Abstract
Diabetes is a pro-inflammatory state. We have previously shown increased monocyte pro-inflammatory cytokines in patients with Type 1 and Type 2 diabetes. High glucose induces pro-inflammatory cytokines via epigenetic changes. Curcumin, a polyphenol responsible for the yellow color of the spice turmeric, is known to exert potent anti-inflammatory activity in vitro. Recent studies indicate that it may regulate chromatin remodeling by inhibiting histone acetylation. In this study, we aimed to test the effect of curcumin on histone acetylation and pro-inflammatory cytokine secretion under high-glucose conditions in human monocytes. Human monocytic (THP-1) cells were cultured in presence of mannitol (osmolar control, mannitol) or normoglycemic (NG, 5.5 mmol/L glucose) or hyperglycemic (HG, 25 mmol/L glucose) conditions in absence or presence of curcumin (1.5-12.5μM) for 72 h. Cytokine level, nuclear factor κB (NF-κB) transactivation, histone deacetylases (HDACs) activity, histone acetylases (HATs) activity were measured by western blots, qRT-PCR, ELISA, Immunofluorescence (IF) staining. HG significantly induced histone acetylation, NF-κB activity and pro-inflammatory cytokine (IL-6, TNF-α and MCP-1) release from THP-1 cells. Curcumin suppressed NF-κB binding and cytokine release in THP-1 cells. Also, since p300 histone acetyltransferase is a coactivator of NF-κB, we examined its acetylation. Curcumin treatment also significantly reduced HAT activity, level of p300 and acetylated CBP/p300 gene expression, and induced histone deacetylase 2 (HDAC2) expression by curcumin. These results indicate that curcumin decreases HG-induced cytokine production in monocytes via epigenetic changes involving NF-κB. In conclusion, curcumin supplementation by reducing vascular inflammation may prevent diabetic complications.
Keywords: Acetylation, Deacetylation, p300, HDAC2, NF-κB
Introduction
Diabetes is a pro-inflammatory state. Several studies indicate that hyperglycemia is one of the key factors that contribute to diabetic complications [1-3]. High glucose leads to the activation of the master switch of inflammation, transcription factor nuclear factor κB (NF-κB) and significantly induces the expression of several inflammatory cytokines [4-8]. Although the mechanisms are not fully resolved so far, some recent studies have shown that in diabetic conditions, specific transcription mechanisms and nuclear chromatin remodeling events occur, which regulate monocyte activation via the NF-κB pathway [9-10].
NF-κB plays an important role in the regulation of pro-inflammatory genes that are associated with several inflammatory diseases, including atherosclerosis, insulin resistance, metabolic syndrome, and diabetes and its complications [11-15]. NF-κB consists of homo- or heterodimers of different sub-units, such as p50, p52, p65/RelA, RelB, and c-Rel, with p65/RelA and p50 being the most common and well studied [16-17]. In most unstimulated cells, NF-κB resides in the cytoplasm in an inactive latent form complexed with its inhibitor subunit, IκBα. Multiple extracellular stimuli, including inflammatory cytokines can induce NF-κB activation by promoting IκBα phosphorylation and its proteasomal degradation [14-15]. The released p65-p50 dimer then translocates to the nucleus, where it binds to the promoters of NF-κB-dependent inflammatory genes to induce their expression. p65 protein is a key transcriptionally active component of NF-κB. NF-κB activation is reciprocally regulated by RelA/p65 acetylation and deacetylation, which are mediated by histone acetyltransferases (HATs) and histone deacetylases (HDACs)[18-19].
Histone acetylation/deacetylation is an important epigenetic event that plays an important role in inflammation [20-21]. Acetylation by HAT of specific lysine residues on the N-terminal tail of core histones, results in uncoiling of the DNA and increased accessibility to transcription factor binding. In contrast, histone deacetylation by HDAC represses gene transcription by promoting DNA winding thereby limiting access to transcription factors [21-22]. Altered HAT and HDAC activities can lead to several diseases [23-26] including cancer, diabetes, cardiac hypertrophy, and asthma [27-29].
The antioxidant and/or anti-inflammatory effects of dietary polyphenols, have all been shown to play a role in either controlling NF-κB activation or chromatin remodeling through modulation of HDAC activity and subsequently inflammatory gene expression [30-31]. Recent studies have reported that curcumin is an inhibitor of p300-HAT [32].
Curcumin (diferuloylmethane) is a polyphenol responsible for the yellow color of the curry spice turmeric. It has been used in a variety of diseases in traditional medicine. Modern scientific research has demonstrated its anti-inflammatory, anti-oxidant, anti-carcinogenic, anti-thrombotic, and cardiovascular protective effects [33]. Since NF-κB regulates expression of a wide variety of genes that are intimately involved in the process of inflammation, inhibition of NF-κB by curcumin may be an interesting prospect for controlling chronic inflammatory diseases involving the NF-κB signaling pathway [34]. However, very little is known about the specific molecular transcription mechanisms and nuclear chromatin remodeling events regulated by curcumin treatment under diabetic conditions especially as it relates to monocyte pro-inflammatory cytokines.
Thus, we hypothesize that curcumin suppresses the proinflammatory cytokine secretion through the NF-κB signaling pathway via altering histone acetylation/histone deacetylation balance under high-glucose conditions in human monocytes.
Materials and Methods
Materials
Anti-HDAC2 antibody was procured from Active Motif (Carlsbad, CA, USA) and Anti-NF-κBp65, anti-phospho NF-κBp65, anti-acetyl CBP/p300 and anti-phospho IκBα were procured from Cell Signaling Technology (Beverly, MA, USA), respectively. Anti-acetyl p65 was procured from Abcam Inc. (Cambridge, MA, USA). Transcription ELISA kit (TransAM NF-κBp65), HAT and HDAC assay kit (colorimetric) were purchased from Active motif (Carlsbad, CA, USA) and Biovision (Mountain View, CA, USA), respectively. Anti-p300 antibody and Milliplex ™ MAP ELISA Kit were purchased from Millipore (USA). Curcumin was purchased from Sigma (St. Louis, MO,USA). The BCA™ protein assay kit was purchased from Pierce. Novex pre-cast Tris-Glycine gels were obtained from Invitrogen (Carlsbad, CA,USA). All other chemicals, unless otherwise stated, were obtained from Sigma (St. Louis, MO, USA).
Cell culture and treatment with curcumin
Human monocytic THP-1 cell line was obtained from American Type Culture Collection (Manassas, VA). THP-1 cells were cultured in RPMI medium containing 10% fetal bovine serum and 1% antibiotics at 37 °C and 5% CO2. Curcumin (dissolved in DMSO) was used for the treatment of cells. The final concentration of DMSO used was 0.1% (v/v) for each treatment. THP-1 cells (1 × 105 cells/ml) were cultured in presence of osmolar control (19.5 mmol/l mannitol) or normo glycemic (NG, 5.5 mmol/L glucose) or hyperglycemic (HG, 25 mmol/L glucose) conditions in absence or presence of curcumin (1.5-12μM) for 72 h after which media was saved for cytokine release measurement and cells were washed with phosphate-buffered saline (PBS) and then harvested.
