Abstract
Objective
To assess the cross-sectional association of thiazolidinediones (TZD) with diabetic macular edema (DME).
Methods
The cross-sectional association of DME and visual acuity with TZD was examined using baseline fundus photographs and visual acuity measurements from the Eye Substudy of the ACCORD Trial. Visual acuity was assessed in 9,690 participants in ACCORD, 3,473 of these participants had fundus photographs that were centrally read in a standardized fashion by masked graders to assess DME and retinopathy.
Results
Among the sub-sample, 695 (20.0%) had TZD use while 217 (6.2%) had DME. TZD use was not associated with DME in unadjusted (OR=1.01, 95% CI: (0.71, 1.44), P=0.95) and adjusted analyses (OR=0.97, (0.67, 1.40), P=0.86). Significant associations with DME were found for retinopathy severity (P<0.0001) and age (OR=0.97, (0.952, 0.997), P=0.0298) but not for HbA1c (P=0.06), duration of diabetes (P=0.65), gender (P=0.72), and race (P=0.20). TZD use was associated with slightly greater visual acuity (0.79 letters, (0.20, 1.38), P=0.0091) of uncertain clinical significance.
Conclusions
In a cross-sectional analysis of data from the largest study to date, no association was observed between TZD exposure and DME in patients with type 2 diabetes; however, we cannot exclude a modest protective or harmful association.
Keywords: Type 2 Diabetes, macular edema, visual acuity, thiazolidinediones, Avandia, Actos, retinopathy, visual acuity, rosiglitazone, pioglitazone
Introduction
Diabetic macular edema (DME) is one of the main causes of visual impairment in persons with diabetic retinopathy. A small number of case reports have raised the possibility that use of thiazolidinediones (TZD), which can cause fluid retention generally, might exacerbate DME (1,2). These observations led to clinician alerts or label changes by the manufacturers to providers in Canada and the United States (3,4,5,6). One report (1) described a patient who developed vision loss and DME after an increase of rosiglitazone dose from 2 mg/day to 8 mg/day. After the dose was decreased back to 2 mg/day, his vision improved and his DME resolved. Ryan et al. reported results from a retrospective chart review of 30 patients who presented to a single practice of retinal specialists with use of pioglitazone or rosiglitazone and both lower extremity edema and DME (2). The authors contended that fluid overload due to TZD use contributed to DME in at least 19 of 30 patients, and that, in two, there was evidence of a direct cause-and-effect relationship. Finally, a case report of macular edema being resolved with systemic furosemide suggests that TZDs may exacerbate macular edema (7). In a larger study of 292 patients, Shen, et al, found no association of rosiglitazone with DME (8).
These reports are limited by the absence of comparison groups of patients who have not been exposed to TZDs, and cannot control for confounding between TZD exposure and other risk factors for DME, including gender (9), age (9), race (10), longstanding diabetes (9,11), insulin use (12), increased severity of diabetic retinopathy (9, 11), elevated serum cholesterol (13,14,15,16), hypertension (9), and poor glycemic control (9,11).
Interest in this issue has been heightened by broader concerns about the safety of the currently available thiazolidinediones, pioglitazone and rosiglitazone. The PROactive Study, which tested the hypothesis that pioglitazone can reduce cardiovascular risk, showed benefit in an important secondary cardiovascular endpoint, but also significant increases of both peripheral edema and congestive heart failure (17). A meta-analysis of trials of pioglitazone suggested reductions of cardiovascular death, myocardial infarction, and stroke, but also increased risk of congestive heart failure (18). Rosiglitazone has been associated in a meta-analysis (19) with greater risk for myocardial infarction (OR=1.43, P=0.03) and death (OR=1.64, P=0.06). An unplanned interim analysis by the Rosiglitazone Evaluated for Cardiac Outcomes and Regulation of Glycaemia in Diabetes (RECORD) Study investigators followed (20), yielding an estimated hazard ratio for the cardiovascular endpoint of 1.11 (95% CI: 0.93, 1.32). A series of editorials (21,22,23,24,25) ensued. The US Food and Drug Administration (FDA) has required the package label for pioglitazone to include a black box warning about risk of congestive heart failure and that for rosiglitazone a similar warning about risk of congestive heart failure or myocardial ischemia (26).
The Eye Substudy of the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial was designed to test the effect of the ACCORD interventions (strategies to control blood glucose, blood lipids, and blood pressure) on the development and progression of diabetic retinopathy. This study provides us the opportunity to evaluate the potential association of TZD with DME. In addition, we report the relationship between TZD use and visual acuity, which is assessed in all participants enrolled in the ACCORD study.
Methods
The ACCORD study (27,28) and the ACCORD Eye Substudy (29) have been described previously. In brief, ACCORD is a multi-center, randomized, controlled, double 2 × 2 factorial trial in 10,251 patients with type 2 diabetes mellitus. The trial was designed to test the effects of intensive glycemia control, of treatment to increase HDL-cholesterol and lower triglycerides, and of intensive blood pressure control on major cardiovascular disease (CVD). Visual acuity was assessed at baseline on 9,690 people using a standardized Early Treatment Diabetic Retinopathy Study (ETDRS) logarithmic chart (30).
