Abstract
Medium spiny neurons (MSNs) constitute most of the striatal neurons and are known to be vulnerable to ischemia; however, the mechanisms of the vulnerability remain unclear. Activated forms of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (NOX), which require interaction between cytosolic and membrane-bound subunits, are among the major sources of superoxide in the central nervous system. Although increasing evidence suggests that NOX has important roles in neurodegenerative diseases, its roles in MSN injury after transient global cerebral ischemia (tGCI) have not been elucidated. To clarify this issue, C57BL/6 mice were subjected to tGCI by bilateral common carotid artery occlusion for 22 minutes. Western blot analysis revealed upregulation of NOX subunits and recruitment of cytosolic subunits to the cell membrane at early (3 to 6 hours) and late (72 hours) phases after tGCI. Taken together with immunofluorescent studies, this activation arose in MSNs and endothelial cells at the early phase, and in reactive microglia at the late phase. Pharmacological and genetic inhibition of NOX attenuated oxidative injury, microglial activation, and MSN death after tGCI. These findings suggest that NOX has pivotal roles in MSN injury after tGCI and could be a therapeutic target for brain ischemia.
Keywords: global cerebral ischemia; medium spiny neuron, microglia; NADPH oxidase; oxidative stress; striatum
Introduction
The striatum is the largest nucleus of the basal ganglia and is crucial not only for control of movement but also for higher brain function, such as cognition and learning. The structure of the striatum is heterogeneous, and striatal neurons anatomically fall into two main classes: GABAergic spiny projection neurons and aspiny interneurons. GABAergic spiny projection neurons, also known as medium spiny neurons (MSNs), represent ∼95% of the striatal neurons. The remaining 5% is made up of various aspiny interneurons, which can be further categorized into medium GABAergic interneurons and large cholinergic interneurons (Kreitzer, 2009). The MSNs are vulnerable to transient global cerebral ischemia (tGCI), as are hippocampal CA1 neurons and cerebellar Purkinje cells (Brown, 1977; Pulsinelli et al, 1982). Many studies have suggested various factors that contribute to this vulnerability, such as ion imbalance, excitotoxic damage, oxidative stress, and apoptosis (Pisani et al, 2004). However, the mechanisms of the vulnerability are not well understood.
Reactive oxygen species (ROS) have been implicated in brain injury after ischemia. The majority of studies on the role of ROS in brain ischemia have focused on the pathological effects of mitochondrial ROS production (Niizuma et al, 2010). However, much attention has recently been paid to the role of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (NOX). The oxidase is a complex enzyme that consists of a membrane subunit, cytochrome b558 (gp91phox [NOX 2] and p22phox), and multiple cytosolic subunits (p47phox, p67phox, p40phox, and Rac-1). On stimulation, the cytosolic subunits migrate to the plasma membrane, whereby a functional complex generating ROS is formed (Bedard and Krause, 2007). Although NOX was originally described in neutrophils, it has subsequently been identified in microglia, vascular cells, and neurons, including MSNs (Bedard and Krause, 2007; Serrano et al, 2003; Sorce and Krause, 2009). Recent studies suggest that ROS generated via NOX have important roles in neurodegenerative diseases such as stroke (Sorce and Krause, 2009). However, the roles of NOX in striatal injury after tGCI remain unknown.
The purpose of this study was to test the hypothesis that NOX has important roles in the ischemic vulnerability of MSNs. We investigated the differences in ischemic vulnerability among striatal neuronal subpopulations, and examined NOX expression in striatal neurons, endothelial cells, and microglia after ischemia using a mouse tGCI model. Furthermore, we studied the effects of pharmacological and genetic NOX inhibition on oxidative injury and neuronal cell death in the striatum after tGCI.
Materials and methods
Global Cerebral Ischemia
All animals were treated in accordance with Stanford University guidelines, and the animal protocols were approved by Stanford University's Administrative Panel on Laboratory Animal Care. Male gp91phox−/− (gp91 knockout (KO)) mice with a C57BL/6J background and their wild-type (WT) littermates (8 to 12 weeks; The Jackson Laboratory, Bar Harbor, ME, USA) were used. Anesthesia was induced with inhalation of 4% isoflurane and intraperitoneal injection of xylazine (4 mg/kg) and maintained with 1.5% isoflurane in 70% nitrous oxide and 30% oxygen via a face mask. Rectal temperatures were maintained at 37°C±0.5°C with a heating blanket (Harvard Apparatus, Holliston, MA, USA) and a heating lamp. Regional cerebral blood flow (rCBF) was monitored by laser Doppler flowmetry (Laserflo BMP2; Vasamedics, Eden Prairie, MN, USA). The probe was fixed on the skull, 4 mm lateral to the bregma. Changes in rCBF after bilateral common carotid artery occlusion (BCCAO) were expressed as a percentage of the preischemic value. The BCCAO was induced by applying microaneurysm clips to both common carotid arteries. As a preliminary study, we optimized the duration of BCCAO; severity of neuronal damage and survival rates 3 days after surgery were examined after 15 (n=18), 22 (n=24), and 30 (n=27) minutes of BCCAO. Sham-operated animals were treated as described above but without BCCAO. After reperfusion, the skin incision was sutured, and the animals were wrapped with a heating blanket to maintain rectal temperatures above 36.0°C for 24 hours after surgery. Acetate Ringer's solution (0.5 mL) was administered subcutaneously in all animals 30 minutes and 24 hours after reperfusion.
Pharmacological inhibition of NOX was achieved in the WT animals with apocynin (Sigma-Aldrich, St Louis, MO, USA), which was dissolved in dimethylsulfoxide and phosphate-buffered saline, and 2.5 mg/kg body weight or the vehicle were administered intravenously 15 minutes before BCCAO.
In a separate group of mice subjected to 22 minutes of BCCAO, blood pressure was measured through a PE-10 cannula inserted into the left femoral artery. Arterial blood samples were analyzed by a blood gas analyzer (i-STAT; Abbott Laboratories, Abbott Park, IL, USA) (n=4 each group).
Carbon Black Evaluation
The relationship between rCBF after 1 minute of BCCAO and patency of the posterior communicating artery (PcomA) was evaluated in the gp91 KO mice and WT littermates (n=7 each). Carbon black ink was injected as described previously (Murakami et al, 1998a). Patency of the PcomA was assessed by comparing the diameter of the PcomA on each side with the diameter of the basilar artery, and grading it as 0 or 1 (Grade 0, PcomA diameter <1/3 of basilar artery diameter; grade 1, PcomA diameter ≥1/3 of basilar artery diameter). Thus, the sum of the scores from both sides was 0, 1, or 2 (Kitagawa et al, 1998).