Cytokine release measurement
Cytokines were measured with a Milliplex ™ MAP Assay Kit (Millipore,USA) according to the manufacturer's instructions. Values were calculated based on a standard curve constructed for the assay.
Trypan blue exclusion assay for cellular viability
The cytotoxicity of curcumin to cultured high glucose-induced THP-1 cells was measured by trypan blue assay. The effect of curcumin on growth inhibition was assessed as the percentage of inhibition in cell growth where vehicle-treated cells were taken as 100% viable. Cytotoxic effect by curcumin (0.5 to 12.5μM; 72h) treatment was not observed. Curcumin treatment was cytotoxic at ≥ 25μM. To be in the physiologic range (0.75-10μM), only concentrations of curcumin <6μM were used for all further experiments.
Detection of transcription factor NF-κBp65 using ELISA
Following treatment of cells with various concentration of curcumin for 72 h, cells were harvested and nuclear lysates were prepared. The commercially available kit for NF-κB/p65 (Active Motif, Carlsbad, CA) contains the specific oligos with the specific consensus sequence for NF-κB/p65 binding. Five micrograms of nuclear lysate protein from each group was taken for quantification of NF-κB activity. The experiment was done according to the manufacturer's instructions. Absorbance was taken at 450 nm by using ELISA reader (Multiscan MCC/340, Fisher Scientific).
Measurement of HDAC and HAT activity using ELISA
Following treatment to cells with various concentrations of curcumin for 72 h, cells were harvested and nuclear lysates were prepared. 10 μg and 50 μg of nuclear lysate protein from each group were taken for determination of HDACs and HATs activity, respectively. The experiment was done according to the manufacturer's instructions. Absorbance was taken at 405 nm and 440 nm.
Preparation of Nuclear and Cytoplasmic Lysates
After treatment of cells with curcumin the medium was aspirated and the cells washed twice in PBS (10 mM, pH 7.4). Nuclear lysates were prepared using NE-PER Nuclear and Cytoplasmic Extraction Reagents (Pierce). The lysates were collected and cleared by centrifugation, and the supernatant was aliquoted and stored at −80°C. The protein content in the lysates was measured by BCA protein assay (Pierce), as per the manufacturer's protocol.
Western Blot Analysis
For Western blot analysis, 12 μg of protein from each treatment was resolved over 10% Trisglycine polyacrylamide gels (Novex), transferred onto nitrocellulose membranes, and subsequently incubated in blocking buffer [5% nonfat dry milk/1% Tween 20; in 20 mmol/L TBS (pH 7.6)] for 2 h. The blots were incubated with appropriate primary antibody (HDAC 2, p300, acetylated p300/CBP, p-NF-κBp65, NF-κBp65 and acetylated p65) washed, and incubated with appropriate secondary horseradish peroxidase–conjugated antibody (Amersham Biosciences). The blots were detected with chemiluminescence (ECL kit, Amersham Biosciences) and autoradiography, using XAR-5 film (Eastman Kodak). Equal loading of protein was confirmed by stripping the blots and reprobing with TATA binding protein (Abcam Inc, MA, USA)
Quantitative RT-PCR
Total RNAs were extracted from cultured cells using TRIZOL reagent (Invitrogen). Template cDNAs were obtained by reverse transcription (RT) using QuantiTect reverse trasnscription kit (Qiagen). Detection of cDNAs was done by PCR reactions using primers designed for candidate transcript detection. HDAC2: sense:5′-GGGAATACTTTCCTGGCACA-3, Antisense;5′ACGGATTGTGTAGCCACCTC-3′,p300:forward:5′-GGTCCACTCCAATCCAG-3′, Reverse: 5′CTCAAGATGTCTCGGAA-3′. Real-time RT-PCR experiments were done in triplicate using SYBR Green chemistry and a Mastercycler ep gradient S (Eppendorf). The quantity in each sample was normalized to the level of GAPDH transcripts. RT-PCR data were calculated by Delta-Delta CT method.
Immunostaining
After treatment of cells with curcumin, the cells were centrifuged and medium was aspirated. Cells were washed twice in PBS (10 mM, pH 7.4) and placed on L-lysine coasted slides, the slides were air dried, fixed with 4% formaldehyde for 30 min at 4°C and stained overnight at 4°C with HDAC2, p300, acetylated p65 antibody (1:1000 dilution). After being air dried, slides were incubated with appropriate secondary antibody for 60 min. The slides were washed as described above, air dried, mounted with mounting medium, and then examined with a fluorescence microscope at 400 × magnification. In order to measure immunostaining intensity of HDAC2, p300 and acetylated p65, images were captured with a Nikon eclipse TE200 camera (Japan). The signal intensity was measured using ImageJ software.
Chromatin immunoprecipitation assays
ChIP assays were performed using Chip-IT™ Express (Active Motif) according to the manufacturer's instructions. After treatment of cells with curcumin, the cells were centrifuged and medium was aspirated. Cells were washed twice in PBS (10 mM, pH 7.4) and fixed with fresh Fixation solution (37% formaldehyde) for 5 sec at room temperature, followed by glycine stop-fix solution. Cells were washed twice with cold PBS, PBS was poured off and discarded and cells were scraped, pelleted by centrifugation for 10 min at 2,500 rpm at 4°C. Cells were resuspended in 1 mL of ice-cold lysis buffer followed by 30 min incubation on ice. Pellets were spun down for 10 min at 5,000 rpm. Chromatin were sheared using 25% power sonication using our optimized condition (10 pulses of 20 sec each with a 30 sec rest on ice between pulses) to an average DNA size of 600 bp and lysates were cleared by centrifugation at 14,000 for 10 min at 4°C. For each ChIP, one-tenth of the total sonicated chromatin volume (500 μL) was used. Immunoprecipitations were performed overnight at 4°C with 5 μL of the p300 antibody. Chromatin–antibody complexes were captured to magnetic beads (25 μL) and chromatin was eluted as described in manufacturer's instructions. The cross-links were reversed and DNA purified by proteinase K. DNA was analyzed by PCR.
PCR
DNA concentration was measured spectrophotometrically at 260 nm. DNA was subjected to PCR. The antibodies against p300 were purchased from Upstate Biotechnology. Primer sequences for the amplification of IL-6 and TNF-α. IL-6 were: Forward: 5′-TTGCGATGCTAAAGGACG-3′ and reverse; 5′- TGTGGAGAAGGAGTTCATAGC- 3′. TNF-α forward:5′-CCCTCCCAGTTCT AGTTCTATC-3′ and reverse:5′-GGGGAAAGAATCATTCAACCAG-3′. PCR was performed after a 4 min denaturation at 94°C, and repeating the cycles of; 94°C, 55°C and 72°C each for 40 sec; the number of cycles was specific for each primer set. PCR products were electrophoresed in a 1.5% agarose gel containing ethidium bromide.
