Abstract
Dantrolene has been shown to be neuroprotective by reducing neuronal apoptosis after brain injury in several animal models of neurological disorders. In this study, we investigated the effects of dantrolene on experimental spinal cord injury (SCI). Forty-six male Wistar rats were laminectomized at T13 and divided in six groups: GI (n = 7) underwent SCI with placebo and was euthanized after 32 h; GII (n = 7) underwent laminectomy alone with placebo and was euthanized after 32 h; GIII (n = 8) underwent SCI with dantrolene and was euthanized after 32 h; GIV (n = 8) underwent SCI with placebo and was euthanized after 8 days; GV (n = 8) underwent laminectomy alone with placebo and was euthanized after 8 days; and GVI (n = 8) underwent SCI with dantrolene and was euthanized after 8 days. A compressive trauma was performed to induce SCI. After euthanasia, the spinal cord was evaluated using light microscopy, TUNEL staining and immunochemistry with anti-Caspase-3 and anti-NeuN. Animals treated with dantrolene showed a smaller number of TUNEL-positive and caspase-3-positive cells and a larger number of NeuN-positive neurons, both at 32 h and 8 days (P ≤ 0.05). These results showed that dantrolene protects spinal cord tissue after traumatic SCI by decreasing apoptotic cell death.
Keywords: apoptosis, caspase-3, dantrolene, immunochemistry, NeuN, spinal cord injury, TUNEL-staining
Traumatic spinal cord injury (SCI) produces primary damage at the injured site that is followed by a delayed secondary lesion extending rostrocaudally, leading to progressive tissue destruction. The neurodegeneration induced by trauma is characterized by the loss of neurons and glia and consequently causes motor, sensory and autonomic functional deficits (Crowe et al. 1997; Liu et al. 1997; Beattie et al. 2000).
The secondary lesion is triggered by a complex series of cellular and molecular events that induces programmed cellular death over a few hours to several days following the initial trauma (Mattson 2000; Tagaki et al. 2003; Xu et al. 2008). The morphological and nuclear changes that occur during apoptosis are initiated and executed through activation of the caspase family of cysteine proteases, and caspase-3 is an especially important effector of the apoptotic process that is active following traumatic SCI (Springer 2002; McEwen & Springer 2005; Springer et al. 2009).
Intracellular calcium (Ca2+) overload and a Ca2+ excitotoxic cascade play essential roles in the induction of neuronal cell death (Happel et al. 1981; Springer et al. 2000; Nakayama et al. 2002). There is current evidence showing that intracellular Ca2+ overload can lead to caspase activation and thereby induce apoptosis (Yano et al. 2001; Orrenius et al. 2003; Tagaki et al. 2003).
Thus, the development of strategies to control intracellular Ca2+ homeostasis and consequently aid in the inhibition of apoptosis may be a means to promote neurological improvement in acute SCI (Fehlings et al. 1989; Thorell et al. 2002). In recent years, much attention has been focused on secondary injury, which is an important potential target for therapeutic intervention (Barut et al. 2005; Solaroglu et al. 2005; Thuret et al. 2006; Shields et al. 2008).
Dantrolene is a drug that inhibits the ryanodine receptor (RyR) Ca2+ channels located on the sarco-endoplasmatic reticulum in skeletal muscle (RyR1) and neuronal cells (RyR3) (Zhao et al. 2001; Kobayashi et al. 2005). It blocks calcium-induced calcium release from intracellular Ca2+ stores, preventing cytosolic Ca2+ overload (Ayar & Kelestimur 2002; Cherednichenko et al. 2008).
Clinically, dantrolene is used as muscle relaxant and in the treatment of malignant hyperthermia (Krause et al. 2004; Muehlschlegel & Sims 2009). It has been shown to have antioxidant (Büyükokuroglu et al. 2001; Uçüncüet al. 2005) and anti-inflammatory (Büyükokuroglu 2002) properties.
Previous investigations have assessed its neuroprotective effects in several models of ischaemic and traumatic brain injury (Frandsen & Schousboe 1991; Zhang et al. 1993; Wei & Perry 1996; Wei et al. 2000; Yano et al. 2001; Nakayama et al. 2002; Popescu et al. 2002; Li et al. 2005; Mori et al. 2005; Gwak et al. 2008), in a traumatic SCI in vitro (Thorell et al. 2002), in an ischaemia/reperfusion model of SCI (Kocogullari et al. 2008). Most recently, a traumatic model of SCI in rabbits showed its benefits against oxidative stress leading to neuronal death (Aslan et al. 2009). Those data suggest a potential role of dantrolene in the prevention of spinal cord apoptotic cell death.
However, the neuroprotective effect of dantrolene, by acting as a potential antiapoptotic drug in an in vivo model of traumatic SCI, had not been shown as yet. Thus, the aim of this study was to investigate whether dantrolene would be effective to prevent programmed cell death and preserve neuronal viability after traumatic SCI in rats.
Materials and methods
This study was approved and performed in agreement with the Ethical Principles in Animal Experimentation, adopted by the Ethics Committee in Animal Experimentation from Federal University of Minas Gerais (CETEA/UFMG, protocol nº059/03).
