Abstract
BACKGROUND AND PURPOSE
Caffeine is consumed extensively in Europe and North America. As a risk factor for osteoporosis, epidemiological studies have observed that caffeine can decrease bone mineral density, adversely affect calcium absorption and increase the risk of bone fracture. However, the exact mechanisms have not been fully investigated. Here, we examined the effects of caffeine on the viability and osteogenesis of rat bone marrow-derived mesenchymal stromal cells (rBMSCs).
EXPERIMENTAL APPROACH
Cell viability, apoptosis and necrosis were quantified using thymidine incorporation and flow cytometry. Sequential gene expressions in osteogenic process were measured by real-time PCR. cAMP, alkaline phosphatase and osteocalcin were assessed by immunoassay, spectrophotometry and radioimmunoassay, respectively. Mineralization was determined by calcium deposition.
KEY RESULTS
After treating BMSCs with high caffeine concentrations (0.1–1 mM), their viability decreased in a concentration-dependent manner. This cell death was primarily due to necrosis and, to a small extent, apoptosis. Genes and protein sequentially expressed in osteogenesis, including Cbfa1/Runx2, collagen I, alkaline phosphatase and its protein, were significantly downregulated except for osteocalcin and its protein. Moreover, caffeine inhibited calcium deposition in a concentration- and time-dependent manner, but increased intracellular cAMP in a concentration-dependent manner.
CONCLUSIONS AND IMPLICATIONS
By suppressing the commitment of BMSCs to the osteogenic lineage and selectively inhibiting gene expression, caffeine downregulated some important events in osteogenesis and ultimately affected bone mass.
Keywords: mesenchymal stromal cells, caffeine, osteogenesis, osteoporosis
Introduction
Osteoporosis, a serious public health issue, is a disease that leads to an increase in bone fragility and susceptibility to bone fracture due to the decrease in bone mass and deterioration in bone structure (Delmas and Fraser, 1999; Keen, 2007). Although there are various therapeutic approaches that are available to relieve the progress of this common disease, such as bisphosphonates, hormone replacement therapy, selective oestrogen receptor modulators and calcitonin, unfavourable side effects have limited their clinical use, and bone mass, once lost, cannot be regained (Rodan and Martin, 2000; Keen, 2007). Therefore, in order to slow down bone loss, it is essential that more attention be given to adjusting unhealthy lifestyles, such as drinking coffee, which has been observed to cause certain risk factors for osteoporosis.
Coffee is a popular beverage in Europe and North America and is consumed in considerable amounts not only in these countries but also worldwide. However, caffeine has been found to be correlated with the decreased bone mineral density (Rapuri et al., 2001; Ilich et al., 2002), increased risk of bone fracture (Cummings et al., 1995; Heaney, 2002) and reduction in calcium absorption (Heaney and Rafferty, 2001; Heaney, 2002; Taylor and Curhan, 2009). Nevertheless, little is known about caffeine-related osteoporosis, apart from the epidemiological studies.
One well-known mechanism involved in the pathological process of osteoporosis is impaired bone formation; thus, any factor that decreases osteoblast numbers and their ability to form bone may have a negative effect on osteoporosis (Rodan and Martin, 2000; Canalis et al., 2007). Because of this, the precursor cells of osteoblasts (Pittenger et al., 1999), mesenchymal stromal cells (MSCs), are important cells in osteoporosis and some harmful factors may result in the impairment of the differentiation of MSCs into osteoblasts.
The differentiation of MSCs into osteoblasts is primarily controlled by Cbfa1/Runx2 (Ducy et al., 1997), which can be regulated by the second messenger, cAMP (Tintut et al., 1999; Yang et al., 2008). Caffeine can suppress the activity of cAMP phosphodiesterase. By inhibiting the phosphodiesterase-mediated degradation of cAMP, caffeine increases the level of intracellular cAMP (Shafer et al., 1998). Therefore, there is a possibility that caffeine could regulate Cbfa1/Runx2 gene expression, alter the rate of MSC differentiation into osteoblasts and finally, affect bone formation. Moreover, a high level of cAMP, as provided by caffeine, can suppress cell proliferation (Kamagata-Kiyoura et al., 1999; Ugland et al., 2008). Thus, caffeine may also suppress growth of MSCs.
Therefore, we postulated that bone marrow-derived mesenchymal stromal cells (BMSCs) may play an important role in caffeine-related osteoporosis. In this work, we examined the possible effects of caffeine on the viability and bone formation ability of BMSCs in order to provide a possible molecular mechanism for caffeine-related osteoporosis.
Methods
Animals
All animals received care in accordance with the Guide for the Care and Use of Laboratory Animals published by the US National Institute of Health (NIH Publication NO. 85-23). All experimental procedures were approved by the Care of Experiment Animals Committee of Huaxi Medical Center, Sichuan University, Chengdu, China. Adult female Sprague–Dawley rats (about 3 months old) were obtained from the Huaxi Medical Center Laboratorial Animal Center of Sichuan University (Chengdu, China).
Isolation and culture of BMSCs
After anaesthetizing rats with nembutal (25 mg·kg−1 i.m.) and disinfecting with iodophor alcohol, the tibias and femurs were removed. BMSCs were harvested by flushing out (with a syringe) the bone marrow in the tibia and femur with sterile low glucose (1%) Dulbecco's modified Eagle's medium (L-DMEM; 4–5 mL per tibia and femur). These cells were then resuspended and placed in culture flasks supplemented with 10% fetal bovine serum (FBS), 100 units·mL−1 penicillin and 100 µg·mL−1 streptomycin and grown in a humidified atmosphere of 5% CO2 at 37°C. The culture solution was replaced every 2 days and the cells that did not adhere to the flask wall were removed. When the cells attached to the flask bottom reached about 80–90% confluency, 0.25% trypsin was used to detach the cells. The cells were then split equally and transferred to a fresh flask; this culture was labelled as passage 1. The procedure was repeated and cells were named as passage 2. All the cells used in our experiments were from passage 2.
Multipotent differentiation of BMSCs
Multipotency was studied by measuring osteogenic differentiation and adipogenic properties. To assess osteogenic differentiation, BMSCs (2 × 104 cells per well) were cultured in an osteogenic differentiation medium (10% FBS, 10−6 M dexamethasone, 10−2 M β-glycerol phosphate and 50 µg·mL−1 ascorbic acid in high glucose DMEM) for 21 days. Cells were fixed with 75% ethanol for 20 min, and then rinsed and stained with Alizarin red solution for 1 h to make the calcium nodules visible on the Nikon Digital Camera DXM 1200 (Tokyo, Japan). To measure the adipogenic properties, BMSCs were (5 × 104 cells per well) cultured in an adipogenic differentiation medium (10% FBS, 10−6 M dexamethasone, 5 µg·mL−1 insulin, 60 µM indomethacin and 0.5 mM isobutyl-methylxanthine in high glucose DMEM) for 1 week. The cells were fixed with 10% formalin and then washed and stained for 30 min with Oil red O solution to detect the lipid-filled vacuoles in the cytoplasm.
