Abstract
BACKGROUND AND PURPOSE
Bradykinin, through its B2 receptor, is involved in inflammatory processes related to arthropathies. In carrageenan and lipopolysaccharide (LPS)-induced arthritis in rat, the anti-inflammatory activity of MEN16132, a potent and selective kinin B2 receptor antagonist, was compared with that of steroidal and nonsteroidal anti-inflammatory drugs. The interaction between MEN16132 and dexamethasone was also investigated.
EXPERIMENTAL APPROACH
Drugs, alone or in combination, were injected into the knee joint 30 min before intra-articular administration of carrageenan or LPS, in pentobarbital anaesthetized rats. Effects on incapacitation, oedema, neutrophil recruitment and kallikrein system activation, in the knee joint, were assessed.
KEY RESULTS
MEN16132 and dexamethasone (10–300 µg per knee) dose-dependently reduced carrageenan-induced joint pain, oedema and neutrophil infiltration, reaching a maximal inhibition of about 50%. Dexketoprofen exerted a similar analgesic activity, whereas it did not affect the other inflammatory responses. MEN16132 showed a partial inhibition of LPS-induced joint pain, whereas dexamethasone produced a full analgesic effect. Combination of MEN16132 and dexamethasone showed a strong synergistic interaction in inhibiting both carrageenan and LPS-induced knee joint inflammation. Dexamethasone did not prevent the contact activation of prekallikrein by carrageenan and the subsequent release of kallikreins and bradykinin in the synovium.
CONCLUSIONS AND IMPLICATIONS
Steroids and kinin B2 receptor antagonists appear to relieve arthritic symptoms induced by carrageenan or LPS and act synergistically to inhibit joint inflammation. This could have interesting therapeutic implications, possibly opening the way for combination therapies in the control of inflammatory arthropathies.
Keywords: MEN16132, B2 receptor antagonist, dexamethasone, steroidal anti-inflammatory drug, bradykinin, carrageenan, lipopolysaccharide, arthritis, synergism
Introduction
Kinins are principally represented by two bioactive peptides: bradykinin, generated in plasma and tissues through the cleavage of kininogen precursors by kallikrein enzymes, and its metabolite, des-Arg9-bradykinin. The constitutively expressed kinin B2 receptor mediates the effects of bradykinin, whereas des-Arg9-bradykinin acts through the kinin B1 receptor whose expression is induced during tissue inflammation and injury (Leeb-Lundberg et al., 2005; receptor nomenclature follows Alexander et al., 2009).
Bradykinin and kallikrein activities have been found in the synovial fluid of patients affected by several arthropathies such as osteoarthritis, rheumatoid arthritis, gout and psoriatic arthritis. Moreover, kinin levels correlate well with the degree of joint disease (Rahman et al., 1995; Nishimura et al., 2002; Meini and Maggi, 2008). Bradykinin potentially affects the joint at the level of either synovium, cartilage or bone, as synovial cells, chondrocytes and osteoblasts all express the kinin B2 receptor. Nociceptive sensory fibres located in the joint capsule, ligaments and subchondral bone, are also possible targets for bradykinin (Brechter and Lerner, 2002; Meini and Maggi, 2008). The intra-articular administration of bradykinin in animals causes an inflammatory response characterized by excitation and sensitization of sensory nerves, evoking pain and hyperalgesia, leucocyte recruitment, increase of vascular permeability and vasodilation, producing local warmth and oedema (Cambridge and Brain, 1995; Lo et al., 1999; Pawlak et al., 2008). Accordingly, joint inflammation can be reduced by blocking bradykinin effects, as already observed in arthritis models induced by carrageenan, Freund's adjuvant, monosodium iodoacetate, lipopolysaccharide (LPS) or urate crystals (Damas and Remacle-Volon, 1992; Sharma and Wirth, 1996; Poole et al., 1999; Tonussi and Ferreira, 1999; Cialdai et al., 2009). In addition to sustaining the inflammatory process, bradykinin can also contribute to the development of structural alterations in the arthritic joints, by stimulating synovial angiogenesis, cartilage destruction and subchondral bone remodelling, as bradykinin is involved in proliferation of endothelial cells, cartilage matrix homeostasis and bone resorption (Colman, 2006; Brechter and Lerner, 2007; Meini and Maggi, 2008).
The arthritic pathologies are currently treated with non-steroidal anti-inflammatory drugs (NSAIDs) and anti-inflammatory steroids to relieve pain and inflammatory symptoms. However, adverse effects may occur with these conventional anti-inflammatory drugs especially when given at high dosage or after repeated administration (Bijlsma et al., 2002; Zhang et al., 2007). Therefore, novel therapeutic approaches are under investigation for the treatment of arthritis focused on pain relief or modification of underlying disease (Dray and Read, 2007). Early clinical trials indicate that intra-articular administration of a kinin B2 receptor antagonist (icatibant) is very well tolerated and reduces pain at rest and during activity in patients affected by osteoarthritis, as do the non-selective cyclooxygenase (COX) inhibitors and selective COX-2 inhibitors (Sorbera et al., 2006; Song et al., 2008). Therefore, bradykinin antagonists could represent a promising therapeutic alternative to the NSAIDs and anti-inflammatory steroids for the treatment of joint pain and inflammation.
The aim of the present study was to test the anti-inflammatory activity of MEN16132, a potent and selective nonpeptide kinin B2 receptor antagonist (Cucchi et al., 2005; Valenti et al., 2005; Meini et al., 2008) in the carrageenan and LPS-induced arthritis models in rats. The effect of MEN16132 was compared with that of both anti-inflammatory steroids and NSAIDs, which are largely used for the treatment of arthritis. Anti-inflammatory interactions between MEN16132 and steroids were also investigated.
Methods
Animals
All animal care and experimental procedures were in accordance with the principles and guidelines of the European Union regulations and approved by the local ethical committee. Experiments were performed in male Wistar rats (Harlan Laboratories, Udine, Italy) weighing 250–300 g.
Induction of inflammatory arthritis
Anaesthetized rats (pentobarbital, 40 mg·kg−1 i.p.) received two intra-articular injections: the first, of anti-inflammatory drugs and the second to induce inflammatory arthritis by carrageenan or LPS. The anti-inflammatory drugs, MEN16132, icatibant, dexketoprofen, dexamethasone 21-phosphate disodium salt (dexamethasone) or hydrocortisone 21-hemisuccinate sodium salt (hydrocortisone), were dissolved in saline and sterilized by filtration (MillexGV 0.22 µm, Millipore, Billerica, MA, USA); 25 µL of these solutions or sterile saline (control group) were injected into the right knee joint. All animals received 25 µL of sterile saline into the contralateral (left) knee. After 30 min, inflammation was induced in the right knee by 25 µL intra-articular injection of 2% λ-carrageenan, previously dissolved in saline and autoclaved, or by 10 µg per knee of LPS from E. Coli 0127:B8 in sterile saline. The left knee of all animals and the right knee of control group received again 25 µL of sterile saline. After each injection the knee joint was flexed and extended repeatedly to allow the dispersion of drugs and inflammatory agents. The animals recovered from anaesthesia within 60–90 min.
