Abstract
Context
Lung cancer is one of the most common cancers in the U.S. and is associated with high levels of symptoms including pain, fatigue, shortness of breath, and psychological distress. Caregivers as well as patients are adversely affected. However, previous studies of coping skills training (CST) interventions have not been tested in patients with lung cancer nor systematically included caregivers.
Objectives
This study tested the efficacy of a caregiver-assisted CST protocol in a sample of patients with lung cancer.
Methods
Two hundred thirty-three lung cancer patients and their caregivers were randomly assigned to receive 14 telephone-based sessions of either caregiver-assisted CST or education/support involving the caregiver. Patients completed measures assessing pain, psychological distress, QOL, and self-efficacy for symptom management; caregivers completed measures assessing psychological distress, caregiver strain, and self-efficacy for helping the patient manage symptoms.
Results
Patients in both treatment conditions showed improvements in pain, depression, QOL, and self-efficacy and caregivers in both conditions showed improvements in anxiety and self-efficacy from baseline to four-month follow-up. Results of exploratory analyses suggested that the CST intervention was more beneficial to patients/caregivers with Stage II and III cancers, whereas the education/support intervention was more beneficial to patients/caregivers with Stage I cancer.
Conclusion
Taken together with the broader literature in this area, results from this study suggest that psychosocial interventions can lead to improvements in a range of outcomes for cancer patients. Suggestions for future studies include the utilization of three-group designs (e.g., comparing two active interventions to a standard-care control) and examining mechanisms of change.
Keywords: Psychosocial interventions, lung cancer, caregivers
Introduction
Lung cancer is the second most common cancer in the United States and also the leading cause of cancer deaths (1). Currently, about half of all lung cancer patients are diagnosed at early stages (Stages I to III). Prognosis is generally poor, with five-year survival rates ranging from 54% for Stage I disease to 10% for Stage III disease (1). Despite the relatively poor prognosis, there are currently over 400,000 lung cancer survivors in the U.S. (1). These patients and their caregivers face significant challenges of coping with symptoms such as pain, fatigue, breathlessness, and psychological distress (2-4) which can persist for months or years following treatment (5-7). In addition, patients with lung cancer report higher levels of distress than patients with other types of cancer (8, 9).
Recent reviews and meta-analyses have supported the efficacy of psychosocial interventions in helping cancer patients symptoms, and psychological distress (10-14). The terms “psychological,” “psychosocial,” and “psychoeducational” have been used to refer to a range of interventions including education, relaxation, guided imagery, music, hypnosis, coping skills training, and supportive counseling (11, 13-15). While few studies have directly compared different intervention strategies, some evidence from meta-analyses suggests that coping skills training (CST) protocols based on cognitive-behavioral principles are among the most effective (10, 14, 16). These interventions are explicitly designed to help patients alter cancer-relevant thoughts, emotions, and behaviors through training in coping skills such as relaxation, cognitive restructuring, and problem solving. Studies have shown that, after completing CST interventions, patients report significant reductions in cancer symptoms as well as improvements in QOL, psychological distress, self-efficacy, and coping (10-14).
Despite the promise of CST interventions in helping cancer patients manage pain, symptoms, and distress, to our knowledge no studies have tested the efficacy of these interventions specifically in lung cancer patients. In addition, although there have been a number of interventions directed at caregivers of cancer patients (17-19), there have been few interventions to include both patients and caregivers together. There has been increasing recognition of the impact of caregiving on family caregivers (17, 20-22) and a number of interventions have been developed specifically for caregivers (17-19). The majority of these interventions have focused on either providing caregivers with information (23, 24) or psychological services such as supportive counseling (25) or problem-solving (26, 27). However, few studies have involved both caregivers and patients together in CST interventions. Doing so may be particularly beneficial for a number of reasons. First, training both patient and caregiver in coping skills together may lead to enhanced communication between the patient and caregiver regarding symptoms and symptom distress, resulting in more efficacious treatment. Second, when caregivers learn coping skills along with the patient they may be more likely to prompt and reinforce the patient’s use of learned skills, leading to enhanced effects for patients. A caregiver’s prompting and reinforcement may be especially useful in helping the patient overcome obstacles to applying coping skills such as a flare in breathlessness, pain symptoms, fatigue, or the onset of a new symptom. Finally, involving caregivers coping skills training may enhance caregivers’ confidence (i.e., self-efficacy) in their ability to help the patient cope with symptoms, and help them manage their own distress.
The current study tested the efficacy of a new, caregiver-assisted CST protocol for patients with early stage lung cancer. We hypothesized that, compared to an education/support control condition, caregiver-assisted CST would lead to (a) improvements in patient outcomes including symptoms, psychological distress, QOL, and self-efficacy for managing symptoms; and (b) improvements in caregiver outcomes including caregiver strain, psychological distress, and self-efficacy for helping the patient manage symptoms. We also conducted exploratory analyses examining whether the intervention effects varied as a function of cancer stage.
Methods
Participants and Setting
Participants were recruited from the Duke University Thoracic Oncology Program as well as several community oncology clinics in the Durham, NC area between December 2002 and March 2005. The entry criteria for patients included having (a) a diagnosis of early stage lung cancer (non-small cell lung cancer Stages I-III, or limited stage small cell lung cancer), (b) no other cancers in the past five years, (c) ability to read and speak English, and (d) a caregiver who was also willing to participate. Patients were recruited at any point in the illness trajectory, from the time of diagnosis through post-treatment. “Caregiver” was broadly defined in this study as any friend or family member who provided practical and/or emotional support to the patient. To identify the patient’s primary caregiver, the patient was asked to list the people they relied on for support with things like getting to the doctor and taking medication. They were then asked to review this list and identify the main person they relied on for support. This person was identified as the primary caregiver and was invited to participate in the study.
