Abstract
Purpose
Rectal cancer is often clinically resistant to radiotherapy and there would be value to identifying molecular markers to define the biological basis for this phenomenon. NF-κB is a potentially anti-apoptotic transcription factor that has been associated with resistance to radiotherapy in model systems. This study was designed to evaluate NF- κB activation in rectal cancers being treated with chemoradiation to determine whether NF- κB activity correlates with outcome in rectal cancer
Methods and Materials
22 patients were biopsied at multiple time points in a prospective study, and another 50 were analyzed retrospectively. Pre-treatment tumor tissue was analyzed for multiple NF- κB subunits by immunohistochemistry (IHC). Serial tumor biopsies were analyzed for NF- κB-regulated gene expression by RT-PCR and for NF-κB subunit nuclear localization by IHC.
Results
Several NF- κB target genes (Bcl-2, cIAP-2, IL-8 and TRAF1) were significantly upregulated by a single fraction of radiotherapy at 24 hours demonstrating for the first time that NF-κB is activated by radiotherapy in human rectal tumors. Baseline NF-κB p50 nuclear expression did not correlate with pathologic response to radiotherapy, but increasing baseline p50 was prognostic for overall survival (HR 2.15, p = 0.040).
Conclusions
NF-κB nuclear expression at baseline in rectal cancer is prognostic for overall survival but not predictive of response to radiotherapy. Larger patient numbers would be needed to assess the effect of NF-κB target gene upregulation on response to RT. Our results suggest that NF-κB may play an important role in tumor metastasis as opposed to resistance to chemoradiotherapy.
Introduction
Rectal cancer is common, representing nearly a third of colorectal cancers, and approximately 40% of rectal cancer patients die from their disease 1. Despite improvement in surgical techniques local control remains a problem with surgery alone, and current combined modality therapy is still inadequate for many patients with locally advanced tumors 2. Radiation and chemotherapy are used to minimize the chance of local recurrence after successful surgery, and to permit sphincter-sparing surgery for low-lying cancers that may otherwise require abdominoperineal resection. Standard radio-chemotherapy with 5-FU results in pathologic complete remission (pCR) only 8%–20% of the time 3, 4; our current understanding of the molecular basis for the relative resistance to radiation of the majority of adenocarcinomas is incomplete.
Evidence of survival pathway activation by radiation has been demonstrated via global gene expression profiling in solid tumor cell lines after single therapeutic-dose fractions of ionizing radiation 5–7. In theory, survival mechanisms may not be detectable if assayed in a tumor at baseline, but rather are activated by stress such as radiotherapy-induced DNA damage.
One such cell survival mechanism involves NF-κB, a transcription factor composed of heterodimers of a family of DNA-binding proteins that has been shown to suppress apoptosis. There are 5 characterized members of the family: p65/RelA, c-Rel, p50, p52 and RelB8. The most intensively studied form of NF-κB is the heterodimer composed of the p50 and p65 subunits. NF-κB exists in the cytoplasm in an inactive form maintained by binding to repressor proteins known as I-kappa B (IκB) proteins. In response to a wide variety of stimuli including TNF, DNA-damaging agents, and ionizing radiation, IκB proteins are phosphorylated, ubiquitinated, and degraded by the ubiquitin-proteasome pathway. Loss of IκB frees NF-κB to enter the cell nucleus where NF-κB acts as a transcriptional activator of a variety of genes encoding proteins involved in immunity, inflammation, cell proliferation, and protection from apoptosis in the case of many cancer cells. The ability of NF-κB to suppress apoptosis occurs at least partly through the transcriptional upregulation of genes encoding proteins that block apoptosis via several mechanisms9, 10. As an example, in an unbiased screen of genes expressed after radiation exposure in CLL cells, cellular inhibitor of apoptosis protein (c-IAP) mRNA was found to be strongly upregulated11 after radiation exposure.
There are data suggesting that NF-κB activation by radiation contributes to radioresistance. In a radioresistant breast cancer model, gene array experiments performed to identify expression changes associated with radioresistance demonstrated increased expression of NF-κB -regulated genes in radioresistant cells compared to parental radiosensitive cells 12. There is also preclinical evidence that constitutive NF-κB activation could influence radiation response. One group demonstrated that high basal NF-κB activity negatively correlates with basal apoptosis of head and neck cancer cell lines, as well as with radiosensitivity13. NF-κB induction by radiation is anti-apoptotic in an array of colorectal cancer cell lines and in a colorectal xenograft model. Furthermore, inhibition of radiation-induced NF-κB induction increases apoptosis as measured by both in vitro and in vivo assays14.
