Abstract
We report the case of a patient treated with living donor-related liver transplantation who suffered from osteomalacia during adefovir dipivoxil (ADV)-containing antiviral therapy for lamivudine-resistant hepatitis B virus infection. The patient had generalized bone pain, with severe hypophosphatemia after 20 mo of ADV therapy. Radiographic studies demonstrated the presence of osteomalacia. The peak plasma ADV level was 38 ng/mL after administration of ADV at 10 mg/d. It was also found that ADV affected the metabolism of tacrolimus, a calcineurin-inhibitor, and caused an increase in the plasma levels of tacrolimus. The disability was reversed with the withdrawal of ADV and with mineral supplementation. ADV can cause an elevation of plasma tacrolimus levels, which may be associated with renal dysfunction. High levels of ADV and tacrolimus can cause nephrotoxicity and osteomalacia. This case highlights the importance of considering a diagnosis of osteomalacia in liver transplantation recipients treated with both ADV and tacrolimus.
Keywords: Hepatitis B virus, Osteomalacia, Adefovir dipivoxil, Living donor-related liver transplantation, Tacrolimus
INTRODUCTION
In hepatitis B virus (HBV) DNA-positive patients, the rate of HBV recurrence after liver transplantation (LT) remains high without prophylaxis using hepatitis B immunoglobulins (HBIG) and lamivudine (LAM)[1-3]. Resistance to LAM is characterized by the substitution of methionine with valine or isoleucine at residue 204 within the tyrosine-methionine-aspartate-aspartate (YMDD) motif of the viral DNA polymerase[4-5]. Adefovir dipivoxil (ADV) is a potent nucleotide analogue against both the wild-type and LAM-resistant HBV[6-7], and it has been reported that ADV can prevent and treat recurrences of HBV infection following LT[8-10].
Hypophosphatemic osteomalacia results from a generalized dysfunction of the proximal renal tubule, leading to impaired reabsorption of amino acids, glucose, urate, and phosphate[11]. The chronic loss of phosphate and the adequate synthesis of 1, 25-dihydroxy vitamin D3 together produce phosphate depletion and failure to properly mineralize bone. Recently, patients with acquired osteomalacia have been reported during treatment of human immunodeficiency virus (HIV) infection using nucleotide analogues such as tenofovir and ADV[12-16]. We report herein a case of osteomalacia that developed during ADV therapy after living donor-related liver transplantation (LDLT).
CASE REPORT
A 48-year-old Japanese man was diagnosed with HBV-related decompensated liver cirrhosis in January 2000 and began undergoing LAM therapy at 100 mg/d. The serum HBs antigen (HBsAg) and HBe antigen (HBeAg) were positive, anti-HBe antibody was negative, and HBV-DNA was detected at 8.0 log genome equivalent (LGE)/mL by transcription-mediated amplification (TMA) before the treatment with LAM. Three months later, his serum HBV-DNA decreased to < 3.7 LGE/mL with a favorable initial response. The response persisted up to February 2001, at which time LAM-resistant mutants (YIDD) emerged with elevated transaminases [alanine aminotransferase (ALT) 374 IU/L (normal; 7-47 IU/L) and aspartate aminotransferase (AST) 444 IU/L (normal; 12-31 IU/L)]. In March 2002, the patient was started on ADV therapy at 10 mg/d combined with LAM, and the antiviral response was progressive, but slow. Although the HBV-DNA was decreased to 4.0 LGE/mL after 2 mo, liver function was not favorably improved. In accordance with our hospital policy, the patient could then be accepted as a liver transplant recipient. His wife volunteered to undergo right hepatectomy for living donation, and he underwent LDLT in May 2002. Serum HBsAg and HBV-DNA were negative, but anti-HBc antibody was positive in the donor. The anti-HBV prophylactic regimen consisted of an intravenous injection of HBIG and peroral administration of LAM (100 mg/d) plus ADV (10 mg/d). Intravenous HBIG was given at a dose of 10 000 IU during the anhepatic phase, and then daily for 6 d. Repeated doses were given to maintain anti-HBs titers above 500 IU/L for the initial 6 mo. The patient remained well with a combination of LAM, ADV, and periodic doses of HBIG injection to maintain anti-HBs greater than 200 IU/L. Anti-HBsAb was always positive, and HBsAg and HBV-DNA were not detected in his serum during observation.