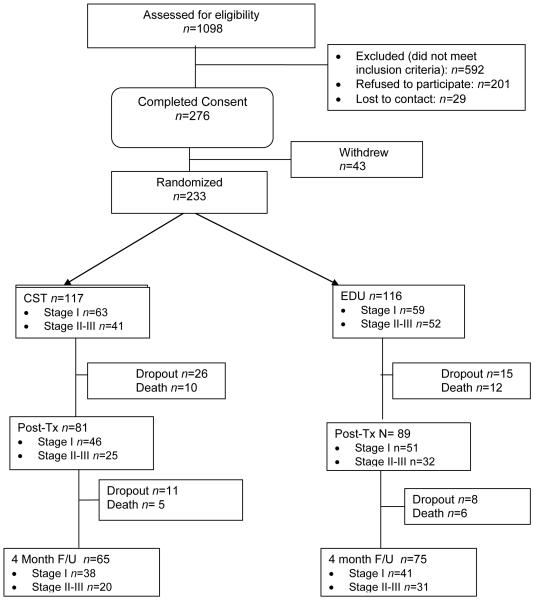
One thousand ninety-eight patients were screened for inclusion. Of these, 592 were deemed ineligible and 506 were approached about participation. Of those approached, 201 (39.7%) declined, 276 (54.5%) consented, and 29 (5.7%) were lost to contact. The most common reasons for declining included lack of interest (42%), “too much trouble” (6%), and lack of time (6%). Of the 276 who consented, 43 dropped out prior to completing baseline questionnaires primarily because of death/declining health (47%), or loss of time or interest (41%). This left a sample of 233 dyads that were included in the current analyses (Figure 1).
Figure 1.
CONSORT flowchart.
Procedures
All participants completed a pre-treatment evaluation and were then randomly assigned to one of two conditions: (a) caregiver-assisted CST (n=117), or (b) cancer education/ support including the caregiver (n=116). Randomization assignments were generated by an individual not involved in the study using a random number table. Assignments were concealed in envelopes that were not opened until participants had completed their pre-treatment evaluation. All participants completed a post-treatment assessment immediately following completion of the treatment sessions and a four-month follow-up assessment. All assessments were conducted over the telephone. Research assistants asked participants the questions and recorded their answers on paper questionnaires. The research assistants conducting the assessments were trained in standardized procedures for administration of questionnaires using didactic instruction and role-plays of common scenarios. They were kept blind to treatment condition. Medical information for the patients, including cancer stage, treatments, and date of diagnosis, were extracted from the medical record. All patients continued with their regular medical care and were followed by their oncologist in an ongoing fashion. This care included medical and surgical treatments, as well as educational information offered during clinic appointments and/or in form of written materials. All procedures were approved by the Duke University Medical Center Institutional Review Board and the Duke Comprehensive Cancer Center Protocol Review Committee.
Treatment Fidelity
Several steps were taken to ensure that the treatment protocols were uniform and that the therapists followed the treatment protocols in a uniform manner. These included therapist training, use of a detailed treatment outline, audiotaping of sessions, weekly supervision of therapists, and assessments of treatment adherence and therapist competence.
General Aspects of Treatment Procedures
Participants in both treatment conditions (Caregiver-assisted CST and Education/Support) participated in 14 45-minute, telephone-based sessions. Sessions were conducted via telephone rather than in person in order to facilitate participation of dyads who lived at a distance from the medical center and patients who were experiencing debilitating symptoms. All sessions were conducted with individual patient-caregiver dyads (rather than in groups), and all participants were provided with speaker phones to facilitate conducting the phone sessions simultaneously with both members of the dyad. All sessions were audiotaped for purposes of supervision. The sessions were scheduled over an eight-month period. The frequency of sessions was tapered from weekly (sessions 1-3) to biweekly (sessions 4-10) to monthly (sessions 11-14) in order to promote skill acquisition and application to symptoms that emerged over the course of the illness trajectory.
Therapist Training
Both treatment conditions were delivered by registered nurses. Nurses were trained by PhD-level psychologists (L.S.P., F.J.K., D.H.B, and D.C.M.,) and medical oncologists (J.G. and L.S.) using didactic instruction, taped illustrations of techniques from model cases, and role-play of common scenarios. Following the initial training, nurses received ongoing weekly supervision with one of the psychologists who reviewed audiotapes of the sessions and provided feedback regarding treatment quality and adherence to the study protocol. Given the variability in factors such as the patient’s symptom severity, types of treatments received, and time since diagnosis, particular attention was given to helping therapists identify the specific needs of each dyad and apply the information and skills training accordingly.
Caregiver-Assisted Coping Skills Training
Participants in this condition received training in symptom management strategies. Sessions were supplemented with written materials (e.g. handouts), provided to the participants in advance of the sessions, that highlighted the major points discussed in the sessions and detailed home practice assignments. Participants also received a CD (or audiotape) with instructions for progressive muscle relaxation (written materials are available from the first author upon request). The primary goals of the intervention were: (a) to teach the patients and caregivers a variety of coping strategies that are effective in managing pain, fatigue, shortness of breath, psychological distress, and other symptoms of lung cancer and its treatment, and (b) to teach the caregiver how to help the patient acquire and maintain coping skills over the illness trajectory. A summary of the coping skills included in the sessions is presented in Table 2.
Table 2.
Summary of Topics Covered in Each Treatment Condition
| Coping Skills Training | Education/Support |
|---|---|
|
|
In the first session, the nurse introduced caregiver-assisted CST as a method to help participants better manage the symptoms associated with lung cancer and its treatment. The coping skills were described as methods that could help in managing the patient’s current identified symptoms, as well as managing and minimizing the effects of other symptoms that the patient may experience during the illness trajectory. The caregiver’s role was described as that of “coach,” with the goal of helping the patient learn the coping skills and apply them on a day-to-day basis. Caregivers were encouraged to learn and practice skills in order to better assist the patient as well as to manage their own stress. In sessions 1-7, the patient and caregiver were trained in specific coping skills including progressive muscle relaxation training, a brief relaxation procedure (mini-practices), pleasant imagery, an activity pacing method (activity-rest cycling), cognitive restructuring, strategies for problem solving, goal setting and pleasant activity scheduling, and strategies for effective communication of thoughts and feelings. In teaching each skill, the nurse used a three-step behavioral rehearsal procedure in which instruction was provided in the skill, the dyad practiced the skill together, and the nurse provided feedback to the dyad on their practice. Each session started with a review of the patient’s and caregiver’s practice of the previous sessions’ skills and ended with discussion of a home practice assignment. Sessions 8-12 focused on training in alternative versions of relaxation and imagery exercises, as well as application of the coping skills to the particular challenges faced by the patient and/or caregiver. In session 8, patients who had not yet quit smoking were also given the alternative of discussing strategies for smoking cessation, including the caregiver’s role in helping the patient in his/her attempts to quit. Session 11 was focused specifically on the caregiver, encouraging the caregiver to explore his/her own resources and sources of stress and discussing how to use coping skills to ease the burden of caregiving. (The patient participated in this session as well.) Sessions 13 and 14 focused on maintenance strategies including a review of the coping skills learned and how to maintain a regular practice of skills in order to prevent and cope with possible setbacks.
