Abstract
The therapeutic utility of oncolytic adenoviruses controlled by a single, tumor-specific regulatory element may be limited by the intra- and inter-tumoral heterogeneity that characterizes many cancers. To address this issue, we constructed an oncolytic adenovirus that uses two distinct tumor-specific promoters (DF3/Muc1 and hTERT) to drive separate E1A expression cassettes, in combination with deletion of the viral E1B region, which confers additional tumor selectivity and increased oncolytic activity. The resultant virus, Adeno-DF3-E1A/hTERT-E1A, induced higher levels of E1A oncoprotein, enhanced oncolysis, and an earlier and higher apoptotic index in infected tumor cells than following infection with Adeno-hTERT-E1A, which harbors a single hTERT promoter-driven E1A cassette. In isolated U251 human gliosarcoma cell holoclones (putative cancer stem cells), where DF3/Muc1 expression is substantially enriched and hTERT expression is decreased compared to the parental U251 cell population, E1A production and oncolysis were strongly decreased following infection with Adeno-hTERT-E1A but not Adeno-DF3-E1A/hTERT-E1A. The strong oncolytic activity of Adeno-DF3-E1A/hTERT-E1A translated into superior anti-tumor activity over Adeno-hTERT-E1A in vivo in a U251 solid tumor xenograft model, where hTERT levels were >90% suppressed and the DF3/Muc1 to hTERT expression ratio was substantially increased compared to cultured U251 cells. The enhanced anti-tumor activity of the dual-targeted Adeno-DF3-E1A/hTERT-E1A was achieved despite premature viral host cell death and decreased production of functional viral progeny, which limited tumor cell spread of the viral infection. These findings highlight the therapeutic benefit of targeting oncolytic viruses to heterogeneous tumor cell populations.
Keywords: Cancer gene therapy, replication-conditional, oncolytic adenovirus, tumor heterogeneity, holoclone, cytochrome P450 prodrug activation
INTRODUCTION
Replication-conditional adenoviruses engineered for cancer specificity have shown promising stand-alone anti-tumor activity as well as potential for therapeutic gene delivery1, 2. Various strategies have been used to improve tumor-specific adenoviral activity while reducing toxicity to non-cancerous host tissues. For example, cancer-specific promoter elements can be used to control expression of the E1A oncoprotein, which is critical for the transcriptional activation and replication of adenoviruses. However, even with these improvements, such tumor-targeted adenoviruses have had limited success in the clinic2-4.
The hTERT promoter displays high activity in a majority of human cancers but not in most host tissues5, 6 and is considered as an ideal tumor-specific regulator for oncolytic adenoviruses7, 8. Previously, we characterized an hTERT promoter fragment, which in the context of the replication-conditional adenovirus Adeno-hTERT-E1A recapitulated high telomerase promoter-based E1A expression and viral activity in cancer cells but not in primary human hepatocytes7. Conceivably, the anti-tumor activity of oncolytic adenoviruses, such as Adeno-hTERT-E1A, might be increased by introducing a second E1A cassette under the control of an independent, cancer-specific promoter element. One such element is derived from the MUC1 gene, which codes for DF3 antigen. DF3/Muc1 is a mucin-like glycoprotein that is overexpressed in ~75% of all human solid tumors, including many late-stage cancers of the pancreas, prostate, breast, ovaries and lungs9-13. DF3/Muc1 functions as an oncogene14, is a determinant of resistance to Herceptin15, regulates intracellular oxidant levels and apoptosis in response to oxidative stress16, and plays a role in cellular adhesion, invasion and metastasis10. DF3/Muc1 is also associated with tumor-forming stem/progenitor cell-like side populations in MCF-7 breast cancer cells17 and regulation of human pluripotent stem cell growth18.
Presently, we investigated the use of two separate tumor-specific promoter elements to regulate adenoviral E1A production, and hence viral replication, in a cancer-specific manner. The goal of this strategy was to increase viral host range to encompass a broad array of human tumors, including tumors with cell subpopulations having large differences in DF3/Muc1 and hTERT activity. Tumor cells infected with the dual E1A cassette virus described here, Adeno-DF3-E1A/hTERT-E1A, are shown to express higher levels of E1A oncoprotein and undergo enhanced lysis with an earlier and higher apoptotic index than tumor cells infected with the corresponding single E1A cassette virus, Adeno-hTERT-E1A. However, the increased apoptosis stimulated by Adeno-DF3-E1A/hTERT-E1A is accompanied by decreases in viral release, viral spread, and activity as a helper virus compared to Adeno-hTERT-E1A. Nevertheless, the increased oncolytic activity of Adeno-DF3-E1A/hTERT-E1A translated into greater anti-tumor activity in a human gliosarcoma tumor xenograft model where putative cancer stem cells, holoclones—clonally isolated cells that have cancer stem cell-like properties and grow with a holoclone morphology19, show elevated expression of DF3/Muc1 compared to hTERT. Thus, the dual E1A cassette adenovirus displays therapeutic potential as a stand-alone anticancer agent against heterogeneous populations of tumor cells, albeit with decreased ability to replicate and spread an adenoviral infection.
MATERIALS and METHODS
Cell lines and reagents
CPA was purchased from Sigma Chemical Co. (St. Louis, MO). Fetal bovine serum (FBS) and RPMI culture medium were purchased from InVitrogen (Frederick, MD). Human tumor cell lines and primary hepatocytes were those used previously7. Human tumor cells were grown at 37°C in a humidified, 5% CO2 atmosphere in RPMI 1640 culture medium containing 5% FBS, 100 units/ml penicillin, and 100 μg/ml streptomycin. U251 gliosarcoma cells implanted in scid mice (see below) were grown in RPMI/10% FBS and antibiotics. Adeno-2B11, a replication-deficient adenovirus encoding the cytochrome P450 gene CYP2B11, was previously described20.
DF3/Muc1 promoter activity assays
A DF3/Muc1 core promoter fragment (nts −699 to +27) was PCR-amplified from MCF-7 genomic DNA cells using the primers: 5′-tactcgagACCCTAGGGTTCATCGGAG-3′ and 5′-tactcgagATTCAGGCAGGCGCTGGCT-3′, where lower case letters designate engineered restriction enzyme sites. The TATA-containing DF3/Muc1 core promoter was verified by sequencing and sub-cloned into the reporter plasmid pGL3-Basic (Promega, Inc.) upstream of the luciferase gene using the restriction enzymes Nhe I and Xho I (New England Biolabs Inc., Beverly, MA). The resulting luciferase reporter vector, pGL3-DF3-Luc, was transfected with TransIT-LT1 (Cat. # MIR 2300; Mirus, Inc.) into individual tumor cell lines followed by dual luciferase analysis7.
qPCR analysis
DF3/Muc1 and hTERT RNAs were quantified in tumor cell lines and in solid tumors grown s.c. in scid mice by qPCR as described7 using with the following human-specific primers: 5′-ACGGCGACATGGAGAACAA-3′ (hTERT sense), 5′-CACTGTCTTCCGCAAGTTCAC-3′ (hTERT antisense), 5′-CTTTCTTCCTGCTGCTGCTCCT-3′ (DF3/Muc1 sense), and 5′-AGCCGAAGTCTCCTTTTCTCCA-3′ (DF3/Muc1 antisense). qPCR analysis of IL8 and ALDH1A1 RNAs used the following primers: 5′-TTGTGTTAGCTGATGCCGACTT-3′ (ALDH1A1 sense), 5′-CCATGGTGTGCAAATTCAACAG-3′ (ALDH1A1 antisense), 5′- CTGGGTGCAGAGGGTTGTGGAGA -3′ (IL-8 sense), and 5′- TGGCAACCCTACAACAGACCCACA -3′ (IL-8 antisense). Results were analyzed using the comparative CT method as described by the manufacturer. Data were normalized to the 18S RNA content of each sample, determined by qPCR as described7.
