Abstract
The medial septum and diagonal band (MSDB) are important in spatial learning and memory. Based on excitotoxic damage of GABAergic MSDB neurons, we have recently suggested a role for these neurons in controlling proactive interference. The present study sought to test this hypothesis in different behavioral procedures using a new GABAergic immunotoxin. GAT1-saporin (GAT1-SAP) was administered into the MSDB of male Sprague Dawley rats. Following surgery, rats were trained in a reference memory water maze procedure for 5 days, followed by a working memory (delayed match to position) water maze procedure. Other rats were trained in a lever press avoidance procedure after intraseptal GAT1-SAP or sham surgery. Intraseptal GAT1-SAP produced extensive damage of GABAergic while sparing most cholinergic MSDB neurons. Rats treated with GAT1-SAP were not impaired in acquiring a spatial reference memory, learning the location of the escape platform as rapidly as sham rats. In contrast, GAT1-SAP rats were slower than sham rats to learn the platform location in a delayed match to position procedure, in which the platform location was changed every day. Moreover, GAT1-SAP rats returned to previous platform locations more often than sham rats. In the active avoidance procedure, intraseptal GAT1-SAP impaired extinction but not acquisition of the avoidance response. Using a different neurotoxin and behavioral procedures than previous studies, the results of the present study paint a similar picture that GABAergic MSDB neurons are important for controlling proactive interference.
Keywords: hippocampus, learning, memory, acetylcholine, basal forebrain, diagonal band of Broca
The medial septum (MS) and diagonal band of Broca (DB) are major afferents to the hippocampus (Amaral and Kurz, 1985; Jakab and Leranth, 1995) and are critical for learning and memory (Kesner et al., 1986; Winson, 1978). Projections to the hippocampus arise mainly from cholinergic, GABAergic and glutamatergic neurons (Amaral and Kurz, 1985; Colom et al., 2005; Freund, 1989), and these projections may be important in the cognitive impairments associated with Alzheimer’s Disease and normal aging (Bartus et al., 1982; Coyle et al., 1983). For example, damage or inactivation of the MSDB impairs performance on a variety of learning and memory tasks (Givens and Olton, 1990; Kesner et al., 1986; Mizumori et al., 1990; Morris et al., 1982). However, selective damage of cholinergic neurons by the immunotoxin 192-IgG saporin results in milder or no impairment in similar tasks (Baxter et al., 1995; Berger-Sweeney et al., 1994; Chappell et al., 1998). These results implicate the importance of noncholinergic MSDB neurons in learning and memory.
The role of noncholinergic MSDB neurons in spatial learning and memory has been investigated using kainic acid. Kainic acid reduced markers of GABAergic neurons in the MSDB, such as glutamic acid decarboxylase (Malthe-Sorenssen et al., 1980) and parvalbumin (Pang et al., 2001; Yoder and Pang, 2005), which is localized in GABAergic septohippocampal neurons (Freund, 1989). In contrast, kainic acid largely spared cholinergic MSDB neurons (Malthe-Sorenssen et al., 1980; Pang et al., 2001; Yoder and Pang, 2005). Behaviorally, intraseptal kainic acid impaired a delayed match to position task (Dwyer et al., 2007) but not acquisition of spatial reference memory in a water maze or spatial working memory in a typical 8-arm radial maze procedure (Pang et al., 2001). In the delayed match to position procedure, rats treated with kainic acid returned to previous goal locations more often than saline treated rats, suggesting increased proactive interference.
In addition to its involvement in spatial learning and memory, the septum has been implicated in avoidance learning. Nonselective lesions of the septum facilitates two-way active avoidance (Kenyon and Krieckhaus, 1965), but impairs passive (Winocur and Mills, 1969) and one-way active avoidance (Olton, 1973). Evaluating the importance of the septum in avoidance learning is made difficult by the finding that septal lesions increase general locomotor activity (Osborne, 1994). In spite of the long history of septal research in avoidance learning, the importance of different medial septum neurons in avoidance learning has not been elucidated.
Although excitotoxins have been useful for investigating noncholinergic neurons of the MSDB, targeted neurotoxins for noncholinergic neurons would greatly stimulate research in this area. Recently, a novel selective GABAergic immunotoxin was used in the bed nucleus of the stria terminalis (Radley et al., 2009). The toxin combines a rabbit polyclonal antibody to the GABA-transporter-1 with the ribosomal toxin saporin (GAT1-saporin). In the present study, we characterized the effects of GAT1-saporin on the MSDB neurons and then used this immunotoxin to investigate the importance of GABAergic MSDB neurons in spatial and avoidance learning.
MATERIALS AND METHODS
Subjects
Male Sprague Dawley rats (n = 45) were 300 – 350 g at the start of the study. Twenty-five rats were tested for spatial learning and memory in the water maze. Another 20 rats were tested for open field activity and then trained in lever press avoidance. All rats were housed individually on a 12-h light/dark cycle with lights turning on at 7:00 a.m. Training and testing were performed during the light phase of the light/dark cycle. All procedures were conducted in accordance with the NIH Guide for the Care and Use of Laboratory Animals and were approved by the IACUC of the Veterans Affairs Medical Center at East Orange, New Jersey.
Surgery
Surgical procedures were as described previously (Pang et al., 2001). Rats were anesthetized with sodium pentobarbital. With leveled skull, the needle of a Hamilton syringe was inserted into the MS to produce a sham lesion or to administer GAT1-SAP (325 ng/µl, 0.5 µl, 0.1 µl/min). The MS coordinates were +0.6 mm and ±1.5 mm lateral from bregma, −6.6 mm from brain surface and 15° toward midline. The tip of the needle was also placed in each DB for sham lesions or GAT1-SAP injections (0.4 µl into each hemisphere); the DB coordinates were +0.6 mm and ±0.5 mm from bregma and −7.8 mm from brain surface. Rats were allowed at least 2 weeks to recover from surgery.
Behavior
Spatial Task
The water maze was a plastic circular container (1.5 m diameter) located at one end of a large room. The container was filled with water to a height of 45 cm, and the water was made opaque with nontoxic acrylic white paint. A clear Lucite platform (10 × 10 cm) was placed in the center of one quadrant (target quadrant) and hidden 1.5 cm below the surface of the water. A variety of extra-maze cues were located around the room, including posters on the wall and a window.
The procedure was divided into 2 phases: 1) acquisition of reference memory and 2) assessment of working memory. During the reference memory phase, rats were trained to find an escape platform that was located in the same location for 4 days (Morris et al., 1982). Each daily session had 4 trials. On each trial, the rat was placed in a non-target quadrant, facing the outer wall of the maze. Each trial ended when the rat found the platform or was led to the platform by the experimenter after 60 s. The rat was allowed to remain on the platform for 15 s and then moved to a plastic holding container for 30 s. The time to reach the platform was recorded as escape latency. If the rat was led to the platform, a latency of 60 s was recorded. Starting locations for subsequent trials were in different locations of non-target quadrants. On the 5th day, memory for the platform location was assessed in a probe trial. The platform was removed for the probe trial, and the probe trial lasted 60 s. The swimming path was recorded on videotape. Total time spent in the target quadrant and the number of times the rat crossed the platform location (platform crossing) were determined from off-line analysis of the videotape.
A re-training session occurred 3 days following the probe trial. During the re-training session, the escape platform was put in the same location as during the reference memory phase. Rats were given one session of 4 trials, starting in non-target quadrants.
