Abstract
The small heat shock protein αB-crystallin (HspB5) is known to be overexpressed in several neurodegenerative disorders. In familial amyloidotic polyneuropathy (FAP), a neurodegenerative disorder characterized by extracellular deposition of mutated transthyretin (TTR), activation of heat shock factor 1 -HSF1- by extracellular TTR deposition has been shown as well as induction of the expression of heat shock proteins, HSP27 and HSP70. Here we investigate the expression of αB-crystallin in FAP. We first detected αB-crystallin in aggregates extracted from tissues of both FAP patients and transgenic mice for the human V30M mutant TTR; however, subsequent studies by confocal fluorescence microscopy did not confirm the association of αB-crystallin with TTR aggregates; thus the presence of αB-crystallin in aggregate extracts might derive from the extraction procedure. Increased levels of αB-crystallin were observed by immunohistochemistry in human FAP skin, as compared to normal skin. Furthermore, skin, stomach and dorsal root ganglia from V30M transgenic mice showed increased expression of αB-crystallin as compared to controls without deposition. A human neuroblastoma cell line incubated with TTR aggregates displayed increased expression of αB-crystallin. Overall, these results show that extracellular TTR deposits induce an intracellular response of αB-crystallin. This small heat shock protein (HSP), which is important for anti-apoptotic and chaperone properties, may have a protective role in FAP.
Keywords: amyloid, crystallin, heat shock response, transthyretin
Heat shock proteins (Hsps) expression is increased in response to heat shock or other stresses including protein misfolding and aggregation. Activation of the heat shock factor-1 (HSF-1) has a major role in this process; small Hsps of low molecular masses (<43 kDa) are a family of chaperones that have a conserved α-crystallin domain in the c-terminal domain; αB-crystallin (HspB5), is a small Hsp (Klemenz et al. 1991) that was primarily found in the eye lens and is constitutively expressed in many tissues. Anti-apoptotic properties of αB-crystallin have been described; thus, it binds to pro-apoptotic Bax, Bcl-xs and p53 and prevents their translocation to mitochondria (Mao et al. 2004; Liu et al. 2007); moreover, αB-crystallin inihibits the activation of pro-caspase-3 (Kamradt et al. 2005); phosphorylation at three serine residues (Ser19, Ser45 and Ser59) in αB-crystallin regulates its chaperone activity (Ecroyd et al. 2007). αB-crystallin can form oligomers with other Hsps, namely with HSP27 and presents ATP-independent chaperone activity. Oligomer size and chaperone activity is modified by phosphorylation (Jakob et al. 1993); ‘in vitro’, αB-crystallin inhibits fibril formation of amyloid β-peptide and β2-microglobulin (Raman et al. 2005). Increased expression of αB-crystallin has been found in Alzheimer disease (AD) (Björkdahl et al. 2008); however, there is no co-localization of αB-crystallin and amyloid β- peptide in senile plaques of AD brains (Wilhelmus et al. 2006). The presence of αB-crystallin in alpha-synuclein inclusions was described as well, but in this case, αB-crystallin co-localized with alpha-synuclein in Lewy bodies (Outeiro et al. 2006). In mouse models of Parkinson disease the levels of αB-crystallin were found to be higher than controls; in Huntington's disease, it was found that mice lacking αB-crystallin had accelerated onset and severity in aggregation (Ecroyd & Carver 2009). All these data shows association of αB-crystallin with neurodegenerative disorders and reveal a probable protection function for αB-crystallin.
Familial amyloid polyneuropathy (FAP) is an autosomal dominant neurodegenerative disorder characterized by the systemic extracellular deposition of mutated transthyretin (TTR) that affects particularly the peripheral nervous system (PNS) (Andrade 1952; Costa et al. 1978). The most common mutation associated with FAP is TTRV30M, (Saraiva et al. 1984). TTR is a tetrameric serum protein of four identical subunits of 14 kDa. Amyloidogenic mutations on TTR favour destabilization and dissociation of the tetrameric structure, leading to misfolded intermediates with high tendency for extracellular aggregation (Cardoso et al. 2002). In asymptomatic carriers (FAP 0) deposition of TTR in an aggregated non-fibrillar form occurs. In later stages of the disease, non-fibrillar and fibrillar deposits co-exist (Sousa et al. 2001a).
