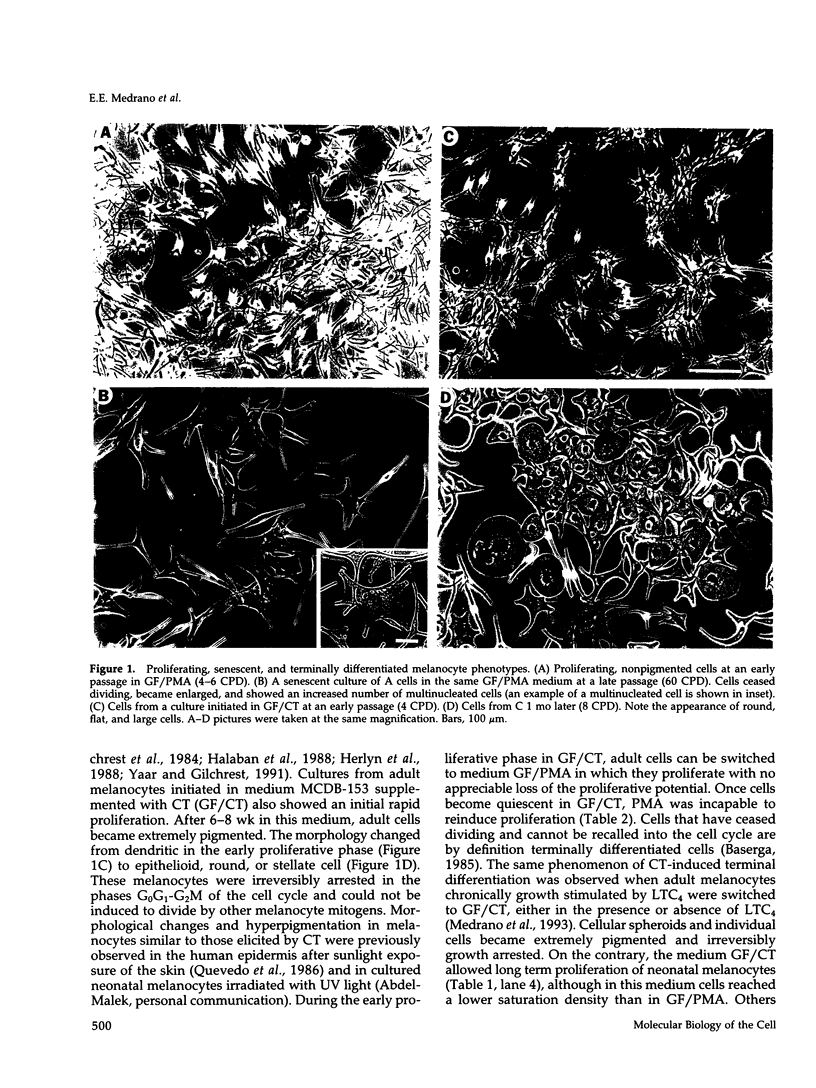
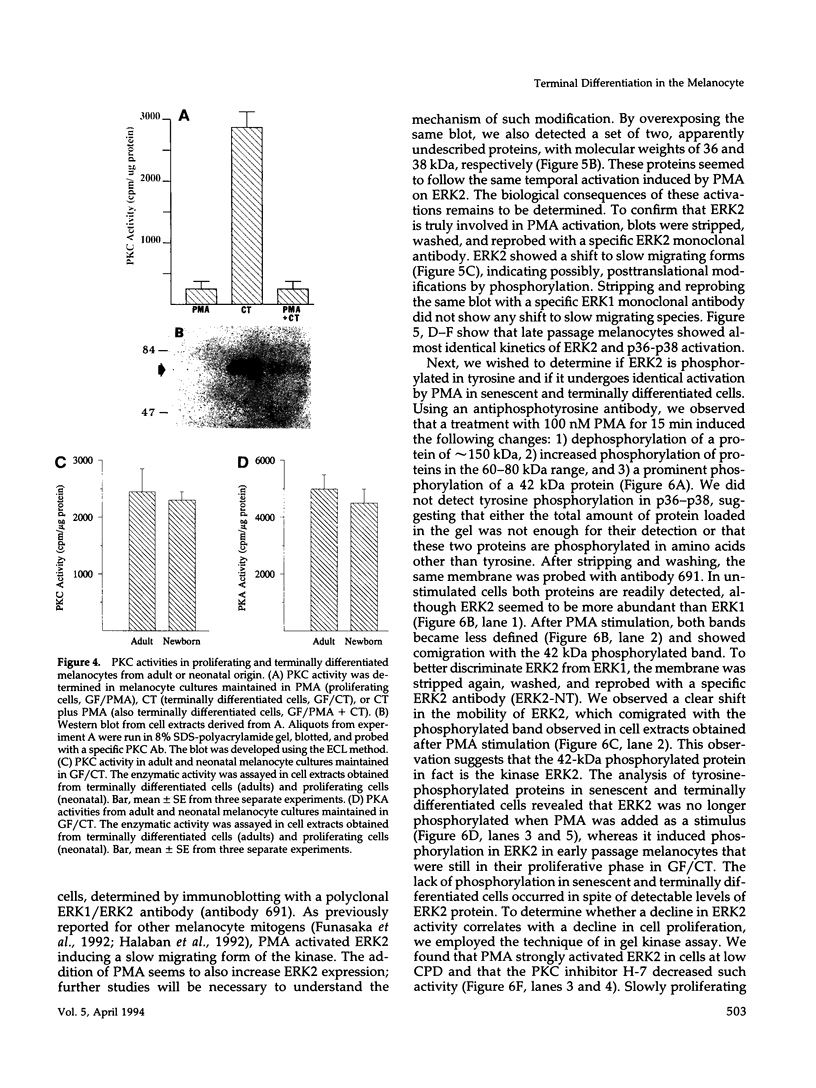
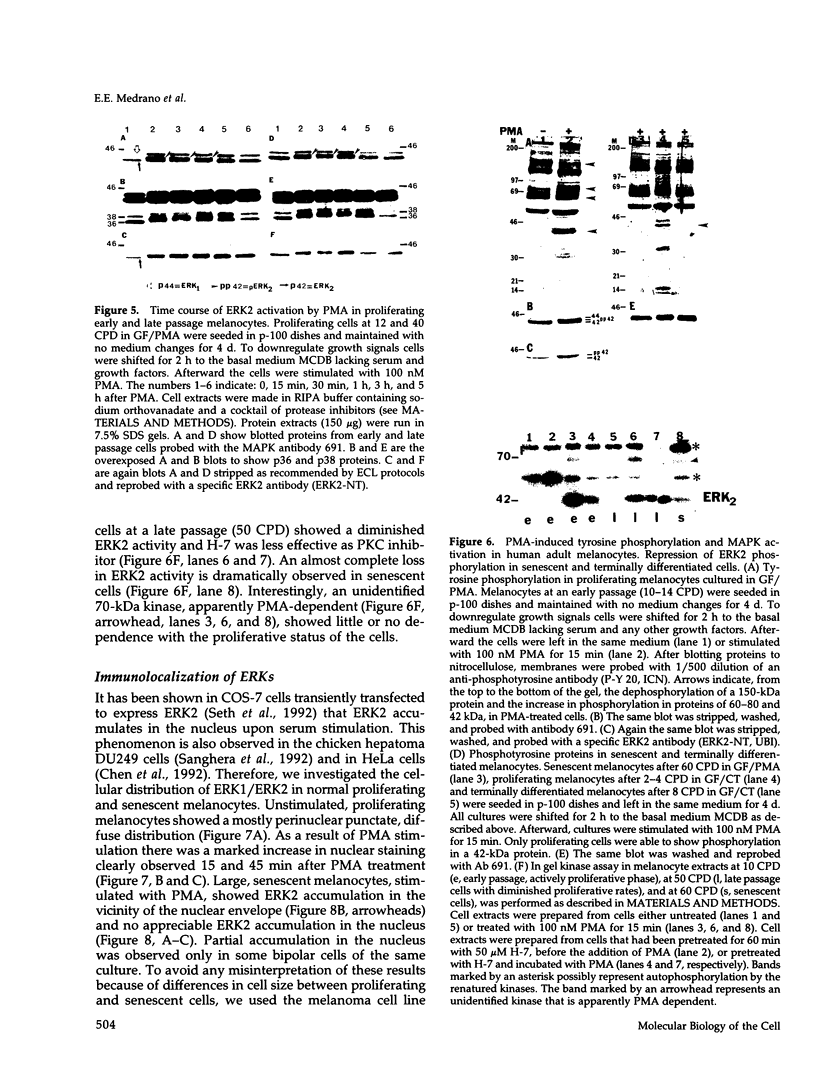
Abstract
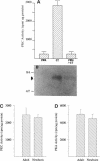
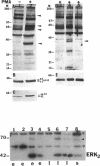
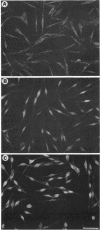
Melanocytes are pigmented cells distributed in humans in several organs like the epidermis, the leptomeninges, the eye, and the inner ear. Epidermal melanocytes, whether derived from adult or neonatal skin, proliferate well in a medium supplemented with phorbol esters and other mitogens before they undergo senescence. Potent cAMP inducers like cholera toxin are also growth promoters for neonatal melanocytes but only transient growth stimulators for cells derived from adults. We used this cellular system to delineate biochemical pathways involved in proliferation and in terminal differentiation. Here we show that after a period of 4-8 wk of sustained proliferation in the presence of cholera toxin, the adult melanocytes became round, flat, and enlarged. These changes were associated with terminal growth and preceded by a five- to sixfold increase in cAMP levels and an 8- to 10-fold increase in melanin content. The simultaneous addition of phorbol esters and cholera toxin did not prevent cells from reaching terminal differentiation. Identified targets for phorbol esters are protein kinase C (PKC) and the mitogen-activated kinases (MAPKs), also called extracellular signal-regulated kinases (ERKs). PKC was found to be similarly regulated in proliferating and in terminally differentiated melanocytes. Proliferating melanocytes in early or late passage showed identical activation of the kinase ERK2. This kinase was rapidly phosphorylated upon phorbol 12-myristate 13-acetate (PMA) addition and specifically accumulated in the nucleus of the cells, whereas in unstimulated cells it had a perinuclear distribution. In contrast, senescent and terminally differentiated cells were