Abstract
Muscular injury associated with local inflammatory reaction frequently occurs in sports medicine, but the individual response and capacity of regeneration vary among subjects. Inflammatory cytokines are probably implicated in activation of repair mechanisms by specifically influencing tissue microenvironment. This work aimed to compare muscle tissue repair in different mouse lineages. We used C57BL/6 and BALB/c mice genetically predisposed to either Type1 or Type2 cytokine production. The role of Type1 cytokines was also investigated in C57IFN-γ (IFNγ-KO) and C57IL-12 (IL12-KO) knockout mice. Participation of T lymphocytes was assessed in athymic BALB/c nude (nu/nu) mice. Muscular lesion was induced with bupivacaine injection in the Triceps brachii muscle. BALB/c mice showed marked collagen deposition and increased TGF-β mRNA content, contrasting with mild fibrosis observed in C57BL/6 mice. C57-IFNγ-KO mice, exhibited pronounced fibrosis, but IL12-KO collagen deposition was similar to that of C57. Twenty-four hours after lesion, C57BL/6 and BALB/cnu/nu presented numerous regenerating myofibres and marked increase of metalloprotease-9 activity compared with BALB/c. These data support that skeletal muscle remodelling is greatly influenced by the genetic backgrounds, shedding light on the molecular mechanisms influencing differential muscular remodelling and tissue regeneration among individuals.
Keywords: bupivacaine, cytokines, inflammation, muscular injury, muscular regeneration
Introduction
Genetic problems, autoimmune diseases and acquired injuries account for up to 30% of all muscle injuries referred in sports medicine (Foster et al. 2003). In general, tissue response to injury takes place in three overlapping defined phases: inflammation, satellite cell activation and tissue remodelling, but the precise mechanisms and factors influencing the outcome of muscular lesion and regenerative process are not yet known (Charge & Rudnicki 2004).
Muscle injury releases chemotactic components that attract leucocyte migration with potential to increase tissue damage or promote repair and regeneration. In vitro and in vivo findings indicate that macrophages may have a dual role depending on muscle loading and lag time after tissue damage. In addition, to amplify muscle injury (Tidball 2005), macrophages also remove necrotic muscle fibres shortly after the occurrence of injuries and, along with infiltrating lymphocytes, secrete many inflammatory mediators that stimulate muscle regeneration (Cantini & Carraro 1995; Yahiaoui et al. 2008). The apparently conflicting roles of macrophages in promoting muscle injury and repair may be related to distinct functions of macrophage subpopulations within muscle microenvironment. Inflammatory macrophages prevail mostly at early stages of tissue damage followed thereafter by a non-phagocytic population mostly distributed nearby regenerative myofibres probably associated with muscle remodelling (McLennan 1996; Tidball & Wehling-Henricks 2007). Local release of cytokines, chemokines and growth factors by inflammatory cells with distinct phenotype may directly influence the pattern of inflammation, muscle repair or fibrosis (Azouz et al. 2004). Cytokine production by different T-lymphocyte subsets may not only influence activation of anti-fibrotic mechanisms with effective tissue remodelling but also favour substitution of functional tissue by dense connective tissue (Sandler et al. 2003). As a variety of other cell types including fibroblasts, macrophages and mast cells can produce these cytokines, it seems appropriate to refer to Type1 and Type2 cytokine responses, rather than Th1 and Th2 responses (Sime & O'Reilly 2001).
Genetic background of immune response influences Type1/Type2 cytokine balance in mice, as observed in C57BL/6 mice that present a Type1 phenotype characterized by high IFNγ and low IL-4 production, whereas the BALB/c strain is a Type2 cytokine producer with low IFNγ and high IL-4 (Watanabe et al. 2004). Such strains are currently used to study the influence of cytokine production in different experimental models: hepatic wound healing (Shi et al. 1997); Leishmania (Tripathi et al. 2007) and cytomegalovirus infection (Geist & Hinde 2001). This study aimed to compare the regenerative capacity of skeletal muscles subjected to injury induced by bupivacaine (Bp) between mouse lineages with different immune genetic background. Intramuscular Bp injection was chosen as established self-limited model that allows detailed analysis of tissue remodelling in a specific period of time (Sandri et al. 2001; Charge & Rudnicki 2004).
Materials and methods
Animals
C57BL/6 and BALB/c mice were chosen as Type1 and Type2-dominant strains respectively (Hsieh et al. 1995; Mills et al. 2000; Yu et al. 2006). Inbred male 6-week-old (wk) C57BL/6, BALB/c and BALB/c nude strains of mice were obtained from the Animal Breeding House from Fluminense Federal University. C57BL/6 IFNγ-KO and IL12-KO mice were kept in Animal Facilities from Oswaldo Cruz Foundation. All procedures were approved by the Institutional Animal Care and were conducted according to Brazilian Ethics Guidelines for Animal Studies (COBEA).
Induction of muscular lesion
Muscular lesion was performed as previously described (Mussini et al. 1987), with 35 μl Bp (bupivacaine; Sigma, St. Louis, MO,USA) 0.05% injected in the Triceps brachii skeletal muscle. Control and sham groups were kept under the same experimental conditions. Mice were sacrificed 1, 4, 8 and 12 days postinjection (dpi).