In contrast to the idea of treatment-induced NF-κB activation, NF-κB nuclear localization indicative of activation has been described at baseline in tissue samples from untreated colorectal cancers, pancreatic, breast, and head and neck cancers, suggesting that constitutive NF-κB activation occurs in a fraction of cancers of many types15–17. In the largest series of human colorectal tumors studied to date, 31% of tumors exhibited activation of NF-κB as measured by detection of nuclear p65/RelA18. These findings lead to questions of which mechanisms are more important for determination of clinical behavior of a cancer; inducible activation or constitutive activation of NF-κB. To clarify these mechanisms we wished to address the following questions in a population of patients receiving preoperative radiotherapy for rectal cancer:
Whether rectal tumors that demonstrate baseline NF-κB activation are less likely to respond clinically to radiation therapy
Whether changes in NF-κB activation status are induced by a single fraction of radiotherapy
Whether radiation-induced activation of NF-κB can be measured in vivo, and if so whether such changes occur differentially in radiotherapy responders and non-responders.
In order to address these questions we designed a study in which serial biopsies were taken during standard fluorouracil-based pre-operative chemoradiation. These biopsies were assayed for multiple aspects of NF-κB activity, and results correlated with pathologic response assessed at the time of surgery.
Methods and Materials
Patients
Two patient cohorts were utilized for this study; the first was a group of patients prospectively enrolled to a biopsy protocol that is described below. The second cohort was a sequential, retrospective cohort of patients who underwent preoperative chemoradiation and surgery at our institution.
Cohort 1 Protocol eligibility
Eligible patients had clinically staged T3-4N0 or T2-4N1-2, pathologically-documented adenocarcinoma of the rectum who were thought by the treating team to be fit for preoperative chemoradiotherapy. All patients were staged using endoscopic ultrasonography and CT or MRI of the abdomen and pelvis.
Cohort 2 eligibility. A group of patients treated at UNC Hospitals with preoperative chemoradiation therapy for rectal cancer to the same doses and radiation fields was identified from our institutional Tumor Registry. Criteria for selection included availability of a pre-operative biopsy suitable for immunhistochemical analysis and surgery performed at UNC. This consisted of 49 patients.
Both portions of the study were approved by our Institutional Review Board and we followed all standard procedures for protection of human research subjects.
Treatment
Patients were treated with standard pelvic radiotherapy to 45 Gy with a 5.4 Gy boost beginning on day one in 180cGy fractions. Concurrent continuous infusion 5-fluorouracil-based chemotherapy began on day 2 after the final biopsy was performed. Biopsies were obtained using a rigid sigmoidoscopy and alligator forceps at three time points: 1) prior to initiation of treatment (at the time of planned endoscopic ultrasonography), 2) 2, 4 or 6 hours after the first fraction of radiation therapy and 3) 24 hours after the first fraction of radiation therapy. Six biopsies were collected at each time, half of the samples were frozen immediately in liquid nitrogen, the other half preserved in formalin for paraffin embedding.
Dose modification and treatment delays were performed per standard of care. All patients who were candidates for surgery underwent resection of their rectal tumors four to eight weeks after the end of radiation therapy.
Clinical Endpoints
The primary clinical outcome measure for this study was pathologic response, which was divided into three categories for purposes of data analysis: limited response (NR: gross disease present), major response (MR: only microscopically visible disease remaining) or complete response (CR: no evidence of residual tumor cells pathologically). AJCC pathologic stage at surgery was also assessed.
Molecular Endpoints
Immunohistochemical (IHC) analysis
For patients 1–24 (prospective study cohort) tissue microarrays (TMA) were constructed from the biopsies using previously described methods 19. Forty-nine additional patients were identified retrospectively. For these patients, sections were taken from whole archived biopsy tissues. Samples were blocked for endogenous peroxidase activity with hydrogen peroxide. Antigen recovery was done by steaming of slides for 25 min in DakoCytomation Target Retrieval Solution (S1699 DakoCytomation, Inc. Carpinteria, CA 93013) for each primary antibody utilized. Sections were then exposed to the primary antibodies (table 1) for 30 min. at room temperature, rinsed in Dako wash buffer (S3006 DakoCytomation, Inc. Carpinteria, CA 93013), incubated with the Dako LSAB2 kit according to the manufacturer’s instructions for 10 min at room temperature (K0675, DakoCytomation, Inc. Carpinteria, CA 93013). Detection of the antibody/antigen complex was visualized using 3–3 diaminobenzidine for 5 min (K3468 DakoCytomation, Inc. Carpinteria, CA 93013). Slides were then rinsed in water, lightly counterstained in filtered Mayer’s hematoxylin (S3309 DakoCytomation, Inc. Carpinteria, CA 93013), rinsed, dehydrated, cleared, and mounted.
Table 1.