Animals and surgical procedure
Forty-six male Wistar rats aged 12 weeks and weighing 320–350 g were used in this study. Rats were kept under a 12/12 h light-dark cycle for 14 days of acclimation with commercial rodent food and water ad libitum. Pre-anaesthetic medication was performed with tramadol (2 mg/kg, orally), and induction and maintenance was carried out with isoflurane administered by mask in a semi-opened system. The animals were positioned in prone position, were prepared for aseptic surgery and received prophylactic antibiotic therapy with cephalothin (30 mg/kg, intravenous). Skin and subcutaneous tissue were incised in the dorsal midline extending from T6 to L1, the paravertebral muscles dissected, and laminectomy of T13 performed with a pneumatic drill. After visualization of the spinal cord covered by the intact dura, a compressive model of SCI was performed as previously described (Allen 1911; Barut et al. 2005; Solaroglu et al. 2005), using a weigh of 70 g/cm loading to the dorsal surface of the spinal cord. Afterwards, the site was irrigated with saline, the muscles approximated and the reduced dead space and skin sutured using an unabsorbed suture. During anaesthetic recovery, the animals were kept warm in a box heated approximately to 37 °C. They received tramadol (2 mg/kg, orally), every 8 h for 3 days.
Treatment
The therapeutic protocol consisted of 10 mg/kg of dantrolene (Cristália Lab. Itapira, SP, Brazil) diluted in 15 ml of water for injection given in single dose, intraperitoneally 1 h after laminectomy. The control groups received only water for injection as placebo given in single dose, intraperitoneally 1 h after laminectomy.
Experimental groups
The animals were randomly divided into six groups according to the protocol of treatment and the time of euthanasia. GI (n = 7) underwent laminectomy followed by SCI, was treated with placebo and was euthanized after 32 h; GII (n = 7) underwent laminectomy alone, was treated with placebo and was euthanized after 32 h; GIII (n = 8) underwent laminectomy followed by SCI, was treated with dantrolene and was euthanized after 32 h; GIV (n = 8) underwent laminectomy followed by SCI, was treated with placebo and was euthanized after 8 days; GV (n = 8) underwent laminectomy alone, was treated with placebo and was euthanized after 8 days; and GVI (n = 8) underwent laminectomy followed by SCI, was treated with dantrolene and was euthanized after 8 days. The study and its results were carried out by investigators who were blind to the experimental conditions.
Macroscopic and light microscopy examination
The rats were euthanized with an overdose of thiopental by intraperitoneal injection and necropsied to evaluate the integrity of the spinal cord. The vertebral column segment between T3 and L3 was collected and fixed in 10% phosphate-buffered formalin (pH 7.4) for 7 days and then dissected for collection of the spinal cord. Transverse cuts were made in the epicentre and at 3 mm cranially and caudally.
TUNEL staining and positive cells counting
TUNEL technique was performed using an in situ apoptosis detection kit (ApopTag Plus - Oncor. Gaithersburg, MD, USA). Four-micron paraffin-embedded transversal spinal cord sections were deparaffinized and incubated sequentially in xylene, 100% ethanol, 95% ethanol, 70% ethanol and phosphate buffered solution (PBS). Protein was digested with proteinase K (20 g/ml PBS) at room temperature for 15 min. Endogenous peroxidase activity was blocked with 3% H2O2 methanol PBS at room temperature for 5 min. Sections were incubated in TdT buffer solution for 10 min, and then incubated with a mixture containing TdT (TdT tagged Biotin-dNTP) at 37 °C for 1 h. The reaction was stopped by incubating sections in termination buffer for 10 min at room temperature. After three washes in PBS, sections were incubated with streptavidin–horseradish peroxidase (HRP) for 30 min at room temperature. Following washes in PBS, sections were incubated with peroxidase substrate, 0.05% diaminobenzidine (DAB), at room temperature for 3–6 min. Sections were washed three times in distilled water and then counterstained with 0.5% Harri's haematoxylin. The slides were washed in running water for 5 min, dehydrated and mounted in a synthetic medium. An immersion objective was used to determine the average number of TUNEL-positive cells counted in 10 fields within the grey matter in a section 3 mm rostral to the epicentre. The sections 3 mm caudal to the epicentre were not counted as there was large amount of necrosis generating non-specific markings. The results were expressed as average number of TUNEL-positive cells per field in each group.
Anti-Caspase-3 staining and positive cells counting
Immunohistochemistry using the polyclonal antibody anti-caspase-3 (CPP32) (Neomarkers, Cat #RB-1197-R7. Fremont, CA, USA) was performed to evaluate programmed cellular death. Four-micron paraffin-embedded transversal spinal cord sections were deparaffinized and incubated sequentially in xylene, 100% ethanol, 95% ethanol, 70% ethanol, and PBS, and then submitted to heat-induced antigen retrieval (water bath at 98 °C) with antigen retrieval solution (DAKO. pH 6.0). Block of endogenous peroxidase activity was performed with 3% hydrogen peroxidase in methanol. Primary antibodies were diluted in PBS and incubated for 60 min at room temperature. Primary antibodies used for immunohistochemical evaluation included a polyclonal anti-caspase-3 (diluted 1:300). The reaction was amplified using a streptavidin-biotin-peroxidase complex method. Diaminobenzidine was used as a chromogen. Slides were counterstained with Mayer's haematoxylin, washed in running water for 5 min, dehydrated and mounted in a synthetic medium. An immersion objective was used to determine the average number of caspase-3-positive cells counted in 10 fields within the grey matter in a section 3 mm rostral to the epicentre. The sections 3 mm caudal to the epicentre were not counted as there was large amount of necrosis generating non-specific immunohistochemical markings. The results were expressed as average number of caspase-3-positive cells per field in each group.