Histological staining and quantitative analysis
Triceps brachii muscles were carefully removed and fixed in Millonig fixative. The 5-μm sections of paraplast-embedded tissue (Sigma) were stained with syrius red to analyse histological alterations and collagen deposition. Degenerating and necrotic fibres were identified by homogeneous pale eosinophilic sarcoplasm, whereas regenerating fibres by strong basophilia and centrally located nuclei. Percentage of collagen in the lesion area was determined using colour view XS digital video camera (Olympus Soft Image Solution GMBH, Munster, Germany) adapted to a Zeiss microscope (Zeiss, Oberkolchen, Germany). The images were analysed using the AnaliSIS 3.2 Soft Image (Soft Imaging System Corp., Lakewood, CO, USA). Each experimental group was composed of at least three animals; three slices were analysed per animal and an area of 0.6 mm2 was analysed per slide.
Immunohistochemistry analysis
Skeletal muscle was removed from each mouse strain at 4 dpi. Tissues were embedded in OCT (Tissue-TEK; Elkhard, IN, USA) and frozen in isopentane cooled by liquid nitrogen. The thick, frozen 5-μm sections were allowed to settle on poly-L-lysine (Sigma) precoated slides, fixed in acetone and blocked for endogenous peroxidase activity with 3% hydrogen peroxide in PBS for 5 min.
For characterization of the inflammatory cell infiltrate monoclonal rat IgG anti-Mac-1.biotin (clone M1/70, Pharmingem; BD-Pharmingen Biosciences, San Diego, CA, USA), monoclonal rat IgG anti-F4/80 (clone CI;A3-1; Serotec, Oxford, UK), monoclonal rat IgG anti-CD4.biotin and anti-CD8.biotin (clone RM2515.3 and RM2215.3; Caltag, Buckingham, UK) were used. Optimal concentration of specific primary antibody diluted in phosphate-buffered saline (PBS), pH 7.4, was applied to the sections followed by incubation at room temperature in a moist chamber for 60 min. Thereafter, sections were incubated for 40 min with biotinylated anti-rat antibody (Sigma) or with streptavidin-peroxidase complex (Sigma) and washed with PBS. Enzyme activity was revealed with aminoethyl-carbazole (Sigma) in the presence of hydrogen peroxide. All sections were lightly counterstained with Mayer′s haematoxylin (Sigma). For histological studies, at least three animals were examined. Percentage of areas with positive immunolabelling for Mac-1 and F4/80 in the lesion was determined using the AnaliSIS 3.2 Soft Image. We used 3–5 mice per experimental group and analysed three frozen sections per animal.
Tissue extract preparation
Muscles were weighed, immediately frozen in liquid nitrogen and homogenized (1/10 w/v) in extraction buffer (100 mM Tris-HCl, pH 7,6, 200 mM NaCl, 100 mM CaCl2 and 1% Triton X-100) at 4 °C. After centrifugation (15,000 × g; 10 °C; 10 min), protein concentration in supernatant aliquots was determined according to the method of Lowry et al. (Lowry et al. 1951), and equal amounts of total protein were loaded for zymography (60 μg/lane).
Gelatin zymography
Zymogram gels consisted of 7.5% (w/v) polyacrylamide impregnated with 2 mg/ml type A gelatin from porcine skin (Sigma) and 5% (w/v) polyacrylamide for stacking gels. Gels were further washed twice for 30 min in 2.5% Triton X-100 solution at room temperature, then incubated at 37 °C for 24 h in substrate buffer (10 mM Tris–HCl buffer, pH 7.5 with CaCl2 5 mM, ZnCl2 1μM). Thereafter, gels were stained with 30% methanol/10% acetic acid containing 0.5% (w/v) brilliant blue R-250. Gelatinase activity was visualized as unstained bands on a blue background, representing areas of proteolysis of the substrate protein. Semi-quantitative analysis was performed using image-analysis software (scion Program National Institutes of Health, Image Program; NIH, Bethesda, MD, USA).
RT-PCR
Total RNA from skeletal muscles was extracted using Trizol Reagent (Invitrogen, Carlsbad, CA, USA) following the manufacturer's suggestions. Total RNA (1 μg) was reverse transcribed using the SuperScript One-Step RT-PCR with Platinum Taq (Invitrogen). PCR was performed in presence of 2.5 mM MgSO4 for 30 cycles (15 s at 94 °C, 30 s at 60 °C, and 3 min at 72 °C). cDNA was amplified using specific primers as previously described (Masli et al. 2002) to target the following sequences: TGF-β1 (260-bp), forward CAAGGAGACGGAATACAGGGCT, reverse GCACACAGCAGTTCTTCTCTGT and GAPDH (245-bp) forward GGTGAAGGTCGGTGTGAACGGA and reverse TGTTAGTGGGGTCTCGCTCCTG. PCR products were separated by 1.5% agarose gel electrophoresis and visualized after staining with ethidium bromide.
Statistical analysis
GraphPad Prism 5 (GraphPad software Inc., La Jolla, CA, USA) was used to calculate mean and standard deviations and Student's t-test was applied for pair-wise comparison among groups and one-way anova Kruskal–Wallis. Error bars depict the standard error of the mean (SEM), P-values below 0.05 were considered significant.
