Abstract
BACKGROUND AND PURPOSE
Adenosine and inosine accumulate extracellularly during hypoxia/ischaemia in the brain and may act as neuroprotectants. In spinal cord, there is pharmacological evidence for increases in extracellular adenosine during hypoxia, but no direct measurements of purine release. Furthermore, the efflux pathways and origin of extracellular purines are not defined. To characterize hypoxia-evoked purine accumulation, we examined the effect of acute hypoxia on the extracellular levels of adenosine and inosine in isolated spinal cords from rats.
EXPERIMENTAL APPROACH
Extracellular adenosine and inosine concentrations were assayed in an in vitro preparation of the isolated spinal cord of the neonatal rat by HPLC.
KEY RESULTS
The extracellular level of inosine was about 10-fold higher than that of adenosine. Acute hypoxia (10 min) caused a temperature-dependent increase in these two purines, which were inhibited by an increase in external Ca2+, but not by several inhibitors of efflux pathways or metabolic enzymes of adenine nucleotides. Inhibitors of adenosine deaminase or the equilibrative nucleoside transporter (ENT) abolished the hypoxia-evoked increase in inosine but not adenosine. The inhibition of glial metabolism abolished the increase of both purines evoked by hypoxia but not by oxygen-glucose deprivation, hypercapnia or an adenosine kinase inhibitor.
CONCLUSIONS AND IMPLICATIONS
Our data suggest that hypoxia releases adenosine itself from intracellular sources. Inosine formed intracellularly may be released through ENTs. During hypoxia, astrocytes appear to play a key role in purine release from neonatal rat spinal cord.
Keywords: hypoxia, adenosine, inosine, spinal cord, fluoroacetate
Introduction
Adenosine accumulates in the extracellular space during cerebral hypoxia/ischaemia (Latini and Pedata, 2001; Pearson et al., 2003), and it acts as a neuroprotectant under this pathological condition, mainly by inhibiting excessive neuronal excitation by actions at adenosine A1 receptors (Waradas, 2002; Pedata et al., 2007). However, the exact mechanisms responsible for adenosine accumulation are unknown. In the rat brain, oxygen-glucose deprivation (OGD) or hypercapnia was reported to release ATP (Parkinson and Xiong, 2004; Dulla et al., 2005; Liu et al., 2008), resulting in the increase in extracellular adenosine due to their degradation by a series of ecto-enzymes such as ecto-5′-nucleotidase (Matsuoka and Ohkubo, 2004; Gödecke, 2008). On the other hand, there is also evidence showing that adenosine itself is directly released by hypoxia (Martín et al., 2007) and OGD (Frenguelli et al., 2007). In rat hippocampal slices, Ca2+ inhibits adenosine release during hypoxia, indicating non-exocytotic release of adenosine (Dale et al., 2000; Martín et al., 2007). An inhibition of glial metabolism is also reported to inhibit hypoxia-evoked adenosine release, suggesting the involvement of astrocytes (Martín et al., 2007).
Inosine also accumulates during hypoxia/ischaemia and elicits protective effects (Haun et al., 1996; Litsky et al., 1999; Chen et al., 2002; Shen et al., 2007). In rat hippocampal slices, most of the inosine increase occurring during hypoxia is due to the extracellular degradation of adenosine mediated by ecto-adenosine deaminase (Frenguelli et al., 2003). On the other hand, the increase in inosine during OGD is due to the formation of inosine intracellularly and its subsequent release by equilibrative nucleoside transporters (ENTs) in cultured rat cortical neurons and astrocytes (Parkinson and Xiong, 2004). The mechanism of adenosine and inosine accumulation in the extracellular space thus depends on the experimental conditions and/or the region of the CNS studied.
Various diseases or surgical procedures cause hypoxic/ischemic conditions in the spinal cord that impair spinal function (Cheshire et al., 1996; Tator and Koyanagi, 1997;Rowland et al., 2008). It has been reported that acute hypoxia depresses spinal synaptic transmission which is reversed by adenosine A1 receptor antagonists (Lloyd et al., 1988; 1989; Czéh and Somjen, 1990; Park et al., 2002). However, there is no direct measurement of extracellular adenosine levels during hypoxia, and the efflux pathways and the origin of extracellular adenosine in response to hypoxia are still not defined, for the spinal cord. In addition, the extracellular concentration of inosine is also expected to show a considerable change because adenosine deaminase plays a significant role in adenosine metabolism in the rat spinal cord (Golembiowska et al., 1995; 1996;).
To investigate the effect of brief exposure to hypoxia on the accumulation of adenosine and inosine in the spinal cord, we measured the extracellular concentration of purines in spinal cord preparations, isolated from neonatal rats, in which acute hypoxia immediately resulted in adenosine A1 receptor-mediated synaptic depression (Lloyd et al., 1989).
Methods
Preparations
All animal care and experimental procedures were approved by the Animal Care and Use Committee of the Graduate School of Veterinary Medicine, Hokkaido University. All efforts were made to minimize animal suffering and to reduce the number of animals used. Both male and female neonatal rats (Wistar, 0–7 days old) were used.
Neonatal rats were killed by decapitation, and the spinal cords were isolated. The composition of artificial cerebrospinal fluid (ACSF) was as follows (mM): NaCl 138; NaHCO3 21; NaH2PO4 0.6; CaCl2 1.25; KCl 3.5; MgCl2 2.0; glucose 10; gassed with 95% O2 and 5% CO2; pH∼7.3. Hypoxic ACSF was gassed with 95% N2 and 5% CO2 (pH∼7.3) at least 1 h before the experiments were started. The partial pressures of O2 (pO2), measured with a dissolved oxygen meter (ISO2, World Precision Instruments, Sarasota, FL, USA), were 65.8 ± 1.4% (n = 3) and 5.0 ± 0.9% (n = 3) in normal and hypoxic ACSF respectively. Hypercapnic ACSF was prepared by gassing with 80% O2 and 20% CO2 (pH∼6.7). For OGD, glucose was substituted by equimolar amounts of sucrose in the hypoxic ACSF. For Ca2+-free ACSF, CaCl2 was removed and 1 mM EGTA was added.
Experimental protocols
The isolated spinal cord was cut into several pieces and equilibrated in ACSF for 1 h at 35°C. The solution (1 mL) was changed every 10 min and the sample solutions were collected. In some experiments, tissues were treated with ACSF containing fluoroacetate (FA) for 30 min or other drugs for 20 min before exposure to hypoxia or other stimulants. The purine level in the presence of drugs was compared with that in its absence in preparations obtained from littermates.