Postoperative immunosuppression consisted of tacrolimus (target trough level 10-15 ng/mL) and steroids (intravenous methylprednisolone 500 mg/d tapered to oral prednisolone 20 mg/d from day 7). The tacrolimus trough level was lowered to 5 to 10 ng/mL, and the dosage of prednisolone was reduced to 2.5 mg/d during the 6th mo after transplantation.
The patient began to complain of right ankle pain in November 2003. His bone pain gradually increased and involved his knees and shoulders. He also began to experience weakness in his leg muscles, with difficulty in walking in July 2004. A neurological evaluation, an electromyogram (EMG), and a muscle biopsy were performed in February 2005, which revealed almost normal muscular fibers. He was admitted for further examination in May 2006. The laboratory data obtained on admission are shown in Table 1. He demonstrated persistent hypophosphatemia with a phosphate level of 1.4 mg/dL (normal range; 2.4-5.1 mg/dL). In addition, he showed significantly elevated levels (3410 IU/L) of alkaline phosphatase (ALP) (normal range; 116-280 IU/L). His serum creatinine level was 1.1 mg/dL and calculated creatinine clearance was 37.9 mL/min, indicating moderate renal insufficiency. Urinalysis and 24-h urine collection confirmed phosphate wasting, which can be caused by impairment of proximal renal tubular reabsorption of phosphate. Although his serum level of intact parathyroid hormone (intact-PTH) was slightly increased, his serum level of 1, 25-dihydroxy vitamin D3 was 21.3 pg/mL (normal range; 20-60 pg/mL). Indications were that the impairment of reabsorption of phosphate could be mainly brought about by the proximal renal tubular injury, and not by vitamin D deficiency, in this patient.
Table 1.
Laboratory data on admission
| Blood chemistry | Arterial blood gas | Urinary chemistry | |||
| Total protein | 6.3 g/dL | pH | 7.375 | Urinary Na | 0.78 g/d |
| Albumin | 3.9 g/dL | PaO2 | 86.8 mmHg | Urinary K | 0.20 g/d |
| AST | 39 IU/L | PaCO2 | 37.1 mmHg | Urinary Cl | 0.67 g/d |
| ALT | 22 IU/L | HCO3- | 21.2 mmol/L | Urinary Ca | 0.14 g/d |
| ALP | 3410 IU/L | Anion gap | 11.3 mmol/L | Uurinary phosphate | 0.43 g/d |
| γ-GTP | 116 IU/L | Base excess | - 3.0 mmol/L | % TRP | 53.7 % |
| Total bilirubin | 0.6 mg/dL | Urinary creatinine | 0.45 g/d | ||
| Direct bilirubin | 0.2 mg/dL | Ccreatinine clearance | 37.9 mL/min | ||
| Urea nitrogen | 36 mg/dL | Uurinary glucose | 2.57 g/d | ||
| Creatinine | 1.1 mg/dL | Urinary protein | 1.44 g/d | ||
| Uric acid | 1.7 mg/dL | Urinary NAG | 49.0 U/L | ||
| Plasma glucose | 90 mg/dL | Urinary BMG | 129, 220 μg/L | ||
| Na | 142 mEq/L | Generalized aminoaciduria | (+) | ||
| K | 3.5 mEq/L | ||||
| Cl | 113 mEq/L | ||||
| Ca | 8.0 mg/dL | ||||
| P | 1.4 mg/dL | ||||
| Mg | 2.1 mg/dL | ||||
| Prothrombin time (INR) | 0.96 | ||||
| Serum BMG | 3.5 mg/L | ||||
| Bone-type ALP | 551 μg/L | ||||
| Intact-PTH | 85 pg/mL | ||||
| 1, 25-dihydroxy vitamin D3 | 21.3 pg/mL | ||||
AST: aspartate aminotransferase; ALT: alanine aminotransferase; ALP: alkaline phosphatase; γ-GTP: γ-glutamyl transferase; P: phosphate; BMG: β2-microglobulin; %TRP: % tublar reabsorption of phosphate; NAG: N-acetyl-β-D-glucosaminidase; Intact-PTH: intact parathyroid hormone.