Results
Histomorphological analysis
Centro-nucleation of muscle fibres was used as a parameter of muscle regeneration. At 4 dpi, C57 mice showed great numbers of regenerate fibres evidenced by basophilic cytoplasm and central nucleation (Figure 1a). In contrast, BALB/c mice had loss of myofibre structural organization and intense inflammatory infiltrate (Figure 1d). At 8 dpi, inflammatory infiltrate was scarce in C57 muscular lesion (Figure 1b), but BALB/c mice presented regenerated myofibres close to areas with intense inflammatory infiltrate (Figure 1e). At 12 dpi, both C57 and BALB/c presented regenerate myofibres with central nucleation (Figure 1c,f).
Figure 1.
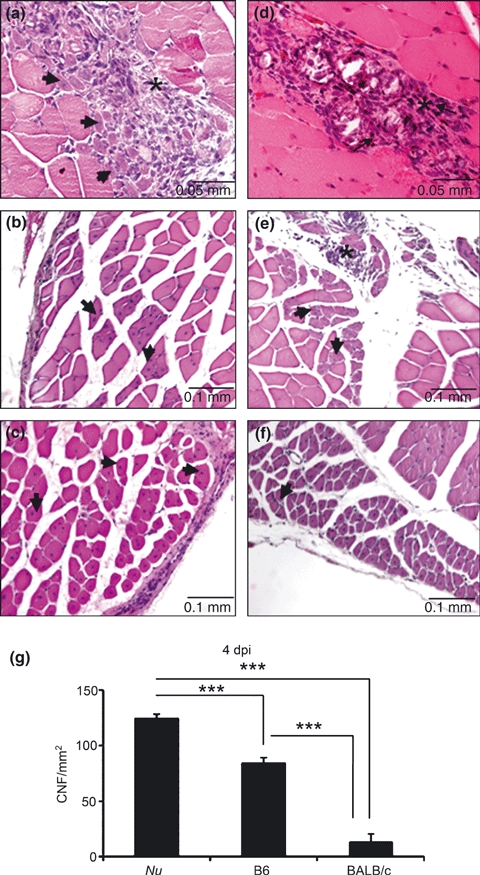
Histological analysis of Triceps brachii stained by H–E at 4 (a, d), 8 (b, e), and 12 (c, f) days after intramuscular Bp injection in C57 (a, b, c) and BALB/c (d, e, f) mice. Arrows show regenerating myofibres and asterisks, the inflammatory infiltrate. (g) quantitative analysis of centrally nucleated fibres (CNFs) in the lesion area. Nu = BALB/cnu/nu, B6 = C57BL/6. Results are expressed as mean (±SD). Three animals per group were included. ***P < 0.0001.
Quantitative analysis (Figure 1g) showed a 6-fold increase (P < 0.001) in centro-nucleated myofibres per lesion area in C57 mice in comparison with BALB/c mice at 4 dpi. Athymic BALB/cnu/nu showed a 9.5-fold increase (P < 0.0001) in central-nucleated fibres in muscle cross-sections in comparison with wild-type BALB/c.
Analysis of collagen deposition
Syrius red staining was used to detect collagen deposition, a parameter used to correlate a putative influence of Type1 and Type2 cytokine background with skeletal muscle remodelling. Discrete collagen deposition was consistently observed at 1 dpi in all groups C57, BALB/c, IFNγ-KO, IL12-KO and BALB/cnu/nu (data not shown). At 4 dpi, C57 showed discrete deposition of collagen, but BALB/c mice presented increased deposition within areas of myonecrosis (Figure 2a,e,b). IFNγ-KO mice which default to Type2 cytokine pathway exhibited pronounced collagen deposition compared with C57 wild-type animals (Figure 2c) similar to BALB/cnu/nu that showed intense collagen deposition in areas with regenerating myofibres (Figure 2e). No difference was observed in collagen deposition between BALB/c and C57 mice at 8 and 12 dpi (data not shown). Morphometric analysis confirmed immunohistochemical analysis and further showed at 4 dpi, a 5-fold increase in (Figure 2e) collagen deposition in the lesion of BALB/c and BALB/cnu/nu in comparison with C57 (P < 0.05). Interestingly, IFNγ-KO mice showed intermediate degree of collagen deposition, while fibrosis in IL12-KO was similar to C57 mice (Figure 2f, P > 0.05).
Figure 2.
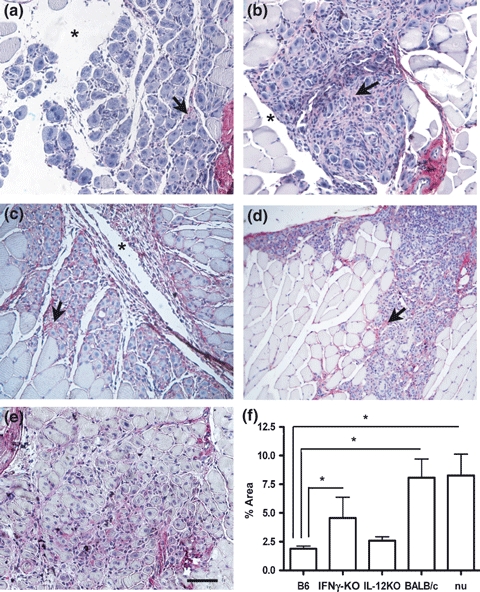
Triceps brachii stained by Syrius red at 4 days after Bp injection in C57BL/6 (a), BALB/c (b), C57 IFNγ-KO (c), C57 IL12-KO (d) and BALB/cnu/nu(e) mouse. The arrows show the collagen expression. Line = 0.1 mm. F = Representation of collagen quantification in the muscular lesion at 4 dpi. Results are expressed as mean ± SEM. Three slices per animal and three animals per group were analysed. *P < 0.05. B6 = C57BL/6, nu = BALB/cnu/nu.