Recently, the heat shock response was investigated in FAP through expression analyses of heat shock factor 1 (HSF1), HSP27 and HSP70. It was demonstrated that in FAP, extracellular TTR deposition induces intracellular activation of HSF1 and increases expression of HSP27 and HS70 (Santos et al. 2008). Here, we investigate the presence of αB-crystallin in TTR tissue aggregate extracts from human FAP and transgenic mice for human V30M TTR. We also analyzed the expression of αB-crystallin in FAP biopsies, in tissues from transgenic mice and in a human neuroblastoma cell line incubated with TTR aggregates.
Materials and methods
Human tissue samples
Autopsy kidney tissues from V30M FAP patients and normal controls were available at the Hospital Geral de Santo António, Porto, Portugal. Skin from FAP patients and normal controls was obtained as part of the clinical diagnosis and evaluation of FAP, prior to the current use of less invasive molecular diagnostic methods. The use of these materials was approved by the ethical committee of Hospital Geral de Santo António, Porto, Portugal. Human tissue processing and characterization was performed as described previously (Santos et al. 2008) and included both asymptomatic carriers (FAP 0) and patients (FAP). Control biopsies were from FAP relatives who were later characterized as non-V30M carriers.
Mouse tissue samples
Different strains of transgenic mice for TTR V30M, all in the Sv129 background were used in this study: (i) TTRV30M without endogeneous TTR, labelled hTTR (Kohno et al. 1997); (ii) this strain crossed to homozygous or heterozygous heat shock transcription factor 1 (HSF1) null mice, labelled either hTTR HSF1 or hTTR HSF1-het respectively (Santos et al. 2010).
Animals age ranged from 6 to 18 months. The animals were housed in pathogen-free conditions in a controlled-temperature room, maintained under a 12 h light/dark period. Water and food were freely available. Animal experiments were carried out in accordance with the European Communities Council Directive. Tissues were processed for immunohistochemistry, as previously described (Santos et al. 2008) or frozen for further analysis. Mice tissues were analyzed for TTR deposition by immunohistochemistry as previously described (Sousa et al. 2002) and classified by the presence and types of deposits: TTR–/– no TTR deposits; TTR+/− only non-fibrillar TTR deposits (congo red negative); TTR+/+ non-fibrillar and fibrillar TTR deposition (congo red positive)
Aggregate extraction
For aggregate extraction, we used the Kaplan method (Kaplan et al. 1994); 100 mg of autopsy samples of human FAP and control kidney and stomach from transgenic mice (n = 3) were homogenized in ice cold phosphate buffered solution (PBS; 1 ml), centrifuged at 19,000 g for 10 min and 1 ml of PBS added to the pellet. This procedure was repeated twice; the pellet was then rinsed with distilled water and resuspended in 20% acetonitrile/0.1% trifluoracetic acid (TFA), incubated for 1 h at room temperature with mixing and centrifuged at 19,000 g for 10 min; the supernatant was saved and the extraction repeated twice. The supernatants were pooled and lyophilized. The samples were resuspended in Laemeli SDS sample buffer, applied in a 15% SDS-PAGE gel and analyzed by western blotting for αB-crystallin (1:1000; abcam, Cambridge, UK); parallel silver stained bands were subjected to mass spectrometry analysis.
Mass spectrometry
Human kidney aggregate extracts were run in SDS-PAGE and silver stained. After silver stain, a band with molecular weight above the normal migration of the TTR monomer and with molecular weight corresponding to αB-crystallin, was excised from the gel and digested with endoproteinase Lys-C. MALDI mass spectroscopic analysis was performed on a PerSeptive Voyager mass spectrometer in the linear mode (Henzel et al. 1993).
Western blot
Transgenic mice stomach samples (n = 4) were homogeneized in lysis buffer: 20 mM MOPS, 2 mM EGTA, 5 mM EDTA, 30 mM sodium fluoride, 40 mM β-glycerophosphate, 20 mM sodium pyrophosphate, 1 mM sodium orthovanadate, 1 mM phenylmethylsulphonyl fluoride, 0.5% Triton X-100 and 1x protease inhibitors mixture (Amersham Bioscience, Little Chalfont, UK). Total protein concentration was determined using the Bio-Rad assay kit (Bio-Rad, Hercules, CA, USA). 40 μg of total protein was applied to 12% SDS-PAGE and transferred onto a nitrocellulose Hybond-C membrane (Amersham Bioscience) using a semidry system. The primary antibodies used were rabbit anti-αB-crystallin (1:1000; abcam) and rabbit anti-β-actin (1:250; Sigma-Aldrich, St. Louis, MO, USA) for normalization. To develop the blots, enhanced chemiluminescence (ECL, Amersham Bioscience) was performed. Quantitative analysis of the western blots was performed with ImageQuant software.