Construction of Adeno-DF3-E1A/hTERT-E1A
The single E1A cassette Adeno-hTERT-E1A was prepared as described7. To construct a DF3-E1A expression cassette, luciferase cDNA was excised from pGL3-DF3-Luc using HindIII and XbaI and the vector was religated to generate pGL3-DF3. Next, the viral E1A gene was PCR-amplified from the plasmid pXC1 (Microbix, Inc., Toronto, Canada) using the primers 5′-tagagatctGCCACTCTTGAGTGCCAGCGAG-3′ (forward) and 5′-tagagatctCACCACTCTATCACCCACTGC-3′ (reverse). The PCR product was BglII digested and ligated into BglII-digested pGL3-DF3 to yield pGL3-DF3-E1A. The DF3- and hTERT-E1A cassettes used to construct the dual E1A cassette adenovirus both contain the same E1A gene sequence and were verified for sequence integrity and functionality as described7. To generate the final adenoviral construct, Adeno-DF3-E1A/hTERT-E1A, the entire DF3-E1A cassette, including an SV40 late polyA signal, was excised using BamHI, blunt-ended, digested with MluI for complete excision and inserted into a SpeI-digested, blunt-ended, and then MluI-digested pShuttle-hTERT-E1A7. This cloning step ensured directionality of the incorporated DF3-E1A-polyA cassette and insertion ~2 kb upstream of the previously positioned hTERT-E1A-polyA cassette. The entire ICeuI-PISceI pShuttle fragment containing the DF3-E1A and hTERT-E1A expression cassettes was then ligated by Dr. Y. Jounaidi of this laboratory into the adenoviral genome using the Adeno-X expression system, per the manufacturer′s instructions (Clontech Labs). The Adeno-X genome is E1- and E3-region deleted, leaving room for up to ~8.3 kb of sequence insertion; the cassettes for DF3-E1A-polyA (~2.1 kb), hTERT-E1A-polyA (~1.75 kb), and pShuttle (~1.1 kb, including an unused CMV promoter insertion site) constitute ~4.95 kb of that space, allowing for up to 3 kb additional sequence downstream of the CMV promoter (Fig. 1C). Adenoviral stocks were propagated, amplified, and purified21. Viral titers were quantified and verified in triplicate in 293 cells using the Adeno-X™ Rapid Titer kit (Clontech Labs); as such, they represent functional titers based on final hexon production. Virus aliquots were stored at −80°C. Genomic integrity of the final virus preparation was verified by PCR and DNA sequencing.
Figure 1.


DF3/Muc1 promoter characterization and Adeno-DF3-E1A/hTERT-E1A genome construction. A, Luciferase reporter activity of the DF3/Muc1 core promoter in a panel of human tumor cell lines. B, qPCR analysis of endogenous DF3/Muc1 RNA for the same cell lines presented in panel A, with the addition of human hepatocytes. RNA levels are calculated relative to that of human hepatocytes, mean ± SD for n = 3 replicates. C, Transcription factor binding sites in the DF3/Muc1 core promoter fragment (−699 to +27) and in the hTERT promoter sequence described previously 7 were cloned upstream of the adenoviral E1A gene (step 1). The DF3-E1A-polyA and hTERT-E1A-polyA cassettes were then inserted into the Shuttle vector (step 2) and finally into the adenoviral genome (step 3). ITR, inverted terminal repeat; B.E., blunt-ended.
Cell line infectivity and oncolysis
The intrinsic adenoviral infectivity of each cell line was determined by infection with Adeno-β-Gal7. To compare the cytolytic activity of Adeno-hTERT-E1A to that of Adeno-DF3-E1A/hTERT-E1A, cells were plated overnight in triplicate at 14,000 cells/well of a 24-well plate. After 24 hr the cells were infected for a 4 hr period with virus in 200 μl culture medium containing the indicated MOI of Adeno-hTERT-E1A or Adeno-DF3-E1A/hTERT-E1A, after which 1 ml of fresh medium was added to each well. Cells remaining 3 or 6 days later were stained and quantified using crystal violet22. Data are expressed as cell number (A595) relative to uninfected cell controls, mean ± SD for triplicate samples.
Western blotting and caspase assays
Western blotting for E1A and PARP-p85 protein7 used extracts prepared from adenovirus-infected cells seeded at 300,000 cells/well in a 6-well plate 24 hr prior to infection. To assay virus-induced apoptosis, tumor cells were seeded in triplicate at 5,000 cells/well in a 96-well plate. The next day, cells in each well were infected with Adeno-hTERT-E1A or Adeno-DF3-E1A/hTERT-E1A at MOIs of 0, 1, 10, and 100 in a total vol of 50 μl/well. After a 30 hr infection, 50 μl of reconstituted Promega Caspase-Glo luminescence assay reagent (Cat#G8091, Promega Corp., Madison, WI) was added to each well, incubated for 1 hr, and then luminescence readings were taken on a Wallac VICTOR3 1420 Multilabel Counter plate-reader (Cat#1420-032, Perkin Elmer Inc., Waltham, MA). Cell line-specific background activity, determined from 0 MOI control samples, was subtracted from each sample.
Human tumor xenografts
Five-wk-old (24 to 26 g) male ICR/Fox Chase scid mice (Taconic Farms, Germantown, NY) were housed in the Boston University Laboratory of Animal Care Facility and treated in accordance with approved protocols and federal guidelines. Mice were injected s.c. on each posterior flank with 6 × 106 U251(NIH) cells in 0.2 ml serum-free RPMI using a 0.5-inch 29-gauge needle and 1 ml insulin syringe. Tumor areas (length × width) were measured twice weekly using Vernier calipers (VWR, Cat#62379-531) and tumor vol was calculated based on Vol = (π/6)*(L*W)3/2. When the tumors reached ~150 mm3 in vol, the mice were divided into three groups (5 mice, 10 tumors per group): (a) Control group, PBS (vehicle) injected intratumorally (i.t.), 90 μl/tumor/day on two consecutive days; (b) Adeno-hTERT-E1A, i.t., 5 × 108 plaque forming units (pfu) injected at a single site in a vol of 90 μl/tumor/day on 2 consecutive days (total 1 × 109 pfu); and (c) Adeno-DF3-E1A/hTERT-E1A, i.t., 5 × 108 pfu injected in 90 μl/tumor/day on 2 consecutive days (total 1 × 109 pfu). A second series of two daily virus or vehicle injections was administered to each tumor 15 days after the first treatment cycle. Tumor growth rates prior to virus administration were similar in all three groups. One-way ANOVA analysis using the Bonferroni multiple comparison was carried out using GraphPad Prism 4 software (San Diego, CA).
U251 holoclone studies
Holoclones were identified based on colony morphology after single cell clonal seeding in RPMI + 7% FBS (1 cell/well in 96-well plates)19. Holoclones represented ~10% of the original U251 cell population used to select the holoclones; the other U251 cells displayed a meroclone (~30%) or paraclone morphology (~60%). RNA analysis of individual holoclones (clones designated A1, B3 and B6) was carried out in comparison to U251 parental cells and mixed meroclone/paraclone subpopulations by qPCR using RNA extracted from 300,000 cells/well of a 6-well plate for each clone. The intrinsic adenoviral infectivity of the U251 clones, and the parental U251 cells, was determined by X-gal staining after a 48 hr infection with Adeno-β-gal at MOIs of 0, 0.25, 1, and 5. X-gal-stained cells were eluted was DMSO and A650 was determined.
Titration of functional virus particles released into culture supernatant
Adenovirus titers in culture supernatants were determined by hexon staining of 293 cells incubated for 30 hr with the culture supernatant from adenovirus-infected U251 and A549 cells7 with the following modifications. Cells were infected for 4 hr with either Adeno-hTERT-E1A or Adeno-DF3-E1A/hTERT-E1A at an MOI of 5, washed with 1 ml of fresh RPMI/5% FBS growth medium, refreshed with 1 ml of RPMI/5% FBS and incubated for the indicated times (1, 3, or 5 days for U251 cells, and 3, 5, or 7 for A549 cells). At each time point, supernatant from each well (~ 1 ml) was used to prepare 10-fold serial dilutions (100, 10−1, and 10−2 for U251 supernatants; 10−2, 10−3, and 10−4 for A549 supernatants). Each dilution (0.5 ml) was used to infect 293 cells (seeded in 24 well plates 24 hr earlier at 100,000 cells/well) for a 4 hr period, followed by the addition of 1 ml of fresh RPMI/5% FBS medium per well. The infected 293 cells were incubated for a further 26 hr (30 hr total infection), methanol-fixed, stored at −20°C and processed for hexon immunostaining7.