Anti-NeuN staining and positive neurons counting
Immunohistochemistry using the monoclonal antibody anti-NeuN (Chemicon, cat# MAB377. Temecula, CA, USA) was performed to evaluate neuronal viability. Four-micron paraffin-embedded transversal spinal cord sections were deparaffinized and incubated sequentially in xylene, 100% ethanol, 95% ethanol, 70% ethanol, and PBS. They were submitted to heat-induced antigen retrieval (water bath at 98 °C) with antigen retrieval solution (DAKO. pH 6.0). Block of endogenous peroxidase activity was performed with 3% H2O2 in PBS. Primary antibody (diluted 1:1600) was incubated with the sections overnight in a refrigerator. The reaction was amplified using a streptavidin-biotin-peroxidase complex method. Diaminobenzidine/H2O2 was used as a chromogen. Slides were counterstained with haematoxylin, washed in running water for 5 min, dehydrated and mounted in a synthetic medium. Immersion objective was used to determine the average number of NeuN-positive neurons counted in 10 fields within the grey matter in a section 3 mm rostral to the epicentre. The sections 3 mm caudal to the epicentre were not counted as there was large amount of necrosis generating non-specific immunohistochemical markings. The results were expressed as average number of NeuN-positive neurons per field in each group.
Statistical analysis
All data collected were analysed using Prism 5 for Windows (GraphPad Software. La Jolla, CA, USA). Data from number of TUNEL-positive cells, caspase-3-positive cells and NeuN-positive neurons were evaluated using one-way anova with a post hoc Student-Newman-Keuls test. These data were presented as mean ± SD values. In all analyses, P value ≤ 0.05 was considered statistically significant.
Results
Macroscopic and light microscopy findings
There were no complications attributable to intraperitoneal injection or side effects related to the use of dantrolene (data not shown). Histological examination of the cord samples from animals subjected only to laminectomy (GII and GV) revealed no changes. At 32 h, the animals from GI and GIII displayed patterns of lesions with similar characteristics at the epicentre. Diffuse microvascularization and multifocal areas of acute haemorrhage with Wallerian degeneration and necrosis were observed in the white matter. In most areas, large quantities of axonal balloons and some Gitter cells, characterizing digestion chambers, were detected. There was a severe loss of grey matter, several foci of haemorrhage and much cellular debris, the latter representing remnants of necrotic neurons surrounding with mild multifocal infiltration of Gitter cells. In both white and grey matters, a mild, diffuse infiltration of neutrophils was noted. At 8 days, the animals from GIV and GVI presented patterns with similar characteristics at the epicentre as well. There were several foci of severe and extensive malacia in both white (Wallerian degeneration) and grey matters, affecting half of the cord diameter. A marked infiltration of Gitter cells was likewise observed in these areas. In most segments of the white matter moderate amounts of axonal balloons and the mild presence of digestion chambers were noted. These results suggest an advanced stage of spinal cord degeneration. In the areas adjacent to the epicentre, axonal degeneration was observed in the white matter, whereas the grey matter displayed marked and diffuse increased cellularity, neuronophagia, mild and multifocal neuronal necrosis and satellitosis. Furthermore, these areas contained neuronal bodies with morphological features of apoptosis, such as increased cytoplasm and chromatin condensation and formation of apoptotic bodies, which appeared to be more frequent in GIV.
TUNEL staining and positive cells counting
Cases were interpreted as positive if nuclei were labelled. Non-positive cells had preserved morphological features, whereas all those that were labelled displayed pathological features of necrosis or apoptosis. The number of TUNEL-positive cells was significantly lower in the animals from GIII and GVI than those from GI and GIV, respectively at 32 h (P < 0.01) and 8 days (P < 0.001). The staining was similar between animals treated with dantrolene and those that did not receive spinal cord trauma at 32 h and 8 days (P>0.05) (Figure 1).
Figure 1.
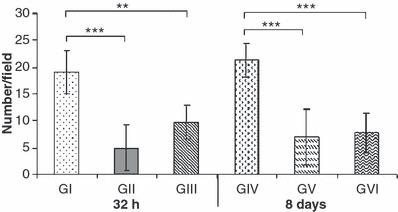
Means ± SD of the average number per field (100×) of TUNEL-positive cells in the spinal cord of Wistar rats. GI [spinal cord injury (SCI) with placebo - 32 h]; GII (laminectomy only with placebo - 32 h); GIII (SCI with dantrolene - 32 h); GIV (SCI with placebo - 8 days); GV (laminectomy only with placebo – 8 days) and GVI (SCI with dantrolene - 8 days). *P < 0.05, **P < 0.01, ***P < 0.001.