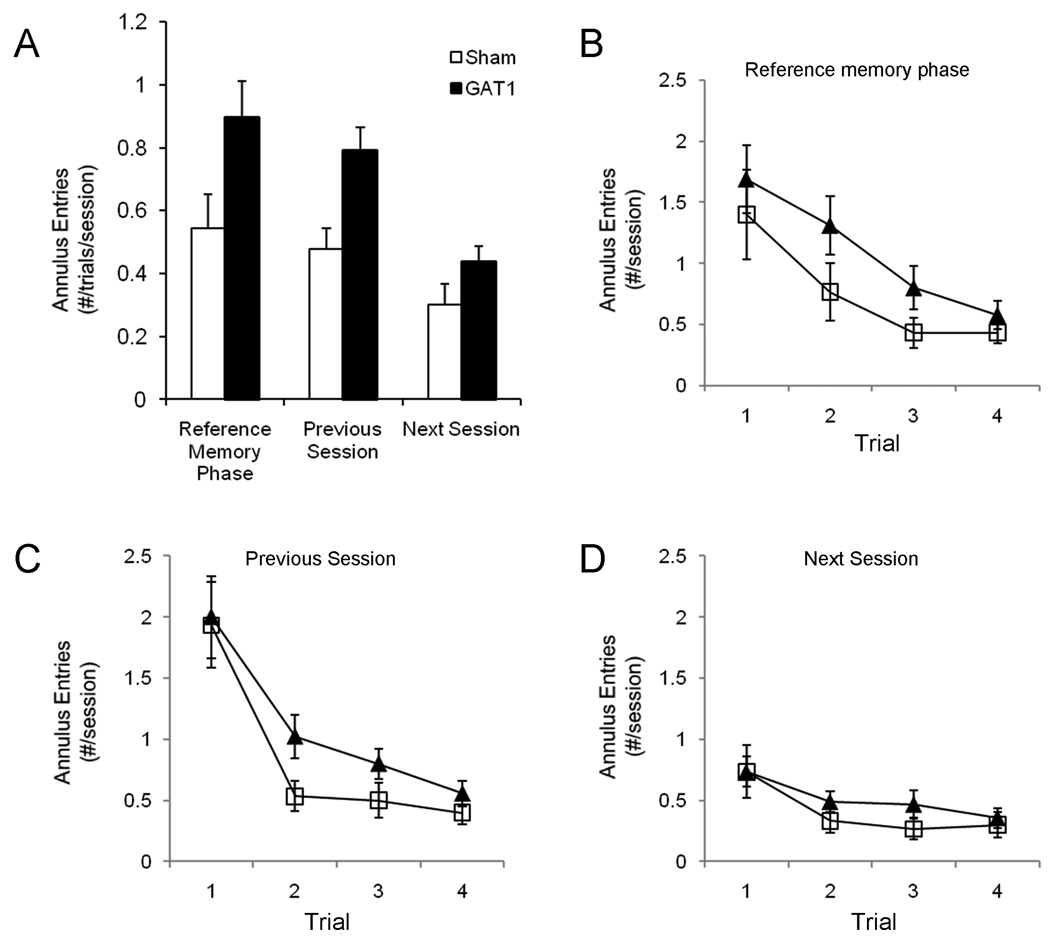
The working memory phase tested the ability of the rat to learn a new platform location during each session (Whishaw, 1985). This phase started the day following the re-training session. During this phase, the platform was moved to a new location at the start of each session (4 sessions). Except for moving the escape platform at the start of each day, all other procedures during the working memory phase were identical to those in the reference memory phase. Swim paths were videotaped and analyzed off-line using a computerized tracking system (ANY-maze, version 4.71, Stoelting Co., Wood Dale, IL). Escape latency, path length and number of entries into previous and future platform locations were assessed. An annulus (20 cm diameter) was centered at the platform locations used 1) on the previous session and 2) during the reference memory phase, and 3) the future platform location for the next session. Annulus entries were segregated by location (previous session, reference memory phase, or next session) and analyzed separately.
For the reference memory phase, session means for escape latency were calculated for each rat. A mixed design ANOVA was performed with session as a within subject factor and treatment as a between subjects factor. Performance measures for the probe trial were analyzed using t-tests.
For the working memory phase, escape latency and path length during sessions 1 – 3 were subjected to a mixed design ANOVA with trials as a within subject factor and treatment as a between subjects factor. Annulus entries were similarly analyzed. Malfunction of the video recorder caused the loss of session 4 data for three animals; therefore, results from session 4 were not analyzed and presented. However, the platform location for session 4 was used in the analysis of next session annulus entries during session 3.
Open Field
General open field activity was evaluated as described previously (Servatius et al., 1995). A rat was placed in the center of a circular open field (75 cm diameter, 40 cm high) under a bright light (3040 lux measured at the floor of the open field). A ventilation fan in the room produced a constant background noise (74 dB). Latency to leave the center, line segments crossed, rearing, fecal boli, and grooming were scored by observers blind to the experimental manipulations. Open field activity was scored for 2 minutes. The arena was wiped with a mild soap solution after testing of each rat. Measures of open field behavior for sham and GAT1-SAP groups were compared using t-tests.
Avoidance Learning
After open field testing, rats were trained in operant chambers (30 cm × 25 cm × 30 cm) (Coulbourn Instruments, Langhorn, PA) enclosed in sound-attenuating boxes. The operant chambers had a lever (10.5 cm above the floor), a cue light (20.5 cm above the grid floor) and a speaker (26 cm above the grid floor) on one wall. On the opposite wall, a light (14 W, 26 cm above grid floor) was constantly lit during the session. The warning signal was a 1000-Hz 75-dB tone (10 dB above background noise). Scrambled footshocks were delivered through the grid floor (Coulbourn Instruments, Langhorn, PA).
Behavioral training during the acquisition phase occurred in sessions of 20 trials. Each session was separated by 2 – 3 days (3 sessions/week). Each session began with a 60-s stimulus-free period, followed by 20 trials. A trial started with the presentation of the warning signal. If a lever response was made in the initial 60 s of the trial, the warning signal was immediately terminated, and a 3-minute intertrial interval was initiated; the response in this case was coded as an “avoidance response”, as the rat avoided the footshock. If an avoidance response was not made, foot shocks (1 mA, 0.5 s duration, 3 s intershock interval) were delivered starting at 60 s and continue until a lever response was made (scored as an “escape response”) or 99 shocks were delivered. Immediately following an escape response or the maximum number of foot shocks, the warning signal was terminated and a 3-minute intertrial interval was initiated. All intertrial intervals were signaled by a flashing light (0.5 Hz, 50% duty cycle). The acquisition phase consisted of 10 sessions.
During the extinction phase, all procedures were the same as in the acquisition phase except the foot shock was omitted. Although shocks were omitted, responses during the first 60 s of the trial were designated as ‘avoidance’ responses, and those with latencies greater than 60 s were designated as ‘escape’ responses. The extinction phase consisted of 6 sessions.
Performance was assessed by calculating the proportion of trials in each session with an avoidance response. A mixed design ANOVA with session as the within subject factor and treatment as the between subjects factor was performed. Although the proportion of trials with an avoidance response is a good measure for determining whether a rat received a footshock, response latency is a sensitive measure to observe changes in escape and avoidance behavior following treatment. Response latency was analyzed by session (after averaging across trials) or by trial (after averaging across sessions). Mixed design ANOVAs were performed with session or trials as the within subject factor and treatment as the between subjects factor. Separate analyses were performed for the acquisition and the extinction phases. To determine whether nonspecific responding might be increased by MSDB lesions, lever presses during the 3 minute intertrial interval was analyzed. Mean lever presses per trial was determined for the intertrial interval and assessed statistically using a mixed design ANOVA with session as a within subject factor and treatment as a between subjects factor.
Immunocytochemistry
At the end of behavioral testing, all animals were perfused intracardially with saline followed by formalin. Brains were extracted and submerged overnight in formalin followed by 30% sucrose. The procedures for immunocytochemstry have been described previously (Pang et al., 2001). Briefly, brain sections through the MSDB area were incubated in antibodies to choline acetyltransferase (ChAT, 1:500 dilution, AB144P, Chemicon International, Temecula, CA, USA), parvalbumin (PV, 1:1000 dilution, P3088, Sigma Immunochemicals, St. Louis, MO, USA), and glutamic acid decarboxylase (GAD67, 1:1000 dilution, MAB5406, Chemicon International). Sections were then incubated in appropriate biotinylated secondary antibodies (1:200 dilution, Jackson ImmunoResearch Laboratory). Visualization was performed using the avidin-biotin method (Standard Vectastain ABC Kit, Vector Laboratory, Burlingame, CA) with nickel-enhanced diaminobenzidine.
Estimates of the number of ChAT-immunoreactive (ir), PV-ir, and GAD67-ir MSDB neurons were determined using standard stereology procedures (West, 1999). Brains of 7 sham and 8 GAT1-SAP rats were lost during the histology procedures and not available for cell counting. Five of the remaining rats in each treatment condition (Sham: n= 11; GAT1-SAP: n = 19) were randomly selected for cell counting PV-ir and ChAT-ir neurons. Four sham and four GAT1-SAP rats were used to quantify the loss of GAD67-ir MSDB neurons. Stereology was performed by a person who was blind to the treatment of the rats. Every third section of the entire MSDB was counted from the anterior pole of the MS to the crossing of the anterior commissure, and included the MS, vertical limb of the DB, and portions of the horizontal limb of the DB. Stereology was performed using the optical fractionator method (Stereo Investigator v.7.0, MicroBrightField, Colchester, VT) on a microscope with an x-, y-, z-axis motorized stage (Bio Point 30, Ludl Electronic Products, Hawthorne, NY). Leading edges of ChAT-ir, PV-ir or GAD67-ir somas were counted using a 40X objective lens (Carl Zeiss, NeoFluar, 0.75 NA). Cells in the uppermost focal plane (2µm) were not counted. The counting frame had a height of 10 µm and was 150 µm × 100µm in size. Numbers of cells in the sham and GAT1-SAP groups were compared using separate t-tests for each neuronal type.
Data Analysis
All data are expressed as mean ± standard error of the mean. Statistical analysis was performed with α = 0.05 using SPSS for Windows (version 12.0.1, SPSS, Inc., Chicago, IL). Mixed design ANOVA and independent sample t-test were used to compare groups. Mauchly’s test was used to determine violations in the assumptions of sphericity for repeated measure factors and Greenhouse-Geisser correction was used in the appropriate situations to correct for violations (Geisser and Greenhouse, 1958). Corrected statistics are only reported when the uncorrected and corrected p-values disagreed with regard to significance; otherwise only the uncorrected values are reported.