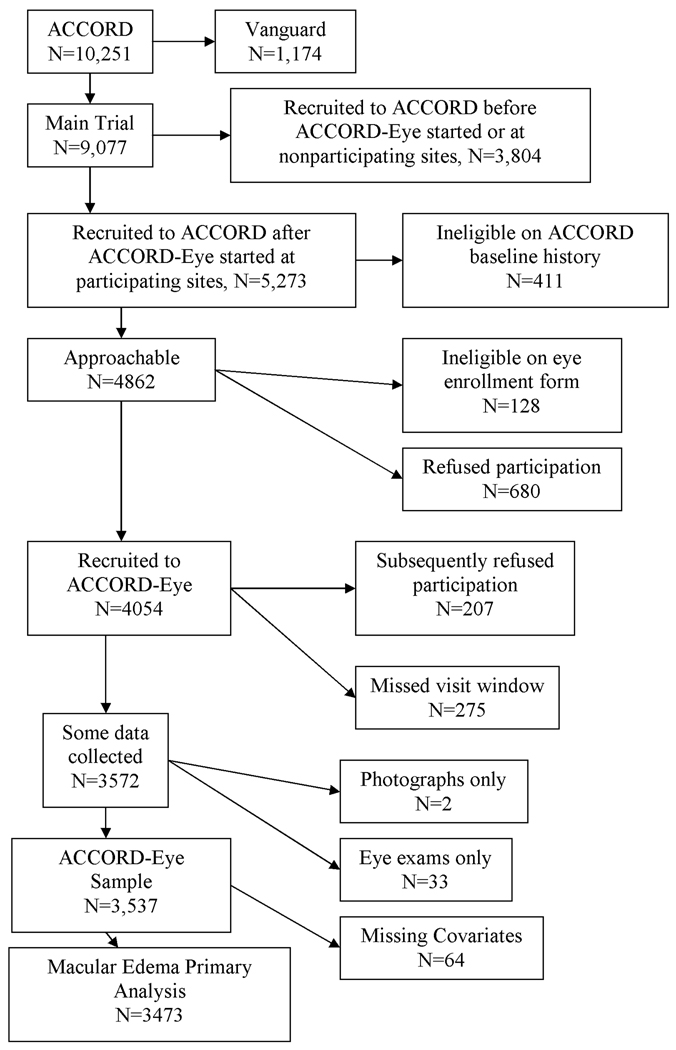
The ACCORD Eye study recruited 3,537 participants in whom baseline stereoscopic retinal photographs of 7 standard fields and ophthalmologic examinations were obtained. Participants were eligible to enroll in the eye study if they had not had laser photocoagulation or vitrectomy for diabetic retinopathy in either eye. A summary of recruitment of ACCORD participants into ACCORD Eye is shown in Figure 1. Masked evaluators at the Fundus Photograph Reading Center (University of Wisconsin) graded all photographs for the severity of diabetic retinopathy and the presence of DME. The severity of diabetic retinopathy was graded using the ETDRS scale (31) and categorized into five levels: none, mild, moderate nonproliferative, severe nonproliferative, and proliferative using the International Clinical Diabetic Retinopathy Severity scale (32). DME was scored separately for each eye on a scale from 0 to 3. Specifically, the scale was 0 = “none,” 1 = “questionable,” 2 = “zone of retinal thickness ≥1 disc area and within ≤ 1 disc diameter from center of the macula,” and 3 = “retinal thickness or adjacent hard exudates within 500 µm of the center of the macula.” This scale was collapsed into absence (0) or presence (1–3) of DME. Those eyes graded as grades 2 or 3 would be considered as having clinically significant macular edema (CSME) because the center of fovea is involved or threatened (33).
Figure 1.
ACCORD-Eye recruitment flow chart. ACCORD-Eye began recruitment after initiation of the main trial.
Thiazolidinedione use was assessed based on self-report at the baseline examination and included current use of rosiglitazone or pioglitazone on a regular basis. The duration of TZD exposure prior to baseline is not known but the ACCORD inclusion criteria required that no new antihyperglycemic drugs were added within 3 months of baseline.
Race/ethnicity was categorized into non-exclusive categories of white, black, and Hispanic. Other therapy for diabetes, a surrogate for severity, was measured by the number of types of diabetes medications used at baseline, excluding TZDs and insulin. These medications included 1) sulfonylureas, 2) alpha-glucosidase inhibitors, 3) biguanides, and 4) meglitinides. The number of medications ranged between 0 and 4 and was treated as a categorical variable. Insulin and diuretic use at baseline were recorded. Mean arterial pressure was calculated as (systolic+2×diastolic)/3. Pretibial edema in either foot was assessed on clinical examination. A history of foot ulcer requiring antibiotics or presence of ulceration on either foot by exam was documented. Foot amputation due to diabetes was assessed by examination.