Education/Support Condition
The primary goal of this intervention was to provide participants with information regarding lung cancer and its treatment in a supportive environment in which patients and caregivers were encouraged to discuss the patient’s treatment and symptoms. Caregivers were encouraged to participate fully in all discussions. Sessions were supplemented with handouts summarizing the major points and listing additional resources (e.g., websites, books) that the participants could access if they desired further information about a topic (again, written materials are available from the first author upon request). Table 2 includes an overview of the topics covered in the sessions. Participants in this condition did not receive any training in coping skills.
The education/support sessions were delivered to participants using a presentation and discussion format. Handouts and discussion sessions centered on presenting information on:
Description of lung cancer: information on the prevalence and incidence of the disease; risk factors and etiology; forms of lung cancer; diagnostic procedures, and symptomatology.
Treatment of lung cancer: goals of treatment were discussed including how staging is done and how prognosis is determined. Information about various treatments including surgery, chemotherapy, radiation therapy, medications, and alternative types of treatment were discussed. Also included were the side effects of the various treatment modalities and how these side effects are treated medically.
Understanding the physical aspects of lung cancer and how they are managed: information about metastasis of the cancer, nutritional needs, physical comfort measures, and medical approaches to pain and symptom management.
Palliative vs. curative care: information on palliative care and how palliative care differs from curative treatment. Hospice care was discussed.
Measures
All evaluation measures were collected through a telephone interview. At each evaluation session (baseline, post-test, and four-month follow-up), measures were collected from both patient and caregiver.
Measures Collected from Patients
Pain
Pain was assessed using two items from the Brief Pain Inventory (BPI) (28) in which participants rate their usual pain and their worst pain in the past week on a scale from 1 (“no pain”) to 10 (“pain as bad as you can imagine”). The worst and usual BPI pain intensity ratings have demonstrated good test-retest reliability (worst, r=0.93; usual, r=0.78) (29). The validity of the BPI has also been supported by studies that have shown a significant relationship between higher pain ratings and increased analgesic and narcotic use (29).
Psychological Distress
Psychological distress was assessed using the Beck Depression Inventory (BDI) (30) and the trait anxiety version of the State Trait Anxiety Inventory (STAI) (31). The BDI is a 21-item self-report inventory assessing current degree of depression through items pertaining to affective, cognitive, motivational, and physiologic areas of depressive symptomatology. The BDI has high internal consistency in clinical and nonclinical populations, and good discriminant, construct, and concurrent validity (30). The possible range on the BDI is from 0 to 63. Cronbach’s alpha in the current study was 0.86. The STAI was developed as a tool for investigating anxiety in normal (non-psychiatric) adults, but has been used in assessing anxiety in neuropsychiatric, medical, and surgical patients. The scale has demonstrated good psychometric properties (31). The possible range on the STAI is from 20 to 80. Cronbach’s alpha in the current study was 0.92.
Quality of Life (QoL)
QoL was measured using the Functional Assessment of Cancer Therapy – Lung Cancer (FACT-L) (32). The FACT-L consists of four general and one lung cancer symptom-specific subscale. The present study utilized three general subscales (physical well-being, functional well-being, and social well-being) along with the lung cancer specific subscale which includes items assessing shortness of breath, coughing, weight loss, and loss of appetite. These subscales were chosen as they assess constructs that are conceptually distinct from those assessed by the other measures used in this study (with the exception of one item of the physical well-being subscale assessing pain). The FACT is widely used in cancer studies and both the general measure and the lung cancer specific measure have been shown to possess adequate psychometric properties (32, 33). The possible range on each scale is from 0 to 28. Cronbach’s alphas in the present study were 0.85 (physical well-being), 0.86 (functional well-being), 0.70 (social well-being), and 0.73 (lung cancer symptoms).
Self-Efficacy
Self-efficacy for managing pain, symptoms, and function was assessed with a modified version of a standard self-efficacy scale (34). The original scale was modified by removing nine items relevant to patients with arthritis but not cancer, and adding seven items regarding management of pain (from the Chronic Pain Self-Efficacy scale) (35) and other common cancer symptoms such as shortness of breath. Patients rated 16 items regarding their perceived ability to manage a variety of symptoms on a scale of 10 (not at all certain) to 100 (completely certain). The scale contains three subscales: self-efficacy for managing pain, self-efficacy for managing other symptoms (e.g., fatigue, nausea, depression), and self-efficacy for function. Because the three subscales were highly correlated with each other (r’s=0.71-0.86 for patients; 0.80-0.86 for caregivers), the total score was utilized for this report (possible range=10-100). Prior studies using this instrument to assess self-efficacy in cancer patients and their caregivers have demonstrated evidence of its internal consistency and construct validity (36, 37). Cronbach’s alpha for the total scale score was 0.95.
Measures Collected from Caregivers
Caregiver Mood
Caregiver mood was assessed using a brief version of the Profile of Mood States-B (POMS-B(38)). Eighteen adjectives were used to rate average mood on scales from 0=very much unlike this to 3=very much like this. These items were selected from the larger number appearing on the POMS-B because of their high item-total correlations for their respective subscales (39). The POMS-B has six subscales: tension/anxiety (range 0-36), depression (range 0-60), anger/hostility (range 0-48), vigor/activity (range 0-32), fatigue (range 0-28), and confusion/bewilderment (range 0-28). A total mood disturbance scale is computed as a sum of all of the subscales (range 0-200). Cronbach’s alpha for the total mood disturbance score was 0.89.
