Abstract
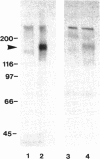
Thrombospondin-1 (TSP-1) is an extracellular matrix glycoprotein that may play important roles in the morphogenesis and repair of skeletal muscle. To begin to explore the role of thrombospondin-1 in this tissue, we have examined the interactions of three rodent skeletal muscle cell lines, C2C12, G8, and H9c2, with platelet TSP-1. The cells secrete thrombospondin and incorporate it into the cell layer in a distribution distinct from that of fibronectin. Myoblasts attach and spread on fibronectin- or thrombospondin-coated substrates with similar time and concentration dependencies. Whereas cells adherent on fibronectin organize actin stress fibers, cells adherent on TSP-1 display prominent membrane ruffles and lamellae that contain radial actin microspikes. Attachment to thrombospondin-1 or the 140-kDa tryptic fragment is mediated by interactions with the type 1 repeats and the carboxy-terminal globular domain. Attachment is not inhibited by heparin, GRGDSP peptide, or VTCG peptide but is inhibited by chondroitin sulphate A. Integrins of the beta 1 or alpha V subgroups do not appear to be involved in myoblast attachment to TSP-1; instead, this process depends in part on cell surface chondroitin sulphate proteoglycans. Whereas the central 70-kDa chymotryptic fragment of TSP-1 does not support myoblast attachment, the carboxy-terminal domain of TSP-1 expressed as a fusion protein in the bacterial expression vector, pGEX, supported myoblast attachment to 30% the level of intact TSP-1. Thrombospondin-4 (TSP-4) is also present in skeletal muscle and a fusion protein containing the carboxy-terminal domain of TSP-4 also supported myoblast adhesion, although this protein was less active on a molar basis than the TSP-1 fusion protein. Thus, the carboxyterminal domain of TSP-1 appears to contain a primary attachment site for myoblasts, and this activity is present in a second member of the thrombospondin family.
Full text
PDF













Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adams J. C., Lawler J. Diverse mechanisms for cell attachment to platelet thrombospondin. J Cell Sci. 1993 Apr;104(Pt 4):1061–1071. doi: 10.1242/jcs.104.4.1061. [DOI] [PubMed] [Google Scholar]
- Adams J. C., Watt F. M. An unusual strain of human keratinocytes which do not stratify or undergo terminal differentiation in culture. J Cell Biol. 1988 Nov;107(5):1927–1938. doi: 10.1083/jcb.107.5.1927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Adams J., Lawler J. Extracellular matrix: the thrombospondin family. Curr Biol. 1993 Mar;3(3):188–190. doi: 10.1016/0960-9822(93)90270-x. [DOI] [PubMed] [Google Scholar]
- Asch A. S., Tepler J., Silbiger S., Nachman R. L. Cellular attachment to thrombospondin. Cooperative interactions between receptor systems. J Biol Chem. 1991 Jan 25;266(3):1740–1745. [PubMed] [Google Scholar]
- Bernfield M., Sanderson R. D. Syndecan, a developmentally regulated cell surface proteoglycan that binds extracellular matrix and growth factors. Philos Trans R Soc Lond B Biol Sci. 1990 Mar 12;327(1239):171–186. doi: 10.1098/rstb.1990.0052. [DOI] [PubMed] [Google Scholar]
- Blau H. M., Pavlath G. K., Hardeman E. C., Chiu C. P., Silberstein L., Webster S. G., Miller S. C., Webster C. Plasticity of the differentiated state. Science. 1985 Nov 15;230(4727):758–766. doi: 10.1126/science.2414846. [DOI] [PubMed] [Google Scholar]
