Abstract
BACKGROUND AND PURPOSE
Haem oxygenase 1 (HO-1) is an inducible protein that plays a major protective role in conditions such as ischaemia-reperfusion injury and inflammation. In this study, we have investigated the role of haem arginate (HA) in human male subjects in the modulation of HO-1 expression and its correlation with the GT length polymorphism (GTn) in the promoter of the HO-1 gene.
EXPERIMENTAL APPROACH
In a dose-escalation, randomized, placebo-controlled trial, seven healthy male subjects with a homozygous short (S/S) and eight with a long (L/L) GTn genotype received intravenous HA. HO-1 protein expression and mRNA levels in peripheral blood monocytes, bilirubin, haptoglobin, haemopexin and haem levels were analysed over a 48 h observation period.
KEY RESULTS
We found that the baseline mRNA levels of HO-1 were higher in L/L subjects, while protein levels were higher in S/S subjects. HA induced a dose-dependent increase in the baseline corrected area under the curve values of HO-1 mRNA and protein over 48 h. The response of HO-1 mRNA was more pronounced in L/L subjects but the protein level was similar across the groups.
CONCLUSIONS AND IMPLICATION
HA is an effective inducer of HO-1 in humans irrespective of the GTn genotype. The potential therapeutic application of HA needs to be evaluated in clinical trials.
Keywords: haem oxygenase, ischaemia-reperfusion injury, healthy volunteers, polymorphism
Introduction
Haem oxygenase (HO) is the rate-limiting enzyme that catalyzes the degradation of haem b (Fe-protoporphyrin-IX) into carbon monoxide (CO), biliverdin (which is rapidly converted to bilirubin) and free iron (Fe2+, which stimulates the induction of the iron-binding compound ferritin). In humans, two genetically distinct isoenzymes of HO have been characterized: a constitutively expressed form (HO-2) and an inducible form (HO-1) (Ryter et al., 2006). HO-1 is a member of the heat-shock protein family (HSP 32) that is expressed in several organs (e.g. spleen and liver) and cell types, including endothelial, epithelial, mononuclear and smooth muscle cells (Otterbein et al., 2003). HO-1 is induced by a variety of stimuli, including oxidative stress. Up-regulation of HO-1 is mostly dependent on transcriptional modulation of the HO-1 gene. HO-1 has emerged as a major ‘protective’ gene, that is, a gene that when expressed restores homeostasis in many situations by its anti-inflammatory, anti-apoptotic and anti-proliferative actions (Otterbein et al., 2003). The suggested use of HO-1 as a therapeutic target is based on these attributes. The cytoprotective action of HO-1 is mediated in part by its three products, CO, ferritin and biliverdin/bilirubin, which are partly overlapping in their mechanisms and effects.
The GT length polymorphism (GTn) dinucleotide repeat polymorphism is specific for humans and has been identified in the proximal promoter region of the HO-1 gene (Lavrovsky et al., 1994). This GTn repeat is highly polymorphic and modulates gene transcription by means of oxidative challenge (Yamada et al., 2000). It has been shown in vitro that a longer GTn repeat corresponds to lower transcriptional activity of the HO-1 promoter (Chen et al., 2002; Hirai et al., 2003; Rueda et al., 2007) and is associated with a susceptibility to various diseases (Exner et al., 2004), such as coronary artery disease (Chen et al., 2002; 2008;), aortic aneurysm (Schillinger et al., 2002), emphysema (Yamada et al., 2000), pneumonia (Yasuda et al., 2006), asthma (Islam et al., 2008), idiopathic recurrent miscarriage (Denschlag et al., 2004), rheumatoid arthritis (Rueda et al., 2007) and cerebral malaria (Takeda et al., 2005). The GTn repeats show a bimodal distribution, with peaks at 22–23 and 27–30 repeats depending on the cohort. Alleles are usually classified into two subgroups based on the number of GTn repeats: the shorter component (<25 or <27 repeats), which is designated as ‘class S’, and the upper component (≥25 or ≥27), also known as ‘class L’.
It has been shown that haem acts as an inducer of HO-1 in human cells, including macrophages, in vitro (Yoshida et al., 1988; Salahudeen et al., 2001; Nakaso et al., 2003; Devadas and Dhawan, 2006; Jazwa et al., 2006). The molecular steps and signal transduction pathways underlying HO-1 up-regulation in general, and by haem in particular, remain largely undefined. A recent study in humans showed that the administration of 3 mg kg−1 hemin (Panhematin) increased the plasma level of HO-1 (Bharucha et al., 2010). However, no studies have been done so far in humans to investigate the responsiveness of HO-1 to haem arginate (HA) and the influence of the human specific GTn polymorphism on HO-1 induction. HA is a ferriporphyrin with less vasculotoxic and thrombotic side effects than other hemin preparations (Balla et al., 2000), and it is an approved treatment (in Europe and South Africa) for acute porphyric attacks (Mustajoki and Nordmann, 1993). In animal studies, HA has protective effects against oxidative stress, which are probably mediated by HO-1 induction (Kubulus et al., 2005; 2008; Maeshima et al., 2005; Sasaki et al., 2006; Jadhav and Ndisang, 2009). No human studies with HA have been done thus far in this field. Currently, a study is investigating HA in non-ST-elevating myocardial infarction (clinicaltrials.gov registration number: NCT00483587, http://clinicaltrials.gov/ct2/show/NCT00682370?term=HEMAHS&rank=1).
Hence, the aim of this study was to demonstrate the ability of intravenous HA to induce HO-1 in humans in vivo and to investigate the role of the GTn polymorphism.
Methods
Subjects
Following approval of the study protocol by the Ethics Commission of the Medical University of Vienna and after written informed consent was obtained, 132 healthy white European male subjects were screened for the GTn in the promoter region of the HO-1 gene. Eight subjects who were homozygous for the long GTn and seven subjects who were homozygous for the short GTn (for classification, see next) were exposed to haem infusion in a clinical study. These subjects were aged between 22 and 43 years, weighed between 61 and 91 kg (see Table 1), and were non-smokers and drug free. Each participant passed a screening examination that included medical history, a physical examination, vital sign measurement, a 12-lead electrocardiogram, laboratory tests, drug screening, and a urine test strip between 3 and 14 days before the first drug infusion. Studies were performed after an overnight fast in rooms with an ambient temperature of 22°C. The trial protocol is registered at the European Clinical Trials database (EudraCT no: 2007-003790-11) and ClinicalTrials.gov (no: NCT00682370).
Table 1.
