Abstract
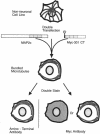
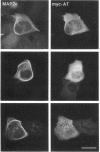
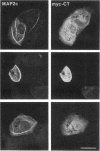
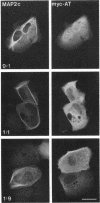
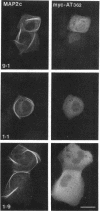
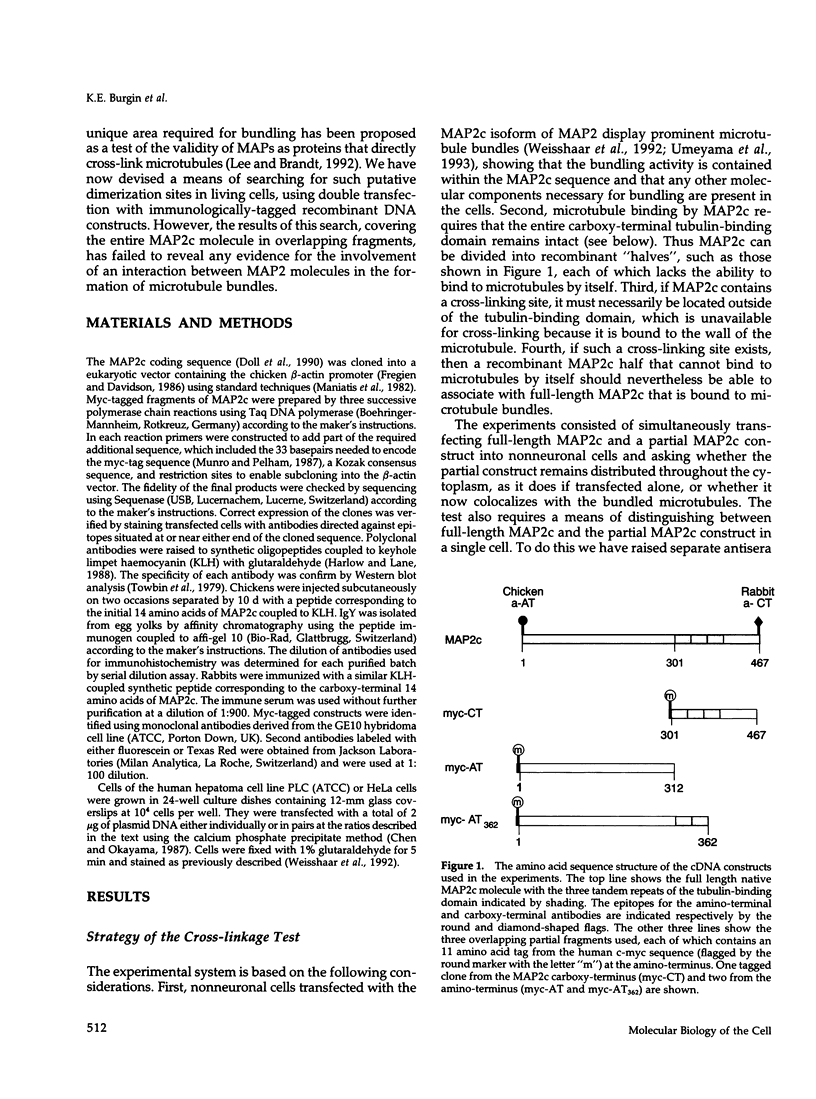
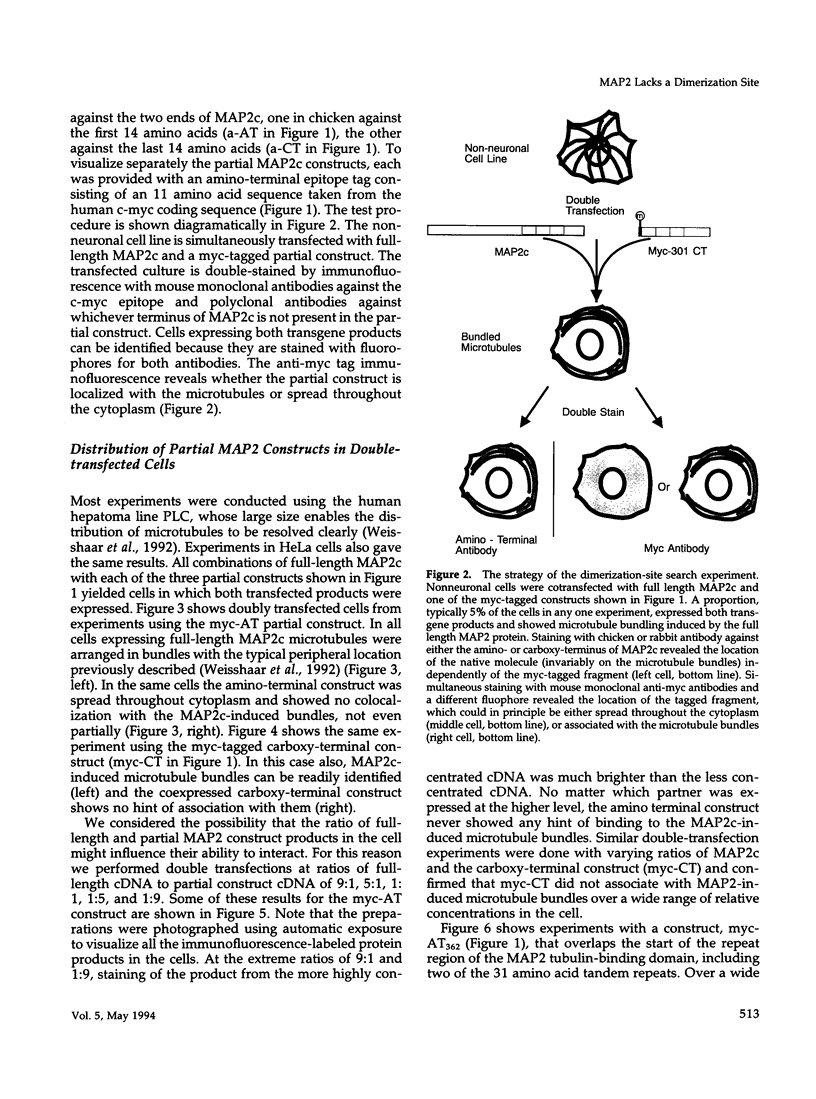
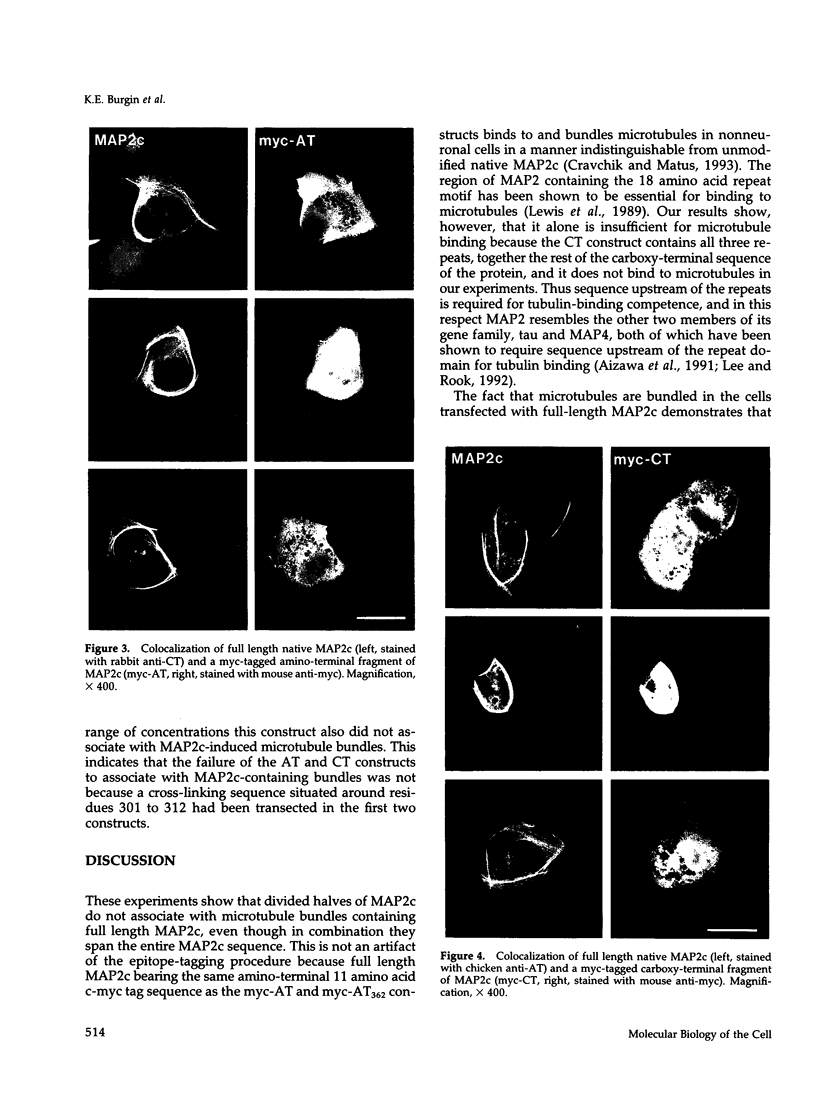
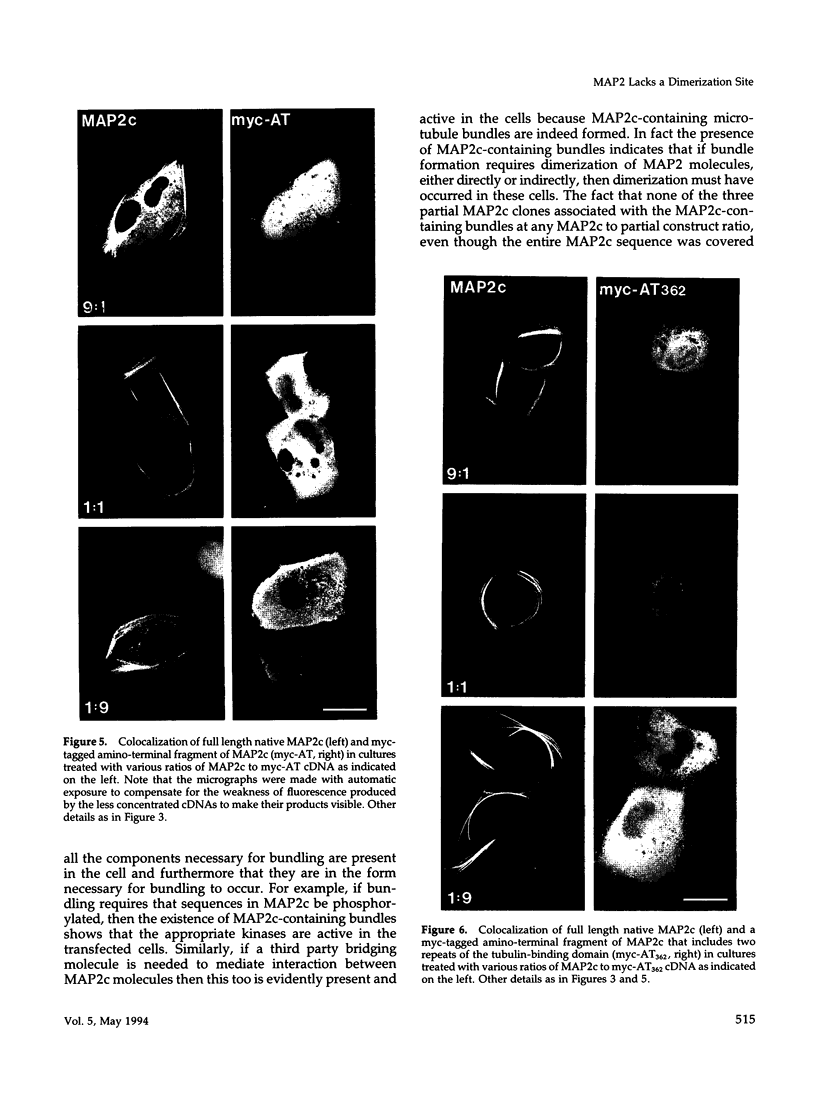
We have searched for putative dimerization sites in microtubule-associated protein 2 (MAP2) that may be involved in the bundling of microtubules. An overlapping series of fragments of the embryonic form MAP2c were created and immunologically "tagged" with an 11 amino acid sequence from human c-myc. Nonneuronal cells were transfected simultaneously with one of these myc-tagged fragments and with full-length native MAP2c. Immunolabeling with site-specific antibodies allowed the two transgene products to be located independently within the cytoplasm of a single double-transfected cell. All transfected cells contained bundled microtubules to which the full-length native MAP2 was bound. The distribution of the tagged MAP2 fragment relative to these MAP2-induced bundles was determined by the anti-myc staining. None of the fragments tested, representing all of the MAP2c sequence in overlapping pieces, were associated with MAP2-induced microtubule bundles. These results suggest that MAP2-induced bundle formation in cells does not involve an autonomous dimerization site within the MAP2 sequence.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Aizawa H., Emori Y., Mori A., Murofushi H., Sakai H., Suzuki K. Functional analyses of the domain structure of microtubule-associated protein-4 (MAP-U). J Biol Chem. 1991 May 25;266(15):9841–9846. [PubMed] [Google Scholar]