Radiographic studies including X-rays, MRI, and bone scans were performed. 99mTc-HMDP whole-body bone scintigraphy showed multiple foci of increased radiotracer uptake in the thoracic spine, the sacroiliac region, the rib cage, the shoulders, the knees, and the ankles (Figure 1). X-rays and MRI findings showed pseudofractures (Looser’s zones) in the right femoris, which could indicate osteomalacia.
Figure 1.
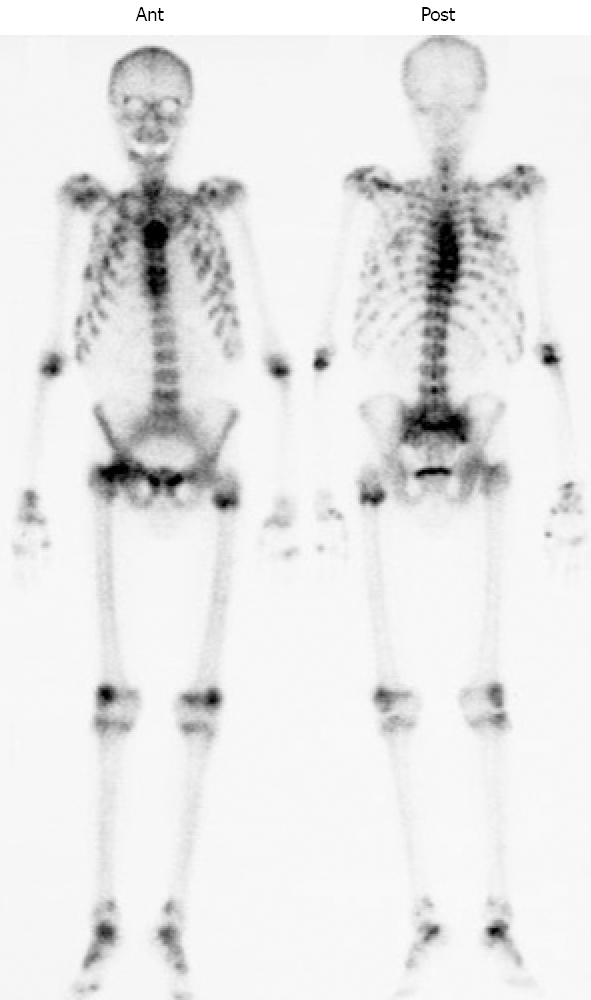
Whole body bone scintigraphy shows multiple foci of increased radiotracer uptake in the thoracic spine, the sacroiliac region, the rib cage, the shoulders, the knees, and the ankles.
The highest plasma level of ADV in the patient was 38 ng/mL, after administration of ADV at 10mg/day, which was approximately 3 times higher than that in patients with normal renal function. We also found that ADV contributed to the elevation of plasma tacrolimus levels in this patient, as the trough levels of tacrolimus with administration of ADV were 1.5 times higher than those without ADV. These results suggest that ADV could affect the metabolism of tacrolimus, and cause increases in the plasma levels of tacrolimus. In this context, high levels of both ADV and tacrolimus could contribute to nephrotoxicity and hypophosphatemia.
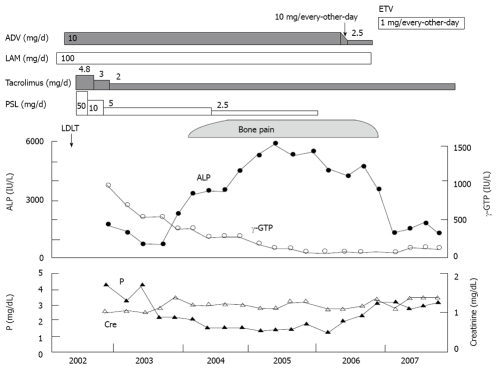
The patient was initially treated with phosphate supplementation and decreasing doses of ADV (10 mg/every other day → 5mg/every other day → 2.5mg/every day). After switching from ADV plus LAM to entecavir hydrate (ETV) at 1 mg/every other day, several laboratory parameters improved, including serum levels of phosphorus and ALP. He was maintained on phosphate replacement, and his bone pain also decreased dramatically (Figure 2).
Figure 2.