unable to phosphorylate tyrosine residues of the ERK2 gene product in spite of presenting normal amounts of ERK2 protein. In addition, ERK2 did not show the nuclear accumulation observed in proliferating melanocytes after PMA activation and remained localized in the perinuclear area. These results demonstrate that senescent and terminally differentiated melanocytes share a common block in a critical pathway thought to integrate multiple intracellular signals transmitted by various second messengers and specifically prevent the continuation of the signal transduction cascade initiated by PMA activation of PKC.
Full text
PDF




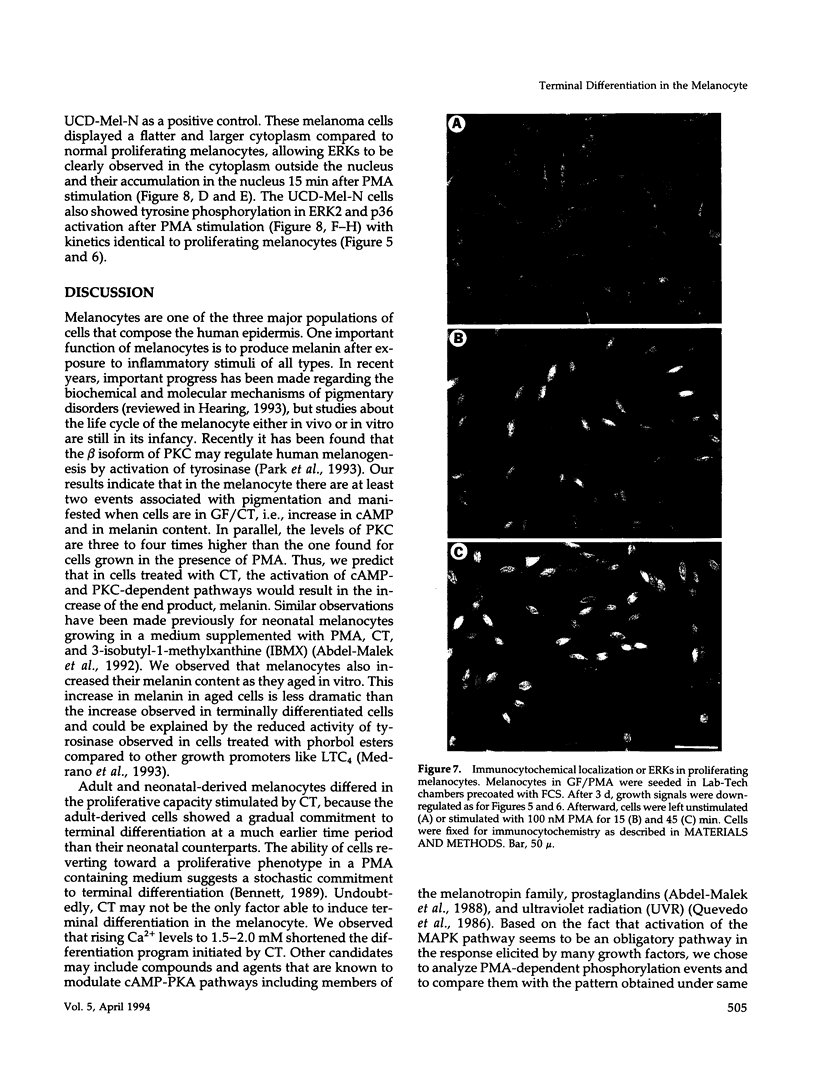
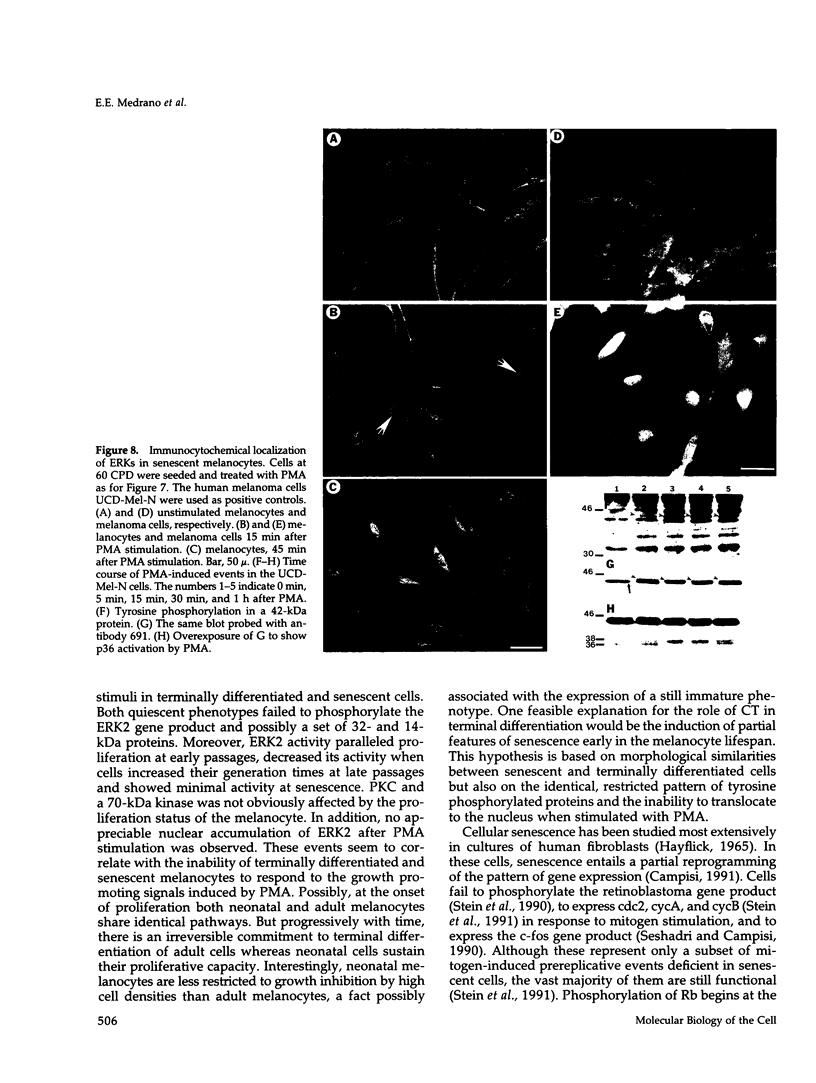



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdel-Malek Z. A., Swope V. B., Trinkle L. S., Ferroni E. N., Boissy R. E., Nordlund J. J. Alteration of the Cloudman melanoma cell cycle by prostaglandins E1 and E2 determined by using a 5-bromo-2'-deoxyuridine method of DNA analysis. J Cell Physiol. 1988 Aug;136(2):247–256. doi: 10.1002/jcp.1041360206. [DOI] [PubMed] [Google Scholar]
- Abdel-Malek Z., Swope V. B., Pallas J., Krug K., Nordlund J. J. Mitogenic, melanogenic, and cAMP responses of cultured neonatal human melanocytes to commonly used mitogens. J Cell Physiol. 1992 Feb;150(2):416–425. doi: 10.1002/jcp.1041500226. [DOI] [PubMed] [Google Scholar]
- Arita Y., O'Driscoll K. R., Weinstein I. B. Growth of human melanocyte cultures supported by 12-O-tetradecanoylphorbol-13-acetate is mediated through protein kinase C activation. Cancer Res. 1992 Aug 15;52(16):4514–4521. [PubMed] [Google Scholar]
- Bennett D. C. Mechanisms of differentiation in melanoma cells and melanocytes. Environ Health Perspect. 1989 Mar;80:49–59. doi: 10.1289/ehp.898049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boissy R. E., Liu Y. Y., Medrano E. E., Nordlund J. J. Structural aberration of the rough endoplasmic reticulum and melanosome compartmentalization in long-term cultures of melanocytes from vitiligo patients. J Invest Dermatol. 1991 Sep;97(3):395–404. doi: 10.1111/1523-1747.ep12480976. [DOI] [PubMed] [Google Scholar]