- Brown P. A., Berlin R. D. Packing volume of sedimented microtubules: regulation and potential relationship to an intracellular matrix. J Cell Biol. 1985 Oct;101(4):1492–1500. doi: 10.1083/jcb.101.4.1492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cassimeris L., Pryer N. K., Salmon E. D. Real-time observations of microtubule dynamic instability in living cells. J Cell Biol. 1988 Dec;107(6 Pt 1):2223–2231. doi: 10.1083/jcb.107.6.2223. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapin S. J., Bulinski J. C., Gundersen G. G. Microtubule bundling in cells. Nature. 1991 Jan 3;349(6304):24–24. doi: 10.1038/349024a0. [DOI] [PubMed] [Google Scholar]
- Chen C., Okayama H. High-efficiency transformation of mammalian cells by plasmid DNA. Mol Cell Biol. 1987 Aug;7(8):2745–2752. doi: 10.1128/mcb.7.8.2745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen J., Kanai Y., Cowan N. J., Hirokawa N. Projection domains of MAP2 and tau determine spacings between microtubules in dendrites and axons. Nature. 1992 Dec 17;360(6405):674–677. doi: 10.1038/360674a0. [DOI] [PubMed] [Google Scholar]
- Cravchik A., Matus A. A novel strategy for the immunological tagging of cDNA constructs. Gene. 1993 Dec 27;137(1):139–143. doi: 10.1016/0378-1119(93)90262-2. [DOI] [PubMed] [Google Scholar]
- Doll T., Papandrikopoulou A., Matus A. Nucleotide and amino acid sequences of embryonic rat MAP2c. Nucleic Acids Res. 1990 Jan 25;18(2):361–361. doi: 10.1093/nar/18.2.361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edson K., Weisshaar B., Matus A. Actin depolymerisation induces process formation on MAP2-transfected non-neuronal cells. Development. 1993 Feb;117(2):689–700. doi: 10.1242/dev.117.2.689. [DOI] [PubMed] [Google Scholar]