Immunohistochemistry
Tissue paraffin slides of human and transgenic mice skin were deparaffinated and hydrated. Epitope exposure with citrate buffer was performed; endogenous peroxidase activity was blocked with 3% hydrogen peroxide in methanol for 30 min. The slides were incubated in blocking buffer (4% foetal bovine serum and 1% bovine serum albumin in PBS) for 1 h at 37 ºC in a humidified chamber. Incubation with primary rabbit alphaB-crystallin antibody (1:1000; Calbiochem, San Diego, CA, USA) in blocking buffer was performed overnight at 4 ºC. Antibody binding was detected by a biotin-extravidin-peroxidase kit (Sigma-Aldrich) using as substrate 3-amino-9-ethyl carbaxole, AEC, (Sigma). Semi-quantitative analysis of the immunostaining was performed in four different areas of the tissue with Image Pro-Plus software; obtained results represent the percentage of occupied area by the substrate reaction colour normalized with the total area occupied by the tissue. The observer was blind to treatment/control samples statistical significance was determined using Student's t-test, a minimal P value of <0.05 was required for significance. Experiments were carried out in four tissue samples from transgenic mice and in five samples from human skin tissues.
For double-immunofluorescence, paraffin slides of human skin were treated as previously described for immunohistochemistry. Primary antibodies were: mouse anti-human TTR (mab15 1 μg/ml) kindly provided by Dr Erik Lundgren from Umea University (Pokrzywa et al. 2007) and rabbit αβ-crystallin (1:200; abcam), incubated overnight at 4 ºC in blocking buffer. The secondary antibodies used were Alexa Fluor 568 anti-mouse and Alexa Fluor 488 anti-rabbit (1:1000; Molecular Probes, Eugene, OR, USA) which were incubated for 1 h at room temperature protected from light. Slides were mounted with Vectashield (Vector, Burlingame, CA, USA) and visualized by confocal microscopy (Leica TCS SP2 AOBS).
Protein
Recombinant wild-type TTR was produced in an Escherichia coli BL21 expression system and purified as previously described (Furuya et al. 1991). TTR was dialyzed against water, diluted to 0.250 mg ml−1 and then stirred at room temperature for 7 days. Oligomeric preparations were tested by thioflavin T spectrofluorometric assay and were stable in serum free media as previously described (Teixeira et al. 2006).
Cell culture assays
Cells were maintained at 37 ºC in a 5% CO2 humidified atmosphere. A SH-SY5Y human neuroblastoma cell line (European Collection of Cell Cultures) was cultured in MEM:F12 (Gibco; Invitrogen, Grand Island, NY, USA) supplemented with 15% foetal bovine serum (Gibco, Invitrogen), 2 mmol−1 of glutamine (Gibco, Invitrogen), 100 Uml−1 penincillin/streptavidin (Gibco, Invitrogen) and 1 mmol−1 of non-essential amino acids (Sigma-Aldrich). One hour before incubation, cell medium was changed to serum free medium. Cells were incubated for 8 h with oligomeric and soluble TTR (0.110 mg ml−1) and collected in Trizol (Invitrogen) for RNA extraction which was performed according to the manufacturer's instructions. Synthesis of cDNA was performed using the SuperScript double-stranded cDNA Kit (Invitrogen). Reverse-transcriptase polymerase chain reaction was performed for human αB-crystallin with primers: forward 5′-AGCTGGTTTGACACTGGACT-3′ (annealing from nucleotide 200–219, exon 1) and reverse 5′-GCAATTCAAGAAAGGGCATC-3′ (annealing from nucleotide 572–553, exon 3); for human β-actin primers were: forward 5′-ATGGATGATGATATCGCCGCG-3′ (annealing from nucleotide 85–105, exon 2) and reverse 5′-TCTCCATGTCGTCCCAGTTG-3′ (annealing from nucleotide 334–315, exon 3). The amplification products were quantified with the ImageQuant software. Three independent experiments with duplicates were performed.
Results
Human αB-crystallin in aggregate preparations
We analyzed TTR aggregates extracted from human kidney samples and from stomach of transgenic mice. Human kidney aggregate extracts from FAP and control human kidney were separated by SDS-PAGE followed by silver staining; a band at approximately 20 kDa migrating above the TTR monomer (Figure 1a) was subjected to mass spectrometry and was identified as human αB-crystallin. To confirm the presence of αB-crystallin in the preparation, the same extract was then analyzed by western blot for αB-crystallin; as seen in Figure 1b i), αB-crystallin was detected in the FAP preparation and was absent in normal kidney extracts. TTR preparations from the stomach of transgenic mice with or without TTR deposition (TTR+ and TTR− respectively) were also analysed by western blot for the presence of αB-crystallin; this protein was detected in the preparations containing TTR aggregates, as opposed to the preparation derived from tissues without TTR deposits (Figure 1b ii), indicating that αB-crystallin might be a component of TTR deposits.