- Boulton T. G., Nye S. H., Robbins D. J., Ip N. Y., Radziejewska E., Morgenbesser S. D., DePinho R. A., Panayotatos N., Cobb M. H., Yancopoulos G. D. ERKs: a family of protein-serine/threonine kinases that are activated and tyrosine phosphorylated in response to insulin and NGF. Cell. 1991 May 17;65(4):663–675. doi: 10.1016/0092-8674(91)90098-j. [DOI] [PubMed] [Google Scholar]
- Breathnach A. S., Nazzaro-Porro M., Passi S., Picardo M. Ultrastructure of melanocytes in chronically sun-exposed skin of elderly subjects. Pigment Cell Res. 1991 Mar;4(2):71–79. doi: 10.1111/j.1600-0749.1991.tb00318.x. [DOI] [PubMed] [Google Scholar]
- Brooks G., Wilson R. E., Dooley T. P., Goss M. W., Hart I. R. Protein kinase C down-regulation, and not transient activation, correlates with melanocyte growth. Cancer Res. 1991 Jun 15;51(12):3281–3288. [PubMed] [Google Scholar]
- Burchill S. A., Marks J. M., Thody A. J. Tyrosinase synthesis in different skin types and the effects of alpha-melanocyte-stimulating hormone and cyclic AMP. J Invest Dermatol. 1990 Nov;95(5):558–561. doi: 10.1111/1523-1747.ep12504908. [DOI] [PubMed] [Google Scholar]
- Cantley L. C., Auger K. R., Carpenter C., Duckworth B., Graziani A., Kapeller R., Soltoff S. Oncogenes and signal transduction. Cell. 1991 Jan 25;64(2):281–302. doi: 10.1016/0092-8674(91)90639-g. [DOI] [PubMed] [Google Scholar]
- Chao-Hsing K. A., Hsin-Su Y. U. A study of the effects of phorbol 12-myristate-13-acetate on cell differentiation of pure human melanocytes in vitro. Arch Dermatol Res. 1991;283(2):119–124. doi: 10.1007/BF00371620. [DOI] [PubMed] [Google Scholar]