Western Blot Analysis
Both sides of the striatum were removed 3, 6, 12, 24, or 72 hours after BCCAO. For whole cell lysate samples, tissues were homogenized and sonicated in ice-cold 1 × lysis buffer (Cell Signaling Technology, Beverly, MA, USA) with 1% protease inhibitor mixture (Sigma-Aldrich). The homogenate was centrifuged (900g for 10 minutes at 4°C), and the resulting supernatant was used for quantification. For preparation of membrane and cytosolic fractions, the striatum was homogenized in ice-cold homogenization buffer (20 mmol/L HEPES, 10 mmol/L KCl, 2 mmol/L ethylene-diaminetetraacetate (EDTA), 1.5 mmol/L MgCl2, 250 mmol/L sucrose, 1% protease inhibitor mixture). The nuclei and cell debris were removed from the homogenate by centrifugation at 900g for 10 minutes at 4°C. The supernatant was further centrifuged at 110,000g for 75 minutes at 4°C. The resulting supernatant was collected as the cytosolic fraction, and membrane pellet was solubilized in buffer (20 mmol/L HEPES, 10 mmol/L KCl, 2 mmol/L EDTA, 1.5 mmol/L MgCl2, 0.5% Triton X-100, 1% protease inhibitor mixture) for 1 hour at 4°C. Equal amounts of samples were loaded per lane. Sodium dodecyl sulfate-polyacrylamide gel electrophoresis was performed on a 10% NuPAGE Bis-Tris gel (Invitrogen, Carlsbad, CA, USA) and then immunoblotted. Anti-p47phox (1:1,000, #07-500; Millipore, Billerica, MA, USA), anti-p67phox (1:250, #610912; BD Biosciences, San Jose, CA, USA), anti-gp91phox (1:1,000, #07-024; Millipore), anti-β-actin (1:100,000, #A5441; Sigma-Aldrich), or anti-sodium potassium ATPase (1:5,000, #ab7671; Abcam, Cambridge, MA, USA) primary antibodies were used. After incubation with an appropriate horseradish peroxidase-conjugated secondary antibody (Cell Signaling Technology), the bounded antibodies were detected by a chemiluminescence system (Pierce, Rockford, IL, USA). Images were scanned, and the results were quantified using Multi-Analyst software (Bio-Rad Laboratories, Hercules, CA, USA).
Immunohistochemistry
Anesthetized animals were perfused with 10 U/mL heparin saline and subsequently with 4% paraformaldehyde in phosphate-buffered saline after 6, 24, or 72 hours of BCCAO. Brains were removed, postfixed for 24 hours, and cut on a vibratome into slices 30 μm thick at the level of the striatum (from bregma +1.1 to −0.1 mm). For diaminobenzidine immunohistochemistry, the avidin–biotin technique was used. Nuclei were counterstained with hematoxylin solution.
Double immunostaining was performed with immunofluorescence. Brain sections were reacted with a primary antibody, then incubated with appropriate Alexa 488- or 594-conjugated immunoglobulin G antibodies (Invitrogen). To identify endothelial cells, sections were incubated with biotinylated lycopersicon esculentum lectin (1:200, #B-1175; Vector Laboratories, Burlingame, CA, USA), and the reaction products were detected with Texas-Red Avidin-D (#A-2006; Vector Laboratories). For evaluation of recruitment of p47phox to the cell membrane, microtubule-associated protein-2 immunostaining was used to demarcate the neuronal cytoplasmic space (Brennan et al, 2009). Negative controls were treated with similar procedures, except that the primary antibody was omitted. The sections were covered with VECTASHIELD mounting medium with 4′,6-diamidino-2-phenylindole (Vector Laboratories) and examined under an LSM510 confocal laser scanning microscope or an Axioplan 2 microscope (Carl Zeiss, Thornwood, NY, USA).
We used the following as primary antibodies: anti-p47phox antibody (1:50, #sc-7660; Santa Cruz Biotechnology, Santa Cruz, CA, USA), anti-gp91phox antibody (1:50, #sc-5827; Santa Cruz Biotechnology), anti-dopamine and cyclic AMP-regulated phosphoprotein of relative molecular weight 32,000 (DARPP-32) antibody (MSN marker) (1:400, #2306; Cell Signaling Technology), anti-choline acetyltransferase antibody (cholinergic interneuron marker) (1:200, #AB143; Millipore), anti-somatostatin antibody (GABAergic interneuron marker) (1:100, #AB5494; Millipore), anti-microtubule-associated protein-2 antibody (1:500, #MAB3560; Millipore), and anti-ionized calcium-binding adaptor molecule 1 (Iba-1) antibody (1:500, #019-19741; Wako Pure Chemical Industries, Osaka, Japan).
In Situ Detection of Superoxide Anion Production
Superoxide production after tGCI was investigated with the use of hydroethidine as previously described (Murakami et al, 1998b). Hydroethidine is diffusible into the central nervous system parenchyma after intravenous injection and is selectively oxidized to ethidium by superoxide anions. Hydroethidine solution (200 μL of 1 mg/mL in 1% dimethyl sulfoxide with saline) was administered intravenously 15 minutes before ischemia induction. A sample was prepared as described in the immunohistochemistry method. Sections were covered with VECTASHIELD mounting medium with 4′,6-diamidino-2-phenylindole and observed with a fluorescence microscope.
Detection of Oxidative Protein Damage
Whole cell lysate samples were prepared as described for the Western blot analysis. With the use of a commercial kit (#S7150; Millipore), we observed the carbonyl groups as indicators of oxidative protein damage. The samples were incubated with 2,4-dinitrophenylhydrazone, and the 2,4-dinitrophenylhydrazone-derivatized carbonyl groups were specifically detected by Western blotting with an anti-2,4-dinitrophenylhydrazone antibody. The image was scanned and quantified as described for the Western blot analysis.
Histological Analysis of Striatal Injury
Histological injury of the striatum was evaluated 72 hours after BCCAO by cresyl violet and terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL) staining. We used a commercial kit (#11684817910; Roche Molecular Biochemicals, Indianapolis, IN, USA) and followed the manufacturer's protocol. Nuclei were counterstained with hematoxylin solution.
Five subregions (central, dorsomedial, dorsolateral, ventromedial, and ventrolateral) were assigned for quantification of TUNEL staining, each consisting of a rectangle of 250 × 174 μm2. The TUNEL-positive cells in each subregion on both sides of the striatum were counted by two masked counters.
Cell Death Assay
For quantification of apoptosis-related DNA fragmentation in the whole striatum, we used a commercial enzyme immunoassay (#11774425001; Roche) to determine cytoplasmic histone-associated DNA fragments, which detect apoptotic but not necrotic cell death. A sample was prepared as described for the Western blotting method. A cytosolic volume containing 20 μg of protein was used for the enzyme-linked immunosorbent assay, according to the manufacturer's protocol.
Statistical Analysis
Statistical analyses were performed by analysis of variance with Dunnett's multiple comparison post hoc test (SigmaStat; Systat Software Inc., Chicago, IL, USA). Comparisons between two groups were achieved with a Student's unpaired t-test. Data are expressed as mean±s.d. and significance was accepted with P<0.05.