Specific antibodies to evaluate NF-κB activation.
| Target | Ab | Manufacturer | Catalog No. | Dilution | Positive Control Tissue |
|---|---|---|---|---|---|
| P50 | p50NLS | Santa Cruz | sc-114NLS | 1:100 | Breast Ca, Panc-1 cell block* |
| P65 | F-6 | Santa Cruz | sc-8008 | 1:1000 | Tonsil, Panc-1 cell block* |
| P52 | c-5 | Santa Cruz | sc-7386 | 1:50 | Tonsil, Panc-1 cell block* |
Panc-1 is a pancreatic cancer cell line stained before and after treatment with TNF-α that increases nuclear p50 and p65. A positive control has baseline staining that increases significantly after TNF treatment.
Scoring of Tissue Microarrays
Individual TMA cores were scored visually and semi-quantitatively by the study pathologist (WKF) who was blinded to all relevant patient clinical and pathologic data. The intensity “score” included measure of signal strength (0–3+) multiplied by percentage of cells of interest showing nuclear immunoreactivity (decade increments, 0–100%). Where possible, two separate biopsies were processed for each patient at each time point to minimize the possibility that localized differences in gene expression might affect results. For NF-κB subunits, nuclear translocation occurs after activation, so increases in nuclear staining are an acceptable surrogate for NF-κB activation.
Quantitative fluourescence RT-PCR
Total RNA was isolated from single frozen biopsy samples using an RNeasy kit (Qiagen, cat. #74104). DNA contamination was resolved by treating all total RNA isolates with DNase I RNase free (Ambion, cat #AM2222). Samples were diluted to 2ng/ul and 5ul/reaction was used. Samples were run in triplicate.
Applied Biosystem’s Primer Express software was used to design primers for the following genes: Bcl-2, Bcl-xL, cIAP1, cIAP2, TRAF1, TRAF2, IκBα, IKKε, IL-8, and cyclin D1 (primers Biosearch Technologies cat. # RPH-5). Probes (Biosearch Technologies cat. #DLO-FB1-5) were labeled with 5’FAM-3’BHQ-1. One step RT-PCR was performed (40 cycles). Values for relative mRNA expression were derived by calculating the mean cycle number, normalizing by subtracting 40 from the mean (40 = the maximum # of cycles) then calculating en where n is the normalized cycle number value.
Statistical Methods
The study was initially designed to accrue 60 patients prospectively. This was eventually limited by slow accrual, likely because of the requirement for multiple biopsies. The initial sample size calculation was based on the primary objective for estimating the probability of activation of NF-κB and of testing for independence between NF-κB activation and therapeutic outcome as follows: The probability of activation of NF-κB will be estimated as a binomial proportion. An exact binomial 95% confidence interval based on n=60 patients has a maximum width of 0.13 on either side of the point estimate, with the interval being widest when 50% of the patients show activation. For example, if 6/60 patients show activation, the confidence interval will be (0.038, 0.21), if 12/60 activate it will be (0.11, 0.33), if 18/60 activate it will be (0.19, 0.43) and if 30/60 activate it will be (0.37, 0.63). The final N was based on availability of prospectively accrued patients and on availability of archived tumor biopsies at our institution.
For analysis, the natural log transformation of the gene scores was the most appropriate for these data; thus, results are reported in terms of a fold difference. Generalized estimating equations, which account for correlated data, were used to estimate the differences in gene scores and NF-κB subunits at different time points. To compare changes in gene scores among the three pathologic response groups (NR, MR, CR), Kruskal-Wallis tests were performed, and nominal p-values are reported; additionally, the NR and MR pathologic response groups were combined and compared to the CR group, using Wilcoxon Rank-Sum tests. The Kruskal-Wallis and Wilcoxon Rank Sum tests were also used to compare the NF-κB subunit expression at baseline among the pathologic response groups for the larger sample of 72 patients. Survival curves were constructed using the Kaplan-Meier method, and Cox regression models were fit to see if the NF-κB subunits were significantly associated with overall survival. Statistical analyses were performed with SAS statistical software (SAS Institute Inc, Cary, NC) and R software (http://www.r-project.org/).
Results
Evidence of NF-κB activation by a single fraction of RT can be visualized by RT-PCR for NF-κB target genes
Twenty-five patients were enrolled to the prospective biopsy collection study. Of these, 24 were successfully biopsied. Two patients’ tumors yielded poor quality RNA. Thus, 22 patients’ tumor samples were assayed by RT-PCR. Eleven genes known to be transcriptional targets of activated p50/p65 were evaluated (inhibitors of apoptosis Bcl-2, Bcl-xL, cellular inhibitor of apoptosis protein [cIAP]1, cIAP2, Tumor necrosis factor receptor-associated factor [TRAF]1, TRAF2, the NF-κB regulated cytokine interleukin-8 [IL-8], the cell-cycle regulator cyclin D1, and regulators of NF-κB activation I-kappa kinase (IKK)ε, IκBα).