Anti-Caspase-3 staining and positive cells counting
Cases were interpreted as positive if either the cytoplasm or the nuclei or both were labelled. Non-positive cells had preserved morphological features, whereas the ones that were labelled displayed pathological features of apoptosis, such as cytoplasm or chromatin condensation and formation of apoptotic bodies. The number of caspase-positive cells was significantly lower in the animals from GIII and GVI than those from GI and GIV, respectively at 32 h (P < 0.05) and 8 days (P < 0.01). The staining was similar between animals treated with dantrolene and those that did not receive spinal cord trauma at 32 h or 8 days (P>0.05) (Figure 2).
Figure 2.
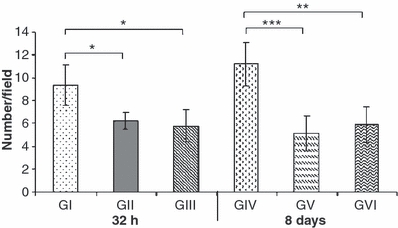
Means ± SD of the average number per field (100×) of caspase-3-positive cells in the spinal cord of Wistar rats. GI (SCI with placebo - 32 h); GII (laminectomy only with placebo - 32 h); GIII [spinal cord injury (SCI) with dantrolene - 32 h]; GIV (SCI with placebo - 8 days); GV (laminectomy only with placebo – 8 days) and GVI (SCI with dantrolene - 8 days). *P < 0.05, **P < 0.01, ***P < 0.001.
Anti-NeuN staining and positive neurons counting
Cases were interpreted as positive if neuronal nuclei were labelled. There was no staining of glial cells and the stained neurons invariably had preserved morphological characteristics. Although the number of NeuN-positive neurons was similar at 32 h between GI and GIII (P>0.05), it was higher in the animals from GVI than those from GIV at 8 days after SCI (P < 0.01). Moreover, the number of stained neurons was similar between animals treated with dantrolene and those that did not receive spinal cord trauma both at 32 h and 8 days (P>0.05) (Figures 3 and 4).
Figure 3.
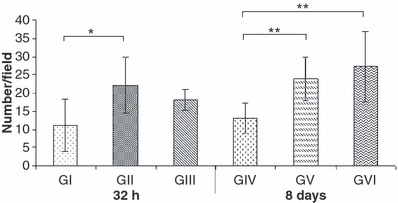
Means ± SD of the average number per field (100×) of NeuN-positive neurons in the spinal cord of Wistar rats. GI (SCI with placebo - 32 h); GII (laminectomy only with placebo - 32 h); GIII [spinal cord injury (SCI)with dantrolene - 32 h]; GIV (SCI with placebo - 8 days); GV (laminectomy only with placebo – 8 days) and GVI (SCI with dantrolene - 8 days). *P < 0.05, **P < 0.01, ***P < 0.001.
Figure 4.
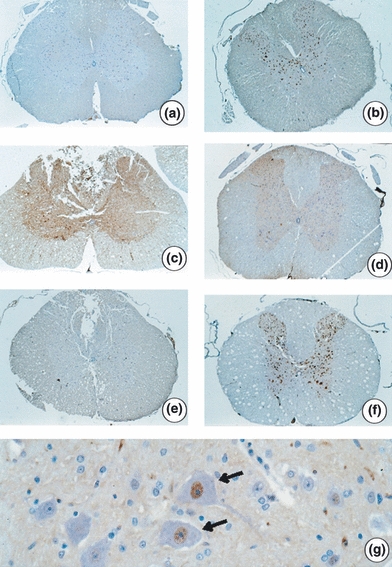
Photomicroscopy of spinal cord cross-sections adjacent to the epicentre of injury in Wistar rats. Immunohistochemistry with anti-NeuN. (a) Nuclei staining of intact neuronal cell bodies (NeuN-positive) in the grey matter in animal from GII (laminectomy only with placebo - 32 h) - 23.5×; (b) NeuN-positive neurons in the animals from GV (laminectomy only with placebo - 8 days) - 26×; (c) Mild staining of NeuN-positive neurons in animals from GI [spinal cord injury (SCI) with placebo - 32 h] - 24×; (d) Moderate staining of NeuN-positive neurons in animal from GIII (SCI with dantrolene - 32 h) – 21.8×; (e) Mild staining of NeuN-positive neurons in animal from GIV (SCI with placebo - 8 days) - 23.9×; (f) NeuN-positive neurons similar to the groups without injury. Animal VI (SCI with dantrolene - 8 days) - 23.3×; (g) Detail of NeuN-positive neurons (arrows) with nucleus staining by monoclonal anti-NeuN - 374.6×.
Discussion
Neuronal cell death following traumatic SCI is a delayed process that contributes to progressive secondary degeneration and, ultimately, results in spinal cord dysfunction below the injury site (Crowe et al. 1997; Liu et al. 1997; Springer 2002). This secondary process, characterized by apoptotic neuronal death in areas far from the initial trauma, is triggered by deleterious substances produced in response to the primary insult (Beattie et al. 2000; Xu et al. 2008).