- Bornstein P., O'Rourke K., Wikstrom K., Wolf F. W., Katz R., Li P., Dixit V. M. A second, expressed thrombospondin gene (Thbs2) exists in the mouse genome. J Biol Chem. 1991 Jul 15;266(20):12821–12824. [PubMed] [Google Scholar]
- Bornstein P. Thrombospondins: structure and regulation of expression. FASEB J. 1992 Nov;6(14):3290–3299. doi: 10.1096/fasebj.6.14.1426766. [DOI] [PubMed] [Google Scholar]
- Bradford M. M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976 May 7;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Burridge K., Fath K., Kelly T., Nuckolls G., Turner C. Focal adhesions: transmembrane junctions between the extracellular matrix and the cytoskeleton. Annu Rev Cell Biol. 1988;4:487–525. doi: 10.1146/annurev.cb.04.110188.002415. [DOI] [PubMed] [Google Scholar]
- Chiquet-Ehrismann R. Anti-adhesive molecules of the extracellular matrix. Curr Opin Cell Biol. 1991 Oct;3(5):800–804. doi: 10.1016/0955-0674(91)90053-2. [DOI] [PubMed] [Google Scholar]
- Christian C. N., Nelson P. G., Peacock J., Nirenberg M. Synapse formation between two clonal cell lines. Science. 1977 May 27;196(4293):995–998. doi: 10.1126/science.193191. [DOI] [PubMed] [Google Scholar]
- Clezardin P., Jouishomme H., Chavassieux P., Marie P. J. Thrombospondin is synthesized and secreted by human osteoblasts and osteosarcoma cells. A model to study the different effects of thrombospondin in cell adhesion. Eur J Biochem. 1989 May 15;181(3):721–726. doi: 10.1111/j.1432-1033.1989.tb14783.x. [DOI] [PubMed] [Google Scholar]
- Corless C. L., Mendoza A., Collins T., Lawler J. Colocalization of thrombospondin and syndecan during murine development. Dev Dyn. 1992 Apr;193(4):346–358. doi: 10.1002/aja.1001930408. [DOI] [PubMed] [Google Scholar]
- Couchman J. R., Rees D. A. The behaviour of fibroblasts migrating from chick heart explants: changes in adhesion, locomotion and growth, and in the distribution of actomyosin and fibronectin. J Cell Sci. 1979 Oct;39:149–165. doi: 10.1242/jcs.39.1.149. [DOI] [PubMed] [Google Scholar]
- Dixit V. M., Haverstick D. M., O'Rourke K. M., Hennessy S. W., Grant G. A., Santoro S. A., Frazier W. A. A monoclonal antibody against human thrombospondin inhibits platelet aggregation. Proc Natl Acad Sci U S A. 1985 May;82(10):3472–3476. doi: 10.1073/pnas.82.10.3472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dreyfus M., Lahav J. The build-up of the thrombospondin extracellular matrix. An apparent dependence on synthesis and on preformed fibrillar matrix. Eur J Cell Biol. 1988 Dec;47(2):275–282. [PubMed] [Google Scholar]
- Frazier W. A. Thrombospondins. Curr Opin Cell Biol. 1991 Oct;3(5):792–799. doi: 10.1016/0955-0674(91)90052-z. [DOI] [PubMed] [Google Scholar]
- Good D. J., Polverini P. J., Rastinejad F., Le Beau M. M., Lemons R. S., Frazier W. A., Bouck N. P. A tumor suppressor-dependent inhibitor of angiogenesis is immunologically and functionally indistinguishable from a fragment of thrombospondin. Proc Natl Acad Sci U S A. 1990 Sep;87(17):6624–6628. doi: 10.1073/pnas.87.17.6624. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham M. F., Diegelmann R. F., Elson C. O., Bitar K. N., Ehrlich H. P. Isolation and culture of human intestinal smooth muscle cells. Proc Soc Exp Biol Med. 1984 Sep;176(4):503–507. doi: 10.3181/00379727-176-4-rc1. [DOI] [PubMed] [Google Scholar]