No significant differences in expression were noted for any of the genes between the baseline and 2–6 hour time points. At 24 hours, statistically significant increases in gene expression were noted for 4 of the 11 NF-κB-regulated genes (table 2). For two of the genes (IL-8, TRAF1), the difference between 2–6 hour gene expression and 24 hour gene expression was also significant, supporting the assertion that these observations were not made by chance. The general trend was for increased expression of all 11 genes, even where statistical significance was not met with a range of 1.03 fold increase to a 7.44 fold increase between 0 and 24 hours. None of the genes studied decreased in expression on average, but they did decrease in individual patients (Data not shown).
Table 2.
NF-κB target gene expression differences from baseline
| Fold Increase (95% CI) | p-value | ||
|---|---|---|---|
| Bcl-2 | Baseline to 24hrs | 3.68 (1.429, 9.479) | 0.007 |
| cIAP-2 | Baseline to 24hrs | 1.78 (1.029, 3.089) | 0.039 |
| IL-8 | Baseline to 24hrs | 7.44 (1.576, 35.135) | 0.01 |
| 2–6 hrs to 24hrs | 11.61 (3.759, 35.848) | <0.0001 | |
| TRAF1 | Baseline to 24hrs | 1.67 (1.057, 2.733) | 0.029 |
| 2–6 hrs to 24hrs | 2.12 (1.275, 3.537) | 0.004 |
Among the 22 patients assayed by RT-PCR, 4 had a pathologic CR, 8 had microscopic residual disease (major response) and 10 had gross residual disease (considered non-response for this analysis). Given the small sample size for each pathologic response group, we were unable to demonstrate any association between specific NF-κB target gene expression and pathologic outcome. The difference in gene expression between biopsies obtained 4 and 24 hours after RT was statistically significant for cIAP2 when the CR group was compared to the MR and NR groups combined (p=0.048), and there was a similar trend for IKK_epsilon (p=0.06). When comparing all three groups separately, a borderline significant difference was found for cyclin D1 from T0 to T24 (p=0.07). Baseline gene expression values were not predictive of pathologic response nor were any predictive of overall survival.
Use of IHC for NF-κB nuclear localization is insufficient to observe changes in NF-κB activation status by a single fraction of RT
As a second method to assess whether NF-κB activation occurs as a result of a single fraction of RT, we utilized IHC for NF-κB activation, as defined by nuclear localization of several well-studied NF-κB subunits (p50, p65 and p52). Samples for 15 of the 24 prospectively enrolled (multiple biopsy) patients were evaluable by IHC for at least two timepoints. Sample dropout occurred primarily due to lost or uninformative (for example only adenoma seen in a given core) spots on the TMA, which occurred in spite of having as many as 6 cores placed on the TMA for each time-point sample. Based on this sample of 15 patients, we were unable to demonstrate significant changes in nuclear NF-κB expression at either the early (2–6 hour) or 24-hour time-point compared to baseline, suggesting that IHC is insufficiently sensitive to demonstrate acute changes in NF-κB activation status resultant from radiotherapy.
Baseline NF-κB status as measured by IHC is prognostic for OS, but does not predict response to radiotherapy
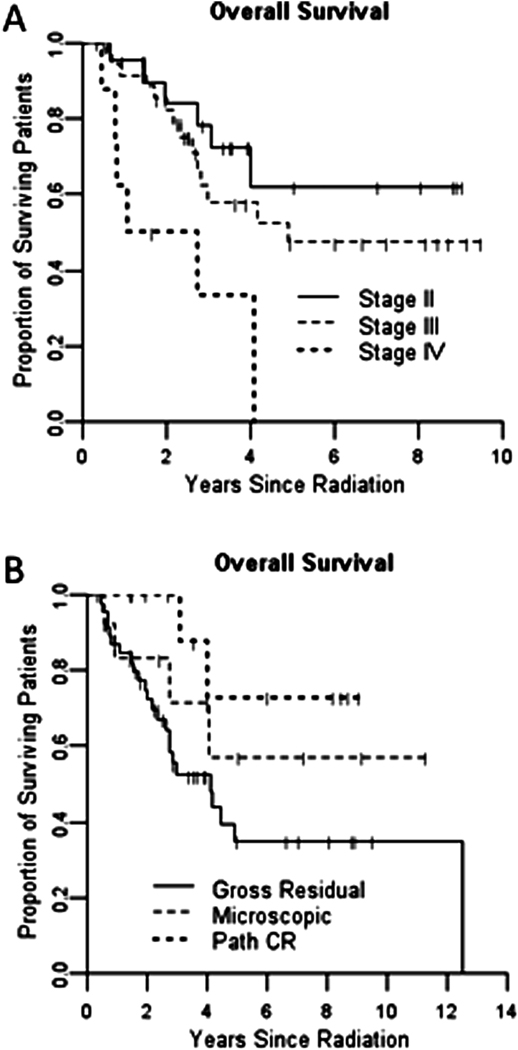
In order to increase our patient numbers for selected end-points, we chose to study NF-κB baseline expression retrospectively in 49 additional patients who had pre-treatment biopsy tissue available in our pathology archives and who were treated with the same standard chemoradiotherapy followed by surgery. These samples were combined with those of the patients who had pre-treatment biopsies for the prospective component of the study. Demographics and response percentages for the entire group are shown in table 3. The entire group of 72 patients demonstrated overall survivals similar to historically reported figures based on pathologic stage and response to radiotherapy (Figure 1)
Table 3.