Caregiver Strain
Caregiver strain was assessed with the Caregiver Strain Index (CSI) (40), a 13-item scale that assesses a variety of stressors commonly experienced by caregivers. The CSI has demonstrated high internal consistency (41) and construct validity (40). The possible range on the CSI is from 0 to 13. In the current study, Cronbach’s alpha was 0.84.
Caregivers’ Self-Efficacy in Symptom Management
To assess caregivers’ confidence regarding their ability to help the patient manage symptoms, a modified version of a standard self-efficacy scale (34) was used. The caregiver version of the instrument is identical to that used with patients except that caregivers are asked to rate how confident they are that they can help the patient manage symptoms (e.g., “How certain are you that you can help the patient decrease his/her pain quite a bit?,” “How certain are you that you can do something to help the patient feel better if he/she is feeling blue?”). Cronbach’s alpha for the total scale score was 0.96.
Statistical Analyses
Data were analyzed by intent-to-treat. Hierarchical linear modeling (HLM) (42) was used to evaluate group differences over time. Each model estimated time, intervention (coping skills training versus education/support), and time X intervention effects. The time effect assessed whether the outcome changed across the baseline, post-test, and four-month follow-up assessments. Significant effects of the intervention would be indicated by a significant time X intervention effect.
In addition, we were interested in whether the effects of the interventions differed according to the severity of the patient’s illness. Despite the fact that all patients in the study were diagnosed with what is defined as “early stage” lung cancer, there was a great deal of variability in the severity of their illness. For example, patients with Stage I cancers were often asymptomatic at diagnosis, treated with surgery only, and told that their disease had been cured. In contrast, patients with Stage II or III cancers were often quite ill at the time of diagnosis, underwent multiple aggressive treatments including surgery, chemotherapy, and/or radiation over a period of many months, and were given a much more guarded prognosis. In order to determine whether patients with Stage I versus Stage II and III cancers and their caregivers responded differently to the two interventions, we conducted exploratory moderator analyses. These models estimated the effects of time, intervention, cancer stage, time X intervention, time X cancer stage, intervention X cancer stage, and time X intervention X cancer stage (Stage I versus Stage II-III). The three-way interaction (time X intervention X cancer stage), if significant, would indicate that the effect of the interventions over time varied according to the between patients with Stage I cancer (and their caregivers) and those with Stage II-III cancer.
Examination of the distribution of the outcome variables indicated that a number of them were skewed including the patient’s usual and worst pain, physical, social, and emotional well-being, and depressive symptoms. These variables were log transformed, and analyses were performed with the raw and log transformed values. The results of both sets of analyses were identical, thus raw values were used in results reported below.
Results
Participant Characteristics
Participant characteristics are presented in Table 1. Slightly more than half of the patients (52.8%) were men, and the majority of caregivers (69%) were women. Both patients and caregivers were predominantly Caucasian and well-educated. Seventy-six percent of the caregivers were spouses of the patient, 14% were sons or daughters, and 8% were sisters, brothers, and friends. In most cases (73%), the patients and caregivers lived in the same household. There were no significant differences between patients and caregivers randomized to the CST group and those randomized to education/support in demographic variables (e.g., age, gender, race) or medical variables (e.g., days since diagnosis, receipt of chemotherapy and radiation therapy, cancer stage).
Table 1.
Participant Characteristics
| Patients (n=233) |
Caregivers (n=233) |
|
|---|---|---|
| Mean age (SD) | 65.3 (9.5) | 59.3 (12.3) |
| Gender (% male) | 52.8 | 31.0 |
| Race | ||
| Caucasian | 84.5% | 82.0% |
| African American | 11.6% | 11.2% |
| Other/unknown | 3.9% | 6.4% |
| Education | ||
| <12 years | 14.2% | 8.2% |
| High school graduate | 30.9% | 32.3% |
| Some college | 29.6% | 30.2% |
| College graduate | 16.3% | 13.8% |
| Post-graduate | 9.0% | 15.5% |
| Median days since diagnosis (IQR) a | 207.5 (668) | |
| 25th percentile | 105 | |
| 75th percentile | 773 | |
| Cancer treatments | ||
| Surgery | 80.5% | |
| Chemotherapy | 39.5% | |
| Radiation | 26.2% | |
| Cancer stage b | ||
| Non-small cell Stage I | n=122 (52.6%) | |
| Non-small cell Stage II | n=37 (15.9%) | |
| Non-small cell Stage III | n=60 (25.9%) | |
| Small cell limited stage | N=10 (4.3%) | |
IQR = interquartile range.
Date of diagnosis was not available for three patients.
Staging information was not available for four patients.
Patient Outcomes
Analyses of patient outcome measures indicated significant main effects of time for ratings of worst pain (B=−0.15, SE=0.13, P=0.02), physical well-being (B= 0.84, SE=0.22, P=0.0002), functional well-being (B=0.55, SE=0.22, P=0.03), lung cancer symptoms (B=0.76, SE=0.21, P=0.0003), depression (B=−0.55, SE=0.28, P=0.05), and self-efficacy (B=2.31, SE=1.03, P=0.02). Patients in both CST and education/support reported improvements over time in their worst pain ratings, their physical and functional well-being, their lung cancer symptoms, their depressive symptoms, and their self-efficacy for controlling symptoms. There were no significant time X intervention interactions.