Statistical Analyses
All analyses were prespecified before analysis began. Descriptive statistics were calculated, including proportions for categorical variables and means, medians, standard deviations, and ranges for continuous variables. Comparisons of proportions between two groups were made using a chi-square test. Almost all participants had measurements on both eyes. To account for the within-participant correlation and thus utilize data from both eyes, our primary model was a generalized estimating equation (GEE) model (34,35) predicting DME, using TZD at baseline as a predictor. Odds ratios (OR), p-values, and 95% confidence intervals (CIs) are reported. P-values were calculated using generalized score statistics, and CIs presented are Wald intervals. Six factors known to be associated with DME were included as covariates: HbA1c (9, 11), diabetes duration (9, 11), retinopathy severity (9, 11), gender (9), age (9), and race (10). In a secondary analysis, a modified version of the model selection approach presented by Hosmer and Lemeshow (36) was employed to examine other potential covariates (Tables 1 and 2). Included in all models were TZD use at baseline, gender, race/ethnicity, diabetes duration, age, HbA1c, and retinopathy. All other variables were examined for possible inclusion using the variable selection method described below. Three interactions of TZD use were specified a priori and were examined in order: TZD use with diabetes duration, insulin use (only if insulin was in the model as a main effect), and HbA1c. We screened potential covariates at P≤0.25 in a series of unadjusted models and comparing TZD users to non-users. These variables and those in the primary model were included, and backwards selection (P>0.1) was used to delete variables from this model. Linearity was examined using generalized additive models (GAM) (37,38) and by categorizing the covariates at their quartiles. Interactions were examined at P≤0.05. Model adequacy was examined using the techniques of Lin, Wei, and Ying (39,40) to assess linearity and adequacy of the logit link. We examined two additional sets of GEE models: one for moderate or severe CSME (defined as scores of 2 or 3) and another for severe CSME (defined as a score of 3). The relationship of baseline visual acuity with TZD exposure was analyzed with a mixed-model of covariance with HbA1c, diabetes duration, gender, and age. A random effect for subject was used to account for within-person correlation.
Table 1.
Participant-specific baseline characteristics of the sample used for the primary analysis (n=3473). Comparisons were made using chi-square test for categorical variables and two-sample t-tests for continuous variables.
| Mean (SD)/Percentage |
||||||
|---|---|---|---|---|---|---|
| Variable | Overall | TZD− | TZD+ | |||
| Type | Variable | (n=3473) | (n=2778) | (n=695) | P | |
| Demographic | History of Cardiovascular Disease | 32.3% | 33.1% | 29.2% | 0.07 | |
| Body Mass Index (kg/m2) | 32.5 (5.4) | 32.3 (5.4) | 33.3 (5.5) | <0.0001 | ||
| Gender (female) | 38.5% | 39.6% | 34.2% | 0.0113 | ||
| Race/Ethnicity | White | 70.0% | 68.1% | 77.6% | <0.0001 | |
| Black | 16.4% | 17.5% | 12.2% | 0.0012 | ||
| Hispanic | 6.8% | 7.0% | 6.0% | 0.39 | ||
| Education (n=3469/2774/695) | 0.0339 | |||||
| Less than high school | 12.9% | 13.7% | 9.9% | |||
| High school grad/GED | 24.3% | 24.1% | 25.3% | |||
| Some college/tech school | 34.7% | 34.1% | 36.7% | |||
| College grad or more | 28.1% | 28.1% | 28.1% | |||
| Diabetes duration (years) | 10.1 (7.1) | 9.8 (7.3) | 11.0 (6.5) | <0.0001 | ||
| Age (years) | 61.7 (6.5) | 61.7 (6.5) | 61.7 (6.4) | 0.97 | ||
| Smoking (n=3462/2768/694) | 0.73 | |||||
| Never | 40.8% | 40.8% | 40.6% | |||
| Former | 45.2% | 44.9% | 46.3% | |||
| Current | 14.0% | 14.2% | 13.1% | |||
| Clinical Center Networks | <0.0001 | |||||
| Canada | 17.0% | 17.5% | 15.3% | |||
| Western | 17.1% | 18.6% | 10.9% | |||
| Minnesota/Iowa | 13.9% | 12.7% | 18.7% | |||
| Ohio/Michigan | 21.1% | 19.9% | 26.2% | |||
| Northeast | 7.8% | 7.4% | 9.5% | |||
| Southeast | 14.7% | 15.2% | 12.4% | |||
| Veteran’s Administration | 8.4% | 8.8% | 7.1% | |||
| Histories | Congestive Heart Failure | 0.89 | ||||
| Yes | 3.8% | 3.9% | 3.6% | |||
| No | 95.2% | 95.1% | 95.5% | |||
| Unknown | 1.0% | 1.0% | 0.9% | |||
| Medication | TZD | 20.0% | 0% | 100% | - | |
| Other Oral Diabetes Medications | <0.0001 | |||||
| 0 | 23.8% | 26.0% | 15.1% | |||
| 1 | 39.8% | 39.0% | 43.2% | |||
| 2 | 35.8% | 34.3% | 41.6% | |||
| 3 | 0.6% | 0.7% | 0.1% | |||
| Insulin | 30.4% | 31.8% | 24.5% | 0.0001 | ||
| Diuretics | 34.2% | 34.3% | 34.0% | 0.93 | ||