- Guo N. H., Krutzsch H. C., Nègre E., Vogel T., Blake D. A., Roberts D. D. Heparin- and sulfatide-binding peptides from the type I repeats of human thrombospondin promote melanoma cell adhesion. Proc Natl Acad Sci U S A. 1992 Apr 1;89(7):3040–3044. doi: 10.1073/pnas.89.7.3040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guo N. H., Krutzsch H. C., Nègre E., Zabrenetzky V. S., Roberts D. D. Heparin-binding peptides from the type I repeats of thrombospondin. Structural requirements for heparin binding and promotion of melanoma cell adhesion and chemotaxis. J Biol Chem. 1992 Sep 25;267(27):19349–19355. [PubMed] [Google Scholar]
- Hantaï D., Rao J. S., Reddy B. R., Festoff B. W. Developmental appearance of thrombospondin in neonatal mouse skeletal muscle. Eur J Cell Biol. 1991 Aug;55(2):286–294. [PubMed] [Google Scholar]
- Holt G. D., Pangburn M. K., Ginsburg V. Properdin binds to sulfatide [Gal(3-SO4)beta 1-1 Cer] and has a sequence homology with other proteins that bind sulfated glycoconjugates. J Biol Chem. 1990 Feb 15;265(5):2852–2855. [PubMed] [Google Scholar]
- Iruela-Arispe M. L., Liska D. J., Sage E. H., Bornstein P. Differential expression of thrombospondin 1, 2, and 3 during murine development. Dev Dyn. 1993 May;197(1):40–56. doi: 10.1002/aja.1001970105. [DOI] [PubMed] [Google Scholar]
- Kimes B. W., Brandt B. L. Properties of a clonal muscle cell line from rat heart. Exp Cell Res. 1976 Mar 15;98(2):367–381. doi: 10.1016/0014-4827(76)90447-x. [DOI] [PubMed] [Google Scholar]
- Klar A., Baldassare M., Jessell T. M. F-spondin: a gene expressed at high levels in the floor plate encodes a secreted protein that promotes neural cell adhesion and neurite extension. Cell. 1992 Apr 3;69(1):95–110. doi: 10.1016/0092-8674(92)90121-r. [DOI] [PubMed] [Google Scholar]
- Kohno T., Carmichael D. F., Sommer A., Thompson R. C. Refolding of recombinant proteins. Methods Enzymol. 1990;185:187–195. doi: 10.1016/0076-6879(90)85018-j. [DOI] [PubMed] [Google Scholar]
- Kolega J., Shure M. S., Chen W. T., Young N. D. Rapid cellular translocation is related to close contacts formed between various cultured cells and their substrata. J Cell Sci. 1982 Apr;54:23–34. doi: 10.1242/jcs.54.1.23. [DOI] [PubMed] [Google Scholar]
- Kosfeld M. D., Frazier W. A. Identification of a new cell adhesion motif in two homologous peptides from the COOH-terminal cell binding domain of human thrombospondin. J Biol Chem. 1993 Apr 25;268(12):8808–8814. [PubMed] [Google Scholar]
- Kosfeld M. D., Frazier W. A. Identification of active peptide sequences in the carboxyl-terminal cell binding domain of human thrombospondin-1. J Biol Chem. 1992 Aug 15;267(23):16230–16236. [PubMed] [Google Scholar]
- Kosfeld M. D., Pavlopoulos T. V., Frazier W. A. Cell attachment activity of the carboxyl-terminal domain of human thrombospondin expressed in Escherichia coli. J Biol Chem. 1991 Dec 25;266(36):24257–24259. [PubMed] [Google Scholar]
- LaBell T. L., Milewicz D. J., Disteche C. M., Byers P. H. Thrombospondin II: partial cDNA sequence, chromosome location, and expression of a second member of the thrombospondin gene family in humans. Genomics. 1992 Mar;12(3):421–429. doi: 10.1016/0888-7543(92)90430-z. [DOI] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Laherty C. D., O'Rourke K., Wolf F. W., Katz R., Seldin M. F., Dixit V. M. Characterization of mouse thrombospondin 2 sequence and expression during cell growth and development. J Biol Chem. 1992 Feb 15;267(5):3274–3281. [PubMed] [Google Scholar]