Figure 1.
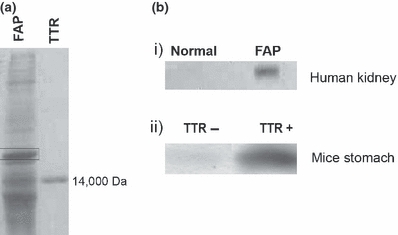
(a) SDS-PAGE silver stain of FAP human kidney aggregate extracts and TTR standard; the αB-crystallin band is pointed out by a rectangle. (b) αB-crystallin western blot of i) human normal and FAP kidney; ii) transgenic mice stomach aggregate extracts (n = 3 each) with and without TTR deposition (TTR+ and TTR− respectively)
αB-crystallin localization in human FAP skin
Because αB-crystallin was detected in human TTR aggregate preparations we examined by double immunofluorescence staining the localization of αB-crystallin and TTR deposits in human skin from FAP patients; no co-localization was found; thus, αB-crystallin stained intracellularly whilst TTR deposits were found extracellularly (Figure 2).
Figure 2.
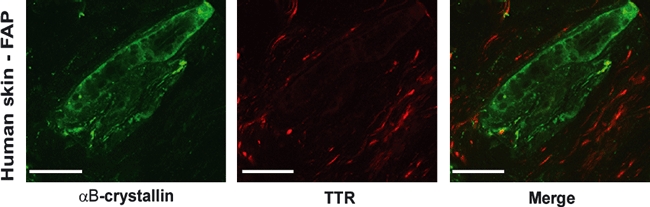
Immunofluorescence in human FAP skin for TTR (red) and αB-crystallin (green) showing no co-localization of the two proteins, with αB-crystallin staining intracellularly and TTR extracellularly (bar = 50μm).
Increased αB-crystallin expression in human FAP tissues and in transgenic mouse tissues with TTR deposition
We investigated the expression of αB-crystallin in human skin biopsies of FAP patients with proven TTR deposits, of asymptomatic carriers of TTR V30M (FAP0) and of control biopsies by semi-quantitative immunohistochemistry. Both FAP 0 and FAP samples showed a significant increase of αB-crystallin expression when compared to normal individuals (Figure 3a).
Figure 3.
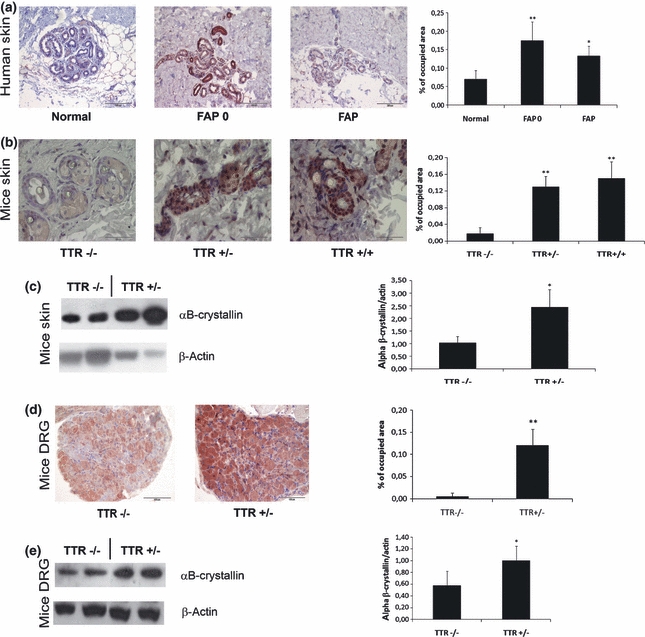
TTR deposition induces αB-crystallin expression. (a) immunohistochemistry of human skin, showing increased expression in FAP 0 (n = 5) and FAP patients (n = 3) when compared to controls (n = 5) (bar = 200 μm); (b) immunohistochemistry of transgenic mice skin: increased expression of αB-crystallin in TTR+/− (n = 4) and TTR+/+ (n = 4) transgenic mice when compared to mice without TTR deposits, TTR−/− (n = 5) (bar = 50 μm); (c) western blot of transgenic mice skin for αB-crystallin: increased expression of αB-crystallin in animals with TTR deposits (TTR+/−, n = 4) when compared with animals without TTR deposits (TTR−/−, n = 4); (d) immunohistochemistry of hTTR HSF1-het transgenic mice DRG, showing increased expression of αB-crystallin in mice with TTR deposits (TTR+/−, n = 4) when compared to mice without TTR deposition (TTR−/−, n = 4) (bar = 100μm); (e) Western blot analysis of αB-crystallin expression in DRG of hTTR HSF1-het showing increased expression of αB-crystallin in mice with TTR deposits (TTR+/−, n = 7) when compared to mice without TTR deposition (TTR−/−, n = 7). Quantification of images and blots are shown on the charts on the right (*P < 0.01, **P < 0.005).