In another series of experiments, dexamethasone was given 2 h before carrageenan in order to increase the time for the induction of its effects at transcriptional level.
Incapacitance test
The pain related to the knee joint incapacitation was assessed by measuring the weight exerted on the two hind limbs with the Incapacitance Tester MkV (Linton Instrumentation, Norfolk, UK). Animals were positioned standing on their hind limbs into a restraint and the weight (in grams) exerted by both the carrageenan-injected (right) leg and the contralateral (saline-injected) leg, was averaged over a 5 s period. This measure was repeated eight times for each animal at different times (6 h, 1–7 days) after carrageenan or LPS administration and the mean value was used for calculation. Data are expressed as per cent of total weight exerted on the right leg.
Knee size measurement, synovial lavage and joint capsule collection
Animals were anaesthetized with urethane (1.5 g·kg−1 i.p.) 6 or 24 h after carrageenan or LPS injection. The knee diameter was measured with callipers and then skin and patellar ligament were removed to perform the synovial lavage. A 30-G needle was inserted into the joint cavity and 100 µL of sterile saline were infused at the rate of 100 µL·min−1 through a peristaltic pump (Ismatec SA, Glattbrugg, Switzerland) connected to the needle via a silicone tubing; another 27-G needle was inserted into the joint space to allow the fluid outflow, driven by the intra-articular pressure. Saline infusion was restarted and maintained until 250–300 µL were collected; synovial lavage fluid was centrifuged (10 000×g, for 5 min, at 4°C) and the supernatant was recovered. Finally, the anterior side of the knee joint capsule was removed, minced with a scalpel and weighed. All samples were stored at –80°C until analysis.
Myeloperoxidase assay
Myeloperoxidase (MPO) activity was assayed in the knee joint capsule homogenates to evaluate the infiltration of neutrophils into the tissues, according to the method described by Schneider and Issekutz (1996). Joint capsule was added to 0.5 mL of cold lysis buffer (6 mM hexadecyltrimethylammonium chloride in 10 mM citric acid/sodium citrate buffer, pH 5) and homogenized in a Ultra-Turrax homogenizer (Ultra-Turrax T25, Janke & Kunkel, Staufen, Germany), twice for 30 s. Homogenates were sonicated twice for 30 s and centrifuged at 25 000×g, for 10 min, at 4°C. Seventy-five microlitre of supernatant or standard solutions (MPO from human leukocytes 0.02–5 iu·mL−1), appropriately diluted in lysis buffer, were dispensed in a 96 well plate; 75 µL of lysis buffer were added in the wells of blank. The reaction was started by adding 75 µL per well of substrate solution containing 3 mM 3',5,5'-tetramethylbenzidine dihydrochloride, 120 µM resorcinol and 2.2 mM H2O2 in distilled water. Immediately after mixing, the absorbance at 620 nm was followed with a microplate reader (CERES UV900C, Bio-Tek Instruments, Winooski, VT, USA) in order to measure the optical density change per min (ΔOD) in the linear range of the reaction kinetics. The ΔOD of blank was subtracted from that of each sample and the MPO activity was calculated by interpolation with the standard curve and expressed as international units (iu) per mg of tissue.
Bradykinin assay
Bradykinin levels in the synovial lavage fluid were measured using an enzyme-linked immunosorbent assay (elisa). Bradykinin standards (0.008–2.5 µg·mL−1) or samples, diluted appropriately in phosphate buffered saline (PBS), were added 100 µL per well to MaxiSorp immunoplates (Nalge Nunc International, Rochester, NY, USA) and incubated overnight at 4°C; all remaining steps were performed at room temperature. The plate was washed three times with PBS containing 0.1% Tween 20 (PBS-T) and incubated, first with 300 µL per well of PBS-T containing 3% bovine albumin serum (BSA) for 30 min and then with 100 µL per well of goat anti-bradykinin antibody (Santa-Cruz Biotechnology, CA, USA) 1:500 in blocking buffer (PBS-T containing 1% BSA) for 1 h. After four washes with PBS-T, 100 µL of biotinylated donkey anti-goat IgG (Santa-Cruz Biotechnology, CA, USA) 1:1000 in blocking buffer were added to the wells for 1 h. Washes were repeated and followed by a 30 min incubation with 100 µL per well of streptavidin-conjugated horseradish peroxidase 1:1000 in blocking buffer. The plate was washed again, and afterwards 100 µL per well of o-phenylenediamine peroxidase substrate (Sigma Fast OPD) were added and incubated for 15 min; finally the reaction was stopped by adding 3 M H2SO4 (50 µL per well) and the absorbance at 490 nm, against a reference wavelength of 620 nm, was read. Bradykinin levels were expressed as total µg recovered in the synovial lavage fluid.
Plasma and tissue kallikrein assay in the synovial lavage fluid
Plasma kallikrein activity was detected using an amidolytic substrate assay kit according to manufacturer's instructions (CoaChrom Diagnostica, Vienna, Austria). Briefly 100 µL of standards (0.26–1.6 iu·mL−1) or samples, diluted appropriately in 0.05 M Tris-HCl buffer, pH 8, were transferred in a 96 well polystyrene plate. After 5 min incubation at 37°C, the specific plasma kallikrein chromogenic substrate MBz-Pro-Phe-Arg-pNA in distilled water was added (2 mM; 50 µL per well).
Tissue kallikrein activity was measured photometrically using a chromogenic substrate specific for this enzyme in the presence of soybean trypsin inhibitor (Jenzano et al., 1988). Standards (porcine pancreatic kallikrein, 0.004–0.125 iu·mL−1) or samples (50 µL) were incubated with 25 µL of trypsin (30 µg·mL−1) in a 96 well plate for 15 min, at 37°C, to activate prekallikrein (Geiger and Miska, 1988). Soybean trypsin inhibitor (4 mg·mL−1; 100 µL per well) was added in the absence or presence of 50 µM PPACK II, a specific inhibitor of kallikreins (Calbiochem, La Jolla, CA, USA). After 20 min incubation at room temperature, 50 µL·per well of 1 mM H-D-Val-Leu-Arg-pNA (Bachem, Bubendorf, Switzerland) were added; all solutions were made in 0.2 M Tris buffer, pH 8.2.
For both plasma and tissue kallikrein, the ΔOD at 405 nm was recorded and sample values were calculated by interpolation with the standard curves. In order to avoid the interference of other tryptic-like proteases, the tissue kallikrein activity recorded for each sample was corrected for the corresponding values measured in the presence of PPACK II. Kallikrein levels were expressed as total enzymatic units recovered in the synovial lavage fluid.