Patient demographics
| Age | 58 (range: 26, 89) |
|---|---|
| Sex | |
| Male | 43 (60%) |
| Female | 29 (40%) |
| Race | |
| White | 53 (75%) |
| Black | 16 (22%) |
| Other | 2 (3%) |
| Stage | |
| II | 23 (34%) |
| III | 37 (54%) |
| IV | 8 (12%) |
| Pathologic Stage | |
| Gross Residual | 46 (64%) |
| Microscopic | 14 (19%) |
| Pathologic CR | 12 (17%) |
Figure 1.
Kaplan-Meier curves for overall survival by stage (A) and pathologic response to radiotherapy (B)
The majority of cases were positive (nuclear) to some extent for both p50 (58/63, 92%) and p65 (49/61, 80/%) based on a cutoff of 100 points, approximating a 1+ average staining intensity. P52 subunit positivity was less frequent, with many samples displaying minimal (0–10 point) staining (38/64, 59%) and 31/64 (48%) displaying high (100 or greater) intensity nuclear staining.
There was no association between nuclear staining for any of the 3 studied NF-κB subunits and pathologic response to radiotherapy (CR vs. micro residual versus gross residual- table 4), refuting a primary hypothesis of our study.
Table 4.
Baseline NF-κB subunit expression versus response to therapy
| NF-κB subunit | P values comparing all 3 response groups |
P values for Gross Resid vs (Micro + CR) |
|---|---|---|
| P50 | 0.7 | 0.7 |
| P52 | 0.4 | 0.4 |
| P65 | 0.8 | 1 |
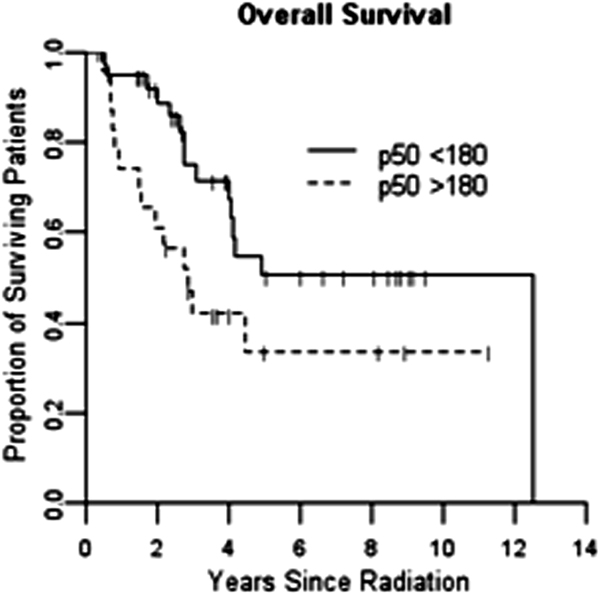
There was however, a significant association between p50 nuclear expression (above or below the median of 180 units) and overall survival (HR 2.15, p = 0.040) (figure 2) with high levels of nuclear expression associated with a poor prognosis There was a similar trend for p65 (HR 1.5, p = 0.28), but this did not demonstrate statistical significance. There was no apparent association between baseline p52 nuclear expression and survival.
Figure 2.
Kaplan-Meier curve for overall survival by p50 nuclear staining above or below median
Conclusions
There has been increasing interest in defining molecular markers of radiation response or resistance and long-term outcome for patients with solid tumors such as rectal cancer. A number of studies have attempted to provide guidance in this determination. Unfortunately, many of these studies have used tissue samples obtained at the time of surgical resection, after the delivery of the radiation therapy. This approach is suboptimal, as molecular changes produced by the radiation therapy itself may significantly affect the analysis. Additionally, tissue samples obtained after a course of radiation therapy are lacking the population of cells that have been killed by the radiation therapy, and this may represent the population of cells of most interest.