Measurement of purine concentration
Collected sample solutions (500 µL) were immediately chilled on ice, and 180 µL of 0.1 M citrate-phosphate buffer (pH 4.0) and 50 µL solution of 4 µM α,β-methylene ADP (internal standard) were added. Then a 365 µL aliquot of the mixture was separated and 10 µL of 45% chloroacetaldehyde was added to it for the measurement of adenosine and adenine nucleotides. The remainder of the mixture was used for the measurement of inosine. The concentration of adenosine and adenine nucleotides was determined by HPLC with a fluorescence detector according to the method of Kawamoto et al. (1998) with some modifications as previously described (Otsuguro et al., 2009). The inosine concentration was determined according to the method described by Ferraris et al. (1991) with the following modifications: the samples were separated by reverse-phase HPLC with an ODS column (Cosmosil 5C18-MS, 4.6 × 150 mm, Nacalai Tesque Inc., Kyoto, Japan) and monitored at 254 nm wavelength with a UV detector (UV-2075, JASCO, Tokyo, Japan). The mobile phase buffer consisted of 100 mM KH2PO4 and 2.0% CH3CN (pH 3.3 with H3PO4). The flow rate was 1.0 mL·min−1. The calibration curves for adenine nucleotides, adenosine and inosine were constructed by plotting the peak height ratio of standard mixtures to that of the internal standard after the same treatment as that for sample solutions. Representative chromatograms of a standard mixture are shown in Figure 1A,B. The amount of purines in a sample was quantified by direct comparison of the peak height to that of the internal standard. The detection limit for all purines was about 20 fmol. The concentration of cAMP was assayed using a commercially available enzyme immunoassay kit (cAMP EIA, non-acetylation, RPN2251, GE Healthcare Japan, Tokyo, Japan). The amount of purines accumulated in 10 min was expressed as the extracellular amount per milligram of tissue wet weight (pmol·mg−1). The increments of adenosine and inosine in response to the stimulants (i.e. hypoxia, OGD, hypercapnia or ABT-702) were estimated by subtracting the resting release (release in 10 min incubation without stimulants) from the subsequent evoked release (10 min incubation with stimulants), and were expressed as Δadenosine and Δinosine respectively.
Figure 1.

Chromatograms of purines obtained by reverse-phase HPLC. A standard mixture (2.0 pmol) of ATP, ADP, AMP and adenosine was injected with internal standard (IS) after ethenopurine derivatization and monitored with fluorescence detector (A). A standard solution of inosine (2.0 pmol) was injected with IS and monitored with UV detector (B). Sample solutions collected 10 min after incubation of isolated spinal cord with normoxic or hypoxic ACSF were injected and monitored with fluorescence detector (C) and UV detector (D). Superimposed chromatograms in normoxia and hypoxia were normalized to the peak amplitude of IS.
Data analysis
Results are expressed as mean ± SEM (n = number of observations). Statistical comparisons between two samples from the same preparation and between those from littermates were performed by the paired and unpaired Student's t-test respectively. For multiple comparisons, analysis of variance with Dunnett's -test was used. A P value of less than 0.05 was considered significant.
Materials
ABT-702 dihydrochloride, arachidonic acid sodium salt (AA), Brilliant Blue G (BBG), carbenoxolone disodium salt, cGMP sodium salt, ARL 67156 trisodium salt, 1,3-dipropyl-8-(p-sulphophenyl)xanthine (DPSPX), dipyridamole, S-(4-nitrobenzyl)-6-thioinosine (NBTI), α,β-methylene ADP sodium salt, rolipram and (+/−)-sulfinpyrazone were purchased from Sigma Chemical Co. (St. Louis, MO, USA). Erythro-9-(2-Hydroxy-3-nonyl) adenine hydrochloride (EHNA) was from Tocris (Bristol, UK). Sodium FA was from Wako Pure Chemical Industries (Osaka, Japan). All drugs were mixed in ACSF and applied to preparations. Drug and molecular target nomenclature follows Alexander et al. (2009).
Results
The isolated spinal cord was incubated with normal ACSF under normoxic conditions, and the extracellular amounts of adenine nucleotides (ATP, ADP, AMP), adenosine and inosine in the ACSF were measured using HPLC (Figure 1C,D). ATP and ADP could not be detected. The level of inosine was approximately 10 or more times higher than the levels of adenosine and AMP. Brief (10 min) exposure of the isolated spinal cord to hypoxic ACSF evoked a significant increase in the concentration of adenosine and inosine but not that of AMP. ATP and ADP were not detected during hypoxia (Figure 2A). The effect of OGD on the rat spinal cord was examined because OGD has been reported to release adenine nucleotides in rat-cultured forebrain astrocytes (Parkinson and Xiong, 2004) and hippocampal slices (Frenguelli et al., 2007). OGD (10 min) evoked significant increases in adenosine and inosine but not AMP in the rat spinal cord. ATP and ADP were not detected during OGD (Figure 2B). The effect of OGD (10 min) on the increase in purines was reversible for adenosine (pre: 0.43 ± 0.12 pmol·mg−1, OGD: 1.28 ± 0.18 pmol·mg−1, P < 0.01, post: 0.70 ± 0.07 pmol·mg−1, n = 4) and inosine (pre: 5.30 ± 0.57 pmol·mg−1, OGD: 17.69 ± 2.73 pmol·mg−1, P < 0.01, post: 6.55 ± 1.46 pmol·mg−1, n = 4) (P < 0.01 vs. pre, Dunnett's test) levels.
Figure 2.

Amounts of extracellular purine during normoxia, hypoxia and oxygen-glucose deprivation (OGD) in the rat spinal cord. The isolated spinal cords were incubated in normal ACSF and then in hypoxic (A) or OGD ACSF (B) for 10 min each. Each column and error bar represents the mean ± SEM (n = 10–11). **P < 0.01 significantly different from values in normoxia (paired Student's t-test).
The adenosine and inosine appearing in the ACSF in response to longer exposure times of the spinal cord to hypoxic ACSF were also examined. The increases in adenosine and inosine during hypoxia gradually declined, but they were significantly larger than control throughout the exposure for 30 min (Figure 3). After return to normoxia, the levels of these purines returned to baseline within 10 min.
Figure 3.
