Abstract
Nesfatin-1 is well established to reduce food intake upon brain injection in rats, while in mice its anorexigenic action and brain expression are largely unexplored. We characterized the influence of intracerebroventricular (icv) and peripheral (intraperitoneal, ip, subcutaneous, sc) injection of nesfatin-1 on dark phase ingestive behavior using an automated feeding monitoring system and mapped NUCB2/nesfatin-1 immunoreactivity in the mouse brain. Nesfatin-1 (0.3, 1 or 3 μg/mouse, icv) caused a dose-related reduction of 4-h dark phase food intake by 13%, 27%, and 46% respectively. Nesfatin-1 (3 μg/mouse, icv) action had a 2-h delayed onset, 82% peak inhibition occurring at 3-4 h post injection and was long lasting (30% reduction for 12h period post injection). Nesfatin-1 (3 μg/mouse, icv)-treated mice had a 46% lower meal frequency associated with 2-times longer inter-meal intervals and a 35% reduction in meal size compared to vehicle during the 1-4 h post injection (p<0.05). NUCB2/nesfatin-1-immunopositive neurons were found in hypothalamic (supraoptic, paraventricular, arcuate, lateral) and brainstem (dorsal vagal complex) feeding-regulatory nuclei. When injected peripherally, neither food intake nor any feeding microstructure parameters were altered. These results demonstrate that NUCB2/nesfatin-1 is prominently expressed in mouse hypothalamus and medulla and acts in the brain to curtail the dark phase feeding by inducing satiation and satiety indicated by reduced meal size and prolonged inter-meal intervals respectively. The lack of nesfatin-1 effect when injected peripherally at a 23-times higher dose indicates a primarily central site of the anorexigenic action for nesfatin-1 in mice.
Keywords: food intake, hypothalamus, meal pattern, mouse, nesfatin-1, satiation, satiety
1. Introduction
Nesfatin-1 is a recently discovered peptide that was identified by Oh-I and colleagues in the rat hypothalamus from the gene encoding nucleobindin2 (NUCB2) which can be potentially processed into nesfatin-1, nesfatin-2 and nesfatin-3 [16]. In their initial study, the authors demonstrated a dark phase food intake reducing effect following third ventricular injection of nesfatin-1, unlike nesfatin-2 and -3 in rats [16]. A physiological relevance of hypothalamic nesfatin-1 to suppress food intake in rats was additionally suggested by the regulation of NUCB2 expression upon changes in metabolic status with a decrease in NUCB2 mRNA expression as well as NUCB2/nesfatin-1 concentration selectively in the paraventricular nucleus (PVN) after 24-h fasting and an increase of daily food consumption and cumulative body weight gain after blockade of endogenous NUCB2 by third ventricular injection of anti-NUCB2 antisense oligonucleotide [16]. Following this seminal report, several independent groups confirmed the reduction of nocturnal feeding induced by nesfatin-1 injected into the cerebrospinal fluid at the level of the lateral, 3rd or 4th ventricle, or cisterna magna as well as directly into the PVN in rats [14, 26, 27, 36, 37]. However, the majority of these studies have been performed in rats and only one study investigated the effects of nesfatin-1 on food intake in mice. Similar to rats, nesfatin-1 injected into the 3rd ventricle decreases dark phase food intake in chronically cannulated mice at the one dose tested [14]. The analysis of the meal pattern is of primary importance to assess the mechanisms regulating feeding behavior [9]. However, so far the food intake microstructure altered by central injection of nesfatin-1 in rats and mice remains to be established. Therefore, in the present study we explored the dose-related influence of nesfatin-1 injected into the lateral brain ventricle on the dark phase meal pattern in freely fed mice using an automated food intake monitoring system developed for mice.
Similarly to the studies on food intake, the expression of NUCB2/nesfatin-1 immunoreactivity has been extensively mapped in the rat brain [3, 5, 7, 10, 13, 16, 27, 35], whereas there is only one report in mice that was focused on the Edinger-Westphal nucleus [17]. Based on the key role of the hypothalamus and brainstem in the regulation of food intake [2, 22], we assessed the occurrence of NUCB2/nesfatin-1-immunoreactive (ir) cells in the mouse hypothalamus and medulla oblongata using immunohistochemistry.
Lastly, besides its dense expression in specific rat brain nuclei, NUCB2/nesfatin-1 is also prominently expressed in peripheral organs, particularly in gastric and pancreatic endocrine cells in both rats and mice [6, 11, 30, 38] which most likely contributes to NUCB2/nesfatin-1 detected in the circulation [27] reported so far in rats. We previously showed that a change in metabolic status induced by a 24-h fast decreases gastric NUCB2 mRNA and NUCB2/nesfatin-1 plasma levels in rats [27, 30] suggestive of a potential food intake regulatory role of peripheral nesfatin-1 as found for other gut peptides [29]. However, up to date only one study indicated a reduction of dark phase food intake following intraperitoneal injection of nesfatin-1 in mice [23] while in rats one report showed no effect [27]. Therefore, we also compared the effects of nesfatin-1 injected icv versus intraperitoneally (ip) or subcutaneously (sc) on the dark phase food intake and feeding microstructure in ad libitum fed mice.
2. Materials and Methods
2.1. Animals
Adult male C57Bl/6 mice (6-8 weeks of age, Harlan Laboratories) were group housed 4/cage under controlled illumination (06:00 – 18:00 h) and temperature (21-23 °C). Animals had ad libitum access to purified standard rodent diet (AIN-93M, Research Diets, Inc., Jules Lane, New Brunswick, NJ) and tap water. Protocols were approved by the Veterans Administration Institutional Animal Care and Use Committee (# 99127-07). All injections were performed directly before the dark phase starting at 18:00 h.
2.2. Peptide
Mouse nesfatin-1(1-82) [NH2-VPIDVDKTKVHNTEPVENARIEPPDTGLYYDEYLKQVIEVLETDPHFREKLQKADIEEIRSGRLSQELDLVSHKVRTRLDEL-CONH2] was synthesized by Abgent Technologies (San Diego, CA) and purified by high pressure liquid chromatography (HPLC) to ≥ 95% purity and the mass assessed by mass spectrometry (manufacturer's information). We independently assessed the mass of nesfatin-1 by mass spectrometry analysis which confirmed the correct mass of the peptide of 9.61 kDa (CURE: Digestive Diseases Research Center, Peptide Biochemistry Core, data not shown). The peptide was stored in powder form at -80 °C and weighed and dissolved in vehicle immediately before administration.
2.3. Intracerebroventricular injection
Injection into the lateral brain ventricle (5 μl) was performed under short isoflurane anesthesia (2-3 min, 4.5% vapor concentration in oxygen; VSS, Rockmart, GA) as in our previous studies [15, 24]. The site of injection was localized at the apex of the equal triangle between the eyes and the back of the head. The site was cleaned with Povidone-Iodine 10% (Aplicare Inc., Meriden, CT) and the skull punctured manually at the point of least resistance with a 30-gauge needle equipped with a polyethylene tube leaving 4-4.5 mm of the needle tip exposed and attached to a Hamilton syringe. On average, mice completely recovered from anesthesia within 5 min. The accuracy of the injections was confirmed in our previous studies by injecting cresyl violet dye intracerebroventricularly (icv) under similar conditions in 50 mice [15].