Clinical course of the present case. After switching from ADV plus LAM to ETV, serum levels of P and ALP improved, and the patient’s bone pain also decreased dramatically. ADV: adefovir dipivoxil; LAM: lamivudine; ETV: entecavir hydrate; PSL: prednisolone; P: phosphate; APL: alkaline phosphatase; γ-GTP: γ-glutamyl transferase; LDLT: living donor-related liver transplantation.
DISCUSSION
We report herein a case with renal tubular injury, hypophosphatemia, and osteomalacia all of which developed during ADV therapy after LDLT. ADV is known to cause renal tubulopathy in patients with HIV or HBV infection[13-14], as is tenofovir, a major antiretroviral medication[15-16]. The pathophysiology of the proximal tubular dysfunction caused by ADV is thought to be due to the concentration of ADV in the mitochondria[17], and, consequently, mitochondrial toxicity and the inhibition of several ATP-dependent critical transporters in proximal tubular cells[18]. Although every-other-day administration of ADV (10 mg/2 d) is generally recommended when a patient has moderate levels of renal dysfunction, we recommend complete cessation of ADV treatment and a change to another antiviral reagent such as ETV[19-20], as the continued every-other-day administration of ADV may continue to injure the proximal tubular cells. It is unclear why, if all patients accumulate ADV in the proximal tubule, that only a small percentage of patients experience the renal complications seen in this case.
Tacrolimus is a calcineurin-inhibitor (CNI), which are immunosuppressive agents for liver transplantation[21]. Renal dysfunction is common after liver transplantation[22-25], and it has been reported to be associated with high levels of CNI[26]. ADV contributed to the elevation of plasma tacrolimus levels in this patient, and this elevation may have been associated with his renal dysfunction. In cases such as this one, tacrolimus levels should be reduced as far as possible and the interaction between tacrolimus and ADV should be given strong consideration in liver-transplant patients with HBV infection.
Although the patient had typical clinical features such as bone pain, hypophosphatemia, and elevated serum APL levels for osteomalacia, the diagnosis of osteomalacia was delayed. It was initially difficult to distinguish bone-derived ALP and liver-derived ALP because the patient had persistently high levels of serum ALP and γ-glutamyl transferase (γ-GTP) associated with chronic rejection after LDLT.
Hypophosphatemic osteomalacia is a potential adverse effect of ADV[13], and patients treated with ADV should be monitored by measuring serum ALP and phosphorus levels. If patients develop bone pain or myopathy in response to ADV treatment, serum hypophosphatemia and phosphate wasting into urine should be confirmed. Initial treatment with phosphate supplementation and decreasing doses of ADV should be performed. Discontinuing administration of ADV and switching to ETV may be recommended for patients after LT, because these patients often have renal insufficiency associated with the use of CNI.
Footnotes
Peer reviewers: Pierluigi Toniutto, Professor, Internal Medicine, Medical Liver Transplant Unit, University of Udine, P. zale S.M. della Misericordia 1, Udine 33100, Italy; Cheng-Shyong Chang, MD, Assistant Professor, Attending physician, Division of Hemato-oncology, Department of Internal Medicine, Changhua Christian Hospital, Nan-Hsiao St., Changhua 500, Taiwan, China
S- Editor Zhang HN L- Editor Roemmele A E- Editor Liu N
References