Determination of contact activation of plasma prekallikrein
Blood was obtained by cardiac puncture from three anaesthetized rats (pentobarbital; 60 mg·kg−1 i.p.) and mixed (9:1 vol) with 0.1 M sodium citrate. Citrated blood was centrifuged (2000×g, for 20 min, at room temperature) and the supernatant plasma was collected and used as source of rat prekallikrein. A series of 1:10 serial dilutions was made for carrageenan and LPS, starting at the same concentration as used for their intra-articular administration (in the range of 2 ng–20 mg·mL−1 and 40 pg–400 µg·mL−1 respectively). These solutions and a commercially available prekallikrein activator (Coachrom Diagnostica) or water (control sample, to assess spontaneous amidolytic activity of plasma) were dispensed (50 µL per well) in a 96 well polystyrene plate and incubated at 37°C, for 3 min. The rat plasma diluted 1:50 in 0.05 M tris-HCl, pH 8, was added (50 µL per well) and the plate was again incubated at 37°C, for 2 min. The activation of the rat prekallikrein to plasma kallikrein was followed by adding the specific tri-peptide chromogenic substrate MBz-Pro-Phe-Arg-pNA in water (1 mM; 50 µL per well) and measuring photometrically (405 nm, at 37°C, for 30 min) the rate at which p-nitroaniline was released. The interference due to the viscosity (for carrageenan 2–20 mg·mL−1) or opalescence (for LPS 40–400 µg·mL−1 and prekallikrein activator) of the solutions was eliminated by subtracting the absorbance of the corresponding blanks (Tris-HCl buffer instead of rat plasma) from that of the experimental samples.
Statistical analysis
Statistical analysis of differences among groups was carried out using one-way anova followed by Dunnett's multiple comparison test. Differences were considered statistically significant at a level of P < 0.05.
Drug combination analysis
The analysis of drug combination was based on the dose–effect curves generated by plotting the dose of drug versus its inhibitory effect. The type and degree of the pharmacological interaction were assessed calculating the combination index (CI) through the median-effect method of Chou and Talalay (1984), using the CalcuSyn software (Biosoft, Ferguson, MO, USA). Values of CI = 1, <1 or >1, indicated an additive effect, synergism or antagonism between drugs respectively.
Materials
MEN16132 (4-(S)-amino-5-(4-{4-[2,4-dichloro-3-(2,4-dimethyl-8-quinolyloxymethyl)phenylsulfonamido]-tetrahydro-2H-4-pyranylcarbonyl}piperazino)-5-oxopentyl(trimethyl)ammonium chloride hydrochloride) and icatibant (Hoe-140, H2N-D-Arg-Arg-Pro-Hyp-Gly-Thi-Ser-D-Tic-Oic-Arg, acetate) were synthesized at the Chemistry Departments of Menarini Ricerche in Florence and Pomezia, Italy. Dexketoprofen was obtained by A. Menarini Manufacturing Logistics and Services, Florence, Italy. Dexamethasone 21-phosphate disodium salt, hydrocortisone 21-hemisuccinate sodium salt, LPS from E. Coli 0127:B8 and all other reagents, if not specified, were purchased from Sigma-Aldrich Co. (St. Louis, MO, USA).
Results
Carrageenan-induced knee joint arthritis
In the control group, rats distributed their weight equally on the two hind limbs, whereas 6 h after carrageenan administration, animals exerted only 24.4 ± 1.2% (n = 12) of weight on the inflamed (right) hind limb, transferring the remaining weight to the contralateral leg. This value recovered to that of control group within 7 days from carrageenan injection, indicating the gradual relief of the joint pain (Figure 1A).
Figure 1.
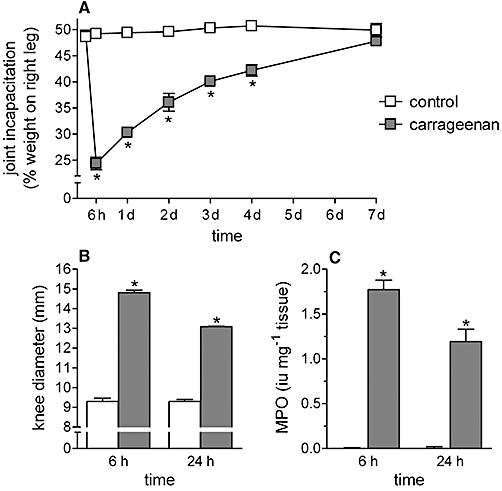
Effect of intra-articular carrageenan on the knee joint incapacitation (A), oedema (B) and neutrophil infiltration (C). λ-Carrageenan (2%, 25 µL) or saline (control group) was injected into the right knee of rats. Knee joint incapacitation, oedema and myeloperoxidase activity were measured at various times after carrageenan administration. Data are expressed as the mean ± SEM of 12 experiments. *P < 0.05 significantly different from the respective control value (one-way anova followed by Dunnett's post-test).
Intra-articular carrageenan also induced a significant oedema and recruitment of neutrophils in the joint tissues at 6 h after its administration, as indicated by the increased knee diameter (59.4 ± 1.3%) and MPO activity (463.3 ± 27.4%, n = 12). The intensity of these responses was lower 24 h after carrageenan administration (Figure 1B and C). The 6 h time-point, corresponding to the maximal intensity of carrageenan-induced inflammatory responses, was chosen for the subsequent experiments.
Effect of kinin B2 receptor antagonists, anti-inflammatory steroids and NSAIDs on carrageenan-induced knee joint incapacitation
Intra-articular administration of MEN16132 was ineffective at 10 µg per knee, whereas from 30–300 µg per knee, it dose-dependently reduced the joint pain, reaching a maximum inhibition of about 50%. Icatibant (30–300 µg per knee) was equipotent to MEN16132 in inhibiting the knee incapacitation (Figure 2). The effect of both dexamethasone and dexketoprofen was comparable to that of the kinin B2 receptor antagonists (Figure 2).
Figure 2.

Effect of kinin B2 receptor antagonists (MEN16132 or icatibant), dexamethasone or dexketoprofen on the carrageenan-induced knee joint incapacitation. Rats received drugs intra-articularly in the right knee at the doses indicated, 30 min before injection of saline (control group) or λ-carrageenan (2%, 25 µL) and the knee joint incapacitation was measured 6 h later. Dotted lines indicate the control value (upper) and the response after carrageenan treatment (lower). Data are expressed as the mean ± SEM of 6–12 experiments. *P < 0.05 and °P < 0.05, significantly different from the carrageenan-treated group and control group respectively (one-way anova followed by Dunnett's post-test).
Effect of kinin B2 receptor antagonists, anti-inflammatory steroids and NSAIDs on carrageenan-induced knee oedema
MEN16132 (10–300 µg per knee) dose-dependently reduced the carrageenan-induced knee oedema with a maximal inhibitory effect of 56.2 ± 4.3% (Figure 3A). Icatibant, at 100 µg per knee, was as effective as MEN16132 (Table 1). Dexamethasone showed a maximal inhibitory effect of 52.4 ± 2.5%, comparable to that of MEN16132 (Figure 3A). This inhibitory effect of dexamethasone (100 µg per knee) was maintained when the pretreatment period was prolonged to 2 h before intra-articular carrageenan administration (43.5 ± 8.0% inhibition, n = 6). Dexketoprofen did not inhibit carrageenan-induced oedema up to a dose of 300 µg per knee (Figure 3A).