2.4. Automated food intake monitoring
To investigate the microstructure of feeding, the BioDAQ episodic Food Intake Monitor for mice (BioDAQ, Research Diets, Inc., New Brunswick, NJ) was used for continuous monitoring of meal pattern in undisturbed mice that ingest a regular rodent diet (AIN-93M, Research Diets, Inc.) as detailed in our previous studies [28]. This diet was used because it causes less spillage than the standard Prolab diet (Prolab RMH 2500; LabDiet). Mice were habituated for one week to single housing and feeding through a low spill food hopper placed on an electronic balance mounted on the animals' regular housing cage that contained enrichment and bedding material. Water was provided ad libitum from regular water bottles placed on the cover of the cage. Mice became accustomed to the new environment within 3-4 days and showed normal food intake and regular body weight gain thereafter.
The BioDAQ system weighs the hopper with food (± 0.01g) every second and algorithmically detects “not eating” as weight stable and “eating” as weight unstable. Feeding bouts (changes in stable weight before and after a bout) are recorded as feeding bout vectors with a start time, duration, and amount consumed. Bouts are separated by an inter-bout interval (IBI), and meals consist of one or more bouts separated by an inter-meal interval (IMI). The IBI was defined as 5 sec, the IMI as 5 min and the minimum meal amount as 0.02 g, meaning food intake was considered as one meal when the feeding bouts occurred within 5 min of the previous response and their sum was equal to or greater than 0.02 g. If bouts of feeding were >5 min apart, they were considered as a new meal. Meal parameters assessed encompassed the meal frequency (number/period), bout frequency (number/period), meal size (g/meal), meal duration (min/meal), total meal time (min/period), time spent in meals (%/period), inter-meal interval (min), latency to first meal (min), duration of first meal (min), eating rate of first meal (mg/min), eating rate/period (mg/min) and the satiety ratio. Parameters were calculated by the software provided by the manufacturer (BioDAQ Monitoring Software 2.2.02) and satiety ratio was calculated as the average inter-meal interval divided by the average meal size (min/g food eaten).
2.5. Food intake experiments
In the first experiment, ad libitum fed mice were injected into the lateral brain ventricle (icv) with nesfatin-1 (0.3, 1, or 3 μg/mouse in pyrogen-free distilled water) or vehicle (pyrogen-free distilled water). Following recovery from anesthesia, food intake microstructure was assessed automatically for a period of 24 h. The doses of nesfatin-1 were based on a previous report of an effective dose injected into the third brain ventricle of chronically cannulated mice [14]. In another study, nesfatin-1 (70 μg/mouse dissolved in sterile saline) or vehicle (sterile saline) was injected ip (100 μl) and food intake pattern was monitored for 24 h thereafter. The dose was based on a previous dose-response study showing the reduction of 3-h cumulative dark phase food intake in ICR mice [23]. Lastly, nesfatin-1 (70 μg/mouse dissolved in 10% dimethyl sulfoxide, DMSO and 90% purified sesame oil) or vehicle (10% DMSO and 90% purified sesame oil) was injected sc (50 μl) at the onset of the dark phase and food intake microstructure was assessed for the subsequent 24-h period. This vehicle was chosen based on a previous report showing enhanced duration of action when the peptide corticotropin releasing factor (CRF) receptor1/2 antagonist, astressin-B was dissolved in DMSO/oil versus water [21]. The experiments were repeated in a crossover design in the same batch of mice for each route of administration.
2.6. Nesfatin-1 immunostaining
Ad libitum fed mice (n=3) were deeply anesthetized with an ip injection of sodium pentobarbital (Nembutal, Abbott Lab., Chicago, IL) during the early dark phase. Thereafter, transcardial perfusion was performed as described before [25] with 40-50 ml of 4% paraformaldehyde and 14% saturated picric acid in 0.1 M phosphate buffer (pH 7.2). Brains were removed and post-fixed in the same fixative. Afterwards, whole brains were rinsed and cryoprotected in 10% sucrose for 24 h and snap-frozen in dry ice-cooled 2-methylbutane. Forebrain at the hypothalamic level (-0.14 to -2.54 mm from bregma) and medulla oblongata (-7.2 to -7.6 mm from bregma) [8] were sectioned at 25 μm using a cryostat (Microm International GmbH, Germany) and collected in three sets. One set of free floating sections was stained using the avidin-biotin-peroxidase complex (ABC) method as detailed before [10]. Briefly, sections were incubated in 0.3 % hydrogen peroxide in phosphate buffered saline (PBS) for 30 min to inactivate endogenous peroxidase followed by incubation overnight at 4 °C in rabbit polyclonal anti-nesfatin-1 antibody (1:10,000; raised against rat nesfatin-1(1-82); Phoenix Pharmaceuticals, Inc., Burlingame, CA). Since rat and mouse nesfatin-1 differ only in 2 amino acids (aa 13 and 18) and this antibody has been used before in mice to stain the Edinger-Westphal nucleus [17], it is expected that this antibody cross-reacts with mouse nesfatin-1. Afterwards, sections were incubated with biotinylated goat anti-rabbit IgG (1:1,000; Jackson Immuno Research, West Grove, PA) for 1 h at room temperature, followed by ABC (Vector, Vermont, CA) for 1 h at room temperature. Sections were developed with 3,3′-diaminobenzidine tetrahydrochloride hydrate and hydrogen peroxide (Sigma Chemical Co, St. Louis, MO) and the progress of color development checked by light microscope until the reaction was stopped after 10 min. Each step of incubation was followed by 3 × 15 min washing in PBS. Sections were mounted on slides (Fisher Super Frost Plus, Fisher Scientific, Pittsburgh, PA), air-dried for 24 h and dehydrated through a gradient of ethanol and cleared in xylene before coverslipping. In a control staining, the same protocol was applied after pre-absorption of the antibody with nesfatin-1. Rat nesfatin-1 (10 μg, Phoenix Pharmaceuticals, Inc.) was incubated with rabbit anti-rat nesfatin-1 at 1:10,000 in 0.3% Triton X in PBS for 2 h at room temperature followed by 22 h at 4 °C. The solution was centrifuged for 15 min at 16,000 g and the supernatant was used for staining as described above.
Brain anatomical correlations were made according to landmarks given in Franklin & Paxinos' mouse brain stereotaxic atlas [8]. NUCB2/nesfatin-1 immunoreactivity was examined in hypothalamic, pontine and medullary sections in nuclei throughout their rostro-caudal extent using a light microscope (Axioscop II, Carl Zeiss, Germany). Images were taken by a digital camera (Hamamatsu, Bridgewater, NJ) using the image acquisition system SimplePCI (Hamamatsu Corporation, Sewickley, PA).
2.7. Statistical analysis
Data are expressed as mean ± SEM and were analyzed by one-way analysis of variance (ANOVA) followed by Tukey post hoc test or two-way ANOVA followed by Holm-Sidak method. Differences between groups were considered significant when p < 0.05.