| Calcium Channel Blockers | 15.9% | 15.9% | 15.8% | 0.96 | ||
| Oral Steroids | 0.2% | 0.2% | 0.1% | 0.84 | ||
| Niacin and nicotinic acid | 1.9% | 1.7% | 2.6% | 0.15 | ||
| Nonsteroidal anti-inflammatories (NSAIDS) | 7.9% | 8.4% | 6.0% | 0.0412 | ||
| Laboratory | HbA1c (%) | 8.3 (1.0) | 8.3 (1.0) | 8.1 (0.9) | <0.0001 | |
| Fasting Plasma Glucose (mg/dL) (n=3462/2767/695) | 176.6 (55.7) | 179.5 (57.1) | 165.0 (48.0) | <0.0001 | ||
| Triglycerides (mg/dL) (n=3465/2770/695) | 195.1 (159.4) | 195.8 (162.6) | 192.5 (145.9) | 0.56 | ||
| Total cholesterol (mg/dL) (n=3465/2770/695) | 180.5 (42.0) | 180.2 (41.7) | 181.6 (43.1) | 0.49 | ||
| HDL (mg/dL) (n=3465/2770/695) | 42.0 (11.2) | 41.7 (11.3) | 43.2 (10.8) | 0.0005 | ||
| LDL (mg/dL) (n=3465/2770/695) | 101.5 (33.3) | 101.6 (33.5) | 101.1 (32.3) | 0.69 | ||
| Proteinuria (albumin/creatinine ratio, mg/mg) (n=3319/2665/664) | 102.0 (92.5) | 101.0 (91.7) | 106.0 (95.4) | 0.22 | ||
| Creatinine (mg/dL) (n=3456/2764/692) | 124.6 (66.5) | 125.6 (67.4) | 120.4 (63.0) | 0.07 | ||
| Pretibial edema | 20.9% | 19.6% | 25.8% | 0.0004 | ||
| Physical Exam | Mean Arterial Pressure (mm Hg) (n=3457/2766/691) | 94.8 (11.2) | 95.1 (11.4) | 93.9 (10.3) | 0.0108 | |
| Systolic Blood Pressure (mm Hg) (n=3457/2766/691) | 134.7 (17.0) | 135.0 (17.3) | 133.8 (15.5) | 0.09 | ||
| Diastolic Blood Pressure (mm Hg) (n=3457/2766/691) | 74.9 (10.6) | 75.1 (10.7) | 74.0 (10.1) | 0.0067 | ||
| Pretibial edema | 20.9% | 19.6% | 25.8% | 0.0004 | ||
| Foot ulcers | 4.2% | 4.1% | 4.2% | 0.88 | ||
| Foot amputation | 2.1% | 2.3% | 1.3% | 0.11 | ||
| Eye Exams | Time since baseline (days) | 67.0 (36.7) | 66.8 (37.0) | 67.6 (35.6) | 0.52 | |
Table 2.
Eye-specific baseline characteristics of the sample used for the primary analysis (6875 eyes). Comparisons were made using a multinomial GEE model to account for correlated eyes.
| Percentage | ||||||
|---|---|---|---|---|---|---|
| Variable Type |
Variable | Overall (6875 eyes) |
TZD− (5498 eyes) |
TZD+ (1377 eyes) |
P | |
| Eye | Retinopathy (ETDRS scale (31)) | 0.0081 | ||||
| Exams | None (<20) | 57.8% | 59.0% | 53.2% | ||
| Mild (20) | 15.6% | 15.2% | 17.4% | |||
| Moderate nonproliferative (35–47) | 25.1% | 24.3% | 28.1% | |||
| Severe nonproliferative (53) | 0.2% | 0.2% | 0.3% | |||
| Proliferative (60+) | 1.3% | 1.3% | 1.1% | |||
| Macular Edema | 0.95 | |||||
| None | 95.9% | 95.9% | 95.8% | |||
| Questionable | 0.9% | 0.9% | 0.9% | |||
| Zone of retinal thickness≥1 disc area part ≤ 1 disc diameter from center | 0.3% | 0.3% | 0.4% | |||
| Retinal thickness or adjacent hard exudates ≤ 500 µm from center | 2.9% | 2.9% | 2.9% | |||
Results
Table 1 depicts baseline characteristics of the 3,473 participants included in the primary analysis. No adjustment for multiple testing was made. These results are presented as guides to potential relationships. A difference in pretibial edema prevalence was observed by exposure to TZDs (P=0.0004). Eye-specific prevalence of DME and retinopathy on the 6,875 eyes included in the primary analysis are presented by TZD exposure in Table 2. All subsequent results use both eyes in statistical models accounting for correlation.
Diabetic Macular Edema
The analyses are summarized in Table 3. In unadjusted analysis, TZD was not associated with DME. The primary analysis provided consistent results. Retinopathy and age were associated and HbA1c was marginally associated with DME. There was evidence that the relationship of HbA1c to DME was nonlinear, but this had no impact on the estimated effect of TZD (data not shown). After the variable selection procedure described above for the secondary analysis, four additional variables were added to the primary model: the logarithm of triglyceride level, cholesterol, the logarithm of the albumin/creatinine ratio, and smoking status, and there was no association between TZD and DME. Former and current smokers had lower prevalence of DME than did non-smokers. Interestingly, the association of HbA1c with DME was attenuated from the primary (OR=1.15, P=0.06) to the secondary model (OR=1.08, P=0.29), perhaps due to confounding by other variables. As in the primary analysis, there was evidence that the relationship between HbA1c and DME was nonlinear (data not shown). When we fit HbA1c as a 4-level category the estimated association between TZD use and DME was essentially unchanged (data not shown). When the primary and unadjusted models were refit using only the data available for the secondary model, the results were substantially unchanged (data not shown). Examining outcomes other than any DME (i.e., moderate/severe or severe) did not substantially change the results in any model (data not shown).
Table 3.