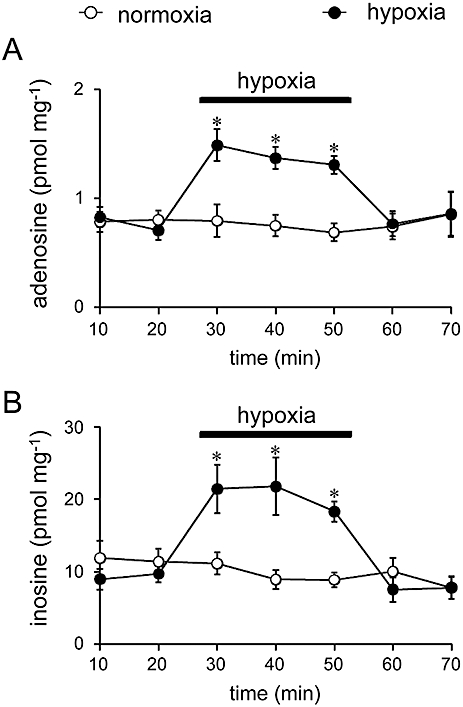
Time course of adenosine (A) and inosine increase (B) evoked by hypoxia. ACSF was changed every 10 min. The isolated spinal cords were exposed to hypoxic ACSF for 30 min. Spinal cord preparations isolated from littermates were used as controls without hypoxic exposure. Each column and error bar represents the mean ± SEM (n = 5–9) *P < 0.05 significantly different from control values at same time point (unpaired Student's t-test).
We next examined the dependency of the extracellular adenosine and inosine increases on temperature and external Ca2+ concentration. The basal level of adenosine in the ACSF at 25°C (0.17 ± 0.01 pmol·mg−1, n = 8, P < 0.05, unpaired Student's t-test) was significantly lower than that at 35°C (0.35 ± 0.05 pmol·mg−1, n = 8), while there was no significant difference in the basal level of inosine between 25°C (5.13 ± 1.20 pmol·mg−1, n = 8) and 35°C (6.39 ± 0.54 pmol·mg−1, n = 8). The increase in adenosine and inosine in response to hypoxic ACSF (10 min) was markedly lower at 25°C than at 35°C (Figure 4A,B). The hypoxia-evoked increases in adenosine and inosine were suppressed by extracellular Ca2+ in a concentration-dependent manner and abolished by 2.5 mM Ca2+ (Figure 4C,D). The extracellular Ca2+ concentration used did not significantly affect the basal levels of adenosine (0 mM Ca2+: 0.59 ± 0.17 pmol·mg−1, 1.25 mM Ca2+: 0.38 ± 0.06 pmol·mg−1, 2.5 mM Ca2+: 0.46 ± 0.07 pmol·mg−1, n = 6) and inosine (0 mM Ca2+: 12.29 ± 2.65 pmol·mg−1, 1.25 mM Ca2+: 15.95 ± 1.51 pmol·mg−1, 2.5 mM Ca2+: 12.07 ± 1.33 pmol·mg−1, n = 6).
Figure 4.

Effects of temperature and extracellular Ca2+ on adenosine and inosine levels. The hypoxia-evoked increment in adenosine (A) and inosine (B) were measured at 25 and 35°C. Each column and error bar represents the mean ± SEM (n = 8). **P < 0.01 significantly different from values at 25°C (unpaired Student's t-test). The hypoxia-evoked increment in adenosine (C) and inosine (D) in 0, 1.25 and 2.5 mM extracellular Ca2+ was measured. Each symbol and error bar represents the mean ± SEM. (n = 6).
The contribution of the purine metabolic pathways to the hypoxia-evoked increase in adenosine and inosine was investigated by using several inhibitors of enzymes involved in these pathways. EHNA (10 µM), an inhibitor of adenosine deaminase that prevents the conversion of adenosine to inosine, significantly increased the basal adenosine level (control: 0.79 ± 0.12 pmol·mg−1 vs. EHNA: 1.71 ± 0.26 pmol·mg−1, n = 6, P < 0.01, unpaired Student's t-test) and decreased the basal inosine level (EHNA: 6.66 ± 1.06 pmol·mg−1 vs. control: 13.88 ± 3.29 pmol·mg−1, n = 6, P < 0.05, unpaired Student's t-test). In the presence of EHNA, the hypoxia (10 min)-evoked adenosine increase was significantly enhanced, while hypoxia failed to increase inosine levels (Figure 5A,B), indicating that the increase in inosine was largely due to the metabolism of adenosine by adenosine deaminase.
Figure 5.
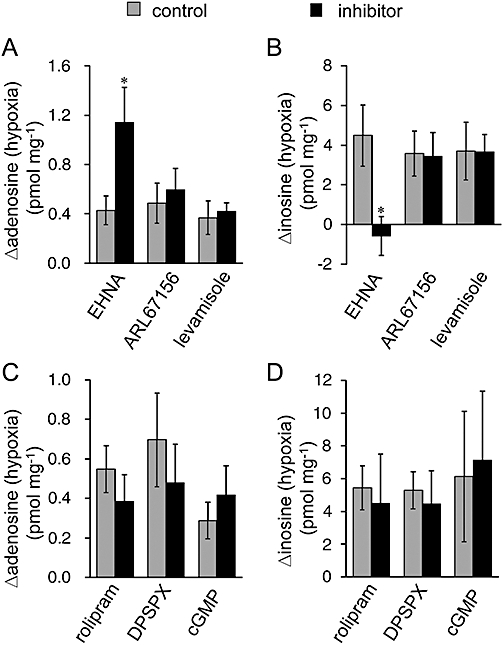
Effect of inhibitors of purine metabolism. The hypoxia-evoked increment in adenosine (A and C) and inosine (B and D) was measured in the presence and absence (control) of 10 µM erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA, n = 6), 100 µM ARL67156 (n = 6), 1 mM levamisole (n = 5), 100 µM rolipram (n = 8), 1 mM 1,3-dipropyl-8-(p-sulphophenyl)xanthine (DPSPX, n = 6) or 1 mM cGMP (n = 6). Each column and error bar represents the mean ± SEM. *P < 0.05, significantly different from control values (unpaired Student's t-test).