- 1.Lok AS. Prevention of recurrent hepatitis B post-liver transplantation. Liver Transpl. 2002;8:S67–S73. doi: 10.1053/jlts.2002.35780. [DOI] [PubMed] [Google Scholar]
- 2.Shouval D, Samuel D. Hepatitis B immune globulin to prevent hepatitis B virus graft reinfection following liver transplantation: a concise review. Hepatology. 2000;32:1189–1195. doi: 10.1053/jhep.2000.19789. [DOI] [PubMed] [Google Scholar]
- 3.Karasu Z, Akyildiz M, Kilic M, Zeytunlu M, Aydin U, Tekin F, Yilmaz F, Ozacar T, Akarca U, Ersoz G, et al. Living donor liver transplantation for hepatitis B cirrhosis. J Gastroenterol Hepatol. 2007;22:2124–2129. doi: 10.1111/j.1440-1746.2006.04782.x. [DOI] [PubMed] [Google Scholar]
- 4.Ling R, Mutimer D, Ahmed M, Boxall EH, Elias E, Dusheiko GM, Harrison TJ. Selection of mutations in the hepatitis B virus polymerase during therapy of transplant recipients with lamivudine. Hepatology. 1996;24:711–713. doi: 10.1002/hep.510240339. [DOI] [PubMed] [Google Scholar]
- 5.Tipples GA, Ma MM, Fischer KP, Bain VG, Kneteman NM, Tyrrell DL. Mutation in HBV RNA-dependent DNA polymerase confers resistance to lamivudine in vivo. Hepatology. 1996;24:714–717. doi: 10.1002/hep.510240340. [DOI] [PubMed] [Google Scholar]
- 6.Perrillo R, Schiff E, Yoshida E, Statler A, Hirsch K, Wright T, Gutfreund K, Lamy P, Murray A. Adefovir dipivoxil for the treatment of lamivudine-resistant hepatitis B mutants. Hepatology. 2000;32:129–134. doi: 10.1053/jhep.2000.8626. [DOI] [PubMed] [Google Scholar]
- 7.Schiff ER, Lai CL, Hadziyannis S, Neuhaus P, Terrault N, Colombo M, Tillmann HL, Samuel D, Zeuzem S, Lilly L, et al. Adefovir dipivoxil therapy for lamivudine-resistant hepatitis B in pre- and post-liver transplantation patients. Hepatology. 2003;38:1419–1427. doi: 10.1016/j.hep.2003.09.040. [DOI] [PubMed] [Google Scholar]
- 8.Bárcena R, Del Campo S, Moraleda G, Casanovas T, Prieto M, Buti M, Moreno JM, Cuervas V, Fraga E, De la Mata M, et al. Study on the efficacy and safety of adefovir dipivoxil treatment in post-liver transplant patients with hepatitis B virus infection and lamivudine-resistant hepatitis B virus. Transplant Proc. 2005;37:3960–3962. doi: 10.1016/j.transproceed.2005.10.061. [DOI] [PubMed] [Google Scholar]
- 9.Marzano A, Lampertico P, Mazzaferro V, Carenzi S, Vigano M, Romito R, Pulvirenti A, Franchello A, Colombo M, Salizzoni M, et al. Prophylaxis of hepatitis B virus recurrence after liver transplantation in carriers of lamivudine-resistant mutants. Liver Transpl. 2005;11:532–538. doi: 10.1002/lt.20393. [DOI] [PubMed] [Google Scholar]
- 10.Lo CM, Liu CL, Lau GK, Chan SC, Ng IO, Fan ST. Liver transplantation for chronic hepatitis B with lamivudine-resistant YMDD mutant using add-on adefovir dipivoxil plus lamivudine. Liver Transpl. 2005;11:807–813. doi: 10.1002/lt.20416. [DOI] [PubMed] [Google Scholar]
- 11.Clarke BL, Wynne AG, Wilson DM, Fitzpatrick LA. Osteomalacia associated with adult Fanconi's syndrome: clinical and diagnostic features. Clin Endocrinol (Oxf) 1995;43:479–490. doi: 10.1111/j.1365-2265.1995.tb02621.x. [DOI] [PubMed] [Google Scholar]
- 12.Verhelst D, Monge M, Meynard JL, Fouqueray B, Mougenot B, Girard PM, Ronco P, Rossert J. Fanconi syndrome and renal failure induced by tenofovir: a first case report. Am J Kidney Dis. 2002;40:1331–1333. doi: 10.1053/ajkd.2002.36924. [DOI] [PubMed] [Google Scholar]
- 13.Earle KE, Seneviratne T, Shaker J, Shoback D. Fanconi's syndrome in HIV+ adults: report of three cases and literature review. J Bone Miner Res. 2004;19:714–721. doi: 10.1359/jbmr.2004.19.5.714. [DOI] [PubMed] [Google Scholar]
