursor mRNA in the pituitary in rats. Endocrinology. 1985;117:1314–1320. doi: 10.1210/endo-117-4-1314. [DOI] [PubMed] [Google Scholar]
- 47.Ivell R, Richter D. Structure and comparison of the oxytocin and vasopressin genes from rat. Proc Natl Acad Sci U S A. 1984;81:2006–2010. doi: 10.1073/pnas.81.7.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]