Extracellular adenosine is formed through the degradation of extracellular ATP by a series of ecto-nucleotidases, including ecto-ATPase and ecto-alkaline phosphatase. ARL67156 (100 µM) and levamisole (1 mM), inhibitors of ecto-ATPase and alkaline phosphatase, respectively, did not affect the basal levels of adenosine (ARL67156: 0.40 ± 0.14 pmol·mg−1 vs. control: 0.37 ± 0.09 pmol·mg−1, n = 6; levamisole: 0.33 ± 0.06 pmol·mg−1 vs. control: 0.42 ± 0.05 pmol·mg−1, n = 5) and inosine (ARL67156: 3.73 ± 0.64 pmol·mg−1 vs. control: 4.47 ± 1.12 pmol·mg−1, n = 6; levamisole: 5.12 ± 1.23 pmol·mg−1 vs. control: 5.37 ± 1.51 pmol·mg−1, n = 5) or their hypoxia (10 min)-evoked increase (Figure 5A,B). Extracellular adenosine is also produced from extracellular cAMP degradation, which is initiated by ecto-phosphodiesterase (PDE). PDE4 is cAMP-specific and a major PDE subtype in the CNS (Jin et al., 1999; Nikulina et al., 2004). However, the PDE4 inhibitor rolipram (100 µM), and the ecto-PDE inhibitors DPSPX (1 mM) and cGMP (1 mM), did not show any significant effect on the hypoxia (10 min)-evoked increase in adenosine and inosine (Figure 5C,D). To confirm the contribution of cAMP to purine accumulation, we measured the extracellular level of cAMP. The level of cAMP was very low and there was no significant difference in the cAMP level between normoxic (4.16 ± 0.41 fmol·mg−1, n = 4) and hypoxic (4.13 ± 0.56 fmol·mg−1, n = 4) conditions for 10 min. These results suggest that the extracellular degradation of ATP and cAMP does not contribute to the accumulation of adenosine and inosine during hypoxia. Rolipram, DPSPX and cGMP did not significantly affect the basal levels of adenosine (rolipram: 0.48 ± 0.10 pmol·mg−1 vs. control: 0.52 ± 0.11 pmol·mg−1, n = 8; DPSPX: 0.51 ± 0.01 pmol·mg−1 vs. control: 0.63 pmol·mg−1, n = 6; cGMP: 0.33 ± 0.04 pmol·mg−1 vs. control: 0.39 ± 0.05 pmol·mg−1, n = 6) and inosine (rolipram: 12.72 ± 2.64 pmol·mg−1 vs. 14.47 ± 1.48 pmol·mg−1, n = 8; DPSPX: 11.50 ± 4.84 pmol·mg−1 vs. control: 11.94 ± 3.33 pmol·mg−1, n = 6; cGMP: 8.24 ± 1.58 pmol·mg−1 vs. control: 10.67 ± 2.63 pmol·mg−1, n = 6). We also examined the effect of homocysteine thiolactone on the accumulation of adenosine and inosine during hypoxia because this compound reduced adenosine accumulation during OGD by trapping intracellular adenosine, in rat hippocampus (Lloyd et al., 1993; Frenguelli et al., 2007). However, homocysteine thiolactone had no effect on the hypoxia-evoked purine increase in the spinal cord (data not shown).
To determine the possible pathways for the transport of purines across the plasma membrane, the effects of inhibitors of several pathways were assessed. A mixture of ENT inhibitors NBTI (5 µM) and dipyridamole (10 µM) significantly increased the basal level of adenosine (NBTI + dipyridamole: 0.92 ± 0.19 pmol·mg−1 vs. control: 0.72 ± 0.18 pmol·mg−1, n = 8, P < 0.05, unpaired Student's t-test) but not that of inosine (NBTI + dipyridamole: 16.06 ± 3.56 pmol·mg−1 vs. control: 15.20 ± 2.73 pmol·mg−1, n = 8). On the other hand, ENT inhibitors significantly suppressed the hypoxia (10 min)-evoked increase in inosine but not adenosine (Figure 6). These results indicate that ENTs play important roles in regulating extracellular adenosine concentration during normoxia and in the efflux pathway of inosine during hypoxia, but not normoxia. As cAMP transporters and channels with large conductance, such as gap junction channels, P2X7 receptors and maxi-anion channels, are possible efflux pathways for adenine nucleotides (Darby et al., 2003; Dutta et al., 2004; Suadicani et al., 2006; El-Sheikh et al., 2008; Kang et al., 2008; Lin et al., 2008; Liu et al., 2008; Russel et al., 2008), the inhibitors of these transporters and channels were examined. Sulfinpyrazone (2 mM), a cAMP transporter inhibitor, carbenoxolone (100 µM), a gap junction channel inhibitor, BBG (5 µM), a P2X7 receptor antagonist and AA (20 µM), a maxi-anion channel inhibitor, had no effect on the basal levels of adenosine (sulfinpyrazone: 0.70 ± 0.06 pmol·mg−1 vs. control: 0.89 ± 0.16 pmol·mg−1, n = 6; carbenoxolone: 0.82 ± 0.31 pmol·mg−1 vs. control: 0.89 ± 0.28 pmol·mg−1, n = 6; BBG: 1.14 ± 0.63 pmol·mg−1 vs. control: 0.93 ± 0.54 pmol·mg−1, n = 6; AA: 0.28 ± 0.09 pmol·mg−1 vs. control: 0.29 ± 0.09 pmol·mg−1, n = 6) and inosine (sulfinpyrazone: 7.07 ± 1.58 pmol·mg−1 vs. control: 10.51 ± 0.90 pmol·mg−1, n = 6; carbenoxolone: 4.91 ± 0.65 pmol·mg−1 vs. control: 6.58 ± 2.17 pmol·mg−1, n = 6; BBG: 5.29 ± 1.23 pmol·mg−1 vs. 4.59 ± 1.14 pmol·mg−1, n = 6; AA: 9.92 ± 2.95 pmol·mg−1 vs. control: 10.78 ± 3.60 pmol·mg−1, n = 6) or on hypoxia (10 min)-evoked increases in these purines (Figure 6).
Figure 6.