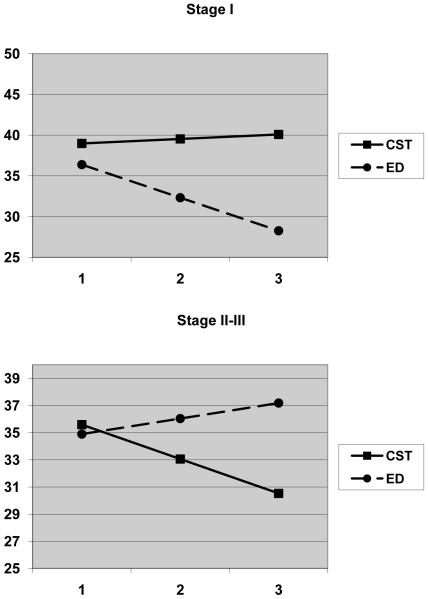
Exploratory moderator analyses were conducted by dichotomizing patient’s cancer stage (I vs. II and III) and including the three-way interaction term (time X intervention X cancer stage) in the equations. The three-way interactions were significant for self-efficacy (B=6.26, SE=3.20, P=0.05), depressive symptoms (B=−2.38, SE=0.86, P=0.006), trait anxiety (B=−8.28, SE=2.85, P=0.005), and the functional well-being subscale of the FACT-L (B=2.22, SE=0.80, P=0.006). These interactions were graphed according to the strategies recommended by Preacher et al. (43). Similar patterns of findings were observed for all four of the outcome variables (trait anxiety, depressive symptoms, self-efficacy, and functional well-being). In each case, it appeared that patients with Stage I cancer benefited more from the education/support intervention and patients with Stage II-III cancer benefited more from CST intervention. The graph for trait anxiety is presented in Figure 2 as an example.
Figure 2.
Estimated trajectories of change in the patients’ scores on the trait version of the State Trait Anxiety Inventory by treatment condition and lung cancer stage.
Caregiver Outcomes
Analyses of caregiver outcome measures indicated significant main effects of time for the anxiety subscale of the POMS-B (B=−0.21, SE=0.21, P=0.02), and self efficacy (B=2.39, SE=0.97, P=0.01). Caregivers in both CST and education/support reported decreases in anxiety and increases in their self-efficacy for helping the patient manage symptoms. There were no significant time X intervention interactions.
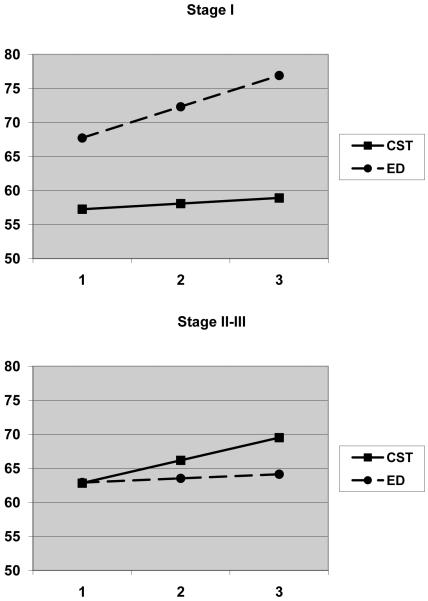
Moderator analyses were conducted by dichotomizing patient’s cancer stage (I vs. II and III) and including the three-way interaction term (time X intervention X cancer stage) in the equations. The three-way interactions were significant for caregiver strain (B=−0.99, SE=0.50, P=0.05) and self-efficacy (B=6.48, SE=3.03, P=0.03). These interactions were graphed according to the strategies recommended by Preacher et al. (43). As with the patient outcomes, the pattern of results for both caregiver strain and self-efficacy suggested that caregivers of patients with Stage I cancer benefited more from the education/support intervention while caregivers of patients with Stage II-III cancer benefited more from CST intervention. The graph for self-efficacy is presented in Figure 3 as an example.
Figure 3.
Estimated trajectories of change in the caregivers’ scores on the self-efficacy scale by treatment condition and the patient’s lung cancer stage.
Discussion
To our knowledge, the current study is the first randomized clinical trial investigating the efficacy of psychosocial interventions focused specifically on lung cancer patients. Lung cancer is one of the most common yet understudied types of cancer and is associated with particularly high levels of symptoms and distress, thus it is particularly important to develop effective supportive care interventions for these patients (44). This study is also one of the few rigorous tests of psychosocial protocols for cancer patients that systematically included caregivers in the interventions and that compared two active interventions. The first, caregiver-assisted CST, was based on cognitive-behavioral principles and taught patients a variety of coping skills for managing their symptoms while teaching their partners how to assist them in acquiring and maintaining these skills. The comparison condition was an education/support intervention that provided patients and caregivers with educational information about lung cancer and its management and as well as opportunities to ask questions and discuss concerns related to the patient’s disease.
The main finding of this study was that both caregiver-assisted CST and the education/support intervention were associated with improvements for lung cancer patients and their caregivers. Patient improvements were seen in a variety of domains including their pain, depression, physical well-being and lung cancer symptoms, their functional well-being, and their self-efficacy for controlling symptoms. Caregivers reported increases in their self-efficacy for helping the patients control symptoms and decreases in anxiety. Because this study lacked a no treatment or standard care control condition, it is not possible to draw definitive conclusions about the impact of these two interventions. It is possible that the effects obtained could be due to time. This seems unlikely, however, given that recent research has documented the severity and persistence of symptoms and psychological distress experienced by lung cancer patients (2, 6, 7, 9, 44, 45).
The findings obtained in this study are consistent with previous studies that have found CST interventions (10, 16) and educational interventions (10, 13, 14) effective in helping cancer patients reduce symptoms and distress. To our knowledge, no previous studies have directly compared CST and education/support interventions. In fact, the majority of previous studies testing the effects of psychosocial interventions with cancer patients have not utilized any active control condition (e.g., 46-52). The findings of the current study suggest that a comprehensive caregiver-CST intervention produced similar results to an education/support intervention involving the caregiver. This raises questions regarding the active ingredients of the interventions (e.g., training in coping skills versus providing information), or whether the improvements can be attributed to non-specific therapeutic effects (e.g., time and attention). Ideally, future studies testing psychosocial interventions should utilize a three group design (e.g., CST versus education/support versus usual care) in order to better answer these questions.
Another important question is which intervention works best for which patients and caregivers. Because “early stage” lung cancer encompasses patients with a wide range of illness severity, we examined in exploratory analyses whether the patient’s cancer stage moderated the treatment effects. We found a pattern of results that suggested the caregiver-assisted CST condition was most beneficial to patients with Stage II and III cancers and their caregivers, whereas the education/support intervention was more beneficial to patients with Stage I cancer and their caregivers. Among patients with Stage II and III cancers, those who received caregiver-assisted CST were more likely to experience increases in self-efficacy and physical function and decreases in depressive symptoms and anxiety than those who received education/support. Caregiver-assisted CST also appeared to be helpful for caregivers of patients with Stage II and III cancers as they showed increases in self-efficacy and decreases in caregiver strain in response to this intervention. In contrast, patients with Stage I cancers and their caregivers were more likely to demonstrate improvements in response to the education/support intervention than to the caregiver-assisted CST intervention.