Statistical analysis
Each experiment was performed at least three times. Results are expressed as the mean ± standard deviation (SD). Statistical analysis was performed using Student's t-test and statistical significance is expressed as *, P < 0.05, **, P < 0.01.
Results
Inflammatory cytokine secretion is decreased by curcumin treatment under hyperglycemic conditions in monocytes
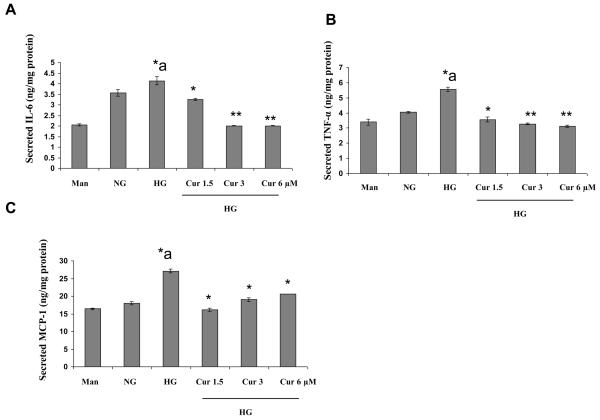
We first examined the effect of hyperglycemia (HG, 25 mmol/L glucose) compared to normo glycemia (NG, 5.5 mmol/L glucose) on cytokine release from monocytes by ELISA (Fig. 1A-1C). Under HG, inflammatory cytokine IL-6, TNF-α and MCP-1 release were significantly increased compared to NG. Mannitol was used as hyperosmolar control and did not significantly affect cytokine release. As shown in Figure 1A-1C, inflammatory cytokines from high glucose-induced THP-1 cells were significantly downregulated by curcumin (1.5μM, 3μM and 6 μM) treatment.
Figure 1. Curcumin treatment suppresses cytokine release in HG-induced THP-1cells.
Human monocytic (THP-1) cells(1 × 105 cells/ml) were cultured in presence of osmolar control (19.5 mmol/L mannitol) or normal glycemic (NG, 5.5 mmol/L glucose ) or hyperglycemic ( HG, 25 mmol/L) conditions in absence or presence of curcumin for 72 h as described in Methods. After that the media was saved. Cytokines were measured with a Milliplex ™ MAP Assay Kit (Millipore,USA)) according to the manufacturer's instructions. Values were calculated based on a standard curve constructed for the assay. Results were shown as mean ± SD of 5 different experiments. *a: p<0.05 compared to NG; *p<0.05; **p<0.01 compared to HG
Curcumin treatment significantly modulates HAT and HDACs activity in monocytes under hyperglycemia
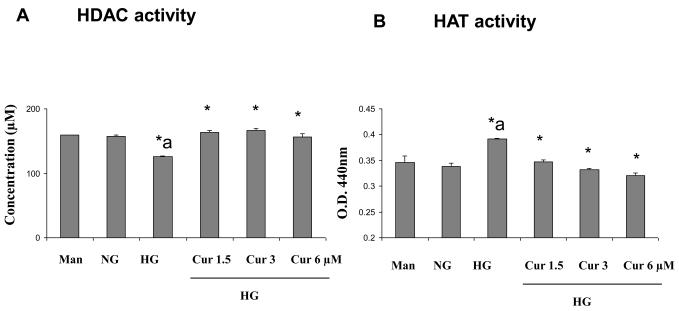
Next, we studied its specific regulation mechanisms. Acetylation of histone protein is associated with increased binding of the transcription factor NF-κB [35]. HDACs and HATs play an important role in regulating pro-inflammatory response. Thus, to obtain further insights into the mechanisms of curcumin-induced downregulation of inflammatory cytokines, as a first step, we examined whether curcumin treatment modulates HDACs activity and HAT activity using ELISA. As shown in Figure 2, under HG conditions, there was a significant increase in the HATs activity and decrease in HDACs activity when compared to normal glucose-treated cells (p<0.01). Furthermore, curcumin treatment results in a significant downregulation of HAT and upregulation of HDAC activity (p<0.01).
Figure 2. Modulation of HDACs and HATs activity by curcumin treatment in HG-induced THP-1 cells.
Following treatment of cells with various concentrations of curcumin for 72 h as described in Methods, cells were harvested and nuclear lysates were prepared. 10 μg and 50 μg of nuclear lysate protein from each group were taken for determination of HDACs and HATs activity, respectively. The experiment was done according to the manufacturer's instructions. Absorbance was taken at 405 nm and 440 nm by using ELISA reader. Results were shown as mean ± SD of 5 different experiments. *a: p<0.05 compared to NG; *p<0.05; **p<0.01 compared to HG
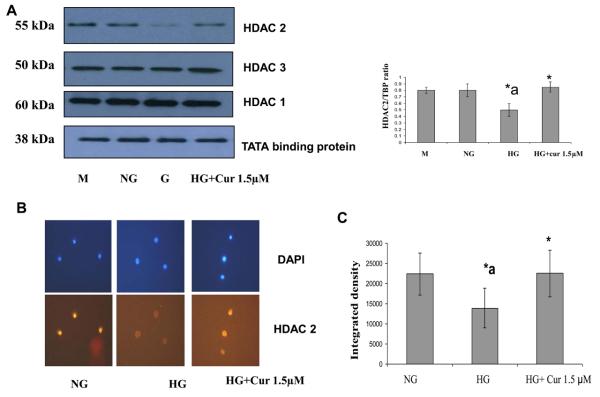
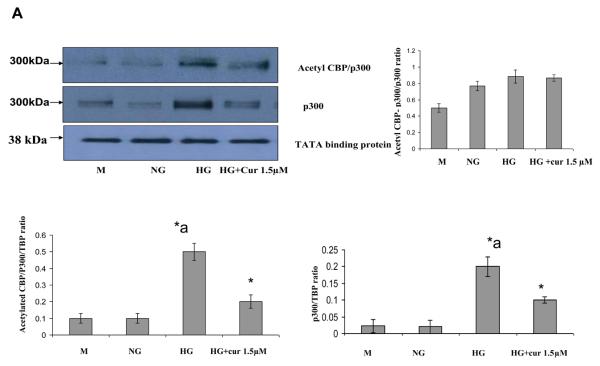
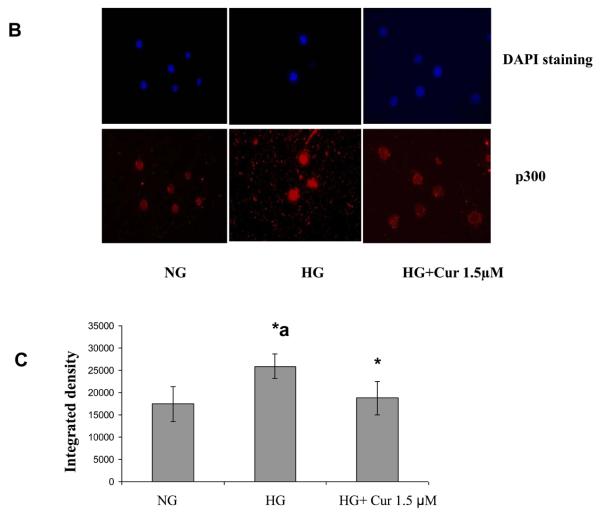
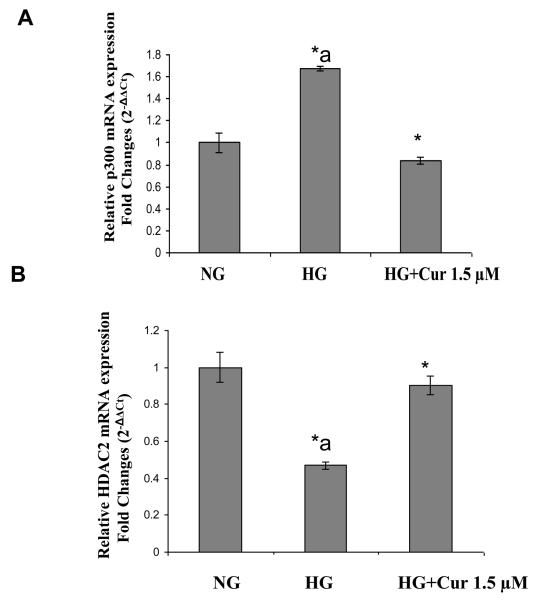
High glucose activates transcription factors, such as NF-κB, by recruitment of transcriptional coactivator molecules p300, which possess intrinsic HAT activity, resulting in histone acetylation and DNA unwinding, allowing DNA polymerases access to the DNA leading to proinflammatory gene expression (inflammatory cytokines). Histone deacetylase type II (HDAC2) forms a bridge with HAT and RelA/p65 to inhibit gene transcription [36]. Thus we tested the effect the expression level of related HDACs and HAT using immunoblots. As shown in Figure 3A, under HG conditions, there was a marked downregulation of HDAC2 when compared with normal glucose-treated cells. This downregulation of HDAC2 was attenuated by curcumin (1.5μM) treatment. Similar results were obtained by using Immunofluorescence (Fig. 3B). There was no significant change in HDAC1 and 3 (Figure 3A). As shown in Figure 4, HG treatment of THP-1 cells resulted in a marked upregulation of p300 which is coactivator including intrinsic HAT and acetylated CBP/p300 expression when compared with normal glucose-treated cells. D-mannitol had no effect on the regulation. In addition, acetylation of Lys1499 p300 has been demonstrated to enhance its HAT activity and affect a wide variety of signaling events [37]. The p300 activation was reduced completely by curcumin (1.5μM) treatment. No effects were seen with DMSO (0.1%) used as vehicle control. Similar results were obtained by using Immunofluorescence (Fig. 4C). mRNA expression level of HDAC2 and p300 also were affected by HG. The downregulation of HDAC2 and upregulation of p300 under HG conditions were abrogated by curcumin treatment (Figure 5)