Demographic data and baseline levels of bilirubin, haem, haem binding proteins, HO-1 mRNA and protein across treatment periods (GTn genotype cut-off level = 27)
| S/S | L/L | |
|---|---|---|
| GTn repeats | 23.5 ± 1.0 | 31.5 ± 3.1 |
| age (years) | 30.7 ± 6.0 | 25.3 ± 3.7 |
| weight (kg) | 74.8 ± 8.1 | 75.1 ± 12.3 |
| body mass index (kg m−2) | 22.7 ± 1.5 | 22.8 ± 2.2 |
| direct bilirubin (mg L−1)* | 1.2 ± 0.3# | 2.2 ± 0.8‡ |
| indirect bilirubin (mg L−1)* | 4.9 ± 1.4# | 9.6 ± 4.4‡ |
| haem (µM) | 23.4 ± 4.2# | 266 ± 6.5‡ |
| haptoglobin (mg L−1) | 975 ± 435# | 766 ± 283‡ |
| haemopexin (mg L−1)† | 653 ± 66# | 725 ± 85‡ |
| HO-1 mRNA (ΔCT)* | 2.76 ± 0.88# | 3.38 ± 0.50‡ |
| HO-1 protein (HO-1/β-actin)* | 0.16 ± 0.14# | 0.06 ± 0.05‡ |
Data are presented as means ± SD, and statistical differences are indicated (* P < 0.01, †P = 0.014; test: generalized estimating equations)
Baseline values from all investigational periods: #19 measurements from seven subjects; ‡23 measurements from eight subjects.
GTn, GT length polymorphism; HO, haem oxygenase.
Study protocol
In an open-label, stratified, three-period, placebo-controlled dose-escalation study, subjects were randomised to one of four dosage sequences (Figure 1). The dose-escalation design was chosen for the participants' safety. Three dosage sequences contained one placebo dose, and one sequence contained only active doses. Each dosage sequence consisted of three investigation periods that included three days (0, 1 and 2). On trial day 0, the study drug was administered followed by a 6 h in-hospital stay. Blood samples were performed before and 30, 60, 180, 240 and 360 min, and 24 and 48 h after the end of the study drug administration. Washout between investigational periods was at least 10 days. Adverse events were recorded throughout the study.
Figure 1.
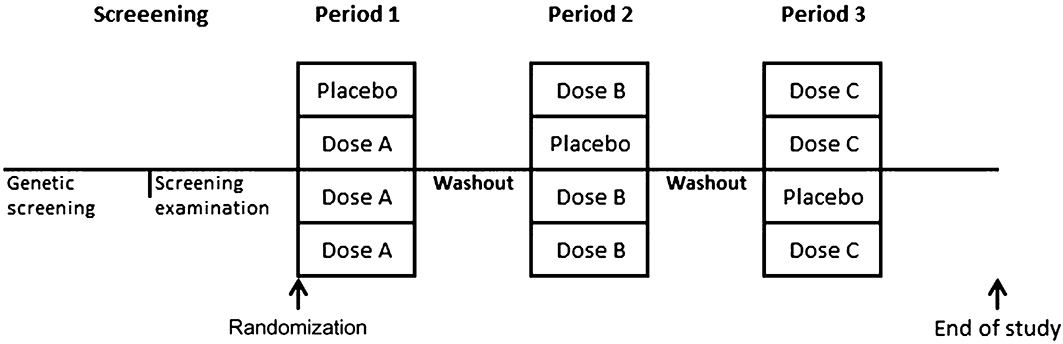
Study design: randomization to one of the four dosage sequences with three periods (1–3) consisting of either placebo or treatment doses A, B or C, comprising 0.3, 1.0 and 3.0 mg kg−1 BW haem arginate respectively.
Study drugs (HA and placebo)
Haem arginate was selected because it is a well-tolerated and a stable haem compound, which contains human haemin (ferriporphyrin with the iron atom in the ferric state), and because it is commercially available and labelled for human use (NORMOSANG®, Orphan Europe, Paris, France). HA was administered at increasing doses of 0.3, 1.0 and 3.0 mg kg−1 body weight (BW) in investigational periods 1, 2 and 3 respectively. The drug was diluted to 100 mL with 0.9% sodium chloride solution and administered i.v. over 15 min with a post-treatment rinsing infusion for 15 min with 0.9% sodium chloride solution. The dosing was based on the maximal approved dose of 3 mg kg−1 BW in the treatment of acute episodes of certain types of porphyria. As a placebo, infusions of 100 mL 0.9% sodium chloride solution were used.
Adverse events
The adverse event profile was evaluated according to known and published side effects (e.g. injection site pain, phlebitis at injection site and elevated serum ferritin concentration) by means of standardized questions, observation, vital signs, ECG and ferritin measurements on each study day.
HO-1 genotype assessment
HO-1 promoter polymorphism analysis was carried out as previously described (Denschlag et al., 2004). Genomic DNA was isolated from peripheral blood mononuclear cells (PBMCs) using commercially available kits, according to the vendors' protocols. The 5′-flanking region of the HO-1 gene containing a GTn repeat was amplified by the PCR using a fluorescent-labelled sense primer (5′-FAM-AGA GCC TGC AGC TTC TCA GA-3′) and an unlabelled antisense primer (5′-ACA AAG TCT GGC CAT AGG AC-3′). The sizes of the PCR products were analysed using an internal size-standard (GeneScan ROX 350 size standard, Applied Biosystems, Foster City, CA, USA), on a laser-based ABI Prism®3100 automated DNA capillary sequencer (Applied Biosystems). The fragment length determination and GT-repeat length attribution were completed semi-automatically using ABI Prism Software (Gene Scan Analysis Version 3.7 and Genotyper Software Version 3.7, both from Applied Biosystems). Allelic repeats were divided into two subclasses using the two different previously described classification systems: short repeats, with <27 GTn, were designated as allele class S (short), and longer repeats with ≥27 GTn as allele class L (long) (Kaneda et al., 2002; Chen et al., 2008). Alternatively, a length of 25 repeats was used as a cut-off (Endler et al., 2004; Exner et al., 2004; Schillinger et al., 2004) in post hoc analysis.
HO-1 protein and mRNA levels in PBMCs
HO-1 levels were analysed in PBMCs because these cells are considered effectors of oxidative stress-mediated injury and influenced by HO-1 expression. PBMCs were isolated from EDTA-blood in Ficoll-Plaque® (Amersham BioSciences, Buckinghamshire, UK) prefilled tubes (Leusosep®, Greiner bio-one, Frickenhausen, Austria). Random samples of differential blood counts of the PBMCs have shown the following subpopulations: approximately 63% lymphocytes (42% CD3+ lymphocytes), 18% monocytes (17% CD14+ monocytes), and 7% granulocytes. Cell pellets for the HO-1 mRNA analysis were treated with lysis buffer (Buffer RLT, Qiagen Sciences, MD, USA) and immediately frozen on liquid nitrogen. Lysates and cell pellets were stored at −80°C until analysis. First-strand cDNAs were synthesized from approximately 1 µg of total RNA by the use of MLV reverse transcriptase and random hexamer primers according to the manufacturer's instructions (RT-PCR Core Kit; Takara Bio, Otsu, Shiga, Japan). For quantitative real-time PCR analyses, sense and antisense primers (Invitrogen, Paisley, Scotland, UK) and fluorogenic probes (Eurogentec, Herstal, Belgium) for HO-1 and the Abelson gene (ABL) were used as previously described (Beillard et al., 2003). ABL was chosen as a reference gene because it was previously shown that it is one of the most consistently expressed housekeeping genes in haematopoietic cells (Beillard et al., 2003). The ABI PRISM 7700 (Applied Biosystems) was used for PCR. Results are expressed as the target/reference ratio. The difference between the HO-1 mRNA levels of HA and those of vehicle-treated cells was considered to reflect the capacity of cells to upregulate HO-1 mRNA and is expressed as ΔHO-1 mRNA.