Figure 3.
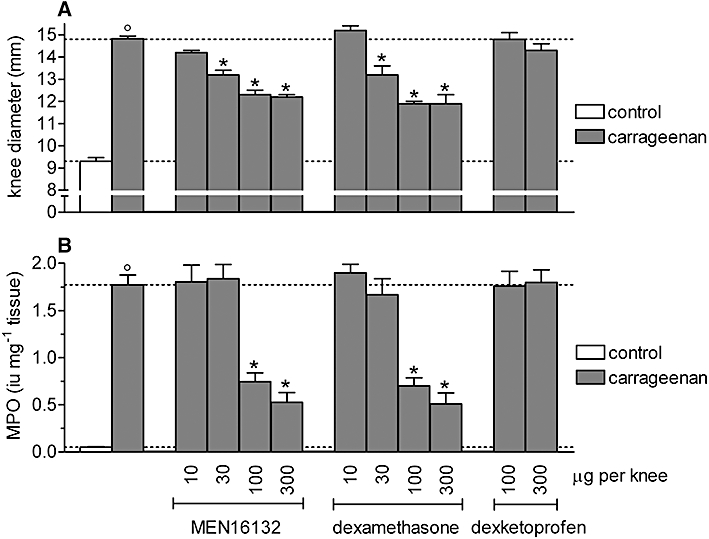
Effect of MEN16132, dexamethasone and dexketoprofen on the carrageenan-induced knee joint oedema (A) and neutrophil infiltration (B). Rats received drugs intra-articularly in the right knee at the doses indicated, 30 min before injection of saline (control group) or λ-carrageenan (2%, 25 µL) and the knee joint diameter and myeloperoxidase activity were measured 6 h later. Dotted lines indicate the control value (lower) and the response after carrageenan treatment (upper). Data are expressed as the mean ± SEM of 6–12 experiments. *P < 0.05 and °P < 0.05, significantly different from the carrageenan-treated group and control group respectively (one-way anova followed by Dunnett's post-test).
Table 1.
Combination between kinin B2 receptor antagonists (MEN16132 or icatibant) and anti-inflammatory steroids (dexamethasone or hydrocortisone) on carrageenan-induced inflammation
| oedema | MPO | ||
|---|---|---|---|
| treatment | µg per knee | % inhibition | |
| MEN16132 | 100 | 46.3 ± 3.3* | 67.5 ± 5.6* |
| icatibant | 100 | 46.2 ± 6.3* | 27.7 ± 19.3 |
| dexamethasone | 100 | 52.4 ± 2.5* | 60.3 ± 4.8* |
| hydrocortisone | 100 | 0.0 ± 3.3 | 0.0 ± 8.6 |
| Icatibant + dexamethasone | 100 + 100 | 90.4 ± 3.8* | 71.9 ± 5.2* |
| MEN16132 + hydrocortisone | 100 + 100 | 84.6 ± 6.3* | 78.3 ± 0.6* |
Data are expressed as the mean ± SEM of six experiments.
P < 0.05 significant inhibition of the carrageenan-treated group (one-way anova followed by Dunnett's post-test).
Effect of kinin B2 receptor antagonists, anti-inflammatory steroids and NSAIDs on neutrophil infiltration in the synovium induced by carrageenan
Both MEN16132 and dexamethasone inhibited the carrageenan-induced neutrophil infiltration in an almost all-or-nothing manner, as they were ineffective at 30 µg per knee but they produced 60–70% inhibition of this response at 100–300 µg per knee (Figure 3B). As observed earlier for carrageenan-induced joint pain and oedema, MEN16132 and dexamethasone were also equipotent in inhibiting infiltration of neutrophils into the joint tissues. Icatibant (100 µg per knee) was less potent than MEN16132, as it did not exert a significant inhibitory effect (Table 1). Dexketoprofen did not affect the MPO activity in the joint tissues up to a dose of 300 µg per knee (Figure 3B).
Effect of combining kinin B2 receptor antagonists and anti-inflammatory steroids on carrageenan-induced knee joint arthritis
MEN16132 and dexamethasone were combined using two different approaches. In the first, MEN16132 and dexamethasone were given at a fixed 1:1 ratio, co-administering equipotent doses of the two drugs (10, 30 or 100 µg per knee, n = 6). In the second, drugs were co-administered at a non-constant dose ratio, giving a fixed dose of MEN16132 (100 µg per knee) while varying the dose of dexamethasone (1, 10 or 30 µg per knee, n = 6).
Combination of MEN16132 with dexamethasone at their maximally effective doses (100 µg per knee) abolished carrageenan-induced knee joint incapacitation, oedema and neutrophil infiltration (Figure 4), showing that the inhibitory effects (about 50%) recorded for each drug alone were additive when they were given together. However, the inhibitory effects of MEN16132 and dexamethasone fixed dose combinations using lower doses (10 and 30 µg per knee) were greater than the sum of the effects of each drug given alone (Figure 4). These results disclosed an anti-inflammatory synergism between the two drugs. Moreover, this synergism was again observed when a maximally effective dose of MEN16132 (100 µg per knee) was combined with doses of dexamethasone ineffective by themselves (3 or 10 µg per knee). On reducing the dose of dexamethasone still further to 1 µg per knee, no interaction with MEN16132 was observed (Figure 4).
Figure 4.
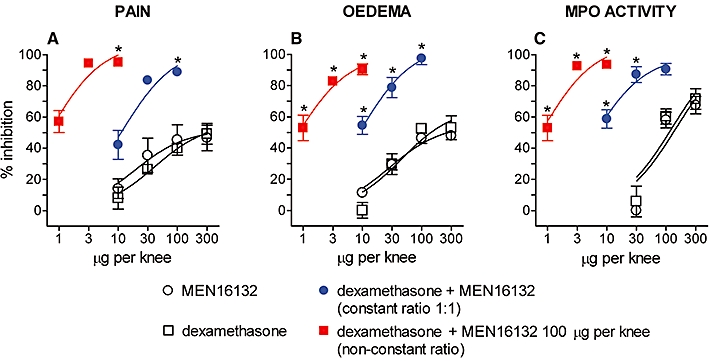
Effect of MEN16132 and dexamethasone co-administration on carrageenan-induced knee joint incapacitation (A), oedema (B) and neutrophil infiltration (C). Rats received intra-articularly in the right knee MEN16132 alone (10–300 µg), dexamethasone alone (10–300 µg), or their combinations at a constant 1:1 dose ratio (10, 30 or 100 µg). The non-constant dose ratio represents a fixed dose of MEN16132 (100 µg) with different doses of dexamethasone (1–10 µg). After 30 min, animals were injected with saline (control group) or λ-carrageenan into the right knee. Inhibitory effects on knee joint incapacitation, oedema and myeloperoxidase activity were measured 6 h after carrageenan administration. Data are expressed as the mean ± SEM of six experiments. *P < 0.05 significant inhibition of carrageenan-induced responses (one-way anova followed by Dunnett's post-test).