- Chen R. H., Sarnecki C., Blenis J. Nuclear localization and regulation of erk- and rsk-encoded protein kinases. Mol Cell Biol. 1992 Mar;12(3):915–927. doi: 10.1128/mcb.12.3.915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobb M. H., Boulton T. G., Robbins D. J. Extracellular signal-regulated kinases: ERKs in progress. Cell Regul. 1991 Dec;2(12):965–978. doi: 10.1091/mbc.2.12.965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eisinger M., Marko O. Selective proliferation of normal human melanocytes in vitro in the presence of phorbol ester and cholera toxin. Proc Natl Acad Sci U S A. 1982 Mar;79(6):2018–2022. doi: 10.1073/pnas.79.6.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erickson C. A. From the crest to the periphery: control of pigment cell migration and lineage segregation. Pigment Cell Res. 1993 Oct;6(5):336–347. doi: 10.1111/j.1600-0749.1993.tb00611.x. [DOI] [PubMed] [Google Scholar]
- Funasaka Y., Boulton T., Cobb M., Yarden Y., Fan B., Lyman S. D., Williams D. E., Anderson D. M., Zakut R., Mishima Y. c-Kit-kinase induces a cascade of protein tyrosine phosphorylation in normal human melanocytes in response to mast cell growth factor and stimulates mitogen-activated protein kinase but is down-regulated in melanomas. Mol Biol Cell. 1992 Feb;3(2):197–209. doi: 10.1091/mbc.3.2.197. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilchrest B. A., Vrabel M. A., Flynn E., Szabo G. Selective cultivation of human melanocytes from newborn and adult epidermis. J Invest Dermatol. 1984 Nov;83(5):370–376. doi: 10.1111/1523-1747.ep12264638. [DOI] [PubMed] [Google Scholar]
- Gille H., Sharrocks A. D., Shaw P. E. Phosphorylation of transcription factor p62TCF by MAP kinase stimulates ternary complex formation at c-fos promoter. Nature. 1992 Jul 30;358(6385):414–417. doi: 10.1038/358414a0. [DOI] [PubMed] [Google Scholar]
- Gordon P. R., Gilchrest B. A. Human melanogenesis is stimulated by diacylglycerol. J Invest Dermatol. 1989 Nov;93(5):700–702. doi: 10.1111/1523-1747.ep12319900. [DOI] [PubMed] [Google Scholar]