For Western blot analyses, the dry cell pellets were transferred from −80°C to dry ice, and the cells were immediately lysed with a lysis buffer (5× extraction reagent diluted with water) containing protease inhibitors. After centrifugation to remove cell debris (18 000×g for 15 min at 4°C), the resulting supernatant was used for measurements using a bicinchoninic acid protein assay kit using a BSA protein standard (Thermo Scientific, Waltham, MA, USA). Then, 30 µL of a solution containing SDS loading buffer and 2-mercaptoethanol was combined with 50 µg of the sample protein and lysis buffer, bringing the volume up to 100 µL. Samples were heated at 93°C for 5 min and loaded onto a 4–12% gradient SDS/polyacrylamide gel (Invitrogen) for protein separation. The gel was run at 120 V for 1.5–2 h and then transferred (20 V for 1.5 h) to a polyvinylidene fluoride-Western blot membrane (GE Healthcare, Amersham, Buckinghamshire, UK) using the semi-dry method. The membranes were blocked in 5% milk in tris-buffered saline containing 0.05 % Tween 20 (TBST; Bio-Rad, Hercules, CA, USA) for 1 h, washed three times for 5 min with TBST, incubated with the primary mouse monoclonal anti-human HO-1 antibody (Clone OSA110, Assay Designs, Ann Arbor, MI, USA) overnight at 4°C, and washed and incubated with the second antibody (anti-mouse Ig horseradish peroxidase linked, Amersham; 1:20.000) for 1 h. The membranes were incubated for 5 min in the substrate solution (ECL Plus Western Blotting Detection System; Amersham Pharmacia Biotech, Piscataway, NJ, USA) and imaged using clear X-ray films (Thermo Scientific). The antibody staining and development procedure was repeated again using a mouse monoclonal anti-human-β-actin antibody (Abcam, Cambridge, MA, USA) for normalization of the results.
Haem, haemopexin and haptoglobin levels
The plasma haem concentration was measured in 96-well plates at 405 nm using a colorimetric haem assay kit (DIHM-250, BioAssay Systems, CA, USA). This assay reduces all haemin to ferrous haem and measures free and protein bound haem to give ‘total’ haem. Serum haemopexin and haptoglobin were measured by immunonephelometry (BN II System, Siemens Health Care Diagnostics, Eschborn, Germany) at the central laboratory facility of the General Hospital of Vienna.
Statistical analysis
Mean and SD were used to describe variables of interest. Pharmacokinetic and pharmacodynamic data (AUC, Cmax and tmax) were calculated using the validated software Kinetica™ (Version 4.4, Innaphase Corporation, Philadelphia, PA, USA). Differences between baseline characteristics were assessed by use of Student's t-test.
Differences in pharmacokinetic and pharmacodynamic parameters were assessed by generalized estimating equations models (Zeger and Liang, 1986), with identity as the link function. An AR(1) working correlation matrix for repeated observations within one patient was assumed. Numbers of observations were different between groups due to the inability to recruit the eighth subject for the S/S group and a few subjects that did not complete all three periods. Missing observations were regarded as missing at random. To test a possible interaction (effect modification) between genetics and treatment, the product of the predictors was added to the model term. For the statistical analyses, SPSS Statistics 17.0 (SPSS Inc., Chicago, IL, USA) was used.
All P values are results of two-sided tests, and P values <0.05 were considered statistically significant. In this exploratory study, no adjustment for multiple testing was performed.
Results
HO-1 genotype characteristics
A total of 132 subjects were screened for the GT length polymorphism in the HO-1 promoter region. The GTn repeats spread between 21 and 37, with 23 and 30 being the most common alleles (Figure 2A). Depending on the cut-off used (27 or 25 repeats), the prevalence of the genotypes for homozygous S/S carriers was 9.1% or 7.6%, for L/L 40.2 or 45.5.%, and for heterozygous S/L 50.8% or 47.0%, respectively (see Figure 2B). With the 25 cut-off level, only five of the recruited S/S subjects were included in the data analysis. Only data for the 27 cut-off level are presented in the following analysis because statistical analysis yielded results similar to those of the 25 cut-off.
Figure 2.
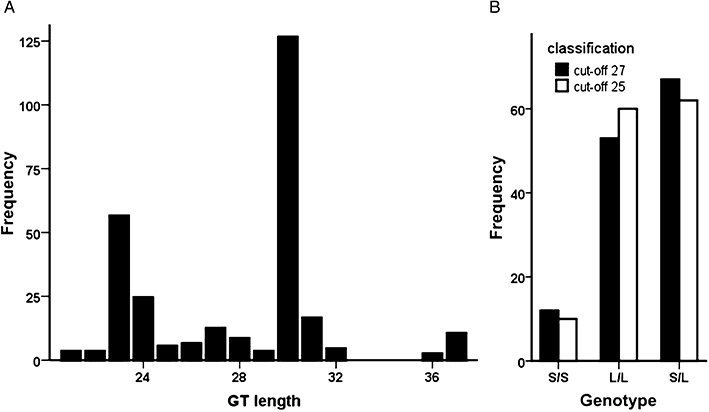
GTn genotype. (A) Allele distribution of the haem oxygenase gene from 132 subjects. The GT length is shown on the x-axis and allele frequency on the y-axis. (B) Prevalence of the GT length genotypes for the two classification cut-offs, 25 and 27 (S: < 25 or 27; L: ≥ 25 or 27).
Baseline characteristics
Eight subjects with the L/L and seven subjects carrying the S/S genotype participated in the drug infusion studies. The demographic data for these subjects are summarized in Table 1. Notably, mean bilirubin (total and sub-fractions) and haemopexin baseline levels before the investigational periods were lower in the S/S group compared with the L/L group (see Table 1). The detailed numbers of subjects available for a particular investigational period are listed in Table 2.
Table 2.