There is a large body of information demonstrating that insults to a cell will impact gene expression patterns, particularly by inducing expression of cellular damage response genes such as those regulated by NF- κB. Radiation represents such an insult, and it is has previously been demonstrated that cancer cells facilitate repair of injury induced by a radiation exposure by the transcription of a large number of genes aimed at repair and survival20–22. It is likely that even a single dose of radiation, as is delivered clinically during an extended 5–6 week course of therapy, could influence the gene expression pattern in a major way. Thus, the gene expression profile of a cell before the initiation of a radiation course may not be relevant to predicting the response of the tumor during the course of radiation therapy.
In order to better define these issues as it relates to rectal cancer, we initiated a clinical trial in a group of patients with rectal cancers that required pelvic radiation therapy combined with concurrent 5-fluorouracil chemotherapy. We obtained biopsies for immunohistochemistry and RT-PCR at three points: before the initiation of treatment, 2–6 hours after the first dose of radiation therapy and 24 hours after the first dose of radiation therapy. No significant changes in expression of NF- κB -related genes were seen at any early time-point (2–6 hours) suggesting that some time between 6 hours and 24 hours (or perhaps more) is optimal to assess gene expression changes in this setting.
We initially hoped to correlate information on gene expression changes with outcome as measured by pathological complete response, or near pathological complete responses. We are not aware of this type of study having ever been done before specifically evaluating radiation response or the NF-κB pathway. Debucquoy23 recently reported on 41 rectal cancer patients in whom biopsies were obtained before and after the administration of cetuximab. They found that cetuximab exposure down-regulated genes involved in proliferation and invasion and up-regulated inflammatory gene expression. This is the only other study, to our knowledge, where gene expression was evaluated during therapy in rectal cancer patients to determine the effect of a therapeutic intervention. Given the numbers of patients we were able to accrue to our prospective study, we were not able to achieve the goal of demonstrating that NF- κB -related gene induction per se was correlated with radiation response.
Our group previously performed studies that suggested the importance of the NF-κB pathway in colorectal cancer response to radiotherapy. In-vitro and in-vivo animal studies by Russo et al.14 demonstrated that NF- κB was induced by radiotherapy, that this induction led to poor response, and that abrogation of NF- κB induction by several means could lead to radiosensitization. We thus designed the present study to emphasize evaluation of the NF-κB pathway, and selectively measured portions of this pathway to correlate with the clinical end-points as well as other genes that were relevant to that pathway.
Eleven genes known to be transcriptional targets of activated p50/p65 were evaluated (inhibitors of apoptosis Bcl-2, Bcl-xL, cellular inhibitor of apoptosis protein [cIAP]1, cIAP2, Tumor necrosis factor receptor-associated factor [TRAF]1, TRAF2, the NF- κB regulated cytokine interleukin-8 [IL-8], the cell-cycle regulator cyclin D1, and regulators of NF- κB activation IKKε, IκBα). Four genes were shown to increase significantly after radiotherapy in our study including Bcl-2, cIAP-2, IL-8 and TRAF1; these showed 1.7–7.4 fold increases over baseline 24 hours after a single dose of irradiation. Bcl-2, cIAP-2 and TRAF1 are all well-described inhibitors of apoptosis. Upregulation of these genes supports the general idea that radiation induces NF- κB in spite of the fact that we could not demonstrate this directly utilizing the relatively crude method of immunohistochemistry.
Although studies of this type are difficult to perform, we were able to demonstrate the feasibility of obtaining the necessary biopsies. This required coordination between the study team, and a dedicated team of surgeons and radiation oncologists. Presumably because of the difficulty of this protocol for patients, we were not able to accrue the originally desired number of patients, and therefore added to the study an additional group of patients who were previously treated and had pre-treatment tumor tissue available to look at constitutive NF- κB expression at baseline. This turned out to be fruitful, as NF- κB p50 was in fact a prognostic factor for overall survival, but not a predictive factor for response to radiotherapy.
Previous studies have demonstrated the importance of NF-κB in advanced colorectal cancer outcomes, and these data are consistent with those studies. In fact, a study by Scartozzi et al. 24 suggests NF- κB expression as a predictive factor of response to a combination of irinotecan and cetuximab. Our data suggest that NF- κB may simply be prognostic given that our patients were treated only with 5-FU based adjuvant therapy. These conclusions are limited by relatively small numbers, and analysis of a larger dataset is planned.