- Gotoh Y., Nishida E., Matsuda S., Shiina N., Kosako H., Shiokawa K., Akiyama T., Ohta K., Sakai H. In vitro effects on microtubule dynamics of purified Xenopus M phase-activated MAP kinase. Nature. 1991 Jan 17;349(6306):251–254. doi: 10.1038/349251a0. [DOI] [PubMed] [Google Scholar]
- HAYFLICK L. THE LIMITED IN VITRO LIFETIME OF HUMAN DIPLOID CELL STRAINS. Exp Cell Res. 1965 Mar;37:614–636. doi: 10.1016/0014-4827(65)90211-9. [DOI] [PubMed] [Google Scholar]
- Halaban R., Langdon R., Birchall N., Cuono C., Baird A., Scott G., Moellmann G., McGuire J. Basic fibroblast growth factor from human keratinocytes is a natural mitogen for melanocytes. J Cell Biol. 1988 Oct;107(4):1611–1619. doi: 10.1083/jcb.107.4.1611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halaban R., Rubin J. S., Funasaka Y., Cobb M., Boulton T., Faletto D., Rosen E., Chan A., Yoko K., White W. Met and hepatocyte growth factor/scatter factor signal transduction in normal melanocytes and melanoma cells. Oncogene. 1992 Nov;7(11):2195–2206. [PubMed] [Google Scholar]
- Hearing V. J. Unraveling the melanocyte. Am J Hum Genet. 1993 Jan;52(1):1–7. [PMC free article] [PubMed] [Google Scholar]
- Herlyn M., Mancianti M. L., Jambrosic J., Bolen J. B., Koprowski H. Regulatory factors that determine growth and phenotype of normal human melanocytes. Exp Cell Res. 1988 Dec;179(2):322–331. doi: 10.1016/0014-4827(88)90271-6. [DOI] [PubMed] [Google Scholar]
- Howe L. R., Leevers S. J., Gómez N., Nakielny S., Cohen P., Marshall C. J. Activation of the MAP kinase pathway by the protein kinase raf. Cell. 1992 Oct 16;71(2):335–342. doi: 10.1016/0092-8674(92)90361-f. [DOI] [PubMed] [Google Scholar]
- Imokawa G., Yada Y., Miyagishi M. Endothelins secreted from human keratinocytes are intrinsic mitogens for human melanocytes. J Biol Chem. 1992 Dec 5;267(34):24675–24680. [PubMed] [Google Scholar]