Pharmacodynamic parameters for HO-1 mRNA and protein levels after a single treatment with different doses of HA
| Treatment dose | Number of subjects | Cmax | tmax[h] | ΔAUC48* | |||||
|---|---|---|---|---|---|---|---|---|---|
| S/S | L/L | S/S | L/L | S/S | L/L | S/S | L/L | ||
| HO-1 mRNA | Placebo | 5 | 6 | – | – | – | – | −2.0 ± 14.9 | −6.5 ± 7.8 |
| 0.3 mg kg−1 | 5 | 5 | 3.3 ± 0.58 | 4.1 ± 0.50 | 8.3 ± 8.5 | 4.4 ± 0.9 | 0.35 ± 13.8 | 11.4 ± 3.5 | |
| 1.0 mg kg−1 | 4 | 6 | 3.6 ± 0.82 | 4.7 ± 1.00 | 4.3 ± 1.2 | 11.0 ± 9.7 | 14.9 ± 12.6 | 24.9 ± 13.0 | |
| 3.0 mg kg−1 | 4 | 6 | 4.2 ± 1.2 | 5.1 ± 1.2 | 19.4 ± 9.0 | 20.7 ± 8.2 | 17.8 ± 11.9 | 47.6 ± 15.6 | |
| HO-1 protein | Placebo | 5 | 6 | – | – | – | – | −2.1 ± 8.4 | −0.44 ± 5.4 |
| 0.3 mg kg−1 | 5 | 5 | 0.24 ± 0.095 | 0.19 ± 0.10 | 16.2 ± 19.8 | 15.6 ± 11.2 | 1.7 ± 4.1 | 3.2 ± 3.8 | |
| 1.0 mg kg−1 | 5 | 6 | 0.39 ± 0.097 | 0.25 ± 0.074 | 11.6 ± 11.5 | 29.4 ± 21.4 | 1.6 ± 7.9 | 5.3 ± 3.0 | |
| 3.0 mg kg−1 | 4 | 5 | 0.48 ± 0.17 | 0.31 ± 0.15 | 35.7 ± 13.5 | 20.2 ± 18.9 | 8.4 ± 10.5 | 8.2 ± 5.4 | |
Data are presented as means ± SD, and statistical differences are reported in the text; Cmax: maximal concentration (mRNA [ΔCT]; protein [(HO-1/β-actin]); tmax time to maximal concentration; ΔAUC48: area under the concentration versus time curve over 48 h as change from the individual baseline level
mRNA [ΔCT × h], protein [(HO-1/β-actin) × h].
On the screening day, there was no difference in the biochemical parameters between the two genetic groups except in the mean triglyceride levels (S/S vs. L/L: 150.3 ± 53.7 vs. 74.1 ± 24.1; P < 0.01), which might be due to differences in their fasting states (S/S vs. L/L: 1 of 7 vs. 5 of 8; ns).
Effect of HA infusion
The plasma haem and haemopexin concentrations versus time curves after a single HA administration are shown in Figure 3. The dose-dependent increase in the area under the curve (AUC) over 48 h, calculated as a change from the individual baseline level (ΔAUC48) of haem (P < 0.001), was paralleled by a decrease in the ΔAUC48 of haemopexin (P < 0.001). No genetic effect was observed regarding HO-1 GT-repeat length for haem or haemopexin (P = 0.389 and P = 0.397 respectively). The ΔAUC48 of haptoglobin did not change after HA infusion.
Figure 3.
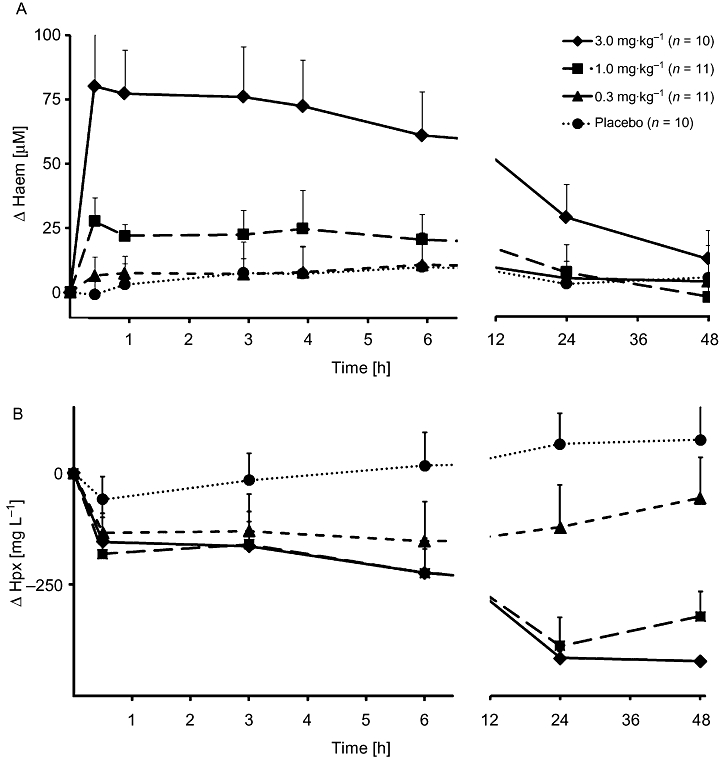
Plasma concentrations of total haem and haemopexin after a single infusion of haem arginate (HA). HA was infused at the indicated doses. Haem (A) and haemopexin (Hpx) (B) are plotted as a change from the individual baseline with bars showing SD. Plots summarize both genotypes (L/L and S/S). Numbers of subjects per group are indicated.
Interestingly, baseline levels of HO-1 mRNA were lower and protein concentrations were higher in genotype S/S compared with L/L (Table 1). HA infusion resulted in a dose-dependent increase in the ΔAUC48 of protein (P = 0.016). No effect of the HO-1 GTn genotype on protein levels could be shown (P = 0.252) (for details, see Table 2 and Figures 4 and 5).
Figure 4.
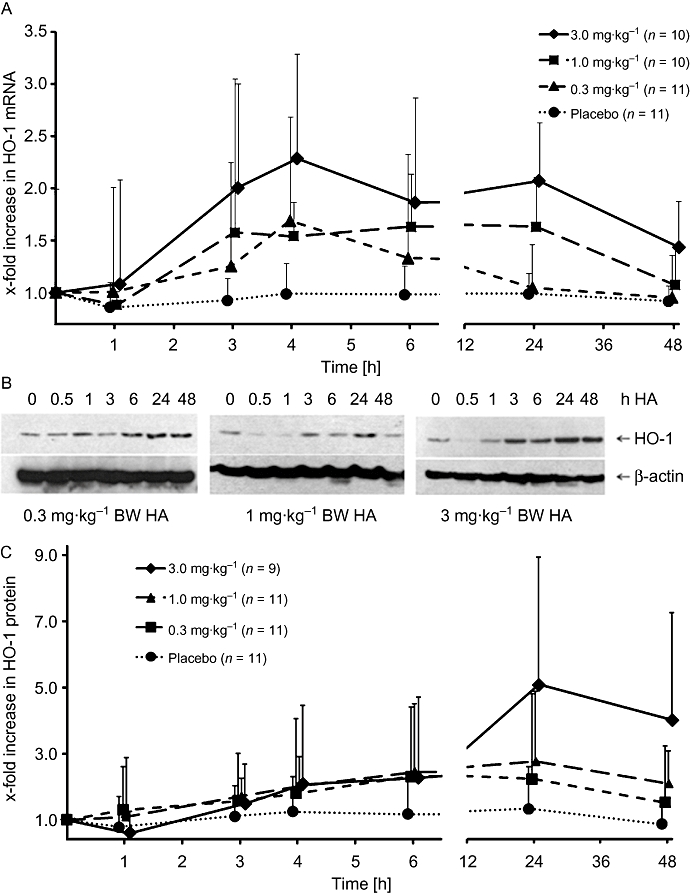
Haem arginate (HA) infusion induces haem oxygenase (HO-1) expression in peripheral blood mononuclear cell in vivo. HO-1 mRNA (A) and protein (C) expression after treatment with different doses of HA; bars show SD. (B) Representative HO-1 Western blots for the three treatment doses. β-Actin was used as a loading control. Plots summarize both genotypes (L/L and S/S). Numbers of subjects per group are indicated. BW, body weight.