Figure 3. Effect of curcumin on HDAC2 gene expression level in HG –induced THP-1 cells.
(A) Following treatment of cells with various concentrations of curcumin for 72 h as described in Methods, cells were harvested and nuclear lysates were prepared. protein was subjected to SDS-PAGE as detailed in Materials and Methods Section. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for TATA binding protein (TBP). The immunoblot shown here are representative of three independent experiments with similar results. (B) Cells were fixed with 4% formaldehyde for 30 min at 4°C and stained overnight at 4 °C with p300 as described in Methods. Data from a typical experiment of 3 are shown; Magnification × 400. *a: p<0.05 compared to NG; *p<0.05 compared to HG.. (C) In order to measure Immunostaining intensity of HDAC2, p300 and acetylated p65, images were captured with a Nikon eclipse TE200 camera (Japan). The signal intensity was measured using ImageJ software. *a: p<0.05 compared to NG; *p<0.05 compared to HG..
Figure 4. Effect of curcumin on p300 and acetylated CBP/p300 gene expression level in HG –induced THP-1 cells.
(A) Following treatment of cells with various concentrations of curcumin for 72 h as described in Methods, cells were harvested and nuclear lysates were prepared. Protein was subjected to SDS-PAGE as detailed in Materials and Methods Section. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for TATA binding protein (TBP). The immunoblot shown here is representative of three independent experiments with similar results. (B) Cells were fixed with 4% formaldehyde for 30 min at 4°C and stained overnight at 4 °C with p300 as described in Methods. Data from a typical experiment of 3 are shown; Magnification × 400. *a: p<0.05 compared to NG; *p<0.05 compared to HG. (C) In order to measure Immunostaining intensity of p300, images were captured with a Nikon eclipse TE200 camera (Japan). The signal intensity was measured using ImageJ software *a: p<0.05 compared to NG; *p<0.05 compared to HG.
Figure 5. Effect of curcumin on HDAC2 and p300 mRNA level in HG –induced THP-1 cells.
Following treatment of cells with various concentrations of curcumin for 72 h as described in Methods, cells were harvested and total RNA were prepared. Template cDNAs are obtained by reverse transcription (RT) using QuantiTect reverse trasnscription kit (Qiagen). Detection of cDNAs was done by PCR reactions using primers designed from for candidate transcript detection. HDAC2 and p300. Real-time RT-PCR experiments was done in triplicate using SYBRR Green chemistry and a Mastercycler ep gradient S (eppendorf). The quantity of each sample was normalized to the levels of GAPDH transcripts. qPCR data were calculated by Delta-Delta CT method. Values are expressed as mean ± standard deviation (n = 3; technical replicates). Data represent mean ± SD. *a: p<0.05 compared to NG; *p<0.05 compared to HG..
Curcumin inhibits NF-κBp65 activation in high glucose-induced THP-1 cells
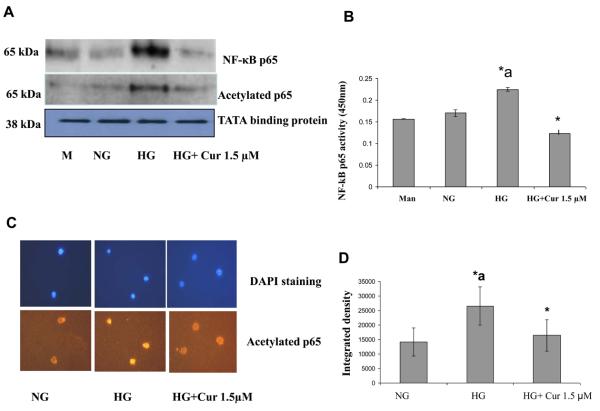
Acetylation of histone protein is associated with increased binding of the transcription factor NF-κB [38]. Increased histone acetylation was associated with increased activation of NF-κB, leading to increased acetylation of RelA/p65 subunit of NF-κB [38]. Therefore, we studied the effects of curcumin on acetylation of NF-κBp65, transactivation and translocation of NF-κBp65 in high glucose induced-THP-1 cells. NF-κB activation was analyzed by measuring inhibitory κBα factor (IκBα) phosphorylation and subsequent translocation of NF-κB (p65) to the nucleus and acetylation of NF-κBp65 using Western blot assays of nuclear protein. We observed that curcumin (1.5 μM) treatment of cells resulted in decreased NF-κBp65 in the nuclear fraction (Fig. 6A). We further confirmed the inhibition of NF-κB/p65 transactivation by performing ELISA (Fig. 6B). We also observed that curcumin (1.5 μM) resulted in significantly decreased acetylation and phosphorylation of NF-κBp65 in the nuclear fraction (Fig.6A). Similar results on acetylation of p65 were obtained by using Immunofluorescence (Fig.6C). Thus, curcumin appears to suppress inflammatory cytokines through at least in part the NF-κB signaling pathway via inducing HDAC activity (HDAC2) and suppressing HAT activity (p300) under HG conditions.