Helper virus studies
U251 cells were seeded at 40,000 cells/well in 12-well plates. 24 hr later the cells were infected with Adeno-2B11 (at MOI 8) in combination with Adeno-hTERT-E1A, Adeno-DF3-E1A/hTERT-E1A, or ONYX-015 (at MOIs of 0, 1.5, and 5), and in a total vol of 0.4 ml for an initial period of 4 hr, after which 1 ml of fresh medium was added/well. After 72 hr, U251 supernatants (~1.5 ml/well) were extracted, mixed thoroughly and divided into 7 aliquots of 0.2 ml each. Aliquots were applied to fresh U251 cells, seeded 24 hr earlier in 24 well plates at 4,000 cells/well, to assay replicating virus-dependent CPA cytotoxicity. A sample of each supernatant (0.2 ml) was also applied to fresh 293 cells to titrate adenovirus release, as described above. Each aliquot of U251 culture supernatant was applied to the fresh U251 cells for a 4 hr period, after which 0.5 ml fresh medium was added per well. The next day (day 4), the culture medium was aspirated and replaced by 1 ml of fresh medium containing CPA at 0-1000 μM, final concentrations. Cells were incubated for 48 hr, at which time the culture medium was changed to fresh-CPA-containing medium and the cells were incubated for another 4 days (10 days total). Cells were stained with crystal violet and relative cell numbers were determined by A595.
Adeno-2B11 spread and HPLC analysis of 4-OH-CPA production
Cells were seeded in 6-well plates at 75,000 cells/well. 24 hr later, the cells were infected for 4 hr with Adeno-2B11 (MOI 0 (control), MOI 8 (U251 cells) or MOI 32 (MCF-7/4HC and MDA-MB-231 cells), based on each cell line's intrinsic adenoviral infectivity) in combination with either Adeno-hTERT-E1A or ONYX-015 at a range of MOIs in 1 ml RPMI 1640 containing 5% FBS. Fresh culture medium (2 ml) was then added directly to each well and the incubation with virus was continued for 48 hr total. The cells were then treated for 4 hr with 250 μM CPA in 3 ml fresh RPMI 1640 containing 5% FBS and 5 mM semicarbazide. Culture medium (0.5 ml) was then removed and assayed for 4-OH-CPA by HPLC20. The remaining cells were quantified by crystal violet staining. Controls, either incubated without CPA or uninfected cells, were set to 100% cell survival.
RESULTS
Tumor cell transcriptional activity of core DF3-promoter
The DF3/Muc1 core promoter (nts −699 to +27) contains an E-box, several GC-rich regions and Sp1 sites, and a TATA box23-25. To ascertain whether this promoter fragment could confer strong expression in tumor cells, a luciferase reporter plasmid driven by this promoter was transfected into a panel of human tumor cell lines (Fig. 1A). DF3/Muc1 promoter-regulated luciferase activity had a cell line expression profile similar to that of endogenous DF3/Muc1 RNA (Fig. 1B) in most of the cell lines tested, suggesting that the core promoter activity is an important determinant of cellular DF3/Muc1 RNA levels. However, in the case of BT-549 and A549 cells, high luciferase promoter activity but low endogenous RNA was observed, suggesting negative regulatory sequences outside of the core DF3 promoter construct may be important for DF3 expression in these two cell lines. DF3/Muc1 RNA varied between cell lines but was most highly expressed in the breast cancer cell lines (Fig. 1B; first six cell lines). DF3/Muc1 RNA was barely detectable in human hepatocytes, where adenoviruses have a strong natural tropism, supporting the use of DF3/Muc1 core promoter to facilitate cancer-specific adenoviral expression.
Oncolytic profile of Adeno-DF3-E1A/hTERT-E1A
We constructed Adeno-DF3-E1A/hTERT-E1A (Fig. 1C), which includes a DF3/Muc1 promoter-driven E1A cassette in addition to the hTERT promoter driven E1A cassette found in Adeno-hTERT-E1A7. The oncolytic profile of this dual E1A cassette virus was compared to that of the single EIA cassette Adeno-hTERT-E1A (Fig. 2). U251 human gliosarcoma cells infected with Adeno-DF3-E1A/hTERT-E1A but not Adeno-hTERT-E1A showed extensive cell death within 24-48 hr (not shown). Moreover, higher MOIs of Adeno-hTERT-E1A were required to achieve a comparable level of oncolysis, as seen after 3 and 6 days (Fig. 2A). Although U251 cells have high endogenous hTERT activity7 and are deficient in DF3/Muc1 RNA (Fig. 1B), the activity of the core DF3/Muc1 promoter incorporated into our adenovirus (Fig. 1A) although low, is apparently sufficient to increase both the rate and the extent of Adeno-DF3-E1A/hTERT-E1A-dependent oncolysis compared to Adeno-hTERT-E1A-infected cells. The increase in activity in the other tumor cell line tested is, however, much greater than in U251 cells, whose very high hTERT expression7 is apparently responsible for the majority of the observed cell death (Fig. 2A). A greater increase in oncolytic activity with the dual E1A cassette virus was seen in A549 lung cancer cells and in MCF-7/4HC breast cancer cells (Fig. 2B, 2C), where DF3/Muc1 promoter activity is high (Fig. 1A). Oncolysis was weaker in MDA-MB-231 cells (Fig. 2D), which show low DF3/Muc1 promoter activity (Fig. 1A) and low intrinsic adenoviral infectivity7.
Figure 2.

Oncolytic activity of Adeno-DF3-E1A/hTERT-E1A and Adeno-hTERT-E1A − Four human tumor cell lines were infected with Adeno-DF3-E1A/hTERT-E1A or Adeno-hTERT-E11A at the indicated MOIs. The cells were stained with crystal violet 3 or 6 days later to quantify remaining cells. Data point represents mean ± SD values based on n = 3 replicates, with the uninfected cell controls set = 100%.
Increased expression of E1A and enhanced tumor cell apoptosis by dual E1A cassette virus
E1A protein production, a prerequisite for adenovirus replication and an indicator of an adenovirus' potential to lyse infected cells, was monitored in tumor cells infected with Adeno-DF3-E1A/hTERT-E1A and Adeno-hTERT-E1A. In accord with the oncolytic activity profiles of Adeno-DF3-E1A/hTERT-E1A shown in Fig. 2, E1A protein levels were higher in cells infected with the dual cassette virus (Fig. 3). Thus, addition of the second, DF3 promoter-regulated E1A cassette resulted in increased E1A expression in each cell line.
Figure 3.


E1A proteins levels and apoptosis induction assayed by PARP cleavage and caspase-3/7 activity in adenovirus-infected cells − A and B, Western blots of E1A protein in total cell lysates (50 μg/lane) of U251 and A549 cells infected for 24 hr, or MDA-MB-231 and MCF-7/4-HC cells infected for 48 hr with either Adeno-hTERT-E1A or Adeno-DF3-E1A/hTERT-E1A at the indicated MOIs. Blots for A549 and MCF-7/4HC were reprobed for the PARP cleavage product p85. Western blots were exposed in parallel for all cell lines; thus, antibody signal intensities are directly comparable. Higher MOIs and later time points were used for MCF-7 and MDA-MB-231 cells due to their lower intrinsic adenoviral infectivity 7. C, to assay virus-induced apoptosis, each tumor cell line was infected with the indicated viruses and MOIs and assayed for caspase 3/7 activity 30 hr later using a Caspase-Glo assay kit. Statistical analysis by two-tailed unpaired t-test with 95% confidence intervals identified significant increases in apoptosis (*, p < 0.05; **, p < 0.001). Caspase-Glo background activity values (subtracted from each sample) were: U251, 11,200; MDA-MB-231, 3,400; MCF-7/4HC, 830; and A549, 1820.