Results
Striatal Injury Induced by 22 Minutes of Bilateral Common Carotid Artery Occlusion
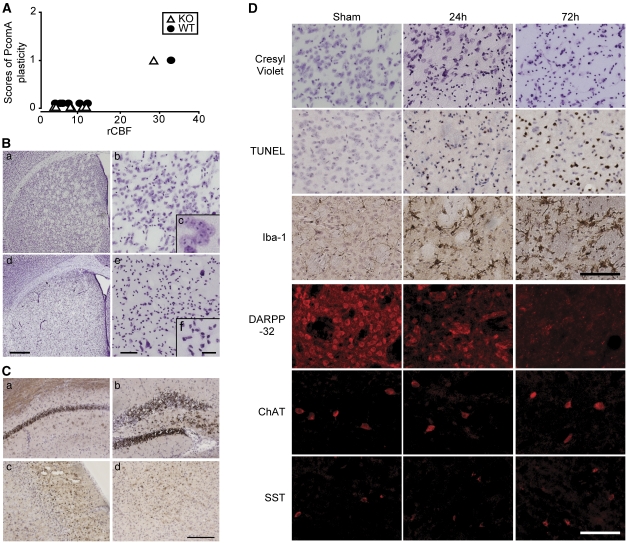
For a preliminary study, we developed a model of consistent striatal injury after tGCI. First, we evaluated the relationship between patency of the PcomA and rCBF after BCCAO. The rCBF after BCCAO was reduced to <13% of the preischemic value in 6 of the 7 littermate mice and in 6 of the 7 gp91 KO mice. In those 12 mice, no patent PcomA was identified (Figure 1A). According to these results, in further studies we used only animals whose rCBF decreased to <13% of the preischemic value.
Figure 1.
A transient global cerebral ischemia (tGCI) model of induction of bilateral common carotid artery occlusion (BCCAO). (A) Relationship between patency of the posterior communicating artery (PcomA) and regional cerebral blood flow (rCBF) after 1 minute of BCCAO. The degree of patency was divided into no patent PcomA on either side (grade 0), patent PcomA on one side only (grade 1), and patent PcomA on both sides (grade 2). No gp91 knockout (KO) mouse or wild-type (WT) littermate whose rCBF decreased to <13% of the preischemic value possessed a patent PcomA. (B) Representative photomicrographs of the striatum (a–c, sham animal; d–f, 3 days after 22 minutes of BCCAO). Brain sections were stained with cresyl violet. Scale bars=500 μm (d), 50 μm (e), 10 μm (f). (C) Terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL)-positive cells were also observed in the hippocampal CA1 subregion (a), CA4 subregion and dentate gyrus (b), cerebral cortex (c), and thalamus (d). Scale bar=200 μm. (D) Time course of cresyl violet, TUNEL, Iba-1, and striatal neuronal subpopulation marker (DARPP-32, anti-somatostatin (SST), anti-choline acetyltransferase (ChAT)) staining. Scale bars=100 μm.
Next, we optimized the duration of BCCAO. Severity of ischemic injury and mortality rate 3 days after surgery were examined after 15, 22, and 30 minutes of BCCAO. The mortality rate was 11.1%, 16.7%, and 51.9%, respectively. In the 15-minute group, many of the ischemic injuries were mild and only a few mice showed severe injuries. In the 22-minute group, severe and consistent injuries were observed; small- and medium-sized cells in the striatum, especially, were consistently injured (Figure 1B). The striatum was uniformly injured, and there were no significant differences in severity among the five subregions (central, dorsomedial, dorsolateral, ventromedial, and ventrolateral) (Figure 1Bd). We also observed ischemic injuries in the hippocampus, the cerebral cortex (mainly the second to fourth layers), and the thalamus (Figure 1C); however, these injuries were not as consistent as those in the striatum. The severity of injury in the 30-minute group was similar to that in the 22-minute group, but the former had a higher rate of mortality. According to these results, we adopted the 22-minute BCCAO model for our further studies.
Figure 1D shows the time course of ischemic injury in the striatum. In the cresyl violet-stained sections, shrunken cells and irregularly condensed nuclei were observed 24 hours after tGCI, and these changes extended to 72 hours. The TUNEL-positive cells appeared at 24 hours and increased at 72 hours. Immunohistochemistry of striatal neuronal markers proved the difference in vulnerability among the neuronal subpopulations. Immunostaining of DARPP-32, a specific marker of MSNs (Arvidsson et al, 2002; Zhu et al, 2006), was present in neuronal cell bodies and dendrites in sham animals, and almost all DARPP-32 staining vanished 72 hours after tGCI. However, anti-choline acetyltransferase-positive cells (cholinergic interneurons) and anti-somatostatin-positive cells (GABAergic interneurons) were preserved 72 hours after tGCI. In the sham samples, Iba-1-positive cells were distributed throughout the striatum, and their processes showed highly ramified structures (ramified microglia). Seventy-two hours after tGCI, expression of Iba-1 became stronger, and the positive cells, resembling reactive microglia, retracted their fine ramified processes and showed plump cell bodies. Physiological parameters and rCBF during ischemia are shown in Table 1. There were no significant differences among the groups.
Table 1. Physiological parameters and rCBF.
| MAPB (mm Hg) | pH | PaCO2 (mm Hg) | Pao2 (mm Hg) | rCBF (%) | |
|---|---|---|---|---|---|
| Before ischemia | |||||
| WT | 64.8±2.2 | 7.31±0.01 | 36.1±3.7 | 146.3±9.0 | 100 |
| Vehicle | 67.3±1.2 | 7.38±0.02 | 31.4±1.5 | 145.0±15.9 | 100 |
| gp91 KO | 65.0±1.0 | 7.33±0.01 | 35.1±3.0 | 146.4±6.4 | 100 |
| Apocynin | 65.3±1.5 | 7.33±0.03 | 33.4±1.6 | 143.7±6.4 | 100 |
| During ischemia (10 minutes) | |||||
| WT | 84.5±14.6 | 7.32±0.01 | 36.1±0.9 | 149.0±9.2 | 6.8±3.4 |
| Vehicle | 89.7±11.6 | 7.30±0.01 | 34.4±4.2 | 150.3±1.5 | 7.0±1.9 |
| gp91 KO | 86.3±14.0 | 7.33±0.01 | 35.3±1.5 | 149.0±2.0 | 7.1±3.4 |
| Apocynin | 90.1±8.9 | 7.32±0.01 | 37.5±3.4 | 155.0±7.2 | 7.5±2.8 |
KO, knockout; MABP, mean arterial blood pressure; rCBF, regional cerebral blood flow; WT, wild type.