Apoptosis can be induced by a variety of stimuli, but execution of the apoptotic program involves a common mechanism that relies on activation of caspases, which are calcium-dependent cysteine proteases (Liu et al. 1997; Mattson 2000). Cytosolic Ca2+ concentration starts to increase about 30 min after SCI, reaches its maximum value approximately 8 h later and remains elevated for a week (Happel et al. 1981). This is the reason to have opted for a single application of dantrolene 1 h after the trauma. Calcium overload can trigger a range of calcium-dependent processes that will lethally alter the metabolism of remaining cells (Zhang et al. 1993; Yano et al. 2001; Nakayama et al. 2002). The control of intracellular Ca2+ homeostasis protects against programmed cellular death, and can thus, in long-term evaluation, be beneficial to neurological improvement after SCI (Fehlings et al. 1989; Springer et al. 2000; Orrenius et al. 2003).
To achieve this therapeutic target, drugs that affect calcium homeostasis could be employed. Dantrolene, a ryanodine receptor antagonist, inhibits Ca2+ efflux from the endoplasmic reticulum to the cytosol, resulting in a documented neuroprotective effect (Zhao et al. 2001; Ayar & Kelestimur 2002; Krause et al. 2004; Kobayashi et al. 2005; Mori et al. 2005). It was hypothesized that this drug would have a neuroprotective effect by reducing the progression of secondary injury mechanisms after SCI (Kocogullari et al. 2008; Aslan et al. 2009). Thus, dantrolene was a promising option to be tested in in vivo traumatic SCI models once its antiapoptotic properties had not been studied in this model.
In this study, dantrolene decreased caspase-3 activity, as demonstrated by immunohistochemistry, at 32 h and 8 days after SCI and, consequently, attenuated nervous cells apoptosis. It has been previously shown that caspase-3 and its upstream and downstream components are activated after traumatic SCI (Springer et al. 2009). These events occur early in neurons and glia at the injury site and hours to days later in areas distant from the primary site (Springer 2002; Tagaki et al. 2003). Our data revealed that caspase-3 staining was localized in the cell nucleus, cytoplasm or in both regions following contusion SCI, as shown by previous studies (McEwen & Springer 2005). This variability in the cellular localization of caspase-3 suggests that during SCI, different pathways of caspase-3 activation are initiated by different insults (Xu et al. 2008), and among these, the overload of cytosolic Ca2+ is one of the most important (Wei & Perry 1996; Popescu et al. 2002; Li et al. 2005; Solaroglu et al. 2005).
Another reliable method to evaluate apoptosis is TUNEL staining, which identifies DNA fragmentation. This technique has been used commonly as a tool for apoptotic cell identification (Negoescu et al. 1996; Barut et al. 2005; Aslan et al. 2009). Our findings indicated that nervous tissue apoptosis could be detected by TUNEL from 32 h to 8 days following traumatic SCI, and that animals treated with dantrolene had fewer TUNEL-positive cells. In addition, the number of anti-NeuN immunoreactive neurons was inversely proportional to the number of TUNEL or caspase-3 positive cells in all groups. Anti-NeuN labels a neuronal nuclear protein that is expressed both in the nucleus and to a lesser degree in the cytosol of non-injured neurons. Interestingly, dantrolene-treated rats showed a greater number of NeuN-positive neurons than the untreated groups, at 8 days after SCI. NeuN immunoreactivity may decrease in several pathological conditions that affect neuronal viability, such as ischaemia, hypoxia and trauma (Mullen et al. 1992; Xu et al. 2002). Thereby, TUNEL and immunohistochemical findings confirmed that dantrolene can significantly attenuate cell apoptosis and protect cord tissue of rats with traumatic spinal injury, emphasizing the potential neuroprotective role of this drug after SCI.
The neuroprotective effect of dantrolene presented in this study, evidenced by decreasing apoptotic features, corroborates the findings of several authors who have reported similar properties after experimental induction of neuronal death (Frandsen & Schousboe 1991; Zhang et al. 1993; Gwak et al. 2008; Muehlschlegel & Sims 2009). Dantrolene prevented abnormal Ca2+ release from intracellular reserves in cultured hypothalamic or hippocampal neurons after transient ischaemia and decreased the apoptotic cell death in these cells (Wei & Perry 1996; Yano et al. 2001; Nakayama et al. 2002). In addition, dantrolene prevented the collapse of mitochondrial membrane potential and blocked the stimuli for mitochondrial activation of the caspase cascade in neurons (Wei et al. 2000). Dantrolene also decreased apoptotic cell death induced by kainic acid in cultured cerebellar granular cells (in vitro) and brain neurons in rats (in vivo) (Popescu et al. 2002). It has been reported that this drug has a neuroprotective effect against ischaemic damage by reducing endoplasmatic reticulum stress-mediated apoptotic signal pathway activation after transient middle cerebral artery occlusion in rats (Li et al. 2005).