While adenoviral infection typically leads to cell death by a non-apoptotic mechanism26, the oncolytic viruses described here both lack E1B region genes that code for anti-apoptotic factors, E1B-19 kDa and E1B-55 kDa. Deletion of these genes may increase selectivity for cancer cells, where apoptotic pathways are mutated or suppressed. The increased expression of E1A in tumor cells infected with Adeno-hTERT-E1A, combined with the E1B gene deletion, leads to tumor cell killing by an E1A-induced apoptotic mechanism7. Tumor cells infected with Adeno-DF3-E1A/hTERT-E1A were assayed and compared to cells infected with Adeno-hTERT-E1A for PARP cleavage, an early apoptotic event. PARP cleavage to yield PARP-p85 occurred at lower MOIs of Adeno-DF3-E1A/hTERT-E1A as compared to Adeno-hTERT-E1A in MCF-7/4HC and A549 cells (Fig. 3A, 3B). In U251 cells, PARP-p85 levels (data not shown) and caspase 3/7 activity (Fig. 3C), which reflects downstream apoptotic events, were very similar after infection with either virus, consistent with the similar, and near complete, oncolytic activity of each virus seen in Fig. 2A. Addition of the second E1A cassette significantly increased caspase-3/7 activity in MDA-MB-231, MCF-7/4HC and A549 cells (Fig. 3C), consistent with the more substantial increases in PARP cleavage and oncolysis seen in these cells.
Activity in a U251 tumor xenograft model
The anti-tumor activity of Adeno-DF3-E1A/hTERT-E1A was compared to that of Adeno-hTERT-E1A in scid mice bearing s.c. human U251 tumor xenografts (Fig. 4A). Significant tumor growth inhibition was achieved with Adeno-DF3-E1A/hTERT-E1A (23.4 ± 7.6% inhibition after treatment period 1 (days 0 to 14) (p<0.05 vs. control; one-way ANOVA analysis with Bonferroni multiple comparison) and 36.9 ± 11.2% inhibition (p<0.01 vs. control) by the end of treatment period 2 (days 14 to 37)), but not with Adeno-hTERT-E1A. The aggressive growth of U251 tumor cells resulted in only a short period of growth stasis, which was apparent after the second series of Adeno-DF3-E1A/hTERT-E1A injections. The tumor growth trends were significantly different for each virus (p<0.05) but only the Adeno-DF3-E1A/hTERT-E1A treatment group was significantly different from the virus-free control (p<0.01). No significant host toxicity was observed with either virus, as determined by monitoring body weights (data not shown).
Figure 4.


Anti-tumor activity of Adeno-DF3-E1A/hTERT-E1A and Adeno-hTERT-E1A: impact of changes in DF3/Muc1 and hTERT expression between solid tumors and cultured tumor cells of Scid mice bearing U251 tumor xenografts were treated with PBS (vehicle control) or 5 × 108 pfu/tumor/day of either Adeno-DF3-E1A/hTERT-E1A (‘Ad-DF3/hTERT’) or Adeno-hTERT-E1A (‘Ad-hTERT’) on each of two consecutive days (arrows), with the virus treatment repeated 14 days later. A, Relative tumor vol, normalized to the tumor vol on the first day of virus injection (day 0) (mean ± SE for n = 5 mice and n = 10 tumors per group). Results of one-way ANOVA analysis of tumor vol data with Bonferroni multiple test correction are as shown (*, p < 0.05; **, p < 0.001). B-D, DF3/Muc1 (left) and hTERT (right) RNA were assayed by qPCR in solid tumor xenografts grown in scid mice from U251, A549, and MDA-MB-231 cells (4 different tumor RNA samples) and in 4 separate RNA preparations from the corresponding cell lines grown in culture. RNA levels were set relative to the mean RNA level of either the four cell-culture (for DF3) or the four tumor (for hTERT) samples. Data shown are mean ± SD for n = 3 replicates. Statistical analysis by two-tailed unpaired t-test with 95% confidence intervals comparing grouped tumor RNA levels to grouped cell culture RNA levels: p<0.001 (**); p<0.0001 (***); and p<0.05 (*).
Next, we investigated why the dual E1A cassette virus was more active than the single, hTERT-E1A cassette virus in U251 tumors, given the high hTERT promoter activity7 and low DF3/Muc1 promoter activity seen in U251 cells (Fig. 1A). In contrast to our finding, above, that cultured U251 cells express DF3/Muc1 RNA at a low level, we observed ~43-fold higher levels of DF3/Muc1 RNA in U251 tumor xenografts (Fig. 4B). Conversely, hTERT RNA was ~10-fold lower in U251 tumors than in cultured U251 cells (Fig. 4B). These tumor microenvironment-dependent increases in DF3/Muc1 and decreases in hTERT expression explain the enhanced in vivo anti-tumor activity of Adeno-DF3-E1A/hTERT-E1A as compared to Adeno-hTERT-E1A. A very similar pattern of increased DF3/Muc1 expression (~21-fold) and decreased hTERT expression (~15-fold) was seen in A549 tumors compared to cells (Fig. 4C). In MDA-MB-231 tumors, DF3/Muc1 expression was also increased, by ~10-fold, however, hTERT expression was unchanged compared to cultured tumor cells (Fig. 4D).
Activity of Adeno-DF3-E1A/hTERT-E1A against U251 holoclones
Cultured tumor cells that grow with a holoclone morphology are enriched in cancer stem-like cells19. U251 holoclones were isolated and characterized with respect to DF3/Muc1 and hTERT expression. Fig. 5A shows a 10-fold increase in DF3/Muc1 RNA and a 5-fold decrease in hTERT RNA for three independent U251 holoclones as compared to either the parental U251 cell line or a mixed U251 meroclone/paraclone subpopulation, which comprises ~90% of the parental cell population. DF3/Muc1 is associated with tumor stem-like cells in breast cancer17. Expression of ALDH1A1, an established tumor stem-like cell marker27, was also enriched ~10-fold in all three holoclones, whereas the very high parental cell expression of IL-8, which is associated with tumor progression and metastasis28, was abolished (data not shown), consistent with the cancer stem cell-like nature of these holoclones. In agreement with the observed changes in hTERT and DF3/Muc1 expression, E1A protein was substantially lower in U251 holoclones infected with Adeno-hTERT-E1A as compared to Adeno-DF3-E1A/hTERT-E1A (Fig. 5B). Adeno-hTERT-E1A also showed substantially reduced oncolysis of the holoclones compared to parent U251 cells (Fig. 5C), whereas Adeno-DF3-E1A/hTERT-E1A oncolytic activity showed a small decrease in activity (Fig. 5D), which can be explained by the reduced adenoviral infectivity of the holoclones, as determined by Adeno-βgal infectivity (data not shown). Given the putative tumor stem cell potential of holoclones, these findings raise the possibility that the superior anti-tumor activity observed for Adeno-DF3-E1A/hTERT-E1A (Fig. 4) in part results from its targeting this cell population.
Figure 5.

Gene expression and adenoviral activity in U251 holoclones – A, qPCR analysis of DF3/Muc1 (left) and hTERT (right) RNA in U251-derived holoclones (designated A1, B3 and B6), the parental U251 cell population, and a meroclone/paraclone (M/P) mixed cell population. RNA levels were set relative to the U251 parental cells and are mean ± SD values for n = 3 replicates. Statistical analysis of RNA levels in holoclones vs. U251 parental and meroclone/paraclone population: **, p < 0.01; ***, p < 0.001. B, Western blot analysis of E1A (top) and PARP p85 protein (bottom) from total cell lysates (50 μg/lane) prepared from U251 holoclones and parental cells infected for 24 hr with Adeno-hTERT-E1A (‘hT’) or Adeno-DF3-E1A/hTERT-E1A (‘D/hT’), as indicated. C and D, U251 holoclones and parental cells were seeded 24 hr prior to infection with Adeno-hTERT-E1A (C) or Adeno-DF3-E1A/hTERT-E1A (D) at the indicated MOIs. The cells were stained with crystal violet 3 days later to quantify remaining cells. Data shown are mean ± SD values based on n = 3 replicates, with the uninfected cell controls set = 100%. One-way ANOVA with Bonferroni multiple test correction indicated no significant difference in Adeno-DF3-E1A/hTERT-E1A-induced cytotoxicity profiles for the holoclones vs. parental U251 cells (panel D), whereas cytotoxicity profiles were significantly different for Adeno-hTERT-E1A-infected holoclones B3 and B6 (but not holoclone A1) compared to parental U251 cells (p < 0.05) (panel C). Adeno-hTERT-E1A-induced cytotoxicity profiles were significantly different for holoclone A1 compared to U251 parental cells when analyzed at individual MOIs, ranging from 5 to 20 MOI (p <0.05).