- Fregien N., Davidson N. Activating elements in the promoter region of the chicken beta-actin gene. Gene. 1986;48(1):1–11. doi: 10.1016/0378-1119(86)90346-x. [DOI] [PubMed] [Google Scholar]
- Fridén B., Nordh J., Wallin M., Deinum J., Nordén B. Effects of proteolysis of the extending parts of the high-molecular-weight microtubule-associated proteins on interactions between microtubules. Biochim Biophys Acta. 1988 Jul 20;955(2):135–142. doi: 10.1016/0167-4838(88)90187-2. [DOI] [PubMed] [Google Scholar]
- Hernández M. A., Avila J., Andreu J. M. Physicochemical characterization of the heat-stable microtubule-associated protein MAP2. Eur J Biochem. 1986 Jan 2;154(1):41–48. doi: 10.1111/j.1432-1033.1986.tb09356.x. [DOI] [PubMed] [Google Scholar]
- Hirokawa N., Shiomura Y., Okabe S. Tau proteins: the molecular structure and mode of binding on microtubules. J Cell Biol. 1988 Oct;107(4):1449–1459. doi: 10.1083/jcb.107.4.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanai Y., Takemura R., Oshima T., Mori H., Ihara Y., Yanagisawa M., Masaki T., Hirokawa N. Expression of multiple tau isoforms and microtubule bundle formation in fibroblasts transfected with a single tau cDNA. J Cell Biol. 1989 Sep;109(3):1173–1184. doi: 10.1083/jcb.109.3.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim H., Binder L. I., Rosenbaum J. L. The periodic association of MAP2 with brain microtubules in vitro. J Cell Biol. 1979 Feb;80(2):266–276. doi: 10.1083/jcb.80.2.266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kindler S., Schulz B., Goedert M., Garner C. C. Molecular structure of microtubule-associated protein 2b and 2c from rat brain. J Biol Chem. 1990 Nov 15;265(32):19679–19684. [PubMed] [Google Scholar]