Upregulation of NADPH Oxidase Subunits After Ischemia
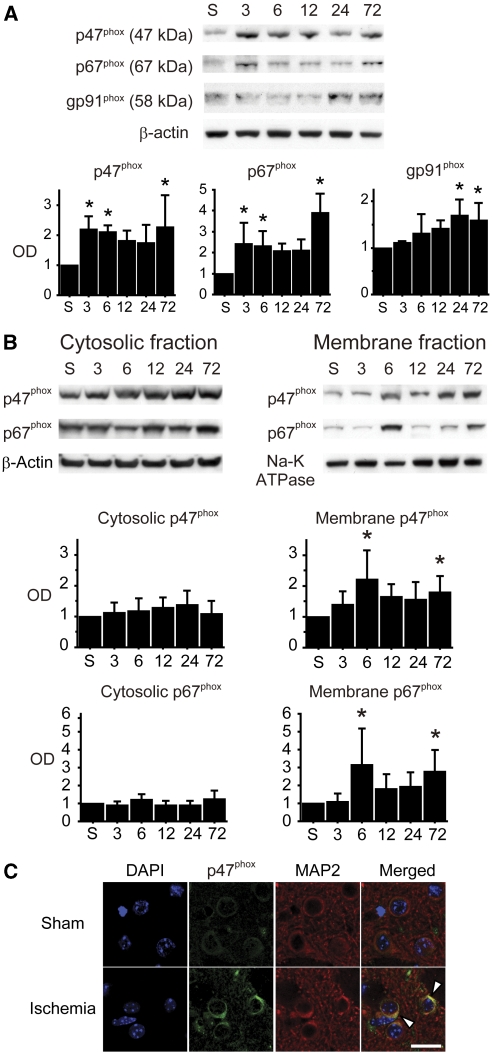
To evaluate the change in expression of NOX subunits in the striatum after tGCI, we performed Western blot analysis using whole cell lysate samples. Bands of p47phox, p67phox, and gp91phox were detected at 47, 67, and 58 kDa, respectively. Cytosolic subunits (p47phox and p67phox) showed biphasic upregulation at early (3 to 6 hours) and late (72 hours) phases after tGCI. In contrast, membrane-bound subunit gp91phox upregulated 24 and 72 hours after tGCI (Figure 2A).
Figure 2.
Upregulation and activation of nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (NOX) after transient global cerebral ischemia (tGCI). (A) Western blots using whole cell lysate samples show biphasic upregulation of the cytosolic subunits (p47phox and p67phox) at the early (3 to 6 hours) and late (72 hours) phases after tGCI. In contrast, the membrane-bound subunit gp91phox upregulated 24 and 72 hours after tGCI (n=4, *P<0.05 compared with sham). S, sham; OD, optical density. (B) Western blot analysis showed increase of cytosolic subunits (p47phox and p67phox) in the membrane fraction 6 and 72 hours after ischemia (n=5, *P<0.05 compared with sham). In the cytosolic fraction, no significant differences were seen at any time point. β-Actin and Na-K ATPase were used as internal controls for the cytosolic and membrane fractions, respectively. (C) Representative confocal images of immunostaining. Expression of p47phox became more intense, especially on the cell surface (arrowheads), 6 hours after ischemia. Microtubule-associated protein-2 (MAP2) immunostaining demarcates the neuronal cytoplasmic space. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Scale bar=20 μm.
Recruitment of Cytosolic NADPH Oxidase Subunits to the Cell Membrane After Ischemia
Recruitment of cytosolic NOX subunits to the cell membrane indicates NOX activation (Brennan et al, 2009; Cross and Segal, 2004). To evaluate NOX activation after tGCI, we performed Western blot analysis using fractionated samples. Western blot analysis showed a significant increase in cytosolic NOX subunits in the membrane fraction 6 and 72 hours after tGCI (P<0.05). In the cytosolic fractions, no significant differences were observed at any time point (Figure 2B). This finding in the neurons was also confirmed by immunofluorescent studies using a confocal microscope. In the sham mice, immunostaining for p47phox was diffusely distributed throughout the cytoplasm. However, 6 hours after tGCI, expression of p47phox became more intense, especially on the cell surface (Figure 2C).
Expression of NADPH Oxidase Subunits in the Striatum
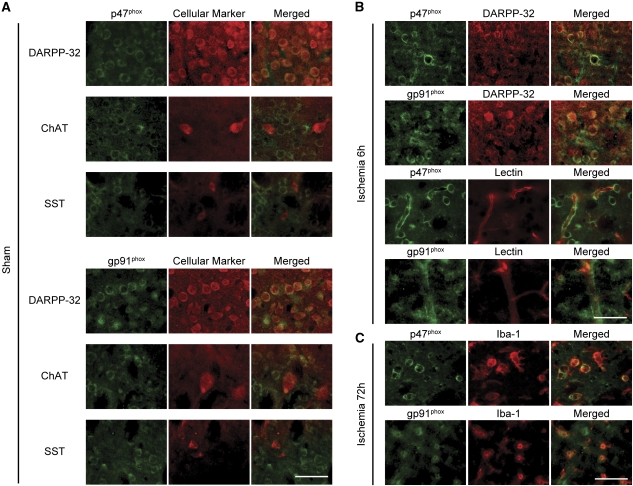
To investigate the expression of NOX subunits in the striatal subpopulations, we performed double immunofluorescence of NOX subunits (p47phox or gp91phox) and cell markers. In the sham animals, staining of both NOX subunits colocalized with that of DARPP-32. However, their expression was weak in anti-choline acetyltransferase- and anti-somatostatin-positive cells (Figure 3A). Both NOX subunits also expressed in lectin-positive endothelial cells. Obvious expression was not detected in resting microglia (data not shown). Negative controls, in which the primary antibody was omitted, were completely blank, and preabsorption with the peptide provided by the manufacturer abolished the staining.
Figure 3.
Immunostaining for the nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (NOX) subunits. (A) In the sham animals, p47phox and gp91phox were expressed in the DARPP-32-positive cells; however, their expression was weak in the anti-choline acetyltransferase (ChAT)- and anti-somatostatin (SST)-positive cells. (B) Six hours after transient global cerebral ischemia (tGCI), expression of p47phox became more intense in the DARPP-32-positive cells and the lectin-positive endothelial cells. (C) Seventy-two hours after tGCI, p47phox and gp91phox were expressed in reactive microglia. Scale bars=100 μm.
p47phox staining became more intense 6 hours after tGCI and appeared to redistribute to the cell surface. At this time point, p47phox and gp91phox were expressed in DARPP-32-positive cells and lectin-positive endothelial cells (Figure 3B). Microglia were activated 72 hours after tGCI, and NOX subunits were expressed in these reactive microglia (Figure 3C). The results of Western blot analysis and immunofluorescent studies indicate that NOX is upregulated and activated in MSNs and endothelial cells at the early phase after tGCI and in reactive microglia at the late phase.
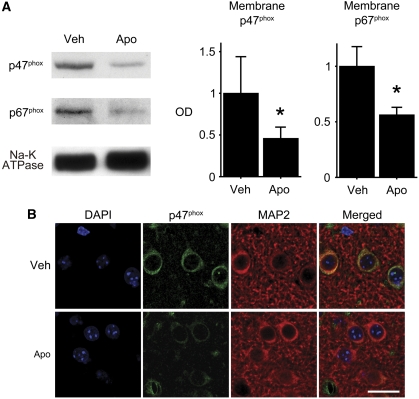
Apocynin Inhibited Recruitment of Cytosolic NADPH Oxidase Subunits to the Cell Membrane
Apocynin blocks migration of cytosolic NOX subunits to the membrane, thus interfering with assembly of a functional NOX complex (Simons et al, 1990). We examined the effect of apocynin on recruitment of cytosolic NOX subunits to the plasma membrane. Western blot analysis of the membrane fraction 6 hours after tGCI showed that treatment with apocynin significantly attenuated recruitment of p47phox and p67phox to the membrane compared with vehicle treatment (P<0.05) (Figure 4A). Immunofluorescent studies also confirmed the effect of apocynin treatment in neurons (Figure 4B).