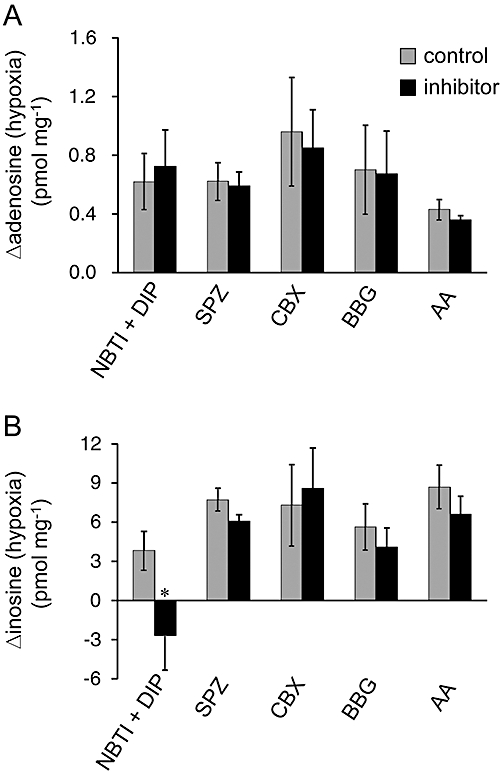
Effect of inhibitors of the purine efflux pathway. The hypoxia-evoked increment in adenosine (A) and inosine (B) was measured in the presence and absence (control) of 5 µM S-(4-nitrobenzyl)-6-thioinosine (NBTI) + 10 µM dipyridamole (DIP, n = 8), 2 mM sulfinpyrazone (SPZ, n = 6), 100 µM carbenoxolone (CBX, n = 6), 5 µM Brilliant Blue G (BBG, n = 6) and 20 µM arachidonic acid (AA, n = 6). Each column and error bar represents the mean ± SEM. *P < 0.05, significantly different from values in the absence of inhibitors (unpaired Student's t-test).
In rat-cultured cortical astrocytes, hypoxia-evoked adenosine release was suppressed in the presence of an inhibitor of glial metabolism (Martín et al., 2007). We investigated the effect of FA, which inhibits glial metabolism, on the hypoxia-evoked increase in adenosine and inosine in the rat spinal cord. Although the application of FA (5 mM) for 30 min did not affect the basal levels of adenosine and inosine, the significant increase in adenosine and inosine during hypoxia disappeared in the presence of FA (Figure 7). The increase in inosine seemed to be more sensitive to FA than that of adenosine.
Figure 7.

Effect of an inhibitor of glial metabolism on the amount of extracellular adenosine (A) and inosine (B). ACSF was changed every 10 min and samples were collected. The preparations were exposed to hypoxic ACSF for 40 min. Control preparations obtained from littermates were incubated in normal ACSF for 50 min. Fluoroacetate (FA, 5 mM) was applied to all preparations for 30 min. Each column and error bar represents the mean ± SEM. (n = 4–8). *P < 0.05 significantly different from control values at the same time point (unpaired Student's t-test).
To confirm the effect of FA, the isolated spinal cords were pre-incubated with 5 mM FA for 30 min and then exposed to hypoxia or other stimulants for 10 min in the presence of FA. The hypoxia-evoked increases in adenosine and inosine were significantly suppressed by this treatment with FA (Figure 8). OGD is reported to cause adenosine and inosine release from both astrocytes and neurons (Parkinson and Xiong, 2004). However, the exposure of OGD for 10 min increased the levels of adenosine and inosine regardless of the presence or absence of FA. We have previously reported that hypercapnia causes the release of adenosine by inhibiting the activity of adenosine kinase in the rat spinal cord (Otsuguro et al., 2006; 2009;). In the present study, we found that the extracellular level of inosine was also increased by exposure (10 min) to hypercapnia (20% CO2). ABT-702 (10 µM), an adenosine kinase inhibitor, also evoked increases in adenosine and inosine (Figure 8). After pre-incubation (30 min), FA failed to suppress these purine increases in response to hypercapnia or ABT-702.
Figure 8.

Effect of an inhibitor of glial metabolism on the adenosine (A) and inosine (B) increase caused by different stimulants. Preparations were pre-incubated with fluoroacetate (FA) for 30 min. Then, in the presence and absence (control) of FA, purine accumulation was induced by hypoxia (n = 7–8), oxygen-glucose deprivation (OGD, n = 6), hypercapnia (n = 8) or 10 µM ABT-702 (n = 7). Each column and error bar represents the mean ± SEM. *P < 0.05 significantly different from values in the absence of FA (unpaired Student's t-test).
As ABT-702 increased not only adenosine but also inosine, the effect of EHNA on the ABT-702-evoked increase in these purines was examined. EHNA (10 µM) markedly enhanced the ABT-702 (10 µM)-evoked increase of adenosine, but abolished that of inosine (Figure 9).
Figure 9.

Effect of erythro-9-(2-hydroxy-3-nonyl)adenine (EHNA) on purine accumulation by ABT-702. The ABT-702 (10 µM)-evoked increment in adenosine (A) and inosine (B) was measured in the presence and absence of 10 µM EHNA. Each column and error bar represents the mean ± SEM. *P < 0.05 significantly different from values in the absence of EHNA (unpaired Student's t-test).
Discussion and conclusions
In the present experiments, the accumulation of purines in the extracellular space during hypoxia was studied in preparations of the rat spinal cord. Our results suggest that the increase in extracellular adenosine concentration during hypoxia occurs through the release of intracellular adenosine, and that astrocytes play a key role in the release of purines from the rat spinal cord.
Extracellular ATP and cAMP can be sources of adenosine because of the activity of ecto-enzymes such as ecto-ATPase, ecto-alkaline phosphatase and ecto-PDE (Matsuoka and Ohkubo, 2004; Gödecke, 2008). However, brief exposure (<30 min) to hypoxia and OGD has been shown to release adenosine itself from rat-cultured cortical astrocytes (Martín et al., 2007) and the hippocampus (Frenguelli et al., 2007) respectively. Our data also suggest that hypoxia directly releases adenosine itself in the rat spinal cord for the following reasons. Firstly, increases in extracellular ATP and cAMP did not occur during hypoxia. Secondly, the increase in adenosine was insensitive to the inhibitors of ecto-metabolic enzymes. In cultured rat forebrain astrocytes, a 60 min exposure to OGD released adenine nucleotides into the extracellular space, where they were then converted to adenosine (Parkinson and Xiong, 2004). In the rat hippocampus, ATP release required longer exposures (>10 min) to OGD in young animals (11–15 days old) than in the older rats (Frenguelli et al., 2007). This age-related sensitivity to OGD may be one reason why ATP release was not observed in our experiments using spinal cords from neonatal rats (0–7 days old).