Metalloprotease activity
Consistently with previous results published by our group (Bani et al. 2008), skeletal muscles showed four bands in gelatine zymograms corresponding to 100-kDa (MMP-9), 66-kDa (pre-pro MMP-2), 60-kDa (pro MMP-2) and 55-kDa (active MMP-2). Control mice without Bp injection showed discrete activity of pre-pro MMP-2 and pro-MMP-2, but no evidence of MMP-9 and active MMP-2 activities. Active MMP-2 was solely observed in T.brachii of mdx dystrophic mice used as a positive control (Figure 3a).
Figure 3.
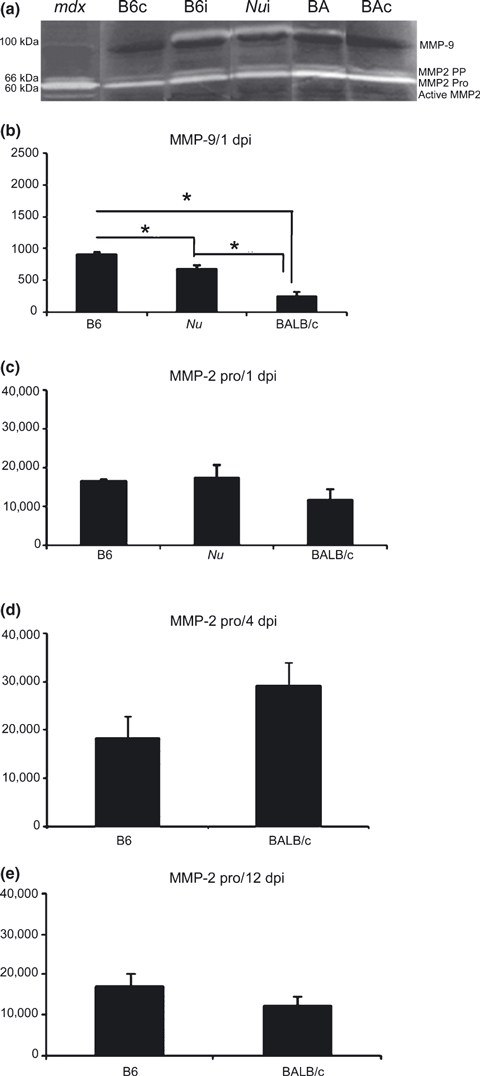
MMP-9 and MMP-2-pro activity in skeletal muscle lesion. (a) Illustrative gelatin zymogram of skeletal muscle (a) 24 h after Bp injection (1 dpi). Mdx = control of muscular lesion, B6 = C57BL/6, BAi = BALB/c, Nu = BALB/cnu/nu, BAc and B6C = control, BAi and B6i = Bp-injection. (b) Semi-quantitative analysis of MMP-9 (b) and MMP-2 (c, d, e) at 1 (a, c), 4 (d) and (e) 12 days after Bp-injection. Activities were quantified by scion Software. Results are expressed as mean (±SD). *P < 0.05.
MMP-9 activity commonly associated with inflammatory process was only evidenced at 1 dpi (Figure 3b). C57 had a 2.5-fold increase in MMP-9 activity (P < 0.05) in relation to BALB/cnu/nu and 1.3-fold compared with BALB/c (Figure 3b). All analysed strains presented similar pro-MMP-2 activity at 1, 4 and 12 dpi (Figure 3 c,d,e).
Immunohistochemical analysis of the cellular infiltrate
Mac-1, a marker of inflammation, is expressed at different levels in a variety of cell types, including macrophages, neutrophils and dendritic cells (Ross 2002). In comparison with C57 mice, BALB/c (P < 0.05) and BALB/cnu/nu showed more Mac-1 positive cells, but few F4/80 positive macrophages in the lesion site (Figure 4). Both lineages showed few scattered CD4 and CD8 positive cells in the muscular lesion (data not shown).
Figure 4.
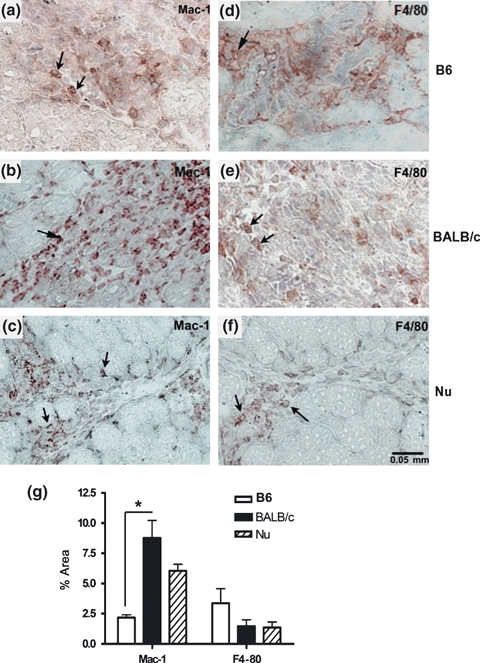
Immunohistochemistry for Mac-1 and F4/80 in the muscular lesion induced by bupivacaine (4 dpi) in C57BL/6 (a, d), BALB/c (b, e) and BALB/cnu/nu (c, f). The arrows show the positive cells. (e) Quantitative analysis of percentage of areas with positive immunolabelling for Mac-1 and F4/80 in the lesion area. Results are expressed as mean (±SD). Three animals per group were included. *P < 0.05. B6 = C57BL/6, Nu = BALB/cnu/nu.