Next, we investigated the expression of αB-crystallin in transgenic mice. Mice transgenic for TTR V30M in a wild type background (hTTR) present systemic TTR deposition in several tissues, including skin, gastrointestinal track but not in the peripheral nervous system, as humans do. Skin and stomach tissues with and without TTR deposition were used to investigate αB-crystallin expression by semi-quantitative immunohistochemistry. Increased expression of αB-crystallin was detected in both skin (Figure 3b) and stomach (data not shown) of mice with deposited TTR, independently of the type of deposit, i.e., it occurs in tissues with fibrillar or non-fibrillar deposits. To confirm this result we performed western blot analysis of skin homogenates from TTR V30M transgenic mice with and without TTR deposition; the level of αB-crystallin was significantly increased in transgenic animals with TTR deposition (Figure 3c).
Next, we investigated αB-crystallin expression in dorsal root ganglia (DRG) of transgenic mice for TTR V30M in a heterozygotic HSF1 background (hTTR HSF1-het); this strain of mice present TTR deposition in the peripheral nervous system. Therefore, immunohistochemistry and western blot was performed in mice DRG with and without deposition of TTR. The results show increased expression of αB-crystallin in DRG with TTR deposition (Figures 3d and e). Transgenic mice for TTR V30M without HSF1 (hTTR HSF1) do not show any αB-crystallin response to TTR deposition, as expected, since αB-crystallin is under the control of HSF1 (data not shown).
Increased αB-crystallin expression is induced by small TTR oligomers
The human neuroblastoma SH-SY5Y cell line was used to analyze the effect of small TTR oligomers on αB-crystallin expression. We found that oligomeric TTR (oTTR) increases αB-crystallin mRNA (Figure 4a) and protein expression (Figure 4b), as compared to non-treated cells (NT), or cells incubated with soluble TTR (sTTR).
Figure 4.
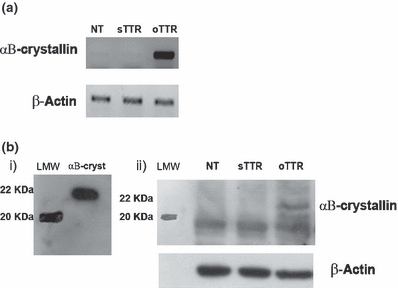
αB-crystallin expression in human neuroblastoma cells (SH-SY5Y) (a) αB-crystallin reverse transcriptase polymerase chain reaction of SH-SY5Y cells incubated with TTR oligomers for 8 h. Up-regulation of αB-crystallin mRNA is observed in cells incubated with TTR oligomers (oTTR) when compared to controls. (b) Western Blot analysis of αB-crystallin in SH-SY5Y cells incubated with TTR oligomers for 8 h. i) Western blot of recombinant αB-crystallin (22 kDa). ii) Increase expression of αB-crystallin in cells incubated with TTR oligomers when compared to controls. This result was confirmed by three independent experiments. (LMW represents low molecular weight standard); an unspecific band below 20 kDa is observed in all samples.
Discussion
Different reports point out neuroprotection properties of HSPs in neurodegenerative disorders (Sittler et al. 2001; Magranéet al. 2004; Shen et al. 2005). In AD, HSPs are known to be associated with senile plaques (Wilhelmus et al. 2006), which might impact in delaying AD pathology. Concerning the small Hsp αB-crystallin a study showed increased expression in AD patients brains, mainly in astrocytes, microglia, and oligodendrocytes restricted to areas with senile plaques and neurofibrillary tangles (Renkawek et al.1994); other study, showed that three αB-crystallin-related small Hsps co-localized with intracellular β amyloid peptide indicating that chaperone activity might modulate intracellular β amyloid peptide metabolism and toxicity (Fonte et al. 2002). These reports, point out to an important role of αB-crystallin in neurodegenerative disorders.