Macular edema results. The primary model was pre-specified and included factors known to be associated with macular edema as listed below.
| Unadjusted Model N=3510 (6934 eyes) |
Primary Adjusted Model n=3473 (6875 eyes) |
Secondary Adjusted Model n=3306 (6533 eyes) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| OR | 95% CI | P | OR | 95% CI | P | OR | 95% CI | P | ||
| TZD | 1.01 | (0.71,1.44) | 0.95 | 0.97 | (0.67,1.40) | 0.86 | 0.95 | (0.65,1.39) | 0.80 | |
| HbA1c | 1.15 | (1.00,1.32) | 0.06* | 1.08 | (0.94,1.25) | 0.29 | ||||
| Diabetes Duration | 0.995 | (0.98,1.02) | 0.65 | 1.00 | (0.98,1.02) | 0.92 | ||||
| Retinopathy | <0.0001 (4 df) | <0.0001 (4 df) | ||||||||
| None | 1 | ref | 1 | ref | ||||||
| Mild | 3.73 | (1.05,13.3) | 3.6 | (1.01,13.0) | ||||||
| Moderate nonproliferative | 147 | (56,387) | 127 | (48,333) | ||||||
| Severe nonproliferative | 338 | (69,1664) | 263 | (55.6,1243) | ||||||
| Proliferative | 224 | (75,673) | 196 | (65,596) | ||||||
| Female | 1.06 | (0.78,1.43) | 0.72 | 0.91 | (0.66,1.25) | 0.56 | ||||
| Age | 0.97 | (0.952,0.997) | 0.0298 | 0.98 | (0.954,1.001) | 0.07 | ||||
| White | 0.85 | (0.55,1.32) | 0.20 (3 df) | 1.00 | (0.65,1.56) | 0.71 (3 df) | ||||
| Black | 1.15 | (0.70,1.90) | 1.17 | (0.69,1.97) | ||||||
| Hispanic | 1.43 | (0.87,2.36) | 1.30 | (0.77,2.19) | ||||||
| log(Triglycerides)** | 0.73 | (0.53.,0.99) | 0.0437 | |||||||
| Cholesterol | 1.006 | (1.002,1.010) | 0.0043 | |||||||
| log(Albumin/Creatinine)** | 0.886 | (0.803,0.976) | 0.0210 | |||||||
| Smoking | Never | 1 | ref | 0.0170(2 df) | ||||||
| Former | 0.61 | (0.43,0.86) | ||||||||
| Current | 0.87 | (0.54,1.38) | ||||||||
Due to the slight differences between the Wald confidence intervals and the generalized score statistics used for p-values there is an apparent discrepancy between the CI and P-value; we prefer the generalized score test over the Wald test (43).
Denotes natural logarithm used as a predictor.
Visual Acuity
In the adjusted analysis, TZD use was associated with marginally better visual acuity (0.79 letters, 95% CI for the difference in means: (0.20, 1.38), P=0.0091) in 9,690 participants (19,239 eyes). That is, those who used TZDs prior to baseline had, on average, visual acuity scores less than one letter (0.79) better on the 0–100 scale. HbA1c (β=−0.85 per 1%, (−1.08, −0.63), P<0.0001), diabetes duration (β=−0.18 per year, (−0.21, −0.15), P<0.0001), female gender (β=−2.66, (−3.14, −2.18), P<0.0001), and age (β=−0.28 per year, (−0.32, −0.25), P<0.0001) were all inversely associated with visual acuity. That is, a ten-year longer diabetes duration is associated with a worse visual acuity by 1.8 letters. Similarly, a ten-year older age is associated with worse acuity by 2.8 letters. A 1% higher HbA1c (above 7.5%, the lower inclusion limit) is associated with a worse acuity by 0.85 letters. That is, a 2% greater HbA1c is approximately equivalent to a 10-year older age (1.9 vs. 1.8 letters). TZD use is approximately equivalent to a 3-year younger age (0.79 vs. 0.84). In an unadjusted model in 9,795 participants (19,446 eyes), the association between visual acuity and use of TZD was similar (0.90 letters, 95% CI: (0.30, 1.51), P=0.0035).
Discussion
TZD use was not associated with the presence of clinically significant or any DME among ACCORD participants at baseline. The ACCORD Eye Substudy provided an opportunity to examine the relationship in a large sample with a comparable untreated group in whom retinopathy, including DME, was graded in a standardized fashion by a centralized reading center. Visual acuities were also collected in all patients using a common protocol. The analyses enabled adjustment for multiple potential confounding variables collected in a standardized protocol.
These findings are reassuring in that they do not support the concern from case reports of DME with TZD use. However there are limitations to this analysis. Perhaps longer-term exposure to a TZD is necessary for risk to develop; we only know that participants had TZD exposure for at least 3 months. It is also possible that there is an idiosyncratic association between TZD use and DME that occurs rarely. As others have previously reported, we observed relationships between HbA1c (9, 11), retinopathy (9, 11), and age (9) with DME. We were unable to confirm previous reports of associations of DME with diabetes duration (9, 11), gender (9), and race/ethnic category (10), perhaps because participants with previous laser photocoagulation, representing the most severe end of the retinopathy scale, were excluded from the Eye Substudy. Additionally, ACCORD was restricted to people with fairly advanced diabetes and many had a fairly long duration of diabetes at randomization, thus we may not have the ability to detect an association with diabetes duration.
Adverse associations were found between DME and elevated cholesterol and non-smoking status and beneficial associations were found between DME and greater albumin/creatinine ratio and triglycerides. Others have reported an association between DME and both triglycerides and cholesterol (16). We believe that the adverse relationship between DME and triglycerides seen in this study may be due to the high collinearity between cholesterol and triglycerides. The biological plausibility of current and former smokers having lower prevalence of DME is unclear and is perhaps due to chance or the inclusion process for ACCORD although the results are consistent with the unadjusted analysis (data not shown). However, a previous study (41) showed an association between high-risk PDR and smoking, which is contrary to the results for DME in this study.