RESULTS
Histology
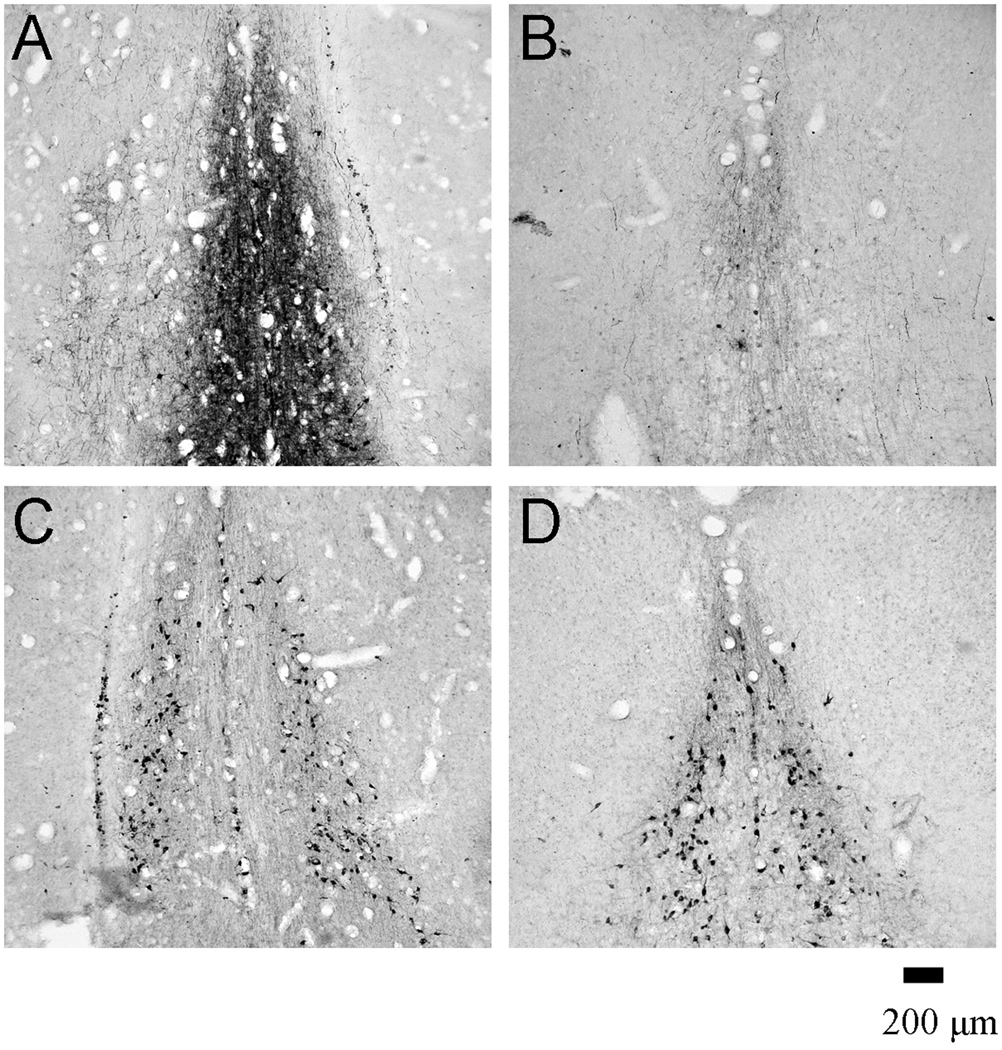
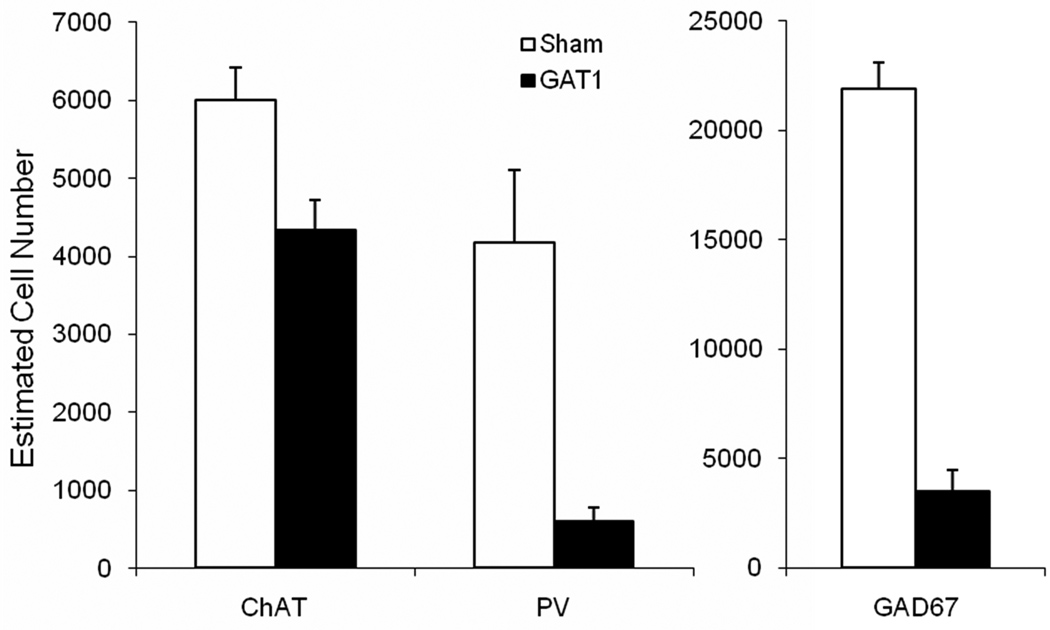
Intraseptal GAT1-SAP preferentially reduced GABAergic neurons as compared to ChAT-ir neurons in the MSDB (Figure 1 and 2). GAT1-SAP reduced the number of GAD67-ir MSDB neurons by 84% (sham: 21875 ± 1198; GAT1-SAP: 3492 ± 982; t(6) = 11.87, p < .001). The effect of GAT1-SAP was also assessed on a subpopulation of GABAergic MSDB neurons. The loss of PV-ir neurons, representing GABAergic septohippocampal neurons, was similar to the reduction of GAD67-ir neurons following intraseptal GAT1-SAP (86% loss; sham: 4176 ± 928; GAT1-SAP: 602 ± 174; t(8) = 3.79, p = .005). Counts of cholinergic neurons made in the same rats used to assess PV-ir neurons demonstrated a mild reduction following GAT1-SAP (sham: 6003 ± 413; GAT-SAP: 4330 ± 392). Although the difference was statistically significant, t(8) = 2.94, p = .02, the reduction of ChAT-ir neurons represented a loss of only 28%. Thus, GAT1-SAP when infused into the MSDB extensively damaged GABAergic MSDB neurons and spared most cholinergic MSDB neurons.
Figure 1.
Photomicrographs of the medial septum and diagonal band of Broca following a sham surgery (A, C) and GAT1-saporin administration (B, D). Immunoreactivity for parvalbumin (A, B) and choline acetyltransferase (C, D) was used to visualize GABAergic and cholinergic MSDB neurons, respectively.
Figure 2.
Intraseptal GAT1-saporin reduced the number of paravalbumin-ir and GAD67-ir neurons by 86% and 84%, respectively. In contrast, cholinergic neurons (ChAT) were only mildly affected by GAT1-saporin (28% reduction).
Spatial Learning
Phase 1 – Spatial Reference Memory
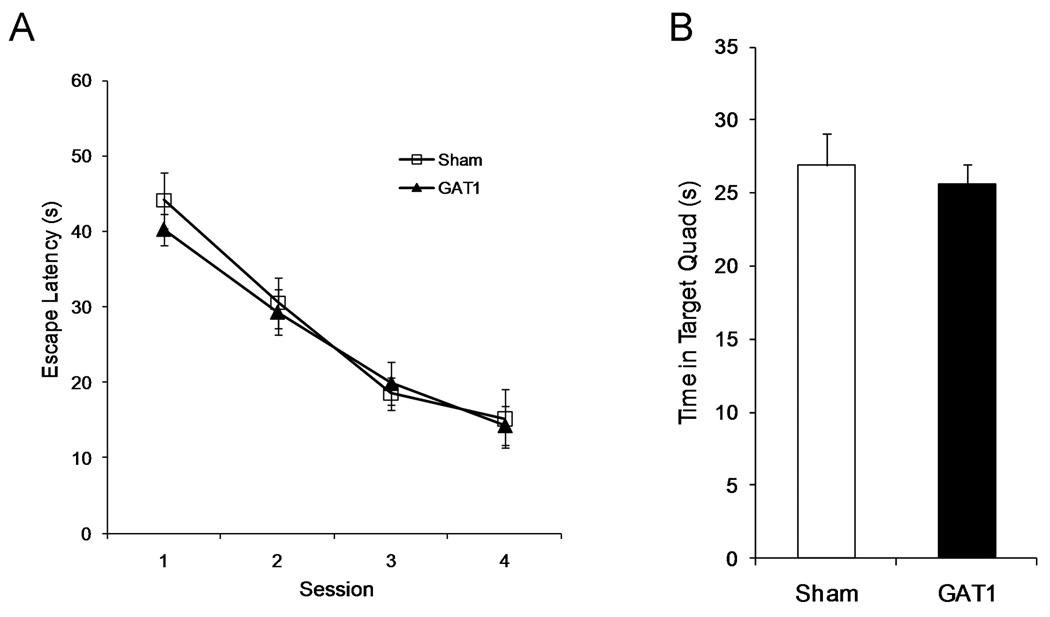
Learning the location of an escape platform was not impaired by intraseptal GAT1-SAP (Figure 3A). During this phase, the escape platform remained in the same location for 4 sessions. Both sham (n = 10) and GAT1-SAP (n=15) rats learned the location of the escape platform with training, F(3,69) = 61.5, p < .001. Moreover, GAT1-SAP treatment did not impair learning, as neither main effect of treatment, F(1,23) = .13, nor session × treatment interaction, F(3,69) = .49, was significant.