Figure 4.
Effect of apocynin on recruitment of cytosolic subunits to the cell membrane. (A) Western blot analysis of the membrane fraction 6 hours after transient global cerebral ischemia (tGCI) showed that apocynin treatment significantly attenuated recruitment of cytosolic subunits p47phox and p67phox to the membrane compared with vehicle treatment (n=4, *P<0.05). Na-K ATPase was used as an internal control. (B) An immunofluorescent study using a confocal microscope also showed the effect of apocynin treatment. Microtubule-associated protein-2 (MAP2) immunostaining demarcates the neuronal cytoplasmic space. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI). Apo, apocynin; Veh, vehicle. Scale bar=20 μm.
NADPH Oxidase Inhibition Alleviated Oxidative Injury
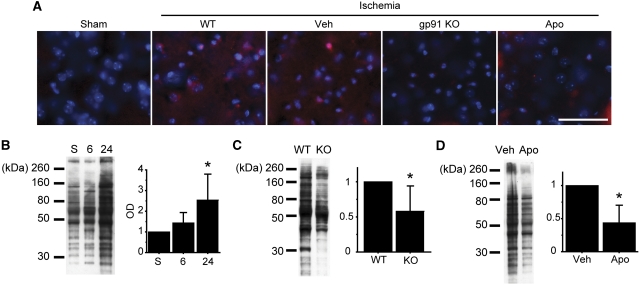
We investigated whether NOX inhibition influences oxidative injury after tGCI. First, we evaluated superoxide production after tGCI using hydroethidine. In the WT (nontreated) and vehicle-treated mice, ethidium signals were diffusely increased 24 hours after tGCI compared with the sham animals; however, this increase was attenuated in the gp91 KO and apocynin-treated mice (Figure 5A). We then quantitatively measured oxidative protein damage in the striatum by detection of the carbonyl groups introduced into proteins. As shown in Figure 5B, the level of the carbonyl groups significantly increased 24 hours after tGCI compared with the nonischemic brains in the nontreated animals (P<0.05). The increases in the carbonyl groups significantly decreased in the gp91 KO and apocynin-treated mice compared with the WT and vehicle-treated mice (P<0.05) (Figures 5C and 5D).
Figure 5.
Nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (NOX) inhibition attenuated oxidative injury after transient global cerebral ischemia (tGCI). (A) Representative photomicrographs of in situ detection of superoxide anion production. In the wild-type (WT) mice, ethidium signals (red) increased diffusely after ischemia; however, this increase was attenuated by NOX inhibition. Nuclei were counterstained with 4′,6-diamidino-2-phenylindole (DAPI) (blue). Scale bar=50 μm. (B) Quantitative evaluation of oxidative protein damage by detection of the carbonyl groups introduced into proteins. The level of the carbonyl groups significantly increased 24 hours after tGCI compared with the nonischemic brains in the nontreated animals (n=8, *P<0.05). (C, D) Increases in the carbonyl groups significantly decreased in the gp91 knockout (KO) and apocynin-treated mice compared with the WT and vehicle-treated mice (n=6, *P<0.05). Apo, apocynin; OD, optical density; Veh, vehicle.
Microglial Activation Was Suppressed by NADPH Oxidase Inhibition
The effect of NOX inhibition on microglial activation was evaluated by Iba-1 immunostaining. As shown in Figure 1D, microglia were activated in the striatum 72 hours after tGCI. However, microglial activation was suppressed in the gp91 KO and apocynin-treated mice compared with the WT and vehicle-treated mice (Figure 6A).
Figure 6.
Nicotinamide-adenine dinucleotide phosphate (NADPH) oxidase (NOX) inhibition alleviated microglial activation and medium spiny neuron (MSN) injury. (A) Representative photomicrographs of Iba-1 staining of the striatum 72 hours after transient global cerebral ischemia (tGCI). Microglial activation was suppressed by NOX inhibition. (B) Representative photomicrographs of the striatum 72 hours after tGCI. Sections were stained with cresyl violet, terminal deoxynucleotidyl transferase-mediated uridine 5′-triphosphate-biotin nick end labeling (TUNEL), and a DARPP-32 antibody. Scale bars=100 μm. (C) Cell counting study showed a significant decrease in TUNEL-positive cells in the gp91 knockout (KO) and apocynin-treated mice compared with the wild-type (WT) and vehicle-treated mice (n=5, *P<0.05). (D) Apoptosis-related DNA fragmentation assay. In the WT mice, DNA fragmentation increased significantly in the striatum 72 hours after tGCI compared with the sham animals (n=5, *P<0.01). (E) DNA fragmentation in the gp91 KO and apocynin-treated mice 72 hours after tGCI decreased significantly compared with the WT and vehicle-treated mice (n=6, *P<0.05). Apo, apocynin; Veh, vehicle.
NADPH Oxidase Inhibition Attenuated Striatal Neuronal Injury After Ischemia
Finally, we examined the effect of NOX inhibition on striatal neuronal injury after tGCI. The mortality rate of animals 3 days after tGCI was 16.7%, 14.3%, 15.4%, and 13.0% in the WT, gp91 KO, vehicle-treated, and apocynin-treated mice, respectively, and there were no significant differences among them. In the cresyl violet-stained sections, more viable neurons were observed in the gp91 KO and apocynin-treated animals 72 hours after tGCI. We also observed that more DARPP-32-positive cells survived by NOX inhibition. These observations were confirmed by the results of TUNEL staining. The TUNEL-positive cells of the gp91 KO and apocynin-treated mice were significantly decreased compared with the WT and vehicle-treated mice (P<0.01) (Figures 6B and 6C).
An apoptotic DNA fragmentation quantitative assay demonstrated that DNA fragmentation in the whole striatum significantly increased 72 hours after tGCI compared with the sham animals (P<0.01) (Figure 6D). DNA fragmentation 72 hours after tGCI was significantly reduced in the gp91 KO and apocynin-treated mice compared with the WT and vehicle-treated mice (P<0.05) (Figure 6E).
Discussion
Important Role of NADPH Oxidase in Reactive Oxygen Species Production After Brain Ischemia
The majority of previous studies linked ROS production with mitochondria dysfunction; however, results of recent research suggest a major role for NOX in brain ischemia (Chen et al, 2009; Kim et al, 2009; Suh et al, 2008; Walder et al, 1997; Zhang et al, 2009). Our current study supports this suggestion. We showed that NOX was upregulated and activated in MSNs and endothelial cells at the early phase (3 to 6 hours) after tGCI and in reactive microglia at the late phase (72 hours). Superoxide production and oxidative protein damage, after NOX activation at the early phase, were suppressed by NOX inhibition. Furthermore, microglial activation and MSN injury were also attenuated by NOX inhibition. Taken together, we conclude that NOX has a pivotal role in ROS production and subsequent ischemic injury in the striatum after tGCI.