Analysis of the degree of drug interaction showed values of CI < 1, confirming the strong synergistic interaction between MEN16132 and dexamethasone in inhibiting carrageenan-induced knee joint incapacitation, oedema and neutrophil infiltration (Table 2).
Table 2.
Interactions between MEN16132 and dexamethasone in inhibiting carrageenan-induced joint inflammation
| drug combination | ||||
|---|---|---|---|---|
| MEN16132 | dexamethasone | pain | oedema combination index (CI) | MPO activity |
| µg per knee | ||||
| 10 | 10 | 0.129 | 0.097 | 0.116 |
| 30 | 30 | 0.007 | 0.119 | 0.154 |
| 100 | 10 | 0.005 | 0.031 | 0.214 |
| 100 | 3 | 0.001 | 0.040 | 0.217 |
Recommended descriptions of the degrees of synergism: CI < 0.1 very strong synergism, 0.1 < CI < 0.3 strong synergism, 0.3 < CI < 0.7 synergism, 0.7 < CI < 0.85 moderate synergism, 0.85 < CI < 0.9 slight synergism, 0.9 < CI < 1.1 nearly additive effect.
A similar drug interaction was also observed with combinations of icatibant with dexamethasone or MEN16132 with hydrocortisone (Table 1). The synergism in inhibiting carrageenan-induced joint inflammation appears to be a general property of the combinations between kinin B2 receptor antagonists and anti-inflammatory steroids.
Effect of kinin B2 receptor antagonists and anti-inflammatory steroids, alone or in combination, on knee joint incapacitation, induced by LPS
Lipopolysaccharide-induced joint pain was maximal at 6 h after its intra-articular administration, when treated rats reduced the weight borne by the right hind paw to 27.1 ± 1.4%, from 47.5 ± 1.4% in the control group (n = 6, Figure 5). This LPS-induced effect was lost and weight-bearing returned to the control value within 6–7 days (data not shown). The intensity, as well as the time-course, of pain in the LPS-induced model of arthritis was similar to those observed in the carrageenan model. MEN16132 and dexamethasone dose-dependently inhibited this pain response, but MEN16132 showed a maximal inhibitory effect of 61.3 ± 9.4%, while dexamethasone produced a full analgesic effect (Figure 5).
Figure 5.
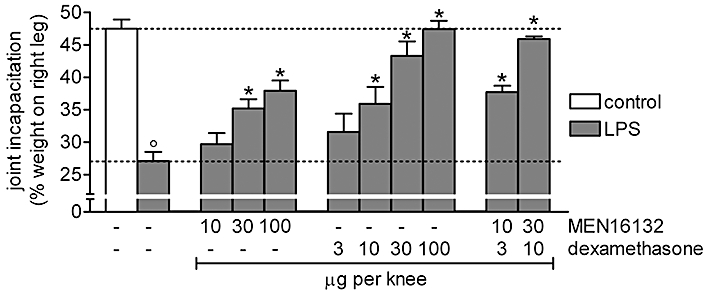
Effect of MEN16132 and dexamethasone on LPS-induced knee joint incapacitation. Rats received intra-articularly in the right knee, anti-inflammatory drugs, alone or in combination at the doses indicated, 30 min before injection of saline (control group) or lipopolysaccharide (LPS) (10 µg, 25 µL). Knee joint incapacitation was measured 6 h later. Dotted lines indicate the control value (upper) and the response after LPS treatment (lower). Data are expressed as the mean ± SEM of 6–12 experiments. *P < 0.05 and °P < 0.05, significantly different from the LPS-treated group and control group respectively (one-way anova followed by Dunnett's post-test).
When given together, MEN16132 (30 µg per knee) and dexamethasone (10 µg per knee) were additive in inhibiting the pain response. Reducing the doses to 10 and 3 µg per knee, respectively, allowed a greater than additive inhibitory effect to be observed (CI = 0.46, Figure 5), showing a synergism between the kinin B2 receptor antagonist and the anti-inflammatory steroid also in the joint pain model induced by LPS.
Carrageenan-induced release of kallikreins and bradykinin in synovial fluid
The synovial lavage fluid of saline-injected knees (control group) contained low levels of both plasma and tissue kallikrein which were significantly increased in the corresponding lavage fluids from carrageenan-injected knees (Figure 6A and B). Consistent with this finding, the levels of bradykinin in the synovial fluid were increased by 3.5-fold, 6 h after with carrageenan (Figure 6C). Dexamethasone (100 µg per knee) did not significantly affect the plasma and tissue kallikrein activity or the release of bradykinin, showing that this steroid was unable to prevent the carrageenan-induced contact activation of the kinin-kallikrein system (Figure 6).
Figure 6.

Effect of dexamethasone on carrageenan-induced activation of the kinin-kallikrein system in the rat knee joint. Carrageenan (2%, 25 µL) or saline (control group) were injected intra-articularly into the right knee. After 6 h, a joint lavage was performed by saline perfusion, and synovial levels of either plasma kallikrein (A), tissue kallikrein (B) or bradykinin (C) were measured. Dexamethasone (100 µg per knee) was given 30 min before carrageenan administration. Data are expressed as the mean ± SEM of 8–12 experiments. *P < 0.05 significantly different from the saline-treated group (one-way anova followed by Dunnett's post-test). n.s., not significant.
Activation of plasma prekallikrein by carrageenan and LPS
Carrageenan (2 µg·mL−1) activated rat plasma prekallikrein at very much lower concentrations than that used to induce knee joint arthritis (20 mg·mL−1). Moreover, its activity in contact activation was very high and comparable to that of a reference activator composed by a mixture of ellagic acid, phospholipids, Hageman factor and high molecular weight kininogen (Figure 7). By contrast, LPS was unable to activate plasma prekallikrein at any concentration from 40 pg·mL−1 to 400 µg·mL−1 which was the concentration injected intra-articularly to induce arthritis.
Figure 7.
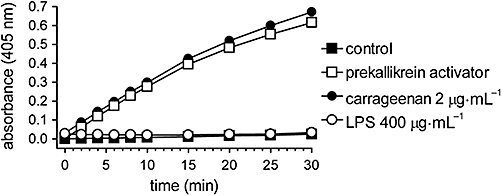
Contact activation of prekallikrein in vitro by carrageenan or lipopolysaccharide (LPS). Rat plasma was added to carrageenan, LPS or saline (control) and the amidolytic cleavage of a specific kallikrein substrate (MBz-Pro-Phe-Arg-pNA) was recorded during 30 min. Data are expressed as the mean ± SEM of four experiments.