- Lawler J. W., Slayter H. S., Coligan J. E. Isolation and characterization of a high molecular weight glycoprotein from human blood platelets. J Biol Chem. 1978 Dec 10;253(23):8609–8616. [PubMed] [Google Scholar]
- Lawler J., Connolly J. E., Ferro P., Derick L. H. Thrombin and chymotrypsin interactions with thrombospondin. Ann N Y Acad Sci. 1986;485:273–287. doi: 10.1111/j.1749-6632.1986.tb34589.x. [DOI] [PubMed] [Google Scholar]
- Lawler J., Derick L. H., Connolly J. E., Chen J. H., Chao F. C. The structure of human platelet thrombospondin. J Biol Chem. 1985 Mar 25;260(6):3762–3772. [PubMed] [Google Scholar]
- Lawler J., Duquette M., Urry L., McHenry K., Smith T. F. The evolution of the thrombospondin gene family. J Mol Evol. 1993 Jun;36(6):509–516. doi: 10.1007/BF00556355. [DOI] [PubMed] [Google Scholar]
- Lawler J., Duquette M., Whittaker C. A., Adams J. C., McHenry K., DeSimone D. W. Identification and characterization of thrombospondin-4, a new member of the thrombospondin gene family. J Cell Biol. 1993 Feb;120(4):1059–1067. doi: 10.1083/jcb.120.4.1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Ferro P., Duquette M. Expression and mutagenesis of thrombospondin. Biochemistry. 1992 Feb 4;31(4):1173–1180. doi: 10.1021/bi00119a029. [DOI] [PubMed] [Google Scholar]
- Lawler J., Hynes R. O. The structure of human thrombospondin, an adhesive glycoprotein with multiple calcium-binding sites and homologies with several different proteins. J Cell Biol. 1986 Nov;103(5):1635–1648. doi: 10.1083/jcb.103.5.1635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lawler J., Weinstein R., Hynes R. O. Cell attachment to thrombospondin: the role of ARG-GLY-ASP, calcium, and integrin receptors. J Cell Biol. 1988 Dec;107(6 Pt 1):2351–2361. doi: 10.1083/jcb.107.6.2351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Legrand C., Dubernard V., Kieffer N., Nurden A. T. Use of a monoclonal antibody to measure the surface expression of thrombospondin following platelet activation. Eur J Biochem. 1988 Jan 15;171(1-2):393–399. doi: 10.1111/j.1432-1033.1988.tb13803.x. [DOI] [PubMed] [Google Scholar]
- Long M. W., Dixit V. M. Thrombospondin functions as a cytoadhesion molecule for human hematopoietic progenitor cells. Blood. 1990 Jun 15;75(12):2311–2318. [PubMed] [Google Scholar]
- Majack R. A., Cook S. C., Bornstein P. Control of smooth muscle cell growth by components of the extracellular matrix: autocrine role for thrombospondin. Proc Natl Acad Sci U S A. 1986 Dec;83(23):9050–9054. doi: 10.1073/pnas.83.23.9050. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Majack R. A., Goodman L. V., Dixit V. M. Cell surface thrombospondin is functionally essential for vascular smooth muscle cell proliferation. J Cell Biol. 1988 Feb;106(2):415–422. doi: 10.1083/jcb.106.2.415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mansfield P. J., Boxer L. A., Suchard S. J. Thrombospondin stimulates motility of human neutrophils. J Cell Biol. 1990 Dec;111(6 Pt 2):3077–3086. doi: 10.1083/jcb.111.6.3077. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Hök M. Thrombospondin modulates focal adhesions in endothelial cells. J Cell Biol. 1989 Sep;109(3):1309–1319. doi: 10.1083/jcb.109.3.1309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murphy-Ullrich J. E., Westrick L. G., Esko J. D., Mosher D. F. Altered metabolism of thrombospondin by Chinese hamster ovary cells defective in glycosaminoglycan synthesis. J Biol Chem. 1988 May 5;263(13):6400–6406. [PubMed] [Google Scholar]