Figure 5.
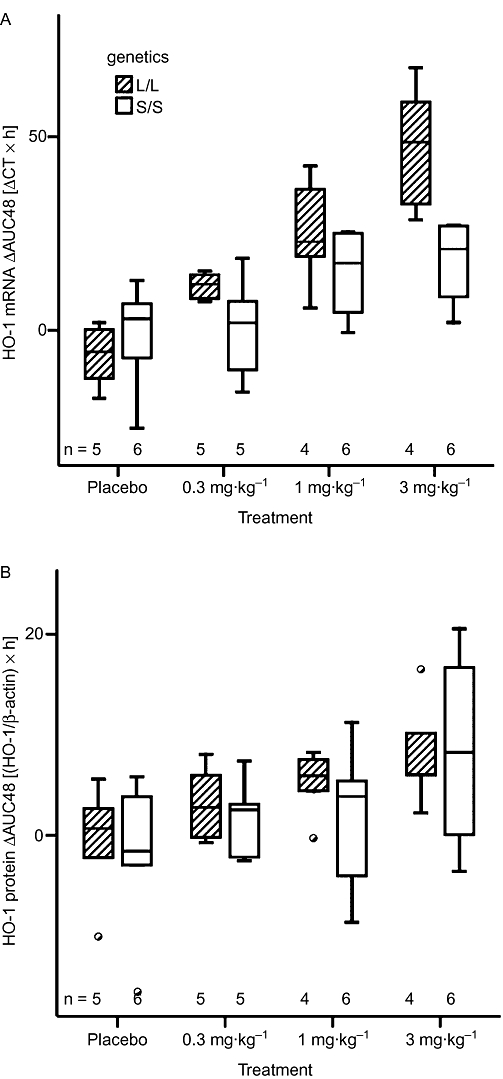
Area under the curve (AUC) over 48 h showing changes from individual baseline levels (ΔAUC48) for haem oxygenase (HO-1) mRNA and protein levels. Box and whisker plots of the ΔAUC48 for the HO-1 mRNA (A) and of the ODs of the protein levels normalized to β-actin (B) in peripheral blood mononuclear cells for the L/L and S/S genotypes. Numbers of subjects per group are indicated.
HA infusion resulted in a dose-dependent increase in the ΔAUC48 of HO-1 mRNA, but this effect depended strongly on the genetic group (P < 0.001 for the treatment-genetics interaction). As can be seen from Table 2 and Figure 5A, there is a much steeper dose-effect in the L/L group than the S/S group. Note that even the weaker dose effect of the S/S group is significant (P < 0.001 from subgroup analysis). The HO-1 induction curves for the individual subjects are presented in Figure S1.
The maximal concentration (Cmax) was also dose-dependent (P < 0.001 for mRNA and protein), with a higher Cmax for higher doses (Table 2). Cmax in the S/S group was lower for mRNA (P < 0.001) and higher for protein (P < 0.001) levels compared with the L/L group. No specific differences were observed for the time to maximal concentration (tmax).
Adverse effects
In total, 21 adverse events were documented. All were mild or moderate in intensity and resolved without sequelae. Injection site pain and headache were the most common reports (7 and 8, respectively) and were regarded as drug related. Injection site pain was found to be dose-related but headache was not. Common cold/flu-like symptoms (rhinitis, sore throat, cough, sweating, arthralgia) and gastrointestinal symptoms (diarrhoea, nausea, abdominal pain) were regarded as not drug related.
Discussion
Induction of HO-1 expression has been shown to be highly effective in various animal models of the ischaemia-reperfusion injury (IRI) (Tsuchihashi et al., 2004; Ryter et al., 2006; Peterson et al., 2009). However, similar studies have not been done in in humans. In the present work, we showed that HO-1 mRNA and protein levels can be augmented in PBMCs by a single i.v. dose of HA infused into healthy subjects.
HO-1 expression was measured in PBMCs because these cells are regarded as important mediators of inflammation in many vascular diseases and prime targets of the cytoprotective actions of HO-1 (Bilban et al., 2008). The total amount, as well as the time to reach the maximal HO-1 mRNA and protein levels (tmax), was increased with higher HA dosing (Table 2).
When considering the HO-1 genotype, the induction (ΔAUC48) of HO-1 mRNA was higher in the L/L group (Figures 4A and 5A). This finding does not accord with other published data (Yamada et al., 2000; Chen et al., 2002; Kronke et al., 2007). Several explanations for the apparent difference are proposed.
Published data regarding functional analyses were based on in vitro luciferase reporter assays using truncated fragments of the HO-1 promoter or by assessment of HO-1 mRNA levels in specific cell cultures (Yamada et al., 2000; Chen et al., 2002; Hirai et al., 2003; Rueda et al., 2007). However, a promoter assay in vitro does not necessarily recapitulate the gene expression in vivo. Of note, a recent study demonstrated that a longer GTn dinucleotide repeat did not inhibit HO-1 promoter activity (Zhang et al., 2006). Additional regulatory sequences located upstream of the transcription initiation site, as well as in the intronic region of the HO-1 gene, are required for haem-induced HO-1 mRNA expression.
Regulation of promoter activity by the GTn repeat length may differ in cell types and in response to different stimuli (Yamada et al., 2000; Rueda et al., 2007). We cannot exclude the possibility that the proportion of HO-1 expressing cells varied across PBMCs tested in S/S and L/L groups. Further studies are needed to determine whether different PBMC subpopulations respond differently to HA.
With regard to the HO-1 protein level, by measuring tmax or ΔAUC48, we did not observe significant differences in expression between S/S and L/L carriers; only Cmax was higher in S/S carriers (Figure 4C and Table 2). Thus, the number of GTn repeats does not seem to affect HA-induced HO-1 protein expression in vivo to a major degree under the conditions tested.
In several reports where HO-1 protein levels have been associated with clinical outcomes, HO-1 protein expression was measured after the onset of disease, thus reflecting induced rather than basal HO-1 expression. We noted higher basal (i.e. inducer-independent) HO-1 protein levels in S/S subjects (Table 2). In a murine model of liver IRI, basal rather than induced HO-1 protein levels were predictive of the antioxidant cytoprotection conferred by HO-1 (Tsuchihashi et al., 2006). Such regulation is likely to be mediated, at least in part, by transcription factors distinct from those used by HA. Further studies are needed to determine whether higher basal HO-1 protein levels are also protective in humans.
Higher basal HO-1 mRNA expression in L/L subjects may also be explained, in part, by the action of a GATA2-SP1 transcription factor module flanking the GTn repeat sequence. This module efficiently operates when the respective binding sites (i.e. GATA2 and SP1) are between 150 and 156 bases apart, as shown for basal eNOS expression in bovine aortic endothelial cells (Zhang et al., 1995). In the human HO-1 5′ flanking sequence, this module exists only when the number of GT repeats is >27 or <32, which is true for most L/L carriers. Whether or not this module contributes to basal and/or induced human HO-1 gene activity needs to be verified experimentally.