Enhanced tumor cell apoptosis suppresses viral release and helper virus activity of dual cassette virus
Next, we investigated whether the earlier and more extensive apoptosis induced by Adeno-DF3-E1A/hTERT-E1A disrupts its ability to produce active, functional viral particles and thereby spread the viral infection within a tumor cell population. Supernatants from tumor cells infected with Adeno-DF3-E1A/hTERT-E1A or Adeno-hTERT-E1A were collected and titered for functional virus. Adeno-hTERT-E1A was more efficient than Adeno-DF3-E1A/hTERT-E1A in terms of the release of functional virus, as seen at various time points after infection, in both U251 cells and A549 cells (Fig. 6A). This finding suggests that the second E1A cassette of Adeno-DF3-E1A/hTERT-E1A induces premature host cell death, reducing the overall yield of functional viral progeny.
Figure 6.
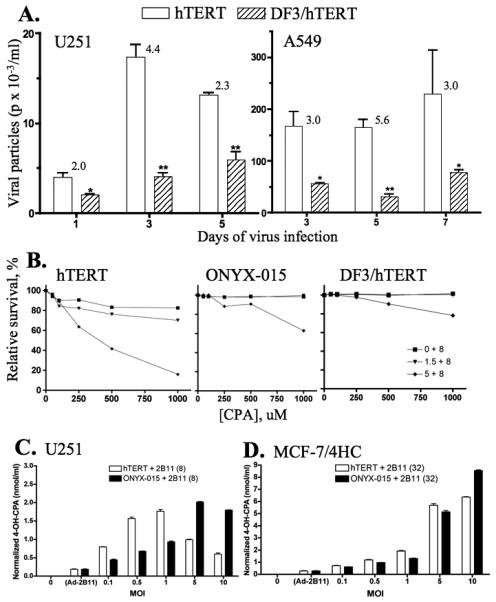
Adeno-DF3-E1A/hTERT-E1A exhibits decreased viral spread and diminished helper virus activity towards Adeno-2B11-mediated CPA prodrug-activation – A, U251 cells (left) and A549 cells (right) were infected with Adeno-hTERT-E1A (hTERT) or Adeno-DF3-E1A/hTERT-E1A (DF3/hTERT) at MOI 5. The cell culture supernatant was removed 1-7 days later, as indicated, and applied to 293 cells to titrate adenoviral particles released into the supernatant. Data shown are mean ± SD values based on duplicate titerings from three 10-fold serial dilutions (100, 10−1, and 10−2) of the virus supernatants. Numbers over each bar indicate the ratio of adenoviral titer between Adeno-hTERT-E1A and Adeno-DF3-E1A/hTERT-E1A. Statistical analysis by two-tailed unpaired t-test with 95% confidence intervals for Adeno-hTERT-E1A vs. Adeno-DF3-E1A/hTERT-E1A: *, p<0.05; **, p<0.001. B, CPA dose-dependent killing of U251 cells infected with cell culture supernatants from U251 cells previously infected with Adeno-2B11 (MOI 8) in combination with Adeno-hTERT-E1A (left), ONYX-015 (middle), or Adeno-DF3-E1A/hTERT-E1A (right) at MOIs of 0, 1.5 and 5. Cells treated with virus in the absence of CPA were set as 100%. C and D, the active metabolite of CPA, 4-OH-CPA, was assayed by HPLC in U251 and MCF-7/4HC cells infected with Adeno-2B11 + Adeno-hTERT-E1A (white bars) or with Adeno-2B11 + ONYX-015 (black bars) for 2 days followed by a 4 hr incubation with 250 μM CPA + semicarbazide (5 mM). Data shown in B and C are mean ± SD values based on n = 2 replicates. All experiments were performed at least in duplicate.
To determine how the decrease in adenovirus release affects the expression of a co-administered replication-deficient adenovirus, studies were carried out with Adeno-2B1120, which codes for the prodrug-activating cytochrome P450 enzyme 2B11 (CYP2B11), an efficient catalyst of CPA activation29. U251 cells were infected with Adeno-2B11 in combination with Adeno-hTERT-E1A, Adeno-DF3-E1A/hTERT-E1A, or another oncolytic virus, ONYX-01530, at various MOIs. Three days later, the culture supernatants were applied to fresh U251 cells in the presence of CPA, a CYP2B11-activated prodrug. Adeno-hTERT-E1A was the most effective in facilitating Adeno-2B11 replication, resulting in CPA-induced cell death, whereas ONYX-015 showed moderate activity (Fig. 6B). Adeno-DF3-E1A/hTERT-E1A was almost inactive (Fig. 6B), consistent with the early oncolysis and reduced viral release (Fig. 6A) comprising the Adeno-2B11 helper virus activity. Analysis of culture supernatants for the release of 4-OH-CPA, the active metabolite of CPA, confirmed the superior ability of Adeno-hTERT-E1A to serve as a helper virus in U251 cells, as evidenced by the lower MOIs required to produce high levels of 4-OH-CPA (Fig. 6C). At higher MOIs, Adeno-hTERT-E1A induced substantial cell death (data not shown), thereby decreasing the number of cells capable of converting CPA to 4-OH-CPA. Adeno-hTERT-E1A and ONYX-015 exhibited similar helper virus activity in MCF-7/4HC cells (Fig. 6D), whereas in MDA-MB-231 cells, which are intrinsically difficult to infect, Adeno-hTERT-E1A showed low helper virus activity while ONYX-015 was inactive (data not shown).
DISCUSSION
Cancer-specific promoters can be used to control viral regulatory genes, such as adenoviral E1A, to restrict the replication of oncolytic adenoviruses to malignant cells and tissues. Dual-specificity adenoviral promoters that regulate E1A expression in response to multiple stimuli, e.g., estrogens and hypoxia, have also been described31. The cancer-specific promoters DF3/Muc1 and hTERT can both confer tumor-specific replication, and several tumor cell-replicating, DF3/Muc1-driven and hTERT-driven adenoviruses have been described8, 32. However, none of these viruses combines both promoter elements into a single virus to regulate E1A expression and viral replication. As hTERT is expressed in >90% of cancers5, 6, and DF3/Muc1 displays increased activity in 75% of solid tumors and in cancer stem cell-associated subpopulations17, 18, an oncolytic virus that combines both of these features has the potential to induce oncolytic activity across a broad range of human tumors and tumor cell populations. The heterogeneity of DF3 and hTERT expression within a single tumor, discussed below, could further increase the utility of such a virus. Presently, we describe a novel adenovirus, Adeno-DF3-E1A/hTERT-E1A, where DF3/Muc1 and hTERT gene regulatory sequences are combined into a single virus to develop a therapeutic approach to targeting heterogeneous populations of tumor cells, including cancer stem-like cells, within a given patient's tumor.