Discussion and conclusions
In this study we have compared the anti-inflammatory activity of MEN16132, a selective non-peptide kinin B2 receptor antagonist, with that of icatibant, a peptide kinin B2 receptor antagonist, an anti-inflammatory steroid, dexamethasone and a NSAID dexketoprofen, in carrageenan-induced model of knee joint arthritis in rats. Carrageenan, because of its negatively charged surface, is able to initiate contact activation of the kinin forming system, thus inducing the release of bradykinin (Ferreira et al., 1993). The intra-articular administration of carrageenan is therefore a suitable experimental model of inflammatory arthritis to study the role of bradykinin antagonists in joint diseases.
Bradykinin may induce inflammatory effects via the direct stimulation of the B2 receptor, such as activation and sensitization of nociceptors or increase of vascular permeability (Cambridge and Brain, 1995; Cheng and Ji, 2008), but it may also act indirectly through the production of prostanoids, leukotrienes and cytokines (Sharma and Buchanan, 1994). Ferreira et al. (1993) reported that intra-articular carrageenan stimulates the bradykinin-mediated release of tumour necrosis factor (TNF)-α, which in turn induces production of IL-1β, IL-6 and IL-8, activating a cytokine cascade. Also des-Arg9-bradykinin, the metabolite of bradykinin, is involved in carrageenan-induced responses through stimulation of the inducible kinin B1 receptor (Sharma and Buchanan, 1994). However, the release of bradykinin is not the only event triggering inflammatory and nociceptive responses to carrageenan. Cytokines, as well as prostaglandins and leukotrienes deriving from their stimulatory activity (Cunha et al., 1992), can be released independently from bradykinin, if the inflammatory stimulus is of sufficient strength as seen in our experimental conditions where intra-articular carrageenan was given at a high dose (Ferreira et al., 1993). Moreover, a residual response to carrageenan, unaffected by kinin B2 receptor antagonists, was observed in rats unable to produce bradykinin following treatment with kallikrein inhibitors or genetically deficient in kininogens (Damas and Remacle-Volon, 1992; Majima et al., 1997).
Accordingly, we observed that the kinin B2 receptor antagonists did not reach a full inhibition of the carrageenan-induced knee joint inflammation. MEN16132 or icatibant reduced, by about 50%, carrageenan-induced knee joint incapacitation, swelling and MPO activity, indicating that bradykinin was involved in eliciting pain, oedema and neutrophil recruitment. Dexketoprofen showed a similar analgesic effect, whereas it did not affect the oedema and MPO activity of the knee joint tissues, in agreement with previously reported findings for other COX-inhibitors (Francischi et al., 2002).
As observed with the kinin B2 receptor antagonists, dexamethasone produced a maximal inhibition of about 50% of carrageenan-induced joint inflammation. A similar partial inhibitory effect was obtained when dexamethasone was given 120 min, instead of 30 min, before carrageenan, indicating that its partial effectiveness was not due to insufficient pre-treatment. This was an unexpected result, because dexamethasone should abolish the carrageenan-induced effects in the knee joint, as this anti-inflammatory steroid is potentially able to control all inflammatory mediators involved in this arthritis model. Steroids are known to inhibit the effects of cytokines and des-Arg9-bradykinin, as well as the release of eicosanoids, switching off many activated inflammatory genes and inducing the expression of annexin 1, a phospholipase A2 inhibitor (Yang et al., 1999; Kamal et al., 2005; Zhang et al., 2005; Earp et al., 2008). Moreover, MEN1632 and dexamethasone can produce their effects acting, at least partially, through overlapping molecular targets. In particular, the B2 receptor antagonists prevent the bradykinin-induced release of prostaglandins or cytokines which is also inhibited by anti-inflammatory steroids. However, steroids do not affect the production of bradykinin, as they are not able to inhibit the kallikrein-kininogen system (Davies et al., 1966) or the contact activation of kallikrein, as shown by our data. In our experiments, pre-treatment with dexamethasone did not change the kallikrein activity or the bradykinin levels in the synovial fluid of carrageenan-injected knee. Thus anti-inflammatory steroids can interfere at several levels with the network of carrageenan-released inflammatory mediators, including the inhibition of bradykinin effects indirectly mediated by the release of eicosanoids and/or cytokines, but they cannot control those effects due to the direct stimulation of bradykinin B2 receptors. Therefore, it appears possible that combination of an anti-inflammatory steroid, which controls the effects of carrageenan not mediated by bradykinin, with a kinin B2 receptor antagonist, which controls the direct effects of bradykinin, could provide a complete block of all carrageenan-induced inflammatory stimuli. In support of this hypothesis, a total inhibition of carrageenan-induced inflammation was obtained when MEN16132 and dexamethasone were administered in combination. This result has interesting therapeutic implications because inflammatory effects resistant to anti-inflammatory steroids do occur in pathological conditions, involving the direct activation of the kinin forming system in the joint, mediated by injury, trauma and coagulation factors (Sharma and Buchanan, 1994).
In the LPS-induced model of arthritis, MEN16132 exerted a partial analgesic effect as observed in the carrageenan model, whereas dexamethasone reached a full inhibitory effect in contrast to that observed for carrageenan-induced joint pain. These data suggest that the release of bradykinin contributed to the effects of LPS, but it was not a primary event triggering the inflammatory response, as dexamethasone would not completely inhibit the LPS-induced joint pain, because it did not prevent the production of bradykinin (as reported above). Although LPS may release bradykinin indirectly via cytokines and through the recruitment of neutrophils (Tonussi and Ferreira, 1999; Cassim et al., 2009), it failed to induce contact activation of plasma prekallikrein. Therefore, in LPS-induced arthritis, in contrast to that observed with carrageenan, dexamethasone can control the bradykinin-mediated nociceptive effect of LPS by preventing the primary inflammatory events, which leads to bradykinin release.
Interestingly, a clear synergism between MEN16132 and dexamethasone was observed as their combinations exerted inhibitory effects greater than the sum of each drug acting alone. This was particularly noticeable when effective doses of MEN16132 were co-administered with dexamethasone at doses almost ineffective by themselves, in both the arthritis models investigated. In the carrageenan-induced arthritis, the synergism was also apparent with combinations of MEN16132 with hydrocortisone or icatibant with dexamethasone. These results would imply that synergistic inhibition of joint inflammation is a general property of the combinations of kinin B2 receptor antagonists and anti-inflammatory steroids.
Several mechanisms could underlie this synergism because reciprocal reinforcements of inflammatory effects are known among bradykinin and some other mediators released by carrageenan and LPS. For example, TNF-α is able to trigger kinin formation in the joints and bradykinin can, in turn, release TNF-α generating a positive feedback loop (Tonussi and Ferreira, 1999). Moreover, TNF-α enhances the synthesis of prostaglandins in response to bradykinin and vice versa (Burch and Tiffany, 1989; Brechter and Lerner, 2007), and a synergistic interaction between bradykinin and IL-1 in stimulating prostaglandin synthesis in synovial cells has been also described (Bathon et al., 1996). Consequently, in the presence of a kinin B2 receptor antagonist, the activity of inflammatory mediators other than bradykinin, such as cytokines and prostaglandins, can be reduced as a result of the removal of the potentiation that bradykinin exerts on their effects. In this way, anti-inflammatory steroids could control arthritis symptoms at lower doses, if given together with a kinin B2 receptor antagonist.