Here, we first detected the presence of αB-crystallin in human and transgenic mice TTR aggregate extracts suggestive of a direct interaction between extracellular deposited TTR and this chaperone; an in vitro study showed that specific interactive sequences of human αB-crystallin modulate the fibrillation of TTR (Ghosh et al. 2008), hypothesizing a possible important role for this protein in FAP; however, in view of our analyses in human FAP skin by confocal fluorescence microscopy that did not reveal any co-localization between TTR deposits and αB-crystallin, a direct in vivo TTR (or TTR aggregates)–αB-crystallin interaction is highly unlikely to occur. This discrepancy in the results of aggregate analysis and confocal microscopy might be due to the extraction procedure; thus, during this procedure, cells burst and release intracellular proteins, such as chaperones that then contact with extracellular aggregates.
We also analyzed the expression of αB-crystallin in tissues that do not synthesize TTR. Increased levels of αB-crystallin were detected both in human tissues as well as in transgenic mice when extracellular TTR aggregates were evident; this increased expression was already detected in initial stages of deposition, when non-fibrillar TTR material (mice classified TTR+/− and asymptomatic FAP carriers) is present and mature fibrillar material is still absent. Thus, the αB-crystallin response is an early event in FAP. Intracellular αB-crystallin increase in the presence of TTR aggregates was also evident in our cellular studies in a neuroblastoma cellular model.
Recently, it was observed that extracellular TTR deposition is capable of exerting an intracellular stress response by increasing the expression of hsp 27 and hsp70 (Santos et al. 2008, 2010). Like hsp27 and hsp70, αB-crystallin is probably being over-expressed as one of the molecular chaperones involved in this general stress response to extracellular TTR deposition. A recent study showed that UPS (ubiquitin-proteasome system) is impaired in FAP and that unfolded/aggregated proteins accumulate inside cells (Santos et al. 2007); it is possible that αB-crystallin, as a molecular chaperone, is being over-expressed in order to deal with the increase of intracellular aggregates. Like other HSPs, αB-crystallin is known to be anti-apoptotic; it can interact with proteins in the apoptotic caspase dependent pathway modulating cell death (Mao et al. 2004; Kamradt et al. 2005). This fact is relevant in FAP since cellular death occurs, at least in part, through caspase-3 activation (Sousa et al. 2001b). In FAP there is also evidence of oxidative stress and inflammation (Sousa et al. 2001a; b; Macedo et al. 2007); published data show that αB-crystallin modulates inflammatory (TNFα) and oxidative stress pathways (Mehlen et al. 1996). It is plausible that αB-crystallin, as a defense mechanism, enhances survival or delay cell death by all these different pathways.
Acknowledgments
We thank Tânia Ribeiro, Rossana Correia, Paul Moreira and Paula Sampaio from IBMC for animal maintenance, tissue processing, TTR production, and confocal microscopy respectively. The authors also thank João Sousa now at Minho University (Portugal) for support in aggregate extraction studies. This study was supported by Fundação para a Ciência e Tecnologia (FCT) through fellowships SFRH/BD/35980/2007 (JM) and SFRH/BPD/20738/2004 (SDS) and grant PIC/IC/82824/2007.
References
- Andrade C. A peculiar form of peripheral neuropathy. Familial atypical generalized amyloidosis with special involvement of the peripheral nerves. Brain. 1952;75:408–427. doi: 10.1093/brain/75.3.408. [DOI] [PubMed] [Google Scholar]
- Björkdahl C, Sjögren MJ, Zhou X, et al. Small heat shock proteins Hsp27 or αB-crystallin and the protein components of neurofibrillary tangles: tau and neurofilaments. J. Neurosci. Res. 2008;86:1343–1352. doi: 10.1002/jnr.21589. [DOI] [PubMed] [Google Scholar]
- Cardoso I, Goldsbury CS, Müller SA, et al. Transthyretin fibrillogenesis entails the assembly of monomers: a molecular model for in vitro assembled transthyretin amyloid-like fibrils. J. Mol. Biol. 2002;317:683–695. doi: 10.1006/jmbi.2002.5441. [DOI] [PubMed] [Google Scholar]