In this study, data analyses were performed for two commonly used cut-offs (≤25 or ≤27) for the S/S classification. Applying the 25 cut-off resulted in the exclusion of two S/S subjects. However, similar statistical results were obtained irrespective of the cut-off level. The prevalence of the S/S genotype in the cohort under study is in agreement with published data from Austria (Funk et al., 2004) and Germany (Hausmann et al., 2008; Lublinghoff et al., 2009), whereas the prevalence of the S/S genotype seems to be more frequent in Asian populations (17–23 %) (Chen et al., 2002; 2008; Kaneda et al., 2002). Previous reports described higher serum bilirubin levels in a large sample of S/S carriers (Endler et al., 2004; Immenschuh et al., 2007; Chen et al., 2008). Differences in dietary conditions could have affected serum bilirubin concentrations in the subjects under study (Ishihara et al., 2001; Zucker et al., 2004).
HA infusion rapidly increased haem plasma concentrations (up to approximately 120 µM with 3 mg kg−1), which is required for transcriptional induction of HO-1 in PBMC (Balla et al., 2000) and is well above the haem levels used to stimulate cells in vitro (5–100 µM) (Kawamura et al., 2005; Lang et al., 2005; Devadas and Dhawan, 2006). However, in vivo haem is rapidly complexed by the high-affinity (Kd < 1 pmol L−1) ‘free haem’ scavenger haemopexin (Delanghe and Langlois, 2001), which is quickly depleted, followed by ‘free haem’ being bound by the low-affinity albumin. Therefore, in vivo and in vitro haem levels are not comparable because measurement of the ‘total’ haem cannot differentiate between free unbound and complexed haem. The haem–haemopexin complex has nearly a sixfold lower activity regarding HO-1 induction in monocytes in vitro than free haem (Hvidberg et al., 2005).
Consistently, HA infusion decreased circulating haemopexin levels. Interestingly, haem as well as haemopexin levels were found to decrease after a plateau phase of about 4–6 h. The haem–haemopexin complex is degraded via a LRP/CD91-mediated endocytosis pathway, mainly in macrophages and hepatocytes (Hvidberg et al., 2005). As shown previously (Tokola et al., 1986; Volin et al., 1988; Kumar and Bandyopadhyay, 2005), a single infusion of 3 mg kg−1 HA-sustained induced lower levels of haemopexin for more than 48 h (Figure 3B), which ultimately may lead to a longer half-life of HA. Although plasma haem concentrations peaked above 100 µM, no signs of haem toxicity (e.g. haemolysis) were observed, as indicated by unchanged haptoglobin levels.
During the preparation of this manuscript, Bharucha et al. (2010) reported induction of plasma HO-1 protein by haemin (3 mg kg−1 i.v.) in humans, in addition to an increase in HO-1 enzymatic activity in PBMC. However, a potential HO-1 GTn genotype effect was not investigated and plasma HO-1 protein might not be a good measure for induced HO-1 in healthy individuals. In our study, we report HO-1 mRNA and protein levels in PBMCs, representing important mediators of inflammation in many vascular diseases and prime targets of the cytoprotective actions of HO-1 (Bilban et al., 2008). Nonetheless, the findings of our study are in good agreement with the HO-1 activity levels in PBMCs following haemin infusion.
In conclusion, this study demonstrates that a single dose of HA induces HO-1 mRNA and protein expression in PBMCs in vivo. The genetic background of the GTn length polymorphisms in the HO-1 promoter (S/S or L/L) does not have clinically relevant influence on this HO-1 induction. Further clinical studies addressing potentially beneficial effects of HA-induced HO-1 expression will be able to evaluate whether HA can be used therapeutically in IRI.
Acknowledgments
This study was funded by the Wiener Wissenschafts-, Forschungs- und Technologiefonds, project number LS07-031. We thank Mathias Roth for his great support in laboratory work.
Glossary
Abbreviations
- AUC
area under the curve
- BW
body weight
- CO
carbon monoxide
- GEE
generalised estimating equations
- HA
haem arginate
- HO
haem oxygenase
- IRI
ischaemia-reperfusion injury
- L/L
homozygous for the long GT repeat length polymorphism in the promoter of the HO-1 gene
- PBMCs
perhipheral blood mononuclear cells
- S/S
homozygous for the short GT repeat length polymorphism in the promoter of the HO-1 gene
Conflict of interest
The authors state no conflict of interest.
Supporting information
Additional Supporting Information may be found in the online version of this article:
Figure S1 Individual plots of HO-1 expression in PBMCs. Relative increase of HO-1 mRNA and protein expression after treatment with different doses of HA or placebo is plotted for all individuals (eight subjects with the L/L and seven subjects with the S/S genotype).
Please note: Wiley-Blackwell are not responsible for the content or functionality of any supporting materials supplied by the authors. Any queries (other than missing material) should be directed to the corresponding author for the article.
References
- Balla J, Balla G, Jeney V, Kakuk G, Jacob HS, Vercellotti GM. Ferriporphyrins and endothelium: a 2-edged sword-promotion of oxidation and induction of cytoprotectants. Blood. 2000;95:3442–3450. [PubMed] [Google Scholar]
- Beillard E, Pallisgaard N, van der Velden VH, Bi W, Dee R, van der Schoot E, et al. Evaluation of candidate control genes for diagnosis and residual disease detection in leukemic patients using ‘real-time’ quantitative reverse-transcriptase polymerase chain reaction (RQ-PCR) – a Europe against cancer program. Leukemia. 2003;17:2474–2486. doi: 10.1038/sj.leu.2403136. [DOI] [PubMed] [Google Scholar]
- Bharucha AE, Kulkarni A, Choi KM, Camilleri M, Lempke M, Brunn GJ, et al. First-in-Human Study demonstrating pharmacological activation of heme oxygenase-1 in humans. Clin Pharmacol Ther. 2010;87:187–190. doi: 10.1038/clpt.2009.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bilban M, Haschemi A, Wegiel B, Chin B, Wagner O, Otterbein L. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med. 2008;86:267–279. doi: 10.1007/s00109-007-0276-0. [DOI] [PubMed] [Google Scholar]