- Kameshita I., Fujisawa H. A sensitive method for detection of calmodulin-dependent protein kinase II activity in sodium dodecyl sulfate-polyacrylamide gel. Anal Biochem. 1989 Nov 15;183(1):139–143. doi: 10.1016/0003-2697(89)90181-4. [DOI] [PubMed] [Google Scholar]
- Lange-Carter C. A., Pleiman C. M., Gardner A. M., Blumer K. J., Johnson G. L. A divergence in the MAP kinase regulatory network defined by MEK kinase and Raf. Science. 1993 Apr 16;260(5106):315–319. doi: 10.1126/science.8385802. [DOI] [PubMed] [Google Scholar]
- Lin B. T., Wang J. Y. Cell cycle regulation of retinoblastoma protein phosphorylation. Ciba Found Symp. 1992;170:227–243. [PubMed] [Google Scholar]
- Matsumoto K., Tajima H., Nakamura T. Hepatocyte growth factor is a potent stimulator of human melanocyte DNA synthesis and growth. Biochem Biophys Res Commun. 1991 Apr 15;176(1):45–51. doi: 10.1016/0006-291x(91)90887-d. [DOI] [PubMed] [Google Scholar]
- Medrano E. E., Farooqui J. Z., Boissy R. E., Boissy Y. L., Akadiri B., Nordlund J. J. Chronic growth stimulation of human adult melanocytes by inflammatory mediators in vitro: implications for nevus formation and initial steps in melanocyte oncogenesis. Proc Natl Acad Sci U S A. 1993 Mar 1;90(5):1790–1794. doi: 10.1073/pnas.90.5.1790. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medrano E. E., Nordlund J. J. Successful culture of adult human melanocytes obtained from normal and vitiligo donors. J Invest Dermatol. 1990 Oct;95(4):441–445. [PubMed] [Google Scholar]
- Moodie S. A., Willumsen B. M., Weber M. J., Wolfman A. Complexes of Ras.GTP with Raf-1 and mitogen-activated protein kinase kinase. Science. 1993 Jun 11;260(5114):1658–1661. doi: 10.1126/science.8503013. [DOI] [PubMed] [Google Scholar]
- Morelli J. G., Hake S. S., Murphy R. C., Norris D. A. Leukotriene B4-induced human melanocyte pigmentation and leukotriene C4-induced human melanocyte growth are inhibited by different isoquinolinesulfonamides. J Invest Dermatol. 1992 Jan;98(1):55–58. doi: 10.1111/1523-1747.ep12494602. [DOI] [PubMed] [Google Scholar]