Flow cytometry
Surface antigen analysis of BMSCs
To analyse surface antigens of BMSCs, cells (about 106 cells per flask) were collected using trypsin and EDTA and incubated with fluorescein isothiocyanate (FITC)-conjugated monoclonal antibodies against rat CD29, CD31, CD34, CD44H, CD45, CD54, CD73 and CD90 as described previously (Kamiya et al., 2007). Membranous antigens expressed on BMSCs were characterized using flow cytometry (Becton, Dickinson and Company, Franklin Lakes, NJ, USA).
Cell viability study
To study the effects of caffeine on cell viability, BMSCs (5 × 105 cells per flask) were treated with different concentrations of caffeine (0, 0.1 and 1 mM) for 48 h and collected using trypsin. Once the cells were stained using the propidium iodide/annexin-V-fluorescein kit, the cell apoptosis and necrosis rates were assayed through flow cytometry.
Thymidine incorporation assay
BMSCs (4 × 104 cells per well) cultured in six-well plates (Corning, Union City, CA, USA) were treated with different concentrations of caffeine (0, 0.1, and 1 mM). After 48 h, 1 µCi of [3H]-thymidine was added to each well, and the cells were incubated for 5 h at 37°C. The culture medium was then removed, and the cells were washed with phosphate-buffered saline (PBS). Aliquots of cell lysis solution [10 mM Tris-HCl, 1 mM EDTA, 1% TritonX-100 and 0.1% sodium doclecyl sulfate (SDS)] were added into each well. Once the cells were solubilized, the solution was added onto glass-fibre filter paper and dried at 37°C. The paper was then transferred to scintillation vials and a 5 mL scintillation solution [5 mg·mL−1 2,5-diphenyl oxazole and 0.3 g·mL−1 1,4-bis(5-phenyl-2-oxazolyl) benzene dissolved in dimethyl benzene] was added into each vial. Two hours after the vials were placed in the dark room, the incorporation of [3H]-thymidine was analysed using a liquid scintillation analyser (Beckman, Los Angeles, CA, USA). The results are shown as counts per minute (CPM) per well.
Quantitative real-time RT–PCR
BMSCs cultured in the osteogenic medium were incubated with different concentrations of caffeine (0, 0.1, 0.5 and 1 mM) for 3, 7, 11 and 16 days. Cells (about 106 cells) were trypsinized and total mRNA was extracted by adding 1 mL of RNAiso plus. Next, 1 µg RNA was subjected to cDNA synthesis by using PrimeScript™ RT Enzyme Mix I (Shiga, Japan) and oligo dT Primer in a 20 µL reaction volume according to the manufacturer's instructions, with the addition of 2.0 µL PrimeScript™ Buffer and 0.5 µL Random 6 mers (TAKARA, Shiga, Japan). SYBY Green PCR assays were then performed on these cDNA samples with iCycler iQ™ Multicolor real-time PCR Detection System (Bio-Rad, Hercules, CA, USA). Amplification was carried out in a 25 µL final volume containing 2 µL cDNA, 1 µL forward primer, 1 µL reverse primer, 12.5 µL SYBR® Premix Ex Taq™ (TAKARA), and 8.5 µL sterile water. The specific primers used are shown in Table 1 and PCR parameters for the amplification program included initial denaturation at 95°C for 10 s, followed by 40 cycles of denaturation at 95°C for 5 s and annealing and extension at 60°C for 45 s. At the end of each run, fluorescence data was analysed using Optical system software version 3.1 (Bio-Rad) in order to obtain cycle threshold (CT, the number of cycles required for the fluorescent signal to cross the threshold) values. Gene expression was calculated using the 2-ΔΔCT method, where CT values from samples were averaged and calibrated in relation to GADPH CT values.
Table 1.
Real-time RT–PCR primers for expression of specific genes in rat BMSCs
| Genes | Sequences of primer(5′- 3′) | Amplicon (bp) | Tm (°C) | Genbank access number |
|---|---|---|---|---|
| Cbfa1 | Forward: tcccagtatgagagtaggtgtcc | 164 | 52.17 | NM_053470 |
| Reverse: ggctcagataagaggggtaagac | 52.17 | |||
| Col-I | Forward: tctgactggaagagcggagag | 112 | 57.14 | NM_053304 |
| Reverse: gagtggggaacacacaggtct | 57.14 | |||
| ALP | Forward: cctagacacaagcactcccacta | 138 | 60.72 | NM_013059 |
| Reverse: gtcagtcaggttgttccgattc | 60.92 | |||
| OC | Forward: gaccctctctctgctcactctg | 124 | 59.09 | NM_013414 |
| Reverse: caccttactgccctcctgctt | 57.04 |
ALP, alkaline phosphatase; BMSC, bone marrow-derived mesenchymal stromal cell; bp, base pair; Col-I, collagen I; OC, osteocalcin.
Alkaline phosphatase assessed by spectrophotometry
BMSCs (2 × 104 cells per well) cultured in the osteogenic medium were incubated with different concentrations of caffeine (0, 0.1 and 1 mM) for 5, 10 and 15 days. After being rinsed with PBS, the cells were harvested with cell lysis solution and sonicated. Alkaline phosphatase (ALP) activity was measured by adding aliquots of supernatant to 100 µL 50 mM p-nitrophenylphosphate containing 1 mM MgCl2, 50 mM glycine, at 37°C for 30 min. The absorbance of p-nitrophenol was monitored at 405 nm using SpectraMax 190 spectrophotometer (Molecular Devices, Silicon Valley, CA, USA), while ALP activity was normalized to the total protein of the cell lysate. The BCA protein kit used to quantify the protein in the cell lysate was purchased from Bio-Rad.
Quantitation of osteocalcin by [125I ] radioimmunoassay
BMSCs (2 × 104 cells per well) cultured in the osteogenic medium were incubated with different concentrations of caffeine (0, 0.1 and 1 mM). After treatment for 7, 14 and 21 days, cells were washed and solubilized. The total osteocalcin (OC) in the cell lysis solution was measured with radioimmunoassay by using the procedures described previously (Gundberg et al., 1998).