On the other hand, few studies have been performed to investigate the effects of dantrolene on SCI. Thorell et al. (2002) examined the role of intracellular calcium in mediating posttraumatic abnormalities in axonal conduction and demonstrated that dantrolene improved electrophysiological recovery in an in vitro model of compressive injury to an isolated spinal cord dorsal column segment. Most recently, dantrolene afforded neuroprotection by antioxidative status in a model of spinal cord ischaemia/reperfusion injury induced by abdominal aortic occlusion in rabbits (Kocogullari et al. 2008) and in a traumatic ballon model, also in rabbits, that showed decreased TUNEL-positive cells counting after dantrolene injection (Aslan et al. 2009). However, to the best of our knowledge, this is the first direct evidence connecting dantrolene with caspase-3 activity inhibition and neuronal viability preservation on spinal cord in an in vivo model of traumatic SCI, strengthening evidence of its neuroprotective effect via antiapoptotic mechanisms in neurological disorders.
It has been shown that sparing as few as 5–10% of the fibres at the lesion epicentre is sufficient to preserve locomotor function after SCI in long-term evaluation (Eidelberg et al. 1977; Fehlings & Tator 1995; Basso et al. 1996). In this context, efforts must be undertaken to minimize tissue damage from the mechanisms of secondary injury, as we have demonstrated in this study. We believe that studies to evaluate whether dantrolene could allow better motor recovery, after SCI in long-term clinical evaluation, must be the object of future investigation.
In summary, we demonstrate here that dantrolene has neuroprotective effects in rats suffering from traumatic SCI. Systemically injected, this drug decreases apoptotic cell death and preserves neuronal viability following SCI. These findings suggest that dantrolene may provide a promising therapeutic strategy for the management of SCI and future investigation, such as concerning long-term clinical evaluation, must be carried out to elucidate the broad potential of this drug on SCI.
Acknowledgments
The study was supported by grants from Fundação de Amparo a Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Cientifico e Tecnologico (CNPq) and Cristalia Lab.
References
- Allen AR. Surgery of experimental lesion of spinal cord equivalent to crush injury of fracture dislocation of spinal column. A preliminary report. JAMA. 1911;57:878–880. [Google Scholar]
- Aslan A, Cemek M, Buyukokuroglu ME, et al. Dantrolene can reduce secondary damage after spinal cord injury. Eur. Spine J. 2009;18:1442–1451. doi: 10.1007/s00586-009-1033-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ayar A, Kelestimur H. The inhibitory effects of dantrolene on action potential-induced calcium transients in cultured rat dorsal root ganglion neurons. Physiol. Res. 2002;51:341–346. [PubMed] [Google Scholar]
- Barut S, Unlu YA, Karaoglan A, et al. The neuroprotective effects of z-DEVD.fmk, a caspase-3 inhibitor, on traumatic spinal cord injury in rats. Surg. Neurol. 2005;64:213–220. doi: 10.1016/j.surneu.2005.03.042. [DOI] [PubMed] [Google Scholar]
- Basso DM, Beattie MS, Bresnahan JC. Graded histological and locomotor outcomes after spinal cord contusion using the NYU weight-drop device versus transaction. Exp. Neurol. 1996;139:244–256. doi: 10.1006/exnr.1996.0098. [DOI] [PubMed] [Google Scholar]
- Beattie MS, Farooqui AA, Bresnahan JC. Review of current evidence for apoptosis after spinal cord injury. J. Neurotrauma. 2000;17:915–925. doi: 10.1089/neu.2000.17.915. [DOI] [PubMed] [Google Scholar]
- Büyükokuroglu ME. Anti-inflamatory and antinociceptive properties of dantrolene sodium in rats and mice. Pharmacol. Res. 2002;45:455–460. doi: 10.1006/phrs.2002.0970. [DOI] [PubMed] [Google Scholar]
- Büyükokuroglu ME, Gulçin I, Oktay M, Kufrevioglu OI. In vitro antioxidant properties of dantrolene sodium. Pharmacol. Res. 2001;44:491–494. doi: 10.1006/phrs.2001.0890. [DOI] [PubMed] [Google Scholar]
- Cherednichenko G, Ward CW, Feng F, et al. Enhanced excitation-coupled calcium entry in myotubes expressing malignant hyperthermia mutation R163c is attenuated by dantrolene. Mol. Pharmacol. 2008;73:1203–1212. doi: 10.1124/mol.107.043299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crowe MJ, Bresnahan JC, Shuman SL, Masters JN, Crowe MS. Apoptosis and delayed degeneration after spinal cord injury in rats and monkeys. Nat. Med. 1997;3:73–76. doi: 10.1038/nm0197-73. [DOI] [PubMed] [Google Scholar]