3. Results
3.1. Nesfatin-1 injected intracerebroventricularly reduces the dark phase food intake and alters meal structure
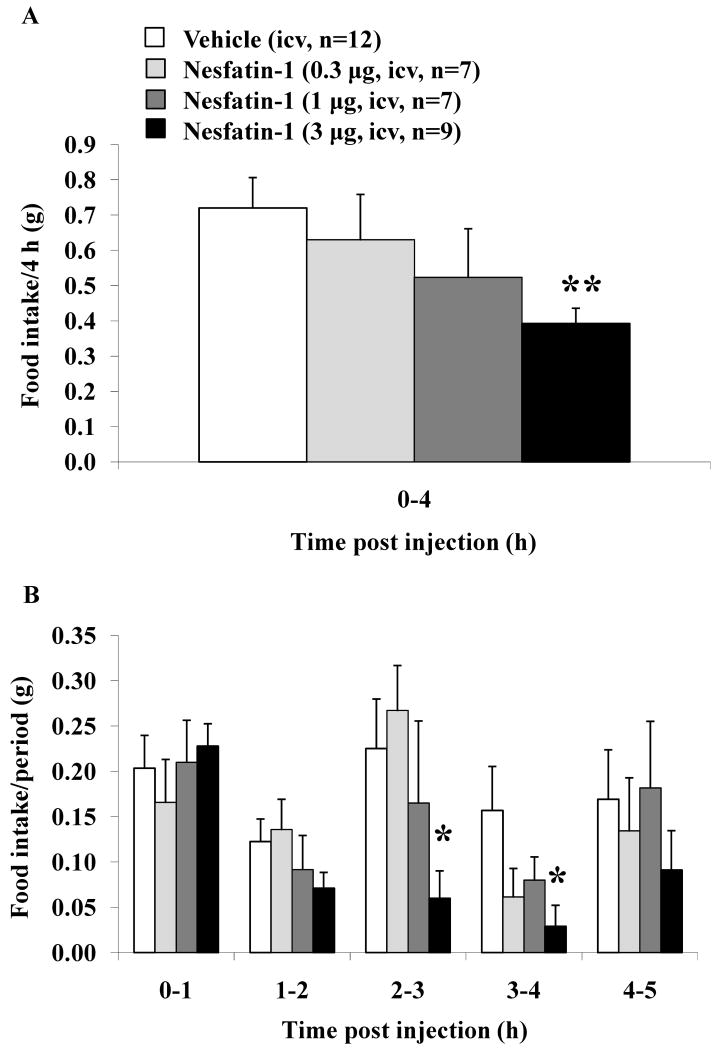
Nesfatin-1 (0.3, 1 and 3 μg/mouse) injected icv induced a dose-related decrease in dark phase food intake during the first 4 h post injection compared to vehicle (g/4 h: 0.63 ± 0.13, 0.52 ± 0.14, and 0.39 ± 0.04 respectively vs. 0.72 ± 0.09) assessed using an automated food intake monitoring system for mice (Fig. 1A). The monitoring of hourly food intake at different times post icv injection of nesfatin-1 at 3 μg/mouse showed that the inhibition was mainly occurring during the 2-3 h (0.06 ± 0.03 vs. 0.23 ± 0.05 g/h, p < 0.05) and 3-4 h periods (0.03 ± 0.02 vs. 0.16 ± 0.05/h g, p < 0.05) compared to vehicle, while no effect was observed thereafter at the 4-5 h period post injection (Fig. 1B). There was also no change during the 1st h post injection and a non-significant trend observed occurring during the 1-2 h period or at lower doses during the 2-3 h and 3-4 h periods (p > 0.05, Fig. 1B). Two-way ANOVA showed a significant influence of treatment (F(3,150) = 3.2, p < 0.05) and time (F(3,150) = 4.5, p < 0.01).
Fig. 1.
Nesfatin-1 injected intracerebroventricularly induces a dose-related decrease in the dark phase food intake in ad libitum fed mice. Nesfatin-1 (0.3, 1 or 3 μg/mouse) or vehicle was injected icv under short anesthesia in ad libitum fed non-cannulated mice at the onset of the dark phase. Food intake was assessed for the first 4 h post icv injection and expressed as cumulative food intake/4 h (A) or food intake/h period (B). Each bar represents the mean ± SEM of 7-12 mice/group. * p < 0.05 and ** p < 0.01 vs. vehicle.
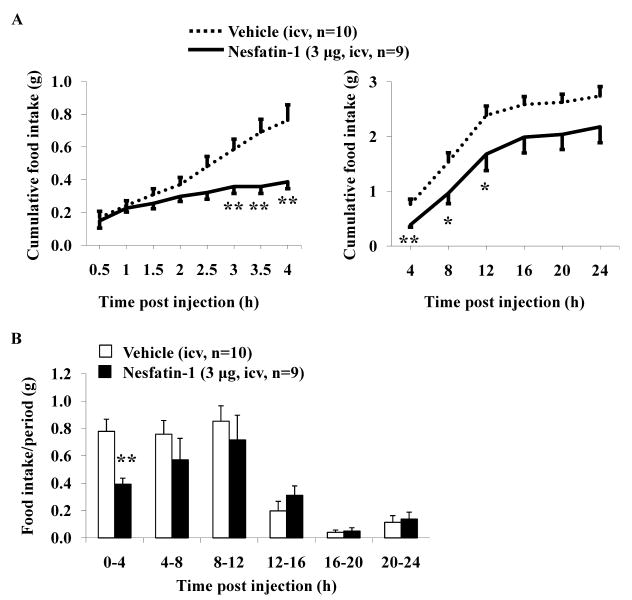
As only the highest dose significantly reduced food ingestion during the first 4-h period compared to vehicle (p < 0.01; Fig. 1A), this dose was used in all further studies. The time course of the icv nesfatin-1 effect on cumulative food intake over a 24-h period is illustrated in Fig. 2. The cumulative food intake was significantly reduced at 3 h post nesfatin-1 injection compared to vehicle-treated mice (0.36 ± 0.04 vs. 0.59 ± 0.06 g/3h, p < 0.01; Fig. 2A). The inhibitory effect was maintained during the whole 12-h dark phase (1.68 ± 0.30 vs. 2.39 ± 0.17 g/12h, p < 0.05) and only visible as a trend at 24 h post injection compared to vehicle (2.18 ± 0.29 vs. 2.74 ± 0.17 g/24h, p > 0.05; Fig. 2A). The food intake monitored as separate 4-h periods for 24-h post icv nesfatin-1 injection was no longer significantly reduced after the first 4-h compared to icv vehicle (p > 0.05; Fig. 2B). Two-way ANOVA indicated a significant influence of treatment (F(1,102) = 23.9, p < 0.001) and time (F(5,102) = 27.5, p < 0.001).
Fig. 2.
Nesfatin-1 injected intracerebroventricularly decreases the dark phase food intake in ad libitum fed mice over a period of 12 h. Ad libitum fed non-cannulated mice were injected with nesfatin-1 (3 μg/mouse) or vehicle icv at the onset of the dark phase under short anesthesia and food intake was monitored and expressed as cumulative food intake (A) and food intake/4-h periods (B). Each line or bar represents the mean ± SEM of 9-10 mice/group. * p < 0.05 and ** p < 0.01 vs. vehicle.