Quantitation of calcium deposition
BMSCs (2 × 104 cells per well) cultured in the osteogenic medium were supplied with different concentrations of caffeine (0, 0.1, 0.5 and 1 mM) for 7, 14 and 21 days. Calcification of BMSCs was measured using the Wada procedure (Xiao et al., 2001). Once the cells were washed, aliquots of 0.6 N HCl were added into each well to decalcify the cultures for 24 h. Calcium in the HCl supernatant was assessed using the o-cresolphthalein complexone (OCPC) method. Then aliquots of 0.1 NaOH N/0.1% SDS were added to solubilize the cells. The calcium content of the cell layers was normalized to the total protein of the cell lysate.
cAMP assay
BMSCs (105) were seeded into six-wells plates in DMEM containing 10% FBS, penicillin and streptomycin. Near confluence, cultures were rinsed three times with serum-free phenol red-free DMEM. Cells were then treated for 5 min with 0.5 mM isobutyl-methylxanthine, to inhibit endogenous phosphodiesterase activity, prior to a 15 min treatment with different concentrations of caffeine (0, 0.1, 0.5 and 1 mM). The treatment was terminated by aspirating the medium in cultures. Intracellular cAMP concentration was assessed by immunoassay, following the manufacturer's instructions. The parallel wells were utilized for cell number count by using a haemacytometer. cAMP content was expressed as pmol per 106 cells.
Statistical analysis
All values were expressed as mean ± standard deviation and statistically analysed using one-way anova. P < 0.05 was considered to be significant. Except for the BMSC surface antigen analysis which was carried out only once and the real-time PCR, cell viability study and cAMP assay which were carried out three times, all other experiments were carried out in triplicate with three independent experiments.
Materials
Caffeine was purchased from Alexis Biochemicals, San Diego, CA, USA. Penicillin, streptomycin, dexamethasone, insulin, indomethacin, isobutyl-methylxanthine, FITC-conjugated monoclonal antibodies CD34, CD45, 2,5-diphenyl oxazole, p-nitrophenylphosphate, glycine, o-cresolphthalein complexone, 1,4-bis(5-phenyl-2-oxazolyl) benzene, ascorbic acid, β-glycerol phosphate, Alizarin red, formalin, Oil red O, NaOH, HCl, Tris-HCl, TritonX-100, SDS, MgCl2, dimethyl benzene and nembutal were all purchased from Sigma-Aldrich, St. Louis, MO, USA. DMEM, FBS and trypsin were purchased from Gibco BRL, Gaithersburg, MD, USA. EDTA was purchased from Sanland chemical Co., Ltd, San Jose, CA, USA. [3H]-thymidine was purchased from Shanghai Institute of Nuclear Research, Shanghai, China. 125Iodine was purchased from Beijing Puer Weiye Biotechnology Company Limited, Beijing, China. Propidium iodide and annexin-V-fluorescein were purchased from Roche Applied Science, Penzberg, Germany. RNAiso plus, PrimeScript™ Buffer, Random 6 mers, oligo dT Primer and SYBR® Premix Ex Taq™ were purchased from TAKARA, Japan. FITC-conjugated monoclonal antibodies against rat CD29, CD31, CD44H, CD54, CD73 were purchased from Biolegend, San Diego, CA, USA. FITC-conjugated monoclonal antibodies CD90 was purchased from eBioscience, USA. cAMP Kit was purchased from R&D Systems, Minneapolis, MN, USA.
Results
Characterization of BMSCs
Most cells that attached to the flask from passage 2 showed shapes of asters or spindles with slim bodies resembling fibroblasts (Figure S1A). Flow cytometry analysis indicated that the majority of cells expressed the MSC surface markers CD29, CD44H, CD54, CD73 and CD90, but only few cells expressed CD31, CD34 and CD45 (Figure S2). After osteogenic induction of BSMCs for 3 weeks, mineralization nodules were observed with Alizarin Red S staining. After adipogenic induction of BSMCs for 7 days, intracytoplasmic lipid vesicles were also observed through oil red O staining (Figure S1B,C).
Caffeine suppresses viability of BMSCs by inducing cell necrosis and apoptosis
As shown in Figure 1, caffeine significantly decreased BMSCs' ability to incorporate thymidine in a concentration-dependent manner (P < 0.05). We further tested whether caffeine-induced cell death represented apoptosis or necrosis. The percentage of apoptotic cell population increased significantly in cultures when exposed to 1 mM caffeine, and the percentage of necrotic cell population also simultaneously rose at the high caffeine concentration group (P < 0.01). However, the decline in BMSC survival following the treatment with 0.1 mM caffeine could not be attributed to increased apoptosis or necrosis (P > 0.05; Figure 2A–C)
Figure 1.
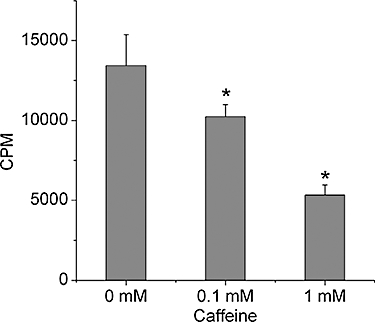
Viability of bone marrow-derived mesenchymal stromal cells (BMSCs) was decreased by caffeine. BMSCs were treated with different concentrations of caffeine (0, 0.1 and 1 mM) for 48 h and growth assessed by thymidine incorporation (shown as CPM per well). Data show that cell viability was decreased in a concentration-dependent manner. Bars represent the mean ± standard deviation (n = 9). Comparisons between groups were performed using anova. *P < 0.05, significantly different from the control group. CPM, counts per minute.
Figure 2.