- Müller R., Bravo R., Burckhardt J., Curran T. Induction of c-fos gene and protein by growth factors precedes activation of c-myc. Nature. 1984 Dec 20;312(5996):716–720. doi: 10.1038/312716a0. [DOI] [PubMed] [Google Scholar]
- Nishizuka Y. Intracellular signaling by hydrolysis of phospholipids and activation of protein kinase C. Science. 1992 Oct 23;258(5082):607–614. doi: 10.1126/science.1411571. [DOI] [PubMed] [Google Scholar]
- Nordlund J. J. The lives of pigment cells. Dermatol Clin. 1986 Jul;4(3):407–418. [PubMed] [Google Scholar]
- Pagès G., Lenormand P., L'Allemain G., Chambard J. C., Meloche S., Pouysségur J. Mitogen-activated protein kinases p42mapk and p44mapk are required for fibroblast proliferation. Proc Natl Acad Sci U S A. 1993 Sep 15;90(18):8319–8323. doi: 10.1073/pnas.90.18.8319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park H. Y., Campisi J. Posttranslational control of cyclic AMP-dependent protein kinase by phorbol ester in normal but not in chemically transformed 3T3 cells. Cancer Res. 1990 Nov 15;50(22):7145–7152. [PubMed] [Google Scholar]
- Pittelkow M. R., Shipley G. D. Serum-free culture of normal human melanocytes: growth kinetics and growth factor requirements. J Cell Physiol. 1989 Sep;140(3):565–576. doi: 10.1002/jcp.1041400323. [DOI] [PubMed] [Google Scholar]
- Rose D. W., McCabe G., Feramisco J. R., Adler M. Expression of c-fos and AP-1 activity in senescent human fibroblasts is not sufficient for DNA synthesis. J Cell Biol. 1992 Dec;119(6):1405–1411. doi: 10.1083/jcb.119.6.1405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rozengurt E., Erusalimsky J., Mehmet H., Morris C., Nånberg E., Sinnett-Smith J. Signal transduction in mitogenesis: further evidence for multiple pathways. Cold Spring Harb Symp Quant Biol. 1988;53(Pt 2):945–954. doi: 10.1101/sqb.1988.053.01.109. [DOI] [PubMed] [Google Scholar]