There was evidence of a positive association (0.79 letters) between TZD exposure and visual acuity. We do not know whether this finding is clinically significant.
The ACCORD Eye study exclusion criteria of previous laser photocoagulation or vitrectomy for diabetic retinopathy may have limited our analysis as laser is also a therapy for DME. It is possible that some ACCORD participants with laser-treated DME would have been excluded from the ACCORD-Eye study resulting in decreased power to detect an association. Unfortunately, no data on DME were collected in these participants. The possibility of recent prior exposure to TZD in some patients in the control group could potentially weaken any differences between groups cannot be excluded, but seems unlikely, because that exposure, if any, should have ended at least 3 months prior to baseline. However, there was no association observed between concurrent TZD exposure and any type of eye surgery including retinal laser photocoagulation and vitrectomy at baseline (data not shown).
The cross-sectional analysis presented here is likely to be less informative than an examination of incident macular edema which will be possible at the end of ACCORD, at which point the dose and duration of TZD exposure during the period between baseline and follow-up photographs can be examined. Other additional covariates could include post-baseline values of HbA1c, fasting plasma glucose, and exposure to insulin, diuretics, calcium channel blockers, oral steroids, niacin and nicotinic acid, and NSAIDs. The current analysis also does not take into account duration of exposure, past exposure, or type of TZD (rosiglitazone or pioglitazone), which could be important causal considerations.
Many recent clinical trials of DME have employed optical coherence tomography (OCT) measured central retinal thickness as an endpoint, which was not employed in this study. Historically, DME measured from stereoscopic fundus photographs has been well accepted as a clinical endpoint and is moderately correlated with OCT measurements (42); therefore results would not likely have been different using OCT.
Conclusion
No association between recent TZD exposure and DME was observed in the ACCORD-Eye baseline population. Due to the size of the confidence interval (OR: 0.67–1.40), and the uncertain duration of exposure, we cannot rule out the possibility of either a modest protective or deleterious association of TZD exposure with DME. A more definitive answer may be provided from the 4-year follow-up data which will enable us to examine prospectively for the relationship between TZD exposure and DME incidence.
Acknowledgements
This study was funded by the National Eye Institute and the National Heart, Lung, and Blood Institute, of the National Institutes of Health. Dr. Ambrosius has full access to the data and takes responsibility for the integrity of the data and the accuracy of the data analysis.
Footnotes
Disclosure: Dr. Gerstein has received honoraria for providing advice and for speaking on thiazolidinediones and rosiglitazone from GlaxoSmithKline-the manufacturer of rosiglitazone. He has also received research grants from this company for independent research related to this drug. Dr. Goff has received research funding from Merck for a trial involving a glucose lowering medication.
Trial Registration: ClinicalTrials.gov, NCT00542178, http://www.clinicaltrials.gov/ct2/show/NCT00542178
References
- 1.Colucciello M. Vision Loss Due to Macular Edema Induced by Rosiglitazone Treatment of Diabetes Mellitus. Arch Ophthalmol. 2005;123:1273–1275. doi: 10.1001/archopht.123.9.1273. [DOI] [PubMed] [Google Scholar]
- 2.Ryan EH, Han DP, Ramsay RC, Cantrill HL, Bennett SR, Dev S, Williams DF. Diabetic macular edema associated with glitazone use. Retina. 2006;26:562–570. doi: 10.1097/00006982-200605000-00011. [DOI] [PubMed] [Google Scholar]
- 3.Wooltorton E, Kendall C. Rosiglitazone (Avandia) and macular edema. CMAJ. 2006;174(5):623. doi: 10.1503/cmaj.060074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. [accessed February 13, 2006];GlaxoSmithKline, “Dear Doctor”. letter, http://www.hc-sc.gc.ca/dhpmps/medeff/advisories-avis/prof/2005/avandia_avandamet_hpc-cps_e.html.
- 5. [accessed February 13, 2006];GlaxoSmithKline, “Dear Doctor”. letter, http://www.fda.gov/medwatch/safety/2006/Avandia_DHCPletter.pdf.
- 6.Takeda Pharmaceuticals America, Inc. [accessed July 21, 2008];Actos Prescirbing Information. http://www.actos.com/actospro/prescribinginfo.aspx.