- Kumagai H., Sakai H. A porcine brain protein (35 K protein) which bundles microtubules and its identification as glyceraldehyde 3-phosphate dehydrogenase. J Biochem. 1983 May;93(5):1259–1269. doi: 10.1093/oxfordjournals.jbchem.a134260. [DOI] [PubMed] [Google Scholar]
- Lee G., Brandt R. Microtubule-bundling studies revisited: is there a role for MAPs? Trends Cell Biol. 1992 Oct;2(10):286–289. doi: 10.1016/0962-8924(92)90106-w. [DOI] [PubMed] [Google Scholar]
- Lee G., Rook S. L. Expression of tau protein in non-neuronal cells: microtubule binding and stabilization. J Cell Sci. 1992 Jun;102(Pt 2):227–237. doi: 10.1242/jcs.102.2.227. [DOI] [PubMed] [Google Scholar]
- Lewis S. A., Ivanov I. E., Lee G. H., Cowan N. J. Organization of microtubules in dendrites and axons is determined by a short hydrophobic zipper in microtubule-associated proteins MAP2 and tau. Nature. 1989 Nov 30;342(6249):498–505. doi: 10.1038/342498a0. [DOI] [PubMed] [Google Scholar]
- Matus A. Microtubule-associated proteins and neuronal morphogenesis. J Cell Sci Suppl. 1991;15:61–67. doi: 10.1242/jcs.1991.supplement_15.9. [DOI] [PubMed] [Google Scholar]
- Munro S., Pelham H. R. A C-terminal signal prevents secretion of luminal ER proteins. Cell. 1987 Mar 13;48(5):899–907. doi: 10.1016/0092-8674(87)90086-9. [DOI] [PubMed] [Google Scholar]
- Papandrikopoulou A., Doll T., Tucker R. P., Garner C. C., Matus A. Embryonic MAP2 lacks the cross-linking sidearm sequences and dendritic targeting signal of adult MAP2. Nature. 1989 Aug 24;340(6235):650–652. doi: 10.1038/340650a0. [DOI] [PubMed] [Google Scholar]
- Sammak P. J., Borisy G. G. Direct observation of microtubule dynamics in living cells. Nature. 1988 Apr 21;332(6166):724–726. doi: 10.1038/332724a0. [DOI] [PubMed] [Google Scholar]