Figure 3.
Spatial reference memory was not altered by intraseptal GAT1-saporin. GAT1-saporin treated rats learned the location of an escape platform at a similar rate and to a similar degree as sham treated rats (left). One day after session 4, rats were tested in a probe trial without the escape platform (60 s maximum). Sham and GAT1-saporin rats swam in the target quadrant to a similar extent during this probe trial (right).
One day after the 4th session, rats were tested in a probe trial in which the escape platform was removed (Figure 3B). Sham and GAT1-SAP treated rats spent similar amounts of time searching in the target quadrant (maximum 60s; Sham: 26.9 ± 2.1 s; GAT1-SAP: 25.6 ± 1.3 s; t(23) = .54). The time spent in the target quadrant for both groups was significantly greater than that expected for a random search pattern (i.e., 15s; sham: t(9) = 5.58, p < .001; GAT1-SAP: t(14) = 7.86, p < .001). Rats in both groups also had similar numbers of platform crossings (Sham: 3.8 ± 0.7; GAT1-SAP: 3.0 ± 0.5; t(23) = .92). Thus, performance on both platform and probe trials demonstrate that intraseptal GAT1-SAP did not impair acquisition of a spatial reference memory.
Following the probe trial, rats were retrained in a single session to the platform location used prior to the probe trial. Mean escape latencies on this retraining session was not different between groups (Sham: 12.4 ± 1.7 s; GAT1: 11.4 ± 1.3; t(23) = .50).
Phase 2 – Spatial Working Memory
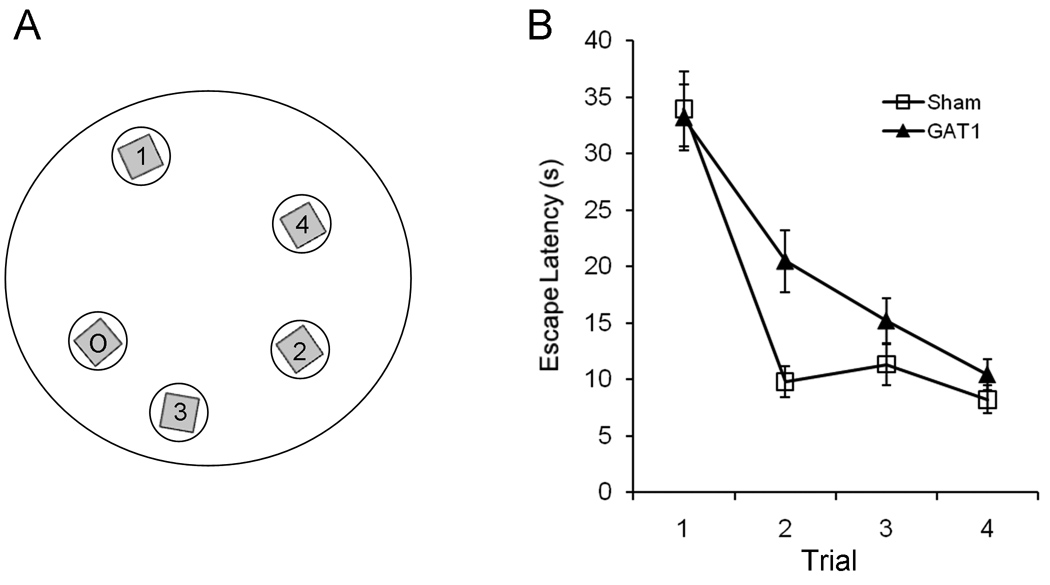
GAT1-SAP impaired performance in a delayed match to position task. After the retraining session, a delayed non-match to position procedure was used to assess spatial working memory. At the start of each daily session, the escape platform was moved to a new location (Figure 4A). Both groups demonstrated within-session learning of the re-located platform position, as demonstrated by reduced escape latencies across trials, F(3,69) = 44.7, p < .001 (Figure 4B) and reduced path length across trials, F(3,69) = 34.4, p < .001 (data not shown). Intraseptal GAT1-SAP impaired learning the new platform locations, as demonstrated by a main effect of treatment, F(1,23) = 4.63, p = .04 for escape latency (Figure 4B). The main effect of treatment just missed significance for path length, F(1,23) = 4.06, p = .056. The trial × treatment interaction for escape latency trended toward significance F(3,69) = 2.25, p = .09, but the interaction for path length did not F(3,69) =1.79. Finally, swim speed was not affected by either trial or treatment, main effect of trial F(3,69) = 1.29, main effect of treatment F(1,23) = .25 and trial × treatment interaction F(3,69) = 1.66.
Figure 4.
Intraseptal GAT1-saporin impaired spatial working memory in a delayed match to position procedure. One of two configurations used for the delayed match to position task (A). The letter O refers to the platform position during the reference memory phase. Numbers refer to the platform location during each daily session of the working memory phase. The circles surrounding the square platform depicts the annulus (20 cm) used in the analysis. The other configuration was similar. At the start of each daily session, the escape platform was moved to a new location. Sham and GAT1-SAP rats learned the location of the platform within a session, as assessed by escape latency (B) or path length (not shown). However, GAT1-saporin treated rats were slower than sham rats to learn the new location.
Rats treated with GAT1-SAP returned to previous goal locations more often than rats with sham surgery, as observed following intraseptal kainic acid (Dwyer et al., 2007). Swim paths were analyzed to determine how often rats returned to the previous platform locations. Entries into a 20 cm annulus surrounding the platform location of the previous session and the location used during the reference memory phase were quantified. To distinguish between a preferential search bias for previous platform locations or a more general search strategy, entries into the platform location for the next session were also quantified (20 cm annulus). This analysis included trials 2 – 4; trial 1 data were not used because this was the sample trial. Overall, intraseptal GAT1-SAP entered previous goal locations but not future goal locations more often than sham rats (Figure 5A). GAT1-SAP significantly increased entries into the reference memory phase annulus, main effect of treatment F(1,23) = 4.41, p = .047 (Figure 5B) and the previous session annulus, main effect of treatment F(1,23) = 0.09, p = .006 (Figure 5C). Trial number influenced entries into the reference memory annulus, F(2,46) = 5.21, p = .009, but not entries into the previous session annulus F(2,46) = 2.13. Trial × treatment interactions did not reach significance for entries into either the reference memory or the previous session annuli, Fs(2,46) < .66.
Figure 5.
Rats treated with GAT1-SAP searched previous platform locations more often than sham rats. Annuli (20 cm) were constructed around previous and future platform locations. GAT1-SAP rats made more entries into the reference memory and previous session annuli compared to sham rats, but entries into the next session annulus were similar between treatment groups (A). Mean entries per trial per session were determined from trials 2 – 4; trial 1 was not included in the analysis because it was a sampling trial on which the rat learned the new platform location. When examined by trials, treatment differences were especially apparent on trials 2 and 3 for entries into the reference memory (B) and the previous session annuli (C). In contrast, GAT1-SAP and sham rats made similar number of entries into the next session annulus for all trials (D). The enhanced entries made by GAT1-SAP rats into previous goal locations but not future goal locations demonstrate a search strategy biased to previously rewarded locations and is consistent with increased proactive interference following GABAergic MSDB damage.
In contrast to the previous platform locations, entries into the next session annulus did not differ between sham and GAT1-SAP rats (Figure 5D). Neither main effect of treatment F(1,23) = 2.80 nor the trial × treatment interaction F(2,46) = .29 reached significance. Trial number also did not influence entries into the next session annulus, F(2,46) = .29.
In total, the results show that rats treated with GAT1-SAP are slower to learn a new platform location. This impairment by intraseptal GAT1-SAP is associated with searching more in previous goal locations than sham rats, consistent with an enhancement of proactive interference.