There are several possible explanations for these differences in response. First, the caregiver-assisted CST intervention may be much more salient to patients with Stage II and III cancer and their caregivers as they are more likely to be experiencing disease-related symptoms and may be more cognizant of the poor prognosis associated with the patient’s disease. Second, because patients with Stage II and III cancers and their caregivers are dealing with persistent symptoms, they have more opportunities to apply coping skills to symptom management and to experience that these skills can actually help them control symptoms. In contrast, patients with Stage I disease may be asymptomatic and feel quite optimistic about their long-term prognosis. Because they and their caregivers are coping with fewer treatment, symptoms, and stressors, they may be less likely to see the relevance of learning and applying coping skills or to benefit from this approach. An education/support intervention may be better matched to these patients and caregivers because it provides them with helpful information in a supportive manner, but unlike caregiver-assisted CST, places few demands on them to change their behavior or consider the possibility of future cancer-related challenges. The type of detailed education provided in this intervention may be beneficial to these individuals by alleviating concerns that come from lack of understanding.
The results from this study suggest several potential avenues for future research. First, future studies could examine the possibility that a stepped approach to intervention leads to the greatest benefits for the greatest number of patients and caregivers. Second, future studies could examine whether patients with early stage disease and their caregivers may benefit from briefer interventions. Third, future studies could compare the efficacy of interventions targeted to patient-caregiver dyads to those delivered to caregivers alone. Finally, future studies should be designed to examine the mechanisms contributing to the efficacy of various interventions. Developing a better understanding of the critical elements of interventions and how they operate will enhance our ability to optimize therapeutic effects and translate intervention research into clinical practice (53). To date, few studies have examined therapeutic processes in the context of psychosocial interventions for cancer patients.
This study had several limitations. First, of those approached to participate, approximately 54% chose to do so. While this participation rate is lower than that reported in some psychosocial intervention studies (46-48, 51, 52), it is somewhat higher than that obtained in other studies which required the participation of a caregiver or spouse (range 34% (50, 54) to 43% (49)). A second limitation of the study was the attrition rate (27% at post-test and 40% at four-month follow-up). However, most of the attrition in both arms was due to the patient’s declining health or death. Interestingly, in prior studies researchers have noted lower rates of retention for patients with lung cancer relative to patients with other types of cancer (50), and lower rates of retention for lung cancer patients in the intervention versus standard care (49). A third limitation was that this study was conducted with patients who were primarily being seen in a tertiary care cancer center and who were predominantly Caucasian. It is not clear that the results obtained are generalizable to patients seen in community-based cancer treatment settings or those from other ethnic and cultural groups. Finally, patients in this study varied in their stage in the illness trajectory. Future studies should evaluate the optimal timing of CST and educational interventions (e.g., shortly after diagnosis, during active treatment, or following treatment in the survivorship phase).
This study adds to the existing literature on psychosocial interventions for cancer patients by its focus on patients with lung cancer. It is notable for the systematic inclusion of caregivers in the interventions, and for the comparison of two active treatment conditions. Results suggest that the interventions may have led to improvements in psychological distress, symptoms, and self-efficacy, although it is also possible these changes were due to time. Given the wide range of psychosocial interventions for cancer patients that have been tested and the few studies that have compared two active interventions, little is known about which interventions are most effective. There has also been little research examining therapeutic processes which would shed light on how interventions produce change. The next wave of studies in this field should strongly consider utilizing three-group designs (e.g., comparing two active interventions to a standard-care control) and planning for analyses of mechanisms of change. This will lead to significant contributions to the knowledge base as well as providing information that can be used more readily to enhance the clinical care of patients with cancer and their loved ones (53).
Disclosures and Acknowledgments
This work was supported by National Cancer Institute grant R01 CA91947.
The authors thank Heidi Suarez, BS, RN, Carole Cain, PhD, Lauren Portnow, and the physicians and staff of the Duke Thoracic Oncology clinic for assistance with participant recruitment, and all of the study participants for their time and effort.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society . Cancer facts and figures 2008. American Cancer Society; Atlanta: 2008. [Google Scholar]
- 2.Cooley ME. Symptoms in adults with lung cancer. A systematic research review. J Pain Symptom Manage. 2000;19(2):137–153. doi: 10.1016/s0885-3924(99)00150-5. [DOI] [PubMed] [Google Scholar]