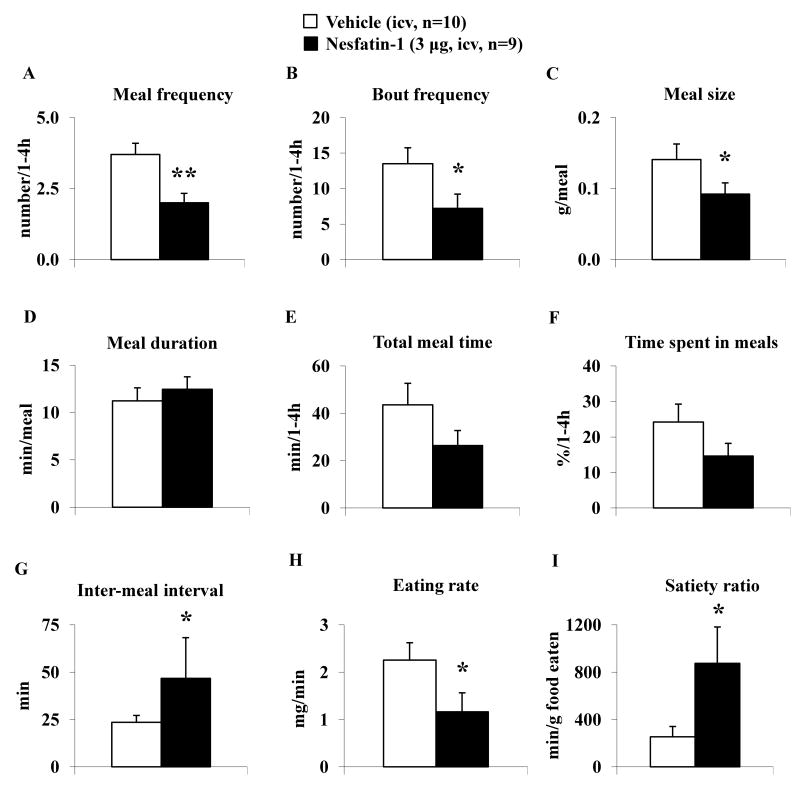
Since nesfatin-1 decreased food intake during the 1-4 h period post icv injection, the analysis of feeding microstructure was performed for this period. Nesfatin-1 injected icv significantly decreased the meal frequency (2.00 ± 0.33 vs. 3.70 ± 0.40 meals/1-4 h, p < 0.01; Fig. 3A), bout frequency (7.22 ± 1.99 vs. 13.50 ± 2.25 bouts/1-4 h, p < 0.05; Fig. 3B) and meal size (0.09 ± 0.02 vs. 0.14 ± 0.02 g/meal, p < 0.05; Fig. 3C), whereas meal duration (12.46 ± 1.31 vs. 11.23 ± 1.38 min/meal, p > 0.05; Fig. 3D) was not significantly altered compared with icv vehicle. Nesfatin-1 injected mice showed a trend towards a reduced total meal time (26.35 ± 6.36 vs. 43.58 ± 9.06 min/1-4 h, p > 0.05; Fig. 3E) and time spent in meals (14.64 ± 3.53 vs. 24.21 ± 5.04 %/1-4 h, p > 0.05; Fig. 3F) compared to vehicle, without reaching statistical significance. Mice treated with nesfatin-1 icv had a significantly prolonged inter-meal interval compared to vehicle icv (46.68 ± 21.59 vs. 23.46 ± 3.65 min, p < 0.05; Fig. 3G). The icv nesfatin-1 response was not immediate as indicated by an unchanged latency to the first meal (22.72 ± 1.83 vs. 19.08 ± 1.51 min, p > 0.05; Table 1), duration of the first meal (23.98 ± 4.68 vs. 23.52 ± 5.07 min, p > 0.05; Fig. Table 1) and eating rate of the first meal (4.22 ± 0.98 vs. 5.85 ± 0.92 mg/min, p > 0.05; Table 1), whereas the eating rate of the full 1-4 h period was reduced compared to vehicle (1.16 ± 0.40 vs. 2.26 ± 0.37 mg/min, p < 0.05; Fig. 3H). In addition, nesfatin-1 icv significantly increased the satiety ratio during the 1-4 h period post injection compared to vehicle (874.34 ± 308.09 vs. 254.08 ± 87.23 min/g food eaten, p < 0.05; Fig. 3I).
Fig. 3.
Nesfatin-1 injected intracerebroventricularly decreases meal frequency while not altering meal size during the 1-4 h period post injection. Nesfatin-1 (3 μg/mouse) or vehicle was icv injected in ad libitum fed mice at the onset of the dark phase and feeding microstructure including meal frequency (A), bout frequency (B), meal size (C), meal duration (D), total meal time (E) and time spent in meals (F), inter-meal interval (G), eating rate (H) and the satiety ratio (I) was assessed using an automated episodic food intake monitoring device. Each bar represents the mean ± SEM of 9-10 mice/group. * p < 0.05 and **p < 0.01 vs. vehicle.
Table 1.
Nesfatin-1 injected icv before the dark phase did not influence the structure of the first meal in ad libitum fed mice.
| Parameters | Vehicle (icv) | Nesfatin-1 (3 μg/mouse, icv) |
|---|---|---|
| Latency to 1st meal (min) | 19.1 ± 1.5 | 22.7 ± 1.8 |
| Duration 1st meal (min) | 23.5 ± 5.1 | 24.0 ± 4.7 |
| Eating rate 1st meal (mg/min) | 5.8 ± 0.9 | 4.2 ± 1.0 |
Data are mean ± SEM; n=9-10 mice/group. p > 0.05.
3.2. NUCB2/nesfatin-1 immunoreactivity in the mouse hypothalamus and dorsal vagal complex
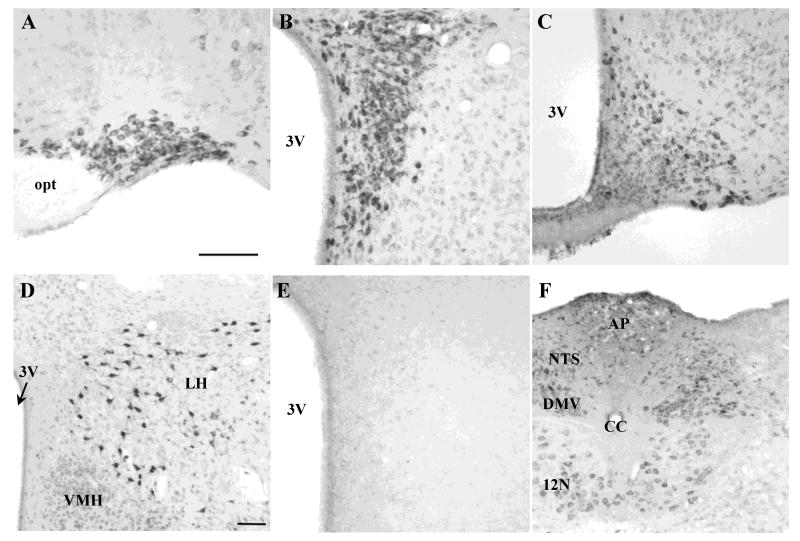
NUCB2/nesfatin-1-ir neurons were found in the hypothalamus in feeding regulatory nuclei. In the supraoptic nucleus (SON, Fig. 4A), NUCB2/nesfatin-1-ir neurons were distributed throughout the rostrocaudal extent. In the paraventricular nucleus (PVN, Fig. 4B), NUCB2/nesfatin-1-immunopositive neurons were dense in the magnocellular but scattered in the parvicellular part. The arcuate nucleus (Arc, Fig. 4C) showed NUCB2/nesfatin-1 immunoreactivity in the ventromedial and dorsolateral regions, with stronger staining in the dorsolateral part. Very intense NUCB2/nesfatin-1-ir neurons were scattered throughout the lateral hypothalamic area (LH, Fig. 4D). In the medulla, NUCB2/nesfatin-1 immunoreactivity was located in the nucleus of the solitary tract (NTS) and dorsal motor nucleus of the vagus nerve (DMV, Fig. 4F). As observed in a previous mouse study [17], NUCB2/nesfatin-1 immunoreactivity was also detected in the Edinger-Westphal nucleus (data not shown). The pattern of immunostaining was consistent across all animals investigated. The nesfatin-1 antibody specifically stains NUCB2/nesfatin-1-containing neurons since pre-absorption of the nesfatin-1 antibody with nesfatin-1 peptide did not yield immunostaining in the PVN (Fig. 4E).