Medium Spiny Neurons
As NOX subunits were expressed in MSNs, endothelial cells, and reactive microglia, NOX might have a particular role in each cellular constituent. Although NOX expression in neurons was considered unlikely for a long time, recent studies have demonstrated that NOX is expressed in neurons and has functions such as modulation of neuronal activity and alteration of cell fate (Bedard and Krause, 2007). Furthermore, some authors have recently reported an important role for neuronal NOX in ROS production in neurodegenerative disease, including brain ischemia (Kim et al, 2009; Suh et al, 2008; Zhang et al, 2009). In the current study, we showed that NOX was upregulated and activated in MSNs after ischemia. After activation of NOX in MSNs at the early phase, oxidative injury was increased 24 hours after tGCI, and was reversed by NOX inhibition. Therefore, neuronal NOX could be a major source of deteriorative ROS in brain ischemia, though we cannot rule out the importance of other sources such as mitochondria.
In this study, we did not resolve the question of how ROS generated via neuronal NOX injure neurons. Mitochondrial ROS not only cause macromolecular damage but also activate cell death signals through release of proapoptotic proteins from mitochondria (Niizuma et al, 2010). Further studies are needed to elucidate whether NOX-produced ROS could activate apoptotic signaling in the ischemic brain.
Interestingly, NOX expression was observed in vulnerable MSNs, but was weak in ischemia-resistant interneurons. Recently, Zhang et al (2009) reported that NOX subunits were observed in the hippocampal CA1 subregion, which is well known as a vulnerable region in rat tGCI models, but not in the ischemia-resistant CA3/dentate gyrus regions. Although the cell death mechanism after ischemia may be different between MSNs and CA1 pyramidal neurons, both vulnerable neurons expressed NOX. Therefore, we suggest that neuronal NOX might cause, at least in part, selective ischemic vulnerability.
Endothelial Cells
Besides MSNs, our immunofluorescent studies showed NOX expression in cerebral endothelial cells at the early phase after tGCI. Endothelial cells are critical in maintaining the structural and functional integrity of the blood–brain barrier. NOX is an important source of ROS in endothelial cells. Excessive ROS injure endothelial cells, which could lead to blood–brain barrier breakdown and exacerbation of ischemic injury (Kahles et al, 2007; Liu et al, 2008). Although transient focal ischemia models have been used in many studies to elucidate endothelial injury and blood–brain barrier breakdown after ischemia, tGCI models have also induced endothelial injury and blood–brain barrier breakdown, especially if ischemic duration is prolonged (Preston and Webster, 2004). Therefore, endothelial cell injury induced by NOX-produced ROS may contribute to striatal injury in this study.
Microglia
As microglia are professional phagocytes, key functions of ROS in microglia are host defense and removal of debris from the central nervous system. In addition, mounting evidence indicates that ROS generated via microglial NOX are neurotoxic in neurodegenerative disease (Block et al, 2007). In Alzheimer's disease, amyloid precursor protein fragments released from neurons activate NOX in neighboring microglia, and the consequent ROS generation by microglial NOX leads to death of neighboring neurons (Qin et al, 2006). In ischemic brains, the precise roles of microglial NOX are not understood. We showed that NOX was activated in reactive microglia and that pharmacological and genetic inhibition of NOX attenuated microglial activation, as well as striatal injury. Wang et al (2006) similarly reported that apocynin administration attenuated microglial activation and delayed neuronal death in the gerbil hippocampus after tGCI. There is a possibility that ROS generated via microglial NOX may contribute to exacerbation of ischemic injury. However, as these studies did not specifically inhibit NOX in microglia, further studies are needed to elucidate whether microglial NOX aggravates ischemic injury. In addition, we did not evaluate the effect of NOX inhibition on ROS production beyond 24 hours after ischemia. As microglial NOX was activated at the late phase (72 hours) after ischemia, we need to evaluate this effect in future studies, which will help to elucidate the roles of microglial NOX.
We did not investigate the mechanisms of microglial activation after ischemia in this study. However, based on previous studies, we can suppose the following scenario. The ROS generated by microglial NOX mediate microglial activation (Min et al, 2004). Nuclear factor-κB activated by oxidative stress leads to further ROS production via NOX and further microglial activation in a positive feedback loop (Anrather et al, 2006). The suppression of microglial activation in our study might have been achieved via inhibition of this positive feedback loop.
Our immunofluorescent study did not detect obvious expression of NOX subunits in resting microglia. This finding is unexpected, as NOX function in microglia is important for normal physiology of the central nervous system (Sorce and Krause, 2009). However, some authors also did not detect NOX expression in resting microglia. Immunohistochemical approaches in rat and mouse brains did not detect NOX in microglia under normal conditions (Kim et al, 2005; Serrano et al, 2003). gp91phox was not detected in resting microglia, though it was detected in activated forms after ischemia (Green et al, 2001). Expression of NOX in resting microglia may be insufficient to be detected by an immunohistochemical approach.
Effects of Apocynin
Although apocynin has been used as a NOX inhibitor in numerous studies, Heumüller et al (2008) recently reported that activation of apocynin needs myeloperoxidase and that apocynin acts as a nonspecific oxidative scavenger in cells that are not rich in myeloperoxidase. Their results suggest that apocynin may not act as an inhibitor in neurons, which are not rich in myeloperoxidase. However, other peroxidases, such as horseradish peroxidase, can also induce an activated form of apocynin (Touyz, 2008; Vejražka et al, 2005). In addition, myeloperoxidase secreted by neutrophils that accumulate after ischemia can metabolize apocynin to an activated form (Chen et al, 2009; Touyz, 2008). In this study, we showed that apocynin inhibited recruitment of cytosolic NOX subunits to the cell membrane in neurons. Taken together, we think that apocynin acted as an NOX inhibitor in this study. However, we will elucidate the effects of apocynin on gp91 KO mice in a future study to evaluate the nonspecific oxidative-scavenger effect of apocynin.
It has been reported that some neuroprotectants only delay ischemic injury and cannot show long-term efficacy (Chaulk et al, 2003). Therefore, in future studies we also need to evaluate the long-term efficacy of apocynin, although we have shown the neuroprotective effects 3 days after ischemia, when ischemic injury was maximal in our model.
Differences in Expression Patterns Between Membrane and Cytosolic NADPH Oxidase Subunits
Western blot analysis of the current study showed the different time courses of protein expression between membrane and cytosolic NOX subunits. Expression of gp91phox peaked 24 to 72 hours after tGCI. In contrast, expression of p47phox and p67phox increased biphasically at 3 to 6 hours and 72 hours, though the time courses of p47phox and p67phox expression were similar. The reasons for this discrepancy are uncertain. The NOX subunits were reported to upregulate after ischemia in previous studies (Chen et al, 2009; Hong et al, 2006; Kim et al, 2009; Kusaka et al, 2004; Liu et al, 2008). The time points when NOX subunits upregulated ranged from 1 to 24 hours after transient focal cerebral ischemia. Most studies evaluated protein expression at one or two time points, and there are no reports that evaluated expression of multiple NOX subunits at many time points. As we evaluated NOX subunit expression at many time points in this study, many factors from various cellular components might complicate the total amounts of protein expression. Kusaka et al (2004) also reported that there were differences in protein expression of each NOX subunit among time courses. The expression pattern may be different in each NOX subunit in each cellular component.