In conclusion, our present results show that kinin B2 receptor antagonists are as effective as anti-inflammatory steroids and NSAIDs in the control of inflammatory joint pain in rats, whereas they are more potent than NSAIDs in reducing the non-algogenic components of inflammation. Moreover, the synergistic interaction between bradykinin antagonists and steroids could have interesting therapeutic implications, possibly opening the way for combination therapies in the control of inflammatory arthritis with lower doses of steroids.
Glossary
Abbreviations
- CI
combination index
- COX
cyclooxygenase
- IL
interleukin
- LPS
lipopolysaccharide
- MPO
myeloperoxidase
- NSAID
non-steroidal anti-inflammatory drug
- PBS
phosphate buffered saline
- PBS-T
PBS 0.1% Tween 20
- TNF
tumour necrosis factor
Conflicts of interest
The authors are employees of Menarini Ricerche S.p.A.
References
- Alexander SPH, Mathie A, Peters JA. Guide to Receptors and Channels (GRAC) Br J Pharmacol. (4th edn.) 2009;158(Suppl 1):S1–S254. doi: 10.1111/j.1476-5381.2011.01649_1.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bathon JM, Chilton FH, Hubbard WC, Towns MC, Solan NJ, Proud D. Mechanisms of prostanoid synthesis in human synovial cells: cytokine-peptide synergism. Inflammation. 1996;20:537–554. doi: 10.1007/BF01487045. [DOI] [PubMed] [Google Scholar]
- Bijlsma JW, Van Everdingen AA, Huisman M, De Nijs RN, Jacobs JW. Glucocorticoids in rheumatoid arthritis: effects on erosions and bone. Ann N Y Acad Sci. 2002;966:82–90. doi: 10.1111/j.1749-6632.2002.tb04205.x. [DOI] [PubMed] [Google Scholar]
- Brechter AB, Lerner UH. Characterization of bradykinin receptors in a human osteoblastic cell line. Regul Pept. 2002;103:39–51. doi: 10.1016/s0167-0115(01)00325-1. [DOI] [PubMed] [Google Scholar]
- Brechter AB, Lerner UH. Bradykinin potentiates cytokine-induced prostaglandin biosynthesis in osteoblasts by enhanced expression of cyclooxygenase 2, resulting in increased RANKL expression. Arthritis Rheum. 2007;56:910–923. doi: 10.1002/art.22445. [DOI] [PubMed] [Google Scholar]
- Burch RM, Tiffany CW. Tumor necrosis factor causes amplification of arachidonic acid metabolism in response to interleukin 1, bradykinin, and other agonists. J Cell Physiol. 1989;141:85–89. doi: 10.1002/jcp.1041410113. [DOI] [PubMed] [Google Scholar]
- Cambridge H, Brain SD. Mechanism of bradykinin-induced plasma extravasation in the rat knee joint. Br J Pharmacol. 1995;115:641–647. doi: 10.1111/j.1476-5381.1995.tb14980.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassim B, Shaw OM, Mazur M, Misso NL, Naran A, Laglands DR, et al. Kallikreins, kininogens and kinin receptors on circulating and synovial neutrophils: role in kinin generation in rheumatoid arthritis. Rheumatology. 2009;48:490–496. doi: 10.1093/rheumatology/kep016. [DOI] [PubMed] [Google Scholar]
- Cheng JK, Ji RR. Intracellular signaling in primary sensory neurons and persistent pain. Neurochem Res. 2008;33:1970–1978. doi: 10.1007/s11064-008-9711-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chou TC, Talalay P. Quantitative analysis of dose-effect relationships: the combined effects of multiple drugs or enzyme inhibitors. Adv Enzyme Regul. 1984;22:27–55. doi: 10.1016/0065-2571(84)90007-4. [DOI] [PubMed] [Google Scholar]
- Cialdai C, Giuliani S, Valenti C, Tramontana M, Maggi CA. Effect of intra-articular MEN16132, a kinin B2 receptor antagonist, on nociceptive response in monosodium iodoacetate-induced experimental osteoarthritis in rats. J Pharmacol Exp Ther. 2009;331:1025–1032. doi: 10.1124/jpet.109.159657. [DOI] [PubMed] [Google Scholar]
- Colman RW. Regulation of angiogenesis by the kallikrein-kinin system. Curr Pharm Des. 2006;12:2599–2607. doi: 10.2174/138161206777698710. [DOI] [PubMed] [Google Scholar]
- Cucchi P, Meini S, Bressan A, Catalani C, Bellucci F, Santicioli P, et al. MEN16132, a novel potent and selective nonpeptide antagonist for the human bradykinin B2 receptor. In vitro pharmacology and molecular characterization. Eur J Pharmacol. 2005;528:7–16. doi: 10.1016/j.ejphar.2005.10.014. [DOI] [PubMed] [Google Scholar]
- Cunha FQ, Poole S, Lorenzetti BB, Ferreira SH. The pivotal role of tumour necrosis factor α in the development of inflammatory hyperalgesia. Br J Pharmacol. 1992;107:660–664. doi: 10.1111/j.1476-5381.1992.tb14503.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damas J, Remacle-Volon G. Influence of a long-acting bradykinin antagonist, Hoe 140, on some acute inflammatory reactions in the rat. Eur J Pharmacol. 1992;211:81–86. doi: 10.1016/0014-2999(92)90266-7. [DOI] [PubMed] [Google Scholar]
- Davies GE, Holman G, Johnston TP, Lowe JS. Studies on kallikrein: failure of some anti-inflammatory drugs to affect release of kinin. Br J Pharmacol Chemother. 1966;28:212–217. doi: 10.1111/j.1476-5381.1966.tb01887.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dray A, Read SJ. Arthritis and pain. Future targets to control osteoarthritis pain. Arthritis Res Ther. 2007;9:212. doi: 10.1186/ar2178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earp JC, DuBois DC, Molano DS, Pyszczynski NA, Almon RR, Jusko WJ. Modeling corticosteroid effects in a rat model of rheumatoid arthritis II: mechanistic pharmacodynamic model for dexamethasone effects in Lewis rats with collagen-induced arthritis. J Pharmacol Exp Ther. 2008;326:546–554. doi: 10.1124/jpet.108.137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferreira SH, Lorenzetti BB, Poole S. Bradykinin initiates cytokine-mediated inflammatory hyperalgesia. Br J Pharmacol. 1993;110:1227–1231. doi: 10.1111/j.1476-5381.1993.tb13946.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Francischi JN, Chaves CT, Moura ACL, Lima AS, Rocha OA, Ferreira-Alves DL, et al. Selective inhibitors of cyclo-oxygenase-2 (COX-2) induce hypoalgesia in a rat paw model of inflammation. Br J Pharmacol. 2002;137:837–844. doi: 10.1038/sj.bjp.0704937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geiger R, Miska W. Human tissue kallikrein. Methods Enzymol. 1988;163:120–115. doi: 10.1016/0076-6879(88)63012-6. [DOI] [PubMed] [Google Scholar]