- Chen YH, Lin SJ, Lin MW, Tsai HL, Kuo SS, Chen JW, et al. Microsatellite polymorphism in promoter of heme oxygenase-1 gene is associated with susceptibility to coronary artery disease in type 2 diabetic patients. Human Genet. 2002;111:1–8. doi: 10.1007/s00439-002-0769-4. [DOI] [PubMed] [Google Scholar]
- Chen YH, Chau LY, Chen JW, Lin SJ. Serum bilirubin and ferritin levels link heme oxygenase-1 gene promoter polymorphism and susceptibility to coronary artery disease in diabetic patients. Diabetes Care. 2008;31:1615–1620. doi: 10.2337/dc07-2126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Delanghe JR, Langlois MR. Hemopexin: a review of biological aspects and the role in laboratory medicine. Clinica Chimica Acta. 2001;312:13–23. doi: 10.1016/s0009-8981(01)00586-1. [DOI] [PubMed] [Google Scholar]
- Denschlag D, Marculescu R, Unfried G, Hefler LA, Exner M, Hashemi A, et al. The size of a microsatellite polymorphism of the haem oxygenase 1 gene is associated with idiopathic recurrent miscarriage. Mol Hum Reprod. 2004;10:211–214. doi: 10.1093/molehr/gah024. [DOI] [PubMed] [Google Scholar]
- Devadas K, Dhawan S. Hemin activation ameliorates HIV-1 infection via heme oxygenase-1 induction. J Immunol. 2006;176:4252–4257. doi: 10.4049/jimmunol.176.7.4252. [DOI] [PubMed] [Google Scholar]
- Endler G, Exner M, Schillinger M, Marculescu R, Sunder-Plassmann R, Raith M, et al. A microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with increased bilirubin and HDL levels but not with coronary artery disease. Thromb Haemost. 2004;91:155–161. doi: 10.1160/TH03-05-0291. [DOI] [PubMed] [Google Scholar]
- Exner M, Minar E, Wagner O, Schillinger M. The role of heme oxygenase-1 promoter polymorphisms in human disease. Free Radic Biol Med. 2004;37:1097–1104. doi: 10.1016/j.freeradbiomed.2004.07.008. [DOI] [PubMed] [Google Scholar]
- Funk M, Endler G, Schillinger M, Mustafa S, Hsieh K, Exner M, et al. The effect of a promoter polymorphism in the heme oxygenase-1 gene on the risk of ischaemic cerebrovascular events: the influence of other vascular risk factors. Thromb Res. 2004;113:217–223. doi: 10.1016/j.thromres.2004.03.003. [DOI] [PubMed] [Google Scholar]
- Hausmann M, Paul G, Kellermeier S, Frey I, Scholmerich J, Falk W, et al. GT)N dinucleotide repeat polymorphism of haem oxygenase-1 promotor region is not associated with inflammatory bowel disease risk or disease course. Clin Exp Immunol. 2008;153:81–85. doi: 10.1111/j.1365-2249.2008.03674.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hirai H, Kubo H, Yamaya M, Nakayama K, Numasaki M, Kobayashi S, et al. Microsatellite polymorphism in heme oxygenase-1 gene promoter is associated with susceptibility to oxidant-induced apoptosis in lymphoblastoid cell lines. Blood. 2003;102:1619–1621. doi: 10.1182/blood-2002-12-3733. [DOI] [PubMed] [Google Scholar]
- Hvidberg V, Maniecki MB, Jacobsen C, Hojrup P, Moller HJ, Moestrup SK. Identification of the receptor scavenging hemopexin-heme complexes. Blood. 2005;106:2572–2579. doi: 10.1182/blood-2005-03-1185. [DOI] [PubMed] [Google Scholar]
- Immenschuh S, Shan Y, Kroll H, Santoso S, Wossmann W, Bein G, et al. Marked hyperbilirubinemia associated with the heme oxygenase-1 gene promoter microsatellite polymorphism in a boy with autoimmune hemolytic anemia. Pediatrics. 2007;119:e764–e767. doi: 10.1542/peds.2006-1385. [DOI] [PubMed] [Google Scholar]
- Ishihara T, Kaito M, Takeuchi K, Gabazza EC, Tanaka Y, Higuchi K, et al. Role of UGT1A1 mutation in fasting hyperbilirubinemia. J Gastroenterol Hepatol. 2001;16:678–682. doi: 10.1046/j.1440-1746.2001.02495.x. [DOI] [PubMed] [Google Scholar]
- Islam T, McConnell R, Gauderman WJ, Avol E, Peters JM, Gilliland FD. Ozone, oxidant defense genes, and risk of asthma during adolescence. Am J Respir Crit Care Med. 2008;177:388–395. doi: 10.1164/rccm.200706-863OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jadhav A, Ndisang JF. Heme arginate suppresses cardiac lesions and hypertrophy in deoxycorticosterone acetate-salt hypertension. Exp Biol Med. 2009;234:764–778. doi: 10.3181/0810-RM-302. [DOI] [PubMed] [Google Scholar]
- Jazwa A, Loboda A, Golda S, Cisowski J, Szelag M, Zagorska A, et al. Effect of heme and heme oxygenase-1 on vascular endothelial growth factor synthesis and angiogenic potency of human keratinocytes. Free Radic Biol Med. 2006;40:1250–1263. doi: 10.1016/j.freeradbiomed.2005.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaneda H, Ohno M, Taguchi J, Togo M, Hashimoto H, Ogasawara K, et al. Heme oxygenase-1 gene promoter polymorphism is associated with coronary artery disease in Japanese patients with coronary risk factors. Arterioscler Thromb Vasc Biol. 2002;22:1680–1685. doi: 10.1161/01.atv.0000033515.96747.6f. [DOI] [PubMed] [Google Scholar]
- Kawamura K, Ishikawa K, Wada Y, Kimura S, Matsumoto H, Kohro T, et al. Bilirubin from heme oxygenase-1 attenuates vascular endothelial activation and dysfunction. Arterioscler Thromb Vasc Biol. 2005;25:155–160. doi: 10.1161/01.ATV.0000148405.18071.6a. [DOI] [PubMed] [Google Scholar]
- Kronke G, Kadl A, Ikonomu E, Bluml S, Furnkranz A, Sarembock IJ, et al. Expression of heme oxygenase-1 in human vascular cells is regulated by peroxisome proliferator-activated receptors. Arterioscler Thromb Vasc Biol. 2007;27:1276–1282. doi: 10.1161/ATVBAHA.107.142638. [DOI] [PubMed] [Google Scholar]
- Kubulus D, Rensing H, Paxian M, Thierbach JT, Meisel T, Redl H, et al. Influence of heme-based solutions on stress protein expression and organ failure after hemorrhagic shock. Crit Care Med. 2005;33:629–637. doi: 10.1097/01.ccm.0000156295.48075.49. [DOI] [PubMed] [Google Scholar]
- Kubulus D, Mathes A, Pradarutti S, Raddatz A, Heiser J, Pavlidis D, et al. Hemin arginate-induced heme oxygenase 1 expression improves liver microcirculation and mediates an anti-inflammatory cytokine response after hemorrhagic shock. Shock. 2008;29:583–590. doi: 10.1097/SHK.0b013e318157e526. [DOI] [PubMed] [Google Scholar]
- Kumar S, Bandyopadhyay U. Free heme toxicity and its detoxification systems in human. Toxicol Lett. 2005;157:175–188. doi: 10.1016/j.toxlet.2005.03.004. [DOI] [PubMed] [Google Scholar]
- Lang D, Reuter S, Buzescu T, August C, Heidenreich S. Heme-induced heme oxygenase-1 (HO-1) in human monocytes inhibits apoptosis despite caspase-3 up-regulation. Int Immunol. 2005;17:155–165. doi: 10.1093/intimm/dxh196. [DOI] [PubMed] [Google Scholar]