Striatal Injury Model After Transient Global Cerebral Ischemia
Striatal MSNs are vulnerable to tGCI. In rat or gerbil models, MSNs are frequently injured after tGCI if the ischemic duration is relatively prolonged (20 to 30 minutes) (Pulsinelli et al, 1982). In a mouse model, MSNs were also reported to be vulnerable. Some authors reported that in mice, MSNs were more vulnerable than even hippocampal CA1 pyramidal cells (Gillingwater et al, 2004; Olsson et al, 2003; Terashima et al, 1998), which is consistent with our results. As mice are commonly used in the production of transgenic animals, a reproducible mouse tGCI model is exceedingly important for elucidating the mechanism of ischemic neuronal injury. The animal model used in this study can produce consistent neuronal injury by a simple technique and can be highly useful for studying the in vivo molecular mechanism of ischemic injury.
Conclusion
NADPH oxidase is activated in MSNs, endothelial cells, and reactive microglia in the striatum after tGCI. The ROS generated via NOX induce oxidative injury and microglial activation, which culminate in the death of vulnerable MSNs. These deteriorative effects were reversed by NOX inhibition. We propose that NOX could be a therapeutic target for brain ischemia.
Acknowledgments
The authors thank Liza Reola and Bernard Calagui for technical assistance, Cheryl Christensen for editorial assistance, and Elizabeth Hoyte for assistance with the figures.
The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH. The authors declare no conflict of interest.
Footnotes
This work was supported by National Institutes of Health Grants P50 NS014543, RO1 NS025372, RO1 NS036147, and RO1 NS038653.
References
- Anrather J, Racchumi G, Iadecola C. NF-κB regulates phagocytic NADPH oxidase by inducing the expression of gp91phox. J Biol Chem. 2006;281:5657–5667. doi: 10.1074/jbc.M506172200. [DOI] [PubMed] [Google Scholar]
- Arvidsson A, Collin T, Kirik D, Kokaia Z, Lindvall O. Neuronal replacement from endogenous precursors in the adult brain after stroke. Nat Med. 2002;8:963–970. doi: 10.1038/nm747. [DOI] [PubMed] [Google Scholar]
- Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- Block ML, Zecca L, Hong J-S. Microglia-mediated neurotoxicity: uncovering the molecular mechanisms. Nat Rev Neurosci. 2007;8:57–69. doi: 10.1038/nrn2038. [DOI] [PubMed] [Google Scholar]
- Brennan AM, Suh SW, Won SJ, Narasimhan P, Kauppinen TM, Lee H, Edling Y, Chan PH, Swanson RA. NADPH oxidase is the primary source of superoxide induced by NMDA receptor activation. Nat Neurosci. 2009;12:857–863. doi: 10.1038/nn.2334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AW. Structural abnormalities in neurones. J Clin Pathol Suppl. 1977;11:155–169. doi: 10.1136/jcp.s3-11.1.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chaulk D, Wells J, Evans S, Jackson D, Corbett D. Long-term effects of clomethiazole in a model of global ischemia. Exp Neurol. 2003;182:476–482. doi: 10.1016/s0014-4886(03)00121-3. [DOI] [PubMed] [Google Scholar]
- Chen H, Song YS, Chan PH. Inhibition of NADPH oxidase is neuroprotective after ischemia–reperfusion. J Cereb Blood Flow Metab. 2009;29:1262–1272. doi: 10.1038/jcbfm.2009.47. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross AR, Segal AW. The NADPH oxidase of professional phagocytes–prototype of the NOX electron transport chain systems. Biochim Biophys Acta. 2004;1657:1–22. doi: 10.1016/j.bbabio.2004.03.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gillingwater TH, Haley JE, Ribchester RR, Horsburgh K. Neuroprotection after transient global cerebral ischemia in WldS mutant mice. J Cereb Blood Flow Metab. 2004;24:62–66. doi: 10.1097/01.WCB.0000095798.98378.34. [DOI] [PubMed] [Google Scholar]
- Green SP, Cairns B, Rae J, Errett-Baroncini C, Hongo J-AS, Erickson RW, Curnutte JT. Induction of gp91-phox, a component of the phagocyte NADPH oxidase, in microglial cells during central nervous system inflammation. J Cereb Blood Flow Metab. 2001;21:374–384. doi: 10.1097/00004647-200104000-00006. [DOI] [PubMed] [Google Scholar]
- Heumüller S, Wind S, Barbosa-Sicard E, Schmidt HHHW, Busse R, Schröder K, Brandes RP. Apocynin is not an inhibitor of vascular NADPH oxidases but an antioxidant. Hypertension. 2008;51:211–217. doi: 10.1161/HYPERTENSIONAHA.107.100214. [DOI] [PubMed] [Google Scholar]
- Hong H, Zeng J-S, Kreulen DL, Kaufman DI, Chen AF. Atorvastatin protects against cerebral infarction via inhibition of NADPH oxidase-derived superoxide in ischemic stroke. Am J Physiol Heart Circ Physiol. 2006;291:H2210–H2215. doi: 10.1152/ajpheart.01270.2005. [DOI] [PubMed] [Google Scholar]
- Kahles T, Luedike P, Endres M, Galla H-J, Steinmetz H, Busse R, Neumann-Haefelin T, Brandes RP. NADPH oxidase plays a central role in blood-brain barrier damage in experimental stroke. Stroke. 2007;38:3000–3006. doi: 10.1161/STROKEAHA.107.489765. [DOI] [PubMed] [Google Scholar]
- Kim GS, Jung JE, Niizuma K, Chan PH. CK2 is a novel negative regulator of NADPH oxidase and a neuroprotectant in mice after cerebral ischemia. J Neurosci. 2009;29:14779–14789. doi: 10.1523/JNEUROSCI.4161-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim MJ, Shin K-S, Chung Y-B, Jung KW, Cha CI, Shin DH. Immunohistochemical study of p47Phox and gp91Phox distributions in rat brain. Brain Res. 2005;1040:178–186. doi: 10.1016/j.brainres.2005.01.066. [DOI] [PubMed] [Google Scholar]
- Kitagawa K, Matsumoto M, Tsujimoto Y, Ohtsuki T, Kuwabara K, Matsushita K, Yang G, Tanabe H, Martinou J-C, Hori M, Yanagihara T. Amelioration of hippocampal neuronal damage after global ischemia by neuronal overexpression of BCL-2 in transgenic mice. Stroke. 1998;29:2616–2621. doi: 10.1161/01.str.29.12.2616. [DOI] [PubMed] [Google Scholar]