- Sanghera J. S., Peter M., Nigg E. A., Pelech S. L. Immunological characterization of avian MAP kinases: evidence for nuclear localization. Mol Biol Cell. 1992 Jul;3(7):775–787. doi: 10.1091/mbc.3.7.775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seshadri T., Campisi J. Repression of c-fos transcription and an altered genetic program in senescent human fibroblasts. Science. 1990 Jan 12;247(4939):205–209. doi: 10.1126/science.2104680. [DOI] [PubMed] [Google Scholar]
- Seth A., Gonzalez F. A., Gupta S., Raden D. L., Davis R. J. Signal transduction within the nucleus by mitogen-activated protein kinase. J Biol Chem. 1992 Dec 5;267(34):24796–24804. [PubMed] [Google Scholar]
- Stein G. H., Beeson M., Gordon L. Failure to phosphorylate the retinoblastoma gene product in senescent human fibroblasts. Science. 1990 Aug 10;249(4969):666–669. doi: 10.1126/science.2166342. [DOI] [PubMed] [Google Scholar]
- Stein G. H., Drullinger L. F., Robetorye R. S., Pereira-Smith O. M., Smith J. R. Senescent cells fail to express cdc2, cycA, and cycB in response to mitogen stimulation. Proc Natl Acad Sci U S A. 1991 Dec 15;88(24):11012–11016. doi: 10.1073/pnas.88.24.11012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sun H., Charles C. H., Lau L. F., Tonks N. K. MKP-1 (3CH134), an immediate early gene product, is a dual specificity phosphatase that dephosphorylates MAP kinase in vivo. Cell. 1993 Nov 5;75(3):487–493. doi: 10.1016/0092-8674(93)90383-2. [DOI] [PubMed] [Google Scholar]
- Tassabehji M., Read A. P., Newton V. E., Harris R., Balling R., Gruss P., Strachan T. Waardenburg's syndrome patients have mutations in the human homologue of the Pax-3 paired box gene. Nature. 1992 Feb 13;355(6361):635–636. doi: 10.1038/355635a0. [DOI] [PubMed] [Google Scholar]
- Urabe K., Aroca P., Hearing V. J. From gene to protein: determination of melanin synthesis. Pigment Cell Res. 1993 Aug;6(4 Pt 1):186–192. doi: 10.1111/j.1600-0749.1993.tb00601.x. [DOI] [PubMed] [Google Scholar]