Expression of TGF-β mRNA in the muscular lesion
As collagen deposition was increased in BALB/c mice in relation to C57, it appeared relevant to assess the expression of TGF-β mRNA, a cytokine with fibrogenic activity. At 1 dpi, BALB/c mice showed a significant increase (P < 0.05) in TGF-β mRNA expression in relation to C57 (Figure 5a,c). Such result correlated with further increase on collagen deposition observed in BALB/c at 4 dpi (Figure 2e). No significant difference was observed in TGF-β mRNA expression when comparing C57 with BALB/c mice at 4 dpi (Figure 5b).
Figure 5.
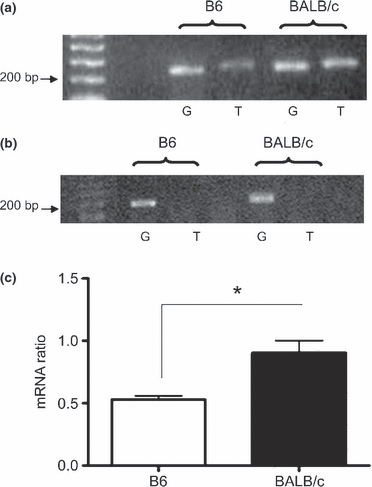
TGF-β mRNA expression in muscular lesion after Bp injection. (a) Illustrative electrophoresis of RT-PCR for TGF-β (T) and constitutive GAPDH (G) in skeletal muscle 1 dpi (a) and 4 dpi (b). TGF-β = 260 bp e GAPDH = 245 bp. (c) Semi-quantitative analysis of TGF-β/GAPDH ratio at 1 dpi. Results are expressed as mean ± SEM of three animals. *P < 0.05 (unpaired t-test).
Discussion
Our findings indicate the importance to consider the genetic background before selecting the appropriate in vivo mouse model for studies related to skeletal muscle remodelling. We observed that mice lineages expressing Type1 (C57) and Type2 (BALB/c) dominant cytokine pattern responded differently to bupivacaine-induced lesion. C57 wild-type and C57 IL12-KO mice presented more areas with regenerating myofibres, but intense myonecrosis and collagen deposition prevailed in BALB/c. In addition, mice lacking IFNγ which default to Type2 cytokine pathway, exhibited pronounced fibrotic lesions in comparison with wild-type animals. BALB/cnu/nu mice showed significant muscle regeneration and increased MMP-9 activity.
In response to injury, tissue repair is partially dependent upon activation of resident cells and locally released factors, such as cytokines (Type1 or Type2), and matrix metaloproteases that influence leucocyte migration and tissue remodelling (Azouz et al. 2004). The genetic background is important for establishing the pattern of cytokine production and activation of specific genes and transcription factors related to tissue remodelling (Henry & Garner 2003; Prisk & Huard 2003; Shäffer & Barbul 1998; Sicard 2002). Splenocytes from C57 mice constitutively produce high levels of IFNγ and low levels of interleukin-4, whereas BALB/c splenocytes are characterized for producing low levels of IFNγ and high levels of interleukin-4 and interleukin-10 (Yu et al. 2006).
Cells and products from the innate immunity are also relevant for tissue repair and the innate response is different in Type1 and Type2 dominant mouse strains (Watanabe et al. 2004). Experimental data show that selective depletion of macrophages impairs wound healing at early stages of injury (Park & Barbul 2004) and that macrophages are essential for skeletal muscle regeneration after myoblast transplantation (Lescaudron et al. 1999). In addition, conditioned medium from peritoneal macrophages increases myoblast proliferation in vitro and the number of Myo-D+ cells (Tidball 2005). In general, macrophages represent the main inflammatory cell present in the muscular lesion (Wehling et al. 2001; Tidball 2005), but differences in the inflammatory cell type present among individuals may influence the outcome of tissue repair. Macrophages are highly versatile and may exert various, and even opposite, functions depending on their activation state. Experimental data show that chemotactic factors released from injured muscle recruit F4/80lo macrophages exhibiting inflammatory characteristic that, within muscle milieu, switch to an anti-inflammatory F4/80hi profile that sustains myogenic differentiation and myofibre repair (Arnold et al. 2007). In the present work, Bp-induced muscular lesion caused distinct changes within muscular microenvironment among mice with different genetic background. The presence of increased numbers of Mac-1 + inflammatory cells and mild F4/80 expression in BALB/c muscular lesion indicate that other cell types participate in the inflammatory infiltrate. In addition, BALB/c macrophages have less phagocytic activity, produce low levels of inflammatory cytokines TNF-α and IL-12 compared with C57 (Watanabe et al. 2004).
Similar to skin injury models, BALB/cnu/nu mice showed significant muscle regeneration and increased MMP-9 activity (Gawronska-Kozak et al. 2006; Manuel & Gawronska-Kozak 2006) – an effect strictly related to their immunodeficiency as previous study showed that athymic nude mice, independent of the genetic background, C57BL/6, BALB/c or corresponding F1, showed potent regenerative features (Mescher & Neff 2005; Gawronska-Kozak et al. 2006). Athymic nude mice possess the capacity to regulate essential steps in the process of wound healing and produce very fine and delicate scars, indistinguishable from the surrounding tissues (Barbul et al. 1982). However, BALB/c nude mice showed an intense deposition of collagen in the muscular lesion, but always associated with regenerating myoblasts.