Our data show an inosine/adenosine ratio that is larger than the ratios previously reported in rat brain (Frenguelli et al., 2003; Parkinson and Xiong, 2004). EHNA, an adenosine deaminase inhibitor, enhanced both the basal and hypoxia-evoked adenosine increase, while it decreased the basal inosine level and abolished the evoked inosine increase. These results indicate that inosine is formed from adenosine during hypoxia through the activity of adenosine deaminase. ABT-702, an adenosine kinase inhibitor, increased not only adenosine but also inosine, to the same extent as observed with hypoxia. In addition, EHNA markedly enhanced the ABT-702-evoked increase of adenosine, while it abolished that of inosine, indicating a rapid conversion from adenosine to inosine by adenosine deaminase. In the rat hippocampus, the intracellular adenosine increase, induced by energy depletion, was mainly regulated by adenosine deaminase (Lloyd and Fredholm, 1995). In the rat spinal cord, co-administration of adenosine kinase and adenosine deaminase inhibitors evoked a marked synergic release of adenosine, suggesting that only a modest inhibition of adenosine kinase is required to recruit the involvement of adenosine deaminase (Golembiowska et al., 1995). In the rat spinal cord, it is likely that the activity of adenosine deaminase is high enough to play a key role in regulating intracellular adenosine concentration, especially when adenosine formation is increased.
The extracellular increases in adenosine and inosine during hypoxia were both temperature-dependent. In the rat hippocampus, a rise in temperature causes an increase in intracellular adenosine concentrations and, thus, adenosine efflux from tissues (Masino et al., 2001). The large increase in extracellular adenosine observed at 35°C in the present study could accelerate the formation of inosine. At 25°C, hypoxia had little effect on adenosine and inosine accumulation, implying the involvement of enzymatic processes in the release of purines and/or reduced demand for oxygen in tissues at lower temperature.
The Ca2+ concentration in CSF is approximately 1.25 mM (Hunter and Smith, 1960), which was the Ca2+ concentration of the ACSF used in the present study. The hypoxia-evoked adenosine and inosine increases were enhanced by the removal of external Ca2+ and greatly inhibited by a high concentration (2.5 mM) of Ca2+, indicating the involvement of non-exocytotic mechanisms. Similar phenomena also occur in rat hippocampal slices (Dale et al., 2000) and cultured cortical astrocytes (Martín et al., 2007). In addition, hypercapnia-evoked adenosine release is also inhibited by Ca2+ (Otsuguro et al., 2006; 2009;). These data emphasize the importance of Ca2+ in the control of purine concentration under pathological conditions. Ca2+ probably acts by inhibiting the formation and/or release of purines in the CNS including the spinal cord.
The ENT inhibitors blocked the hypoxia-evoked increase in inosine but not adenosine, suggesting that ENT is a main pathway for inosine release during hypoxia. FA, an inhibitor of glial metabolism, abolished the increase in adenosine and inosine induced by hypoxia, but did not suppress the increase in these purines by hypercapnia or ABT-702. In rat brain, fluorocitrate, another inhibitor of glial metabolism, also abolished hypoxia-evoked adenosine release (Martín et al., 2007). It would therefore seem likely that the adenosine was released from astrocytes. Alternatively, the activity of astrocytes may affect purine release from other cells such as neurons, as astrocytes are able to control neuronal function under physiological and pathological conditions (Hansson and Rönnbäck, 2003; Haydon and Carmignoto, 2006;Rossi et al., 2007). On the other hand, it has been reported that both astrocytes and neurons release adenosine during OGD (Parkinson and Xiong, 2004). In our study, unlike hypoxia, OGD increased the purine levels in the presence of FA. Therefore, OGD might recruit different mechanisms for purine release than those triggered by hypoxia. Astrocytes have also been proposed to regulate basal adenosine level (Pascual et al., 2005). However, treatment with 5 mM FA for 30 min suppressed hypoxia-evoked release, but did not affect the basal level of purines. In cultured astrocytes, fluorocitrate (1 mM, 1 h) reduced basal adenosine levels (Martín et al., 2007). On the other hand, prolonged treatment (>1 h) of high concentration (20 mM) of FA has been reported to increase adenosine release by itself (Canals et al., 2008). The effects of glial metabolic inhibitors such as FA and fluorocitrate on basal purine release appear to depend on the experimental conditions.
In conclusion, hypoxia induces the release of adenosine itself, which is negatively modulated by Ca2+. Hypoxia also releases inosine, which is mainly produced intracellularly and released through the activity of ENTs (see Figure 10). The concentration of extracellular inosine was markedly higher than that of adenosine during normoxia and hypoxia. Astrocytic function plays an important role in purine accumulation during hypoxia, but not during hypercapnia or OGD in the rat spinal cord.
Figure 10.

Schematic representation of adenosine and inosine production and release in neonatal rat spinal cord. Hypoxia increases intracellular adenosine, which is released via unknown pathways or converted to inosine by adenosine deaminase (ADA). Inosine is released via equilibrative nucleoside transporters (ENTs), which are blocked by S-(4-nitrobenzyl)-6-thioinosine (NBTI) and dipyridamole (DIP). ABT-702 inhibits adenosine kinase (AK), resulting in increase in intracellular adenosine.
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research from the Ministry of Education, Science, Sports and Culture of Japan.
Glossary
Abbreviations
- AA
arachidonic acid
- ACSF
artificial cerebrospinal fluid
- BBG
brilliant blue G
- DPSPX
1,3-dipropyl-8-(p-sulphophenyl)xanthine
- EHNA
erythro-9-(2-hydroxy-3-nonyl)adenine hydrochloride
- ENT
equilibrative nucleoside transporter
- HPLC
high performance liquid chromatography
- NBTI
S-(4-nitrobenzyl)-6-thioinosine
- OGD
oxygen-glucose deprivation
- PDE
phosphodiesterase
Conflict of interest
None.
Supporting Information
Teaching Materials; Figs 1–10 as PowerPoint slide.