Figure 6. Suppression of NF-κBp65 activation pathway by curcumin in HG-induced THP-1 cells.
(A)Following treatment of cells with various concentrations of curcumin for 72 h as described in Methods, cells were harvested and nuclear lysates were prepared. Protein was subjected to SDS-PAGE as detailed in Methods Section. Equal loading of protein was confirmed by stripping the immunoblot and reprobing it for TATA binding protein (TBP). The immunoblot shown here are representative of three independent experiments with similar results. (B) The commercially available kit for NF-κB/p65 (Active Motif, Carlsbad, CA) contains the specific oligos with the specific consensus sequence for NF-κB/p65 binding. Five micrograms of nuclear lysate protein from each group was taken for quantification of NF-κB activity. The experiment was done according to the manufacturer's instructions. Absorbance was taken at 450 nm by using ELISA reader (Multiscan MCC/340, Fisher Scientific). (C) Cells were fixed with 4% formaldehyde for 30 min at 4°C and stained overnight at 4 °C with p300 as described in Methods. Data from a typical experiment of 3 are shown; Magnification × 400. *a: p<0.05 compared to NG; *p<0.05 compared to HG. (D) In order to measure Immunostaining intensity of acetylated p65, images were captured with a Nikon eclipse TE200 camera (Japan). The signal intensity was measured using ImageJ software. *a: p<0.05 compared to NG; *p<0.05 compared to HG..
Curcumin affect chromatin events at the promoters of inflammatory genes
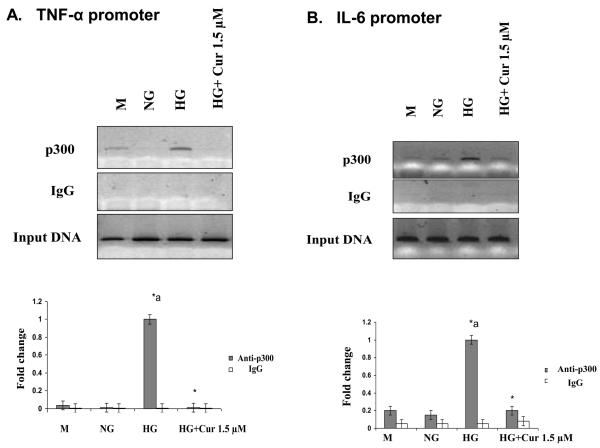
To confirm the epigenetic regulation of curcumin on inflammation, we next used ChIP assays to further investigate whether p300 can be bound to the promoters of NF-κB-related inflammatory cytokine genes under HG conditions. ChIP assays showed that HG increased the recruitment of p300 to the TNF-α and IL-6 promoters. As shown in Figure 7, curcumin reduced the binding of p300 to the promoter region of IL-6 and TNF-alpha. This was associated with decreased IL-6 and TNF transcription in monocytes under HG conditions.
Figure 7. Effect of curcumin on chromatin events at the promoters of inflammatory genes.
ChIP assays were performed using Chip-IT™ Express (Active Motif) according to the manufacturer's instructions. Immunoprecipitations were performed overnight at 4°C with 5 μL of p300 antibody. DNA was subjected to PCR. ChIP assays showed the recruitment of p300 to the TNF-α (A) and IL-6 (B) promoters. Results of 1 typical experiment of 3 are shown. Values from ChIP with anti-p300 antibody represents the fold difference relative to those from IgG control antibody. Data are the average of three independent experiments each, and error bars represent standard deviations. *a: p<0.05 compared to NG; *p<0.05 compared to HG..
Discussion
Hyperglycemia has been implicated as a major contributor to several diabetic complications [38-39]. Several lines of evidence also point to diabetes being a pro-inflammatory state. Monocytes are important in orchestrating effects of hyperglycemia-induced inflammation. We and others have shown that HG induces proinflammatory cytokines (IL-1, IL-6 and TNF-alpha) in monocytes via NF-κB dependent pathway. In correlation with this observation and earlier report, high glucose treatment to the THP-1 cells leads to increased expression of the inflammatory cytokines TNF-alpha, IL-6, IL-8 and IL-1β in a hyperglycemia-induced oxidative stress, nuclear NF-κB and AP-1 transcription factor-dependent manner [4, 10, 40]. TNF-alpha, IL-6, IL-8 and MCP-1 are proinflammatory cytokines and widely recognized markers of vascular inflammation [41-42]. The levels of these cytokines are elevated in diabetic patients [43-46]. We have previously shown increased monocyte pro-inflammatory cytokines in patients with Type 1 and Type 2 diabetes [47].
The antioxidant and/or anti-inflammatory effects of dietary polyphenols, have all been shown to play a role in either controlling NF-κB activation or chromatin remodeling through modulation of HDAC activity and subsequently inflammatory gene expression [30-31].
Curcumin (diferuloylmethane) is a polyphenol responsible for the yellow color of the curry spice turmeric. It has been used in a variety of diseases in traditional medicine. Modern scientific research has demonstrated its anti-inflammatory, anti-oxidant, anti-carcinogenic, anti-thrombotic, and cardiovascular protective effects [33]. Also, curcumin has been shown to be anti-inflammatory via suppression of the NF-κB pathway [34, 40]. Recent studies have reported that curcumin possesses HAT inhibitory activity with specificity for the p300/CREB-binding protein [32]. However, its specific regulation mechanisms at the level of chromatin are not known in diabetic conditions.
The goal of this study was to determine whether curcumin, can be used as a therapeutic agent for inflammation that contributes to diabetes complications. In the present study, we investigated the role of curcumin in regulation of high glucose-mediated proinflammatory cytokines (IL-6 and TNF-α release), chromatin remodeling and posttranslational modification of transcription factor, NFKb in high glucose induced-THP-1cells.