Fig. 4.
Representative microphotographs of NUCB2/nesfatin-1-immunoreactive cells in hypothalamic and medullary brain sections of naïve mice. Ad libitum fed mice were anesthetized and transcardially perfused at the onset of the dark phase and brains processed for nesfatin-1 immunohistochemistry. NUCB2/nesfatin-1 immunoreactivity was observed in the SON (A), PVN (B), Arc (C), LH (D) and NTS and DMV (F). No staining was detected following pre-absorption of the anti-nesfatin-1 antibody with nesfatin-1 peptide in the paraventricular nucleus (D). The scale bars are 100 μm. Scale bars in A and D are representative for B, C, E and F, respectively. Abbreviations: AP, area postrema; CC, central canal; DMV, dorsal motor nucleus of the vagus nerve; LH, lateral hypothalamic area; NTS, nucleus of the solitary tract; opt, optic tract; VMH, ventromedial hypothalamus; 3V, third brain ventricle; 12N, hypoglossal nucleus.
3.3. Nesfatin-1 injected intraperitoneally or subcutaneously has no effect on dark phase food intake
In contrast to icv injection, when nesfatin-1 was injected ip at the dose of 70 μg/mouse at the beginning of the dark phase in ad libitum fed mice, no change in food intake was observed at any time point during the 24-h measurement period post injection compared to vehicle (e.g. 4h: 1.2 ± 0.12 vs. 0.88 ± 0.15 g, p > 0.05). Likewise, when nesfatin-1 was injected sc (70 μg/mouse), the dark phase food intake was similar at all time points post injection compared to vehicle treated mice (e.g. 4h: 1.00 ± 0.08 vs. 0.96 ± 0.05 g/4h, p > 0.05). During this time, the hourly food intake was not changed compared to vehicle (p > 0.05; Table 2). Analysis of the food intake microstructure did not show significant changes of any of the parameters analyzed following ip or sc injection of nesfatin-1 compared to vehicle (p > 0.05; Table 3).
Table 2.
Nesfatin-1 injected ip or sc did not influence the hourly dark phase food intake during the first 4 h post injection in ad libitum fed mice.
| Food intake/period (g) | Intraperitoneal | Subcutaneous | ||
|---|---|---|---|---|
| Vehicle | Nesfatin-1 (70 μg/mouse) | Vehicle | Nesfatin-1 (70 μg/mouse) | |
| 0-1 h | 0.35 ± 0.18 | 0.37 ± 0.09 | 0.24 ± 0.04 | 0.28 ± 0.05 |
| 1-2 h | 0.17 ± 0.10 | 0.24 ± 0.08 | 0.30 ± 0.07 | 0.22 ± 0.03 |
| 2-3 h | 0.18 ± 0.05 | 0.24 ± 0.06 | 0.19 ± 0.06 | 0.29 ± 0.05 |
| 3-4 h | 0.15 ± 0.02 | 0.19 ± 0.09 | 0.20 ± 0.04 | 0.16 ± 0.05 |
Each group had vehicle controls tested at the same time of nesfatin-1 and also injected ip or sc. Data are mean ± SEM; n=6-8 mice/group. p > 0.05.
Table 3.
Nesfatin-1 injected ip or sc did not influence the 0-4 h meal pattern of dark phase feeding in ad libitum fed mice.
| Parameters | Intraperitoneal | Subcutaneous | ||
|---|---|---|---|---|
| Vehicle | Nesfatin-1 (70 μg/mouse) | Vehicle | Nesfatin-1 (70 μg/mouse) | |
| Meal frequency (number/4h) | 5.5 ± 0.6 | 5.7 ± 1.0 | 6.1 ± 0.5 | 6.3 ± 0.5 |
| Bout frequency (number/4h) | 31.8 ± 8.7 | 39.2 ± 5.8 | 20.9 ± 3.1 | 28.6 ± 4.4 |
| Meal size (g/meal) | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 | 0.2 ± 0.0 |
| Meal duration (min/meal) | 26.9 ± 8.2 | 27.2 ± 2.8 | 18.5 ± 2.9 | 22.8 ± 5.6 |
| Total meal time (min/4h) | 116.0 ± 26.5 | 140.0 ± 12.7 | 107.4 ± 11.9 | 111.3 ± 6.5 |
| Time spent in meals (%/4h) | 48.3 ± 11.0 | 58.3 ± 5.3 | 44.8 ± 5.0 | 53.1 ± 7.1 |
| Inter-meal interval (min) | 22.1 ± 4.9 | 19.7 ± 3.7 | 24.6 ± 2.9 | 21.1 ± 2.5 |
| Latency to 1st meal (min) | 23.1 ± 10.7 | 15.1 ± 6.4 | 5.5 ± 1.1 | 4.5 ± 0.4 |
| Duration 1st meal (min) | 14.9 ± 4.5 | 15.1 ± 4.8 | 9.3 ± 3.3 | 10.6 ± 1.9 |
| Eating rate 1st meal (mg/min) | 4.3 ± 1.7 | 4.1 ± 0.6 | 4.5 ± 1.1 | 2.9 ± 0.5 |
| Eating rate/4h (mg/min) | 3.9 ± 0.4 | 4.5 ± 0.5 | 4.1 ± 0.2 | 4.3 ± 0.3 |
| Satiety ratio 1st period (min/g food eaten) | 167.3 ± 48.8 | 113.2 ± 21.8 | 155.8 ± 18.5 | 160.4 ± 16.2 |
Each group had vehicle controls tested at the same time of nesfatin-1 and also injected ip or sc. Data are mean ± SEM; n=6-8 mice/group. p > 0.05.