Open Field
General locomotor activity in an open field was not different following intraseptal GAT1-SAP. Open field behavior was assessed in 20 rats (sham = 8; GAT1 = 12). Data for one rat treated with GAT1-SAP could not be analyzed for line crossing because of videotape failure. However, all other open field measures were obtained for this rat and were included in the data analysis. Treatment groups did not differ on line crossings (sham: 52.3 ± 3.5; GAT1: 44.3 ± 3.4), latency to leave the center (sham: 8.0 ± 1.0 s; GAT1: 9.3 ± 2.0 s) and number of rearings (sham: 5.5 ± 0.8, GAT1: 4.8 ± 1.0) (all p > .13). Number of fecal boli and grooming episodes were virtually non-existent for both groups. Therefore, general activity was not altered by intraseptal GAT1-SAP.
Avoidance Learning
After completing the open field testing, rats were trained in a lever press avoidance task.
Acquisition
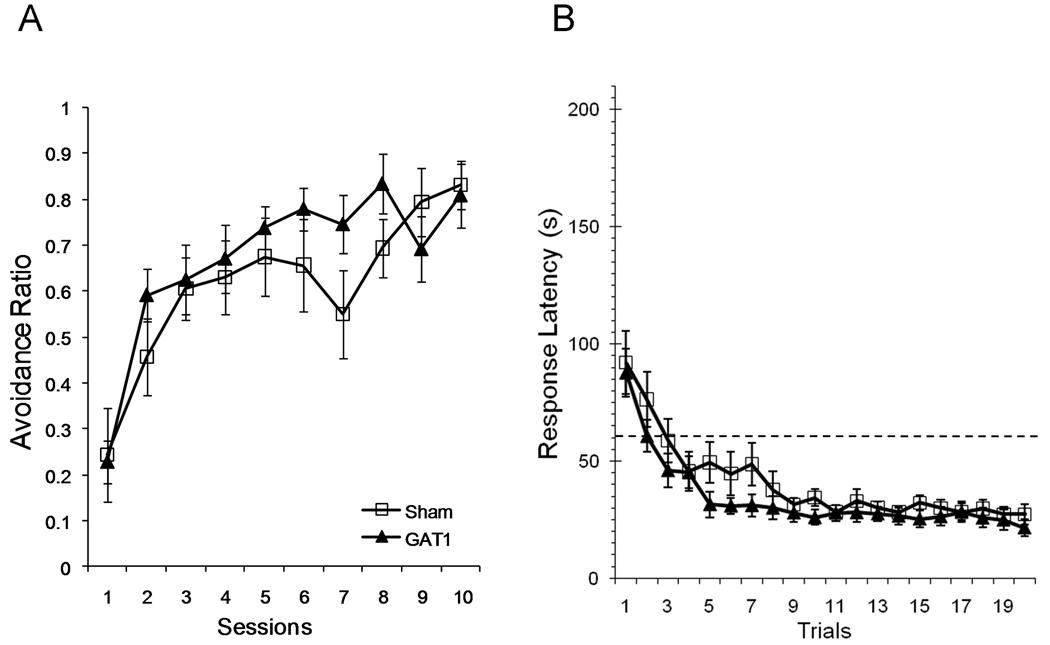
Avoidance learning was not altered by intraseptal GAT1-SAP (Figure 6A). Both treatment groups made more avoidance responses as training proceeded, F(9, 162) = 18.0, p < .001, and the acquisition curve was similar for sham and GAT1-SAP rats (main effect of treatment: F(1,18) = .68; session × treatment interaction: F(9,162) = 1.38).
Figure 6.
Acquisition of lever press avoidance was not altered by intraseptal GAT1-saporin. Proportion of trials with avoidance responses (left) and response latency (right) were used to determine acquisition of avoidance behavior. The proportion of avoidance responses increased across sessions and the response latency decreased across trials. Neither measure was different between treatments. Avoidance responses were responses with latencies less than 60 s (dotted line in right panel).
Analyses of response latency also provided evidence that intraseptal GAT1-SAP did not alter acquisition of lever press avoidance. Response latency shortened as sessions increased, F(9,162) = 13.69, p < .001 (data not shown). Training reduced response latency similarly in sham and GAT1-SAP treated rats (main effect of treatment, F(1,18) = 1.53; session × treatment interaction, F(9,162) = .92).
To determine whether within-session learning was altered by intraseptal GAT1-SAP, response latency was analyzed by trial. At the start of a session, response latency was generally above the avoidance threshold (Figure 6b), but quickly decreased below the threshold and reached an asymptotic latency between 5 – 9 trials. The reduction in latency with increasing trials was significant, F(19,342) = 26.70, p < .001. However, the trajectory of response latency for sham and GAT1-SAP treated rats was similar as neither main effect of treatment F(1,18) = 1.46 nor the trial × treatment interaction F(19,342) = .83 was significant.
Extinction
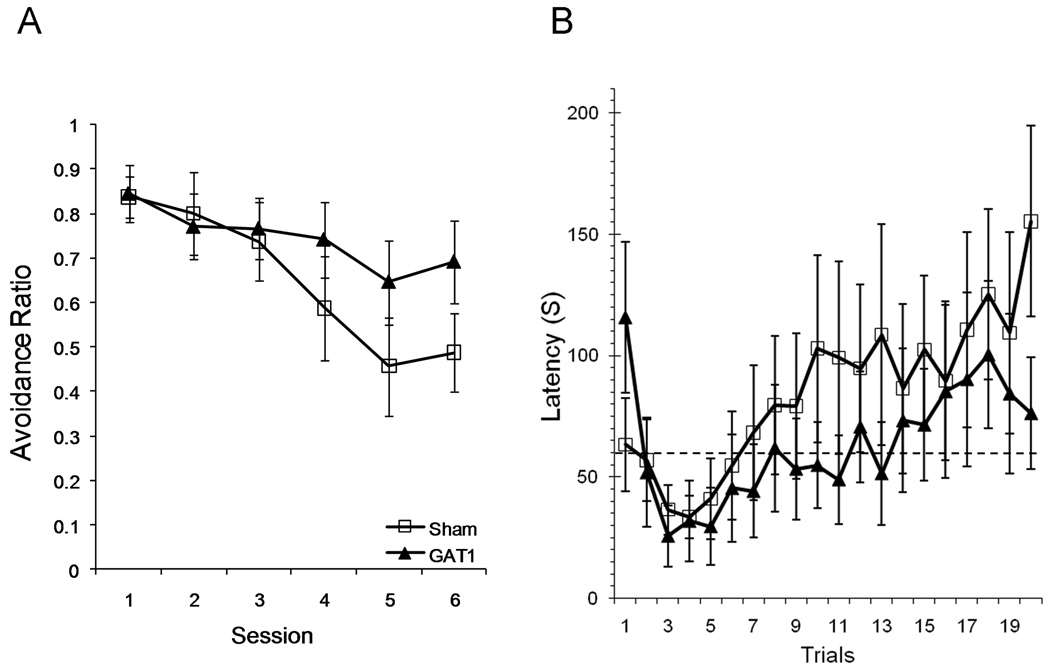
In contrast to acquisition, extinction was impaired by intraseptal administration of GAT1-SAP (Figure 7a). The proportion of avoidance responses decreased with extinction training, F(5,90) = 15.7, p < .001, and GAT1-SAP rats were more resistant to extinction than sham rats, as demonstrated by a significant session × treatment interaction, F(5,90) = 3.08, p = .013. The main effect of treatment was not statistically significant, F(1,18) = .67.
Figure 7.
Intraseptal GAT1-saporin impaired extinction of lever press avoidance. Extinction sessions were identical to acquisition sessions, except the foot shock was never delivered. Although there was no foot shock to “avoid” during extinction trials, all responses with latencies less than 60 s (dotted line in right panel) were designated avoidance responses, similar to the acquisition phase. The proportion of trials with avoidance responses decreased across extinction sessions (left) and response latency increased across trials (right). Rats treated with GAT1-saporin were slower to extinguish avoidance responding in both measures.
Analyses of response latency provided further evidence of an effect of intraseptal GAT1-SAP on extinction. Analysis by session showed that response latency increased following extinction training, F(5,90) = 10.74, p < .001 (data not shown). The general increase in response latency was less in GAT1-SAP rats compared to sham-treated rats, session × treatment interaction F(5,90) = 3.89, p = .003. The main effect of treatment was not significant, F(1,18) = .47.
Analysis by trials also demonstrated a resistance to extinction in GAT1-SAP treated rats (Figure 7b). At the start of an extinction session, response latencies of both groups decreased during the first couple of trials, but increased from trial 4 to the end of the session, F(19,342) = 5.64, p < .001. Differences in the response latencies of sham and GAT1-SAP rats approached significance for the trials × treatment interaction, F(19,342) = 1.59, p = .06. The main effect of treatment was not significant, F(1,18) = .40.