Production of reactive oxygen species has been implicated as a common factor of hyperglycemic damage [48-49]. Reactive oxygen species (ROS) alter nuclear histone acetylation and deacetylation (chromatin remodeling) leading to increased NF-κB-dependent gene expression of proinflammatory mediators [50]. NF-κB plays an important role in the regulation of proinflammatory genes that are associated with several inflammatory diseases, including atherosclerosis, insulin resistance, metabolic syndrome, and diabetes and its complications [11-15]. NF-κB consists of homo- or heterodimers of different sub-units, such as p50, p52, p65/RelA, RelB, and c-Rel, with p65/RelA and p50 being the most common and well studied [16-17]. p65 protein is a key transcriptionally active component of NF-κB whose transactivation potential is enhanced by several coactivators, including CREB-binding protein/p300, p/CAF, and SRC1 [51] which have histones. These cofactors have HAT activity and play key roles in the transcription machinery [52-53]. The p300/CBP-mediated hyperacetylation of RelA (p65) is critical for NF-κB activation. Five main acetylation sites have been identified within p65. Acetylation at Lys221 enhances DNA binding by p65 and impairs its assembly with IκBα, whereas acetylation of Lys316 is required for full transcriptional activity of p65 [54]. Therefore, the attenuation of p65 acetylation is a potential molecular target for the prevention of chronic inflammation. In this study, at a dose of 1.5 μM and 3 μM, curcumin treatment inhibited the expression of NF-κB target genes including IL-6, MCP-1 and TNF-α in high glucose-induced THP-1 cells. We also show novel data that curcumin prevents high glucose-induced p65 translocation to the nucleus, confirming that hyperacetylation is critical for NF-kappaB translocation as well as activity under hyperglycemia. Furthermore we demonstrate that curcumin inhibits HG-induced p65 acetylation thereby resulting in suppressed NF-κB transcription activity via hypoacetylation of p65 at Lys310.
There is also some previous evidence to suggest that HDAC corepressor proteins may function to negatively regulate NF-κB transcriptional activity [55-57]. We observed that curcumin can inhibit inflammation through upregulation of HDAC2 activity in monocytes. Curcumin restored HDAC2 activity in high glucose-induced THP-1 cells. This HDAC2 recruitment may be, at least in part, attenuating NF-κB-mediated chromatin acetylation to exert its corepressor function and subsequent proinflammatory gene expression. Curcumin reduced the binding of p300 to the promoter region of IL-6 and TNF-α genes. Thus, curcumin appears to exert anti-inflammatory effects in monocytes under HG conditions via inhibition of HAT activity, preventing NF-κB-mediated chromatin acetylation and subsequent transcription of cytokines.
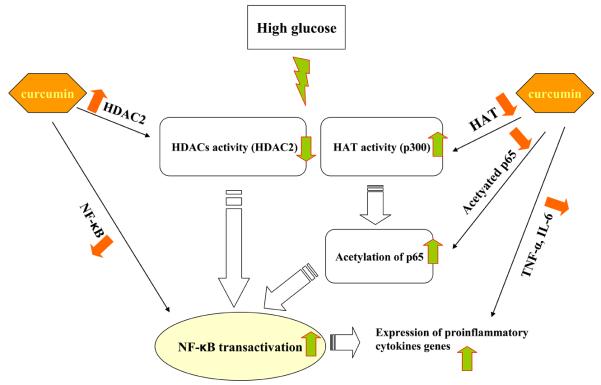
Thus, in summary, high glucose activates HAT (p300) which in turn acetylates p65 and suppresses HDAC2 resulting in induction NF-κB activation and increased transcription of IL-6 and TNF-α in monocytes. Curcumin acts at different levels and induces epigenetic changes by increasing HDAC2, decreasing HAT (p300) activity, thereby resulting in decreased NF-κB activation and inflammatory cytokine release (IL-6, TNF) (Figure 8). All these results were also reproduced using human monocytes. Our results reveal for the first time that in vitro chromatin remodeling by curcumin occurs under diabetic conditions. However, future studies on chromatin events and molecular mechanism in HG conditions are needed in order to understand the effect this compound as natural therapy for chronic inflammation associated with diabetes and its complications.
Figure 8. Proposed epigenetic mechanisms via which curcumin regulates NF-κB signaling pathway leading to decreased expression of pro-inflammatory genes.
High glucose activates the NF-κB signaling pathway leading to pro-inflammatory gene expression. Curcumin treatment of HG-induced cells activates the HDACs activity, especially HDAC2, and suppresses HAT activity, especially p300, leading to deacetylation of p65 NF-κB, subsequently suppressing proinflammatory cytokine release.
Acknowledgements
NIHK24AT 00596 (IJ) and Manpreet Kaur for editorial assistance
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Brownlee M. Glycation and diabetic complications. Diabetes. 1994;43:826–41. doi: 10.2337/diab.43.6.836. [DOI] [PubMed] [Google Scholar]
- 2.Lyons T. Glycation and oxidation: a role in the pathogenesis of atherosclerosis. Am J Cardiol. 1993;71:26B–31B. doi: 10.1016/0002-9149(93)90142-y. [DOI] [PubMed] [Google Scholar]
- 3.Vlassara H, Bucala R, Sturcker L. Pathogenic effects of advanced glycosylation: biochemical, biologic, and clinical implications for diabetes and aging. Lab Invest. 1994;70:138–51. [PubMed] [Google Scholar]
- 4.Guha M, Bai W, Nadler J, Natarajan R. Molecular mechanisms of TNF-alpha gene expression in monocytic cells via hyglycemia-induced oxidant stress dependent and independent pathways. J Biol Chem. 2000;275:17728–39. doi: 10.1074/jbc.275.23.17728. [DOI] [PubMed] [Google Scholar]
- 5.Shanmugam N, Reddy MA, Guha M, Natarajan R. High-glucose–induced expression of proinflammatory cytokine and chemokine genes in monocytic cells. Diabetes. 2003;52:1256–64. doi: 10.2337/diabetes.52.5.1256. [DOI] [PubMed] [Google Scholar]
- 6.Jain SK, Kannan K, Lim G, Matthew-Greer J, McVie R, Bocchini JA. Elevated blood interleukin-6 levels in hyperketonemic type 1 diabetic patients and secretion by acetoacetate-treated cultured U937 monocytes. Diabetes Care. 2003;26:2139–43. doi: 10.2337/diacare.26.7.2139. [DOI] [PubMed] [Google Scholar]
- 7.Igarashi M, Wakasaki H, Takahara N, Ishii H, Jiang ZY, Yamauchi T, Kuboki K, Meier M, Rhodes CJ, King GL. Glucose or diabetes activates p38 mitogen-activated protein kinase via different pathways. J Clin Invest. 1999;103:185–95. doi: 10.1172/JCI3326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dandona P, Chaudhuri A, Ghanim H, Mohanty P. Proinflammatory effects of glucose and anti-inflammatory effect of insulin: relevance to cardiovascular disease. Am J Cardiol. 2007;99:15B–26B. doi: 10.1016/j.amjcard.2006.11.003. [DOI] [PubMed] [Google Scholar]