- 3.Steinberg T, Roseman M, Kasymjanova G, et al. Prevalence of emotional distress in newly diagnosed lung cancer patients. Support Care Cancer. 2009;17(12):1493–1497. doi: 10.1007/s00520-009-0614-6. [DOI] [PubMed] [Google Scholar]
- 4.Sarna L. Lung cancer. In: Holland JC, editor. Psycho-oncology. Oxford University Press; New York: 1998. pp. 340–348. [Google Scholar]
- 5.Sarna L, Padilla G, Holmes C, et al. Quality of life of long-term survivors of non-small-cell lung cancer. J Clin Oncol. 2002;20(13):2920–2929. doi: 10.1200/JCO.2002.09.045. [DOI] [PubMed] [Google Scholar]
- 6.Akechi T, Okuyama T, Akizuki N, et al. Course of psychological distress and its predictors in advanced non-small cell lung cancer patients. Psycho-oncology. 2006;15(6):463–473. doi: 10.1002/pon.975. [DOI] [PubMed] [Google Scholar]
- 7.Sugimura H, Yang P. Long-term survivorship in lung cancer: a review. Chest. 2006;129(4):1088–1097. doi: 10.1378/chest.129.4.1088. [DOI] [PubMed] [Google Scholar]
- 8.Degner LF, Sloan JA. Symptom distress in newly diagnosed ambulatory cancer patients and as a predictor of survival in lung cancer. J Pain Symptom Manage. 1995;10(6):423–431. doi: 10.1016/0885-3924(95)00056-5. [DOI] [PubMed] [Google Scholar]
- 9.Zabora J, Brintzenhofeszoc K, Curbow B, Hooker C, Piantadosi S. The prevalence of psychological distress by cancer site. Psycho-oncology. 2001;10:19–28. doi: 10.1002/1099-1611(200101/02)10:1<19::aid-pon501>3.0.co;2-6. [DOI] [PubMed] [Google Scholar]
- 10.Osborn R, Demoncada A, Feuerstein M. Psychosocial interventions for depression, anxiety, and quality of life in cancer survivors: meta-analysis. Int J Psychiatry Med. 2006;36(1):13–34. doi: 10.2190/EUFN-RV1K-Y3TR-FK0L. [DOI] [PubMed] [Google Scholar]
- 11.Sheard T, McGuire P. The effect of psychological interventions on anxiety and depression in cancer patients: results of two meta-analyses. Br J Cancer. 1999;80:1770–1780. doi: 10.1038/sj.bjc.6690596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Andrykowski MA, Manne SL. Are psychological interventions effective and accepted by cancer patients. I. Standards and levels of evidence. Ann Behav Med. 2006;32(2):93–97. doi: 10.1207/s15324796abm3202_3. [DOI] [PubMed] [Google Scholar]
- 13.Barsevick AM, Sweeney C, Haney E, Chung E. A systematic qualitative analysis of psychoeducational interventions for depression in patients with cancer. Oncol Nurs Forum. 2002;29(1):73–84. doi: 10.1188/02.ONF.73-87. [DOI] [PubMed] [Google Scholar]
- 14.Devine EC. Meta-analysis of the effect of psychoeducational interventions on pain in adults with cancer. Oncol Nurs Forum. 2003;30(1):75–89. doi: 10.1188/03.ONF.75-89. [DOI] [PubMed] [Google Scholar]
- 15.Newell S, Sanson-Fisher R, Savolainen N. Systematic review of psychological therapies for cancer patients: overviews and recommendations for future research. J Natl Cancer Inst. 2002;94(8):558–584. doi: 10.1093/jnci/94.8.558. [DOI] [PubMed] [Google Scholar]
- 16.Graves K. Social cognitive theory and cancer patients’ quality of life: a meta-analysis of psychosocial intervention components. Health Psychol. 2006;22:210–219. [PubMed] [Google Scholar]
- 17.McCorkle R, Pasacreta JV. Enhancing caregiver outcomes in palliative care. Cancer Control. 2001;8:36–45. doi: 10.1177/107327480100800106. [DOI] [PubMed] [Google Scholar]
- 18.Harding R, Higginson IJ. What is the best way to help caregivers in cancer and palliative care? A systematic literature review of interventions and their effectiveness. Palliat Med. 2003;17:63–74. doi: 10.1191/0269216303pm667oa. [DOI] [PubMed] [Google Scholar]
- 19.Honea NJ, Brintnall R, Given B, et al. Putting evidence into practice: nursing assessment and interventions to reduce family caregiver strain and burden. Clin J Oncol Nurs. 2003;12(3):507–516. doi: 10.1188/08.CJON.507-516. [DOI] [PubMed] [Google Scholar]
- 20.Kim Y, Schulz R. Family caregivers’ strains: comparative analysis of cancer caregiving with dementia, diabetes, and frail elderly caregiving. J Aging Health. 2008;20:483–503. doi: 10.1177/0898264308317533. [DOI] [PubMed] [Google Scholar]
- 21.Kim Y, Kashy D, Wellish D, et al. Quality of life of couples dealing with cancer: dyadic and individual adjustment among breast and prostate cancer survivors and their spousal caregivers. Ann Behav Med. 2008;35:230–238. doi: 10.1007/s12160-008-9026-y. [DOI] [PubMed] [Google Scholar]
- 22.O’Mara A. Who’s taking care of the caregiver? J Clin Oncol. 2005;23:6820–6821. doi: 10.1200/JCO.2005.96.008. [DOI] [PubMed] [Google Scholar]
- 23.Ferrell BR, Grant M, Chan J, Ahn C, Ferrell BA. The impact of cancer pain education on family caregivers of elderly patients. Oncol Nurs Forum. 1995;22(8):1211–1218. [PubMed] [Google Scholar]
- 24.Pasacreta JV, Barg F, Nuamah I, McCorkle R. Participant characteristics before and 4 months after attendance at a family caregiver cancer education program. Cancer Nurs. 2000;23(4):295–303. doi: 10.1097/00002820-200008000-00007. [DOI] [PubMed] [Google Scholar]
- 25.Sabo D, Brown J, Smith C. The male role and mastectomy: support groups and men’s adjustment. J Psychosoc Oncol. 1986;4:19–31. [Google Scholar]
- 26.Houts PS, Nezu AM, Nezu CM, Butcher JA. The prepared family caregiver: a problem-solving approach to family caregiver education. Patient Educ Couns. 1996;27(1):63–73. doi: 10.1016/0738-3991(95)00790-3. [DOI] [PubMed] [Google Scholar]
- 27.Blanchard CG, Toseland RW, McCallion P. The effects of a problem solving intervention with spouses of cancer patients. J Psychosoc Oncol. 1997;14:1–21. [Google Scholar]
- 28.Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- 29.Daut RL, Cleeland CS, Flanery RC. Development of the Wisconsin Brief Pain Questionnaire to assess pain in cancer and other diseases. Pain. 1983;17(2):197–210. doi: 10.1016/0304-3959(83)90143-4. [DOI] [PubMed] [Google Scholar]