Intertrial Interval Responding
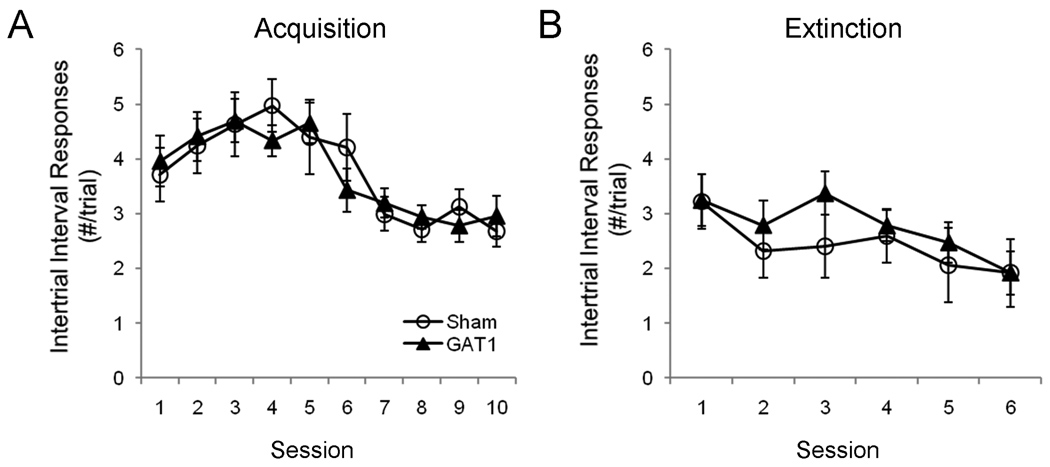
Responding during the intertrial interval was examined to determine whether MSDB treatment affected non-specific responding. During acquisition, intertrial interval responses increased between sessions 1 and 4, then decreased reaching its lowest rate between sessions 8 – 10, F(9,162) = 6.21, p < .001 (Figure 8a). However, GAT1-SAP treatment did not alter intertrial interval responding during the acquisition phase, as neither the main effect of treatment, F(1,18) = .1, nor the session × treatment interaction, F(9,162) = .4, were reliable. During extinction training, intertrial interval responding showed a monotonic decrease, F(5,90) = 6.47, p < .001. Again, GAT1-SAP treatment did not alter responding, with neither the main effect of treatment, F(1,18) = .75, nor the session × treatment interaction, F(5,90) = 1.0, reaching significance.
Figure 8.
GAT1-SAP treatment did not alter intertrial interval responding during avoidance learning. Intertrial interval responses increased through the first half, then decreased during the latter half of the acquisition phase (A). However, treatment groups did not differ. During the extinction phase, intertrial interval responses decreased throughout, and treatment groups were similar.
Overall, intraseptal GAT1-SAP did not alter avoidance acquisition, but had a detrimental effect on extinction of avoidance. The persistence of avoidance responding during the extinction phase was not due to a general enhancement of lever pressing throughout the trials, as GAT1-SAP did not increase intertrial interval responding.
DISCUSSION
The present study utilized a recently developed GABAergic toxin GAT1-SAP to investigate the role of GABAergic MSDB neurons in learning and memory. Administration of GAT1-SAP into the MSDB damaged GABAergic neurons, while sparing most cholinergic cells. The major behavioral effects of intraseptal GAT1-SAP were 1) impaired performance in spatial working memory with no impairment on acquisition of spatial reference memory and 2) slowed extinction of an active avoidance response with no impairment of avoidance acquisition. The results are consistent with an increase in perseveration and proactive interference following GABAergic MSDB lesions.
GAT1-SAP combines a polyclonal antibody to the GABA transporter protein GAT1 with saporin. GAT1 is one of three GABAergic transporters and is present, along with GAT3, in the medial septum (Durkin et al., 1995). GAT1 is more exclusively localized to neurons compared to GAT3, which is present in glia and neurons. Intraseptal administration of GAT1-SAP damaged the majority of GABAergic MSDB neurons while sparing most cholinergic MSDB neurons. The similar loss of GAD67-ir and PV-ir neurons suggests that GAT1-SAP is not selective for different subpopulations of GABAergic MSDB neurons. Further evidence for selective damage of GABAergic neurons by GAT1-SAP was recently obtained in the bed nucleus of the stria terminalis (Radley et al., 2009). While GABAergic neurons were destroyed, little effect of GAT1-SAP was observed on glutamatergic and CRF-containing neurons. Thus, the present results provide further support that GAT1-SAP preferentially damages GABAergic neurons in the MSDB.
Our previous work using intraseptal kainic acid demonstrated the importance of noncholinergic MSDB neurons in learning and memory, and suggested a critical role for these neurons in proactive interference (Dwyer et al., 2007). In that study, a delayed match to position procedure was used in which food was placed in one arm of a radial maze. The reinforced arm was randomly moved to a different arm at the start of each new session. Rats with intraseptal kainic acid were slower to learn the location of the newly reinforced arm and made more re-entries into the reinforced arm of the previous day when compared to rats treated with saline. In the present study, intraseptal GAT1-SAP impaired performance on a similar delayed match to position procedure adapted to the water maze. Rats with intraseptal GAT1-SAP took longer to learn the new location of the escape platform and entered previous goal locations more often than rats with sham lesions. Moreover, GAT1-SAP and sham rats had similar numbers of entries into future goal locations, providing evidence that rats treated with GAT1-SAP had an enhanced bias to search specifically around previous goal locations. Despite differences in task procedures and motivation between the Dwyer et al. and the present studies, intraseptal kainic acid and GAT1-SAP resulted in a similar pattern of impairment, demonstrating a critical role for GABAergic MSDB neurons in spatial working memory. It is unlikely that the impairment is due to reduced working memory capacity or inability to hold information in working memory, as performance following intraseptal kainic acid was not altered in an 8-arm radial maze procedure with delays up to 4 hours (Pang et al., 2001). The persistent entry into previous goal locations following damage of GABAergic MSDB neurons is a reflection of increased proactive interference, possibly due to disruption of hippocampal theta rhythm (Hasselmo, 2005; Yoder and Pang, 2005).
In the present study, GAT1-SAP was associated with an 86% loss of GABAergic neurons and a 28% loss of cholinergic neurons in the MSDB. Thus, associating the behavioral impairments to the loss of GABAergic neurons alone is not straightforward. It is unlikely that the impairment is due solely to loss of cholinergic neurons because rats with more cholinergic damage following 192-IgG saporin were not impaired in a similar delayed match to position procedure (Dwyer et al., 2007). Evidence suggest that a minimum amount of MSDB damage is required to observe learning and memory impairments, as extensive damage of both GABAergic and cholinergic neurons led to spatial reference and working memory impairments that were not observed following each lesion alone (Pang et al., 2001). However, the pattern of impairment was different following combined damage of GABAergic and cholinergic MSDB neurons and after preferential damage of GABAergic MSDB neurons; impaired acquisition of a spatial reference memory was seen after the combined damage but not after preferential GABAergic lesions. Finally, the impairments observed in the present study are not just a function of obtaining a suprathreshold damage of septohippocampal cells; the types of neurons that are damaged are critical. In our previous studies, intraseptal 192-IgG saporin typically damage between 65 – 90% of cholinergic and about 20% of GABAergic MSDB neurons (Dwyer et al., 2007; Pang et al., 2001; Yoder and Pang, 2005). Yet, this damage is not associated with working memory impairments (Dwyer et al., 2007). In contrast, intraseptal kainic acid or GAT1-SAP have typically showed the opposite pattern with 75 – 85% loss of GABAergic and 20 – 30% loss of cholinergic neurons and this type of damage is associated with working memory impairments (present study and Dwyer et al., 2007). Thus, the amount of septohippocampal neurons damaged by 192-IgG, kainic acid and GAT1-SAP are similar, but the effect of intraseptal 192-IgG on working memory is dramatically different than that of intraseptal kainic acid or GAT1-SAP. Thus, while the effects of highly specific damage of the GABAergic MSDB neurons will have to wait for future studies, our present and past studies suggest that damage of GABAergic MSDB neurons alone or with a small amount of cholinergic damage impairs spatial working memory with enhanced perseveration.
Intraseptal GAT1-SAP did not impair the acquisition of a spatial reference memory when the escape platform remained in the same location for multiple sessions. This finding is similar to that observed following intraseptal kainic acid (Pang et al., 2001). However, Cahill and Baxter reported impaired spatial discrimination following intraseptal ibotenic acid that damaged PV-ir and spared cholinergic MSDB neurons (Cahill and Baxter, 2001). While the underlying reason for this impairment is still unclear, our results strongly suggest it is unlikely to be due to damage of GABAergic neurons. Cholinergic neurons at most play a small role in acquiring spatial reference memory, as rats with selective cholinergic MSDB lesions produce no or only a modest impairment (Baxter et al., 1995; Berger-Sweeney et al., 1994; Dornan et al., 1996; Pang et al., 2001). An interesting possibility that needs to be explored is whether glutamatergic septohippocampal neurons are critical for spatial learning. To date, these neurons have been described electrophysiologically and anatomically, but behavioral studies must await selective toxins and methods to selectively activate or inactivate these neurons (Colom et al., 2005; Sotty et al., 2003).