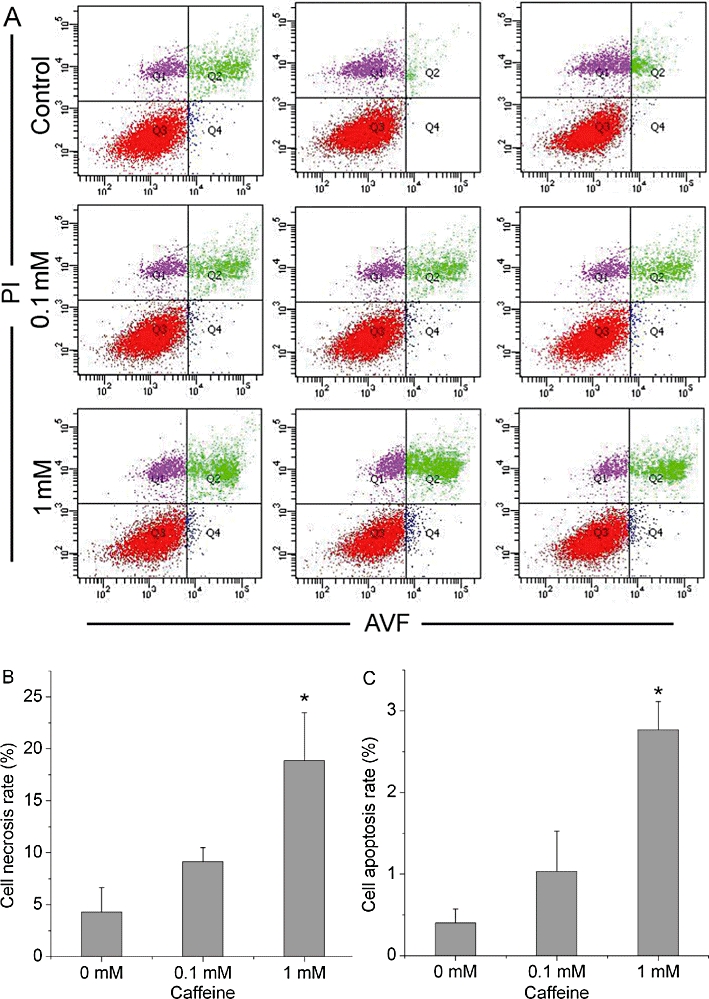
Caffeine inhibits bone marrow-derived mesenchymal stromal cell (BMSC) viability by inducing cell necrosis and apoptosis. BMSCs were treated with different concentrations of caffeine (0, 0.1, 1 mM) for 48 h. (A) Cell apoptosis and necrosis rates were analysed by flow cytometry (n = 3). PI−/AVF− represents viable cells. PI+/AVF+ represents necrotic cells. PI−/AVF+ represents apoptotic cells. (B,C) Data show the ratios of necrotic cells and apoptotic cells in each group. The percentage of apoptotic cells and necrotic cells increased significantly when exposed to 1 mM caffeine, but 0.1 mM caffeine was without effect on BMSC apoptosis and necrosis. Bars represent the mean ± standard deviation (n = 3). Comparisons between groups were performed using anova. *P < 0.01, significantly different from the control (0 mM caffeine) group. PI, propidium iodide. AVF, annexin-V-fluorescein.
Caffeine selectively suppresses bone sequential gene expressions during osteogenesis
Compared with the non-caffeine-treated cells, Cbfa1/Runx2 expression was dose-dependently inhibited by 0.1, 0.5 and 1 mM caffeine (P < 0.01; Figure 3A). Similarly, the expressions of collagen I (Col-I) and ALP showed a marked reduction when exposed to these concentrations of caffeine (P < 0.01; Figure 3B,C). However, caffeine raised the levels of mRNA for OC in each treated group (P < 0.05; Figure 3D).
Figure 3.
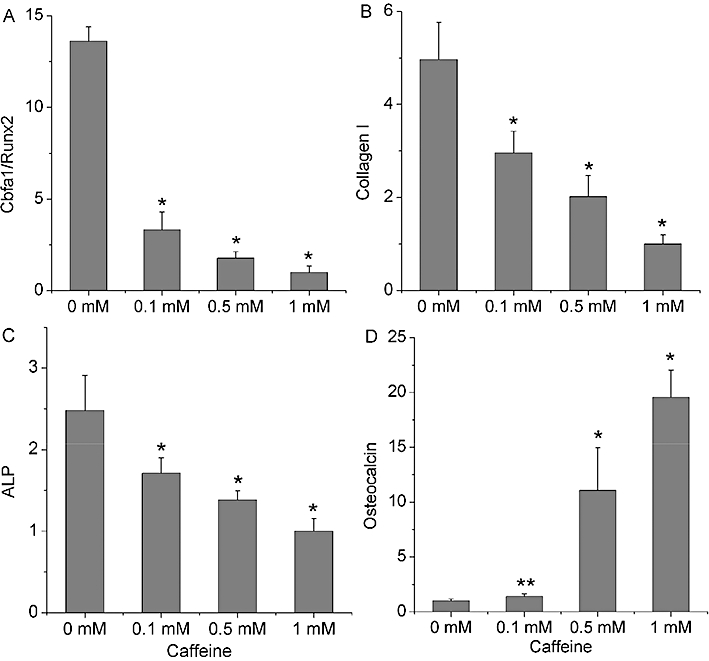
Sequential gene expressions in osteogenesis were selectively inhibited by caffeine. The expressions of Cbfa1/Runx2, collagen I, ALP and osteocalcin were, respectively, detected at following time points: 3, 7, 11 and 16 days. (A–C) Cbfa1/Runx2, collagen I and ALP gene expressions were downregulated in a concentration-dependent manner. (D) Caffeine concentration dependently enhanced osteocalcin gene expression. Bars represent the mean ± standard deviation (n = 3). Comparisons between groups were performed using anova. *P < 0.01, **P < 0.05, significantly different from the control (0 mM caffeine) group. ALP, alkaline phosphatase.
Different responses of ALP activity and OC on caffeine treatment
In the process of osteogenesis, the expressions of ALP and OC in all groups were gradually increased. However, incubation of BMSCs, with different concentrations of caffeine (0.1–1 mM) attenuated ALP activity, but induced an accumulation of OC, compared with the control, without caffeine, group, at each time point (P < 0.01; Figure 4). In the same samples, levels of OC were enhanced on the 14th and the 21st day, although on the 7th day, the levels of OC were not affected by caffeine (P > 0.05; Figure 5).
Figure 4.
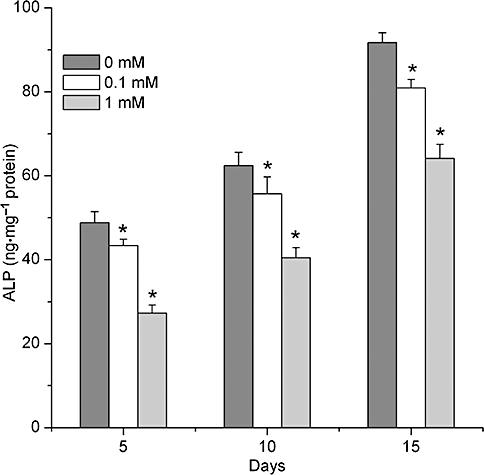
ALP activity was negatively regulated by caffeine in a concentration- and time-dependent manner. ALP was assayed in cell lysates of BMSCs on 5, 10 and 15 days. Data show an attenuation of ALP activity after caffeine treatment at each time point. Each bar represents the mean ± standard deviation (n = 9). Comparisons between groups were performed using anova. *P < 0.01, significantly different from the control group. ALP, alkaline phosphatase; BMSCs, bone marrow-derived mesenchymal stromal cells.