- 14.Parsonage MJ, Wilkins EG, Snowden N, Issa BG, Savage MW. The development of hypophosphataemic osteomalacia with myopathy in two patients with HIV infection receiving tenofovir therapy. HIV Med. 2005;6:341–346. doi: 10.1111/j.1468-1293.2005.00318.x. [DOI] [PubMed] [Google Scholar]
- 15.Zimmermann AE, Pizzoferrato T, Bedford J, Morris A, Hoffman R, Braden G. Tenofovir-associated acute and chronic kidney disease: a case of multiple drug interactions. Clin Infect Dis. 2006;42:283–290. doi: 10.1086/499048. [DOI] [PubMed] [Google Scholar]
- 16.Malik A, Abraham P, Malik N. Acute renal failure and Fanconi syndrome in an AIDS patient on tenofovir treatment--case report and review of literature. J Infect. 2005;51:E61–E65. doi: 10.1016/j.jinf.2004.08.031. [DOI] [PubMed] [Google Scholar]
- 17.Tanji N, Tanji K, Kambham N, Markowitz GS, Bell A, D'agati VD. Adefovir nephrotoxicity: possible role of mitochondrial DNA depletion. Hum Pathol. 2001;32:734–740. doi: 10.1053/hupa.2001.25586. [DOI] [PubMed] [Google Scholar]
- 18.Cihlar T, Lin DC, Pritchard JB, Fuller MD, Mendel DB, Sweet DH. The antiviral nucleotide analogs cidofovir and adefovir are novel substrates for human and rat renal organic anion transporter 1. Mol Pharmacol. 1999;56:570–580. doi: 10.1124/mol.56.3.570. [DOI] [PubMed] [Google Scholar]
- 19.Lai CL, Rosmawati M, Lao J, Van Vlierberghe H, Anderson FH, Thomas N, Dehertogh D. Entecavir is superior to lamivudine in reducing hepatitis B virus DNA in patients with chronic hepatitis B infection. Gastroenterology. 2002;123:1831–1838. doi: 10.1053/gast.2002.37058. [DOI] [PubMed] [Google Scholar]
- 20.Chang TT, Gish RG, Hadziyannis SJ, Cianciara J, Rizzetto M, Schiff ER, Pastore G, Bacon BR, Poynard T, Joshi S, et al. A dose-ranging study of the efficacy and tolerability of entecavir in Lamivudine-refractory chronic hepatitis B patients. Gastroenterology. 2005;129:1198–1209. doi: 10.1053/j.gastro.2005.06.055. [DOI] [PubMed] [Google Scholar]
- 21.Busuttil RW, Lake JR. Role of tacrolimus in the evolution of liver transplantation. Transplantation. 2004;77:S44S–51. doi: 10.1097/01.tp.0000126927.49589.3f. [DOI] [PubMed] [Google Scholar]
- 22.Stratta P, Canavese C, Quaglia M, Balzola F, Bobbio M, Busca A, Franchello A, Libertucci D, Mazzucco G. Posttransplantation chronic renal damage in nonrenal transplant recipients. Kidney Int. 2005;68:1453–1463. doi: 10.1111/j.1523-1755.2005.00558.x. [DOI] [PubMed] [Google Scholar]
- 23.Magee C, Pascual M. The growing problem of chronic renal failure after transplantation of a nonrenal organ. N Engl J Med. 2003;349:994–996. doi: 10.1056/NEJMe038120. [DOI] [PubMed] [Google Scholar]
- 24.Veillon S, Caillard S, Epailly E, Eisenmann B, Hannedouche T, Moulin B. Chronic renal failure after cardiac transplantation: predictive factors and influence on mortality-results of a monocenter study in 141 patients. Transplant Proc. 2002;34:2819–2820. doi: 10.1016/s0041-1345(02)03527-3. [DOI] [PubMed] [Google Scholar]
- 25.Ziolkowski J, Paczek L, Senatorski G, Niewczas M, Oldakowska-Jedynak U, Wyzgal J, Sanko-Resmer J, Pilecki T, Zieniewicz K, Nyckowski P, et al. Renal function after liver transplantation: calcineurin inhibitor nephrotoxicity. Transplant Proc. 2003;35:2307–2309. doi: 10.1016/s0041-1345(03)00786-3. [DOI] [PubMed] [Google Scholar]
- 26.Morard I, Mentha G, Spahr L, Majno P, Hadengue A, Huber O, Morel P, Giostra E. Long-term renal function after liver transplantation is related to calcineurin inhibitors blood levels. Clin Transplant. 2006;20:96–101. doi: 10.1111/j.1399-0012.2005.00447.x. [DOI] [PubMed] [Google Scholar]