The present study also investigated the effects of GABAergic MSDB lesions on avoidance learning. Acquisition of lever press avoidance was not altered by intraseptal GAT1-SAP. Avoidance responding within a session showed the typical warm-up pattern previously described for lever press avoidance (Hoffman et al., 1961), where animals make escape responses in the initial trials of a session and then transition to avoidance responding for the remaining trials. This pattern was observed in both sham and GAT1-SAP groups. Thus, the present study found no evidence of an alteration of lever press avoidance learning following intraseptal GAT1-SAP. Previous studies have reported enhanced active avoidance following nonselective lesions of the septum or MSDB (Hedges et al., 1975; Hepler et al., 1985), and the simplest explanation is that damage of non-GABAergic MSDB neurons is responsible for the facilitated avoidance learning. Studies with the selective cholinergic immunotoxin is the logical next step, but systemic administration of scopolamine impaired, not facilitated, acquisition of lever press escape and lever press avoidance (Kuribara et al., 1985; Mattingly, 1986). These studies point to the possible importance of non-cholinergic, non-GABAergic MSDB neurons in avoidance learning.
In contrast to active avoidance, passive avoidance is impaired following lesions or inactivation of the MSDB (Hepler et al., 1985; Lorenzini et al., 1996). These effects may be influenced by changes in general activity following septal damage (Brito and Brito, 1990). In the present study, intraseptal GAT1-SAP did not alter general open field activity or noncontingent intertrial interval responding, also implicating non-GABAergic neurons of the MSDB in the hyperactivity often seen following non-selective MSDB damage.
Although avoidance acquisition was unaffected, intraseptal GAT1-SAP impaired extinction of avoidance responding. Following acquisition of lever press avoidance, the foot shock was removed to assess extinction. Sham rats decreased their avoidance responding during extinction sessions, especially after the third session. In contrast, rats treated with GAT1-SAP did not appreciably reduce avoidance responding throughout the entire extinction phase. GAT1-SAP rats perseverated and continued to make avoidance responses, while sham rats learned the new contingencies (i.e., lack of foot shook) and reduced their lever presses accordingly. The perseveration of avoidance responding in GAT1-SAP rats was not due to an enhancement of nonspecific lever pressing because the rate of lever presses during the intertrial interval was not different between treatment groups. Therefore, the continued lever presses during extinction by GAT1-SAP treated rats appears to be specific to responding during the warning signal. These results suggest that GABAergic neurons of the MSDB are a critical component in extinction learning. Together, the pattern of impairments in spatial working memory and extinction of avoidance support the hypothesis that GABAergic MSDB neurons are important for controlling proactive interference. How these GABAergic MSDB neurons interact with the extinction learning circuits described by others (Peters et al., 2009) will be an important focus for future studies.
The mechanism by which GABAergic neurons contribute to flexibility, perseveration and proactive interference is unknown. The GABAergic MSDB neurons terminate primarily on hippocampal interneurons, leading to disinhibition of hippocampal pyramidal neurons (Freund, 1989; Toth et al., 1997). Damage of GABAergic MSDB neurons should result in a higher tonic inhibition of hippocampal pyramidal cells, possibly leading to less excitation during learning situations. GABAergic MSDB lesions may also alter the interplay between sharp waves and theta rhythm that is hypothesized to be important in memory formation (Buzsaki, 1989). However, the effects of GABAergic MSDB damage on hippocampal sharp waves have not yet been investigated. Another view is that damage of GABAergic septohippocampal neurons enhances proactive interference through reduction of hippocampal theta rhythm (Hasselmo, 2005; Hasselmo et al., 1996). Hasselmo and colleagues have proposed that hippocampal theta rhythm is important for the segregation of encoding and retrieval processes. We have previously shown that damage of GABAergic MSDB neurons dramatically reduces hippocampal theta rhythm (Yoder and Pang, 2005), which accordingly would lead to a mixing of encoding and retrieval processes and result in enhanced interference. The results of the present study are consistent with this view. However, blocking theta rhythm has also been reported to enhance extinction (Holt and Gray, 1983). Stimulation of the septum with a pattern that blocked hippocampal theta rhythm was followed by acquisition of a lever press response using a fixed ratio (FR-5) reinforcement schedule. Fifteen days of acquisition was followed by 12 days of extinction. Rats given theta blocking stimulation enhanced extinction learning, exactly opposite the pattern that we observed for extinction of avoidance responding. The discrepant results may be attributed to differences in the manner in which theta was blocked, when theta was blocked in the behavioral procedure or to the different behavioral tasks. Unfortunately, with our current state of knowledge future studies will be necessary to delineate the mechanisms by which GABAergic MSDB neurons facilitate flexibility and assist in reducing proactive interference and perseveration.
In summary, GABAergic MSDB neurons were preferentially damaged by GAT1-SAP. Following intraseptal GAT1-SAP, animals had very specific impairments in a delayed match to position task and extinction of avoidance without altering acquisition of spatial reference memory, active avoidance acquisition or open field behavior. Furthermore, rats with damage of GABAergic MSDB neurons were slower to update changes to previous contingencies. Taken together with previous studies, our results make a strong case that GABAergic neurons in the medial septum are critical for controlling proactive interference and perseveration, critical processes that are abnormal in disorders such as drug abuse, anxiety, Alzheimer’s disease and schizophrenia.
Acknowledgements
This work was supported by the Department of Veterans Affairs Medical Research, the National Institutes of Health, and the Stress and Motivated Behavior Institute of the New Jersey Medical School. We thank Amanda Agley for her assistance on this project. GAT1-saporin was a gift from Advanced Targeting System.
References
- Amaral DG, Kurz J. An analysis of the origins of the cholinergic and noncholinergic septal projections to the hippocampal-formation of the rat. Journal of Comparative Neurology. 1985;240(1):37–59. doi: 10.1002/cne.902400104. [DOI] [PubMed] [Google Scholar]
- Bartus RT, Dean RL, Beer B, Lippa AS. The cholinergic hypothesis of geriatric memory dysfunction. Science. 1982;217:408–416. doi: 10.1126/science.7046051. [DOI] [PubMed] [Google Scholar]
- Baxter MG, Bucci DJ, Gorman LK, Wiley RG, Gallagher M. Selective immunotoxic lesions of basal forebrain cholinergic cells: effects on learning and memory in rats. Behavioral Neuroscience. 1995;109(4):714–722. doi: 10.1037//0735-7044.109.4.714. [DOI] [PubMed] [Google Scholar]
- Berger-Sweeney J, Heckers S, Mesulam M-M, Wiley RG, Lappi DA, Sharma M. Differential effects on spatial navigation of immunotoxin-induced cholinergic lesions of the medial septal area and nucleus basalis magnocellularis. Journal of Neuroscience. 1994;14(7):4507–4519. doi: 10.1523/JNEUROSCI.14-07-04507.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brito GN, Brito LS. Septohippocampal system and the prelimbic sector of frontal cortex: a neuropsychological battery analysis in the rat. Behav Brain Res. 1990;36(1–2):127–146. doi: 10.1016/0166-4328(90)90167-d. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Two-stage model of memory trace formation: a role for "noisy" brain states. Neuroscience. 1989;31(3):551–570. doi: 10.1016/0306-4522(89)90423-5. [DOI] [PubMed] [Google Scholar]
- Cahill JF, Baxter MG. Cholinergic and noncholinergic septal neurons modulate strategy selection in spatial learning. Eur J Neurosci. 2001;14(11):1856–1864. doi: 10.1046/j.0953-816x.2001.01807.x. [DOI] [PubMed] [Google Scholar]
- Chappell J, McMahon R, Chiba A, Gallagher M. A re-examination of the role of basal forebrain cholinergic neurons in spatial working memory. Neuropharmacology. 1998;37(4–5):481–487. doi: 10.1016/s0028-3908(98)00032-x. [DOI] [PubMed] [Google Scholar]