Locally produced cytokines and growth factors regulate fibroblast proliferation, collagen synthesis and angiogenesis (Gillery et al. 1992; Granstein et al. 1987; Park & Barbul 2004). In this context, BALB/c mice, but not C57, showed increased collagen deposition, which is in accordance with studies showing that Type2 cytokines (IL-4, IL-13 and TGF-β) cause fibroblast activation, increased collagen production and fibrosis (Wynn 2004; Mescher & Neff 2005). In fact, we observed a high TGF-β mRNA expression in the muscular lesion of BALB/c, but not in C57. In addition, IFNγ-KO mice lacking IFNγ, which default to the Th2 cytokine pathway, exhibited more pronounced fibrotic lesions than did wild-type animals. Moreover, in IL12-KO mice that presented an IL-12-independent IFNγ production (Michailowsky et al. 2001), collagen deposition was comparable to Bp-injected C57. Experiments in the cardiotoxin model of muscular injury carried out in the IFNγ null mouse showed that impaired muscle healing and marked fibrosis were associated with altered macrophage function (Cheng et al. 2008). Similar deposition of collagen between BALB/c wild-type and athymic BALB/c nude mice indicates that development of fibrosis is independent of intrathymically differentiated lymphocyte in this experimental model. Considering that BALB/c macrophages produce lower levels of TNF-α than C57BL/6 (Watanabe et al. 2004), it is conceivable that TNF-α, a Type1 cytokine produced mainly by macrophages, may be inhibiting TGF-β gene activation and ultimately reducing type I collagen and fibrosis, as has been suggested in other models (Verrecchia & Mauviel 2004).
Skeletal muscle remodelling during degeneration and regeneration is accompanied by a high turnover of extracellular matrix components (Kherif et al. 1999; Carmeli et al. 2004). Following cardiotoxin injury, MMP-9 activity is extensively up-regulated during initial phase (72 h) associated with acute inflammatory reaction caused partly by macrophages and neutrophils migration into the lesion (Kherif et al. 1999), and by satellite cells activated in the periphery of the injury, also other possible source of MMP-9 (Roach et al. 2002). Increased MMP-9 activation during the acute stage (1 dpi) of Bp-induced inflammation in C57 mice was important for degradation of collagen and thereby facilitating migration of phagocytic leucocytes and clearing of cell debris. As data from skin injury model, BALB/cnu/nu showed a higher MMP-9 activity than wild BALB/c (Manuel & Gawronska-Kozak 2006). After the fourth day of bupivacaine-induced lesion, reduced MMP-9 but increased pro-MMP-2 activities may be related to increased tissue remodelling, associated with degradation of basement membrane components and stimulation of satellite cell proliferation by growth factors (Kherif et al. 1999). Lack of MMP-2 activity in the bupivacaine model of muscle damage may be related to extension of the lesion and/or lack of sensibility of zymography for detection of small amounts of protease in tissue extracts.
Such data highlight an important issue concerning the cytokine profile that affects not only the outcome of infection or inflammation but also the nature of reparative response within the injured skeletal muscle. Knowledge of regulatory process that mediates muscular remodelling may contribute to development appropriate strategies to promote either regeneration or fibrosis.
Acknowledgments
We are grateful to Nina Cortez and Bartira D. de Oliveira for excellent technical assistance. We are also grateful to Marcella d’Alincourt Salazar from the University of Toledo, Health Science Campus, Toledo, Ohio for the English revision. This study was supported by grants from CAPES (Coordenação de Aperfeiçoamento de Pessoal de Nível Superior), FAPERJ (Fundação de Amparo a Pesquisa do Rio de Janeiro) and CNPq (Brazilian Research Council).
Conflict of interest
The authors declare that they have no competing financial interests.