- 7.Ciardella AP. Partial resolution of diabetic macular oedema after systemic treatment with furosemide. British Journal of Ophthalmology. 2004;88(9):1224–1225. doi: 10.1136/bjo.2004.042580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Shen LQ, Child A, Weber GM, Folkman J, Aiello LP. Rosiglitazone and Delayed Onset of Proliferative Diabetic Retinopathy. Arch Ophthalmol. 2008;126(6):793–799. doi: 10.1001/archopht.126.6.793. [DOI] [PubMed] [Google Scholar]
- 9.Klein R, Klein BEK, Moss SE, Davis MD, DeMets DL. The Wisconsin Epidemiologic Study of Diabetic Retinopathy IV: Diabetic Macular Edema. Ophthalmology. 1984;91(12):1464–1474. doi: 10.1016/s0161-6420(84)34102-1. [DOI] [PubMed] [Google Scholar]
- 10.Wong TY, Klein R, Islam A, Cotch MF, Folsom AR, Klein BEK, Sharrett AR, Shea S for the Multi-Ethnic Study of Atherosclerosis (MESA) Diabetic Retinopathy in a Multi-ethnic Cohort in the United States. Am J Ophthalmology. 2006;141:446–455. doi: 10.1016/j.ajo.2005.08.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klein R, Klein BEK, Moss SE, Cruickshanks KJ. The Wisconsin Epidemiologic Study of Diabetic Retinopathy XV: The Long-term Incidence of Macular Edema. Ophthalmology. 1995;102:7–16. doi: 10.1016/s0161-6420(95)31052-4. [DOI] [PubMed] [Google Scholar]
- 12.Klein R, Moss SE, Klein BE, Davis MD, DeMets DL. The Wisconsin epidemiologic study of diabetic retinopathy. XI. The incidence of macular edema. Ophthalmology. 1989;96(10):1501–1510. doi: 10.1016/s0161-6420(89)32699-6. [DOI] [PubMed] [Google Scholar]
- 13.Chew EY, Klein ML, Ferris FL, 3rd, Remaley NA, Murphy RP, Chantry K, Hoogwerf BJ, Miller D the ETDRS Research Group. Association of elevated serum lipids with retinal hard exudate in diabetic retinopathy. ETDRS Report No. 22. Arch Ophthalmol. 1996;114:1079–1084. doi: 10.1001/archopht.1996.01100140281004. [DOI] [PubMed] [Google Scholar]
- 14.Klein BEK, Moss SE, Klein R, Surawicz TS. The Wisconsin Epidemiologic Study of Diabetic Retinopathy. XIII. Relationship of serum cholesterol to retinopathy and hard exudate. Ophthalmology. 1991;98:1261–1265. doi: 10.1016/s0161-6420(91)32145-6. [DOI] [PubMed] [Google Scholar]
- 15.Roy MS, Klein R. Macular edema and retinal hard exudates in African Americans with type 1 diabetes: the New Jersey 725. Arch Ophthalmol. 2001;119(2):251–259. [PubMed] [Google Scholar]
- 16.Miljanovic B, Glynn RJ, Nathan DM, Manson JE, Schaumberg DA. A prospective study of serum lipids and risk of diabetic macular edema in type 1 diabetes. Diabetes. 2004;53(11):2883–2892. doi: 10.2337/diabetes.53.11.2883. [DOI] [PubMed] [Google Scholar]
- 17.Dormandy JA, Charbonnel B, Eckland DJA, Erdmann E, Massi-Benedetti M, Moules IK, Skene AM, Tan MH, Lefèbvre PJ, Murray GD, Standl E, Wilcox RG, Wilhelmsen L, Betteridge J, Birkeland K, Golay A, Heine RJ, Korányi L, Laakso M, Mokáň M, Norkus A, Pirags V, Podar T, Scheen A, Scherbaum W, Schernthaner G, Schmitz O, Škrha J, Smith U, Tatoň J on behalf of the PROactive investigators. Secondary prevention of macrovascular events in patients with type 2 diabetes in the PROactive Study (PROspective pioglitAzone Clinical Trial In macroVascular Events): a randomised controlled trial. Lancet. 2005;36:1279–1289. doi: 10.1016/S0140-6736(05)67528-9. [DOI] [PubMed] [Google Scholar]
- 18.Lincoff AM, Wolski K, Nicholls SJ, Nissen SE. Pioglitazone and Risk of Cardiovascular Events in Patients With Type 2 Diabetes Mellitus: A Meta-analysis of Randomized Trials. JAMA. 2007;298:1180–1188. doi: 10.1001/jama.298.10.1180. [DOI] [PubMed] [Google Scholar]
- 19.Nissen SE, Wolski K. Effect of Rosiglitazone on the Risk of Myocardial Infarction and Death from Cardiovascular Causes. New Engl J Med. 2007;356(24):2457–2471. doi: 10.1056/NEJMoa072761. [DOI] [PubMed] [Google Scholar]
- 20.Home PD, Pocock SJ, Beck-Nielsen H, Gomis R, Hanefeld M, Jones NP, Komajda M, McMurray JJ RECORD Study Group. New Engl J Med. 2007;357(1):28–38. doi: 10.1056/NEJMoa073394. [DOI] [PubMed] [Google Scholar]
- 21.Psaty BM, Furberg CD. Rosiglitazone and Cardiovascular Risk. New Eng J Med. 2007;356(24):2522–2524. doi: 10.1056/NEJMe078099. [DOI] [PubMed] [Google Scholar]
- 22.Drazen JM, Morrissey S, Curfman GD. Rosiglitazone—Continued Uncertainty about Safety. New Engl J Med. 2007;357(1):63–64. doi: 10.1056/NEJMe078118. [DOI] [PubMed] [Google Scholar]
- 23.Nathan DM. Rosiglitazone and Cardiotoxity—Weighing the Evidence. New Engl J Med. 2007;357(1):64–66. doi: 10.1056/NEJMe078117. [DOI] [PubMed] [Google Scholar]
- 24.Psaty BM, Furberg CD. The Record on Rosiglitazone and the Risk of Myocardial Infarction. New Engl J Med. 2007;357(1):67–69. doi: 10.1056/NEJMe078116. [DOI] [PubMed] [Google Scholar]
- 25.Rosiglitazone: seeking a balanced perspective. Lancet. 2007;369(9576):1834. doi: 10.1016/S0140-6736(07)60787-9. Editors. [DOI] [PubMed] [Google Scholar]
- 26. [accessed August 15, 2007];GlaxoSmithKline, Avandia Prescribing Information. 2007 http://www.fda.gov/cder/drug/infopage/rosiglitazone/rosiglitazone_label20070814.pdf.