- Costa PP, Figueira AS, Bravo FR. Amyloid fibril protein related to prealbumin in familial amyloidotic polyneuropathy. Proc. Natl. Acad. Sci. U S A. 1978;75:4499–4503. doi: 10.1073/pnas.75.9.4499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecroyd H, Carver J. Crystallin proteins and amyloid fibrils. Cell. Mol. Life Sci. 2009;66:62–81. doi: 10.1007/s00018-008-8327-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ecroyd H, Meehan S, Horwitz J, et al. Mimicking phosphorylation of αB-crystallin affects its chaperone activity. Biochem. J. 2007;401:129–141. doi: 10.1042/BJ20060981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fonte V, Kapulkin V, Taft A, Fluet A, Friedman D, Link CD. Interaction of intracellular beta amyloid peptide with chaperone proteins. Proc. Natl. Acad. Sci. U S A. 2002;99:9439–9444. doi: 10.1073/pnas.152313999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Furuya H, Saraiva MJ, Gawinowicz MA, et al. Production of recombinant human transthyretin with biological activities toward the understanding of the molecular basis of familial amyloidotic polyneuropathy (FAP) Biochemistry. 1991;30:2415–2421. doi: 10.1021/bi00223a017. [DOI] [PubMed] [Google Scholar]
- Ghosh JG, Houck SA, Clark JI. Interactive sequences in the molecular chaperone, human αb crystallin modulate the fibrillation of amyloidogenic proteins. Int. J. Biochem. Cell Biol. 2008;40:954–967. doi: 10.1016/j.biocel.2007.10.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henzel WJ, Billeci TM, Stults JT, Wond SC, Grimley C, Watanabe C. Identifying proteins from two-dimensional gels by molecular mass searching of peptide fragments in protein sequence databases. Proc. Natl. Acad. Sci. U S A. 1993;90:5011–5015. doi: 10.1073/pnas.90.11.5011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- Kamradt MC, Lu M, Werner ME, et al. The small heat shock protein αB-crystallin is a novel inhibitor of TRAIL-induced apoptosis that suppresses the activation of caspase-3. J. Biol. Chem. 2005;280:11059–11066. doi: 10.1074/jbc.M413382200. [DOI] [PubMed] [Google Scholar]
- Kaplan B, German G, Ravid M, Pras M. Determination of amyloid type by ELISA using milligram amounts of tissue. Clin. Chim. Acta. 1994;229:171–179. doi: 10.1016/0009-8981(94)90239-9. [DOI] [PubMed] [Google Scholar]
- Klemenz R, Fröhli E, Steiger RH, Schäfer R, Aoyama A. αB-crystallin is a small heat shock protein. PNAS. 1991;88:3652–3656. doi: 10.1073/pnas.88.9.3652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kohno K, Palha JA, Miyakawa K, et al. Analysis of amyloid deposition in a transgenic mouse model of homozygous familial amyloidotic polyneuropathy. Am. J. Pathol. 1997;150:1497–1508. [PMC free article] [PubMed] [Google Scholar]
- Liu S, Li J, Tao Y, Xiao X. Small heat shock protein AlphaB-crystallin binds to p53 to sequester its translocation to mitochondria during hydrogen peroxide-induced apoptosis. Biochem. Biophys. Res. Commun. 2007;354:109–114. doi: 10.1016/j.bbrc.2006.12.152. [DOI] [PubMed] [Google Scholar]
- Macedo B, Batista AR, do Amaral JB, Saraiva MJ. Biomarkers in the assessment of therapies for Familial Amyloidotic Polyneuropathy. Mol. Med. 2007;13:584–591. doi: 10.2119/2007-00068.Macedo. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magrané J, Smith RC, Walsh K, Querfurth HW. Heat shock protein 70 participates in the neuroprotective response to intracellularly expressed beta-amyloid in neurons. J. Neurosci. 2004;24:1700–1706. doi: 10.1523/JNEUROSCI.4330-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mao YW, Liu JP, Xiang H, Li DW. Human AlphaA- and AlphaB-crystallins bind to Bax and Bcl-X(S) to sequester their translocation during staurosporine-induced apoptosis. Cell Death Differ. 2004;11:512–526. doi: 10.1038/sj.cdd.4401384. [DOI] [PubMed] [Google Scholar]
- Mehlen P, Kretz-Remy C, Préville X, Arrigo AP. Human hsp27, Drosophila hsp27 and human αb-crystallin expression-mediated increase in glutathione is essential for the protective activity of these proteins against TNFalpha-induced cell death. EMBO J. 1996;15:2695–2706. [PMC free article] [PubMed] [Google Scholar]