- Neugebauer K. M., Emmett C. J., Venstrom K. A., Reichardt L. F. Vitronectin and thrombospondin promote retinal neurite outgrowth: developmental regulation and role of integrins. Neuron. 1991 Mar;6(3):345–358. doi: 10.1016/0896-6273(91)90244-t. [DOI] [PubMed] [Google Scholar]
- O'Neill C., Jordan P., Riddle P., Ireland G. Narrow linear strips of adhesive substratum are powerful inducers of both growth and total focal contact area. J Cell Sci. 1990 Apr;95(Pt 4):577–586. doi: 10.1242/jcs.95.4.577. [DOI] [PubMed] [Google Scholar]
- O'Rourke K. M., Laherty C. D., Dixit V. M. Thrombospondin 1 and thrombospondin 2 are expressed as both homo- and heterotrimers. J Biol Chem. 1992 Dec 15;267(35):24921–24924. [PubMed] [Google Scholar]
- O'Shea K. S., Dixit V. M. Unique distribution of the extracellular matrix component thrombospondin in the developing mouse embryo. J Cell Biol. 1988 Dec;107(6 Pt 2):2737–2748. doi: 10.1083/jcb.107.6.2737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Shea K. S., Liu L. H., Dixit V. M. Thrombospondin and a 140 kd fragment promote adhesion and neurite outgrowth from embryonic central and peripheral neurons and from PC12 cells. Neuron. 1991 Aug;7(2):231–237. doi: 10.1016/0896-6273(91)90261-w. [DOI] [PubMed] [Google Scholar]
- Oldberg A., Antonsson P., Lindblom K., Heinegård D. COMP (cartilage oligomeric matrix protein) is structurally related to the thrombospondins. J Biol Chem. 1992 Nov 5;267(31):22346–22350. [PubMed] [Google Scholar]
- Osterhout D. J., Frazier W. A., Higgins D. Thrombospondin promotes process outgrowth in neurons from the peripheral and central nervous systems. Dev Biol. 1992 Apr;150(2):256–265. doi: 10.1016/0012-1606(92)90240-h. [DOI] [PubMed] [Google Scholar]
- Pancake S. J., Holt G. D., Mellouk S., Hoffman S. L. Malaria sporozoites and circumsporozoite proteins bind specifically to sulfated glycoconjugates. J Cell Biol. 1992 Jun;117(6):1351–1357. doi: 10.1083/jcb.117.6.1351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Plantefaber L. C., Hynes R. O. Changes in integrin receptors on oncogenically transformed cells. Cell. 1989 Jan 27;56(2):281–290. doi: 10.1016/0092-8674(89)90902-1. [DOI] [PubMed] [Google Scholar]
- Prater C. A., Plotkin J., Jaye D., Frazier W. A. The properdin-like type I repeats of human thrombospondin contain a cell attachment site. J Cell Biol. 1991 Mar;112(5):1031–1040. doi: 10.1083/jcb.112.5.1031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rao J. S., Hantaï D., Festoff B. W. Thrombospondin, a platelet alpha-granule and matrix glycoprotein, is increased in muscle basement membrane of patients with amyotrophic lateral sclerosis. J Neurol Sci. 1992 Nov;113(1):99–107. doi: 10.1016/0022-510x(92)90271-l. [DOI] [PubMed] [Google Scholar]
- Rapraeger A. C., Krufka A., Olwin B. B. Requirement of heparan sulfate for bFGF-mediated fibroblast growth and myoblast differentiation. Science. 1991 Jun 21;252(5013):1705–1708. doi: 10.1126/science.1646484. [DOI] [PubMed] [Google Scholar]
- Raugi G. J., Mumby S. M., Abbott-Brown D., Bornstein P. Thrombospondin: synthesis and secretion by cells in culture. J Cell Biol. 1982 Oct;95(1):351–354. doi: 10.1083/jcb.95.1.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raugi G. J., Olerud J. E., Gown A. M. Thrombospondin in early human wound tissue. J Invest Dermatol. 1987 Dec;89(6):551–554. doi: 10.1111/1523-1747.ep12461198. [DOI] [PubMed] [Google Scholar]