- Colom LV, Castaneda MT, Reyna T, Hernandez S, Garrido-Sanabria E. Characterization of medial septal glutamatergic neurons and their projection to the hippocampus. Synapse. 2005;58(3):151–164. doi: 10.1002/syn.20184. [DOI] [PubMed] [Google Scholar]
- Coyle JT, Price DL, DeLong MR. Alzheimer's disease: a disorder of cortical cholinergic innervation. Science. 1983;219:1184–1190. doi: 10.1126/science.6338589. [DOI] [PubMed] [Google Scholar]
- Dornan WA, McCampbell AR, Tinkler GP, Hickman LJ, Bannon AW, Decker MW, Gunther KL. Comparison of site-specific injections into the basal forebrain on water maze and radial arm maze performance in the male rat after immunolesioning with 192 IgG saporin. Behavioral Brain Research. 1996;82:93–101. doi: 10.1016/s0166-4328(97)81112-6. [DOI] [PubMed] [Google Scholar]
- Durkin MM, Smith KE, Borden LA, Weinshank RL, Branchek TA, Gustafson EL. Localization of messenger RNAs encoding three GABA transporters in rat brain: an in situ hybridization study. Brain Res Mol Brain Res. 1995;33(1):7–21. doi: 10.1016/0169-328x(95)00101-w. [DOI] [PubMed] [Google Scholar]
- Dwyer TA, Servatius RJ, Pang KC. Noncholinergic lesions of the medial septum impair sequential learning of different spatial locations. J Neurosci. 2007;27(2):299–303. doi: 10.1523/JNEUROSCI.4189-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Freund TF. GABAergic septohippocampal neurons contain parvalbumin. Brain Research. 1989;478:375–381. doi: 10.1016/0006-8993(89)91520-5. [DOI] [PubMed] [Google Scholar]
- Geisser S, Greenhouse SW. An extension of Box's results on the use of the F distribution in multivariate analysis. Annals of Mathematical Statistics. 1958;29 85–18. [Google Scholar]
- Givens BS, Olton DS. Cholinergic and GABAergic modulation of medial septal area: effect on working memory. Behavioral Neuroscience. 1990;104(6):849–855. doi: 10.1037//0735-7044.104.6.849. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME. What is the function of hippocampal theta rhythm?--Linking behavioral data to phasic properties of field potential and unit recording data. Hippocampus. 2005;15(7):936–949. doi: 10.1002/hipo.20116. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Wyble BP, Wallenstein GV. Encoding and retrieval of episodic memories: Role of cholinergic and GABAergic modulation in the hippocampus. Hippocampus. 1996;6:693–708. doi: 10.1002/(SICI)1098-1063(1996)6:6<693::AID-HIPO12>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Hedges AS, Van Atta L, Thomas JB. Septal lesions facilitate the shift from conditioned escape to conditioned avoidance behavior in the rat. Physiol Behav. 1975;14(1):25–30. doi: 10.1016/0031-9384(75)90136-5. [DOI] [PubMed] [Google Scholar]
- Hepler DJ, Wenk GL, Cribbs BL, Olton DS, Coyle JT. Memory impairments following basal forebrain lesions. Brain Res. 1985;346(1):8–14. doi: 10.1016/0006-8993(85)91088-1. [DOI] [PubMed] [Google Scholar]
- Hoffman HS, Fleshler M, Chorny H. Discriminated bar-press avoidance. J Exp Anal Behav. 1961;4:309–316. doi: 10.1901/jeab.1961.4-309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holt L, Gray JA. Septal driving of the hippocampal theta rhythm produces a long-term, proactive and non-associative increase in resistance to extinction. Q J Exp Psychol B. 1983;35(Pt 2):97–118. doi: 10.1080/14640748308400897. [DOI] [PubMed] [Google Scholar]
- Jakab R, Leranth C. Septum. In: Paxinos G, editor. The rat nervous system. 2nd ed. San Diego: Academic Press; 1995. pp. 405–442. [Google Scholar]
- Kenyon J, Krieckhaus EE. Enhanced Avoidance Behavior Following Septal Lesions in the Rat as a Function of Lesion Size and Spontaneous Activity. J Comp Physiol Psychol. 1965;59:466–468. doi: 10.1037/h0022028. [DOI] [PubMed] [Google Scholar]
- Kesner RP, Crutcher KA, Measom MO. Medial septal and nucleus basalis magnocellularis lesions produce order memory deficit in rats which mimic symptomology of Alzheimer's disease. Neurobiology of Aging. 1986;7:287–295. doi: 10.1016/0197-4580(86)90009-6. [DOI] [PubMed] [Google Scholar]
- Kuribara H, Haraguchi H, Tadokoro S. Comparisons between discrete lever-press and shuttle avoidance responses in mice: acquisition processes and effects of psychoactive drugs. Jpn J Pharmacol. 1985;38(2):141–151. doi: 10.1254/jjp.38.141. [DOI] [PubMed] [Google Scholar]
- Lorenzini CA, Baldi E, Bucherelli C, Tassoni G. Amnesic effects of preacquisition, postacquisition, or preretrieval tetrodotoxin administration into the medial septal area on rat's passive avoidance memorization. Neurobiol Learn Mem. 1996;66(1):80–84. doi: 10.1006/nlme.1996.0045. [DOI] [PubMed] [Google Scholar]
- Malthe-Sorenssen D, Odden E, Walaas I. Selective destruction by kainic acid of neurons innervated by putative glutamatergic afferents in septum and nucleus of the diagonal band. Brain Research. 1980;182:461–465. doi: 10.1016/0006-8993(80)91204-4. [DOI] [PubMed] [Google Scholar]
- Mattingly BA. Scopolamine disrupts leverpress shock escape learning in rats. Pharmacol Biochem Behav. 1986;24(6):1635–1638. doi: 10.1016/0091-3057(86)90498-3. [DOI] [PubMed] [Google Scholar]
- Mizumori SJY, Perez GM, Alvarado MC, Barnes CA, McNaughton BL. Reversible inactivation of the medial septum differentially affects two forms of learning in rats. Brain Research. 1990;528:12–20. doi: 10.1016/0006-8993(90)90188-h. [DOI] [PubMed] [Google Scholar]
- Morris RGM, Garrund P, Rawlins JNP, O'Keefe J. Place navigation impaired in rats with hippocampal lesions. Nature. 1982;297:681–683. doi: 10.1038/297681a0. [DOI] [PubMed] [Google Scholar]
- Olton DS. Shock-motivated avoidance and the analysis of behavior. Psychol Bull. 1973;79(4):243–251. doi: 10.1037/h0033902. [DOI] [PubMed] [Google Scholar]
- Osborne PG. A GABAergic mechanism in the medial septum influences cortical arousal and locomotor activity but not a previously learned spatial discrimination task. Neurosci Lett. 1994;173(1–2):63–66. doi: 10.1016/0304-3940(94)90150-3. [DOI] [PubMed] [Google Scholar]
- Pang KCH, Nocera R, Secor AJ, Yoder RM. GABAergic septohippocampal neurons are not necessary for spatial memory. Hippocampus. 2001;11:814–827. doi: 10.1002/hipo.1097. [DOI] [PubMed] [Google Scholar]
- Peters J, Kalivas PW, Quirk GJ. Extinction circuits for fear and addiction overlap in prefrontal cortex. Learn Mem. 2009;16(5):279–288. doi: 10.1101/lm.1041309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Gosselink KL, Sawchenko PE. A discrete GABAergic relay mediates medial prefrontal cortical inhibition of the neuroendocrine stress response. J Neurosci. 2009;29(22):7330–7340. doi: 10.1523/JNEUROSCI.5924-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Servatius RJ, Ottenweller JE, Natelson BH. Delayed startle sensitization distinguishes rats exposed to one or three stress sessions: further evidence toward an animal model of PTSD. Biol Psychiatry. 1995;38(8):539–546. doi: 10.1016/0006-3223(94)00369-E. [DOI] [PubMed] [Google Scholar]
- Sotty F, Danik M, Manseau F, Laplante F, Quirion R, Williams S. Distinct electrophysiological properties of glutamatergic, cholinergic and GABAergic rat septohippocampal neurons: novel implications for hippocampal rhythmicity. J Physiol. 2003;551(Pt 3):927–943. doi: 10.1113/jphysiol.2003.046847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Toth K, Freund TF, Miles R. Disinhibition of rat hippocampal pyramidal cells by GABAergic afferents from the septum. Journal of Physiology. 1997;500(2):463–474. doi: 10.1113/jphysiol.1997.sp022033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- West M. Stereological methods for estimating the total number of neurons and synapses: issues of precision and bias. Trends in Neuroscience. 1999;22(2):51–61. doi: 10.1016/s0166-2236(98)01362-9. [DOI] [PubMed] [Google Scholar]
- Whishaw IQ. Formation of a place learning-set by the rat - a new paradigm for neuro-behavioral studies. Physiology & Behavior. 1985;35(1):139–143. doi: 10.1016/0031-9384(85)90186-6. [DOI] [PubMed] [Google Scholar]
- Winocur G, Mills JA. Hippocampus and septum in response inhibition. J Comp Physiol Psychol. 1969;67(3):352–357. doi: 10.1037/h0026764. [DOI] [PubMed] [Google Scholar]
- Winson J. Loss of hippocampal theta rhythm results in spatial memory deficit in the rat. Science. 1978;201:160–163. doi: 10.1126/science.663646. [DOI] [PubMed] [Google Scholar]
- Yoder RM, Pang KCH. Involvement of GABAergic and cholinergic medial septal neurons in hippocampal theta rhythm. Hippocampus. 2005;15:381–392. doi: 10.1002/hipo.20062. [DOI] [PubMed] [Google Scholar]