- Jenzano JW, Coffey JC, Heizer WD, Lundblad RL, Scicli AG. The assay of glandular kallikrein and prekallikrein in human mixed saliva. Arch Oral Biol. 1988;33:641–644. doi: 10.1016/0003-9969(88)90117-3. [DOI] [PubMed] [Google Scholar]
- Kamal AM, Flower RJ, Perretti M. An overview of the effects of annexin 1 on cells involved in the inflammatory process. Mem Inst Oswaldo Cruz. 2005;100:39–48. doi: 10.1590/s0074-02762005000900008. [DOI] [PubMed] [Google Scholar]
- Leeb-Lundberg LMF, Marceau F, Müller-Esterl W, Pettibone DJ, Zuraw BL. Classification of the kinin receptor family: from molecular mechanisms to pathophysiological consequences. Pharmacol Rev. 2005;57:27–77. doi: 10.1124/pr.57.1.2. [DOI] [PubMed] [Google Scholar]
- Lo EJ, Green PG, Miao FJ, Relchling DB, Levine JD. Bradykinin-induced neurogenic migration of neutrophils into the rat knee joint. Neuroreport. 1999;10:3821–3824. doi: 10.1097/00001756-199912160-00018. [DOI] [PubMed] [Google Scholar]
- Majima M, Kawashima N, Hiroshi I, Katori M. Effects of an orally active non-peptide bradykinin B2 receptor antagonist, FR173657, on plasma exudation in rat carrageenin-induced pleurisy. Br J Pharmacol. 1997;121:723–730. doi: 10.1038/sj.bjp.0701194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meini S, Maggi CA. Knee osteoarthritis: a role for bradykinin? Inflamm Res. 2008;57:351–361. doi: 10.1007/s00011-007-7204-1. [DOI] [PubMed] [Google Scholar]
- Meini S, Bellucci F, Catalani C, Cucchi P, Giuliani S, Maggi CA. Bradykinin and B2 receptor antagonism in human knee synoviocytes and chondrocytes. Osteoarthritis Cartilage. 2008;16(Suppl 4):S188. [Google Scholar]
- Nishimura M, Segami N, Kaneyama K, Suzuki T, Miyamaru M. Relationships between pain-related mediators and both synovitis and joint pain in patients with internal derangements and osteoarthritis of the temporomandibular joint. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2002;94:328–332. doi: 10.1067/moe.2002.124106. [DOI] [PubMed] [Google Scholar]
- Pawlak M, Borkiewicz P, Podgòrski T, Schmidt RF. The activity of fine afferent nerve fibres of the rat knee joint and their modulation by inflammatory mediators. Ortop Traumatol Rehabil. 2008;10:63–74. [PubMed] [Google Scholar]
- Poole S, Lorenzetti BB, Cunha JM, Cunha FQ, Ferreira SH. Bradykinin B1 and B2 receptors, tumor necrosis factor α and inflammatory hyperalgesia. Br J Pharmacol. 1999;126:649–656. doi: 10.1038/sj.bjp.0702347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahman MM, Bhoola KD, Elson CJ, Lemon M, Dieppe PA. Identification and functional importance of plasma kallikrein in the synovial fluids of patients with rheumatoid, psoriatic, and osteoarthritis. Ann Rheum Dis. 1995;54:345–350. doi: 10.1136/ard.54.5.345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider T, Issekutz AC. Quantitation of eosinophil and neutrophil infiltration into rat lung by specific assays for eosinophil peroxidase and myeloperoxidase. Application in a Brown Norway rat model of allergic pulmonary inflammation. J Immunol Methods. 1996;198:1–14. doi: 10.1016/0022-1759(96)00143-3. [DOI] [PubMed] [Google Scholar]
- Sharma JN, Buchanan WW. Pathogenic responses of bradykinin system in chronic inflammatory rheumatoid disease. Exp Toxicol Pathol. 1994;46:421–433. doi: 10.1016/S0940-2993(11)80053-9. [DOI] [PubMed] [Google Scholar]
- Sharma JN, Wirth KJ. Inhibition of rats adjuvant arthritis by a bradykinin antagonist Hoe 140 and its influence on kallikreins. Gen Pharmacol. 1996;27:133–136. doi: 10.1016/0306-3623(95)00078-x. [DOI] [PubMed] [Google Scholar]
- Song IH, Althoff CE, Hermann K, Scheel AK, Knetsch T, Burmester G, et al. Contrast-enhanced ultrasound in monitoring the efficacy of a bradykinin receptor-2 antagonist in painful knee osteoarthritis compared to magnetic resonance imaging. Ann Rheum Dis. 2008;68:75–83. doi: 10.1136/ard.2007.080382. [DOI] [PubMed] [Google Scholar]
- Sorbera LA, Fernandez-Forner D, Bayes M. Icatibant acetate. Drugs Future. 2006;31:101–106. [Google Scholar]
- Tonussi CR, Ferreira SH. Tumor necrosis factor-α mediates carrageenin-induced knee-joint incapacitation and also triggers overt nociception in previously inflamed rat knee-joints. Pain. 1999;82:81–87. doi: 10.1016/S0304-3959(99)00035-4. [DOI] [PubMed] [Google Scholar]
- Valenti C, Cialdai C, Giuliani S, Lecci A, Tramontana M, Meini S, et al. MEN16132, a novel potent and selective nonpeptide kinin B2 receptor antagonist: in vivo activity on bradykinin-induced bronchoconstriction and nasal mucosa microvascular leakage in anesthetized guinea pigs. J Pharmacol Exp Ther. 2005;315:616–623. doi: 10.1124/jpet.105.088252. [DOI] [PubMed] [Google Scholar]
- Yang Y, Hutchinson P, Morand EF. Inhibitory effect of annexin I on synovial inflammation in rat adjuvant arthritis. Arthritis Rheum. 1999;42:1538–1544. doi: 10.1002/1529-0131(199907)42:7<1538::AID-ANR29>3.0.CO;2-3. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Adner M, Cardell LO. Glucocorticoids suppress transcriptional up-regulation of bradykinin receptors in a murine in vitro model of chronic airway inflammation. Clin Exp Allergy. 2005;35:531–538. doi: 10.1111/j.1365-2222.2005.02207.x. [DOI] [PubMed] [Google Scholar]
- Zhang W, Moskowitz RW, Nuki G, Abramson S, Altman RD, Arden N, et al. OARSI recommendations for the management of hip and knee osteoarthritis, Part I: critical appraisal of existing treatment guidelines and systematic review of current research evidence. Osteoarthritis Cartilage. 2007;15:981–1000. doi: 10.1016/j.joca.2007.06.014. [DOI] [PubMed] [Google Scholar]