- Rich K. A., George F. W., 4th, Law J. L., Martin W. J. Cell-adhesive motif in region II of malarial circumsporozoite protein. Science. 1990 Sep 28;249(4976):1574–1577. doi: 10.1126/science.2120774. [DOI] [PubMed] [Google Scholar]
- Roberts D. D. Interactions of thrombospondin with sulfated glycolipids and proteoglycans of human melanoma cells. Cancer Res. 1988 Dec 1;48(23):6785–6793. [PubMed] [Google Scholar]
- Roberts D. D., Sherwood J. A., Ginsburg V. Platelet thrombospondin mediates attachment and spreading of human melanoma cells. J Cell Biol. 1987 Jan;104(1):131–139. doi: 10.1083/jcb.104.1.131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosen G. D., Sanes J. R., LaChance R., Cunningham J. M., Roman J., Dean D. C. Roles for the integrin VLA-4 and its counter receptor VCAM-1 in myogenesis. Cell. 1992 Jun 26;69(7):1107–1119. doi: 10.1016/0092-8674(92)90633-n. [DOI] [PubMed] [Google Scholar]
- Sage E. H., Bornstein P. Extracellular proteins that modulate cell-matrix interactions. SPARC, tenascin, and thrombospondin. J Biol Chem. 1991 Aug 15;266(23):14831–14834. [PubMed] [Google Scholar]
- Schwartz N. B. Regulation of chondroitin sulfate synthesis. Effect of beta-xylosides on synthesis of chondroitin sulfate proteoglycan, chondroitin sulfate chains, and core protein. J Biol Chem. 1977 Sep 25;252(18):6316–6321. [PubMed] [Google Scholar]
- Smith D. B., Johnson K. S. Single-step purification of polypeptides expressed in Escherichia coli as fusions with glutathione S-transferase. Gene. 1988 Jul 15;67(1):31–40. doi: 10.1016/0378-1119(88)90005-4. [DOI] [PubMed] [Google Scholar]
- Speziale M. V., Detwiler T. C. Free thiols of platelet thrombospondin. Evidence for disulfide isomerization. J Biol Chem. 1990 Oct 15;265(29):17859–17867. [PubMed] [Google Scholar]
- Stomski F. C., Gani J. S., Bates R. C., Burns G. F. Adhesion to thrombospondin by human embryonic fibroblasts is mediated by multiple receptors and includes a role for glycoprotein 88 (CD36). Exp Cell Res. 1992 Jan;198(1):85–92. doi: 10.1016/0014-4827(92)90152-x. [DOI] [PubMed] [Google Scholar]
- Sun X., Skorstengaard K., Mosher D. F. Disulfides modulate RGD-inhibitable cell adhesive activity of thrombospondin. J Cell Biol. 1992 Aug;118(3):693–701. doi: 10.1083/jcb.118.3.693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki S., Argraves W. S., Pytela R., Arai H., Krusius T., Pierschbacher M. D., Ruoslahti E. cDNA and amino acid sequences of the cell adhesion protein receptor recognizing vitronectin reveal a transmembrane domain and homologies with other adhesion protein receptors. Proc Natl Acad Sci U S A. 1986 Nov;83(22):8614–8618. doi: 10.1073/pnas.83.22.8614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G., Morigi M., Figliuzzi M., Giavazzi R., Zoja C., Remuzzi G. Thrombospondin induces glomerular mesangial cell adhesion and migration. Lab Invest. 1992 Nov;67(5):566–571. [PubMed] [Google Scholar]
- Taraboletti G., Roberts D. D., Liotta L. A. Thrombospondin-induced tumor cell migration: haptotaxis and chemotaxis are mediated by different molecular domains. J Cell Biol. 1987 Nov;105(5):2409–2415. doi: 10.1083/jcb.105.5.2409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taraboletti G., Roberts D., Liotta L. A., Giavazzi R. Platelet thrombospondin modulates endothelial cell adhesion, motility, and growth: a potential angiogenesis regulatory factor. J Cell Biol. 1990 Aug;111(2):765–772. doi: 10.1083/jcb.111.2.765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tucker R. P. The in situ localization of tenascin splice variants and thrombospondin 2 mRNA in the avian embryo. Development. 1993 Jan;117(1):347–358. doi: 10.1242/dev.117.1.347. [DOI] [PubMed] [Google Scholar]