- Eidelberg E, Straehley D, Erspamer R, Watkins CJ. Relationship between residual hindlimb-assisted locomotion and surviving axons after incomplete spinal cord injuries. Exp. Neurol. 1977;56:312–322. doi: 10.1016/0014-4886(77)90350-8. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH. The relationships among the severity of spinal cord injury, residual neurological function, axon counts, and counts of retrogradely labeled neurons after experimental spinal cord injury. Exp. Neurol. 1995;132:220–228. doi: 10.1016/0014-4886(95)90027-6. [DOI] [PubMed] [Google Scholar]
- Fehlings MG, Tator CH, Linden RD. The effect of nimodipine and dextran on axonal function and blood flow following experimental spinal cord injury. J. Neurosurg. 1989;71:403–1416. doi: 10.3171/jns.1989.71.3.0403. [DOI] [PubMed] [Google Scholar]
- Frandsen A, Schousboe A. Dantrolene prevents glutamate cytotoxicity and Ca2+ release from intracellular stores in cultured cerebral cortical neurons. J. Neurochem. 1991;56:1075–1078. doi: 10.1111/j.1471-4159.1991.tb02031.x. [DOI] [PubMed] [Google Scholar]
- Gwak M, Park P, Kim K, et al. The effects of dantrolene on hypoxic-ischemic injury in the neonatal rat brain. Anesth. Analg. 2008;106:227–233. doi: 10.1213/01.ane.0000287663.81050.38. [DOI] [PubMed] [Google Scholar]
- Happel RD, Smith KP, Banik NL, Powers JM, Hogan EL, Balentine JD. Ca2+-accumulation in experimental spinal cord trauma. Brain Res. 1981;211:476–479. doi: 10.1016/0006-8993(81)90976-8. [DOI] [PubMed] [Google Scholar]
- Kobayashi S, Bannister ML, Gangopadhyay JP, Hamada T, Parness J, Ikemoto N. Dantrolene stabilizes domain interactions within the ryanodine receptor. J. Biol. Chem. 2005;280:6580–6587. doi: 10.1074/jbc.M408375200. [DOI] [PubMed] [Google Scholar]
- Kocogullari CU, Emmiler M, Cemek M, et al. Can dantrolene protect spinal cord against ischemia/reperfusion injury? An experimental study. Thorac. Cardiovasc. Surg. 2008;56:406–411. doi: 10.1055/s-2008-1038731. [DOI] [PubMed] [Google Scholar]
- Krause T, Gerbershagen MU, Fiege M, Weisshorn R, Wappler F. Dantrolene – a review of its pharmacology, therapeutic use and new developments. Anaesthesia. 2004;59:364–373. doi: 10.1111/j.1365-2044.2004.03658.x. [DOI] [PubMed] [Google Scholar]
- Li F, Hayashi T, Jin G, et al. The protective effect of dantrolene on ischemic neuronal cell death is associated with reduced expression of endoplasmic reticulum stress markers. Brain Res. 2005;1048:59–68. doi: 10.1016/j.brainres.2005.04.058. [DOI] [PubMed] [Google Scholar]
- Liu XZ, Xu XM, Hu R, et al. Neuronal and glial apoptosis after traumatic spinal cord injury. J. Neurosci. 1997;17:5395–5406. doi: 10.1523/JNEUROSCI.17-14-05395.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mattson MP. Apoptosis in neurodegenerative disorders. Nat. Rev. Mol. Cell Biol. 2000;1:120–129. doi: 10.1038/35040009. [DOI] [PubMed] [Google Scholar]
- McEwen ML, Springer JE. A mapping study of caspase-3 activation following acute spinal cord contusion in rats. J. Histochem. Cytochem. 2005;53:809–819. doi: 10.1369/jhc.4A6467.2005. [DOI] [PubMed] [Google Scholar]
- Mori F, Okada M, Tomiyama M, Kaneko S, Wakabayashi K. Effects of ryanodine receptor activation on neurotransmitter release and neuronal cell death following kainic acid-induced status epilepticus. Epilepsy Res. 2005;65:59–70. doi: 10.1016/j.eplepsyres.2005.04.006. [DOI] [PubMed] [Google Scholar]
- Muehlschlegel S, Sims JR. Dantrolene: mechanisms of neuroprotection and possible clinical applications in the neurointensive care unit. Neurocrit. Care. 2009;10:103–115. doi: 10.1007/s12028-008-9133-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mullen RJ, Buck CR, Smith AM. NeuN, a neuronal specific nuclear protein in vertebrates. Development. 1992;116:201–211. doi: 10.1242/dev.116.1.201. [DOI] [PubMed] [Google Scholar]
- Nakayama R, Yano T, Ushijima K, Abe K, Terasaki H. Effects of dantrolene on extracellular glutamate concentration and neuronal death in the rat hippocampal CA1 region subjected to transient ischemia. Anesthesiol. 2002;96:705–710. doi: 10.1097/00000542-200203000-00029. [DOI] [PubMed] [Google Scholar]
- Negoescu A, Lorimier P, Labat-Moleur F, et al. In situ apoptotic cell labeling by the TUNEL method: improvement and evaluation on cell preparations. J. Histochem. Cytochem. 1996;44:959–968. doi: 10.1177/44.9.8773561. [DOI] [PubMed] [Google Scholar]
- Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: the calcium-apoptosis link. Nat. Rev. Mol. Cell Biol. 2003;4:552–565. doi: 10.1038/nrm1150. [DOI] [PubMed] [Google Scholar]
- Popescu BO, Oprica M, Sajin M, et al. Dantrolene protects neurons against kainic acid induced apoptosis in vitro and in vivo. J. Cell Mol. Med. 2002;6:555–569. doi: 10.1111/j.1582-4934.2002.tb00454.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shields LBE, Zhang WP, Burke DA, Gray R, Shields CB. Benefit of chondroitinase abc on sensory axon regeneration in a laceration model of spinal cord injury in the rat. Surg. Neurol. 2008;69:568–577. doi: 10.1016/j.surneu.2008.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Solaroglu I, Kaptanoglu E, Okutan O, Beskonakli E, Attar A, Kilinc K. Magnesium sulfate treatment decreases caspase-3 activity after experimental spinal cord injury in rats. Surg. Neurol. 2005;64:17–21. doi: 10.1016/j.surneu.2005.07.058. [DOI] [PubMed] [Google Scholar]
- Springer JE. Apoptotic cell death following traumatic injury to the central nervous system. J. Biochem. Mol. Biol. 2002;35:94–105. doi: 10.5483/bmbrep.2002.35.1.094. [DOI] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Nottingham SA, Kennedy SE. Calcineurin mediated BAD dephosphorylation activates the caspase-3 apoptotic cascade in traumatic spinal cord injury. J. Neurosci. 2000;20:7246–7251. doi: 10.1523/JNEUROSCI.20-19-07246.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JE, Azbill RD, Knapp PE. Activation of the caspase-3 apoptotic cascade in traumatic spinal cord injury. Nat. Med. 2009;5:943–946. doi: 10.1038/11387. [DOI] [PubMed] [Google Scholar]
- Tagaki T, Takayasu M, Mizuno M, Yoshimoto M, Yoshida J. Caspase activation in neuronal and glial apoptosis following spinal cord injury in mice. Neurol. Med. Chir. (Tokyo) 2003;43:20–30. doi: 10.2176/nmc.43.20. [DOI] [PubMed] [Google Scholar]
- Thorell WE, Leibrock LG, Agrawal SK. Role of RYRs and IP3 receptors after traumatic injury to spinal cord white matter. J. Neurotrauma. 2002;19:335–342. doi: 10.1089/089771502753594909. [DOI] [PubMed] [Google Scholar]
- Thuret S, Moon LDF, Gage FH. Therapeutic interventions after spinal cord injury. Nat. Rev. Neurosci. 2006;7:628–643. doi: 10.1038/nrn1955. [DOI] [PubMed] [Google Scholar]
- Uçüncü H, Taysi S, Aktan B, Buyukokuroglu ME, Elmastas M. Effect of dantrolene on lipid peroxidation, glutathione and glutathione-dependent enzyme activities in experimental otitis media with effusion in guinea pigs. Hum. Exp. Toxicol. 2005;24:567–571. doi: 10.1191/0960327105ht569oa. [DOI] [PubMed] [Google Scholar]
- Wei H, Perry DC. Dantrolene is cytoprotective in two models of neuronal cell death. J. Neurochem. 1996;67:2390–2398. doi: 10.1046/j.1471-4159.1996.67062390.x. [DOI] [PubMed] [Google Scholar]
- Wei H, Leeds P, Chen RW, et al. Neuronal apoptosis induced by pharmacological concentrations of 3-hydroxykynurenine: characterization and protection by dantrolene and Bcl-2 overexpression. J. Neurochem. 2000;75:81–90. doi: 10.1046/j.1471-4159.2000.0750081.x. [DOI] [PubMed] [Google Scholar]
- Xu GP, Dave KR, Vivero R, Schmidt-Kastner R, Sick TJ, Pérez-Pinzón MA. Improvement in neuronal survival after ischemic preconditioning in hippocampal slice cultures. Brain Res. 2002;952:153–158. doi: 10.1016/s0006-8993(02)02988-8. [DOI] [PubMed] [Google Scholar]
- Xu GY, Liu S, Hughes MG, McAdoo DJ. Glutamate-induced losses of oligodendrocytes and neurons and activation of caspase-3 in the rat spinal cord. Neurosci. 2008;153:1034–1047. doi: 10.1016/j.neuroscience.2008.02.065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yano T, Nakayama R, Imaizumi T, Terasaki H, Ushijima K. Dantrolene ameliorates delayed cell death and concomitant DNA fragmentation in the rat hippocampal Ca1 neurons subjected to mild ischemia. Resuscitation. 2001;50:117–125. doi: 10.1016/s0300-9572(00)00369-5. [DOI] [PubMed] [Google Scholar]
- Zhang L, Andou Y, Masuda S, Mitani A, Kataoka K. Dantrolene protects against ischemic, delayed neuronal death in gerbil brain. Neurosci. Lett. 1993;58:105–108. doi: 10.1016/0304-3940(93)90623-s. [DOI] [PubMed] [Google Scholar]
- Zhao F, Li P, Chen SR, Louis CF, Fruen BR. Dantrolene inhibition of ryanodine receptor Ca2+ release channels. molecular mechanisms and isoform selectivity. J. Biol. Chem. 2001;276:13810–13816. doi: 10.1074/jbc.M006104200. [DOI] [PubMed] [Google Scholar]