Figure 5.
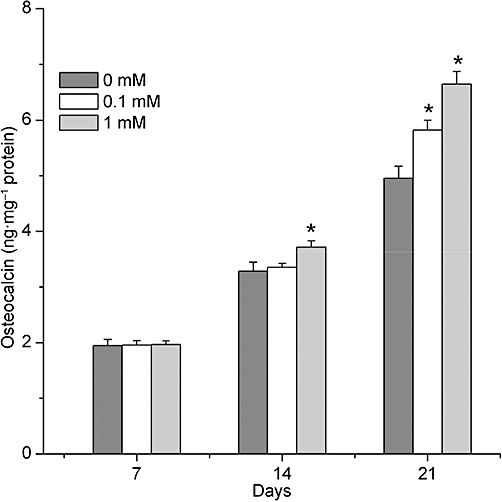
Concentration and time response of osteocalcin upregulation by caffeine. Osteocalcin was assayed in cell lysates of BMSCs on 7, 14 and 21 days. Data show an accumulation of osteocalcin (OC) after caffeine treatment at each time point. Each bar represents the mean ± standard deviation (n = 9). Comparisons between groups were performed using anova. *P < 0.01, significantly different from the control group. BMSCs, bone marrow-derived mesenchymal stromal cells.
Mineralization negatively regulates by caffeine in a concentration- and time-dependent manner
Deposition of calcium was gradually increased in all groups during the process of osteogenesis. However, following exposure to caffeine (0.1–1 mM), the amount of calcium deposition was decreased, compared to the control group, at each time point (P < 0.01; Figure 6).
Figure 6.
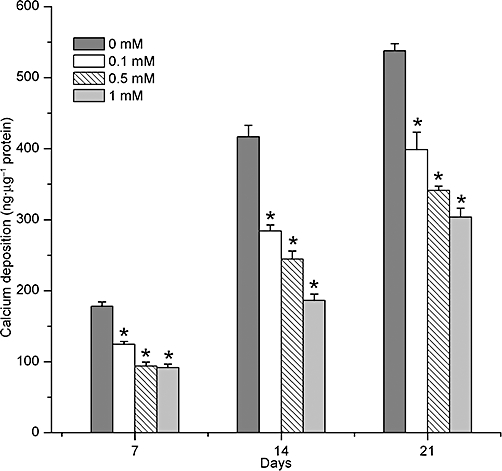
Time and concentration dependence of caffeine-induced inhibition of calcium deposition. Calcification of BMSCs was assayed on 7, 14 and 21 days of caffeine treatment. Data show that decreased calcium deposition was clearly observed at each time after caffeine treatment. Each bar represents the mean ± standard deviation (n = 9). Comparisons between groups were performed using anova. *P < 0.01, significantly different from the control group. BMSCs, bone marrow-derived mesenchymal stromal cells.
Caffeine increases intracellular cAMP in a concentration-dependent manner
cAMP is a key intracellular signalling molecule in osteogenesis (Tintut et al., 1999; Kondo et al., 2002; Yang et al., 2008). Methylxanthine has the ability to increase the level of intracellular cAMP (Yang et al., 2008); hence we tested whether intracellular cAMP in BMSCs could be elevated by the natural methylxanthine, caffeine. As shown in Figure 7, cAMP levels were significantly increased after treatment with all concentrations of caffeine (P < 0.01).
Figure 7.
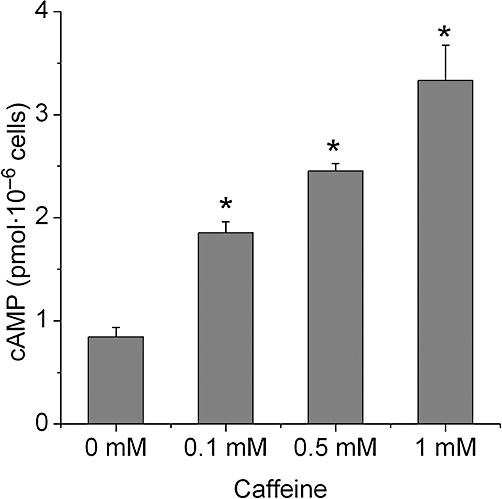
Caffeine concentration dependently increases intracellular cAMP. BMSCs were pre-treated for 5 min with 0.5 mM isobutyl methylxanthine and then treated with caffeine for 15 min. cAMP was measured by immunoassay. Data show an accumulation of cAMP after caffeine treatment. Each bar represents the mean ± standard deviation (n = 3). Comparisons between groups were performed using anova. *P < 0.01, significantly different from the control group. BMSCs, bone marrow-derived mesenchymal stromal cells.
Discussion
Flow cytometry analysis indicated that the majority of detached BMSCs expressed surface markers CD29, CD44H, CD54, CD73 and CD90, but only few cells expressed CD31, CD34 and CD45. This surface expression pattern was similar to human and murine MSCs (Colter et al., 2001; Peister et al., 2004).
Several studies have demonstrated that caffeine is anti-proliferative towards osteoblasts (Tassinari et al., 1996; Kamagata-Kiyoura et al., 1999; Tsuang et al., 2006; Lu et al., 2008). In these studies, it is relevant to note that 0.1–0.4 mM caffeine alone did not affect growth of osteoblasts (Tassinari et al., 1996; Kamagata-Kiyoura et al., 1999; Lu et al., 2008). However, in the presence of prostaglandin E2, 0.1 mM caffeine was able to decrease viability of osteoblasts (Kamagata-Kiyoura et al., 1999) and, in another report, cell growth was also attenuated after osteoblasts were exposed to 0.1 mM caffeine for 7 days (Tsuang et al., 2006). Our study found that the viability of BMSCs was considerably inhibited by incubating BMSCs in 0.1 mM caffeine for only 2 days. Given that BMSCs are more sensitive than osteoblasts to the same concentration of caffeine (Figure S3), it is reasonable to postulate that BMSCs, the precursor cells of osteoblasts, may be the primary target in caffeine-related osteoporosis. Treatment with caffeine induced cell death by two modes in BMSCs, primarily necrosis, and to a smaller extent, apoptosis, although neither was observed at the lowest caffeine concentration (0.1 mM). BMSCs are thought to play a key role in bone physiology and partly participate in the process of osteoporosis. In osteoporotic patients, BMSCs exhibit a lower proliferation rate (Rodriguez et al., 1999; Ocarino et al., 2008). Although comparisons among different species have to be conducted with utmost care, we may infer that if these patients have a coffee-drinking habit, there may be a possibility that the number of MSCs in the bone marrow could be decreased further due to the impairment of cell viability by caffeine. This impairment could lead to the reduction of MSCs numbers that may, in turn, have synergistic effects on the process of osteoporosis.