Our data also suggest that all NF-κB subunits are not equal, and that some may be prognostic while others are not. The lack of correlation between the prognostic marker and the pathological response to preoperative radiation therapy with concurrent chemotherapy may suggest that NF-κB impacts on survival via promotion of the metastatic process and perhaps alters chemotherapy sensitivity rather than by affecting radiation therapy sensitivity primarily.
Lastly, when measuring expression of specific NF-κB-regulated genes, we were clearly able to demonstrate the induction of a subset of genes by a single dose of 180 cGy of radiation therapy. This change was seen only at the 24 hour time-point, and not at 2, 4 or 6 hours, giving some indication of the time course of these changes. The magnitude of these changes were such that they could potentially affect the response to the radiation course, although we were not able to demonstrate that given the small number of patients. Direct changes in nuclear NF- κB localization were not, however, large enough that they could be measured by immunohistochemistry. This has implications for how studies of this type are conducted. Assay sensitivity is an important issue, and an erroneous answer due to poor test sensitivity can impact on our understanding of the biology of tumor response to therapy.
SUMMARY
The reasons for cellular sensitivity to radiation therapy and chemotherapy are complex. Part of that complexity is due to the fact that the use of radiation therapy can induce gene expression that can then alter the response of the tumor to the therapeutic intervention. We have demonstrated, to our knowledge for the first time in patients, that such gene expression change does indeed occur and that the altered expression varies between molecular markers and that the changes do not reflect global up or down gene expression changes. We have also demonstrated that the NF-κB pathway is important in the long-term survival of rectal cancer patients, and that this is likely independent of radiation effect. Further studies of molecular markers of response may need to consider not only the baseline status of those markers, but also their status after the initiation of therapy. We have continued to obtain fresh-frozen biopsies of tumors preoperatively, and our future efforts will be aimed at discovery using both microarray and high-throughput sequencing techniques to try and identify novel, potentially targetable predictors of radiotherapy response.
Acknowlegements
The study is funded by P50 CA106991-06 and K23 CA118431-04. We thank UNC Translational Pathology Laboratory (TPL) for expert technical assistance. The UNC TPL is supported, in part, by grants from the National Cancer Institute (3P30CA016086), National Institute of Environmental Health Sciences (3P30ES010126), Department of Defense (W81XWH-09-2-0042), and the UNC University Cancer Research Fund (UCRF).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.American Cancer Society. Cancer facts & figures. New York: American Cancer Society; 2008. [Google Scholar]
- 2.Kapiteijn E, Kranenbarg EK, Steup WH, et al. Total mesorectal excision (TME) with or without preoperative radiotherapy in the treatment of primary rectal cancer. Prospective randomised trial with standard operative and histopathological techniques. Dutch ColoRectal Cancer Group. Eur J Surg. 1999;165:410–420. doi: 10.1080/110241599750006613. [DOI] [PubMed] [Google Scholar]
- 3.Crane CH, Skibber JM, Birnbaum EH, et al. The addition of continuous infusion 5-FU to preoperative radiation therapy increases tumor response, leading to increased sphincter preservation in locally advanced rectal cancer. International Journal of Radiation Oncology*Biology*Physics. 2003;57:84–89. doi: 10.1016/s0360-3016(03)00532-7. [DOI] [PubMed] [Google Scholar]
- 4.Sauer R, Becker H, Hohenberger W, et al. Preoperative versus Postoperative Chemoradiotherapy for Rectal Cancer. N Engl J Med. 2004;351:1731–1740. doi: 10.1056/NEJMoa040694. [DOI] [PubMed] [Google Scholar]
- 5.Jen KY, Cheung VG. Transcriptional response of lymphoblastoid cells to ionizing radiation. Genome Res. 2003;13:2092–2100. doi: 10.1101/gr.1240103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Liu SS, Cheung AN, Ngan HY. Differential gene expression in cervical cancer cell lines before and after ionizing radiation. Int J Oncol. 2003;22:1091–1099. [PubMed] [Google Scholar]