- 9.Li Y, Reddy MA, Miao F, Shanmugam N, Yee JK, Hawkins D, Ren B, Natarajan R. Role of the Histone H3 Lysine 4 Methyltransferase, SET7/9, in the Regulation of NF-κB-dependent Inflammatory Genes: Relevance To Diabetes And Inflammation. J Biol Chem. 2008;283:26771–81. doi: 10.1074/jbc.M802800200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Miao F, Gonzalo IG, Lanting L, Natarajan R. In vivo chromatin remodeling Events Leading to inflammatory Gene Transcription under diabetic Conditions. J Biol Chem. 2004;23:18091–7. doi: 10.1074/jbc.M311786200. [DOI] [PubMed] [Google Scholar]
- 11.Arkan MC, Hevener AL, Greten FR, Maeda S, Li ZW, Long JM, Wynshaw-Boris A, Poli G, Olefsky J, Karin M. IKK-beta links inflammation to obesity-induced insulin resistance. Nat Med. 2005;11:191–8. doi: 10.1038/nm1185. [DOI] [PubMed] [Google Scholar]
- 12.Cai D, Yuan M, Frantz DF, Melendez PA, Hansen L, Lee J, Shoelson SE. Local and systemic insulin resistance resulting from hepatic activation of IKK-beta and NF-kappaB. Nat Med. 2005;11:183–90. doi: 10.1038/nm1166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.de Winther MP, Kanters E, Kraal G, Hofker MH. Nuclear factor kappaB signaling in atherogenesis. Arterioscler Thromb Vasc Biol. 2005;25:904–14. doi: 10.1161/01.ATV.0000160340.72641.87. [DOI] [PubMed] [Google Scholar]
- 14.Shanmugam N, Kim YS, Lanting L, Natarajan R. Regulation of cyclooxygenase-2 expression in monocytes by ligation of the receptor for advanced glycation end products. J Biol Chem. 2003;278:34834–44. doi: 10.1074/jbc.M302828200. [DOI] [PubMed] [Google Scholar]
- 15.Hayden MS, Ghosh S. Signaling to NF-κB. Genes Dev. 2004;18:2195–224. doi: 10.1101/gad.1228704. [DOI] [PubMed] [Google Scholar]
- 16.Hayden MS, Ghosh S. Shared principles in NF-kappaB signaling. Cell. 2008;132:344–62. doi: 10.1016/j.cell.2008.01.020. [DOI] [PubMed] [Google Scholar]
- 17.Ghosh S, May MJ, Kopp EB. NF-kappa B and Rel proteins: evolutionarily conserved mediators of immune responses. Annu Rev Immunol. 1998;16:225–260. doi: 10.1146/annurev.immunol.16.1.225. [DOI] [PubMed] [Google Scholar]
- 18.Chen LF, Mu Y, Greene WC. Acetylation of RelA at discrete sites regulates distinct nuclear functions of NF-κB. EMBO J. 2002;21:6539–48. doi: 10.1093/emboj/cdf660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kiernan R, Brès V, Ng RW, Coudart MP, El Messaoudi S, Sardet C, Jin DY, Emiliani S, Benkirane M. Post-activation turn-off NF-κB-dependent transcription is regulated by acetylation of p65. J Biol Chem. 2003;278:2758–66. doi: 10.1074/jbc.M209572200. [DOI] [PubMed] [Google Scholar]
- 20.Rahman I, Marwick J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-kappaB and pro-inflammatory gene expression. Biochem Pharmacol. 2004;15:1255–67. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 21.Roth SY, Denu JM, Allis CD. Histone acetyltransferases. Annu. Rev Biochem. 2001;70:81–120. doi: 10.1146/annurev.biochem.70.1.81. [DOI] [PubMed] [Google Scholar]
- 22.Berger SL. Histone modifications in transcriptional regulation. Curr Opin Genet Dev. 2002;12:142–8. doi: 10.1016/s0959-437x(02)00279-4. [DOI] [PubMed] [Google Scholar]
- 23.Marks PA, Rifkind RA, Richon VM, Breslow R, Miller T, Kelly WK. Histone deacetylases and cancer: causes and therapies. Nat Rev Cancer. 2001;1:194–202. doi: 10.1038/35106079. [DOI] [PubMed] [Google Scholar]
- 24.Lyon J, La Thangue NB. Chromatin research gathers pace. Trends Cell Biol. 1997;7:389. doi: 10.1016/S0962-8924(97)01149-5. [DOI] [PubMed] [Google Scholar]
- 25.Wolffe AP. Chromatin remodeling: why it is important in cancer. Oncogene. 2001;20:2988–90. doi: 10.1038/sj.onc.1204322. [DOI] [PubMed] [Google Scholar]
- 26.Rouaux C, Jokic N, Mbebi Boutiller S, Leoffler JP, Boutiller AL. Critical loss of CBP/p300 histone acetylase activity by caspase-6 during neurodegeneration. EMBO J. 2003;22:6537–49. doi: 10.1093/emboj/cdg615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.McKinsey TA, Olson EN. Cardiac histone acetylation--therapeutic opportunities abound. Trends Genet. 2004;20:206–13. doi: 10.1016/j.tig.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 28.Yang XJ. The diverse superfamily of lysine acetyltransferases and their roles in leukemia and other diseases. Nucleic Acids Res. 2004;32:959–76. doi: 10.1093/nar/gkh252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kramer OH, Gottlicher M, Heinzel T. Histone deacetylase as a therapeutic target. Trends Endocrinol Metab. 2001;12:294–300. doi: 10.1016/s1043-2760(01)00438-6. [DOI] [PubMed] [Google Scholar]
- 30.Wolfram S. Effects of Green Tea and EGCG on Cardiovascular and Metabolic Health. J Am Coll Nutr. 2007;26:373S–88S. doi: 10.1080/07315724.2007.10719626. [DOI] [PubMed] [Google Scholar]
- 31.Rahman I, Biswas SK, Kirkham PA. Regulation of inflammation and redox signaling by dietary polyphenols. Biochem Pharmacol. 2006;30:1439–52. doi: 10.1016/j.bcp.2006.07.004. [DOI] [PubMed] [Google Scholar]
- 32.Marcu MG, Jung YJ, Lee S, Chung EJ, Lee MJ, Trepel J, Neckers L. Curcumin is an inhibitor of p300 histone acetylatransferase. Med Chem. 2006;2:169–74. doi: 10.2174/157340606776056133. [DOI] [PubMed] [Google Scholar]
- 33.Wongcharoen W, Phrommintikul A. The protective role of curcumin in cardiovascular diseases. Int J cardiol. 2009;133:145–51. doi: 10.1016/j.ijcard.2009.01.073. [DOI] [PubMed] [Google Scholar]
- 34.Aggarwal BB, Shishodia S. Suppression of the nuclear factor-kappaB activation pathway by spice-derived phytochemicals: reasoning for seasoning. Ann N Y Acad Sci. 2004;1030:434–41. doi: 10.1196/annals.1329.054. [DOI] [PubMed] [Google Scholar]
- 35.Rahman I, Marwich J, Kirkham P. Redox modulation of chromatin remodeling: impact on histone acetylation and deacetylation, NF-κB and proinflammatory gene expression. Biochem Pharmacol. 2004;15:1255–67. doi: 10.1016/j.bcp.2004.05.042. [DOI] [PubMed] [Google Scholar]