- Outeiro TF, Klucken J, Strathearn KE, et al. Small heat shock proteins protect against alpha-synuclein-induced toxicity and aggregation. Biochem. Biophys. Res. Commun. 2006;351:631–638. doi: 10.1016/j.bbrc.2006.10.085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pokrzywa M, Dacklin I, Hultmark D, Lundgren E. Misfolded transthyretin causes behavioral changes in a Drosophila model for transthyretin-associated amyloidosis. Eur. J. Neurosci. 2007;26:913–924. doi: 10.1111/j.1460-9568.2007.05728.x. [DOI] [PubMed] [Google Scholar]
- Raman B, Ban T, Sakai M, et al. ΑlphaB-crystallin, a small heat-shock protein, prevents the amyloid fibril growth of an amyloid beta-peptide and beta2-microglobulin. Biochem. J. 2005;392:573–581. doi: 10.1042/BJ20050339. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Renkawek K, Voorter CE, Bosman GJ, van Workum FP, de Jong WW. Expression of αb-crystallin in Alzheimer's disease. Acta Neuropathol. 1994;87:155–160. doi: 10.1007/BF00296185. [DOI] [PubMed] [Google Scholar]
- Santos SD, Cardoso I, Magalhães J, Saraiva MJ. Impairment of the ubiquitin-proteasome system associated with extracellular transthyretin aggregates in familial amyloidotic polyneuropathy. J. Pathol. 2007;213:200–209. doi: 10.1002/path.2224. [DOI] [PubMed] [Google Scholar]
- Santos SD, Magalhães J, Saraiva MJ. Activation of the heat shock response in familial amyloidotic polyneuropathy. J. Neuropathol. Exp. Neurol. 2008;67:449–455. doi: 10.1097/NEN.0b013e31816fd648. [DOI] [PubMed] [Google Scholar]
- Santos SD, Fernandes R, Saraiva MJ. The heat shock response modulates transthyretin deposition in the peripheral and autonomic nervous systems. Neurobiol. Aging. 2010;31:280–309. doi: 10.1016/j.neurobiolaging.2008.04.001. [DOI] [PubMed] [Google Scholar]
- Saraiva MJ, Birken S, Costa PP, Goodman DS. Family studies of the genetic abnormality in transthyretin (prealbumin) in Portuguese patients with familial amyloidotic polyneuropathy. Ann. N Y Acad. Sci. 1984;435:86–100. doi: 10.1111/j.1749-6632.1984.tb13742.x. [DOI] [PubMed] [Google Scholar]
- Shen HY, He JC, Wang Y, Huang QY, Chen JF. Geldanamycin induces heat shock protein 70 and protects against MPTP-induced dopaminergic neurotoxicity in mice. J. Biol. Chem. 2005;280:39962–39969. doi: 10.1074/jbc.M505524200. [DOI] [PubMed] [Google Scholar]
- Sittler A, Lurz R, Lueder G, et al. Geldanamycin activates a heat shock response and inhibits huntingtin aggregation in a cell culture model of Huntington's disease. Hum. Mol. Genet. 2001;10:1307–1315. doi: 10.1093/hmg/10.12.1307. [DOI] [PubMed] [Google Scholar]
- Sousa MM, Cardoso I, Fernandes R, Guimarães A, Saraiva MJ. Deposition of transthyretin in early stages of familial amyloidotic polyneuropathy: evidence for toxicity of nonfibrillar aggregates. Am. J. Pathol. 2001a;159:1993–2000. doi: 10.1016/s0002-9440(10)63050-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa MM, Du Yan S, Fernandes R, Guimaraes A, Stern D, Saraiva MJ. Familial amyloid polyneuropathy: receptor for advanced glycation end products-dependent triggering of neuronal inflammatory and apoptotic pathways. J. Neurosci. 2001b;21:7576–7586. doi: 10.1523/JNEUROSCI.21-19-07576.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sousa MM, Fernandes R, Palha JA, Taboada A, Vieira P, Saraiva MJ. Evidence for early cytotoxic aggregates in transgenic mice for human transthyretin Leu55Pro. Am. J. Pathol. 2002;161:1935–1948. doi: 10.1016/S0002-9440(10)64469-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teixeira PF, Cerca F, Santos SD, Saraiva MJ. Endoplasmic reticulum stress associated with extracellular aggregates. Evidence from transthyretin deposition in familial amyloid polyneuropathy. J. Biol. Chem. 2006;281:21998–22003. doi: 10.1074/jbc.M602302200. [DOI] [PubMed] [Google Scholar]
- Wilhelmus MM, Otte-Höller I, Wesseling P, de Waal RM, Boelens WC, Verbeek MM. Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer's disease brains. Neuropathol. Appl. Neurobiol. 2006;32:119–130. doi: 10.1111/j.1365-2990.2006.00689.x. [DOI] [PubMed] [Google Scholar]