Adeno-hTERT-E1A is a single hTERT-E1A cassette adenovirus that uses an hTERT core promoter to drive adenoviral E1A expression in a manner similar to that of the endogenous full-length hTERT promoter7. Furthermore, Adeno-hTERT-E1A combines hTERT regulation of E1A with deletion of the E1B region genes E1B-19 kDa and E1B-55 kDa to further increase tumor cell-specificity7. Adeno-DF3-E1A/hTERT-E1A contains the same hTERT-E1A expression cassette and E1B gene deletions as Adeno-hTERT-E1A, but in addition, places a second E1A expression cassette under control of a core DF3/Muc1 promoter element. This promoter element was chosen based upon earlier promoter deletion studies23-25 and is shown here to regulate expression in a cell line-dependent manner similar to that of endogenous DF3/Muc1 RNA. Incorporation of a second E1A gene cassette did not lead to recombination or instability of the dual DF3/hTERT adenovirus genome following viral amplification. Functionally, the second E1A cassette conferred on Adeno-DF3-E1A/hTERT-E1A the ability to induce higher E1A expression, more extensive apoptosis and greater oncolysis as compared to Adeno-hTERT-E1A. The studies reported here were all carried out in parallel using carefully titered batches of the single hTERT and the dual DF3/hTERT adenoviruses, making it highly likely that the additional activity of Adeno-DF3-E1A/hTERT-E1A is due to the second, DF3 promoter-regulated E1A cassette. Efforts to prepare a single DF3 virus to verify this were unsuccessful.
The oncolytic activity of Adeno-DF3-E1A/hTERT-E1A was increased over that of Adeno-hTERT-E1A in several cultured tumor cells, with the increased activity also apparent in vivo in a U251 gliosarcoma solid tumor xenograft model (Fig. 4A). Given the high adenoviral infectivity and high hTERT promoter activity of U251 cells7, Adeno-hTERT-E1A and Adeno-DF3-E1A/hTERT-E1A were both expected to induce strong anti-tumor responses. However, Adeno-hTERT-E1A, which induced extensive U251 cell death in culture, showed no significant anti-tumor activity in U251 tumor xenografts, in contrast to Adeno-DF3-E1A/hTERT-E1A. The ineffectiveness of Adeno-hTERT-E1A, but not Adeno-DF3-E1A/hTERT-E1A, against U251 tumors in vivo may be explained by the 10-fold decrease in hTERT expression, coupled with the 43-fold increase in DF3/Muc1 expression that we observed in U251 solid tumors compared to cultured U251 cells (Fig. 4B). Thus, incorporation of a DF3/Muc1 promoter, which was previously shown to have augmented activity in many cancers including breast, prostate, ovarian, pancreatic, colon and lung, where DF3/Muc1 is overexpressed9-13, might also be beneficial in treating gliosarcomas, as indicated by our finding that DF3/Muc1 expression in low in cultured U251 cells but is strongly increased when U251 cells are grown as solid tumors in vivo. Large increases in DF3/Muc1 expression and large decreases in hTERT expression were also seen in A549 tumors as compared to cultured A549 cells (Fig. 4C). However, hTERT was not down-regulated in MDA-MB-231 tumors (Fig. 4D, right), which may explain why the single-cassette Adeno-hTERT-E1A shows strong anti-tumor activity against MDA-MB-231 xenografts7. The underlying factors that dictate these major changes in DF3/Muc1 and hTERT expression in solid tumor xenografts are unknown, but in the case of DF3/Muc1 may be related to its role in tumor angiogenesis and extracellular matrix remodeling13, functions not manifested in cell culture. Together, these findings highlight the potential advantage of using a dual promoter regulated adenovirus, such as Adeno-DF3-E1A/hTERT-E1A, which is expected to exert anti-tumor activity, independent of whether the target tumor cells overexpress DF3/Muc1 or hTERT. Recently, an hTERT promoter-regulated adenovirus, Telomelysin, was evaluated in a phase I clinical trial and was found to have limited clinical activity with some evidence of viral replication in a subset of the patients studied33. While the heterogeneity of patient responses may relate to the hTERT heterogeneity reported for the patient population33, this possibility was not considered in that study. Given these findings with a single promoter-regulated adenovirus, and considering the heterogeneity of tumor hTERT expression that characterizes individual patients, and perhaps also individual tumor cells within a given patient, future studies using dual promoter-regulated adenovirus, such as Adeno-DF3-E1A/hTERT-E1A, can be expected to be useful and may lead to improved clinical responses.
Tumor cell heterogeneity is widespread, as revealed by genome-wide expression profiling studies34, 35, and may arise through the course of cell differentiation, tumor progression and clonal expansion36. The differentiation and genetic evolution of tumor cell subpopulations in different microenvironments contribute to tumor heterogeneity, affecting both intrinsic and acquired drug resistance37, 38. Moreover, heterogeneity between primary tumors and their metastatic lesions is commonly observed and complicates cancer treatment. Studies of tumor cell heterogeneity related to stem/progenitor cell-like niches, differentiation states and cycling versus non-cycling cell status17 highlight the difficulty in developing therapeutics that effectively target entire tumor cell populations. Presently, we investigated the potential utility of Adeno-DF3-E1A/hTERT-E1A as a therapeutic against heterogeneous tumor cell populations. Heterogeneity of expression has been documented for both DF3/Muc1 and hTERT in individual tumors and between tumor cell lines, and for DF3/Muc1, between cells within a given tumor cell line39, 40. DF3/Muc1 heterogeneity is also observed between stages of cancer differentiation and progression9, 10, 12, and the expression of hTERT also enriched in metastatic lesions as compared to primary tumors41. The potential for such heterogeneity to impact viral oncolytic activity was evident from our examination of U251 holoclones. Holoclones constitute a minor fraction of cultured tumor cell populations and show enrichment for tumor stem- and progenitor-like cells19. Analysis of the U251 gliosarcoma holoclones isolated in this study revealed a substantial (~10-fold) increase in DF3/Muc1 expression coupled with a ~5-fold decrease in hTERT expression compared to the overall population of U251 cells. Correspondingly, substantial decreases in E1A production and oncolysis were observed following infection of the holoclones with Adeno-hTERT-E1A but not Adeno-DF3-E1A/hTERT-E1A. This finding highlights the ability of our dual E1A cassette adenovirus to target a tumor cell subpopulation that may be an important therapeutic target in view of its stem/progenitor cell-like potential. Thus, our work complements recent and proposed efforts to utilize adenoviral vectors to target cancer stem cell populations42, 43.
The adenoviral E1A protein induces p53-related increases in apoptosis and has strong intrinsic cytotoxic activity44-46. EIA can be used as a therapeutic gene to induce anti-tumor responses, including inhibition of angiogenesis46 and can sensitize tumor cells to chemotherapy and radiation47-49. In the case of Adeno-DF3-E1A/hTERT-E1A, the absence of the anti-apoptotic activities of both E1B proteins coupled with the high level of E1A expression resulted in early host-cell apoptosis and increased oncolytic activity. However, this early induction of viral host cell death suppressed viral replication and decreased by 4 to 6-fold the subsequent release of functional progeny available for later rounds of infection and viral spread, compared to tumor cells infected with Adeno-hTERT-E1A. Although adenovirus-induced host cell death typically proceeds by an apoptosis-independent pathway, the absence of both anti-apoptotic E1B proteins and the increased cytotoxicity associated with the elevated expression of E1A by Adeno-hTERT-E1A changes the mechanism of cell death from late-stage viral replication-induced lysis to an E1A-dependent apoptosis, as we reported earlier7. In cells infected with Adeno-DF3-E1A/hTERT-E1A, the second E1A cassette further increases E1A expression, thereby increasing oncolysis via an apoptotic mechanism, which interrupts viral progeny maturation and decreases viral spread. Accordingly, when we investigated the potential of both viruses to facilitate the spread and expression of the replication-deficient Adeno-2B11, which encodes the CPA-activating cytochrome P450 2B1120, 29, Adeno-DF3-E1A/hTERT-E1A was ineffective in facilitating Adeno-2B11/CPA-dependent killing of co-infected tumor cells. We also investigated the oncolytic adenovirus ONYX-015, which has undergone extensive clinical evaluation30. ONYX-015 retains one of the two E1B genes, E1B-19 kDa, and in contrast to Adeno-hTERT-E1A induces little or no detectable tumor cell apoptosis, as judged by PARP cleavage and TUNEL staining7. Nevertheless, Adeno-hTERT-E1A exhibited greater helper virus activity toward Adeno-2B11 than ONYX-015, as evidenced by the enhanced chemosensitization of U251 cells to CPA and by the increased production of active prodrug metabolites (4-OH-CPA) seen in U251 cells. The loss of helper virus activity in the case of Adeno-DF3-E1A/hTERT-E1A demonstrates the importance of balancing E1A production with the extent and the timing of virus-induced apoptosis. Indeed, to be most effective, P450 and other prodrug enzyme-based gene therapy strategies require delayed, rather than accelerated death of tumor cells that express the prodrug-activating enzyme50.