- Tuszynski G. P., Rothman V. L., Deutch A. H., Hamilton B. K., Eyal J. Biological activities of peptides and peptide analogues derived from common sequences present in thrombospondin, properdin, and malarial proteins. J Cell Biol. 1992 Jan;116(1):209–217. doi: 10.1083/jcb.116.1.209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Underhill C. CD44: the hyaluronan receptor. J Cell Sci. 1992 Oct;103(Pt 2):293–298. doi: 10.1242/jcs.103.2.293. [DOI] [PubMed] [Google Scholar]
- Varani J., Dixit V. M., Fligiel S. E., McKeever P. E., Carey T. E. Thrombospondin-induced attachment and spreading of human squamous carcinoma cells. Exp Cell Res. 1986 Dec;167(2):376–390. doi: 10.1016/0014-4827(86)90178-3. [DOI] [PubMed] [Google Scholar]
- Varani J., Nickoloff B. J., Riser B. L., Mitra R. S., O'Rourke K., Dixit V. M. Thrombospondin-induced adhesion of human keratinocytes. J Clin Invest. 1988 May;81(5):1537–1544. doi: 10.1172/JCI113486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vos H. L., Devarayalu S., de Vries Y., Bornstein P. Thrombospondin 3 (Thbs3), a new member of the thrombospondin gene family. J Biol Chem. 1992 Jun 15;267(17):12192–12196. [PubMed] [Google Scholar]
- Watkins S. C., Lynch G. W., Kane L. P., Slayter H. S. Thrombospondin expression in traumatized skeletal muscle. Correlation of appearance with post-trauma regeneration. Cell Tissue Res. 1990 Jul;261(1):73–84. doi: 10.1007/BF00329440. [DOI] [PubMed] [Google Scholar]
- Wight T. N., Raugi G. J., Mumby S. M., Bornstein P. Light microscopic immunolocation of thrombospondin in human tissues. J Histochem Cytochem. 1985 Apr;33(4):295–302. doi: 10.1177/33.4.3884704. [DOI] [PubMed] [Google Scholar]
- Wikner N. E., Dixit V. M., Frazier W. A., Clark R. A. Human keratinocytes synthesize and secrete the extracellular matrix protein, thrombospondin. J Invest Dermatol. 1987 Feb;88(2):207–211. doi: 10.1111/1523-1747.ep12525350. [DOI] [PubMed] [Google Scholar]
- Woods A., Couchman J. R., Johansson S., Hök M. Adhesion and cytoskeletal organisation of fibroblasts in response to fibronectin fragments. EMBO J. 1986 Apr;5(4):665–670. doi: 10.1002/j.1460-2075.1986.tb04265.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yabkowitz R., Dixit V. M., Guo N., Roberts D. D., Shimizu Y. Activated T-cell adhesion to thrombospondin is mediated by the alpha 4 beta 1 (VLA-4) and alpha 5 beta 1 (VLA-5) integrins. J Immunol. 1993 Jul 1;151(1):149–158. [PubMed] [Google Scholar]
- Yabkowitz R., Dixit V. M. Human carcinoma cells bind thrombospondin through a Mr 80,000/105,000 receptor. Cancer Res. 1991 Jul 15;51(14):3648–3656. [PubMed] [Google Scholar]
- Yabkowitz R., Dixit V. M. Human carcinoma cells express receptors for distinct domains of thrombospondin. Cancer Res. 1991 Mar 15;51(6):1645–1650. [PubMed] [Google Scholar]
- von der Mark H., Dürr J., Sonnenberg A., von der Mark K., Deutzmann R., Goodman S. L. Skeletal myoblasts utilize a novel beta 1-series integrin and not alpha 6 beta 1 for binding to the E8 and T8 fragments of laminin. J Biol Chem. 1991 Dec 15;266(35):23593–23601. [PubMed] [Google Scholar]