The differentiation of BMSCs can be generally divided into three stages: commitment to osteogenic lineage, matrix synthesis and the mineralization of matrix (Beck, 2003). During the process of osteogenesis, a number of genes, including Cbfa1/Runx2, ALP, Col-I and OC, are sequentially expressed for these discrete stages of osteogenesis. Cbfa1/Runx2 is an essential transcriptional activator of commitment to the osteogenic lineage (Ducy et al., 1997). Knockdown of the Cbfa1/Runx2 gene leads to impairment of bone formation because of the insufficient number of osteoblasts (Komori et al., 1997). The attenuation of Cbfa1/Runx2 gene expression caused by caffeine suggests that caffeine could affect osteogenesis at an early stage by reducing the commitment of MSCs to osteoblasts. As MSCs have been shown to exhibit a decreased ability to differentiate into osteoblasts in osteoporotic patients (Rodriguez et al., 1999), it may be that osteoporotic patients who like to drink coffee may have less bone formation, due to the decreased number of osteoblasts caused by caffeine. Caffeine-induced reduced matrix synthesis was shown by reduced expressions of the mRNA for early (Col-I), and middle (ALP) stage markers, as well as its protein during osteogenesis.
OC is a marker gene, which is generally highly expressed in the late stage of bone formation. In contrast to the adverse effect of caffeine on Cbfa1/Runx2, Col-I and ALP, the expression of gene OC observed in our study was surprisingly enhanced by caffeine in a concentration-dependent manner accompanied by a parallel enhancement in the amount of OC protein. It is interesting to note that the role of caffeine on OC contradicts that on Cbfa1/Runx2, especially as Cbfa1/Runx2 shows the ability to bind to cis-acting element 2 (OSE2) of the OC promoter region and regulate the expression of OC (Ducy and Karsenty, 1995). One interpretation for this inconsistency is that although Cbfa1/Runx2 is downregulated by caffeine, it may increase the ability of Cbfa1/Runx2 to bind to OSE2 of the OC promoter. The possibility can be supported by the finding that the enhancement of Cbfa1/Runx2 binding to OSEs is not followed by a parallel escalation in the level of Cbfa1/Runx2 mRNA (Xiao et al., 1998). In addition, caffeine is generally considered to have the ability to increase the levels of intracellular cAMP. Given that the elevated cAMP can increase the mRNA expression of OC via cAMP-dependent protein kinase A pathway (Boguslawski et al., 2000; Huang et al., 2005), another explanation for this contradictory phenomenon may be that in the context of caffeine, OC expression can be increased due to the high level of cAMP. The precise mechanism underlying these two possibilities will be studied in future research.
Although the exact role of OC on bone metabolism remains unclear, the functions of OC are many. Thus OC released from the bone matrix can modify bone resorption (Ivaska et al., 2004) and deletion of the OC gene resulted in an increased bone formation, whereas bone resorption and mineralization were not affected (Ducy et al., 1996). These results reveal that one function of OC is to inhibit the osteogenesis of bone-related cells. Thus, we may infer that the high level of OC in the context of caffeine may suppress osteogenesis, rather than promote bone formation by BMSCs.
The ability of extracellular matrix to undergo mineralization requires a sequence of genes to be expressed. After treatment with caffeine, gene expression of Col-I and ALP were markedly downregulated, thereby impairing the capability of BMSCs to promote matrix synthesis and maturation in vitro (Franceschi, 1999). Moreover, the decreased commitment of BMSCs to the osteogenic lineage, induced by caffeine, was also partially responsible for the reduced bone formation (Franceschi, 1999).
cAMP/PKA signalling plays an important role in regulating bone formation and differentiation of BMSCs (Kondo et al., 2002; Yang et al., 2008). In osteoporotic patients, the levels of cAMP in plasma and urine were increased (Neelon et al., 1973). As the main function of cAMP is to activate PKA, our observation that caffeine increased intracellular cAMP in BMSCs could imply that signalling pathways such as cAMP/PKA were involved in the inhibition by caffeine of the osteogenesis in BMSCs. One possible mechanism for the decreased bone formation after caffeine is that caffeine increases glucocorticoid receptor (GR) activity in osteoblasts (Föcking et al., 2005). However, GR activity can also be modulated by PKA (Rangarajan et al., 1992).
In conclusion, our investigation indicates that by acting adversely on the commitment of BMSCs to the osteogenic lineage and inhibiting gene expression at the early and middle stages of osteogenesis, caffeine downregulates some important events in osteogenesis and eventually affects bone formation. Although interspecies interpretation from rats to humans must be done with great caution, the present study investigated the negative effects of caffeine on BMSC viability and bone-forming ability and our results may provide clues to explain the epidemiological finding that habitual consumption of caffeine has harmful effects on bone mass (Rapuri et al., 2001; Ilich et al., 2002).
Acknowledgments
We are grateful to Dr Shang Zhang (State Key Laboratory of Biotherapy and Cancer Center, West China Hospital, Sichuan University, China) for kindly providing his technical supports in several of our experiments. We also wish to acknowledge the help extended by Prof Chih-Ko Yeh (The Dental School at the University of Texas Health Science Center at San Antonio, USA), for his careful correction of grammatical errors in the manuscript. This study was supported by the National Science Foundation of China (Grant NO: 30772449).
Glossary
Abbreviations
- ALP
alkaline phosphatase
- AVF
annexin-V-fluorescein
- Col-I
collagen I
- CPM
counts per minute
- FBS
fetal bovine serum
- GR
glucocorticoid receptor
- L-DMEM
low Dulbecco's Modified Eagle Medium
- MSCs
mesenchymal stromal cells
- OC
osteocalcin
- OCPC
o-cresolphthalein complexone
- OSE2
cis-acting element 2
- PBS
phosphate-buffered saline
- PI
propidium iodide
- rBMSCs
rat bone marrow-derived mesenchymal stromal cells
Conflicts of interest
The authors declare that there are no potential conflicts of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Characterization of the detached mesenchymal stromal cells (MSCs) by observing cell appearance and multipotent differentiantion study. (A) Bone marrow MSCs (BMSCs) from passage 2 appeared as fibroblast-like cells with slim bodies in phase contrast micoscope, scale bar = 500 µM. (B) Mineralization noules were observed with Alizarin Red S staining after osteogenic induciton for 3 weeks, scale bar = 200 µM. (C) After adipogenic induction for 7 days, intracytoplasmic lipid vesicles were seen after oil red O staining, scale bar = 50 µM.