In conclusion, we have engineered a novel dual E1A cassette oncolytic adenovirus that displays improved oncolysis in many cancer cell lines compared to the corresponding single E1A-cassette adenovirus and has the potential to target a heterogeneous tumor cell population, including cancer stem-like cells that display strong DF3/Muc1 promoter activity. Despite the increased activity of the dual E1A cassette virus as a stand-alone anti-cancer agent, the premature apoptosis of infected tumor cells decreased its potential to replicate and to facilitate helper virus activity. Future studies may examine adenoviral genomes that combine the dual promoter E1A gene control described here with the retention of adenoviral E1B and/or E3 region genetic elements to prevent premature host cell apoptosis and thereby facilitate more efficient production of viral progeny while maintaining activity in a wide range of cancer types and tumors with heterogeneous tumor cell populations.
Acknowledgements
The authors thank Dr. Youssef Jounaidi for guidance in adenovirus design and for many useful suggestions in the initial stages of this project, and to Michael Durando for his assistance in generating the holoclones.
Supported in part by NIH grant CA49248 (to D.J.W.).
Abbreviations
- 4-OH-CPA
4-hydroxycyclophosphamide
- CMV
cytomegalovirus
- CPA
cyclophosphamide
- E1A
early adenoviral region 1A
- FBS
fetal bovine serum
- hTERT
human telomerase
- MOI
multiplicity of infection
- PARP
poly (ADP-ribose) polymerase
- pfu
plaque forming units
- qPCR
quantitative PCR
- RPMI
Roswell Park Memorial Institute
REFERENCES
- 1.Bauerschmitz GJ, Barker SD, Hemminki A. Adenoviral gene therapy for cancer: from vectors to targeted and replication competent agents (review) Int J Oncol. 2002;21(6):1161–1174. [PubMed] [Google Scholar]
- 2.Jounaidi Y, Doloff JC, Waxman DJ. Conditionally Replicating Adenoviruses for Cancer Treatment. Curr Cancer Drug Targets. 2007;7(3):285–301. doi: 10.2174/156800907780618301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Everts B, van der Poel HG. Replication-selective oncolytic viruses in the treatment of cancer. Cancer gene therapy. 2005;12(2):141–161. doi: 10.1038/sj.cgt.7700771. [DOI] [PubMed] [Google Scholar]
- 4.Nevins JR. Mechanism of activation of early viral transcription by the adenovirus E1A gene product. Cell. 1981;26(2 Pt 2):213–220. doi: 10.1016/0092-8674(81)90304-4. [DOI] [PubMed] [Google Scholar]
- 5.Kim NW, Piatyszek MA, Prowse KR, et al. Specific association of human telomerase activity with immortal cells and cancer. Science. 1994;266(5193):2011–2015. doi: 10.1126/science.7605428. [DOI] [PubMed] [Google Scholar]
- 6.Mo Y, Gan Y, Song S, et al. Simultaneous targeting of telomeres and telomerase as a cancer therapeutic approach. Cancer Res. 2003;63(3):579–585. [PubMed] [Google Scholar]
- 7.Doloff JC, Waxman DJ, Jounaidi Y. hTERT-promoter driven oncolytic adenovirus with E1B-19 kDa and E1B-55 kDa gene deletions. Hum Gene Ther. 2008 doi: 10.1089/hum.2008.056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Wirth T, Kuhnel F, Kubicka S. Telomerase-dependent gene therapy. Curr Mol Med. 2005;5(2):243–251. doi: 10.2174/1566524053586536. [DOI] [PubMed] [Google Scholar]
- 9.Cozzi PJ, Wang J, Delprado W, et al. MUC1, MUC2, MUC4, MUC5AC and MUC6 expression in the progression of prostate cancer. Clin Exp Metastasis. 2005;22(7):565–573. doi: 10.1007/s10585-005-5376-z. [DOI] [PubMed] [Google Scholar]
- 10.Hinoda Y, Ikematsu Y, Horinochi M, et al. Increased expression of MUC1 in advanced pancreatic cancer. J Gastroenterol. 2003;38(12):1162–1166. doi: 10.1007/s00535-003-1224-6. [DOI] [PubMed] [Google Scholar]
- 11.Ho SB, Niehans GA, Lyftogt C, et al. Heterogeneity of mucin gene expression in normal and neoplastic tissues. Cancer Res. 1993;53(3):641–651. [PubMed] [Google Scholar]
- 12.Nagai S, Takenaka K, Sonobe M, Ogawa E, Wada H, Tanaka F. A novel classification of MUC1 expression is correlated with tumor differentiation and postoperative prognosis in non-small cell lung cancer. J Thorac Oncol. 2006;1(1):46–51. [PubMed] [Google Scholar]
- 13.Khodarev NN, Pitroda SP, Beckett MA, et al. MUC1-induced transcriptional programs associated with tumorigenesis predict outcome in breast and lung cancer. Cancer Res. 2009;69(7):2833–2837. doi: 10.1158/0008-5472.CAN-08-4513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Li Y, Liu D, Chen D, Kharbanda S, Kufe D. Human DF3/MUC1 carcinoma-associated protein functions as an oncogene. Oncogene. 2003;22(38):6107–6110. doi: 10.1038/sj.onc.1206732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Fessler SP, Wotkowicz MT, Mahanta SK, Bamdad C. MUC1* is a determinant of trastuzumab (Herceptin) resistance in breast cancer cells. Breast Cancer Res Treat. 2009 doi: 10.1007/s10549-009-0412-3. [DOI] [PubMed] [Google Scholar]
- 16.Yin L, Li Y, Ren J, Kuwahara H, Kufe D. Human MUC1 carcinoma antigen regulates intracellular oxidant levels and the apoptotic response to oxidative stress. J Biol Chem. 2003;278(37):35458–35464. doi: 10.1074/jbc.M301987200. [DOI] [PubMed] [Google Scholar]
- 17.Engelmann K, Shen H, Finn OJ. MCF7 side population cells with characteristics of cancer stem/progenitor cells express the tumor antigen MUC1. Cancer Res. 2008;68(7):2419–2426. doi: 10.1158/0008-5472.CAN-07-2249. [DOI] [PubMed] [Google Scholar]
- 18.Hikita ST, Kosik KS, Clegg DO, Bamdad C. MUC1* mediates the growth of human pluripotent stem cells. PLoS One. 2008;3(10):e3312. doi: 10.1371/journal.pone.0003312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li H, Chen X, Calhoun-Davis T, Claypool K, Tang DG. PC3 human prostate carcinoma cell holoclones contain self-renewing tumor-initiating cells. Cancer Res. 2008;68(6):1820–1825. doi: 10.1158/0008-5472.CAN-07-5878. [DOI] [PubMed] [Google Scholar]
- 20.Jounaidi Y, Chen CS, Veal GJ, Waxman DJ. Enhanced antitumor activity of P450 prodrug-based gene therapy using the low Km cyclophosphamide 4-hydroxylase P450 2B11. Mol Cancer Ther. 2006;5(3):541–555. doi: 10.1158/1535-7163.MCT-05-0321. [DOI] [PubMed] [Google Scholar]
- 21.Jounaidi Y, Waxman DJ. Use of replication-conditional adenovirus as a helper system to enhance delivery of P450 prodrug-activation genes for cancer therapy. Cancer Res. 2004;64(1):292–303. doi: 10.1158/0008-5472.can-03-1798. [DOI] [PubMed] [Google Scholar]
- 22.Jounaidi Y, Hecht JE, Waxman DJ. Retroviral transfer of human cytochrome P450 genes for oxazaphosphorine-based cancer gene therapy. Cancer Res. 1998;58(19):4391–4401. [PubMed] [Google Scholar]