References
- Alexander SPH, Mathie A, Peters JA. Guide to receptors and channels (GRAC), 4th edn. Br J Pharmacol. 2009;158:S1–S254. doi: 10.1111/j.1476-5381.2009.00499.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canals S, Larrosa B, Pintor J, Mena MA, Herreras O. Metabolic challenge to glia activates an adenosine-mediated safety mechanism that promotes neuronal survival by delaying the onset of spreading depression waves. J Cereb Blood Flow Metab. 2008;28:1835–1844. doi: 10.1038/jcbfm.2008.71. [DOI] [PubMed] [Google Scholar]
- Chen P, Goldberg DE, Kolb B, Lanser M, Benowitz LI. Inosine induces axonal rewiring and improves behavioral outcome after stroke. Proc Natl Acad Sci USA. 2002;99:9031–9036. doi: 10.1073/pnas.132076299. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheshire WP, Santos CC, Massey EW, Howard JF., Jr Spinal cord infarction: etiology and outcome. Neurology. 1996;47:321–330. doi: 10.1212/wnl.47.2.321. [DOI] [PubMed] [Google Scholar]
- Czéh G, Somjen GG. Hypoxic failure of synaptic transmission in the isolated spinal cord, and the effects of divalent cations. Brain Res. 1990;527:224–233. doi: 10.1016/0006-8993(90)91141-3. [DOI] [PubMed] [Google Scholar]
- Dale N, Pearson T, Frenguelli BG. Direct measurement of adenosine release during hypoxia in the CA1 region of the rat hippocampal slice. J Physiol. 2000;526:143–155. doi: 10.1111/j.1469-7793.2000.00143.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darby M, Kuzmiski JB, Panenka W, Feighan D, MacVicar BA. ATP released from astrocytes during swelling activates chloride channels. J Neurophysiol. 2003;89:1870–1877. doi: 10.1152/jn.00510.2002. [DOI] [PubMed] [Google Scholar]
- Dulla CG, Dobelis P, Pearson T, Frenguelli BG, Staley KJ, Masino SA. Adenosine and ATP link PCO2 to cortical excitability via pH. Neuron. 2005;48:1011–1023. doi: 10.1016/j.neuron.2005.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dutta AK, Sabirov RZ, Uramoto H, Okada Y. Role of ATP-conductive anion channel in ATP release from neonatal rat cardiomyocytes in ischaemic or hypoxic conditions. J Physiol. 2004;559:799–812. doi: 10.1113/jphysiol.2004.069245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Sheikh AA, van den Heuvel JJ, Koenderink JB, Russel FG. Effect of hypouricaemic and hyperuricaemic drugs on the renal urate efflux transporter, multidrug resistance protein 4. Br J Pharmacol. 2008;155:1066–1075. doi: 10.1038/bjp.2008.343. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraris SP, Lew H, Elsayed NM. Simultaneous determination of inosine, hypoxanthine, xanthine, and uric acid and the effect of metal chelators. Anal Biochem. 1991;195:116–121. doi: 10.1016/0003-2697(91)90305-d. [DOI] [PubMed] [Google Scholar]
- Frenguelli BG, Llaudet E, Dale N. High-resolution real-time recording with microelectrode biosensors reveals novel aspects of adenosine release during hypoxia in rat hippocampal slices. J Neurochem. 2003;86:1506–1515. doi: 10.1046/j.1471-4159.2003.01957.x. [DOI] [PubMed] [Google Scholar]
- Frenguelli BG, Wigmore G, Llaudet E, Dale N. Temporal and mechanistic dissociation of ATP and adenosine release during ischaemia in the mammalian hippocampus. J Neurochem. 2007;101:1400–1413. doi: 10.1111/j.1471-4159.2006.04425.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gödecke A. cAMP: fuel for extracellular adenosine formation? Br J Pharmacol. 2008;153:1087–1089. doi: 10.1038/bjp.2008.7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golembiowska K, White TD, Sawynok J. Modulation of adenosine release from rat spinal cord by adenosine deaminase and adenosine kinase inhibitors. Brain Res. 1995;699:315–320. doi: 10.1016/0006-8993(95)00926-h. [DOI] [PubMed] [Google Scholar]
- Golembiowska K, White TD, Sawynok J. Adenosine kinase inhibitors augment release of adenosine from spinal cord slices. Eur J Pharmacol. 1996;307:157–162. doi: 10.1016/0014-2999(96)00248-8. [DOI] [PubMed] [Google Scholar]
- Hansson E, Rönnbäck L. Glial neuronal signaling in the central nervous system. FASEB J. 2003;17:341–348. doi: 10.1096/fj.02-0429rev. [DOI] [PubMed] [Google Scholar]
- Haun SE, Segeleon JE, Trapp VL, Clotz MA, Horrocks LA. Inosine mediates the protective effect of adenosine in rat astrocyte cultures subjected to combined glucose-oxygen deprivation. J Neurochem. 1996;67:2051–2059. doi: 10.1046/j.1471-4159.1996.67052051.x. [DOI] [PubMed] [Google Scholar]
- Haydon PG, Carmignoto G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol Rev. 2006;86:1009–1031. doi: 10.1152/physrev.00049.2005. [DOI] [PubMed] [Google Scholar]
- Hunter G, Smith HV. Calcium and magnesium in human cerebrospinal fluid. Nature. 1960;186:161–162. doi: 10.1038/186161a0. [DOI] [PubMed] [Google Scholar]
- Jin SL, Richard FJ, Kuo WP, D'Ercole AJ, Conti M. Impaired growth and fertility of cAMP-specific phosphodiesterase PDE4D-deficient mice. Proc Natl Acad Sci USA. 1999;96:11998–12003. doi: 10.1073/pnas.96.21.11998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang J, Kang N, Lovatt D, Torres A, Zhao Z, Lin J, et al. Connexin 43 hemichannels are permeable to ATP. J Neurosci. 2008;28:4702–4711. doi: 10.1523/JNEUROSCI.5048-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kawamoto Y, Shinozuka K, Kunitomo M, Haginaka J. Determination of ATP and its metabolites released from rat caudal artery by isocratic ion-pair reversed-phase high-performance liquid chromatography. Anal Biochem. 1998;262:33–38. doi: 10.1006/abio.1998.2729. [DOI] [PubMed] [Google Scholar]
- Latini S, Pedata F. Adenosine in the central nervous system: release mechanisms and extracellular concentrations. J Neurochem. 2001;79:463–484. doi: 10.1046/j.1471-4159.2001.00607.x. [DOI] [PubMed] [Google Scholar]