References
- Arnold L, Henry A, Poron F, et al. Inflammatory monocytes recruited after skeletal muscle injury switch into antiinflammatory macrophages to support myogenesis. J. Exp. Med. 2007;204:1057–1069. doi: 10.1084/jem.20070075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azouz A, Razzaque MS, El-Hallak M, Taguchi T. Immunoinflammatory responses and fibrogenesis. Med. Electron Microsc. 2004;37:141–148. doi: 10.1007/s00795-004-0255-2. [DOI] [PubMed] [Google Scholar]
- Bani C, Lagrota-Candido J, Pinheiro DF, et al. Pattern of metalloprotease activity and myofiber regeneration in skeletal muscles of mdx mice. Muscle Nerve. 2008;37:583–592. doi: 10.1002/mus.20970. [DOI] [PubMed] [Google Scholar]
- Barbul A, Sisto D, Rettura G, Levenson SM, Seifter E, Efron G. Thymic inhibition of wound healing: abrogation by adult thymectomy. J. Surg. Res. 1982;32:338–342. doi: 10.1016/0022-4804(82)90110-x. [DOI] [PubMed] [Google Scholar]
- Cantini M, Carraro U. Macrophage-released factor stimulates selectively myogenic cells in primary muscle culture. J. Neuropathol. Exp. Neurol. 1995;54:121–128. doi: 10.1097/00005072-199501000-00014. [DOI] [PubMed] [Google Scholar]
- Carmeli E, Moas M, Reznick AZ, Coleman R. Matrix metalloproteinases and skeletal muscle: a brief review. Muscle Nerve. 2004;29:191–197. doi: 10.1002/mus.10529. [DOI] [PubMed] [Google Scholar]
- Charge SB, Rudnicki MA. Cellular and molecular regulation of muscle regeneration. Physiol. Rev. 2004;84:209–238. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- Cheng M, Nguyen MH, Fantuzzi G, Koh TJ. Endogenous interferon-gamma is required for efficient skeletal muscle regeneration. Am. J. Physiol. Cell Physiol. 2008;294:C1183–C1191. doi: 10.1152/ajpcell.00568.2007. [DOI] [PubMed] [Google Scholar]
- Foster W, Li Y, Usas A, Somogyi G, Huard J. Gamma interferon as an antifibrosis agent in skeletal muscle. J. Orthop. Res. 2003;21:798–804. doi: 10.1016/S0736-0266(03)00059-7. [DOI] [PubMed] [Google Scholar]
- Gawronska-Kozak B, Bogacki M, Rim JS, Monroe WT, Manuel JA. Scarless skin repair in immunodeficient mice. Wound Repair Regen. 2006;14:265–276. doi: 10.1111/j.1743-6109.2006.00121.x. [DOI] [PubMed] [Google Scholar]
- Geist LJ, Hinde SL. Susceptibility to cytomegalovirus infection may be dependent on the cytokine response to the virus. J. Investig. Med. 2001;49:434–441. doi: 10.2310/6650.2001.33788. [DOI] [PubMed] [Google Scholar]
- Gillery P, Serpier H, Polette M, et al. Gamma-interferon inhibits extracellular matrix synthesis and remodeling in collagen lattice cultures of normal and scleroderma skin fibroblasts. Eur. J. Cell Biol. 1992;57:244–253. [PubMed] [Google Scholar]
- Granstein RD, Murphy GF, Margolis RJ, Byrne MH, Amento EP. Gamma-interferon inhibits collagen synthesis in vivo in the mouse. J. Clin. Invest. 1987;79:1254–1258. doi: 10.1172/JCI112945. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry G, Garner WL. Inflammatory mediators in wound healing. Surg. Clin. North Am. 2003;83:483–507. doi: 10.1016/S0039-6109(02)00200-1. [DOI] [PubMed] [Google Scholar]
- Hsieh CS, Macatonia SE, O'Garra A, Murphy KM. T cell genetic background determines default T helper phenotype development in vitro. J. Exp. Med. 1995;181:713–721. doi: 10.1084/jem.181.2.713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kherif S, Lafuma C, Dehaupas M, et al. Expression of matrix metalloproteinases 2 and 9 in regenerating skeletal muscle: a study in experimentally injured and mdx muscles. Dev. Biol. 1999;205:158–170. doi: 10.1006/dbio.1998.9107. [DOI] [PubMed] [Google Scholar]
- Lescaudron L, Peltekian E, Fontaine-Perus J, et al. Blood borne macrophages are essential for the triggering of muscle regeneration following muscle transplant. Neuromuscul. Disord. 1999;9:72–80. doi: 10.1016/s0960-8966(98)00111-4. [DOI] [PubMed] [Google Scholar]
- Lowry OH, Rosebrough NJ, Farr AL, Randall RJ. Protein measurement with the Folin phenol reagent. J. Biol. Chem. 1951;193:265–275. [PubMed] [Google Scholar]
- Manuel JA, Gawronska-Kozak B. Matrix metalloproteinase 9 (MMP-9) is upregulated during scarless wound healing in athymic nude mice. Matrix Biol. 2006;25:505–514. doi: 10.1016/j.matbio.2006.07.008. [DOI] [PubMed] [Google Scholar]
- Masli S, Turpie B, Hecker KH, Streilein JW. Expression of thrombospondin in TGFbeta-treated APCs and its relevance to their immune deviation-promoting properties. J. Immunol. 2002;168:2264–2273. doi: 10.4049/jimmunol.168.5.2264. [DOI] [PubMed] [Google Scholar]
- McLennan IS. Degenerating and regenerating skeletal muscles contain several subpopulations of macrophages with distinct spatial and temporal distributions. J. Anat. 1996;188(Pt 1):17–28. [PMC free article] [PubMed] [Google Scholar]
- Mescher AL, Neff AW. Regenerative capacity and the developing immune system. Adv. Biochem. Eng. Biotechnol. 2005;93:39–66. doi: 10.1007/b99966. [DOI] [PubMed] [Google Scholar]