4. Discussion
In the present study we show that freely fed mice acutely injected with nesfatin-1 into the lateral brain ventricle under short inhalation anesthesia decrease their dark phase food intake. During the first 4 h post icv injection, nesfatin-1 (0.3, 1 or 3 μg/mouse) caused a dose-related reduction of the nocturnal feeding by 13%, 27%, and 46% respectively compared to vehicle. However, values reached significance only at the dose of 3 μg/mouse. Time course studies at such a dose indicate that nesfatin-1 injected icv before the dark phase induces a potent and long-lasting inhibitory action on food consumption with a delayed onset in mice. This was shown by the lack of nesfatin-1 anorexic effect during the 1st h post icv injection, whereas in the second hour there was a non-significant trend towards a reduction and in the following two hours significant 73% and 82% inhibition of hourly food intake, respectively. In the 4-5 h period, no significant reduction of food intake was observed indicating that the peak response occurred during the 2-4 h period post nesfatin-1 icv injection. A significant 30% reduction of 12h-cumulative dark phase food intake was still maintained at 12 h post injection. However, thereafter the 21% reduction of 24-h cumulative food intake was no longer statistically significant. These data expand one previous report showing a reduction of the dark phase food intake starting at 2 h post 3rd ventricular injection of mouse nesfatin-1 at one dose (1 μg/mouse) in chronically third ventricular cannulated mice that resulted in a ∼20% reduction of cumulative food intake up to 18 h post injection [14]. These findings in mice are also consistent with our previous report in chronically cannulated rats injected with rat nesfatin-1 into the lateral brain ventricle showing lower dark phase food intake with a maximum 87% reduction occurring during the 2-3 h period post injection and a decrease of dark phase feeding up to 6 h post injection. However, this response was achieved at a much lower dose in rats (0.05 μg/rat) [27]. In the present and previous studies [27], we used synthetic mouse and rat nesfatin-1 respectively and the mouse nesfatin-1 used was independently assessed by mass spectrometry analysis confirming the correct 9.61 kDa mass of the peptide. Therefore, the 60-fold higher icv dose of mouse nesfatin-1 required to induce a significant reduction of dark phase food intake in mice compared to that after rat nesfatin-1 in rats [16, 27] is not related to species difference in the peptide used and may reflect strain differences in mechanisms influencing peptide availability, stability and/or cellular action.
Microstructural analyses of meal pattern showed that the icv nesfatin-1 anorexigenic effect in mice is due to reduced meal size and decreased meal frequency associated with prolonged inter-meal intervals. In addition, the satiety ratio was increased during the 1-4 h period after icv nesfatin-1 injection. This pattern is indicative of increased satiation as supported by the reduced meal size and increased satiety as reflected by prolonged inter-meal intervals [31]. The nesfatin-1 action is centrally mediated since a 23-times higher dose of nesfatin-1 injected peripherally either ip or sc did not influence the meal pattern of dark phase feeding in mice. These data suggest that exogenous activation of central nesfatin-1 pathways reinforces the satiety value of food ingested during the time of spontaneous nocturnal feeding. This may occur by enhancing the processing of gut derived peptide signaling involved in induction of satiety [34]. The observed dense expression of nesfatin-1 immunoreactivity in the mouse nucleus of the solitary tract which is a site of convergence for gut satiety signaling [1] supports such a possible action of endogenous nesfatin-1. It may also act indirectly through recruitment of other brain pathways inducing satiety. We previously reported in rats that the icv nesfatin-1-induced reduction of food intake is mediated via activation of the CRF receptor 2 (CRF2) signaling pathway as indicated by the complete blockade of icv nesfatin-1 anorexigenic action by the selective CRF2 antagonist, astressin2-B [27]. The food intake pattern induced by the selective CRF2 agonist, urocortin 3 injected icv in rats closely resembles the effects of icv nesfatin-1 observed here. First, the central action of urocortin 3 has a delayed onset with no effect during the first 1-2 h and a reduction of food intake occurring during the 3-4 h period post injection [4]. Likewise, in the present study the inhibitory effect of icv nesfatin-1 was delayed in onset in mice as shown before in mice and rats with the peak reduction of food intake occurring during the third hour post icv injection [14, 27]. Second, icv urocortin 3, like nesfatin-1, decreases meal frequency associated with increased inter-meal intervals indicating an effect on satiety and at higher doses also decreases meal size reflecting increased satiation [4]. Collectively, these data support the mediation of the icv nesfatin-1 action on food intake via a downstream CRF2 signaling pathway which will require further investigations in mice using pharmacologic or genetic approaches.
The specific brain sites of action of nesfatin-1 to reduce the dark phase food intake have been localized in the PVN and brainstem in rats [14, 27]. Studies using 125I-nesfatin-1 in mice showed that the uptake of nesfatin-1 by the hypothalamus was greater than that by the total brain suggesting higher representation of nesfatin-1 binding sites, yet to be identified, in the hypothalamus [18]. Supporting a potential physiological role of hypothalamic and medullary nesfatin-1 pathways in regulating satiety in mice, we found NUCB2/nesfatin-1-immunopositive neurons in key hypothalamic nuclei such as the SON, PVN, Arc, LH and the brainstem (dorsal vagal complex) which are involved in the regulation of food intake in mice [33]. These findings also extend the prominent expression of NUCB2/nesfatin-1 immunoreactivity in these hypothalamic and brainstem (NTS, DMV) nuclei to mice which have been solely reported in rats previously [3, 5, 7, 10, 13, 16, 35]. The chemical coding of NUCB2/nesfatin-1 immunoreactive neurons will need to be further characterized and may be similar to those described in rats which encompasses melanin-concentrating hormone, cocaine-and amphetamine-regulated transcript, α-melanocyte-stimulating hormone, pro-opiomelanocortin, vasopressin, oxytocin, growth hormone-releasing hormone, CRF, thyrotropin-releasing hormone, somatostatin, neurotensin and also neuropeptide Y in the hypothalamus [3, 5, 7, 12-14], and in pons and brainstem urocortin and serotonin [3, 17]. The specificity of the NUCB2/nesfatin-1 immunostaining in mice is indicated by the lack of NUCB2/nesfatin-1 immunosignals following pre-absorption of the anti-nesfatin-1 antibody with nesfatin-1 peptide. However, whereas the antibody raised against nesfatin-1(1-82) does not cross-react with nesfatin-2 and nesfatin-3 or other neuropeptides, it also recognizes full length NUCB2 [3, 5]. We previously showed in rats that the antibody recognized nesfatin-1 as indicated by a 10 kDa band in the Western Blot but also a 50 kDa band corresponding to NUCB2 [30]. Based on these findings, the nesfatin-1 immunosignals described here reflect either nesfatin-1 peptide and/or full length NUCB2 immunoreactivity.
In addition to the brain, there are several reports showing peripheral expression of NUCB2/nesfatin-1 including the gut with the main localization in endocrine cells of the stomach and pancreas in rats [6, 11, 30] and mice [6, 11, 38] and mouse adipose tissue [20]. Moreover, circulating levels of NUCB2/nesfatin-1 can be modulated by feeding status in rats [27]. In mice there is evidence that intravenously injected nesfatin-1 crosses the blood brain barrier by a non-saturable mechanism although at a modest rate [18, 19], although endogenous circulating levels of nesfatin-1 and variations with feeding are still to be established. Likewise, whether peripherally injected nesfatin-1, like a number of other gut peptides [34], can influence food intake is still to be ascertained. In rats, nesfatin-1 injected ip at 2 μg/rat does not influence food intake [27], while one report indicates a reduction of cumulative 3-h dark phase food intake in ad libitum fed mice upon ip injection of 70 μg/mouse [23]. In the present study a 23-times higher dose than the effective icv dose does not influence the hourly dark phase food intake or food intake/4-h periods in ad libitum fed mice when injected ip or sc. Moreover, none of the food intake microstructure parameters assessed were altered by the peripheral injection of nesfatin-1 at 70 μg/mouse. The discrepancy with the previous report in mice [23] may be related to differences in mouse strain used (ICR outbred versus C57BL/6 inbred in the present study) or source of mouse nesfatin-1 (recombinant versus synthetic in the present study). Irrespectively, these data indicate that the peripheral action of nesfatin-1 to influence food intake is not as robustly demonstrated both in rats and mice as its central anorexigenic action and requires further investigations [26]. There is still a paucity of studies in mice using peripheral injection of nesfatin-1. A recent report suggests that nesfatin-1 may influence glucose control as indicated by the reduction of blood glucose levels induced by intravenous injection of nesfatin-1 at a dose of 100 μg/mouse in db/db mice [32] which is also to be confirmed.