- 23.Abe M, Kufe D. Characterization of cis-acting elements regulating transcription of the human DF3 breast carcinoma-associated antigen (MUC1) gene. Proc Natl Acad Sci U S A. 1993;90(1):282–286. doi: 10.1073/pnas.90.1.282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kovarik A, Lu PJ, Peat N, Morris J, Taylor-Papadimitriou J. Two GC boxes (Sp1 sites) are involved in regulation of the activity of the epithelium-specific MUC1 promoter. J Biol Chem. 1996;271(30):18140–18147. doi: 10.1074/jbc.271.30.18140. [DOI] [PubMed] [Google Scholar]
- 25.Kovarik A, Peat N, Wilson D, Gendler SJ, Taylor-Papadimitriou J. Analysis of the tissue-specific promoter of the MUC1 gene. J Biol Chem. 1993;268(13):9917–9926. [PubMed] [Google Scholar]
- 26.Abou El Hassan MA, van der Meulen-Muileman I, Abbas S, Kruyt FA. Conditionally replicating adenoviruses kill tumor cells via a basic apoptotic machinery-independent mechanism that resembles necrosis-like programmed cell death. J Virol. 2004;78(22):12243–12251. doi: 10.1128/JVI.78.22.12243-12251.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Moreb JS. Aldehyde dehydrogenase as a marker for stem cells. Curr Stem Cell Res Ther. 2008;3(4):237–246. doi: 10.2174/157488808786734006. [DOI] [PubMed] [Google Scholar]
- 28.Yuan A, Chen JJ, Yao PL, Yang PC. The role of interleukin-8 in cancer cells and microenvironment interaction. Front Biosci. 2005;10:853–865. doi: 10.2741/1579. [DOI] [PubMed] [Google Scholar]
- 29.Chen CS, Lin JT, Goss KA, He YA, Halpert JR, Waxman DJ. Activation of the anticancer prodrugs cyclophosphamide and ifosfamide: identification of cytochrome P450 2B enzymes and site-specific mutants with improved enzyme kinetics. Mol Pharmacol. 2004;65(5):1278–1285. doi: 10.1124/mol.65.5.1278. [DOI] [PubMed] [Google Scholar]
- 30.Crompton AM, Kirn DH. From ONYX-015 to armed vaccinia viruses: the education and evolution of oncolytic virus development. Curr Cancer Drug Targets. 2007;7(2):133–139. doi: 10.2174/156800907780058862. [DOI] [PubMed] [Google Scholar]
- 31.Hernandez-Alcoceba R, Pihalja M, Qian D, Clarke MF. New oncolytic adenoviruses with hypoxia- and estrogen receptor-regulated replication. Hum Gene Ther. 2002;13(14):1737–1750. doi: 10.1089/104303402760293574. [DOI] [PubMed] [Google Scholar]
- 32.Kurihara T, Brough DE, Kovesdi I, Kufe DW. Selectivity of a replication-competent adenovirus for human breast carcinoma cells expressing the MUC1 antigen. J Clin Invest. 2000;106(6):763–771. doi: 10.1172/JCI9180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Nemunaitis J, Tong AW, Nemunaitis M, et al. A phase I study of telomerase-specific replication competent oncolytic adenovirus (telomelysin) for various solid tumors. Mol Ther. 18(2):429–434. doi: 10.1038/mt.2009.262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Francis P, Fernebro J, Eden P, et al. Intratumor versus intertumor heterogeneity in gene expression profiles of soft-tissue sarcomas. Genes Chromosomes Cancer. 2005;43(3):302–308. doi: 10.1002/gcc.20191. [DOI] [PubMed] [Google Scholar]
- 35.Nagasaki K, Miki Y. Gene expression profiling of breast cancer. Breast Cancer. 2006;13(1):2–7. doi: 10.2325/jbcs.13.2. [DOI] [PubMed] [Google Scholar]
- 36.Leedham SJ, Wright NA. Expansion of a mutated clone: from stem cell to tumour. J Clin Pathol. 2008;61(2):164–171. doi: 10.1136/jcp.2006.044610. [DOI] [PubMed] [Google Scholar]
- 37.Mimeault M, Hauke R, Mehta PP, Batra SK. Recent advances in cancer stem/progenitor cell research: therapeutic implications for overcoming resistance to the most aggressive cancers. J Cell Mol Med. 2007;11(5):981–1011. doi: 10.1111/j.1582-4934.2007.00088.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Tredan O, Galmarini CM, Patel K, Tannock IF. Drug resistance and the solid tumor microenvironment. J Natl Cancer Inst. 2007;99(19):1441–1454. doi: 10.1093/jnci/djm135. [DOI] [PubMed] [Google Scholar]
- 39.Walsh MD, Luckie SM, Cummings MC, Antalis TM, McGuckin MA. Heterogeneity of MUC1 expression by human breast carcinoma cell lines in vivo and in vitro. Breast Cancer Res Treat. 1999;58(3):255–266. doi: 10.1023/a:1006345301364. [DOI] [PubMed] [Google Scholar]
- 40.Yan P, Benhattar J, Seelentag W, Stehle JC, Bosman FT. Immunohistochemical localization of hTERT protein in human tissues. Histochem Cell Biol. 2004;121(5):391–397. doi: 10.1007/s00418-004-0645-5. [DOI] [PubMed] [Google Scholar]
- 41.Hao X, Sun B, Hu L, et al. Differential gene and protein expression in primary breast malignancies and their lymph node metastases as revealed by combined cDNA microarray and tissue microarray analysis. Cancer. 2004;100(6):1110–1122. doi: 10.1002/cncr.20095. [DOI] [PubMed] [Google Scholar]
- 42.Bauerschmitz GJ, Ranki T, Kangasniemi L, et al. Tissue-specific promoters active in CD44+CD24-/low breast cancer cells. Cancer Res. 2008;68(14):5533–5539. doi: 10.1158/0008-5472.CAN-07-5288. [DOI] [PubMed] [Google Scholar]
- 43.Ribacka C, Pesonen S, Hemminki A. Cancer, stem cells, and oncolytic viruses. Ann Med. 2008;40(7):496–505. doi: 10.1080/07853890802021342. [DOI] [PubMed] [Google Scholar]
- 44.Hale TK, Braithwaite AW. The adenovirus oncoprotein E1a stimulates binding of transcription factor ETF to transcriptionally activate the p53 gene. J Biol Chem. 1999;274(34):23777–23786. doi: 10.1074/jbc.274.34.23777. [DOI] [PubMed] [Google Scholar]
- 45.Rao XM, Tseng MT, Zheng X, et al. E1A-induced apoptosis does not prevent replication of adenoviruses with deletion of E1b in majority of infected cancer cells. Cancer gene therapy. 2004;11(9):585–593. doi: 10.1038/sj.cgt.7700739. [DOI] [PubMed] [Google Scholar]
- 46.Zhou Z, Zhou RR, Guan H, Bucana CD, Kleinerman ES. E1A gene therapy inhibits angiogenesis in a Ewing's sarcoma animal model. Mol Cancer Ther. 2003;2(12):1313–1319. [PubMed] [Google Scholar]
- 47.Deissler H, Opalka B. Therapeutic transfer of DNA encoding adenoviral E1A. Recent Pat Anticancer Drug Discov. 2007;2(1):1–10. doi: 10.2174/157489207779561471. [DOI] [PubMed] [Google Scholar]
- 48.Liao Y, Yu D, Hung MC. Novel approaches for chemosensitization of breast cancer cells: the E1A story. Adv Exp Med Biol. 2007;608:144–169. doi: 10.1007/978-0-387-74039-3_11. [DOI] [PubMed] [Google Scholar]
- 49.Martin-Duque P, Sanchez-Prieto R, Romero J, et al. In vivo radiosensitizing effect of the adenovirus E1A gene in murine and human malignant tumors. Int J Oncol. 1999;15(6):1163–1168. doi: 10.3892/ijo.15.6.1163. [DOI] [PubMed] [Google Scholar]
- 50.Waxman DJ, Schwartz PS. Harnessing apoptosis for improved anticancer gene therapy. Cancer Res. 2003;63(24):8563–8572. [PubMed] [Google Scholar]