- Michailowsky V, Silva NM, Rocha CD, Vieira LQ, Lannes-Vieira J, Gazzinelli RT. Pivotal role of interleukin-12 and interferon-gamma axis in controlling tissue parasitism and inflammation in the heart and central nervous system during Trypanosoma cruzi infection. Am. J. Pathol. 2001;159:1723–1733. doi: 10.1016/s0002-9440(10)63019-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills CD, Kincaid K, Alt JM, Heilman MJ, Hill AM. M-1/M-2 macrophages and the Th1/Th2 paradigm. J. Immunol. 2000;164:6166–6173. doi: 10.4049/jimmunol.1701141. [DOI] [PubMed] [Google Scholar]
- Mussini I, Favaro G, Carraro U. Maturation, dystrophic changes and the continuous production of fibers in skeletal muscle regenerating in the absence of nerve. J. Neuropathol. Exp. Neurol. 1987;46:315–331. doi: 10.1097/00005072-198705000-00007. [DOI] [PubMed] [Google Scholar]
- Park JE, Barbul A. Understanding the role of immune regulation in wound healing. Am. J. Surg. 2004;187:11S–16S. doi: 10.1016/S0002-9610(03)00296-4. [DOI] [PubMed] [Google Scholar]
- Prisk V, Huard J. Muscle injuries and repair: the role of prostaglandins and inflammation. Histol. Histopathol. 2003;18:1243–1256. doi: 10.14670/HH-18.1243. [DOI] [PubMed] [Google Scholar]
- Roach DM, Fitridge RA, Laws PE, Millard SH, Varelias A, Cowled PA. Up-regulation of MMP-2 and MMP-9 leads to degradation of type IV collagen during skeletal muscle reperfusion injury; protection by the MMP inhibitor, doxycycline. Eur. J. Vasc. Endovasc. Surg. 2002;23:260–269. doi: 10.1053/ejvs.2002.1598. [DOI] [PubMed] [Google Scholar]
- Ross GD. Role of the lectin domain of Mac-1/CR3 (CD11b/CD18) in regulating intercellular adhesion. Immunol. Res. 2002;25:219–227. doi: 10.1385/IR:25:3:219. [DOI] [PubMed] [Google Scholar]
- Sandler NG, Mentink-Kane MM, Cheever AW, Wynn TA. Global gene expression profiles during acute pathogen-induced pulmonary inflammation reveal divergent roles for Th1 and Th2 responses in tissue repair. J. Immunol. 2003;171:3655–3667. doi: 10.4049/jimmunol.171.7.3655. [DOI] [PubMed] [Google Scholar]
- Sandri M, Sandri C, Brun B, et al. Inhibition of FasL sustains phagocytic cells and delays myogenesis in regenerating muscle fibers. J. Leuk. Biol. 2001;69:482–489. [PubMed] [Google Scholar]
- Shäffer M, Barbul A. Lymphocyte function in wound healing and following injury. Br. J. Surg. 1998;85:444–460. doi: 10.1046/j.1365-2168.1998.00734.x. [DOI] [PubMed] [Google Scholar]
- Shi Z, Wakil AE, Rockey DC. Strain-specific differences in mouse hepatic wound healing are mediated by divergent T helper cytokine responses. Proc. Natl. Acad. Sci. USA. 1997;94:10663–10668. doi: 10.1073/pnas.94.20.10663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sicard RE. Differential inflammatory and immunological responses in tissue regeneration and repair. Ann. NY Acad. Sci. 2002;961:368–371. doi: 10.1111/j.1749-6632.2002.tb03126.x. [DOI] [PubMed] [Google Scholar]
- Sime PJ, O'Reilly KM. Fibrosis of the lung and other tissues: new concepts in pathogenesis and treatment. Clin. Immunol. 2001;99:308–319. doi: 10.1006/clim.2001.5008. [DOI] [PubMed] [Google Scholar]
- Tidball JG. Inflammatory processes in muscle injury and repair. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2005;288:R345–R353. doi: 10.1152/ajpregu.00454.2004. [DOI] [PubMed] [Google Scholar]
- Tidball JG, Wehling-Henricks M. Macrophages promote muscle membrane repair and muscle fibre growth and regeneration during modified muscle loading in mice in vivo. J. Physiol. 2007;578:327–336. doi: 10.1113/jphysiol.2006.118265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tripathi P, Singh V, Naik S. Immune response to leishmania: paradox rather than paradigm. FEMS Immunol. Med. Microbiol. 2007;51:229–242. doi: 10.1111/j.1574-695X.2007.00311.x. [DOI] [PubMed] [Google Scholar]
- Verrecchia F, Mauviel A. TGF-beta and TNF-alpha: antagonistic cytokines controlling type I collagen gene expression. Cell. Signal. 2004;16:873–880. doi: 10.1016/j.cellsig.2004.02.007. [DOI] [PubMed] [Google Scholar]
- Watanabe H, Numata K, Ito T, Takagi K, Matsukawa A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock. 2004;22:460–466. doi: 10.1097/01.shk.0000142249.08135.e9. [DOI] [PubMed] [Google Scholar]
- Wehling M, Spencer MJ, Tidball JG. A nitric oxide synthase transgene ameliorates muscular dystrophy in mdx mice. J. Cell Biol. 2001;155:123–131. doi: 10.1083/jcb.200105110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wynn TA. Fibrotic disease and the T(H)1/T(H)2 paradigm. Nat. Rev. Immunol. 2004;4:583–594. doi: 10.1038/nri1412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yahiaoui L, Gvozdic D, Danialou G, Mack M, Petrof BJ. CC family chemokines directly regulate myoblast responses to skeletal muscle injury. J. Physiol. 2008;586:3991–4004. doi: 10.1113/jphysiol.2008.152090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu Q, Horak K, Larson DF. Role of T lymphocytes in hypertension-induced cardiac extracellular matrix remodeling. Hypertension. 2006;48:98–104. doi: 10.1161/01.HYP.0000227247.27111.b2. [DOI] [PubMed] [Google Scholar]