- Kreitzer AC. Physiology and pharmacology of striatal neurons. Annu Rev Neurosci. 2009;32:127–147. doi: 10.1146/annurev.neuro.051508.135422. [DOI] [PubMed] [Google Scholar]
- Kusaka I, Kusaka G, Zhou C, Ishikawa M, Nanda A, Granger DN, Zhang JH, Tang J. Role of AT1 receptors and NAD(P)H oxidase in diabetes-aggravated ischemic brain injury. Am J Physiol Heart Circ Physiol. 2004;286:H2442–H2451. doi: 10.1152/ajpheart.01169.2003. [DOI] [PubMed] [Google Scholar]
- Liu W, Sood R, Chen Q, Sakoglu U, Hendren J, Çetin Ö, Miyake M, Liu KJ. Normobaric hyperoxia inhibits NADPH oxidase-mediated matrix metalloproteinase-9 induction in cerebral microvessels in experimental stroke. J Neurochem. 2008;107:1196–1205. doi: 10.1111/j.1471-4159.2008.05664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Min K-J, Pyo H-K, Yang M-S, Ji K-A, Jou I, Joe E-H. Gangliosides activate microglia via protein kinase C and NADPH oxidase. Glia. 2004;48:197–206. doi: 10.1002/glia.20069. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Chan PH. The development of a new mouse model of global ischemia: focus on the relationships between ischemia duration, anesthesia, cerebral vasculature, and neuronal injury following global ischemia in mice. Brain Res. 1998a;780:304–310. doi: 10.1016/s0006-8993(97)01217-1. [DOI] [PubMed] [Google Scholar]
- Murakami K, Kondo T, Kawase M, Li Y, Sato S, Chen SF, Chan PH. Mitochondrial susceptibility to oxidative stress exacerbates cerebral infarction that follows permanent focal cerebral ischemia in mutant mice with manganese superoxide dismutase deficiency. J Neurosci. 1998b;18:205–213. doi: 10.1523/JNEUROSCI.18-01-00205.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niizuma K, Yoshioka H, Chen H, Kim GS, Jung JE, Katsu M, Okami N, Chan PH. Mitochondrial and apoptotic neuronal death signaling pathways in cerebral ischemia. Biochim Biophys Acta. 2010;1802:92–99. doi: 10.1016/j.bbadis.2009.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olsson T, Wieloch T, Smith M-L. Brain damage in a mouse model of global cerebral ischemia. Effect of NMDA receptor blockade. Brain Res. 2003;982:260–269. doi: 10.1016/s0006-8993(03)03014-2. [DOI] [PubMed] [Google Scholar]
- Pisani A, Bonsi P, Calabresi P. Calcium signaling and neuronal vulnerability to ischemia in the striatum. Cell Calcium. 2004;36:277–284. doi: 10.1016/j.ceca.2004.02.010. [DOI] [PubMed] [Google Scholar]
- Preston E, Webster J. A two-hour window for hypothermic modulation of early events that impact delayed opening of the rat blood-brain barrier after ischemia. Acta Neuropathol. 2004;108:406–412. doi: 10.1007/s00401-004-0905-4. [DOI] [PubMed] [Google Scholar]
- Pulsinelli WA, Brierley JB, Plum F. Temporal profile of neuronal damage in a model of transient forebrain ischemia. Ann Neurol. 1982;11:491–498. doi: 10.1002/ana.410110509. [DOI] [PubMed] [Google Scholar]
- Qin B, Cartier L, Dubois-Dauphin M, Li B, Serrander L, Krause K-H. A key role for the microglial NADPH oxidase in APP-dependent killing of neurons. Neurobiol Aging. 2006;27:1577–1587. doi: 10.1016/j.neurobiolaging.2005.09.036. [DOI] [PubMed] [Google Scholar]
- Serrano F, Kolluri NS, Wientjes FB, Card JP, Klann E. NADPH oxidase immunoreactivity in the mouse brain. Brain Res. 2003;988:193–198. doi: 10.1016/s0006-8993(03)03364-x. [DOI] [PubMed] [Google Scholar]
- Simons JM, Hart BA, Ip Vai Ching TR, Van Dijk H, Labadie RP. Metabolic activation of natural phenols into selective oxidative burst agonists by activated human neutrophils. Free Radic Biol Med. 1990;8:251–258. doi: 10.1016/0891-5849(90)90070-y. [DOI] [PubMed] [Google Scholar]
- Sorce S, Krause K-H. NOX enzymes in the central nervous system: from signaling to disease. Antioxid Redox Signal. 2009;11:2481–2504. doi: 10.1089/ars.2009.2578. [DOI] [PubMed] [Google Scholar]
- Suh SW, Shin BS, Ma H, Van Hoecke M, Brennan AM, Yenari MA, Swanson RA. Glucose and NADPH oxidase drive neuronal superoxide formation in stroke. Ann Neurol. 2008;64:654–663. doi: 10.1002/ana.21511. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terashima T, Namura S, Hoshimaru M, Uemura Y, Kikuchi H, Hashimoto N. Consistent injury in the striatum of C57BL/6 mice after transient bilateral common carotid artery occlusion. Neurosurgery. 1998;43:900–907. doi: 10.1097/00006123-199810000-00102. [DOI] [PubMed] [Google Scholar]
- Touyz RM. Apocynin, NADPH oxidase, and vascular cells. A complex matter. Hypertension. 2008;51:172–174. doi: 10.1161/HYPERTENSIONAHA.107.103200. [DOI] [PubMed] [Google Scholar]
- Vejražka M, Míček R, Štípek S. Apocynin inhibits NADPH oxidase in phagocytes but stimulates ROS production in non-phagocytic cells. Biochim Biophys Acta. 2005;1722:143–147. doi: 10.1016/j.bbagen.2004.12.008. [DOI] [PubMed] [Google Scholar]
- Walder CE, Green SP, Darbonne WC, Mathias J, Rae J, Dinauer MC, Curnutte JT, Thomas GR. Ischemic stroke injury is reduced in mice lacking a functional NADPH oxidase. Stroke. 1997;28:2252–2258. doi: 10.1161/01.str.28.11.2252. [DOI] [PubMed] [Google Scholar]
- Wang Q, Tompkins KD, Simonyi A, Korthuis RJ, Sun AY, Sun GY. Apocynin protects against global cerebral ischemia-reperfusion-induced oxidative stress and injury in the gerbil hippocampus. Brain Res. 2006;1090:182–189. doi: 10.1016/j.brainres.2006.03.060. [DOI] [PubMed] [Google Scholar]
- Zhang Q-G, Raz L, Wang R, Han D, De Sevilla L, Yang F, Vadlamudi RK, Brann DW. Estrogen attenuates ischemic oxidative damage via an estrogen receptor α-mediated inhibition of NADPH oxidase activation. J Neurosci. 2009;29:13823–13836. doi: 10.1523/JNEUROSCI.3574-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JPQ, Xu W, Angulo JA. Methamphetamine-induced cell death: selective vulnerability in neuronal subpopulations of the striatum in mice. Neuroscience. 2006;140:607–622. doi: 10.1016/j.neuroscience.2006.02.055. [DOI] [PMC free article] [PubMed] [Google Scholar]