In conclusion, icv injection of nesfatin-1 in ad libitum fed mice induced a dose-related delayed in onset and long-lasting reduction of dark phase food intake which was associated with an increase in satiation as reflected in a decreased meal size and increased satiety as indicated by prolonged inter-meal intervals during the 1-4 h period post injection. Nesfatin-1's anorexigenic action was centrally mediated as 23-times higher doses injected peripherally, either intraperitoneally or subcutaneously, did not influence the dark phase food intake and meal pattern. The potential role of brain nesfatin-1 as a satiety signal in mice is supported by the prominent distribution of NUCB2/nesfatin-1 immunoreactivity in the mouse brain at specific hypothalamic (SON, PVN, Arc, LH) and brainstem nuclei (NTS, DMV) regulating food intake and also involved in autonomic control of visceral functions. Although NUCB2/nesfatin-1 is also prominently expressed in peripheral digestive organs in ICR mice, particularly in stomach, duodenum and pancreas [38], the lack of changes in the dark phase food intake in response to ip or sc injection of nesfatin-1 in mice in the present study requires additional investigations to ascertain whether higher circulating levels of nesfatin-1 can influence feeding behavior.
Research highlights.
Anorexigenic action of nesfatin-1 and brain expression in mice is largely unexplored
Nesfatin-1 icv dose-dependently decreases dark phase food intake with delayed onset
Nesfatin-1 icv reduces meal size and prolongs inter-meal intervals
Peripheral injection of nesfatin-1 does not alter food intake or feeding microstructure
NUCB2/Nesfatin-1 immunoreactivity is expressed in mouse feeding regulatory nuclei
Acknowledgments
This work was supported by German Research Foundation Grants STE 1765/1-1 (A.S.), GO 1718/1-1 (M.G.), Veterans Administration Research Career Scientist Award, R01 NIH DK-33061, Center Grant DK-41301 (Animal Core and Supplement Grant, Y.T. and Peptide Biochemistry Core, Joseph R. Reeve, Jr.). We are grateful to Dr. Kym Faull for performing the mass spectral analysis, to Mrs. Honghui Liang for the excellent technical support and thank Ms. Eugenia Hu for reviewing the manuscript.
Footnotes
Disclosure: The authors have nothing to disclose. No conflicts of interest exist.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Berthoud HR. Vagal and hormonal gut-brain communication: from satiation to satisfaction. Neurogastroenterol Motil. 2008;20 1:64–72. doi: 10.1111/j.1365-2982.2008.01104.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Berthoud HR, Morrison C. The brain, appetite, and obesity. Annu Rev Psychol. 2008;59:55–92. doi: 10.1146/annurev.psych.59.103006.093551. [DOI] [PubMed] [Google Scholar]
- 3.Brailoiu GC, Dun SL, Brailoiu E, Inan S, Yang J, Chang JK, et al. Nesfatin-1: distribution and interaction with a G protein-coupled receptor in the rat brain. Endocrinology. 2007;148:5088–94. doi: 10.1210/en.2007-0701. [DOI] [PubMed] [Google Scholar]
- 4.Fekete EM, Inoue K, Zhao Y, Rivier JE, Vale WW, Szucs A, et al. Delayed satiety-like actions and altered feeding microstructure by a selective type 2 corticotropin-releasing factor agonist in rats: intra-hypothalamic urocortin 3 administration reduces food intake by prolonging the post-meal interval. Neuropsychopharmacology. 2007;32:1052–68. doi: 10.1038/sj.npp.1301214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Foo K, Brismar H, Broberger C. Distribution and neuropeptide coexistence of nucleobindin-2 mRNA/nesfatin-like immunoreactivity in the rat CNS. Neuroscience. 2008;156:563–79. doi: 10.1016/j.neuroscience.2008.07.054. [DOI] [PubMed] [Google Scholar]
- 6.Foo KS, Brauner H, Ostenson CG, Broberger C. Nucleobindin-2/nesfatin in the endocrine pancreas: distribution and relationship to glycaemic state. J Endocrinol. 2010;204:255–63. doi: 10.1677/JOE-09-0254. [DOI] [PubMed] [Google Scholar]
- 7.Fort P, Salvert D, Hanriot L, Jego S, Shimizu H, Hashimoto K, et al. The satiety molecule nesfatin-1 is co-expressed with melanin concentrating hormone in tuberal hypothalamic neurons of the rat. Neuroscience. 2008;155:174–81. doi: 10.1016/j.neuroscience.2008.05.035. [DOI] [PubMed] [Google Scholar]
- 8.Franklin KBJ, Paxinos G. The mouse brain in stereotaxic coordinates. San Diego: Academic Press; 1997. [Google Scholar]
- 9.Geary N. A new way of looking at eating. Am J Physiol Regul Integr Comp Physiol. 2005;288:R1444–6. doi: 10.1152/ajpregu.00066.2005. [DOI] [PubMed] [Google Scholar]
- 10.Goebel M, Stengel A, Wang L, Lambrecht NWG, Taché Y. Nesfatin-1 immunoreactivity in rat brain and spinal cord autonomic nuclei. Neurosci Lett. 2009;452:241–6. doi: 10.1016/j.neulet.2009.01.064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Gonzalez R, Tiwari A, Unniappan S. Pancreatic beta cells colocalize insulin and pronesfatin immunoreactivity in rodents. Biochem Biophys Res Commun. 2009;381:643–8. doi: 10.1016/j.bbrc.2009.02.104. [DOI] [PubMed] [Google Scholar]
- 12.Inhoff T, Stengel A, Peter L, Goebel M, Taché Y, Bannert N, et al. Novel insight in distribution of nesfatin-1 and phospho-mTOR in the arcuate nucleus of the hypothalamus of rats. Peptides. 2010;31:257–62. doi: 10.1016/j.peptides.2009.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kohno D, Nakata M, Maejima Y, Shimizu H, Sedbazar U, Yoshida N, et al. Nesfatin-1 neurons in paraventricular and supraoptic nuclei of the rat hypothalamus coexpress oxytocin and vasopressin and are activated by refeeding. Endocrinology. 2008;149:1295–301. doi: 10.1210/en.2007-1276. [DOI] [PubMed] [Google Scholar]
- 14.Maejima Y, Sedbazar U, Suyama S, Kohno D, Onaka T, Takano E, et al. Nesfatin-1-regulated oxytocinergic signaling in the paraventricular nucleus causes anorexia through a leptin-independent melanocortin pathway. Cell Metab. 2009;10:355–65. doi: 10.1016/j.cmet.2009.09.002. [DOI] [PubMed] [Google Scholar]