- 7.Park WY, Hwang CI, Im CN, et al. Identification of radiation-specific responses from gene expression profile. Oncogene. 2002;21:8521–8528. doi: 10.1038/sj.onc.1205977. [DOI] [PubMed] [Google Scholar]
- 8.Baldwin AS. Control of oncogenesis and cancer therapy resistance by the transcription factor NF-kappaB. J Clin Invest. 2001;107:241–246. doi: 10.1172/JCI11991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cusack JC, Jr, Liu R, Houston M, et al. Enhanced chemosensitivity to CPT-11 with proteasome inhibitor PS-341: implications for systemic nuclear factor-kappaB inhibition. Cancer Research. 2001;61:3535–3540. [PubMed] [Google Scholar]
- 10.Wang CY, Guttridge DC, Mayo MW, Baldwin AS., Jr NF-kappaB induces expression of the Bcl-2 homologue A1/Bfl-1 to preferentially suppress chemotherapy-induced apoptosis. Mol Cell Biol. 1999;19:5923–5929. doi: 10.1128/mcb.19.9.5923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Vallat L, Magdelenat H, Merle-Beral H, et al. The resistance of B-CLL cells to DNA damage-induced apoptosis defined by DNA microarrays. Blood. 2003;101:4598–4606. doi: 10.1182/blood-2002-06-1743. [DOI] [PubMed] [Google Scholar]
- 12.Li Z, Li JJ. Effector genes altered in mcf-7 human breast cancer cells after exposure to fractionated ionizing radiation. Radiat Res. 2001;155:543–553. doi: 10.1667/0033-7587(2001)155[0543:egaimh]2.0.co;2. [DOI] [PubMed] [Google Scholar]
- 13.Didelot C, Barberi-Heyob M, Bianchi A, et al. Constitutive NF-kappaB activity influences basal apoptosis and radiosensitivity of head-and-neck carcinoma cell lines. Int J Radiat Oncol Biol Phys. 2001;51:1354–1360. doi: 10.1016/s0360-3016(01)02608-6. [DOI] [PubMed] [Google Scholar]
- 14.Russo SM, Tepper JE, Baldwin AS, Jr, et al. Enhancement of radiosensitivity by proteasome inhibition: implications for a role of NF-kappaB. International Journal of Radiation Oncology, Biology, Physics. 2001;50:183–193. doi: 10.1016/s0360-3016(01)01446-8. [DOI] [PubMed] [Google Scholar]
- 15.Cogswell PC, Guttridge DC, Funkhouser WK, Baldwin AS., Jr Selective activation of NF-kappa B subunits in human breast cancer: potential roles for NF-kappa B2/p52 and for Bcl-3. Oncogene. 2000;19:1123–1131. doi: 10.1038/sj.onc.1203412. [DOI] [PubMed] [Google Scholar]
- 16.Nakayama H, Ikebe T, Beppu M, Shirasuna K. High expression levels of nuclear factor kappaB, IkappaB kinase alpha and Akt kinase in squamous cell carcinoma of the oral cavity. Cancer. 2001;92:3037–3044. doi: 10.1002/1097-0142(20011215)92:12<3037::aid-cncr10171>3.0.co;2-#. [DOI] [PubMed] [Google Scholar]
- 17.Wang W, Abbruzzese JL, Evans DB, Larry L, Cleary KR, Chiao PJ. The nuclear factor-kappa B RelA transcription factor is constitutively activated in human pancreatic adenocarcinoma cells. Clin Cancer Res. 1999;5:119–127. [PubMed] [Google Scholar]
- 18.Evertsson S, Sun XF. Protein expression of NF-kappaB in human colorectal adenocarcinoma. Int J Mol Med. 2002;10:547–550. [PubMed] [Google Scholar]
- 19.O'Neil BH, Buzkova P, Farrah H, et al. Expression of nuclear factor-kappaB family proteins in hepatocellular carcinomas. Oncology. 2007;72:97–104. doi: 10.1159/000111116. [DOI] [PubMed] [Google Scholar]
- 20.Amundson SA, Do KT, Vinikoor LC, et al. Integrating global gene expression and radiation survival parameters across the 60 cell lines of the National Cancer Institute Anticancer Drug Screen. Cancer Res. 2008;68:415–424. doi: 10.1158/0008-5472.CAN-07-2120. [DOI] [PubMed] [Google Scholar]
- 21.Amundson SA, Lee RA, Koch-Paiz CA, et al. Differential responses of stress genes to low dose-rate gamma irradiation. Mol Cancer Res. 2003;1:445–452. [PubMed] [Google Scholar]
- 22.Fornace AJ, Jr, Amundson SA, Bittner M, et al. The complexity of radiation stress responses: analysis by informatics and functional genomics approaches. Gene Expr. 1999;7:387–400. [PMC free article] [PubMed] [Google Scholar]
- 23.Debucquoy A, Haustermans K, Daemen A, et al. Molecular response to cetuximab and efficacy of preoperative cetuximab-based chemoradiation in rectal cancer. J Clin Oncol. 2009;27:2751–2757. doi: 10.1200/JCO.2008.18.5033. [DOI] [PubMed] [Google Scholar]
- 24.Scartozzi M, Bearzi I, Pierantoni C, et al. Nuclear factor-kB tumor expression predicts response and survival in irinotecan-refractory metastatic colorectal cancer treated with cetuximab-irinotecan therapy. J Clin Oncol. 2007;25:3930–3935. doi: 10.1200/JCO.2007.11.5022. [DOI] [PubMed] [Google Scholar]