- Sandoval I. V., MacDonald E., Jameson J. L., Cuatrecasas P. Role of nucleotides in tubulin polymerization: effect of guanylyl 5'-methylenediphosphonate. Proc Natl Acad Sci U S A. 1977 Nov;74(11):4881–4885. doi: 10.1073/pnas.74.11.4881. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sato M., Schwartz W. H., Selden S. C., Pollard T. D. Mechanical properties of brain tubulin and microtubules. J Cell Biol. 1988 Apr;106(4):1205–1211. doi: 10.1083/jcb.106.4.1205. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiff P. B., Horwitz S. B. Taxol stabilizes microtubules in mouse fibroblast cells. Proc Natl Acad Sci U S A. 1980 Mar;77(3):1561–1565. doi: 10.1073/pnas.77.3.1561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schulze E., Kirschner M. Dynamic and stable populations of microtubules in cells. J Cell Biol. 1987 Feb;104(2):277–288. doi: 10.1083/jcb.104.2.277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H., Staehelin T., Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci U S A. 1979 Sep;76(9):4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Umeyama T., Okabe S., Kanai Y., Hirokawa N. Dynamics of microtubules bundled by microtubule associated protein 2C (MAP2C). J Cell Biol. 1993 Jan;120(2):451–465. doi: 10.1083/jcb.120.2.451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weisshaar B., Doll T., Matus A. Reorganisation of the microtubular cytoskeleton by embryonic microtubule-associated protein 2 (MAP2c). Development. 1992 Dec;116(4):1151–1161. doi: 10.1242/dev.116.4.1151. [DOI] [PubMed] [Google Scholar]
- Wille H., Mandelkow E. M., Dingus J., Vallee R. B., Binder L. I., Mandelkow E. Domain structure and antiparallel dimers of microtubule-associated protein 2 (MAP2). J Struct Biol. 1992 Jan-Feb;108(1):49–61. doi: 10.1016/1047-8477(92)90006-v. [DOI] [PubMed] [Google Scholar]