- 36.Ashburner BP, Westerheide SD, Baldwin AS., Jr The p65 (RelA) subunit of NF-kappaB interacts with the histone deacetylase (HDAC) corepressors HDAC1 and HDAC2 to negatively regulate gene expression. Mol Cell Biol. 2001;21:7065–77. doi: 10.1128/MCB.21.20.7065-7077.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Thompson PR, Wang D, Wang L, Fulco M, Pediconi N, Zhang D, An W, Ge Q, Roeder RG, Wong J, Levrero M, Sartorelli V, Cotter RJ, Cole PA. Regulation of the p300 HAT domain via a novel activation loop. Nat Struct Mol Biol. 2004;11:308–15. doi: 10.1038/nsmb740. [DOI] [PubMed] [Google Scholar]
- 38.Ruderman N, Williamson JR, Brwonlee M. Glucose and diabetic vascular disease. FASEB J. 1992;6:2905–14. doi: 10.1096/fasebj.6.11.1644256. [DOI] [PubMed] [Google Scholar]
- 39.Pugliese G, Tilton RG, Williamson JR. Glucose-induced metabolic imbalances in the pathogenesis of diabetic vascular disease. Diabete Metab Rev. 1991;7:35–59. doi: 10.1002/dmr.5610070106. [DOI] [PubMed] [Google Scholar]
- 40.Jain SK, Rains J, Croad J, Larson B, Jones K. Curcumin supplementation lowers TNF-alpha, IL-6, IL-8, and MCP-1 secretion in high glucose-treated cultured monocytes and blood levels of TNF-alpha, IL-6, MCP-1, glucose, and glycosylated hemoglobin in diabetic rats. Antioxid Redox Signal. 2009;11:241–9. doi: 10.1089/ars.2008.2140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Singh U, Devraj S, Jialal I. Vitamin E, oxidative stress and inflammation. Annu Rev Nutr. 2005;25:151–74. doi: 10.1146/annurev.nutr.24.012003.132446. [DOI] [PubMed] [Google Scholar]
- 42.Wellen KE, Hotamisligil GS. Inflammation, stress, and oxidative stress. J Clin Invest. 2005;115:1111–9. doi: 10.1172/JCI25102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Jain SK, Kannan K, Lim G, McVie R, Bocchini JA. Hyperketonemia increases TNF-_ secretion in cultured U937 monocytes and type-1 diabetic patients. Diabetes. 2002;51:2287–93. doi: 10.2337/diabetes.51.7.2287. [DOI] [PubMed] [Google Scholar]
- 44.Nicolls MR, Haskins K, Flores SC. Oxidant stress, immune dysregulation, and vascular function in type I diabetes. Antioxid Redox Signal. 2007;9:879–89. doi: 10.1089/ars.2007.1631. [DOI] [PubMed] [Google Scholar]
- 45.Pennathur S, Heinecke JW. Mechanisms for oxidative stress in diabetic cardiovascular disease. Antioxid Redox Signal. 2007;9:955–69. doi: 10.1089/ars.2007.1595. [DOI] [PubMed] [Google Scholar]
- 46.Evans JL, Maddux BA, Goldfine ID. The molecular basis for oxidative stress-induced insulin resistance. Antioxidant Redox Signal. 2005;7:1040–52. doi: 10.1089/ars.2005.7.1040. [DOI] [PubMed] [Google Scholar]
- 47.Devaraj S, Cheung AT, Jialal I, Griffen SC, Nguyen D, Glaser N, Aoki T. Evidence of increased inflammation and microcirculatory abnormalities in patients with type 1 diabetes and their role in microvascular complications. Diabetes. 2007;56:2790–6. doi: 10.2337/db07-0784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Brownlee M. Biochemistry and molecular cell biology of diabetic complications. Nature. 2001;414:813–20. doi: 10.1038/414813a. [DOI] [PubMed] [Google Scholar]
- 49.Nishikawa T, Edelstein D, Du XL, Yamagishi S, Matsumura T, Kaneda Y, Yorek MA, Beebe D, Oates PJ, Hammes HP, Giardino I, Brownlee M. Normalizing mitochondrial superoxide production blocks three pathways of hyperglycemic damage. Nature. 2000;404:787–90. doi: 10.1038/35008121. 2000. [DOI] [PubMed] [Google Scholar]
- 50.Perkins ND. Post-translational modifications regulating the activity and function of the nuclear factor κB pathway. Oncogene. 2006;25:6717–30. doi: 10.1038/sj.onc.1209937. [DOI] [PubMed] [Google Scholar]
- 51.Zhong H, Voll RE, Ghosh S. Phosphorylation of NF-kappa B p65 by PKA stimulates transcriptional activity by promoting a novel bivalent interaction with the coactivator CBP/p300. Mol Cell. 1998;1:661–71. doi: 10.1016/s1097-2765(00)80066-0. [DOI] [PubMed] [Google Scholar]
- 52.Cheung WL, Briggs SD, Allis CD. Acetylation and chromosomal functions. Curr Opin Cell Biol. 2000;12:326–33. doi: 10.1016/s0955-0674(00)00096-x. [DOI] [PubMed] [Google Scholar]
- 53.Kurdistani SK, Grunstein M. Histone acetylation and deacetylation in yeast. Nat. Rev. Mol. Cell Biol. 2003;4:276–84. doi: 10.1038/nrm1075. [DOI] [PubMed] [Google Scholar]
- 54.Yeung F. Modulation of NF-κB-dependent transcription and Cell survivial by the sirt1 deacetylase. EMBO J. 2004;23:2360–80. doi: 10.1038/sj.emboj.7600244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.El Kharroubi A, Piras G, Zensen R, Martin MA. Transcriptional activation of the integrated chromatin-associated human immunodeficiency virus type 1 promoter. Mol Cell Biol. 1998;18:2535–44. doi: 10.1128/mcb.18.5.2535. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ito K, Barnes P, Adcock IM. Glucocorticoid receptor recruitment of histone deacetylase 2 inhibits interleukin-1beta-induced histone H4 acetylation on lysines 8 and 12. Mol Cell Biol. 2000;20:6891–903. doi: 10.1128/mcb.20.18.6891-6903.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Vanden Berghe W, De Bosscher K, Boone E, Plaisance S, Haegeman G. The nuclear factor-kappaB engages CBP/p300 and histone acetyltransferase activity for transcriptional activation of the interleukin-6 gene promoter. J Biol Chem. 1999;274:32091–8. doi: 10.1074/jbc.274.45.32091. [DOI] [PubMed] [Google Scholar]
- 58.Balasubramanyam K, Altaf M, Varier RA, Swaminathan V, Ravindran A, Sadhale PP, Kundu TK. Polyisoprenylated benzophenone, garcinol, a natural histone acetyltransferase inhibitor, represses chromatin transcription and alters global gene expression. J Biol Chem. 2000;279:33716–26. doi: 10.1074/jbc.M402839200. [DOI] [PubMed] [Google Scholar]
- 59.Balasubramanyam K, Swaminathan V, Ranganathan A, Kundu TK. Small molecule modulators of histone acetyltransferase p300. J Biol Chem. 2003;278:19134–40. doi: 10.1074/jbc.M301580200. [DOI] [PubMed] [Google Scholar]