- 15.Martinez V, Wang L, Rivier J, Grigoriadis D, Taché Y. Central CRF, urocortins and stress increase colonic transit via CRF1 receptors while activation of CRF2 receptors delays gastric transit in mice. J Physiol. 2004;556:221–34. doi: 10.1113/jphysiol.2003.059659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Oh-I S, Shimizu H, Satoh T, Okada S, Adachi S, Inoue K, et al. Identification of nesfatin-1 as a satiety molecule in the hypothalamus. Nature. 2006;443:709–12. doi: 10.1038/nature05162. [DOI] [PubMed] [Google Scholar]
- 17.Okere B, Xu L, Roubos EW, Sonetti D, Kozicz T. Restraint stress alters the secretory activity of neurons co-expressing urocortin-1, cocaine- and amphetamine-regulated transcript peptide and nesfatin-1 in the mouse Edinger-Westphal nucleus. Brain Res. 2010;1317C:92–9. doi: 10.1016/j.brainres.2009.12.053. [DOI] [PubMed] [Google Scholar]
- 18.Pan W, Hsuchou H, Kastin AJ. Nesfatin-1 crosses the blood-brain barrier without saturation. Peptides. 2007;28:2223–8. doi: 10.1016/j.peptides.2007.09.005. [DOI] [PubMed] [Google Scholar]
- 19.Price TO, Samson WK, Niehoff ML, Banks WA. Permeability of the blood-brain barrier to a novel satiety molecule nesfatin-1. Peptides. 2007;28:2372–81. doi: 10.1016/j.peptides.2007.10.008. [DOI] [PubMed] [Google Scholar]
- 20.Ramanjaneya M, Chen J, Brown JE, Tripathi G, Hallschmid M, Patel S, et al. Identification of nesfatin-1 in human and murine adipose tissue: a novel depot-specific adipokine with increased levels in obesity. Endocrinology. 2010;151:3169–80. doi: 10.1210/en.2009-1358. [DOI] [PubMed] [Google Scholar]
- 21.Rivier JE, Kirby DA, Lahrichi SL, Corrigan A, Vale WW, Rivier CL. Constrained corticotropin releasing factor antagonists (astressin analogues) with long duration of action in the rat. J Med Chem. 1999;42:3175–82. doi: 10.1021/jm9902133. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz MW, Woods SC, Porte D, Jr, Seeley RJ, Baskin DG. Central nervous system control of food intake. Nature. 2000;404:661–71. doi: 10.1038/35007534. [DOI] [PubMed] [Google Scholar]
- 23.Shimizu H, Oh-I S, Hashimoto K, Nakata M, Yamamoto S, Yoshida N, Eguchi H, Kato I, Inoue K, Satoh T, Okada S, Yamada M, Yada T, Mori M. Peripheral Administration of Nesfatin-1 Reduces Food Intake in Mice: The leptin-independent mechanism. Endocrinology. 2009;150:662–71. doi: 10.1210/en.2008-0598. [DOI] [PubMed] [Google Scholar]
- 24.Stengel A, Coskun T, Goebel M, Wang L, Craft L, Alsina-Fernandez J, Rivier J, Taché Y. Central injection of the stable somatostatin analog, ODT8-SST induces a somatostatin2 receptor mediated orexigenic effect: role of neuropeptide Y and opioid signaling pathways in rats. Endocrinology. 2010:4224–35. doi: 10.1210/en.2010-0195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Stengel A, Goebel M, Million M, Stenzel-Poore MP, Kobelt P, Mönnikes H, et al. CRF over-expressing mice exhibit reduced neuronal activation in the arcuate nucleus and food intake in response to fasting. Endocrinology. 2009;150:153–60. doi: 10.1210/en.2008-0723. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Stengel A, Goebel M, Taché Y. Nesfatin-1: a novel inhibitory regulator of food intake and body weight. Obes Rev. 2010 doi: 10.1111/j.1467-789X.2010.00770.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, et al. Central nesfatin-1 reduces dark-phase food intake and gastric emptying in rats: differential role of corticotropin-releasing factor2 receptor. Endocrinology. 2009;150:4911–9. doi: 10.1210/en.2009-0578. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Stengel A, Goebel M, Wang L, Rivier J, Kobelt P, Mönnikes H, et al. Activation of brain somatostatin(2) receptors stimulates feeding in mice: Analysis of food intake microstructure. Physiol Behav. 2010 doi: 10.1016/j.physbeh.2010.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Stengel A, Goebel M, Wang L, Taché Y. Ghrelin, des-acyl ghrelin and nesfatin-1 in gastric X/A-like cells: role as regulators of food intake and body weight. Peptides. 2010;31:357–69. doi: 10.1016/j.peptides.2009.11.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Stengel A, Goebel M, Yakubov I, Wang L, Witcher D, Coskun T, et al. Identification and characterization of nesfatin-1 immunoreactivity in endocrine cell types of the rat gastric oxyntic mucosa. Endocrinology. 2009;150:232–8. doi: 10.1210/en.2008-0747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Strubbe JH, Woods SC. The timing of meals. Psychol Rev. 2004;111:128–41. doi: 10.1037/0033-295X.111.1.128. [DOI] [PubMed] [Google Scholar]
- 32.Su Y, Zhang J, Tang Y, Bi F, Liu JN. The novel function of nesfatin-1: anti-hyperglycemia. Biochem Biophys Res Commun. 2010;391:1039–42. doi: 10.1016/j.bbrc.2009.12.014. [DOI] [PubMed] [Google Scholar]
- 33.Williams G, Harrold JA, Cutler DJ. The hypothalamus and the regulation of energy homeostasis: lifting the lid on a black box. Proc Nutr Soc. 2000;59:385–96. doi: 10.1017/s0029665100000434. [DOI] [PubMed] [Google Scholar]
- 34.Woods SC. Gastrointestinal satiety signals I An overview of gastrointestinal signals that influence food intake. Am J Physiol Gastrointest Liver Physiol. 2004;286:G7–13. doi: 10.1152/ajpgi.00448.2003. [DOI] [PubMed] [Google Scholar]
- 35.Xu L, Bloem B, Gaszner B, Roubos EW, Kozicz LT. Sex-specific effects of fasting on Urocortin 1, Cocaine- and Amphetamine-Regulated Transcript peptide and Nesfatin-1 expression in the rat Edinger-Westphal nucleus. Neuroscience. 2009;162:1141–9. doi: 10.1016/j.neuroscience.2009.05.003. [DOI] [PubMed] [Google Scholar]
- 36.Yosten GL, Samson WK. The anorexigenic and hypertensive effects of nesfatin-1 are reversed by pretreatment with an oxytocin receptor antagonist. Am J Physiol Regul Integr Comp Physiol. 2010;298:R1642–7. doi: 10.1152/ajpregu.00804.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Yosten GL, Samson WK. Nesfatin-1 exerts cardiovascular actions in brain: possible interaction with the central melanocortin system. Am J Physiol Regul Integr Comp Physiol. 2009;297:R330–6. doi: 10.1152/ajpregu.90867.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Zhang AQ, Li XL, Jiang CY, Lin L, Shi RH, Chen JD, et al. Expression of nesfatin-1/NUCB2 in rodent digestive system. World J Gastroenterol. 2010;16:1735–41. doi: 10.3748/wjg.v16.i14.1735. [DOI] [PMC free article] [PubMed] [Google Scholar]