- 27.Goff DC, Jr, Gerstein HC, Ginsberg HN, Cushman WC, Margolis KL, Byington RP, Buse JB, Genuth S, Probstfield JL, Simons-Morton DG for the ACCORD Study Group. Prevention of Cardiovascular Disease in Persons with Type 2 Diabetes Mellitus: Current Knowledge and Rationale for the Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial. American Journal of Cardiology. 2007;99 suppl:4i–20i. doi: 10.1016/j.amjcard.2007.03.002. [DOI] [PubMed] [Google Scholar]
- 28.ACCORD Study Group. Action to Control Cardiovascular Risk in Diabetes (ACCORD) Trial: Design and Methods. American Journal of Cardiology. 2007;99 suppl:21i–33i. doi: 10.1016/j.amjcard.2007.03.003. [DOI] [PubMed] [Google Scholar]
- 29.Chew EY, Ambrosius WT, Howard LT, Greven CM, Johnson S, Danis RP, Davis MD, Genuth S, Domanski M for the ACCORD Study Group. Rationale, Design, and Methods of the ACCORD Eye Substudy. American Journal of Cardiology. 2007;99 suppl:103i–111i. doi: 10.1016/j.amjcard.2007.03.028. [DOI] [PubMed] [Google Scholar]
- 30.Ferris FL, 3rd, Kassoff A, Bresnick GH, Bailey I. New visual acuity charts for clinical research. American Journal of Ophthalmology. 1982;94(1):91–96. [PubMed] [Google Scholar]
- 31.Early Treatment Diabetic Retinopathy Study Research Group. Grading Diabetic Retinopathy from Stereoscopic Color Fundus Photographs—An Extension of the Modified Airlie House Classification, ETDRS Report Number 10. Ophthalmology. 1991;98:786–806. [PubMed] [Google Scholar]
- 32.Wilkinson CP, Ferris FL, 3rd, Klein RE, Lee PP, Agardh CD, Davis M, Dills D, Kampik A, Pararajasegaram R, Verdaguer JT. Proposed International Clinical Diabetic Retinopathy and Diabetic Macular Edema Disease Severity Scales. Ophthalmology. 2003;110(9):1677–1682. doi: 10.1016/S0161-6420(03)00475-5. [DOI] [PubMed] [Google Scholar]
- 33.Early Treatment Diabetic Retinopathy Study Research Group. Treatment techniques and clinical guidelines for photocoagulation of diabetic macular edema. Early Treatment Diabetic Retinopathy Study Report Number 2. Ophthalmology. 1987;94(7):761–774. doi: 10.1016/s0161-6420(87)33527-4. [DOI] [PubMed] [Google Scholar]
- 34.Liang KY, Zeger SL. Longitudinal data analysis using generalized linear models. Biometrika. 1986;73:13–22. [Google Scholar]
- 35.Hardin JW, Hilbe JM. Generalized Estimating Equations. Boca Raton: Chapman & Hall/CRC; 2003. [Google Scholar]
- 36.Hosmer DW, Lemeshow S. Applied Logistic Regression. Second Edition. New York: John Wiley & Sons; 2000. [Google Scholar]
- 37.Hastie TJ, Tibshirani RJ. Generalized Additive Models. London: Chapman & Hall; 1990. [Google Scholar]
- 38.Chambers JM, Hastie TJ. Statistical Models. Pacific Grove, CA: S, Wadsworth & Cole; 1992. [Google Scholar]
- 39.Lin DY, Wei LJ, Ying Z. Model-Checking Techniques Based on Cumulative Residuals. Biometrics. 2002;58:1–12. doi: 10.1111/j.0006-341x.2002.00001.x. [DOI] [PubMed] [Google Scholar]
- 40.SAS Institute Inc. SAS/STAT 9.1 User’s Guide. Cary, NC: 2004. [Google Scholar]
- 41.Davis MD, Fisher MR, Gangnon RE, Barton F, Aiello LM, Chew EY, Ferris FL, 3rd, Knatterud GL for the Early Treatment Diabetic Retinopathy Study Research Group. Risk Factors for High-Risk Proliferative Diabetic Retinopathy and Severe Visual Loss: Early Treatment Diabetic Retinopathy Study Report #18. Invest Ophthalmol Vis Sci. 1998;39:233–252. [PubMed] [Google Scholar]
- 42.Davis MD, Bressler SB, Aiello LP, Bressler NM, Browning DJ, Flaxel CJ, Fong DS, Foster WJ, Glassman AR, Hartnett MER, Kollman C, Li HK, Qin H, Scott IU the Diabetic Retinopathy Clinical Research Network Study Group. Comparison of Time-Domain OCT and Fundus Photographic Assessments of Retinal Thickening in Eyes with Diabetic Macular Edema. Invest Ophthalmol Vis Sci. 2008;49(5):1745–1752. doi: 10.1167/iovs.07-1257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Harrell FE., Jr . Regression Modeling Strategies. New York: Springer; 2001. [Google Scholar]