- Lin JHC, Lou N, Kang N, Takano T, Hu F, Han X, et al. A central role of connexin 43 in hypoxic preconditioning. J Neurosci. 2008;28:681–695. doi: 10.1523/JNEUROSCI.3827-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Litsky ML, Hohl CM, Lucas JH, Jurkowitz MS. Inosine and guanosine preserve neuronal and glial cell viability in mouse spinal cord cultures during chemical hypoxia. Brain Res. 1999;821:426–432. doi: 10.1016/s0006-8993(99)01086-0. [DOI] [PubMed] [Google Scholar]
- Liu HT, Sabirov RZ, Okada Y. Oxygen-glucose deprivation induces ATP release via maxi-anion channels in astrocytes. Purinergic Signal. 2008;4:147–154. doi: 10.1007/s11302-007-9077-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lloyd HGE, Fredholm BB. Involvement of adenosine deaminase and adenosine kinase in regulating extracellular adenosine concentration in rat hippocampal slices. Neurochem Int. 1995;26:387–395. doi: 10.1016/0197-0186(94)00144-j. [DOI] [PubMed] [Google Scholar]
- Lloyd HGE, Spence I, Johnston GAR. Involvement of adenosine in synaptic depression induced by a brief period of hypoxia in isolated spinal cord of neonatal rat. Brain Res. 1988;462:391–395. doi: 10.1016/0006-8993(88)90571-9. [DOI] [PubMed] [Google Scholar]
- Lloyd HGE, Perkins A, Spence I. Effect of magnesium on depression of the monosynaptic reflex induced by 2-chloroadenosine or hypoxia in the isolated spinal cord of neonatal rats. Neurosci Lett. 1989;101:175–181. doi: 10.1016/0304-3940(89)90526-0. [DOI] [PubMed] [Google Scholar]
- Lloyd HGE, Lindström K, Fredholm BB. Intracellular formation and release of adenosine from rat hippocampal slices evoked by electrical stimulation or energy depletion. Neurochem Int. 1993;23:173–185. doi: 10.1016/0197-0186(93)90095-m. [DOI] [PubMed] [Google Scholar]
- Martín ED, Fernández M, Perea G, Pascual O, Haydon PG, Araque A, et al. Adenosine released by astrocytes contributes to hypoxia-induced modulation of synaptic transmission. Glia. 2007;55:36–45. doi: 10.1002/glia.20431. [DOI] [PubMed] [Google Scholar]
- Masino SA, Latini S, Bordoni F, Pedata F, Dunwiddie TV. Changes in hippocampal adenosine efflux, ATP levels, and synaptic transmission induced by increased temperature. Synapse. 2001;41:58–64. doi: 10.1002/syn.1060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka I, Ohkubo S. ATP- and adenosine-mediated signaling in the central nervous system: adenosine receptor activation by ATP through rapid and localized generation of adenosine by ecto-nucleotidases. J Pharmacol Sci. 2004;94:95–99. doi: 10.1254/jphs.94.95. [DOI] [PubMed] [Google Scholar]
- Nikulina E, Tidwell JL, Dai HN, Bregman BS, Filbin MT. The phosphodiesterase inhibitor rolipram delivered after a spinal cord lesion promotes axonal regeneration and functional recovery. Proc Natl Acad Sci USA. 2004;101:8786–8790. doi: 10.1073/pnas.0402595101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuguro K, Yamaji Y, Ban M, Ohta T, Ito S. Involvement of adenosine in depression of synaptic transmission during hypercapnia in isolated spinal cord of neonatal rats. J Physiol. 2006;574:835–847. doi: 10.1113/jphysiol.2006.109660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Otsuguro K, Ban M, Ohta T, Ito S. Roles of purines in synaptic modulation evoked by hypercapnia in isolated spinal cord of neonatal rat in vitro. Br J Pharmacol. 2009;156:1167–1177. doi: 10.1111/j.1476-5381.2009.00118.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park YK, Jung SJ, Kwak J, Kim J. Effect of hypoxia on excitatory transmission in the rat substantia gelatinosa neurons. Biochem Biophys Res Commun. 2002;295:929–936. doi: 10.1016/s0006-291x(02)00790-8. [DOI] [PubMed] [Google Scholar]
- Parkinson FE, Xiong W. Stimulus- and cell-type-specific release of purines in cultured rat forebrain astrocytes and neurons. J Neurochem. 2004;88:1305–1312. doi: 10.1046/j.1471-4159.2003.02266.x. [DOI] [PubMed] [Google Scholar]
- Pascual O, Casper KB, Kubera C, Zhang J, Revilla-Sanchez R, Sul JY, et al. Astrocytic purinergic signaling coordinates synaptic networks. Science. 2005;310:113–116. doi: 10.1126/science.1116916. [DOI] [PubMed] [Google Scholar]
- Pearson T, Currie AJ, Etherington LAV, Gadalla AE, Damian K, Llaudet E, et al. Plasticity of purine release during cerebral ischemia: clinical implications? J Cell Mol Med. 2003;7:362–375. doi: 10.1111/j.1582-4934.2003.tb00239.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pedata F, Melani A, Pugliese AM, Coppi E, Cipriani S, Traini C. The role of ATP and adenosine in the brain under normoxic and ischemic conditions. Purinergic Signal. 2007;3:299–310. doi: 10.1007/s11302-007-9085-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossi DJ, Brady JD, Mohr C. Astrocyte metabolism and signaling during brain ischemia. Nat Neurosci. 2007;10:1377–1386. doi: 10.1038/nn2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rowland JW, Hawryluk GWJ, Kwon B, Fehlings MG. Current status of acute spinal cord injury pathophysiology and emerging therapies: promise on the horizon. Neurosurg Focus. 2008;25:E2. doi: 10.3171/FOC.2008.25.11.E2. [DOI] [PubMed] [Google Scholar]
- Russel FG, Koenderink JB, Masereeuw R. Multidrug resistance protein 4 (MRP4/ABCC4): a versatile efflux transporter for drugs and signalling molecules. Trends Pharmacol Sci. 2008;29:200–207. doi: 10.1016/j.tips.2008.01.006. [DOI] [PubMed] [Google Scholar]
- Shen H, Chen GJ, Harvey BK, Bickford PC, Wang Y. Inosine reduces ischemic brain injury in rats. Stroke. 2007;36:654–659. doi: 10.1161/01.STR.0000155747.15679.04. [DOI] [PubMed] [Google Scholar]
- Suadicani SO, Brosnan CF, Scemes E. P2X7 receptors mediate ATP release and amplification of astrocytic intercellular Ca2+ signaling. J Neurosci. 2006;26:1378–1385. doi: 10.1523/JNEUROSCI.3902-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tator CH, Koyanagi I. Vascular mechanisms in the pathophysiology of human spinal cord injury. J Neurosurg. 1997;86:483–492. doi: 10.3171/jns.1997.86.3.0483. [DOI] [PubMed] [Google Scholar]
- Waradas J. Neuroprotective role of adenosine in the CNS. Pol J Pharmacol. 2002;54:313–326. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