Figure S2 Bone marrow-derived mesenchymal stromal cells' (BMSCs) surface antigens CD29, CD31, CD34, CD44H, CD45, CD54, CD73 and CD90 were analysed by flow cytometry. Majority of cells expressed markers of CD29, CD44H, CD54, CD73 and CD90, but didn't express CD31, CD34 and CD45 markers. This surface expression pattern was similar to human and murine MSCs. FITC, fluorescein isothiocyanate.
Figure S3 Osteoblasts viability negatively regulated by caffeine. Osteoblasts treated with different doses of caffeine (0, 0.1, 1 mM) for 48 h and assessed by thymidine incorporation. Caffeine (0.1 mM) exerted no effect on the proliferation of osteoblasts (P > 0.05), but inhibited osteoblasts' growth when caffeine's concentration was 1 mM (*P < 0.05). Each data point represents mean ± standard deviation of nine independent experiments and was analysed by ANOVA. CPM, count per minute.
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Beck GR. Inorganic phosphate as a signaling molecule in osteoblast differentiation. J Cell Biochem. 2003;90:234–243. doi: 10.1002/jcb.10622. [DOI] [PubMed] [Google Scholar]
- Boguslawski G, Hale LV, Yu XP, Miles RR, Onyia JE, Santerre RF, et al. Activation of osteocalcin transcription involves interaction of protein kinase A- and protein kinase C-dependent pathways. J Biol Chem. 2000;275:999–1006. doi: 10.1074/jbc.275.2.999. [DOI] [PubMed] [Google Scholar]
- Canalis E, Giustina A, Bilezikian JP. Mechanisms of anabolic therapies for osteoporosis. N Engl J Med. 2007;357:905–916. doi: 10.1056/NEJMra067395. [DOI] [PubMed] [Google Scholar]
- Colter DC, Sekiya I, Prockop DJ. Identification of a subpopulation of rapidly self-renewing and multipotential adult stem cells in colonies of human marrow stromal cells. Proc Natl Acad Sci USA. 2001;98:7841–7845. doi: 10.1073/pnas.141221698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cummings SR, Nevitt MC, Browner WS, Stone K, Fox KM, Ensrud KE, et al. Risk factors for hip fracture in white women. N Engl J Med. 1995;332:767–774. doi: 10.1056/NEJM199503233321202. [DOI] [PubMed] [Google Scholar]
- Delmas PD, Fraser M. Strong bones in later life: luxury or necessity? Bull World Health Organ. 1999;77:416–422. [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Karsenty G. Two distinct osteoblast-specific cis-acting elements control expression of a mouse osteocalcin gene. Mol Cell Biol. 1995;15:1858–1869. doi: 10.1128/mcb.15.4.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ducy P, Desbois C, Boyce B, Pinero G, Story B, Dunstan C, et al. Increased bone formation in osteocalcin-deficient mice. Nature. 1996;382:448–452. doi: 10.1038/382448a0. [DOI] [PubMed] [Google Scholar]
- Ducy P, Zhang R, Geoffroy V, Ridall AL, Karsenty G. Osf2/Cbfa1: a transcriptional activator of osteoblast differentiation. Cell. 1997;89:747–754. doi: 10.1016/s0092-8674(00)80257-3. [DOI] [PubMed] [Google Scholar]
- Föcking M, Schmiegelt D, Trapp T. Caffeine-mediated enhancement of glucocorticoid receptor activity in human osteoblastic cells. Biochem Biophys Res Commun. 2005;337:435–439. doi: 10.1016/j.bbrc.2005.09.067. [DOI] [PubMed] [Google Scholar]
- Franceschi RT. The developmental control of osteoblast-specific gene expression role of specific transcription factors and the extracellualr matrix enviromment. Crit Rev Oral Biol Med. 1999;10:40–57. doi: 10.1177/10454411990100010201. [DOI] [PubMed] [Google Scholar]
- Gundberg CM, Nieman SD, Abrams S, Rosen H. Vitamin K status and bone health: an analysis of methods for determination of undercarboxylated osteocalcin. J Clin Endocrinol Metab. 1998;83:3258–3266. doi: 10.1210/jcem.83.9.5126. [DOI] [PubMed] [Google Scholar]
- Heaney RP. Effects of caffeine on bone and the calcium economy. Food Chem Toxicol. 2002;40:1263–1270. doi: 10.1016/s0278-6915(02)00094-7. [DOI] [PubMed] [Google Scholar]
- Heaney RP, Rafferty K. Carbonated beverages and urinary calcium excretion. Am J Clin Nutr. 2001;74:343–347. doi: 10.1093/ajcn/74.3.343. [DOI] [PubMed] [Google Scholar]
- Huang WC, Xie ZH, Konaka H, Sodek J, Zhau HE, Chung LWK. Human osteocalcin and bone sialoprotein mediating osteomimicry of prostate cancer cells: role of cAMP-dependent protein kinase A signaling pathway. Cancer Res. 2005;65:2303–2313. doi: 10.1158/0008-5472.CAN-04-3448. [DOI] [PubMed] [Google Scholar]
- Ilich JZ, Brownbill RA, Tamborini L, Crncevic-Orlic Z. To drink or not to drink: how are alcohol, caffeine and past smoking related to bone mineral density in elderly women? J Am Coll Nutr. 2002;21:536–544. doi: 10.1080/07315724.2002.10719252. [DOI] [PubMed] [Google Scholar]
- Ivaska KK, Hentunen TA, Vääräniemi J, Ylipahkala H, Pettersson K, Väänänen HK. Release of intact and fragmented osteocalcin molecules from bone matrix during bone resorption in vitro. J Biol Chem. 2004;279:18361–18369. doi: 10.1074/jbc.M314324200. [DOI] [PubMed] [Google Scholar]
- Kamagata-Kiyoura Y, Ohta M, Cheuk G, Saltzman MJ, Nakamoto T. Combined effects of caffeine and prostaglandin E2 on the proliferation of osteoblast-like cells (UMR106-01) J Periodontol. 1999;70:283–288. doi: 10.1902/jop.1999.70.3.283. [DOI] [PubMed] [Google Scholar]
- Kamiya K, Fujinami Y, Hoya N, Okamoto Y, Kouike H, Komatsuzaki R, et al. Mesenchymal stem cell transplantation accelerates hearing recovery through the repair of injured cochlear fibrocytes. Am J Pathol. 2007;171:214–226. doi: 10.2353/ajpath.2007.060948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keen R. Osteoporosis: strategies for prevention and management. Best Pract Res Clin Rheumatol. 2007;21:109–122. doi: 10.1016/j.berh.2006.10.004. [DOI] [PubMed] [Google Scholar]