- Lavrovsky Y, Schwartzman ML, Levere RD, Kappas A, Abraham NG. Identification of binding sites for transcription factors NF-kappa B and AP-2 in the promoter region of the human heme oxygenase 1 gene. Proc Natl Acad Sci USA. 1994;91:5987–5991. doi: 10.1073/pnas.91.13.5987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lublinghoff N, Winkler K, Winkelmann BR, Seelhorst U, Wellnitz B, Boehm BO, et al. Genetic variants of the promoter of the heme oxygenase-1 gene and their influence on cardiovascular disease (the Ludwigshafen Risk and Cardiovascular Health study) BMC Med Genet. 2009;10:36. doi: 10.1186/1471-2350-10-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maeshima K, Takahashi T, Uehara K, Shimizu H, Omori E, Yokoyama M, et al. Prevention of hemorrhagic shock-induced lung injury by heme arginate treatment in rats. Biochem Pharmacol. 2005;69:1667–1680. doi: 10.1016/j.bcp.2005.03.007. [DOI] [PubMed] [Google Scholar]
- Mustajoki P, Nordmann Y. Early administration of heme arginate for acute porphyric attacks. Arch Intern Med. 1993;153:2004–2008. [PubMed] [Google Scholar]
- Nakaso K, Yano H, Fukuhara Y, Takeshima T, Wada-Isoe K, Nakashima K. PI3K is a key molecule in the Nrf2-mediated regulation of antioxidative proteins by hemin in human neuroblastoma cells. FEBS Lett. 2003;546:181–184. doi: 10.1016/s0014-5793(03)00517-9. [DOI] [PubMed] [Google Scholar]
- Otterbein LE, Soares MP, Yamashita K, Bach FH. Heme oxygenase-1: unleashing the protective properties of heme. Trends Immunol. 2003;24:449–455. doi: 10.1016/s1471-4906(03)00181-9. [DOI] [PubMed] [Google Scholar]
- Peterson SJ, Frishman WH, Abraham NG. Targeting heme oxygenase: therapeutic implications for diseases of the cardiovascular system. Cardiol Rev. 2009;17:99–111. doi: 10.1097/CRD.0b013e31819d813a. [DOI] [PubMed] [Google Scholar]
- Rueda B, Oliver J, Robledo G, Lopez-Nevot MA, Balsa A, Pascual-Salcedo D, et al. HO-1 promoter polymorphism associated with rheumatoid arthritis. Arthritis Rheum. 2007;56:3953–3958. doi: 10.1002/art.23048. [DOI] [PubMed] [Google Scholar]
- Ryter SW, Alam J, Choi AM. Heme oxygenase-1/carbon monoxide: from basic science to therapeutic applications. Physiol Rev. 2006;86:583–650. doi: 10.1152/physrev.00011.2005. [DOI] [PubMed] [Google Scholar]
- Salahudeen AA, Jenkins JK, Huang H, Ndebele K, Salahudeen AK. Overexpression of heme oxygenase protects renal tubular cells against cold storage injury: studies using hemin induction and ho-1 gene transfer. [Article] Transplantation. 2001;72:1498–1504. doi: 10.1097/00007890-200111150-00005. [DOI] [PubMed] [Google Scholar]
- Sasaki T, Takahashi T, Maeshima K, Shimizu H, Toda Y, Morimatsu H, et al. Heme arginate pretreatment attenuates pulmonary NF-kappaB and AP-1 activation induced by hemorrhagic shock via heme oxygenase-1 induction. Med Chem. 2006;2:271–274. doi: 10.2174/157340606776930781. [DOI] [PubMed] [Google Scholar]
- Schillinger M, Exner M, Mlekusch W, Domanovits H, Huber K, Mannhalter C, et al. Heme oxygenase-1 gene promoter polymorphism is associated with abdominal aortic aneurysm. Thromb Res. 2002;106:131–136. doi: 10.1016/s0049-3848(02)00100-7. [DOI] [PubMed] [Google Scholar]
- Schillinger M, Exner M, Minar E, Mlekusch W, Mullner M, Mannhalter C, et al. Heme oxygenase-1 genotype and restenosis after balloon angioplasty: a novel vascular protective factor. J Am Coll Cardiol. 2004;43:950–957. doi: 10.1016/j.jacc.2003.09.058. [DOI] [PubMed] [Google Scholar]
- Takeda M, Kikuchi M, Ubalee R, Na-Bangchang K, Ruangweerayut R, Shibahara S, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to cerebral malaria in Myanmar. Jpn J Infect Dis. 2005;58:268–271. [PubMed] [Google Scholar]
- Tokola O, Tenhunen R, Volin L, Mustajoki P. Pharmacokinetics of intravenously administered haem arginate. Br J Clin Pharmacol. 1986;22:331–335. doi: 10.1111/j.1365-2125.1986.tb02895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsuchihashi S, Fondevila C, Kupiec-Weglinski JW. Heme oxygenase system in ischemia and reperfusion injury. Ann Transplant. 2004;9:84–87. [PubMed] [Google Scholar]
- Tsuchihashi S, Livhits M, Zhai Y, Busuttil RW, Araujo JA, Kupiec-Weglinski JW. Basal rather than induced heme oxygenase-1 levels are crucial in the antioxidant cytoprotection. J Immunol. 2006;177:4749–4757. doi: 10.4049/jimmunol.177.7.4749. [DOI] [PubMed] [Google Scholar]
- Volin L, Rasi V, Vahtera E, Tenhunen R. Heme arginate: effects on hemostasis. Blood. 1988;71:625–628. [PubMed] [Google Scholar]
- Yamada N, Yamaya M, Okinaga S, Nakayama K, Sekizawa K, Shibahara S, et al. Microsatellite polymorphism in the heme oxygenase-1 gene promoter is associated with susceptibility to emphysema. Am J Hum Genet. 2000;66:187–195. doi: 10.1086/302729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yasuda H, Okinaga S, Yamaya M, Ohrui T, Higuchi M, Shinkawa M, et al. Association of susceptibility to the development of pneumonia in the older Japanese population with haem oxygenase-1 gene promoter polymorphism. J Med Genet. 2006;43:e17. doi: 10.1136/jmg.2005.035824. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida T, Biro P, Cohen T, Muller RM, Shibahara S. Human heme oxygenase cDNA and induction of its mRNA by hemin. Eur J Biochem. 1988;171:457–461. doi: 10.1111/j.1432-1033.1988.tb13811.x. [DOI] [PubMed] [Google Scholar]
- Zeger SL, Liang KY. Longitudinal data analysis for discrete and continuous outcomes. Biometrics. 1986;42:121–130. [PubMed] [Google Scholar]
- Zhang J, Ohta T, Maruyama A, Hosoya T, Nishikawa K, Maher JM, et al. BRG1 interacts with Nrf2 to selectively mediate HO-1 induction in response to oxidative stress. Mol Cell Biol. 2006;26:7942–7952. doi: 10.1128/MCB.00700-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang R, Min W, Sessa WC. Functional analysis of the human endothelial nitric oxide synthase promoter. J Biol Chem. 1995;270:15320–15326. doi: 10.1074/jbc.270.25.15320. [DOI] [PubMed] [Google Scholar]
- Zucker SD, Horn PS, Sherman KE. Serum bilirubin levels in the U.S. population: gender effect and inverse correlation with colorectal cancer. Hepatology. 2004;40:827–835. doi: 10.1002/hep.20407. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