- 30.Beck AT, Beamesderfer A. Assessment of depression: the Depression Inventory. Mod Probl Pharmacopsychiatry. 1974;7:151–169. doi: 10.1159/000395074. [DOI] [PubMed] [Google Scholar]
- 31.Spielberger CD. Manual for the State-Trait Anxiety Inventory (Form Y) Consulting Psychologists; Palo Alto, CA: 1983. [Google Scholar]
- 32.Cella DF, Bonomi AE, Lloyd SR, et al. Reliability and validity of the Functional Assessment of Cancer Therapy-Lung (FACT-L) quality of life instrument. Lung Cancer. 1995;12(3):199–220. doi: 10.1016/0169-5002(95)00450-f. [DOI] [PubMed] [Google Scholar]
- 33.Cella DF, Tulsky DS, Gray G, et al. The Functional Assessment of Cancer Therapy scale: development and validation of the general measure. J Clin Oncol. 1993;11(3):570–579. doi: 10.1200/JCO.1993.11.3.570. [DOI] [PubMed] [Google Scholar]
- 34.Lorig K, Chastain RL, Ung E, Shoor S, Holman HR. Development and evaluation of a scale to measure perceived self-efficacy in people with arthritis. Arthritis Rheum. 1989;32(1):37–44. doi: 10.1002/anr.1780320107. [DOI] [PubMed] [Google Scholar]
- 35.Anderson KO, Dowds BN, Pelletz RE, Edwards WT, Peeters-Asdourian C. Development and initial validation of a scale to measure self-efficacy beliefs in patients with chronic pain. Pain. 1995;63(1):77–84. doi: 10.1016/0304-3959(95)00021-J. [DOI] [PubMed] [Google Scholar]
- 36.Porter LS, Keefe FJ, McBride CM, et al. Perceptions of patients’ self-efficacy for managing pain and lung cancer symptoms: correspondence between patients and family caregivers. Pain. 2002;98(1-2):169–178. doi: 10.1016/s0304-3959(02)00042-8. [DOI] [PubMed] [Google Scholar]
- 37.Porter L, Keefe F, Garst J, McBride C, Baucom D. Self-efficacy for managing pain, symptoms, and function in patients with lung cancer and their informal caregivers: associations with symptoms and distress. Pain. 2008;137(2):306–315. doi: 10.1016/j.pain.2007.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Lorr M, McNair D. Profile of Mood States-B. Educational and Industrial Testing Service; San Diego: 1982. [Google Scholar]
- 39.Affleck G, Tennen H, Pfeiffer C, Fifel J. Appraisals of control and predictability in adapting to a chronic diseases. J Pers Soc Psychol. 1988;52:273–279. doi: 10.1037//0022-3514.53.2.273. [DOI] [PubMed] [Google Scholar]
- 40.Robinson BC. Validation of a Caregiver Strain Index. J Gerontol. 1983;38(3):344–348. doi: 10.1093/geronj/38.3.344. [DOI] [PubMed] [Google Scholar]
- 41.Miaskowski C, Kragness L, Dibble S, Wallhagen M. Differences in mood states, health status, and caregiver strain between family caregivers of oncology outpatients with and without cancer-related pain. J Pain Symptom Manage. 1997;13(3):138–147. doi: 10.1016/s0885-3924(96)00297-7. [DOI] [PubMed] [Google Scholar]
- 42.Raudenbush SW, Bryk AS. Hierarchical linear models: Applications and data analysis methods. 2nd ed. Sage; Thousand Oaks, CA: 2002. [Google Scholar]
- 43.Preacher KJ, Curran PJ, Bauer DJ. Computational tools for probing interaction effects in multiple linear regression, multilevel modeling, and latent curve analysis. J Educ Behav Stat. 2006;31:437–448. [Google Scholar]
- 44.Schofield P, Ugalde A, Carey M, et al. Lung cancer: challenges and solutions for supportive care interventions. Palliat Support Care. 2008;6(3):281–287. doi: 10.1017/S1478951508000424. [DOI] [PubMed] [Google Scholar]
- 45.Graves KD, Arnold SM, Love CL, et al. Distress screening in a multidisciplinary lung cancer clinic: prevalence and predictors of clinically significant distress. Lung Cancer. 2007;55:215–224. doi: 10.1016/j.lungcan.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Allen SM, Shah AC, Nezu AM, et al. A problem-solving approach to stress reduction among younger women with breast carcinoma: a randomized controlled trial. Cancer. 2002;94(12):3089–3100. doi: 10.1002/cncr.10586. [DOI] [PubMed] [Google Scholar]
- 47.Antoni MH, Wimberly SR, Lechner SC, et al. Reduction of cancer-specific thought intrusions and anxiety symptoms with a stress management intervention among women undergoing treatment for breast cancer. Am J Psychiatry. 2006;163(10):1791–1797. doi: 10.1176/ajp.2006.163.10.1791. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Boesen EH, Ross L, Frederiksen K, et al. Psychoeducational intervention for patients with cutaneous malignant melanoma: a replication study. J Clin Oncol. 2005;23(6):1270–1277. doi: 10.1200/JCO.2005.05.193. [DOI] [PubMed] [Google Scholar]
- 49.Given C, Given B, Rahbar M, et al. Effect of a cognitive behavioral intervention on reducing symptom severity during chemotherapy. J Clin Oncol. 2004;22(3):507–516. doi: 10.1200/JCO.2004.01.241. [DOI] [PubMed] [Google Scholar]
- 50.Kurtz ME, Kurtz JC, Given CW, Given B. A randomized, controlled trial of a patient/caregiver symptom control intervention: effects on depressive symptomatology of caregivers of cancer patients. J Pain Symptom Manage. 2005;30(2):112–122. doi: 10.1016/j.jpainsymman.2005.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rawl SM, Given BA, Given CW, et al. Intervention to improve psychological functioning for newly diagnosed patients with cancer. Oncol Nurs Forum. 2002;29(6):967–975. doi: 10.1188/02.ONF.967-975. [DOI] [PubMed] [Google Scholar]
- 52.Andersen B, Farrar W, Golden-Drueutz D, et al. Psychological, behavioral, and immune changes after a psychological intervention: a clinical trial. J Clin Oncol. 2004;22:3570–3580. doi: 10.1200/JCO.2004.06.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kazdin A. Mediators and mechanisms of change in psychotherapy research. Annu Rev Clin Psychol. 2007;3:1–27. doi: 10.1146/annurev.clinpsy.3.022806.091432. [DOI] [PubMed] [Google Scholar]
- 54.Manne S, Ostroff J, Winkel G, et al. Couple-focused group intervention for women with early stage breast cancer. J Consult Clin Psychol. 2005;73(4):634–646. doi: 10.1037/0022-006X.73.4.634. [DOI] [PubMed] [Google Scholar]