- Komori T, Yagi H, Nomura S, Yamaguchi A, Sasaki K, Deguchi K, et al. Targeted disruption of Cbfa1 results in a complete lack of bone formation owing to maturational arrest of osteoblasts. Cell. 1997;85:755–764. doi: 10.1016/s0092-8674(00)80258-5. [DOI] [PubMed] [Google Scholar]
- Kondo H, Guo J, Bringhurst FR. Cyclic adenosine monophosphate/protein kinase A mediates parathyroid hormone/parathyroid hormone-related protein receptor regulation of osteoclastogenesis and expression of RANKL and osteoprotegerin mRNAs by marrow stromal cells. J Bone Miner Res. 2002;17:1667–1679. doi: 10.1359/jbmr.2002.17.9.1667. [DOI] [PubMed] [Google Scholar]
- Lu PZ, Lai CY, Chan WH. Caffeine induces cell death via activation of apoptotic signal and inactivation of survival signal in human ostoblasts. Int J Mol Sci. 2008;9:698–718. doi: 10.3390/ijms9050698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neelon FA, Birch BM, Drezner M, Lebovitz HE. Urinary cyclic adenosine monophosphate as an aid in the diagnosis of hyperparathyroidism. Lancet. 1973;1:631–633. doi: 10.1016/s0140-6736(73)92199-5. [DOI] [PubMed] [Google Scholar]
- Ocarino NM, Boeloni JN, Goes AM, Silva JF, Marubayashi U, Serakides R. Osteogenic differentiation of mesenchymal stem cells from osteopenic rats subjected to physical activity with and without nitric oxide. Nitric Oxide. 2008;19:320–325. doi: 10.1016/j.niox.2008.08.004. [DOI] [PubMed] [Google Scholar]
- Peister A, Mellad JA, Larson BL, Hall BM, Gibson LF, Prockop DJ. Adult stem cells from bone marrow (MSCs) isolated from different strains of inbred mice vary in surface epitopes, rates of proliferation, and differentiation potential. Blood. 2004;103:1662–1668. doi: 10.1182/blood-2003-09-3070. [DOI] [PubMed] [Google Scholar]
- Pittenger MF, Mackay AM, Beck SC, Jaiswal RK, Douglas R, Mosca JD, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- Rangarajan PN, Umesono K, Evans RM. Modulation of glucocorticoid receptor function by protein kinase A. Mol Endocrinol. 1992;6:1451–1457. doi: 10.1210/mend.6.9.1435789. [DOI] [PubMed] [Google Scholar]
- Rapuri PB, Gallagher JC, Kinyamu HK, Ryschon KL. Caffeine intake increases the rate of bone loss in elderly women and interacts with vitamin D receptor genotypes. Am J Clin Nutr. 2001;74:694–700. doi: 10.1093/ajcn/74.5.694. [DOI] [PubMed] [Google Scholar]
- Rodan GA, Martin TJ. Therapeutic approaches to bone diseases. Science. 2000;289:1508–1514. doi: 10.1126/science.289.5484.1508. [DOI] [PubMed] [Google Scholar]
- Rodriguez JP, Garat S, Gajardo H, Pino AM, Seitz G. Abnormal osteogenesis in osteoporotic patients is reflected by altered mesenchymal stem cells dynamics. J Cell Biochem. 1999;75:414–423. doi: 10.1002/(sici)1097-4644(19991201)75:3<414::aid-jcb7>3.3.co;2-3. [DOI] [PubMed] [Google Scholar]
- Shafer SH, Phelps SH, Williams CL. Reduced DNA synthesis and cell viability in small cell lung carcinoma by treatment with cyclic AMP phosphodiesterase inhibitors. Biochem Pharmacol. 1998;56:1229–1236. doi: 10.1016/s0006-2952(98)00260-3. [DOI] [PubMed] [Google Scholar]
- Tassinari MS, Gerstenfeld LC, Stein GS, Lian JB. Effect of caffeine on parameters of osteoblast growth and differentiation of a mineralized extracellular matrix in vitro. J Bone Miner Res. 1996;6:1029–1036. doi: 10.1002/jbmr.5650061003. [DOI] [PubMed] [Google Scholar]
- Taylor EN, Curhan GC. Demographic, dietary, and urinary factor and 24-h urinary calcium excretion. Clin J Am Soc Nephrol. 2009;4:1980–1987. doi: 10.2215/CJN.02620409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tintut Y, Parhami F, Le V, Karsenty G, Demer LL. Inhibition of osteoblast-specific transcription factor Cbfa1 by the cAMP pathway in osteoblastic cells. J Biol Chem. 1999;274:28875–28879. doi: 10.1074/jbc.274.41.28875. [DOI] [PubMed] [Google Scholar]
- Tsuang YH, Sun JS, Chen LT, Sun SC, Chen SC. Direct effects of caffeine on osteoblastic cells metabolism: the possible causal effect of caffeine on the formation of osteoporosis. J Orthop Surg. 2006;1:7–16. doi: 10.1186/1749-799X-1-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ugland H, Boquest AC, Naderi S, Collas P, Blomhoff HK. cAMP-mediated induction of cyclin E sensitizes growth-arrested adipose stem cells to DNA damage-induced apoptosis. Mol Biol Cell. 2008;19:5082–5092. doi: 10.1091/mbc.E08-01-0094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xiao GZ, Wang D, Benson MD, Karsenty G, Franceschi RT. Role of the α2-integrin in osteoblast-specific gene expression and activation of the Osf2 transcription factor. J Biol Chem. 1998;273:32988–32944. doi: 10.1074/jbc.273.49.32988. [DOI] [PubMed] [Google Scholar]
- Xiao ZS, Quarles LD, Chen QQ, Yu YH, Qu XP, Jiang CH, et al. Effect of asymmetric dimethylarginine on osteoblastic differentiation. Kidney Int. 2001;60:1699–1704. doi: 10.1046/j.1523-1755.2001.00011.x. [DOI] [PubMed] [Google Scholar]
- Yang DC, Tsay HJ, Lin SY, Chiou SH, Li MJ, Chang TJ, et al. cAMP/PKA regulates osteogenesis, adipogenesis and ratio of RANKL/OPG mRNA expression in mesenchymal stem cells by suppressing leptin. PLoS ONE. 2008;3:e1540–e1549. doi: 10.1371/journal.pone.0001540. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


